SUMMARY
Potyviral helper component‐proteinase (HC‐Pro) is a multifunctional protein involved in plant–virus interactions. In this study, we constructed a Carica papaya L. plant cDNA library to investigate the host factors interacting with Papaya ringspot virus (PRSV) HC‐Pro using a Sos recruitment two‐hybrid system (SRS). We confirmed that the full‐length papaya calreticulin, designated PaCRT (GenBank accession no. FJ913889), interacts specifically with PRSV HC‐Pro in yeast, in vitro and in plant cells using SRS, in vitro protein‐binding assay and bimolecular fluorescent complementation assay, respectively. SRS analysis of the interaction between three PaCRT deletion mutants and PRSV HC‐Pro demonstrated that the C‐domain (residues 307–422), with a high Ca2+‐binding capacity, was responsible for binding to PRSV HC‐Pro. In addition, quantitative real‐time reverse transcriptase‐polymerase chain reaction assay showed that the expression of PaCRT mRNA was significantly upregulated in the primary stage of PRSV infection, and decreased to near‐basal expression levels in noninoculated (healthy) papaya plants with virus accumulation inside host cells. PaCRT is a new calcium‐binding protein that interacts with potyviral HC‐Pro. It is proposed that the upregulated expression of PaCRT mRNA may be an early defence‐related response to PRSV infection in the host plant, and that interaction between PRSV HC‐Pro and PaCRT may be involved in plant calcium signalling pathways which could interfere with virus infection or host defence.
INTRODUCTION
The multiple infection events of positive‐strand RNA viruses are dependent on many host factors (Ahlquist et al., 2003), and these factors are involved in host–virus interactions, viral pathogenicity, evolution and host range (Nagy, 2008; Whitham and Wang, 2004). Increasing evidence has indicated that numerous virus‐ and host‐coded protein interactions play crucial roles in the virus infection process (Nagy, 2008; Scholthof, 2005; Stange, 2006; Whitham, et al., 2006). Unravelling these protein interactions will provide insights into the molecular mechanisms of virus infection and host defence. Potyviral helper component‐proteinase (HC‐Pro) is a multifunctional protein involved in aphid transmission, cell‐to‐cell and systemic movement, polyprotein processing, genome replication, synergism and symptom expression, and suppression of post‐transcriptional gene silencing (PTGS) (Maia et al., 1996; Plisson et al., 2003; Urcuqui‐Inchima et al., 2001). Its multiple functions imply that HC‐Pro may be involved in multiple interactions with host factors in the viral cycle. Moreover, HC‐Pro domains possibly involved in host specificity and virus replication have been found to be less conserved in different potyviruses (Mangrauthia et al., 2008; Saenz et al., 2002), suggesting that host proteins interacting with HC‐Pro may vary with different host plants and potyviruses. To date, a limited number of host proteins, such as calmodulin (CaM)‐related protein (Anandalakshmi et al., 2000), RING finger protein HIP1(Guo et al., 2003), chloroplast division‐related factor NtMinD (Jin et al., 2007a), chloroplast precursor of ferredoxin‐5 (Cheng et al., 2008), 20S proteasome (Ballut et al., 2005) and its three subunits (Jin et al., 2007b), have been reported to interact with HC‐Pro from Tobacco etch virus (TEV), Potato virus A (PVA), Potato virus Y (PVY), Sugar cane mosaic virus (SCMV), Lettuce mosaic virus (LMV) and PVY, respectively. However, most Potyvirus (as the second largest virus genus; Fauquet et al., 2005) host proteins that interact with HC‐Pro, including those of Papaya ringspot virus (PRSV) (genus Potyvirus, family Potyviridae), have not been ascertained.
PRSV is considered to be the most destructive virus occurring in almost all papaya plantation areas of the world (Gonsalves, 1998). There are two types of PRSV: type P, which infects papaya and cucurbits, and type W, which infects cucurbits but not papaya (Roy et al., 1999). Previous studies on PRSV have focused mainly on the molecular characterization of the viral genome and transgenic papaya resistance to PRSV (Tripathi et al., 2008), whereas the host factors involved in PRSV infection have not been reported to date. The yeast two‐hybrid system (YTHS) is the most commonly used method for large‐scale, high‐throughput identification of potential protein–protein interactions (Suter et al., 2008). As mentioned above, host proteins interacting with potyviral HC‐Pro have been identified by YTHS based on transcription activation in the yeast nucleus. However, PRSV HC‐Pro is localized in cytoplasmic amorphous inclusions induced by PRSV (Riedel et al., 1998), and some protein–protein interactions are dependent on post‐translational modifications (Bracha‐Drori et al., 2004; Fang and Kerppola, 2004; Kwon et al., 2006; Ohad et al., 2007). Therefore, a Sos recruitment two‐hybrid system (SRS) detecting protein interactions in yeast cytoplasm was used to screen host proteins interacting with PRSV HC‐Pro from a papaya plant cDNA library in this study. Compared with the conventional YTHS based on transcription activation in the yeast nucleus, SRS uses a temperature‐sensitive cdc25H mutant strain of Saccharomyces cerevisiae, which can grow at the permissive temperature (25°C), but not at the nonpermissive temperature (37°C); when the two proteins interact, the human Sos (hSos) protein (human homologue of yeast cdc25) is recruited to the yeast cell membrane, thereby activating the Ras signalling pathway and complementing the temperature‐sensitive defect of the yeast mutant strain cdc25H to grow at 37°C (Broder et al.,1998). Finally, we identified three different host protein genes from the papaya cDNA library, and it was confirmed that a papaya calreticulin (CRT) protein (PaCRT) could interact specifically with the PRSV HC‐Pro in yeast, in vitro and in living plant cells via SRS, in vitro binding assay and bimolecular fluorescent complementation (BiFC) assay, respectively. The PaCRT C‐domain, with a high calcium‐binding capacity, was identified as a necessary domain for interaction with PRSV HC‐Pro. Furthermore, quantitative real‐time reverse transcriptase‐polymerase chain reaction (qRT‐PCR) assay showed that the expression of PaCRT mRNA was significantly upregulated in the primary stage of PRSV infection relative to that of noninoculated (healthy) papaya plants, and then decreased to near‐basal expression levels in healthy papaya plants with virus accumulation inside host cells. As plant CRT may affect cellular calcium homeostasis and signalling (Jia et al., 2009; Persson et al., 2001; Wyatt et al., 2002), we propose that the interaction between PRSV HC‐Pro and PaCRT may be involved in plant calcium signalling pathways which could interfere with virus infection or host defence.
RESULTS
Isolation of the host factor PaCRT that interacts with PRSV HC‐Pro via SRS
To identify host proteins that interact with PRSV HC‐Pro, pSos‐HC was used as a bait vector to screen the Carica papaya L. plant cDNA expression library fused to the myristoylation signal. First, the yeast strain cdc25H cells were cotransformed with bait vector pSos‐HC and the empty library vector pMyr. The result showed that the cotransformant failed to grow on synthetic galactose minimal medium without leucine and uracil [(SD)/galactose (−UL)] at a nonpermissive temperature (37°C), indicating that PRSV HC‐Pro fused to hSos by itself was unable to activate the Ras pathway to complement the temperature‐sensitive mutant of the yeast strain cdc25H. Moreover, cells cotransformed with positive control vector pMyr sos‐binding protein (SB) encoding a hSos‐interacting protein and pSos‐HC plasmid grew on SD/galactose (−UL) medium at 37°C. This result demonstrated that PRSV HC‐Pro fused to hSos was stable and therefore suitable for use as bait in SRS screen.
Using PRSV HC‐Pro as bait, about 1.86 × 106 independent cDNA library clones were screened as described in Experimental procedures. Four positive candidate clones were identified by two rounds of patching cell tests for galactose‐dependent growth on SD/galactose (−UL) and SD/glucose (−UL) plates at 25 or 37°C. Then, the cDNA plasmids of these four colonies were rescued and the specificity of the interaction was verified by retransformation into yeast strain cdc25H cells in combination with the bait pSos‐HC or control plasmids. Sequence analysis revealed that the cDNA inserts of the four candidate plasmids encoded three different proteins. Two did not encode proteins of known function; the other two clones shared an identical sequence coding for a 157‐amino‐acid protein fragment with high sequence similarity to the C‐terminus of plant CRT, and further analysis was performed.
