SUMMARY
Fungi respond and adapt to different environmental stimuli via signal transduction systems. We determined the function of a yeast SLT2 mitogen‐activated protein (MAP) kinase homologue (AaSLT2) in Alternaria alternata, the fungal pathogen of citrus. Analysis of the loss‐of‐function mutant indicated that AaSLT2 is required for the production of a host‐selective toxin, and is crucial for fungal pathogenicity. Moreover, the A. alternata slt2 mutants displayed hypersensitivity to cell wall‐degrading enzymes and chemicals such as Calcofluor white and Congo red. This implicates an important role of AaSLT2 in the maintenance of cell wall integrity in A. alternata. The A. alternata slt2 mutants were also hypersensitive to a heteroaromatic compound, 2‐chloro‐5‐hydroxypyridine, and a plant growth regulator, 2,3,5‐triiodobenzoic acid. Developmentally, the AaSLT2 gene product was shown to be critical for conidial formation and hyphal elongation. Compared with the wild‐type, the mutants produced fewer but slightly larger conidia with less transverse septae. The mutants also accumulated lower levels of melanin and chitin. Unlike the wild‐type progenitor, the A. alternata slt2 mutants produced globose, swollen hyphae that did not elongate in a straight radial direction. All defective phenotypes in the mutant were restored by transformation and expression of a wild‐type copy of AaSLT2 under the control of its endogenous promoter. This study highlights an important role of the AaSLT2 MAP kinase‐mediated signalling pathway, regulating diverse physiological, developmental and pathological functions, in the tangerine pathotype of A. alternata.
INTRODUCTION
Mitogen‐activated protein (MAP) kinases which belong to the serine/threonine protein family play crucial roles in the transduction of environmental signals and in the regulation of gene expression in eukaryotes (Gustin et al., 1998; Kültz, 1998; Pelech and Sanghera, 1992; Robinson and Cobb, 1997). These systems deliver environmental signals via sequential phosphate transfers from a MAP kinase kinase kinase (MAPKKK) to a MAP kinase kinase (MAPKK) and then to MAP kinase (MAPK). The budding yeast Saccharomyces cerevisiae has five distinct MAPK‐mediated signalling pathways. Filamentous fungi have three MAPK signalling pathways of the yeast, SLT2, FUS3 and HOG1, each with diverse functions (Xu, 2000; Zhou et al., 2007). The SLT2 pathway is primarily involved in pathogenicity, invasion, vegetative growth, conidiation, cell wall integrity, anastomosis and response to oxidative stress. The FUS3 pathway regulates mating, vegetative growth and the formation of filamentous hyphae, conidia, fruiting bodies and appressoria, and is also necessary for the pathogenicity of various fungi. The HOG1 pathway participates in the stress response, toxicity of fungicides and pathogenesis.
Although MAPK signalling pathways are well conserved in eukaryotes, each pathway may have different physiological, developmental and pathological functions within and between species (Bardwell, 2006). In addition, interconnections or cross‐regulations between different transduction pathways further exacerbate the complexity of the signalling network (Chen and Thorner, 2007; Schwartz and Madhani, 2004; Smith and Scott, 2002). For example, expression of the genes whose products are involved in melanin biosynthesis and pigmentation is co‐regulated by FUS3 and SLT2 MAPK‐mediated pathways in the maize pathogen Cochliobolus heterostrophus (Eliahu et al., 2007). HOG1 has been shown to suppress the FUS3/KSS1 signalling pathway in budding yeast in response to hyperosmotic stress (Shock et al., 2009).
SLT2 homologues have been demonstrated to regulate the integrity of cell walls in Alternaria brassicicola, Aspergillus nidulans, Claviceps purpurea, Fusarium graminearum and Magnaporthe grisea (Fujioka et al., 2007; Hou et al., 2002; Mey et al., 2002; Scott, 2009; Xu et al., 1998), but not in Botrytis cinerea, Colletotrichum lagenarium and Mycosphaerella graminicola (Kojima et al., 2002; Mehrabi et al., 2006; Rui and Hahn, 2007). SLT2 homologues are required for the production of secondary metabolites in F. graminearum and Neurospora crassa (Hou et al., 2002; Park et al., 2008). In the human pathogen Aspergillus fumigatus, SLT2 is needed for cell wall integrity and resistance to oxidants, but not for fungal pathogenicity (Valiante et al., 2008). However, SLT2 homologues are required for cell wall integrity, stress response and fungal virulence in other human pathogens, Candida albicans and Cryptococcus neoformans (Kraus et al., 2003; Monge et al., 2006).
