SUMMARY
The HrpZ harpin of Pseudomonas syringae is known to induce a hypersensitive response (HR) in some plants. In P. syringae pv. tabaci (Pta), the harpin gene hrpZ has been spontaneously disrupted by an internal deletion in its open reading frame and a frame shift. The loss of the ability of the recombinant harpin polypeptide of Pta to induce HR despite the high sensitivity of tobacco plants to harpin led us to investigate the meaning of the disrupted hrpZ gene in the virulence of Pta 6605. The hrpZ gene from P. syringae pv. pisi was introduced into wild‐type (WT) Pta. The hrpZ‐complemented Pta secreted harpin into the culture medium, but failed to cause disease symptoms by both infiltration and spray inoculation. Inoculation with the hrpZ‐complemented Pta induced defence responses in tobacco plants, whereas the defence responses of tobacco plants were not prominent on inoculation with WT Pta. These results indicate that an ancestor of Pta might have disrupted hrpZ by an internal deletion to evade plant defences and confer the ability to cause disease in tobacco plants.
The genetic region that causes the hypersensitive response (HR) in nonhost plants and pathogenicity in host plants was first identified in Pseudomonas syringae pv. phaseolicola (Pph) and designated as the hrp gene cluster (Lindgren et al., 1986). Hrp genes, which are found in many Gram‐negative phytopathogenic bacteria, encode a type III secretion apparatus, regulatory proteins and accessory proteins (Jin et al., 2003; Mansfield, 2009). Bacteria inject Hrp outer proteins (Hops) directly into the plant cytoplasm and release harpin proteins from the bacterial cells through the type III secretion system (T3SS) (Alfano and Collmer, 2004; Grant et al., 2006). Unlike other Hops, harpins elicit HR from outside the cells. Harpins are known to be rich in glycine, lacking in cysteine and to have heat‐stable HR‐inducing activity, and thus they are thought to possess one of the pathogen‐associated molecular patterns (PAMPs) (He et al., 1993). We have cloned the harpin genes hrpZ from P. syringae pathovars pisi (Ppi), glycinea (Pgl) and tabaci (Pta), and found that the deduced amino acid sequences of these harpin proteins are well conserved. However, hrpZ in Pta 6605 has a 326‐bp internal deletion in the central region (which corresponds to amino acids 131–239 in the highly homologous harpin from Pgl) of the open reading frame, which results in a significant frame shift (Taguchi et al., 2001). Because all isolates of Pta investigated so far retain the deletion, this genetic feature might be specific to pv. tabaci. These results indicate that the hrpZ gene was evolutionarily disrupted in Pta. To evaluate the elicitor activity of the truncated harpin protein from Pta, a recombinant harpin from Pta (harpinPta) possessing only 130 (N)‐terminal amino acids with 10 unrelated amino acids in the carboxyl terminal extension was expressed in Escherichia coli. The purified recombinant harpinPta did not induce HR in tobacco plants (Taguchi et al., 2001). HarpinPta was not only unable to induce defence responses, but Pta 6605 did not produce harpinPta in the intracellular fraction or secrete it into the extracellular space (Taguchi et al., 2001). Although the role of harpin protein in pathogenesis is not yet clear, mutational analysis of hrpZ in P. syringae pv. tomato (Pto) indicated that harpinPto is not a prerequisite for virulence against the host tomato plant (Charkowski et al., 1998). In this study, an hrpZ‐complemented strain of Pta 6605 was created, and the reduction or enhancement of its virulence was analysed in comparison with the wild‐type (WT) strain. Furthermore, evolutionary aspects of plant‐pathogenic bacteria are discussed.