The full‐length CRT cDNA of papaya, designated PaCRT (GenBank accession no. FJ913889), was obtained by the 5′ rapid amplification of cDNA ends (5′‐RACE) method. PaCRT cDNA is 1637 bp in length, including a 68‐bp 5′ untranslated region (5′‐UTR), a 300‐bp 3′‐UTR and a 1269‐bp open reading frame (ORF) encoding 422 deduced amino acid residues. Sequence alignment showed that the amino acid sequence of PaCRT has a high homology (71%–81% sequence identity) with plant CRT1/2 isoforms from Arabidopsis thaliana, Oryza sativa, Nicotiana tabacum and Triticum aestivum. Furthermore, some conserved regions, zonal characteristics and functional domains of CRT in plant and mammalian species (Jia et al., 2009; Persson et al., 2003) have also been found in the deduced amino acid of PaCRT. As shown in Fig. 1, PaCRT consists of three domain structures typical of CRTs: residues 1–24 of PaCRT comprise a hydrophobic signal sequence, residues 25–209 a globular N‐domain, residues 210–306 a proline‐rich central domain (P‐domain) and residues 307–422 a polyacidic C‐domain terminating with the amino acid motif HDEL, an endoplasmic reticulum (ER) retention sequence.
Figure 1.

Amino acid sequence alignment and domains of Carica papaya calreticulin (PaCRT). Sequence alignment of CRT proteins from Carica papaya (Pa; database accession number ACQ91203), Arabidopsis thaliana (At; CRT1: AAC49695; CRT2: NP172392; CRT3: AAC49697), Oryza sativa (Os; OsCRT1/2: BAA88900; CRT3: BAC06263), Nicotiana tabacum (Nt; CAA59694) and Triticum aestivum (Ta; EF452301) was performed using the sequence manipulation suite (Paul Stothard, University of Alberta, Edmonton, AB, Canada). Residues that were identical in all of these proteins are highlighted on a black background. The arrows indicate the approximate positions of the three domains (N, P and C). The predicted signal peptide, conserved CRT family motifs (1, KHEQKLDCGGGYVKLL; 2, IMFGPDICG) and triplicate repeats (A, PXXIXDPXX KKPEXWDD; B, GXWXAXXIXNPXYK) are underlined (Michalak et al. 1999). The putative nuclear targeting sequence (PPKKIKDPE) is marked with a bold underline. The endoplasmic reticulum retention sequence HDEL is indicated at the C‐terminus.
PRSV HC‐Pro interacts with full‐length PaCRT in yeast
To determine whether the full‐length PaCRT interacts with PRSV HC‐Pro in yeast, the full‐length coding sequence of PaCRT was cloned into the pMyr vector and cotransformed into yeast strain cdc25H with pSos‐HC plasmid; the plasmid combinations pSos‐MAFB/pMyr‐LaminC, pSos‐HC/pMyr and pSos/pMyr‐PaCRT served as negative controls, and pSos‐MAFB/pMyr‐MAFB as a positive control. Only transformants with pSos‐HC/pMyr‐PaCRT and the positive control were able to grow on SD/galactose (−UL) agar plates [but not on SD/glucose (−UL) plates] at 37°C (Fig. 2). The results obtained from these five independent complementation experiments indicate that the specific interaction between full‐length PaCRT and PRSV HC‐Pro occurs in the cytoplasm of the yeast cells.
Figure 2.
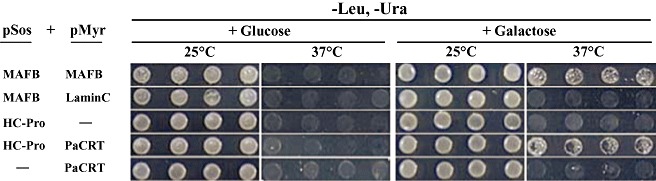
Detection of the interaction between Papaya ringspot virus helper component‐proteinase (PRSV HC‐Pro) and Carica papaya calreticulin (PaCRT) by the Sos recruitment assay. Saccharomyces cerevisiae strain cdc25H was transformed with the indicated plasmid combinations. Four colonies from each transformant were picked up, resuspended and diluted to optical densities at 600 nm of 0.5 in sterile water. An aliquot of 2.5 µL of each dilution was patched in rows onto each of two synthetic glucose minimal medium without leucine and uracil [SD/glucose (−UL)] and two synthetic galactose minimal medium without leucine and uracil [SD/galactose (−UL)] plates, and one of each type of plate was incubated at the permissive temperature or nonpermissive temperature (25 or 37°C) for 5 days to compare the growth of yeast. pMyr‐MAFB and pSos‐MAFB were used as positive controls, and pMyr‐Lamin C and pSos‐MAFB were used as negative controls.
PRSV HC‐Pro interacts with PaCRT in vitro and in planta
To confirm the two‐hybrid results above, an in vitro protein‐binding assay was performed. PRSV HC‐Pro was expressed and purified as a glutathione S‐transferase (GST)‐tag fusion protein (GST‐PRSV HC‐Pro) from Escherichia coli cells. Subsequently, the biotinylated PaCRT was synthesized via the TNT Quick Coupled Transcription/Translation System and mixed with GST‐PRSV HC‐Pro, which was immobilized on MagneGST particles. The nonspecifically bound proteins were washed away, whereas the MagneGST or MagneGST‐PRSV HC‐Pro particle‐bound proteins were captured through protein–protein interactions and then eluted. Finally, in vitro interaction of GST‐PRSV HC‐Pro (78.0 kDa) and biotinylated PaCRT (48.2 kDa) was visualized in lane 5 by Western blotting analysis with streptavidin–alkaline phosphatase (streptavidin–AP) (Fig. 3A) or anti‐GST antibody (Fig. 3B), whereas the control interaction between GST (26.0 kDa) and biotinylated PaCRT was not detected in lane 4 (Fig. 3A,B). Collectively, these results suggest that the interaction between PRSV HC‐Pro and PaCRT is specific and not a result of nonspecific interactions with other cellular proteins in vitro.
Figure 3.
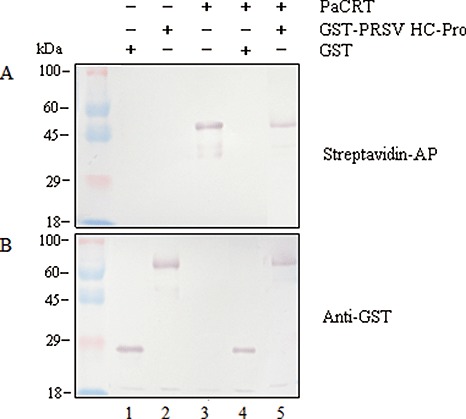
In vitro protein‐binding assay of Papaya ringspot virus helper component‐proteinase (PRSV HC‐Pro) and Carica papaya calreticulin (PaCRT). In vitro translated biotinylated PaCRT was incubated with purified glutathione S‐transferase (GST) or GST‐PRSV HC‐Pro, which was immobilized on MagneGST particles. After incubation, the proteins interacting with GST or GST‐PRSV HC‐Pro were bound to the MagneGST particles. The eluted GST‐bound proteins (lane 4) or GST‐PRSV HC‐Pro‐bound proteins (lane 5) were then analysed by 10% sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) and probed with streptavidin‐alkaline phosphatase (streptavidin‐AP) (A) or anti‐GST antibody (B) (GST, 26.0 kDa; GST‐PRSV HC‐Pro, 78.0 kDa; biotinylated PaCRT, 48.2 kDa). The purified GST (lane 1), purified GST‐PRSV HC‐Pro (lane 2) and in vitro translated biotinylated PaCRT (lane 3) were used as controls, respectively. The prestained dual colour protein size marker (Tiangen) is indicated on the left of the panel.