Alternaria alternata in citrus has two different pathotypes, each producing a host‐selective toxin (HST) with defined host specificity (Ito et al., 2004; Miyamoto et al., 2009). The tangerine pathotype produces an ACT (Alternaria citri Tangerine pathotype) toxin which is toxic to tangerines, grapefruit and some of their hybrids. The rough lemon pathotype produces an ACRL (Alternaria citri Rough Lemon pathotype) toxin which is only toxic to rough lemon and rangpur lime. The production and toxicity of HSTs have long been recognized to be required for pathogenicity by A. alternata. It is equally important to note that cellular detoxification of reactive oxygen species mediated by the redox‐responsive AP1 transcription regulator, a YAP1 homologue, has recently been demonstrated to be essential for the pathogenesis of A. alternata in citrus (Lin et al., 2009; Yang et al., 2009). YAP1 is a leucine zipper‐containing transcription activator belonging to the mammalian AP1 family (Moye‐Rowley, 2003). Further investigation has uncovered a synergistic linkage between AaAP1‐regulated gene expression and AaFUS3 MAPK‐mediated signalling pathways in A. alternata (Lin et al., 2010). AaAP1 has been documented to interfere with AaFUS3 by suppressing post‐translational phosphorylation. In contrast with the AaAP1 gene, AaFUS3 is not essential for resistance to oxidative stress. The study also inadvertently discovered that both AaAP1 and AaFUS3 are involved in resistance to 2‐chloro‐5‐hydroxypyridine (CHP), 2,3,5‐triiodobenzoic acid (TIBA) and many other compounds containing benzene or pyridine backbones. TIBA is a plant regulator, which is often used as a herbicide or inhibitor of indole‐3‐acetic acid transportation. Pyridine containing a heteroaromatic compound is commonly present in many natural compounds. However, the mechanisms that confer CHP or TIBA resistance in A. alternata remain unknown. In addition, AaFUS3 showed an important effect on vegetative growth, conidiation, pathogenicity, production of melanin and hydrolytic enzymes, as well as resistance to copper fungicides and osmotic stress (Lin et al., 2010). Similar defects in conidial maturation were also observed by the disruption of a FUS3 gene homologue in the necrotrophic Brassica pathogen, A. brassicicola (Cho et al., 2007).
The important functions of a HOG1 MAPK homologue (AaHOG1), conferring cellular resistance to oxidative and salt stress, as well as pathogenicity, have been investigated recently in A. alternata (Lin and Chung, 2010). In many fungi and yeasts, the HOG1 MAPK‐mediated signalling pathway often functions collaboratively with the ‘two‐component’ histidine kinase. However, in response to osmotic stress, A. alternata AaHOG1 apparently does not function in the same pathway with a ‘two‐component’ histidine kinase (AaHSK1). AaHSK1, perhaps mediated via a phosphorelay system (Thomason and Kay, 2000), has been shown to be essential for response to sugar, but not to salt, osmolytes. AaHSK1 does not play any role in response to oxidative stress and pathogenicity. However, as with AaAP1 and AaFUS3, AaHSK1 and AaHOG1 are essential for resistance to CHP or TIBA, because disruption of either of these genes yields fungal strains highly sensitive to both compounds (Lin and Chung, 2010; Lin et al., 2010). The role of the SLT2‐type MAPK‐mediated signalling pathway remains largely unknown in A. alternata. We report the cloning and functional characterization of a yeast SLT2 homologue, AaSLT2, to explore its role in pathogenicity, conidiation and other genetic traits. We also determine whether AaSLT2 is required for resistance to CHP or TIBA in A. alternata.
RESULTS
Cloning and characterization of the A. alternata AaSTL2 gene
The AaSLT2 gene, encoding a yeast SLT2 MAPK homologue, was cloned by polymerase chain reaction (PCR) amplification from the genomic DNA of A. alternata. Sequence analysis revealed that AaSLT2 contains a 1677‐bp open reading frame (ORF) with five intervening introns (Fig. S1, see Supporting Information). The AaSLT2 ORF encodes a polypeptide containing 416 amino acids. The other two MAPKs, a high‐osmolarity glycerol homologue (AaHOG1, 355 amino acids) and a pheromone‐responsive homologue (AaFUS3, 352 amino acids) of yeasts, have been cloned and characterized previously in A. alternata (Lin and Chung, 2010; Lin et al., 2010). AaSLT2, AaHOG1 and AaFUS3, sharing 42%–48% amino acid similarity, all contain a protein kinase ATP‐binding signature and a serine/X/threonine (TXY) activation site in the N‐terminus (Fig. S1). Phylogenetic analyses revealed that AaSLT2 is most similar to MAPKs associated with cell wall integrity found in A. brassicicola, C. heterostrophus, Pyrenophora tritici‐repentis and M. graminicola, but shows less similarity to those in Co. lagenarium, Gibberella zeae and Hypocrea lixii (Fig. S2, see Supporting Information).
Generation of AaSTL2 disruption mutant
A split‐marker strategy was used to inactivate the AaSLT2 gene in the wild‐type of A. alternata (Fig. 1A). Two AaSLT2/HYG fusion fragments (2.9 and 1.9 kb), overlapping within the hygromycin phosphotransferase gene (HYG), were generated and co‐transformed into fungal protoplasts. Of six transformants recovered from the hygromycin‐containing medium, five were selected for further analyses because they formed white colonies distinct from that of the wild‐type (Fig. 2A and data not shown). Southern blot hybridization of SalI‐ and StuI‐digested genomic DNA purified from the wild‐type and five putative disruptants to an AaSLT2 gene probe resulted in the expected banding patterns (Fig. 1B), indicating the integration of HYG at the AaSLT2 locus. The 1.2‐kb AaSLT2 transcript was not detected in the RNA samples prepared from three putative transformants (Fig. 1C), thus confirming the successful inactivation of AaSLT2 in A. alternata.
Figure 1.