The hrpZ operon consists of hrpA, hrpZ, hrpB, hrcJ, hrpD and hrpE (He, 1996). To complement the hrpZ gene in the WT strain, a set of primers (pro‐F, 5′‐cgGAATTCgagctcgatatcccacgtcg‐3′; hrpZ‐R, 5′‐cgGAATTCtcaggctgcagcctgattgc‐3′; italic letters are noncomplementary nucleotides and capital letters indicate an EcoRI site) was first used to amplify a DNA fragment containing hrpA, hrpZ and the promoter of the hrpZ operon using a cosmid clone that possesses the entire hrpZ operon of Ppi as the template DNA (Nakada et al., 1999) by polymerase chain reaction (PCR). The PCR product was digested with EcoRI and inserted into an EcoRI‐linearized broad‐host‐range plasmid vector pDSK519 (Keen et al., 1988) to construct phrpAZ. To reveal the effect of exogenous hrpA in the hrpAZ‐complemented strain, we also generated complemented strains with the plasmid containing only hrpA or only hrpZ. Three sets of PCR primers [pro‐F and pro‐R (5′‐cgGGATCCaccgatcgtgttgacgacac‐3′) for the hrpZ promoter; pro‐F and hrpA‐R (5′‐tcagaactggacgaccgagt) for the hrpZ promoter with the hrpA coding region; and hrpZ‐F (5′‐cgGGATCCatgcagagtctcagtattaa‐3′) and hrpZ‐R for the hrpZ coding region; italic letters are noncomplementary nucleotides and capital letters indicate a BamHI site] were used to amplify the respective DNA fragment using a phrpAZ plasmid as a template. PCR products of the hrpZ promoter and hrpZ coding region were ligated after digestion with BamHI. The resulting fragment was subjected to PCR again using primers pro‐F and hrpZ‐R, and introduced into a pDSK519 vector to construct phrpZ. The DNA fragment of the hrpZ promoter with hrpA was also inserted into a pDSK519 vector to construct phrpA. The plasmids phrpA, phrpZ and phrpAZ, and an empty pDSK519 vector as a control, were introduced into the Pta 6605 WT strain by conjugation via E. coli S‐17 using a method described previously (Shimizu et al., 2003).
To confirm the production of harpin protein, WT Pta and hrpAZ‐complemented strains were incubated for 24 h in nutrient‐poor MMMF (50 mm potassium phosphate buffer, 7.6 mm (NH4)2SO4, 1.7 mm MgCl2, 1.7 mm NaCl, 10 mm mannitol, 10 mm fructose, pH 5.7) medium, and each bacterial culture supernatant was concentrated. As shown in Fig. 1, Pta 6605 WT did not produce harpin either inside or outside the bacterium. However, the hrpAZ‐complemented strain produced harpin protein both inside and outside the cells. phrpAZ was also introduced into the T3SS‐defective mutant ΔhrcC strain in Pta 6605 (Marutani et al., 2005). The hrpAZ‐complemented ΔhrcC strain produced harpin protein inside the cells. However, harpin protein was not detected in the culture supernatant, suggesting that the hrpAZ‐complemented ΔhrcC strain failed to secrete harpin protein outside the bacterium via the defective T3SS. HrpA encodes a major structural protein of the Hrp pilus (Roine et al., 1997). Although the hrpA‐complemented strain did not produce or secrete harpin protein, the hrpZ‐complemented strain did. However, the secretion of harpin by the hrpZ‐complemented strain was less than that by the hrpAZ‐complemented strain. This result indicates that exogenous expression of hrpA facilitates the secretion of harpin.
Figure 1.
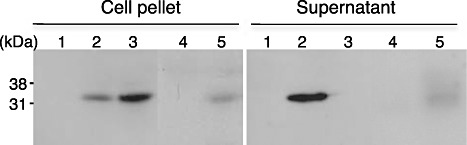
Immunoblot analysis of harpin from each strain of Pseudomonas syringae pv. tabaci (Pta). Each strain was grown in modified KB (2% proteose peptone No. 3, 1.6 nM MgSO4·7H2O, 8.6 mM K2HPO4, 1% glycerol) medium for 24 h and incubated in MMMF (50 mm potassium phosphate buffer, 7.6 mm (NH4)2SO4, 1.7 mm MgCl2, 1.7 mm NaCl, 10 mm mannitol, 10 mm fructose, pH 5.7) medium for 24 h. The bacterial culture was centrifuged, and the supernatant was concentrated 100‐fold. Harpin protein in the cell pellet and supernatant was detected as described previously by Taguchi et al. (2001). 1, Pta (wild‐type, WT); 2, Pta (phrpAZ); 3, PtaΔhrcC (phrpAZ); 4, Pta (phrpA); 5, Pta (phrpZ).
To investigate the effect of hrpAZ complementation in Pta 6605 on tobacco plants, tobacco leaves were inoculated with Pta 6605 WT and the hrpAZ‐complemented strain. The Pta WT strain caused typical and severe wildfire disease in tobacco, whereas the hrpAZ‐complemented strain did not cause any disease symptoms using infiltration and dip inoculation methods (Fig. 2A). Consistent with the inoculation of the hrpAZ‐complemented Pta, inoculation of the solo hrpZ‐complemented Pta did not cause severe disease symptoms; however, Pta WT with the solo hrpA‐complemented Pta and the Pta‐possessing empty plasmid produced typical symptoms of wildfire disease by both infiltration and dip inoculation methods, although the disease symptoms caused by the inoculation of the solo hrpA‐complemented Pta were not as severe as those caused by the Pta WT strain (Fig. 2A). These results indicate that hrpZ‐complemented Pta shows reduced virulence as a result of the expression of exogenous hrpZ, and that the exogenous expression of hrpA also reduces the virulence slightly. Furthermore, bacterial propagation of hrpAZ‐, hrpZ‐ and hrpA‐complemented strains was reduced compared with that of the Pta WT strain and empty plasmid possessing Pta (Fig. 2B).