To further characterize the interaction of PRSV HC‐Pro with PaCRT, BiFC assay was carried out in living plant cells. The BiFC transformation vectors pSAT1‐cEYFP‐C1‐PaCRT (Fig. 4A) and pSAT1‐cEYFP‐N1‐PaCRT (Fig. 4B), expressing PaCRT fused to the C‐ or N‐terminus of the cEYFP fragment (C‐terminal yellow fluorescent protein variant derived from enhanced green fluorescent protein, cEYFP), and pSAT1‐nEYFP‐C1‐HC (Fig. 4A) or pSAT1‐nEYFP‐N1‐HC (Fig. 4B), expressing HC‐Pro fused to the C‐ or N‐terminus of the nEYFP fragment (N‐terminal EYFP, nEYFP), were constructed. The two combinations of transient expression vectors (pSAT1‐cEYFP‐C1‐PaCRT/pSAT1‐nEYFP‐C1‐HC, pSAT1‐cEYFP‐N1–PaCRT/pSAT1‐nEYFP‐N1‐HC) were individually cobombarded into onion tissues, whereas the plasmid pairs pSAT1‐nEYFP‐C1/pSAT1‐cEYFP‐C1(B), pSAT1‐nEYFP‐C1‐HC/pSAT1‐cEYFP‐C1(B), pSAT1‐nEYFP‐C1/pSAT1‐cEYFP‐C1‐PaCRT, pSAT1‐nEYFP‐N1/pSAT1‐cEYFP‐N1, pSAT1‐nEYFP‐N1‐HC/pSAT1‐cEYFP‐N1 and pSAT1‐nEYFP‐N1 /pSAT1‐cEYFP‐N1‐PaCRT were used as negative controls. After 18 h, EYFP fluorescence was observed in the onion epidermal cells bombarded with pSAT1‐cEYFP‐C1–PaCRT/pSAT1‐nEYFP‐C1‐HC or pSAT1‐cEYFP‐N1‐PaCRT/pSAT1‐nEYFP‐N1‐HC by epifluorescence microscopy (Fig. 4C,D), whereas no signal was detected in the negative controls (data not shown). These results provide additional evidence confirming the interaction of PRSV HC‐Pro with PaCRT in living plant cells.
Figure 4.
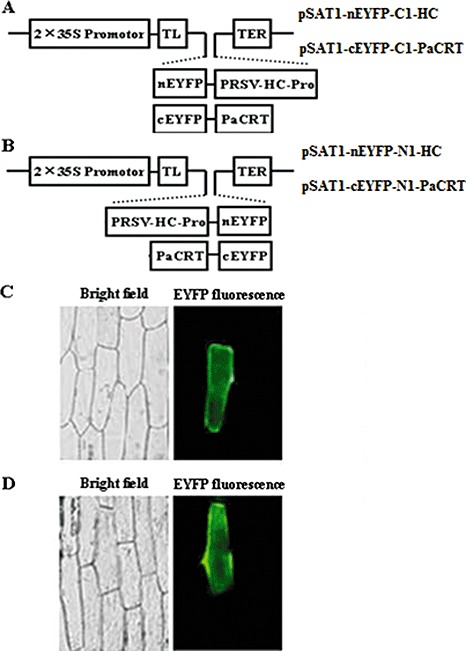
Interaction between Papaya ringspot virus helper component‐proteinase (PRSV HC‐Pro) and Carica papaya calreticulin (PaCRT) by bimolecular fluorescent complementation (BiFC) assay in living plant cells. (A) Schematic structure of pSAT1‐nEYFP‐C1‐HC and pSAT1‐cEYFP‐C1‐PaCRT constructs used for BiFC assay. (B) Schematic structure of pSAT1‐nEYFP‐N1‐HC and pSAT1‐cEYFP‐N1‐PaCRT constructs used for BiFC assay; 2 × 35S, tandem cauliflower mosaic virus (CaMV) 35S promoter; TL, Tobacco etch virus (TEV) translation leader; TER, CaMV 35S poly(A) transcriptional terminator; nEYFP and cEYFP, N‐terminal and C‐terminal fragments of yellow fluorescent protein variant derived from enhanced green fluorescent protein (EYFP); C1 and N1 vectors produce fusion to the C‐ and N‐termini of cEYFP and nEYFP, respectively. (C) The reconstructed YFP signal detected in onion (Allium cepa) epidermal cells by epifluorescence microscopy after bombardment with (A) constructs. (D) The reconstructed YFP signal detected in onion epidermal cells by epifluorescence microscopy after bombardment with (B) constructs. Left panels, bright field; right panels, EYFP fluorescence (green).
Identification of the PaCRT domains necessary for interaction with PRSV HC‐Pro
Three deletion mutants (Fig. 5A) were constructed to determine which of the three above‐mentioned typical domains of the PaCRT protein are necessary for interaction with PRSV HC‐Pro using the SRS assay. The coding sequences of the PaCRT mutants were subcloned individually into pMyr and cotransformed with pSos‐HC into yeast strain cdc25H. As shown in Fig. 5B, only the positive control and the cotransformants of pMyr‐PaCRT (residues 210–422) or pMyr‐PaCRT (residues 307–422) and pSos‐HC were able to grow on SD/galactose (−UL) agar plates [but not on SD/glucose (−UL) plates] at 37°C. However, like the negative controls, the cotransformants of pMyr‐PaCRT (residues 1–306) and pSos‐HC could not grow on SD/galactose (−UL) agar plates at 37°C by the patching yeast cells test. Therefore, the C‐domain (residues 307–422) of PaCRT is the necessary domain for interaction with PRSV HC‐Pro, because the deletion of the N‐domain (residues 1–209) and the P‐domain (residues 210–306) of PaCRT did not affect the interaction between PaCRT and PRSV HC‐Pro.
Figure 5.
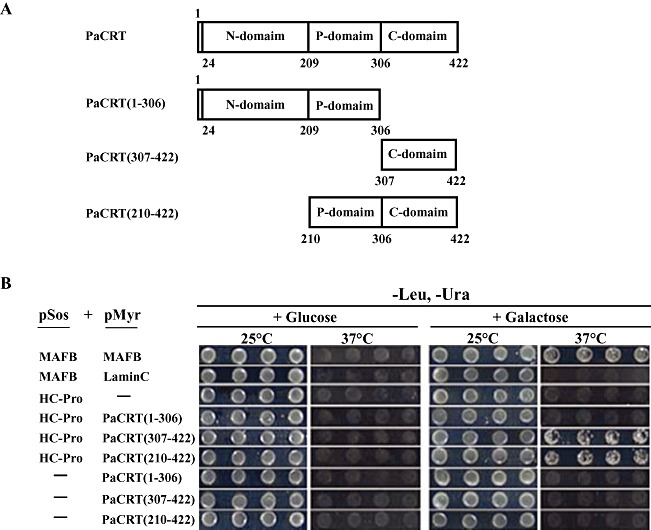
Schematic representation of Carica papaya calreticulin (PaCRT) mutants and identification of domains of PaCRT necessary for interaction with Papaya ringspot virus helper component‐proteinase (PRSV HC‐Pro). (A) Schematic representation of PaCRT and three PaCRT mutants: PaCRT, PaCRT(1–306), PaCRT(307–422) and PaCRT(210–422). (B) Interaction of PRSV HC‐Pro and mutants of PaCRT in Saccharomyces cerevisiae strain cdc25H cells. Four colonies transformed with the indicated plasmid combinations (B) from each transformant were picked up, resuspended and diluted to optical densities at 600 nm of 0.5 in sterile water. An aliquot of 2.5 µL of each dilution was patched in rows onto each of two synthetic glucose minimal medium without leucine and uracil [SD/glucose (−UL)] and two synthetic galactose minimal medium without leucine and uracil [SD/galactose (−UL)] plates, and one of each type of plate was incubated at the permissive temperature or nonpermissive temperature (25 or 37°C) for 5 days to compare the growth of yeast. pMyr‐MAFB and pSos‐MAFB were used as positive controls, and pMyr‐Lamin C and pSos‐MAFB were used as negative controls.
PRSV infection results in upregulated expression of PaCRT mRNA in papaya plants
After the papaya leaves had been mechanically inoculated with PRSV, the appearance of mosaic symptoms on the leaves was observed at 7–8 days post‐inoculation (dpi). To analyse how systemic infection of PRSV influences the expression pattern of PaCRT in papaya plants, the relative expression of PaCRT mRNA in papaya leaves at various times (0 h, 6 h, 12 h, 1 day, 1.5 days, 2 days, 3 days, 4 days, 5 days and 8 days) post‐inoculation with PRSV was evaluated by qRT‐PCR. Moreover, parallel qRT‐PCR testing was used to detect the endogenous basal expression level of PaCRT mRNA in noninoculated (healthy) papaya plants at each time point. As shown in Fig. 6, the expression level of PaCRT mRNA for healthy papaya plants showed a very weak increasing trend from 3 to 8 days, but it was obvious that PaCRT mRNAs were significantly increased at 1.5 dpi and peaked at 3 dpi in PRSV‐inoculated plants relative to that in healthy plants, and then decreased to near‐basal expression levels in healthy plants from 4 to 8 dpi with virus accumulation inside host cells. These results demonstrate that the primary PRSV infection can induce and upregulate the expression of PaCRT mRNA.