Targeted gene disruption of AaSLT2 in Alternaria alternata using a split‐marker approach. (A) Schematic depiction of gene disruption within AaSLT2 via a homologous integration, using truncated but overlapping DNA fragments of the hygromycin phosphotransferase gene (HYG) under the control of the Aspergillus nidulans trpC promoter. Split‐marker fragments 5′AaSLT2::h/YG (2.9 kb) and HY/g::3′AaSLT2 (1.9 kb) were amplified with primers SLT‐pro paired with hyg4 and primers SLT‐taa paired with hyg3, respectively. Oligonucleotide primers used to amplify each fragment are also indicated. (B) Southern blot hybridization of genomic DNA from wild‐type (WT) and five putative disruptants (Aaslt2). Fungal DNA was cleaved with SalI and StuI endonucleases, electrophoresed, blotted to a nylon membrane and hybridized to an AaSLT2 probe as indicated in (A). (C) RNA gel blotting. Fungal RNA was electrophoresed in a formaldehyde‐containing, denaturing gel, blotted and washed at high stringency after hybridization with an AaSLT2 probe.
Figure 2.

(A) Quantitative analysis and comparison of conidia produced by the wild‐type EV‐MIL31 (WT), the Alternaria alternata slt2 mutants (slt2‐D1, D2) and the genetically reverted strains (slt2‐D1/SLT2‐C6, C10) of A. alternata. Means (separation by Waller–Duncan k‐ratio t‐test, P ≤ 0.05) followed by the same letter are not significantly different. (B) Production of protoplasts over time by the A. alternata strains after exposure to cell wall‐degrading enzymes containing lyticase, driselase, β‐d‐glucanase and β‐glucuronidase. The release of protoplasts was determined with a haemocytometer by microscopy. Each column or point represents the mean number of conidia ± the standard error from two independent experiments, with at least three replicates.
AaSLT2 MAPK regulates conidiation
Phenotypic analysis revealed that disruption of the AaSLT2 gene resulted in fungi with reduced growth (data not shown). The A. alternata slt2 mutants accumulated little or no pigmentation compared with the wild‐type, showing fluffy and white colonies on potato dextrose agar (PDA) (Fig. 2A, inset). Quantitative analysis of conidial formation revealed that disruption of the AaSLT2 gene reduced conidiation by as much as 90% (Fig. 2A). Conidiation was restored in strains C6 and C10 by transforming slt2 D1 mutant protoplasts with a functional copy of AaSLT2. The slt2 D1 conidia germinated efficiently on glass slides (data not shown).
The A. alternata slt2 mutant is hypersensitive to cell wall‐degrading enzymes
The integrity of the fungal cell wall was assessed by the formation of protoplasts over time after fungal strains had been exposed to cell wall‐degrading enzymes. The A. alternata slt2 mutants released significantly more protoplasts (≥25%) than the wild‐type strain in the presence of an enzymatic mixture containing lyticase, driselase, β‐d‐glucanase and β‐glucuronidase (Fig. 2B). The genetically reverted strains released protoplasts at a rate and magnitude comparable with those of the wild‐type.
AaSLT2 is required for resistance to cell wall‐degrading compounds
The A. alternata slt2 mutants grew slightly more slowly than the wild‐type on PDA. Impairment of the AaSLT2 gene did not reduce the radial growth of the resulting mutants in the presence of sorbitol, glucose, caffeine, sodium dodecylsulphate (SDS) or H2O2 relative to the mock controls (Fig. 3). Application of NaCl or KCl restored radial growth by the A. alternata slt2 mutants. However, the slt2 mutants showed an elevated sensitivity to Calcofluor white, Congo red, TIBA and CHP. Previous studies discovered serendipitously that disruption of the AaFUS3 gene (encoding a FUS3 MAPK) or the AaHOG1 gene (encoding a HOG1 MAPK) resulted in fungal strains that were hypersensitive to TIBA and CHP (Lin and Chung, 2010; Lin et al., 2010). The A. alternata slt2 mutants did not display increased resistance to the dicarboximide fungicides (iprodione and vinclozolin) or the phenylpyrrole fungicide (fludioxonil) (data not shown). The transformation and expression of a functional copy of AaSLT2 under the control of its endogenous promoter in the A. alternata slt2 mutant fully restored all defective phenotypes (Fig. 3).
Figure 3.

Assays for hypersensitivity of the wild‐type EV‐MIL31 (WT), the Alternaria alternata slt2 mutants (slt2‐D1, D2) and the genetically reverted strains (slt2‐D1/SLT2‐C6, C10) of A. alternata. Fungal growth was measured 4–7 days after incubation on potato dextrose agar (PDA) or PDA containing 2,3,5‐triiodobenzoic acid (TIBA), 2‐chloro‐5‐hydroxypyridine (CHP) or other compounds as indicated (SDS, sodium dodecylsulphate). Only one representative replicate of each treatment is shown.
AaSLT2 plays an important role in fungal development
Light microscopy examination revealed that the wild‐type strain produced abundant multicellular, obclavate to obpyriform conidia with both vertical and transverse septae and dark pigment on PDA (Fig. 4). Many conidia contained short conical beaks at the tip, whereas others contained no beaks at all. In contrast, the A. alternata slt2 mutant generated aberrant, less melanized conidia with fewer vertical septae (Fig. 4). The size of conidia was also different: the wild‐type produced conidia of 8–11 µm × 22–33 µm (n = 10); the mutant produced slightly larger conidia of 10–13 µm × 24–35 µm. Scanning electron microscopy (SEM) analysis also revealed the morphological abnormalities of conidia produced by the A. alternata slt2 mutant, which were less verruculose than those of the wild‐type (Fig. 5). The complementation strain produced conidia with both vertical and transverse septae and size resembling those of the wild‐type. Further analysis by transmission electron microscopy (TEM) revealed that the conidial cell wall was wider in the wild‐type (0.849 µm) than in the A. alternata slt2 mutant (0.0989 µm) (Fig. 6). The conidia produced by the mutant apparently lack distinct vertical and transverse septae compared with those produced by the wild‐type.