Figure 2.
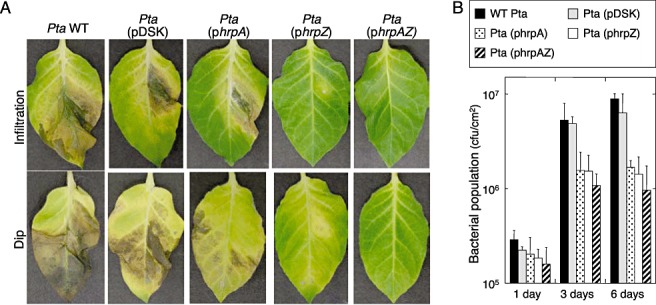
Inoculation of tobacco leaves with Pseudomonas syringae pv. tabaci (Pta) wild‐type (WT), hrpA‐, hrpZ‐ and hrpAZ‐complemented strains. (A) Tobacco leaves (Nicotiana tabacum L. cv. Xanthi NC) were inoculated by infiltration with 2 × 105 colony‐forming units (cfu)/mL (top) and by the dip inoculation method with 2 × 108 cfu/mL (bottom), and were incubated at 23°C for 10 days. (B) The bacterial population of each strain on host tobacco leaves was measured at 1, 3 and 6 days after dip inoculation. Bacterial strains were Pta 6605 WT (Pta WT) and an empty vector possessing Pta[Pta (pDSK)], hrpA complement [Pta (phrpA)], hrpZ complement [Pta (phrpZ)] and hrpAZ complement [Pta (phrpAZ)]. The bars represent standard deviations for at least three independent experiments.
Because tobacco plants are highly sensitive to harpin elicitors and mount strong defence responses, including hypersensitive cell death, oxidative burst and the activation of defence‐related genes (Andi et al., 2001; He et al., 1993; Ichinose et al., 2001), the reduction of virulence in the hrpAZ‐complemented strain might be a consequence of the induction of harpin‐triggered defence responses. To examine tobacco defence responses, callose deposition and harpin‐induced gene expression were investigated. Inoculation of the hrpAZ‐complemented strain induced callose deposition in tobacco leaves, whereas inoculation of Pta WT or empty plasmid possessing Pta did not (Fig. 3A). Pta (phrpA) weakly induced callose deposition, and the induction of callose deposition by Pta (phrpZ) was intermediate between that of Pta (phrpA) and Pta (phrpAZ). Semi‐quantitative reverse transcription (RT)‐PCR analysis was performed to monitor the expression of the harpin‐induced 1 gene (HIN1; Gopalan et al., 1996). HIN1 is a typical plant defence gene, and tobacco HIN1 mRNA is accumulated as a result of treatment with harpins from Erwinia amylovora Ea321 and P. syringae pv. syringae (Pss) 61 (Gopalan et al., 1996). Total RNA was purified from tobacco leaves that had been inoculated with Pta 6605 WT, hrpA‐, hrpZ‐ or hrpAZ‐complemented strains by the infiltration method at 2 × 108 colony‐forming units (cfu)/mL. The induction of HIN1 expression was prominent after inoculation with the hrpAZ‐ and hrpZ‐complemented strains, although HIN1 was not remarkably induced by inoculation with the Pta 6605 WT strain (Fig. 3B).
Figure 3.