Figure 6.
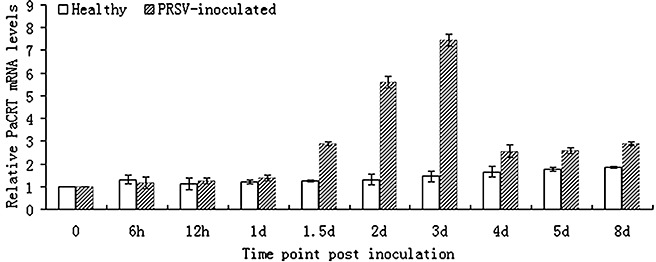
Comparison of relative Carica papaya calreticulin (PaCRT) mRNA levels in healthy and PRSV‐inoculated Carica papaya plants by quantitative real‐time reverse transcriptasepolymerase chain reaction (qRT‐PCR). Data shown are expressed as the mean ± SD of three independent samples (P < 0.05).
DISCUSSION
As described in the Introduction, several plant factors interacting with potyviral protein HC‐Pro have been reported, including a CaM‐like protein (Anandalakshmi et al., 2000). In this study, a new calcium‐binding protein, named PaCRT, was shown to interact specifically with PRSV HC‐Pro in yeast, in vitro and in plant cells by SRS, in vitro protein‐binding assay and BiFC assay, respectively.
Like other plant CRTs, the amino acid sequence of PaCRT contains three distinct structural domains and several conserved sequence features of CRT, such as two well‐conserved CRT family signature motifs, a potential signal peptide sequence located in the N‐terminus, two types of triplicate repeats in the P‐domain and an ER retention signal HDEL at the end of the C‐terminus (Jia et al., 2009; Persson et al., 2003) (Fig. 1). Previous reports have shown that CRT may be divided into distinct plant and mammalian CRT groups, whereas higher plants are clustered into at least two distinct subgroups of CRT: CRT1/2 isoforms and CRT3 isoforms (Jia et al., 2009; Persson et al., 2003). A phylogenetic analysis based on 16 different CRT protein sequences indicated that PaCRT clusters into the plant CRT1/2 isoform subgroup (Fig. 7) and shares higher sequence homology with plant CRT1/2 isoforms (70–85%) than with CRT3 isoforms (54–59%). All of these bioinformational results confirm that the PaCRT cDNA clone encodes a papaya CRT1/2 protein.
Figure 7.
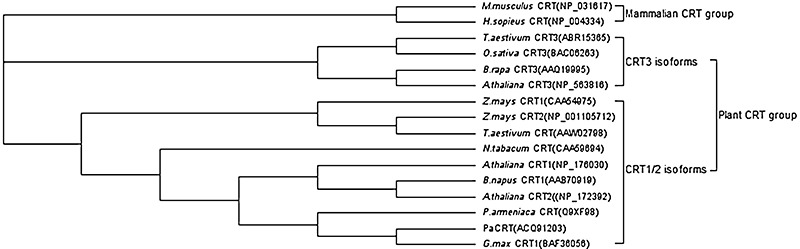
Phylogenetic analysis of calreticulin (CRT) protein sequences in plants and two mammalians. Sequence data for CRT protein were obtained from the GenBank database and the accession numbers are indicated in parentheses. The phylogenetic analysis was performed using Geneious Pro 4.5 software (Biomatters, Auckland, New Zealand). Two distinct groups and two plant subgroups are indicated to the left of the phylogenetic tree.
Plant CRT is a highly conserved calcium‐binding protein with animal homologues (Coughlan et al., 1997; Hassan et al., 1995; Jia et al., 2009; Menegazzi et al., 1993), and several studies have reported that the alteration of the CRT expression level in plants affects cellular calcium ion (Ca2+) storage (Christensen et al., 2008; Persson et al., 2001; Wyatt et al., 2002). As Ca2+ is now firmly established as an essential second messenger and mediates many plant signal pathways, such as defence signalling (Bush, 1993, 1995; Lecourieux et al., 2006; Rudd and Franklin‐Tong, 1999; Sanders et al., 1999; Tuteja and Mahajan, 2007; White and Broadley, 2003), the role played by CRT can be attributed to its potential to affect cellular calcium homeostasis and signalling (Jia et al., 2009). These data suggest that the interaction between PRSV HC‐Pro and PaCRT may be involved in plant calcium signalling pathways, which could interfere with virus infection or host defence. In addition, the C‐domain of CRT has a high calcium‐binding capacity (Michalak et al., 1992; Nakamura et al., 2001). Overexpression of the C‐domain of CRT in Arabidopsis plants resulted in an increase in Ca2+ storage and improved the survival of transgenic seedlings under calcium stress conditions (Wyatt et al., 2002). In this work, the result from SRS assay using deletion mutants indicated that the C‐domain (residues 307–422) of PaCRT was the necessary domain for interaction with PRSV HC‐Pro, which suggests that this interaction may affect the calcium‐binding capacity of PaCRT and, in turn, trigger the activation of plant calcium signalling pathways to disturb virus infection or host defence. A study on the interaction between the plant CaM‐related protein, named rgs‐CaM, and TEV HC‐Pro has identified rgs‐CaM as a cellular suppressor of PTGS, like HC‐Pro, and suggests that calcium may play a role in regulating the activity of the PTGS pathway (Anandalakshmi et al., 2000). CaM and related proteins are also calcium‐binding proteins and play important roles in the calcium signalling network, like CRT (Anandalakshmi et al., 2000; Snedden and Fromm, 1998; Yang and Poovaiah, 2003). However, CaM and related proteins are calcium‐binding proteins with EF‐hands, but CRT is not, and these two proteins play different roles in the plant calcium signalling network (Lecourieux et al., 2006; Tuteja and Mahajan, 2007). Therefore, whether the interaction between PaCRT and PRSV HC‐Pro is also involved in PTGS remains to be ascertained. Furthermore, a tobacco CRT has been shown to bind to the movement protein (MP) of tobacco mosaic virus (TMV) and to impair TMV movement by interfering with plasmodesmal targeting of TMV MP if overexpressed in transgenic plants (Chen et al., 2005). Chen et al. did not discuss the interrelationships between this defence pathway and Ca2+, but it is possible that the interaction between tobacco CRT and MP is also involved in plant calcium signalling pathways during host–virus interaction, as plant CRT may affect cellular calcium homeostasis and signalling (Christensen et al., 2008; Jia et al., 2009; Persson et al., 2001; Wyatt et al., 2002). As the work presented here does not address what kind of functional role is played by the interaction between PaCRT and PRSV HC‐Pro during the process of PRSV infection, these hypotheses remain to be determined through the use of an infectious virus cDNA clone and transgenic papaya plants in which the PaCRT gene is overexpressed or silenced.
The expression of both plant CRT mRNA and protein has been found to be induced and upregulated in response to various biotic (Jelitto‐Van Dooren et al., 1999) and abiotic (Denecke et al., 1995; Jia et al., 2008; Khan et al., 2005; Komatsu et al., 2007; Persson et al., 2003; Shaterian et al., 2005) stresses, which was considered to be a probable conserved self‐protection mechanism acquired during long‐term evolution to facilitate the survival of plants under various stress conditions (Jia et al., 2008). During plant–pathogen interactions, the induction of CRT expression was suggested to be an early signal transduction pathway necessary to rapidly trigger the expression of defence‐related proteins (Jelitto‐Van Dooren et al., 1999). In this study, the result of the qRT‐PCR assay showed that the expression of PaCRT mRNA was significantly upregulated in the primary stage of PRSV infection relative to that of noninoculated papaya plants, which suggests that it may be an early defence‐related response to PRSV infection in host plants.