Figure 4.

Multicellular conidia produced by the wild‐type EV‐MIL31 (WT) and the Alternaria alternata slt2 mutant (slt2) grown on potato dextrose agar (PDA) in the light for 7 days. As observed using a light microscope, the wild‐type conidia contain both vertical and transverse septae, whereas conidia produced by the slt2 strain have no distinct vertical septae. Bar, 10 µm.
Figure 5.

Visualization of conidia produced by the wild‐type EV‐MIL31 (WT), the Alternaria alternata slt2 mutant (slt2‐D1) and the genetically reverted strain (slt2‐D1/SLT2) of A. alternata using scanning electron microscopy. Fungal conidia were harvested from 7‐day‐old cultures grown on potato dextrose agar (PDA) in the light. The wild‐type and genetically reverted strains produce multicellular clavate conidia, showing a warty appearance with both cross and longitudinal septae. Conidia produced by the slt2‐D1 strain apparently lack septae, showing a less warty appearance. Bar, 10 µm.
Figure 6.
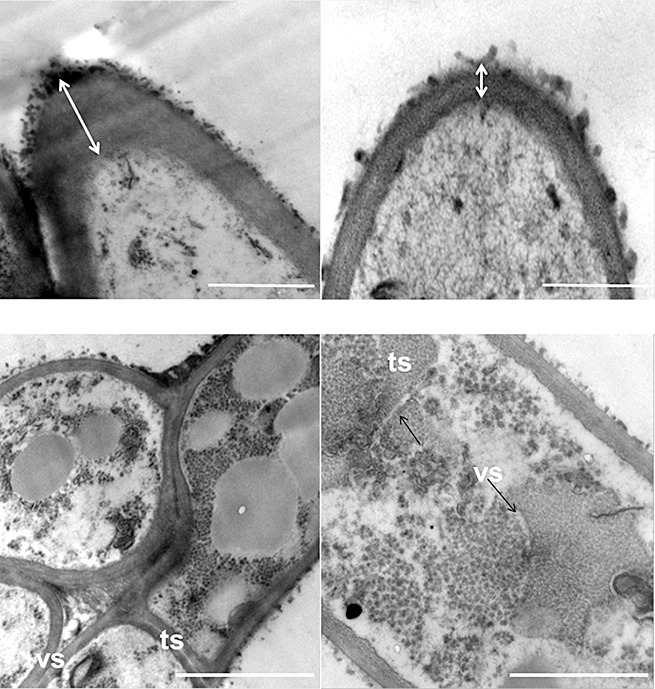
Transmission electron microscopy indicates that conidia produced by the wild‐type (WT) strain of Alternaria alternata contain a conical beak with a thick cell wall and form distinct vertical (vs) and transverse (ts) septae. In contrast, the slt2 strain produces conidia with a round‐shaped beak, a thinner cell wall and abnormal vertical and transverse septae compared with the wild type. Bar, 10 µm.
The wild‐type strain of A. alternata often formed dark‐pigmented colonies, containing typical septate mycelium which grew relatively straight, often branched, on agar medium (Fig. 7). In contrast, the A. alternata slt2 mutant produced round‐shaped hyphae that extended in a crooked pattern. Some hyphal apices tended to form hairpin loops (Fig. 7).
Figure 7.

Scanning electron microscopy reveals that the slt2 mutant of Alternaria alternata produces abnormal hyphae which elongate in a devious path and often form a hairpin loop in the tips (indicated by an arrow) compared with the wild‐type (WT). Bar, 10 µm.
AaSLT2 is involved in the accumulation of melanin and chitin
Compared with the wild‐type, the A. alternata slt2 mutants produced less pigments. Quantification assays revealed that the mutant showed noticeably decreased melanin accumulation: at least five‐fold that of the wild‐type (Fig. 8A). The melanin production ability was fully restored to wild‐type levels by the transformation of mutant protoplasts with a functional copy of AaSLT2. This confirms an important role of the AaSLT2 gene in the production of melanin. The A. alternata slt2 mutants also showed lower chitin content in relation to the levels measured in the wild‐type and the genetically reverted strains (Fig. 8B).
Figure 8.

Quantification of melanin (A) and chitin (B) in the wild‐type EV‐MIL31 (WT), the Alternaria alternata slt2 mutants (slt2‐D1, D2) and the genetically reverted strains (slt2‐D1/SLT2‐C6, C10) of A. alternata. Melanin was purified with 2% NaOH. Chitin was extracted with HCl and determined by measuring the acid‐released glucosamine from chitin using p‐dimethylaminobenzaldehyde as a chromogen. Each column represents the mean value of the compound ± the standard error from two independent experiments with at least three replicates. Means (separation by Waller–Duncan k‐ratio t‐test, P ≤ 0.05) followed by the same letter are not significantly different.