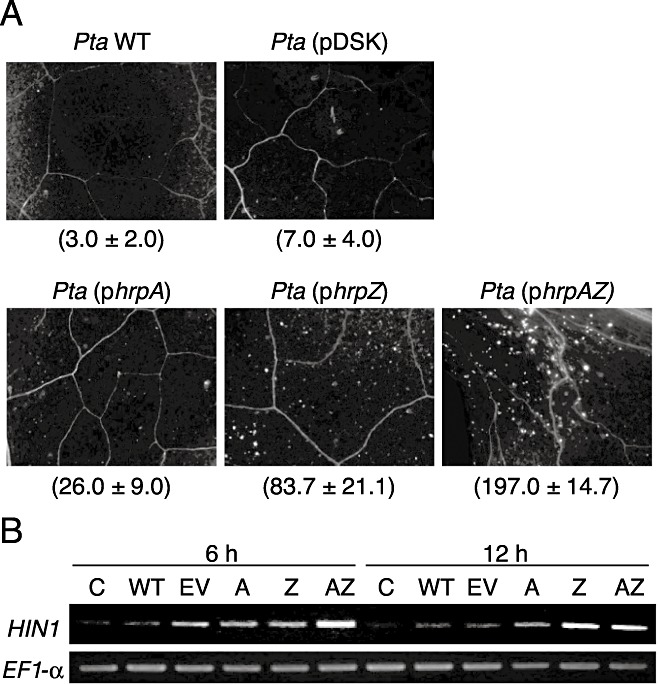
Tobacco defence responses. Tobacco leaves were infiltration inoculated with Pseudomonas syringae pv. tabaci (Pta) 6605 [Pta wild‐type (WT)] or Pta (phrpAZ). (A) Tobacco leaves were incubated for 48 h, and then stained with aniline blue according to the method described by Adam and Somerville (1996) to observe callose deposition. The average number (±standard deviation) of callose deposits per 4‐mm2 area is shown in parentheses. (B) Gene expression of HIN1 (AF212183) was investigated by semi‐quantitative reverse transcription‐polymerase chain reaction (RT‐PCR). One microgram of total RNA was used to synthesize the first‐strand cDNA with AMV RTase according to the manufacturer's protocols (Takara, Otsu, Japan). The PCR programme involved one cycle of 95°C for 2 min, followed by 28 cycles of 95°C for 30 s, 53°C for 30 s and 72°C for 1 min. The expression of elongation factor 1α (EF‐1α, D63396) was determined as a housekeeping gene. Primer sequences used for PCR were as follows: NtHIN1 (5′‐caaggcgagaggtttgatag‐3′, 5′‐gatggcatctggtttcctca‐3′) and NtEF‐1α (5′‐tcacatcaacattgtggtcattggc‐3′, 5′‐tggtagcatccatcttgttacagca‐3′). Experiments were performed in triplicate and representative results are shown.
The recombinant 42‐kDa harpinPss (harpin from Pss) is known to effectively induce defence responses in tobacco (>2.4 µm), potato (>2.4 µm) and tomato (>20 µm), but not in bean at a concentration of 60 µm (He et al., 1993). Similarly, harpinPss, harpinPgl and harpinPto (harpins from Pss, Pgl and Pto, respectively) elicit HR in tomato but not in soybean (Preston et al., 1995). We also observed that the recombinant harpinPto, harpinPgl and harpinPpi (harpin from Ppi) did not elicit pea defence responses at a concentration of 6.8 µm (Tanaka et al., 2001). Thus, the effects of harpin vary among plant species, and leguminous plants seem to be insensitive or slightly sensitive to harpins from P. syringae. The reason for the differential response in plants to harpin protein may depend on the existence of corresponding receptors in plants and quantitative differences in behaviour in harpin recognition. He et al. (1993) also found that a recombinant 32‐kDa harpin protein (harpinPssΔ125), in which 125 N‐terminal amino acids were deleted, induced a rather higher defence response. For example, harpinPssΔ125 induced a defence response in tobacco (>0.6 µm), tomato (>5 µm) and potato (>0.6 µm), indicating that the 125 N‐terminal amino acids in harpinPss are not required to induce the plant defence response (He et al., 1993). To define the smallest fragment responsible for elicitor activity, a variety of sizes and domains of recombinant harpinPph polypeptides (harpins from Pph) were expressed in E. coli, and their elicitor activity was assessed in tobacco (Lee et al., 2001a). The results suggest that the elicitor activity of recombinant harpinPph resides in a carboxy (C)‐terminal fragment corresponding to amino acids 200–300 (Lee et al., 2001a). Furthermore, harpinPss lacking an amino acid sequence (254–298) and harpinPph lacking a C‐terminal sequence (306–345) lost HR‐inducing activity (Haapalainen et al., 2010; He et al., 1993). Haapalainen et al. (2010) also reported that tobacco recognizes a P24 peptide (amino acids 290–313, PNQDLGQLLGGLLQKGLEATLQDA), which resides in the C‐terminal domain from Pph HrpZ and elicits a full HR in tobacco. Because the truncated Pta harpin protein also lacks the C‐terminal domain, including P24 (Fig. S1), our observation is consistent with the results of He et al. (1993), Lee et al. (2001a) and Haapalainen et al. (2010). Although the structurally minimal portion of harpin that elicits plant defence was not clearly identified in other pathovars, a deleted portion of harpinPta might contain the elicitor‐active domain in part.