In addition, BiFC is a powerful tool for the detection, visualization and subcellular localization of protein–protein interactions in living cells (Citovsky et al., 2008). In this work, reconstituted EYFP was observed in onion epidermal cells by BiFC, which provides evidence that PRSV HC‐Pro can interact with PaCRT in living plant cells. Nevertheless, the precise subcellular colocalization of the PRSV HC‐Pro–PaCRT complex remains to be investigated in papaya cells. After all, onion is not the host plant of PRSV and plant CRT has been located not only inside ER, but also outside ER (Chen et al., 2005; Denecke et al., 1995; Jia et al., 2008; Napier et al., 1995; Nardi et al., 2006).Therefore, a papaya leaf protoplast system for studying the subcellular localization of host proteins and PRSV infection is currently being established in our laboratory.
EXPERIMENTAL PROCEDURES
Plant materials and virus sources
Six‐ to eight‐week‐old C. papaya L. seedlings were used and grown in a controlled environment with a cycle of 16 h light at 28°C and 8 h darkness at 25°C. PRSV‐P isolate HN (Lu et al., 2008) was maintained by mechanical inoculation onto C. papaya L. seedlings.
Bait plasmid and cDNA library construction
A Sos recruitment system (SRS)/CytoTrap two‐hybrid system (Stratagene, La Jolla, CA, USA) and temperature‐sensitive mutant yeast (Saccharomyces cerevisiae) strain cdc25H (Stratagene) were used to perform the yeast two‐hybrid assay. The complete nucleotide sequence coding HC‐Pro protein was amplified by PCR from full‐length cDNA of PRSV‐P isolate HN (GenBank: EF1834997) using primers HC1 and HC2 (Table 1). The PCR product was digested and subcloned into the BamHI /SacI site of the bait vector pSos (Leu+) (Stratagene) to be fused with the hSos sequence, resulting in the pSos‐HC vector. The accuracy of the HC‐Pro fusion junction sequence and the reading frame in the bait vector were identified by DNA sequencing.
Table 1.
Primers used in plasmid construction.
| Primers | Primer sequences | Restriction enzyme | Constructs |
|---|---|---|---|
| HC1 | 5′‐CTGGATCCCCAACGATGTCGCTGAGAAATTCT‐3′ | BamHI | pSos‐HC |
| HC2 | 5′‐CTGAGCTCTCAACCAACAATGTAGTGCTTCATT‐3′ | SacI | |
| CRT1 | 5′‐CAGAATTCATGGCGTCCGGGAAATTAAACC‐3′ | EcoRI | pMyr‐PaCRT |
| CRT2 | 5′‐CTCTCGAGTCACAGCTCATCATGTCCTGTGCT‐3′ | XhoI | |
| HC3 | 5′‐CTGAATTCAACGATGTCGCTGAGAAATTCT‐3′ | EcoRI | pGEX‐HC |
| HC4 | 5′‐CTGTCGACTCAACCAACAATGTAGTGCTTCAT‐3′ | SalI | |
| CRT3 | 5′‐CACTGCAGCTCGAGCCACCATGGCGTCCGGGAAATTAAACC‐3′ | PstI; XhoI | pSP73‐PaCRT |
| CRT4 | 5′‐CTGAGCTCTTACAGCTCATCATGTCCTGTGCT‐3′ | SacI | |
| BiFC HC5 | 5′‐CTCTCGAGCTAACGATGTCGCTGAGAAATTCT‐3′ | XhoI | pSAT1‐nEYFP‐C1‐HC |
| BiFC HC6 | 5′‐CTGGATCCTCAACCAACAATGTAGTG CTT‐3′ | BamHI | |
| BiFC CRT5 | 5′‐CACTCGAGCTATGGCGTCCGGGAAATTAAACC‐3′ | XhoI | pSAT1‐cEYFP‐C1‐PaCRT |
| BiFC CRT6 | 5′‐CTGGATCCTCACAGCTCATCATGTCCTGTGCT‐3′ | BamHI | |
| BiFC HC7 | 5′‐CTCTCGAGCAACGATGTCGCTGAGAAAT‐3′ | XhoI | pSAT1‐nEYFP‐N1‐HC |
| BiFC HC8 | 5′‐CTGGATCCCACCAACAATGTAGTGCTTCAT‐3′ | BamHI | |
| BiFC CRT7 | 5′‐CACTCGAGCATGGCGTCCGGGAAATTAAACC‐3′ | XhoI | pSAT1‐cEYFP‐N1‐PaCRT |
| BiFC CRT8 | 5′‐CTGGATCCCCAGCTCATCATG TCCTGTGCT‐3′ | BamHI | |
| mCRT1 | 5′‐TAGAATTCATGGCGTCCGGGAAATTAAAC‐3′ | EcoRI | pMyr‐PaCRT(1–306) |
| mCRT2 | 5′‐CTCTCGAGTCACTTGAAATCTGGGTTGTCAAT‐3′ | XhoI | |
| mCRT3 | 5′‐TAGAATTCGATGACCCTGATCTTTATGT‐3′ | EcoRI | pMyr‐PaCRT(307–422) |
| mCRT4 | 5′‐CTCTCGAGTCACAGCTCATCATGTCCTGTGC‐3′ | XhoI | |
| mCRT5 | 5′‐TAGAATTCCCAAAGAAAATCAAAGATCCTG‐3′ | EcoRI | pMyr‐PaCRT(210–422) |
| mCRT6 | 5′‐CTCTCGAGTCACAGCTCATCATGTCCTGTG‐3′ | XhoI |
Total RNA was extracted from three whole healthy C. papaya L. plants at the seven‐ to eight‐leaf stage using TRIzol (Invitrogen, Carlsbad, CA, USA), and Poly(A)+‐RNA (5.0 µg), isolated with an Oligotex mRNA Kit (Qiagen, Hilden, Germany), was used for cDNA synthesis. cDNAs larger than 500 bp as EcoRI‐XhoI fragments were inserted into the pMyr vector (Ura+) (Stratagene) to be expressed with a myristoylation signal, which anchors the proteins in the membrane, and the pMyr‐cDNAs were transformed into XL10‐Gold Kan supercompetent E. coli cells (Stratagene) as described in the CytoTrap XR Library Construction Kit Instruction Manual (Stratagene). The cDNA library plasmids were isolated by a Plasmid Maxi Kit (Qiagen).
SRS screening
According to the manufacturer's instructions (Stratagene), the yeast cdc25H strains cotransformed with pMyr‐cDNA library plasmids (40.0 µg) and pSos‐HC plasmids (40.0 µg) were initially selected by plating onto SD/glucose (−UL) at the permissive temperature (25°C) until colonies began to appear; then the transformants were replica‐plated onto SD/galactose (−UL) plates using velvet pads to induce the expression of the Sos‐HC‐Pro bait at the nonpermissive temperature (37°C). After 6 days, the growing colonies (candidate interactors) were picked up and dispatched into sterile H2O. The resuspended cotransformants were patched (dotted) onto SD/glucose (−UL) plates again to incubate for 2 days at 25°C using a pin multi‐blot replicator (V&P, San Diego, CA, USA). Subsequently, the putative positives were identified among the candidates by two rounds of patching cell tests for galactose‐dependent growth on SD/galactose (−UL) or SD/glucose (−UL) plates for 5 days at 25 or 37°C. Meanwhile, cdc25H yeast cells cotransformed with pSos‐MAFB (Stratagene) and pMyr‐MAFB (Stratagene) and with pSos‐MAFB and pMyr‐Lamin C (Stratagene) were used as standard positive controls and negative controls, respectively, in all two‐hybrid assays (Stratagene). Finally, the pMyr plasmids were isolated from yeast positive candidate interactors and amplified in E. coli for further retransformation with the pSos‐HC bait construct into yeast cells to perform false positive tests and DNA sequence analysis. The DNA sequences were used further for a blast search within the GenBank database.