Inactivation of AaSLT2 reduces ACT toxin production
To determine whether AaSLT2 plays any role in the production of secondary metabolites, fungal isolates were grown in culture medium conducive for ACT production (Kohmoto et al., 1991). The secretion of host‐selective ACT toxin by A. alternata strains was evaluated by a bioassay on detached citrus leaves dipping in cell‐free culture filtrates (Fig. 9A). Culture filtrates obtained from the wild‐type or the complementation strains resulted in necrotic lesions along the vascular system at day 2. Citrus leaves treated with water or culture filtrates from the A. alternata slt2 mutant resulted in no noticeable lesions (Fig. 9A). The results indicate that the loss of AaSLT2 function correlates with a reduced ability to form lesions. To detect and quantify ACT toxin, culture filtrates were further extracted with Amberlite XAD‐2 resin and ethyl acetate. Analysis by thin layer chromatography (TLC) revealed that the A. alternata slt2 mutants produced smaller amounts of ACT (R f = 0.53) in axenic culture (Fig. 9B). The R f = 0.53 band (indicated by an arrow) has been shown to be toxic to host plants (Lin et al., 2011). Quantification analysis further confirmed a reduced production of ACT toxin by the A. alternata slt2 mutant (Fig. 9C).
Figure 9.

Impairment of the AaSLT2 gene in the tangerine pathotype of Alternaria alternata causes a reduced accumulation of host‐selective toxin. (A) Development of necrotic lesions along the vascular system on detached calamondin leaves after 3 days of soaking in cell‐free culture filtrates obtained from A. alternata wild‐type (WT), slt2 deletion D1 and slt2 D1/SLT2 complementation strains grown in Richard's medium for 24 days. The mock controls were treated with sterile water only. (B) Thin‐layer chromatography (TLC) analysis of the ethyl acetate extract separated with benzene/ethyl acetate/acetic acid, indicating a reduced amount of ACT (Alternaria citri Tangerine pathotype) toxin (R f = 0.53 indicated by an arrow) produced by the A. alternata slt2 mutants. (C) Quantification of host‐selective ACT toxin. The amounts of toxin were determined after ethyl acetate extracts had been completely dried in a preweighed centrifuge tube, confirming further that the slt2 mutant produces less ACT toxin in axenic culture.
AaSLT2 is required for full virulence
Pathogenicity assays using a point‐inoculation method revealed that the A. alternata slt2 mutants produced necrotic lesions similar to those of the wild‐type and the genetically reverted strain on disconnected Minneola leaves at 3 days post‐inoculation (data not shown). However, pathogenicity assessed on detached leaves sprayed uniformly with conidial suspension revealed a reduction in necrotic lesions induced by the A. alternata slt2 mutant relative to those induced by the wild‐type (Fig. 10). Statistical analysis using the t‐test indicated that the mean number of lesions per leaf (40, n = 10) induced by the wild‐type was significantly different from the number induced by an A. alternata slt2 mutant (12.3, n = 10, P ≤ 0.05). The complementation strain induced necrotic lesions at a rate and magnitude similar to those of the wild‐type (Fig. 10).
Figure 10.

Pathogenicity of Alternaria alternata assayed on detached leaves of citrus cv. Minneola. Minneola leaves were uniformly sprayed with conidial suspension (105 conidia/mL). The inoculated leaves were incubated in a mist chamber for lesion development for 3 days post‐inoculation (dpi). The mock controls were treated with water only. Only some representative replicates are shown.
DISCUSSION
All microorganisms utilize a multifaceted signalling transduction system to perceive and respond to a broad diversity of environmental cues. We have characterized a yeast SLT2 MAPK gene homologue, AaSLT2, which plays a versatile role in the tangerine pathotype of A. alternata. Collectively, experimental evidence has revealed that AaSLT2 is necessary for conidiation, formation of septae, hyphal elongation, fungal virulence, maintenance of cell wall integrity and the production of ACT toxin, melanin and chitin.
The tangerine pathotype of A. alternata has no known sexual cycles. Thus, the formation and dispersal of conidia are crucial for the fulfilment of the life cycle and the onset of Alternaria brown spot in citrus. Previous studies have demonstrated that conidiation is positively regulated by the G‐protein‐controlled cyclic AMP (cAMP) level in A. alternata (Wang et al., 2010). Further investigation has revealed that conidiation is also controlled by a FUS3 MAPK‐mediated signalling pathway, as impairment of the AaFUS3 gene in A. alternata completely annuls the production of conidia, either in culture or in planta (Lin et al., 2010). An A. alternata strain disrupted in a G‐protein α‐subunit‐coding gene (AaGα1) showed increased phosphorylation of AaFUS3 (Chung et al., unpublished data). In the present study, we observed that the AaSLT2‐mediated signalling pathway is required for conidiation. Inactivation of the AaSLT2 gene in A. alternata reduced conidial formation by over 95%, but did not affect conidial germination (data not shown). Intriguingly, inactivation of the AaSLT2 gene apparently interferes with the formation of septae and hyphal extension by A. alternata. In addition, AaSLT2 is required for normal growth, as disruption of AaSLT2 in A. alternata resulted in growth retardation. Similarly, disruption of an SLT2 homologue in A. brassicicola, B. cinerea or F. graminearium also interferes with normal fungal growth (Hou et al., 2002; Rui and Hahn, 2007; Scott, 2009). However, the SLT2 homologue is not required for radial growth by Co. lagenarium, Ma. grisea or M. graminicola (Kojima et al., 2002; Mehrabi et al., 2006; Xu et al., 1998).