Previously, Huang et al. (1988) have reported that the cosmid clone pHIR11 containing a 31‐kb DNA from Pss 61 is responsible for the elicitation of plant reactions. A pHIR11 cosmid that contains the entire hrp gene cluster and hrp‐linked hrmA has been reported to contain two regions that alkalinize the medium of tobacco suspension cell cultures (1988, 1995). HrmA was found to encode an Avr‐like protein, HopPsyA, that converts compatible tobacco–pathogen interactions into incompatible interactions (Alfano et al., 1997; Shen et al., 2000). However, the hrmA homologue was absent in Pta 11528, and thus heterologous expression of hrmA from pHIR11 enhanced the tobacco defence response (Alfano et al., 1997). Very recently, it has been revealed that there is also no hrmA homologue in Pta 6605 (D. Studholme, University of Exeter, personal communication). In this study, we found that harpin functions as an avirulence factor when expressed in Pta, although harpin is not strictly an avirulence factor because it is not race–cultivar specific, but rather pathovar–plant species' specific, and the corresponding resistance gene for the hrpZ gene is not known.
Although hrpZ‐ and hrpAZ‐complemented strains did not cause disease symptoms, they were able to survive and multiply to some extent in tobacco leaves, indicating that the disruption of hrpZ is not the only adaptation of Pta that allows this bacterial strain to live in tobacco plants. It is well known that Pta secretes a variety of virulence factors, such as effectors and tabtoxin, that allow its growth in tobacco leaves. These factors might suppress strong harpin‐induced defence responses, including HR. We observed that the inoculation of tobacco leaves with Pta (phrpZ) and Pta (phrpAZ) did not cause HR, and that all bacteria isolated from tobacco leaves 6 days after inoculation with Pta (phrpAZ) were kanamycin resistant. These results indicate that the plasmid was not cured from bacteria during the infection process, and it is possible that the hrpZ‐complemented strains may not secrete sufficient harpin to induce HR cell death.
Comparison of the phenotypes of Pta (phrpAZ) and Pta (phrpZ) shows that the reduction in virulence is more prominent in Pta (phrpAZ) (Fig. 2A), and that Pta (phrpAZ) induces callose deposition and HIN1 gene expression more strongly than Pta (phrpZ) (Fig. 3). It is not known whether or not HrpA has elicitor activity. However, if HrpA has weak elicitor activity, these results can be explained as tobacco defence responses induced by the expression of an elicitor protein HrpA.
In the phylogenetic analyses of P. syringae pathovars, DNA sequences for hrp and its neighbouring regions were investigated, and Pta was classified in the same group with Pgl and Pph (Guttman et al., 2006; Inoue and Takikawa, 2000; Sawada et al., 1999). Guttman et al. (2006) carried out the comparative sequencing analysis of 22 hrpZ operons from P. syringae strains, and reported that hrpA is under diversifying selection to maintain genetic diversity in order to avoid detection by the host's innate immune system, whereas hrpZ is not under strong selective pressure. However, Guttman et al. (2006) did not include hrpZ of Pta in the construction of phylogenetic trees for their analysis. Thus, Guttman's result and our study indicate that, except for Pta, hrpZ is relatively stable among P. syringae pathovars, and that the internal deletion in hrpZ is specific to Pta.
Although the intrinsic function of harpin protein in pathogenesis is not fully understood, based on the evidence that harpinPph binds to lipid bilayers and forms an ion‐conducting pore (Lee et al., 2001b), it is thought that harpin allows nutrient release and/or delivery of virulence factors during bacterial colonization through the host plasma membrane. The HrpNEa harpin of E. amylovora triggered defence responses on its nonhost Arabidopsis thaliana cells but not on host apple cells (Reboutier et al., 2007). Reboutier et al. (2007) hypothesized that HrpNEa harpin assists the translocation of effector proteins into plant cells. Some nonhost plants seem to be able to recognize HrpNEa harpin and trigger defence responses; however, apple cells are probably missing a guard receptor able to recognize harpin and induce defence responses. Furthermore, Bocsanczy et al. (2008) demonstrated that HrpNEa harpin was involved in the delivery of DspA/E, an indispensable virulence factor of E. amylovora. Recently, Engelhardt et al. (2009) reported that the intrinsic function of HrpZ1 harpin from Pph is to permeate membranes during host infection, and that the pore formation and plant immunity‐stimulating activities of HrpZ1 harpin are structurally separable. Namely, a C‐terminal fragment of HrpZ1 harpin retains the ability to activate plant immunity, whereas ion pore formation requires intact HrpZ1.