Cloning the full‐length PaCRT cDNA from C. papaya L. and SRS assay
Total RNA was isolated from C. papaya L. plants as described above. To obtain the full‐length PaCRT cDNA, 5′‐RACE reactions were carried out using a 5′‐Full RACE kit (Takara, Dalian, China) and the primers CRT‐F (5′‐ATTGCAGCACACTCAGATCTACAG‐3′), CRT‐R (5′‐GATCTACAGCTCATCATGTCCTGT‐3′), 5′‐RACE Outer (5′‐CATGGCTACATGCTGACAGCCTA‐3′) and 5′‐RACE Inner (5′‐CGCGGATCCACAGCCTACTGATGATCAGTCGATG‐3′), whereas specific primers CRT‐F/R were designed according to the sequence obtained from our library screen. The full‐length cDNA of PaCRT was cloned into pMD18‐T (Takara), resulting in a pMD18‐PaCRT vector, and verified by sequencing, and the amino acid sequence was analysed in the PROSITE database. Then, the full‐length coding sequence of PaCRT was amplified by PCR using the primers CRT1 and CRT2 (Table 1), and cloned into pMyr via the EcoRI/XhoI sites to form pMyr‐PaCRT. Two plasmids, pMyr‐PaCRT and pSos‐HC, were cotransformed into cdc25H yeast cells, as described above in the SRS screening section and in the manufacturer's instructions (Stratagene) of the CytoTrap two‐hybrid system.
Recombinant protein expression and in vitro GST pull‐down assay
To obtain the GST‐PRSV HC‐Pro fusion protein, HC‐Pro was amplified by PCR with pSos‐HC as template and HC3/HC4 as primers (Table 1). The resultant PCR product was digested with EcoRI and SalI, and subcloned between the corresponding cloning sites of pGEX‐6P‐1 (Amersham Biosciences, Little Chalfont, England, UK) to construct pGEX‐HC. Then, the recombinant plasmid pGEX‐HC, verified by sequencing, was transformed into E. coli expression strain BL21 (Novagen, Madison, WI, USA) to express the GST‐PRSV HC‐Pro fusion protein, as described by the manufacturer (Amersham). The empty vector (pGEX‐6P‐1) was used as control. GST‐fused proteins were purified using the MagneGST Protein Purification System (Promega, Madison, WI, USA) and separated by 10% sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE).
To produce biotinylated PaCRT protein and to optimize translation, the full‐length PaCRT cDNA was amplified by PCR, with pMD18‐PaCRT as template and CRT3/CRT4 as primers (Table 1), to be cloned into the PstI/SacI site of pSP64 (Promega) and then subcloned into the XhoI/EcoRI site of pSP73 (Promega), generating the pSP73‐PaCRT vector in favourable Kozak context and Poly(A) sequence. After the pSP73‐PaCRT constructs had been verified by sequencing and the corresponding plasmids had been purified using the PureYield Plasmid Midiprep System (Promega), in vitro translation of pSP73‐PaCRT (1.0 µg) was carried out directly with the TNT Quick Coupled Transcription/Translation System, according to the manufacturer's protocol (Promega). To allow nonradioactive detection of the proteins synthesized in vitro, biotinylated lysine (2 µL) was added to the TNT Quick Master Mix (40.0 µL) as a precharged epsilon‐labelled biotinylated lysine–tRNA complex (Transcend tRNA, Promega) rather than a free amino acid. After the translation reaction had been incubated at 30°C for 90 min, the translation products were analysed using SDS‐PAGE and electroblotting. The biotinylated proteins can be visualized by binding streptavidin–AP, followed by colorimetric detection, according to the protocol for the Transcend nonradioactive translation detection system (Promega). Subsequently, the translation mixture and purified GST‐HC‐Pro or GST were used for the pull‐down assays as described in the MagneGST Pull‐Down System Instruction Manual (Promega). In vitro binding was detected by Western blotting analysis with streptavidin–AP (Promega) or anti‐GST antibody (Tiangen, Beijing, China).
BiFC assay and epifluorescence microscopy
A full‐length PRSV HC‐Pro fragment was amplified by PCR with pSos‐HC as template and BiFC HC5/HC6 (BiFC HC7/HC8) as primers (Table 1), and cloned into the XhoI/BamHI sites of the pSAT1‐nEYFP‐C1 (pSAT1‐nEYFP‐N1) vector (Citovsky et al., 2006), resulting in the pSAT1‐nEYFP‐C1‐HC (pSAT1‐nEYFP ‐N1‐HC) vector. Similarly, full‐length and truncated mutants of PaCRT were amplified with pMD18‐PaCRT as template and BiFC CRT5/CRT6 (BiFC CRT7/CRT8) as primers (Table 1), and cloned into the XhoI/BamHI sites of the pSAT1‐cEYFP‐C1(B) (pSAT1‐cEYFP‐N1) vector (Citovsky et al., 2006), generating the pSAT1‐cEYFP‐C1‐PaCRT (pSAT1‐cEYFP‐N1‐PaCRT) constructs. All fusion constructs were verified by sequencing.
For the BiFC assay, onion (Allium cepa) tissues were placed on Murashige and Skoog (MS) plates. Various combinations of plasmids encoding cEYFP or nEYFP fusion proteins were mixed at a 1:1 (w/w) ratio, and 5 µg of DNA coated with 3 mg of 1 µm gold particles were microbombarded into the onion epidermal peels using a particle delivery system (model PDS‐1000/He, Bio‐Rad, Hercules, CA, USA). The conditions of bombardment were a vacuum of 28 mmHg, a helium pressure of 1300 psi and a target distance of 6 cm. After bombardment, the tissues were incubated on MS plates for 16–24 h at 25°C.Then, the epidermal cell layers were peeled and observed by epifluorescence microscopy (NIKON ECLIPSE 80i) with a YFP‐specific filter.
Construction of deletion mutants for PaCRT and SRS assay
Three coding sequences of PaCRT (residues 1–306, 307–422 and 210–422) were amplified individually by PCR using pMyr‐PaCRT as template and the primers (mCRT1–6) listed in Table 1. Then, the PCR products of these deletion mutants were individually subcloned into the pMyr vector and cotransformed with pSos‐HC into the yeast strain cdc25H to confirm which domains of PaCRT were necessary for interaction with PRSV HC‐Pro, as described in the SRS screening section and the manufacturer's instructions of the CytoTrap two‐hybrid system (Stratagene).
Virus inoculation and qRT‐PCR analysis
PRSV‐P isolate HN (Lu et al., 2008) was transferred to the leaves of C. papaya (cultivar Suizhonghong) seedlings at the seven‐ to eight‐leaf stage by mechanical inoculation (Bau et al., 2003). Total RNA was isolated from the upper three or four noninoculated leaves of C. papaya plants at various times (0 h, 6 h, 12 h, 1 day, 1.5 days, 2 days, 3 days, 4 days, 5 days and 8 days) post‐inoculation with PRSV using TRIzol reagent, as described above. To produce ultrapure, intact RNA, DNase I digestion (Qiagen) was employed to eliminate possible genomic DNA contamination; UV–visible spectrophotometry (Beckman Coulter, Brea, CA, USA) and denaturing agarose gel electrophoresis were used to assess RNA quality and quantity. qRT‐PCR was performed with a SYBR PrimeScript RT‐PCR Kit II (Takara) on an Mx3000P cycler (Stratagene) using the fluorescent SYBR green method. Primers (Takara) of qPCR were as follows: PaCRT forward primer, 5′‐CTCTCCCTTGTCCTCCTTTCC‐3′; reverse primer, 5′‐TGTAGTTCCATTCCCCAGCA‐3′; actin forward primer, 5′‐TTGATTTTGAGCAGGAGCTTGA‐3′; reverse primer, 5′‐TGAGTGATGGCTGGAAGAGAAC‐3′. The cycling programme was a two‐step cycling method: 95°C for 30 s hold; 40 cycles of 95°C for 3 s and 61°C for 30 s. The qRT‐PCR protocols were initially established and optimized using 10‐fold serial dilutions of purified PCR‐derived PaCRT or papaya actin fragment (GenBank accession no. AY906938). Melting curve analysis was carried out to ensure that the qRT‐PCR products did not include primer dimers or multiple products. All reactions were performed in triplicate and ‘no template’ and ‘no reverse transcriptase’ controls were included for each primer pair. The mean cycle threshold (C t) value and its corresponding standard deviation (SD) were given by MX Pro Stratagene analysis software. Relative gene expressions of PaCRT were calculated using the 2−ΔΔCt method (Livak and Schmittgen 2001). In this method, papaya actin served as a control to normalize the amount of PaCRT mRNA for each sample: ΔΔC t= (C t,PaCRT−C t,actin)Timex− (C t,PaCRT−C t,actin)Time0, where Timex is one of ten time points. The data are presented as the fold change in gene expression normalized to the papaya actin gene and relative to the noninoculated control at time zero. In all experiments, each sample used at least three plants for each time point tested. Moreover, the leaves of noninoculated (healthy) papaya plants at various time points (0 h, 6 h, 12 h, 1 day, 1.5 days, 2 days, 3 days, 4 days, 5 days and 8 days) were simultaneously used as background materials to perform parallel qRT‐PCR testing.