SLT2 homologues have been shown to have an important function in the pathogenicity/virulence of various phytopathogenic fungi, including B. cinerea, Co. lagenarium, Cl. purpurea, F. graminearum, Ma. grisea, M. graminicola and A. brassicicola (Hou et al., 2002; Joubert et al., 2011; Kojima et al., 2002; Mehrabi et al., 2006; Mey et al., 2002; Rui and Hahn, 2007; Scott, 2009; Xu et al., 1998), as well as human pathogens, including Ca. albicans and Cr. neoformans (Kraus et al., 2003; Monge et al., 2006). Our findings also indicate that AaSLT2 is necessary for the full virulence of A. alternata. This pathogenic impairment could be a result, in part, of a reduced production of ACT toxin by the A. alternata slt2 mutant. Growth retardation and deformation of hyphal extension of the mutant could also account for the reduction in fungal virulence. Careful examination of Minneola leaves uniformly sprayed with conidia of the wild‐type or the A. alternata slt2 mutant revealed significant differences in terms of lesion number and progression of necrotic areas. However, infection assessed using point inoculation (10 µL) did not reveal any differences in lesions induced by the wild‐type or the A. alternata slt2 mutant, perhaps because of excessive conidia in a small area. In addition to AaSLT2, A. alternata seemingly depends on the regulatory functions of AaAP1, AaHOG1 and AaFUS3 for full virulence on citrus (Lin and Chung, 2010; Lin et al., 2009, 2010, 2011).
Both AaFUS3 and AaSLT2 are functionally required for conidiation. Fungal strains defective in either AaSLT2 or AaFUS3 displayed retardation in radial growth compared with the wild‐type. Similar to AaFUS3, the A. alternata slt2 mutants formed white colonies with fluffy hyphae on PDA. This probably results from a reduced accumulation of melanin and conidia, as evidenced by the A. alternata fus3 mutants (Lin et al., 2010). However, the A. alternata slt2, but not fus3, mutants were hypersensitive to cell wall‐degrading enzymes and compounds such as Calcofluor white and Congo red. Moreover, we observed that the slt2 mutants produced deformed conidia and accumulated reduced levels of melanin and chitin, which may explain why the mutants were sensitive to cell wall‐degrading agents. Deletion of an SLT2 homologue in A. brassicicola also resulted in aberrant conidia and hyphal growth, and the mutant was nonpathogenic on cabbage (Scott, 2009). Thus, we conclude that AaSLT2 plays an important role in the maintenance of cell wall integrity.
The A. alternata SLT2 homologue apparently plays a critical role in the regulation of the biosynthesis of secondary metabolites because the slt2 mutants accumulated lower levels of ACT toxin and melanin. The reintroduction of a functional copy of AaSLT2 into the mutant restored their production to wild‐type levels. Melanin biosynthesis has also been shown to be regulated by an SLT2 homologue in the maize pathogen C. heterostrophus (Eliahu et al., 2007), as well as in N. crassa (Park et al., 2008). An F. graminearum SLT2 homologue has been demonstrated to regulate the accumulation of deoxynivalenol toxin in plants (Hou et al., 2002).
Sensitivity tests revealed that impairment of AaSLT2 in A. alternata did not alter the cellular sensitivity to osmolytic and oxidative agents, or dicarboximide (iprodione and vinclozolin) and phenylpyrrole (fludioxonil) fungicides. Contrary to our finding, the A. brassicicola mutant deficient at an SLT2 MAPK displayed hypersensitivity to H2O2 (Joubert et al., 2011; Scott, 2009). The B. cinerea slt2 mutant became hypersensitive to the phenylpyrrole fungicide fludioxonil and the oxidizing agent paraquat (Rui and Hahn, 2007). Furthermore, the B. cinerea and Co. lagenarium SLT2 homologues seem to play little or no role in maintaining cell wall integrity, as the impaired mutants are unchanged in sensitivity to cell wall‐degrading enzymes or Calcofluor white (Kojima et al., 2002; Rui and Hahn, 2007). These studies indicate that the SLT2 MAPK‐mediated signalling pathway may be well conserved among fungi, but the biological functions of each component kinase may vary considerably in different species.
One of the most intriguing findings of the present study was that three different MAPK signalling pathways, AaFUS3, AaSLT2 and AaHOG1, the AaAP1‐mediated redox response activator and the AaHSK1 histidine kinase‐mediated signalling pathway had an impact on the cellular resistance to CHP, TIBA and compounds containing benzene or pyridine (Lin and Chung, 2010; Lin et al., 2009, 2010). However, fungal mutants disrupted at a G‐protein α‐subunit‐coding gene (AaGα1) did not display an increased sensitivity to CHP or TIBA (Wang et al., 2010). Signalling transduction pathways mediated by AaAP1, AaHSK1, AaHOG1, AaSLT2 and AaFUS3 play a critical and nonredundant role in the resistance to CHP and TIBA in A. alternata. Pyridine, a heteroaromatic compound composed of five carbons and one nitrogen atom, occurs ubiquitously in natural compounds and acts as a potent carcinogen by accelerating the production of superoxide and hydroxyl radical in the presence of Cu2+ and H2O2 (Nerud et al., 2001; Watanabe et al., 1998). In biological systems, pyridine and its derivatives have important functions: as electron carriers, such as NADP/NADPH and flavin nucleotides (FAD/FADH); as constituents of RNA and DNA; and as energy storage molecules, such as ATP and GTP. However, TIBA is a plant growth regulator, often used as a herbicide or an inhibitor of indole‐3‐acetic acid transportation.