We speculate that, when the ancestor of Pta attempted to invade tobacco plants, harpin might have been one of the virulence factors. However, after tobacco plants acquired a receptor molecule for harpin protein and established the mechanisms to trigger strong and rapid harpin‐mediated defence responses, the ancestor of Pta apparently lost its virulence on tobacco plants. Thus, Pta might have adapted to the tobacco plant by disruption of the hrpZ gene to evade recognition, and might have again acquired virulence on tobacco plants. Such co‐evolution of host plants to overcome pathogenic invasion and of phytopathogens to evade host detection and/or to suppress the expression of defence response is often called an arms race (Ingle et al., 2006). Thus, the deletion of hrpZ in Pta is a typical example of an evolutionary arms race between plants and phytopathogenic bacteria.
Supporting information
Figure S1 Deduced amino acid sequences of harpin proteins from Pseudomonas syringae pv. tabaci (Pta) (Taguchi et al., 2001), P. syringae pv. phaseolicola (Pph) (Lee et al., 2001a) and P. syringae pv. syringae (Pss) (He et al., 1993). The amino acid sequence from Pta is frameshifted by the deletion at position 131, and the nonhomologous extension is represented by lowercase letters. The peptide sequence represented by red letters (P24) in Pph elicits a full hypersensitive response (HR) in tobacco (Haapalainen et al., 2010). Peptide sequences underlined are reported to be necessary for HR induction in Pph (Haapalainen et al., 2010) and Pss (He et al., 1993).
Supporting info item
ACKNOWLEDGEMENTS
We thank the Leaf Tobacco Research Laboratory, Japan Tobacco Inc. and Dr D. Studholme, University of Exeter, UK, for providing Pta 6605 and unpublished genome sequence information of Pta 6605, respectively. This work was supported in part by Grants‐in‐Aid for Scientific Research (B) (No. 18380035) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- Adam, L. and Somerville, S.C. (1996) Genetic characterization of five powdery mildew disease resistance loci in Arabidopsis thaliana . Plant J. 9, 341–356. [DOI] [PubMed] [Google Scholar]
- Alfano, J.R. and Collmer, A. (2004) Type III secretion system effector proteins: double agents in bacterial disease and plant defense. Annu. Rev. Phytopathol. 42, 385–414. [DOI] [PubMed] [Google Scholar]
- Alfano, J.R. , Kim, H.S. , Delaney, T.P. and Collmer, A. (1997) Evidence that the Pseudomonas syringae pv. syringae hrp‐linked hrmA gene encodes an Avr‐like protein that acts in an hrp‐dependent manner within tobacco cells. Mol. Plant–Microbe Interact. 10, 580–588. [DOI] [PubMed] [Google Scholar]
- Andi, S. , Taguchi, F. , Toyoda, K. , Shiraishi, T. and Ichinose, Y. (2001) Effect of methyl jasmonate on harpin‐induced hypersensitive cell death, generation of hydrogen peroxide and expression of defense genes in tobacco suspension cultured BY‐2 cells. Plant Cell Physiol. 42, 446–449. [DOI] [PubMed] [Google Scholar]
- Bocsanczy, A.M. , Nissinen, R.M. , Oh, C.S. and Beer, S.V. (2008) HrpN of Erwinia amylovora functions in the translocation of DspA/E into plant cells. Mol. Plant Pathol. 9, 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charkowski, A.O. , Alfano, J.R. , Preston, G. , Yuan, J. , He, S.Y. and Collmer, A. (1998) The Pseudomonas syringae pv. tomato HrpW protein has domains similar to harpins and pectate lyases and can elicit the plant hypersensitive response and bind to pectate. J. Bacteriol. 180, 5211–5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt, S. , Lee, J. , Gäbler, Y. , Kemmerling, B. , Haapalainen, M.L. , Li, C.M. , Wei, Z. , Keller, H. , Joosten, M. , Taira, S. and Nürnberger, T. (2009) Separable roles of the Pseudomonas syringae pv. phaseolicola accessory protein HrpZ1 in ion‐conducting pore formation and activation of plant immunity. Plant J. 57, 706–717. [DOI] [PubMed] [Google Scholar]
- Gopalan, S. , Wei, W. and He, S.Y. (1996) hrp gene‐dependent induction of hin1: a plant gene activated rapidly by both harpins and the avrPto gene‐mediated signal. Plant J. 10, 591–600. [DOI] [PubMed] [Google Scholar]