ACKNOWLEDGEMENTS
This work was supported by the National Nonprofit Institute Research Grant (ITTBZD07‐32), the National Natural Science Foundation of China (30760134) and the Open Foundation of the Key Laboratory of Protection and Development Utilization of Tropical Crop Germplasm Resources (Hainan University), Ministry of Education, China. We thank Dr Stanton B. Gelvin (Purdue University, West Lafayette, IN, USA) and Dr Lan‐Ying Lee (Purdue University) for providing the vectors used in the BiFC assay.
REFERENCES
- Ahlquist, P. , Noueiry, A.O. , Lee, W.M. , Kushner, D.B. and Dye, B.T. (2003) Host factors in positive‐strand RNA virus genome replication. J. Virol. 77, 8181–8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anandalakshmi, R. , Marathe, R. , Ge, X. , Herr, J.M., Jr , Mau, C. , Mallory, A. , Pruss, G. , Bowman, L. and Vance, V.B. (2000) A calmodulin‐related protein that suppresses posttranscriptional gene silencing in plants. Science, 290, 142–144. [DOI] [PubMed] [Google Scholar]
- Ballut, L. , Drucker, M. , Pugnière, M. , Cambon, F. , Blanc, S. , Roquet, F. , Candresse, T. , Schmid, H.P. , Nicolas, P. , Gall, O.L. and Badaoui, S. (2005) HcPro, a multifunctional protein encoded by a plant RNA virus, targets the 20S proteasome and affects its enzymic activities. J. Gen. Virol. 86, 2595–2603. [DOI] [PubMed] [Google Scholar]
- Bau, H.J. , Cheng, Y.H. , Yu, T.A. , Yang, J.S. and Yeh, S.D. (2003) Broad‐spectrum resistance to different geographic strains of papaya ringspot virus in coat protein gene transgenic papaya. Phytopathology 93, 112–120. [DOI] [PubMed] [Google Scholar]
- Bracha‐Drori, K. , Shichrur, K. , Katz, A. , Oliva, M. , Angelovici, R. , Yalovsky, S. and Ohad, N. (2004) Detection of protein–protein interactions in plants using bimolecular fluorescence complementation. Plant J. 40, 419–427. [DOI] [PubMed] [Google Scholar]
- Broder, Y.C. , Katz, S. and Aronheim, A. (1998) The ras recruitment system, a novel approach to the study of protein–protein interactions. Curr. Biol. 8, 1121–1124. [DOI] [PubMed] [Google Scholar]
- Bush, D. (1993) Calcium regulation in plant cells and its role in signaling. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46, 95–122. [Google Scholar]
- Bush, D. (1995) Regulation of cytosolic calcium in plants. Plant Physiol. 103, 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, M.H. , Tian, G.W. , Gafni, Y. and Citovsky, V. (2005) Effects of calreticulin on viral cell‐to‐cell movement. Plant Physiol. 138, 1866–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, Y.Q. , Liu, Z.M. , Xu, J. , Zhou, T. , Wang, M. , Chen, Y.T. , Li, H.F. and Fan, Z.F. (2008) HC‐Pro protein of sugar cane mosaic virus interacts specifically with maize ferredoxin‐5 in vitro and in planta. J. Gen. Virol. 89, 2046–2054. [DOI] [PubMed] [Google Scholar]
- Christensen, A. , Svensson, K. , Persson, S. , Jung, J. , Michalak, M. , Widell, S. and Sommarin, M. (2008) Functional characterization of Arabidopsis calreticulin1a: a key alleviator of endoplasmic reticulum stress. Plant Cell Physiol. 49, 912–924. [DOI] [PubMed] [Google Scholar]
- Citovsky, V. , Lee, L.Y. , Vyas, S. , Glick, E. , Chen, M.H. , Vainstein, A. , Gafni, Y. , Gelvin, S.B. and Tzfira, T. (2006) Subcellular localization of interacting proteins by bimolecular fluorescence complementation in planta. J. Mol. Biol. 362, 1120–1131. [DOI] [PubMed] [Google Scholar]
- Citovsky, V. , Gafni, Y. and Tzfira, T. (2008) Localizing protein–protein interactions by bimolecular fluorescence complementation in planta. Methods, 45, 196–206. [DOI] [PubMed] [Google Scholar]
- Coughlan, S.J. , Hastings, C. and Winfrey, R. Jr (1997) Cloning and characterization of the calreticulin gene from Ricinus communis L. Plant Mol. Biol. 34, 897–911. [DOI] [PubMed] [Google Scholar]
- Denecke, J. , Carlsson, L.E. , Vidal, S. , Hoglund, A.S. , Ek, B. , Van Zeijl, M.J. , Sinjorgo, K.M. and Palva, E.T. (1995) The tobacco homolog of mammalian calreticulin is present in protein complexes in vivo . Plant Cell, 7, 391–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, D. and Kerppola, T.K. (2004) Ubiquitin‐mediated fluorescence complementation reveals that Jun ubiquitinated by Itch/AIP4 is localized to lysosomes. Proc. Natl. Acad. Sci. USA, 101, 14782–14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauquet, C.M. , Mayo, M.A. , Maniloff, J. , Desselberger, U. and Ball, L.A. (2005) Virus Taxonomy: VIIIth Report of the International Committee on Taxonomy of Viruses. San Diego, CA: Elsevier Academic Press, p. 1259. [Google Scholar]
- Gonsalves, D. (1998) Control of papaya ringspot virus in papaya: a case study. Annu. Rev. Phytopathol. 36, 415–437. [DOI] [PubMed] [Google Scholar]
- Guo, D. , Spetz, C. , Saarma, M. and Valkonen, J.P. (2003) Two potato proteins, including a novel RING finger protein (HIP1), interact with the potyviral multifunctional protein HCpro. Mol. Plant–Microbe Interact. 16, 405–410. [DOI] [PubMed] [Google Scholar]
- Hassan, A.M. , Wesson, C. and Trumble, W.R. (1995) Calreticulin is the major Ca2+ storage protein in the endoplasmic reticulum of the pea plant (Pisum sativum). Biochem. Biophys. Res. Commun. 211, 54–59. [DOI] [PubMed] [Google Scholar]
- Jelitto‐Van Dooren, E.P. , Vidal, S. and Denecke, J. (1999) Anticipating endoplasmic reticulum stress. A novel early response before pathogenesis‐related gene induction. Plant Cell, 11, 1935–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, X.Y. , Xu, C.Y. , Jing, R.L. , Li, R.Z. , Mao, X.G. , Wang, J.P. and Chang, X.P. (2008) Molecular cloning and characterization of wheat calreticulin (CRT) gene involved in drought‐stressed responses. J. Exp. Bot. 59, 739–751. [DOI] [PubMed] [Google Scholar]
- Jia, X.Y. , He, L.H. , Jing, R.L. and Li, R.Z. (2009) Calreticulin: conserved protein and diverse functions in plants. Physiol. Plant, 136, 127–138. [DOI] [PubMed] [Google Scholar]
- Jin, Y. , Ma, D. , Dong, J. , Li, D. , Deng, C. , Jin, J. and Wang, T. (2007a) The HC‐pro protein of potato virus Y interacts with NtMinD of tobacco. Mol. Plant–Microbe Interact. 20, 1505–1511. [DOI] [PubMed] [Google Scholar]
- Jin, Y. , Ma, D. , Dong, J. , Li, D. , Deng, C. , Jin, J. and Wang, T. (2007b) HC‐Pro protein of potato virus Y can interact with three Arabidopsis 20S proteasome subunits in planta. J. Virol. 81, 12881–12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, M. , Takasaki, H. and Komatsu, S. (2005) Comprehensive phosphoproteome analysis in rice and identification of phosphoproteins responsive to different hormones/stresses. J. Proteome Res. 4, 1592–1599. [DOI] [PubMed] [Google Scholar]
- Komatsu, S. , Yang, G. , Khan, M. , Onodera, H. , Toki, S. and Yamaguchi, M. (2007) Over‐expression of calcium‐dependent protein kinase 13 and calreticulin interacting protein 1 confers cold tolerance on rice plants. Mol. Genet. Genomics, 277, 713–723. [DOI] [PubMed] [Google Scholar]