How or why A. alternata recruits different signalling pathways to avoid the toxicity of TIBA or CHP is not yet clear. The functions of the ATP‐binding cassette (ABC) transporters and the major facilitator superfamily (MSF) transporters are often ascribed to multidrug resistance in fungi (Gulshan and Moye‐Rowley, 2007). Both MFS and ABC transporters are probably responsible for toxin secretion, as well as resistance to plant‐derived antifungal compounds, such as phytoalexins (Coleman and Mylonakis, 2009; Kema et al., 2008). Similar mechanisms might also be applicable to A. alternata. Previous studies have identified several gene clones from a suppression subtractive hybridization (SSH) library (Lin et al., 2011). Among these, one contig (#19) encoding a putative MSF transporter was simultaneously regulated by AaAP1, AaHOG1, AaSLT2, AaFUS3 and AaHSK1 (unpublished data). Contig #54, also encoding a putative MFS transporter, was co‐ordinately controlled by AaAP1, AaSLT2, AaFUS3, AaHSK1, but not AaHOG1. Thus, A. alternata apparently has evolved sophisticated regulatory networks involving different signalling pathways in order to adapt to diverse environmental conditions, perhaps, most importantly, to thrive within host plants.
EXPERIMENTAL PROCEDURES
Fungal strains and culture conditions
The wild‐type EV‐MIL31 strain (tangerine pathotype) of A. alternata (Fr.) Keissler was isolated from diseased leaves of Minneola tangelo, and has been characterized elsewhere (Lin and Chung, 2010; Lin et al., 2009, 2010, 2011; Wang et al., 2010). Fungal strains were reproduced on PDA (Difco, Sparks, MD, USA) at 28 °C. Fungal strains were grown in a synthetic medium (Chung, 2003) for protein purification; they were grown on PDA or complete medium with a layer of sterile cellophane for DNA or RNA purification. Fungal strains were cultured in Richard's solution (Kohmoto et al., 1991) for 24 days for the production of host‐specific ACT toxin. Sensitivity assays were conducted by transferring hyphal segments with sterile toothpicks onto a medium containing the test chemicals. Fungal growth was determined by measuring the diameter of colonies in 4–6 days. Sensitivity tests were performed at least twice with multiple replicates. The significance of treatments was determined by analysis of variance, and treatment means were separated by the Waller–Duncan k‐ratio t‐test (P ≤ 0.05).
Conidiation
Fungal isolates were induced to form conidia by incubation on PDA under fluorescent light with an intensity of approximately 40 µE/m2/s for 7–10 days. Conidia were collected by flooding with sterile water and low‐speed centrifugation (5000 g), and examined by microscopy with the aid of a haemocytometer. All isolates were examined at least twice with multiple replicates.
Cloning and sequence analysis of the AaSLT2 gene
Portions of the AaSLT2 gene sequences were amplified by PCR from genomic DNA of the EV‐MIL31 strain with the primers Slt2‐1F (5′‐GCCATCAAGAAGGTCACCAACG‐3′) and Slt2‐1R (5′‐GGGTCGAAAGCGAGCATGC‐3′) that were complementary to an SLT2 gene homologue of A. brassicicola. The 5′ and 3′ nontranslated regions of AaSLT2 were amplified by PCR from an A. alternata genomic library, constructed using a Universal GenomeWalker kit (BD Biosciences, San Jose, CA, USA). Amplicons were cloned into a pGEM‐T easy vector (Promega, Madison, WI, USA) for sequence analysis from both directions at Eton Bioscience (San Diego, CA, USA). blast similarity searches were conducted at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/). ORF and exon/intron positions were determined by comparing genomic and cDNA sequences. Functional domains were identified using the PROSITE database available in the ExPASy Molecular Biology Server (http://us.expasy.org) or the Motif/ProDom and Block programs (http://motif.genome.jp/). Sequence data reported in this article have been deposited with the EMBL/GenBank Data Libraries under accession number GQ414510 (AaSLT2).
Interruption of AaSLT2 in the genome of A. alternata
The AaSLT2 function in A. alternata was inactivated by targeted gene disruption using a split‐marker approach as described previously (Yang and Chung, 2010; You et al., 2009). Split‐marker fragment 5′AaSLT2::h/YG (2.9 kb) was generated by two‐round PCR with the primers SLT‐pro (5′‐GACACGAGTCGAGCCACGTTTTGT‐3′), SLT2‐F2 (5′‐GTCGTGACTGGGAAAACCCTGGCGTGCTTCTCGGACCAGGGGTTTC‐3′), M13F (5′‐CGCCAGGGTTTTCCCAGTCACGAC‐3′) and hyg4 (5′‐CGTTGCAAGAACTGCCTGAA‐3′). Split‐marker fragment HY/g::3′AaSLT2 (1.9 kb) was amplified with the primers hyg3 (5′‐GGATGCTCCGCTCGAAGTA‐3′), M13R (5′‐AGCGGATAACAATTTCACACAGGA‐3′), SLT2‐F3 (5′‐TCCTGTGTGAAATTGTTATCCGCTACTCGTCAACGCCGACTGCGAG‐3′) and SLT‐taa (5′‐TCATCGCATGCGACCGTCAAG‐3′). Primers SLT2‐F2 and SLT2‐F3 contain sequences (italic) complementary to primers M13F and M13R, respectively. The resulting DNA fragments were transformed into protoplasts prepared from the EV‐MIL31 strain, using CaCl2 and polyethylene glycol as described previously (Chung et al., 2002). Fungal transformants were recovered from a regeneration medium supplemented with 200 µg/mL hygromycin (Calbiochem, La Jolla, CA, USA).