- Grant, S.R. , Fisher, E.J. , Chang, J.H. , Mole, B.M. and Dangl, J.L. (2006) Subterfuge and manipulation: type III effector proteins of phytopathogenic bacteria. Annu. Rev. Microbiol. 60, 425–449. [DOI] [PubMed] [Google Scholar]
- Guttman, D.S. , Gropp, S.J. , Morgan, R.L. and Wang, P.W. (2006) Diversifying selection drives the evolution of the type III secretion system pilus of Pseudomonas syringae . Mol. Biol. Evol. 23, 2342–2354. [DOI] [PubMed] [Google Scholar]
- Haapalainen, M. , Engelhardt, S. , Küfner, I. , Li, C.‐M. , Nürnberger, T. , Lee, J. , Romantschuk, M. and Taira, S. (2010) Functional mapping of harpin HrpZ of Pseudomonas syringae reveals the sites responsible for protein oligomerization, lipid interactions and plant defence induction. Mol. Plant Pathol. 12, 151–166. doi: 10.1111/j.1364‐3703.2010.00655.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, S.Y. (1996) Elicitation of plant hypersensitive response by bacteria. Plant Physiol. 112, 865–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, S.Y. , Huang, H. and Collmer, A. (1993) Pseudomonas syringae harpinPss: a protein that is secreted via the Hrp pathway and elicits the hypersensitive response in plants. Cell, 73, 1255–1266. [DOI] [PubMed] [Google Scholar]
- Huang, H.C. , Schuurink, R. , Denny, T.P. , Atkinson, M.M. , Baker, C.J. , Yucel, I. , Hutcheson, S.W. and Collmer, A. (1988) Molecular cloning of a Pseudomonas syringae pv. syringae gene cluster that enables Pseudomonas fluorescens to elicit the hypersensitive response in tobacco plants. J. Bacteriol. 170, 4748–4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H.C. , Lin, R.H. , Chang, C.J. , Collmer, A. and Deng, W.L. (1995) The complete hrp gene cluster of Pseudomonas syringae pv. syringae 61 includes two blocks of genes required for harpinPss secretion that are arranged colinearly with Yersinia ysc homologs. Mol. Plant–Microbe Interact. 8, 733–746. [DOI] [PubMed] [Google Scholar]
- Ichinose, Y. , Andi, S. , Doi, R. , Tanaka, R. , Taguchi, F. , Sasabe, M. , Toyoda, K. , Shiraishi, T. and Yamada, T. (2001) Generation of hydrogen peroxide is not required for harpin‐induced apoptotic cell death in tobacco BY‐2 cell suspension culture. Plant Physiol. Biochem. 39, 771–776. [Google Scholar]
- Ingle, R.A. , Carstens, M. and Denby, K.J. (2006) PAMP recognition and the plant–pathogen arms race. Bioessays, 28, 880–889. [DOI] [PubMed] [Google Scholar]
- Inoue, Y. and Takikawa, Y. (2000) Pseudomonas syringae strains are classified into five groups by comparing DMA homology at the hrp neighboring regions. J. Gen. Plant Pathol. 66, 238–241. [Google Scholar]
- Jin, Q. , Thilmony, R. , Zwiesler‐Vollick, J. and He, S.Y. (2003) Type III protein secretion in Pseudomonas syringae . Microbes Infect. 5, 301–310. [DOI] [PubMed] [Google Scholar]
- Keen, N.T. , Tamaki, S. , Kobayashi, D. and Trollinger, D. (1988) Improved broad‐host‐range plasmids for DNA cloning in Gram‐negative bacteria. Gene, 70, 191–197. [DOI] [PubMed] [Google Scholar]
- Lee, J. , Klessig, D.F. and Nürnberger, T. (2001a) A harpin binding site in tobacco plasma membranes mediates activation of the pathogenesis‐related gene HIN1 independent of extracellular calcium but dependent on mitogen‐activated protein kinase activity. Plant Cell, 13, 1079–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. , Klusener, B. , Tsiamis, G. , Stevens, C. , Neyt, C. , Tampakaki, A.P. , Panopoulos, N.J. , Nöller, J. , Weiler, E.W. , Cornelis, G.R. , Mansfield, J.W. and Nürnberger, T. (2001b) HrpZ (Psph) from the plant pathogen Pseudomonas syringae pv. phaseolicola binds to lipid bilayers and forms an ion‐conducting pore in vitro. Proc. Natl. Acad. Sci. USA, 98, 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren, P.B. , Peet, R.C. and Panopoulos, N.J. (1986) Gene cluster of Pseudomonas syringae pv. phaseolicola controls pathogenicity of bean plants and hypersensitivity on nonhost plants. J. Bacteriol. 168, 512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield, J.W. (2009) From bacterial avirulence genes to effector functions via the hrp delivery system: an overview of 25 years of progress in our understanding of plant innate immunity. Mol. Plant Pathol. 10, 721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marutani, M. , Taguchi, F. , Shimizu, R. , Inagaki, Y. , Toyoda, K. , Shiraishi, T. and Ichinose, Y. (2005) Flagellin from Pseudomonas syringae pv. tabaci induced hrp‐independent HR in tomato. J. Gen. Plant Pathol. 71, 289–295. [Google Scholar]