- Kwon, S.J. , Choi, E.Y. , Choi, Y.J. , Ahn, J.H. and Park, O.K. (2006) Proteomics studies of post‐translational modifications in plants. J. Exp. Bot. 57, 1547–1551. [DOI] [PubMed] [Google Scholar]
- Lecourieux, D. , Ranjeva, R. and Pugin, A. (2006) Calcium in plant defence‐signalling pathways. New Phytol. 171, 249–269. [DOI] [PubMed] [Google Scholar]
- Livak, K.J. and Schmittgen, T.D. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)). Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lu, Y.W. , Shen, W.T. , Zhou, P. , Tang, Q.J. , Niu, Y.M. , Peng, M. and Xiong, Z. (2008) Complete genomic sequence of a Papaya ringspot virus isolated from Hainan Island, China. Arch. Virol. 153, 991–993. [DOI] [PubMed] [Google Scholar]
- Maia, I.G. , Haenni, A. and Bernardi, F. (1996) Potyviral HC‐Pro: a multifunctional protein. J. Gen. Virol. 77, 1335–1341. [DOI] [PubMed] [Google Scholar]
- Mangrauthia, S.K. , Jain, R.K. and Praveen, S. (2008) Sequence motifs comparisons establish a functional portrait of a multifunctional protein HC‐Pro from papaya ringspot potyvirus. J. Plant Biochem. Biol. 17, 201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegazzi, P. , Guzzo, F. , Baldan, B. , Mariani, P. and Treves, S. (1993) Purification of calreticulin‐like protein(s) from spinach leaves. Biochem. Biophys. Res. Commun. 190, 1130–1135. [DOI] [PubMed] [Google Scholar]
- Michalak, M. , Milner, R.E. , Burns, K. and Opas, M. (1992) Calreticulin. Biochem. J. 285, 681–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak, M. , Corbett, E.F. , Mesaeli, N. , Nakamura, K. and Opas, M. (1999) Calreticulin: one protein, one gene, many functions. Biochem. J. 344, 281–292. [PMC free article] [PubMed] [Google Scholar]
- Nagy, P.D. (2008) Yeast as a model host to explore plant virus–host interactions. Annu. Rev. Phytopathol. 46, 217–242. [DOI] [PubMed] [Google Scholar]
- Nakamura, K. , Zuppini, A. , Arnaudeau, S. , Lynch, J. , Ahsan, I. , Krause, R. , Papp, S. , De Smedt, H. , Parys, J.B. , Muller‐Esterl, W. , Lew, D.P. , Krause, K.H. , Demaurex, N. , Opas, M. and Michalak, M. (2001) Functional specialization of calreticulin domains. J. Cell. Biol. 154, 961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napier, R.M. , Trueman, S. , Henderson, J. , Boyce, J.M. , Hawes, C. , Fricker, M.D. and Venis, M.A. (1995) Purification, sequencing and functions of calreticulin from maize. J. Exp. Bot. 46, 1603–1613. [Google Scholar]
- Nardi, M.C. , Feron, R. , Navazio, L. , Mariani, P. , Pierson, E. , Wolters‐Arts, M. , Knuiman, B. , Mariani, C. and Derksen, J. (2006) Expression and localization of calreticulin in tobacco anthers and pollen tubes. Planta, 223, 1263–1271. [DOI] [PubMed] [Google Scholar]
- Ohad, N. , Shichrur, K. and Yalovsky, S. (2007) The analysis of protein–protein interactions in plants by bimolecular fluorescence complementation. Plant Physiol. 145, 1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson, S. , Wyatt, S.E. , Love, J. , Thompson, W.F. , Robertson, D. and Boss, W.F. (2001) The Ca2+ status of the endoplasmic reticulum is altered by induction of calreticulin expression in transgenic plants. Plant Physiol. 126, 1092–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson, S. , Rosenquist, M. , Svensson, K. , Galvão, R. , Boss, W.F. and Sommarin, M. (2003) Phylogenetic analyses and expression studies reveal two distinct groups of calreticulin isoforms in higher plants. Plant Physiol. 133, 1385–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plisson, C. , Drucker, M. , Blanc, S. , German‐Retana, S. , Le Gall, O. , Thomas, D. and Bron, P. (2003) Structural characterization of HC‐Pro, a plant virus multifunctional protein. J. Biol. Chem. 278, 23753–23761. [DOI] [PubMed] [Google Scholar]
- Riedel, D. , Lesemann, D.E. and Maiss, E. (1998) Ultrastructural localization of nonstructural and coat proteins of 19 potyviruses using antisera to bacterially expressed proteins of plum pox potyvirus. Arch. Virol. 143, 2133–2158. [DOI] [PubMed] [Google Scholar]
- Roy, G. , Jain, R.K. , Bhat, A.I. and Varma, A. (1999) Comparative host range and serological studies of papaya ringspot potyvirus isolates. Indian Phytopathol. 62, 14–17. [Google Scholar]
- Rudd, J.J. and Franklin‐Tong, V.E. (1999) Calcium signaling in plants. Cell Mol. Life Sci. 55, 214–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz, P. , Salvador, B. , Simon‐Mateo, C. , Kasschau, K.D. , Carrington, J.C. and Garcia, J.A. (2002) Host‐specific involvement of the HC protein in the long‐distance movement of potyviruses. J. Virol. 76, 1922–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders, D. , Brownlee, C. and Harper, J.F. (1999) Communicating with calcium. Plant Cell, 11, 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholthof, H.B. (2005) Plant virus transport: motions of functional equivalence. Trends Plant Sci. 10, 376–382. [DOI] [PubMed] [Google Scholar]
- Shaterian, J. , Georges, F. , Hussain, A. , Waterer, D. , Jong, H.D. and Tanino, K.K. (2005) Root to shoot communication and abscisic acid in calreticulin (CR) gene expression and salt‐stress tolerance in grafted diploid potato clones. Environ. Exp. Bot. 53, 323–332. [Google Scholar]
- Snedden, W.A. and Fromm, H. (1998) Calmodulin, calmodulin related proteins and plant responses to the environment. Trends Plant Sci. 3, 299–304. [Google Scholar]
- Stange, C. (2006) Plant–virus interactions during the infective process. Cien. Inv. Agr. 33, 1–18. [Google Scholar]
- Suter, B. , Kittanakom, S. and Stagljar, I. (2008) Two‐hybrid technologies in proteomics research. Curr. Opin. Biotechnol. 19, 316–323. [DOI] [PubMed] [Google Scholar]
- Tripathi, S. , Suzuki, J.Y. , Ferreira, S.A. and Gonsalves, D. (2008) Papaya ringspot virus‐P: characteristics, pathogenicity, sequence variability and control. Mol. Plant Pathol. 9, 269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuteja, N. and Mahajan, S. (2007) Calcium signaling network in plants: an overview. Plant Signal. Behav. 2, 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuqui‐Inchima, S. , Haenni, A.L. and Bernardi, F. (2001) Potyvirus proteins: a wealth of functions. Virus Res. 74, 157–175. [DOI] [PubMed] [Google Scholar]
- White, P.J. and Broadley, M.R. (2003) Calcium in plants. Ann. Bot. 92, 487–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham, S.A. and Wang, Y. (2004) Roles for host factors in plant viral pathogenicity. Curr. Opin. Plant Biol. 7, 365–371. [DOI] [PubMed] [Google Scholar]
- Whitham, S.A. , Yang, C. and Goodin, M.M. (2006) Global impact: elucidating plant responses to viral infection. Mol. Plant–Microbe Interact. 19, 1207–1215. [DOI] [PubMed] [Google Scholar]
- Wyatt, S.E. , Tsou, P.L. and Robertson, D. (2002) Expression of the high capacity calcium‐binding domain of calreticulin increases bioavailable calcium stores in plants. Transgenic Res. 11, 1–10. [DOI] [PubMed] [Google Scholar]
- Yang, T. and Poovaiah, B.W. (2003) Calcium/calmodulin‐mediated signal network in plants. Trend Plant Sci. 8, 505–512. [DOI] [PubMed] [Google Scholar]