- Nakada, H. , Tahara, A. , Hayashi, M. , Shimizu, R. , Tanaka, R. , Ichinose, Y. , Shiraishi, T. and Yamada, T. (1999) Cloning and structural characterization of hrp locus of Pseudomonas syringae pv. pisi . Ann. Phytopathol. Soc. Jpn. 65, 147–152. [Google Scholar]
- Preston, G. , Hung, H. , He, S.Y. and Collmer, A. (1995) The HrpZ proteins of Pseudomonas syringae pvs. syringae, glycinea and tomato are encoded by an operon containing Yersinia ysc homologs and elicit the hypersensitive response in tomato but not soybean. Mol. Plant–Microbe Interact. 8, 717–732. [DOI] [PubMed] [Google Scholar]
- Reboutier, D. , Frankart, C. , Briand, J. , Biligui, B. , Laroche, S. , Rona, J.P. , Barny, M.A. and Bouteau, F. (2007) The HrpNea harpin from Erwinia amylovora triggers differential responses on the nonhost Arabidopsis thaliana cells and on the host apple cells. Mol. Plant–Microbe Interact. 20, 94–100. [DOI] [PubMed] [Google Scholar]
- Roine, E. , Wei, W. , Yuan, J. , Nurmiaho‐Lassila, E.L. , Kalkkinen, N. , Romantschuk, M. and He, S.Y. (1997) Hrp pilus: an hrp‐dependent bacterial surface appendage produced by Pseudomonas syringae pv. tomato DC3000. Proc. Natl. Acad. Sci. USA, 94, 3459–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada, H. , Suzuki, F. , Matsuda, I. and Saitou, N. (1999) Phylogenetic analysis of Pseudomonas syringae pathovars suggests the horizontal gene transfer of argK and the evolutionary stability of hrp gene cluster. J. Mol. Evol. 49, 627–644. [DOI] [PubMed] [Google Scholar]
- Shen, S. , Li, Q. , He, S.Y. , Barker, K.R. , Li, D. and Hunt, A.G. (2000) Conversion of compatible plant–pathogen interactions into incompatible interactions by expression of the Pseudomonas syringae pv. syringae 61 hrmA gene in transgenic tobacco plants. Plant J. 23, 205–213. [DOI] [PubMed] [Google Scholar]
- Shimizu, R. , Taguchi, F. , Marutani, M. , Mukaihara, T. , Inagaki, Y. , Toyoda, K. , Shiraishi, T. and Ichinose, Y. (2003) The ?fliD mutant of Pseudomonas syringae pv. tabaci, which secretes flagellin monomers, induces a strong hypersensitive reaction (HR) in non‐host tomato cells. Mol. Genet. Genomics, 269, 21–30. [DOI] [PubMed] [Google Scholar]
- Taguchi, F. , Tanaka, R. , Kinoshita, S. , Ichinose, Y. , Imura, Y. , Andi, S. , Toyoda, K. , Shiraishi, T. and Yamada, T. (2001) HarpinPsta from Pseudomonas syringae pv. tabaci is defective and deficient in its expression and HR‐inducing activity. J. Gen. Plant Pathol. 67, 116–123. [Google Scholar]
- Tanaka, R. , Taguchi, F. , Ichinose, Y. , Toyoda, K. , Shiraishi, T. and Yamada, T. (2001) Effect of harpin from four pathovars of Pseudomonas syringae on pea defense responses. J. Gen. Plant Pathol. 67, 148–151. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Deduced amino acid sequences of harpin proteins from Pseudomonas syringae pv. tabaci (Pta) (Taguchi et al., 2001), P. syringae pv. phaseolicola (Pph) (Lee et al., 2001a) and P. syringae pv. syringae (Pss) (He et al., 1993). The amino acid sequence from Pta is frameshifted by the deletion at position 131, and the nonhomologous extension is represented by lowercase letters. The peptide sequence represented by red letters (P24) in Pph elicits a full hypersensitive response (HR) in tobacco (Haapalainen et al., 2010). Peptide sequences underlined are reported to be necessary for HR induction in Pph (Haapalainen et al., 2010) and Pss (He et al., 1993).
Supporting info item