Genetic complementation
Genetic complementation was conducted by co‐transforming a 2.4‐kb AaSLT2 fragment under the control of its endogenous promoter with a pCB1532 plasmid carrying a sulphonylurea‐resistant gene (Sweigard et al., 1997) into protoplasts of an A. alternata slt2 mutant. Transformants were selected from medium containing 5 µg/mL sulphonylurea (chlorimuron ethyl; Chem Service, West Chester, PA, USA) and tested for phenotypic restoration.
Miscellaneous methods for nucleic acids
A DNeasy Plant kit (Qiagen, Valencia, CA, USA) and Trizol reagent (Molecular Research Center, Cincinnati, OH, USA) were used to isolate fungal DNA and RNA, respectively. Double‐stranded cDNA of AaSLT2 was amplified with gene‐specific primers from total RNA of A. alternata using a cDNA synthesis kit (BD Clontech). Plasmid DNA propagated in Escherichia coli DH5α cells was isolated using a miniprep kit (GenScript, Piscataway, NJ, USA). Probes used for DNA and RNA hybridizations were labelled with digoxigenin (DIG)‐11‐dUTP (Roche Applied Science, Indianapolis, IN, USA) by PCR with gene‐specific primers. The probe was detected by an immunological assay using disodium 3‐[4‐methoxyspiro{1,2‐dioxetane‐3,2′‐(5′‐chloro)tricyclo [3.3.1.1]decan}‐4‐yl]phenyl phosphate as a fluorescent substrate (Roche Applied Science).
Sensitivity to cell wall‐degrading enzymes
The integrity of the fungal cell wall was assessed by determining the number of protoplasts released from fungal isolates over time on exposure to cell wall‐degrading enzymes, including driselase, β‐d‐glucanase, β‐glucuronidase and lyticase. Enzymes were dissolved in an osmotic buffer for fungal protoplasts, as described previously (Chung et al., 2002). The formation of protoplasts was examined at 1‐h intervals by microscopy. All treatments were performed at least twice with multiple replicates.
Fungal pathogenicity and toxin production
Fungal pathogenicity was evaluated on disconnected Minneola, a hybrid between Duncan grapefruit (Citrus paradisi Macfad.) and Dancy tangerine (C. reticulata Blanco), leaves inoculated with conidial suspension (1 × 105 conidia/mL), as described previously (Lin et al., 2009). The conidial suspension was point‐inoculated (10 µL) or uniformly sprayed onto detached Minneola leaves, and the inoculated leaves were incubated in a damp chamber for 3 days for lesion formation.
The production of host‐selective ACT toxin by A. alternata strains was evaluated by a bioassay on detached calamondin (× Citrofortunella mitis J. Ingram and H. E. Moore) leaves. Fungal strains grown in liquid Richard's medium for 24 days were removed by subsequently passing through a paper filter and a 0.45‐µm cellulose acetate membrane. Citrus leaves were dipped in 1.5 mL of cell‐free filtrates and incubated in a mist chamber. The development of necrotic lesions along the vascular system indicates the production of ACT toxin. Fungal toxin was also extracted using Amberlite XAD‐2 resin and ethyl acetate from culture filtrates, and analysed by TLC as described previously (Kohmoto et al., 1993). To quantify ACT toxin, ethyl acetate extract was dried in a preweighed centrifuge tube and each tube was reweighed to determine the amount of toxin.
Extraction and measurement of fungal melanin and chitin
Fungal melanin was extracted with 2% NaOH from 1‐week‐old cultures by boiling at 100 °C for 2 h. The extracts were acidified to pH 2.0 with 5 M HCl and centrifuged at 6000 g for 15 min at room temperature. The resulting pellet was dissolved in 2% NaOH and the solution was measured spectrophotometrically for absorbance at 459 nm (Babitskaya et al., 2000).
To isolate the fungal cell wall, fungal mycelium was ground in liquid nitrogen and extracted with a buffer containing 50 mm Tris‐HCl (pH 7.8), 2% SDS, 0.3 mβ‐mercaptoethanol and 1 mm ethylenediaminetetraacetic acid (EDTA) at 100 °C for 15 min. After centrifugation at 8000 g, the pellet was washed three times with water and dried completely. The fungal cell wall was dissolved in water to make a solution of 25 mg/mL. In total, 5 mg of cell wall was acidified in 6 M HCl at 100 °C for at least 4 h and used for chitin quantification. Chitin was determined by measuring the acid‐released glucosamine from chitin using p‐dimethylaminobenzaldehyde as a chromogen (Selvaggini et al., 2004). The reaction was measured at A 520 and the quantity of glucosamine was calculated by reference to a regression line established using pure glucosamine as a standard.
Microscopy
For SEM analysis, samples were fixed in 1.2% glutaraldehyde and observed using a Hitachi model S‐530 scanning electron microscope (Nissei Sangyo America, Rockville, MD, USA). For TEM, samples were fixed with 3% glutaraldehyde in 0.1 m phosphate buffer (pH 7.2) and 2% osmium tetraoxide, and embedded in Spurr's resin after being dehydrated with an acetone series. Samples were sectioned, examined using a Model 268 FEI Morgagni transmission electron microscope (FEI Company, Hillsboro, OR, USA) after being stained with uranyl acetate and lead citrate, and photographed with an AMT CCD camera (Advanced Microscopy Techniques, Danvers, MA, USA). For light microscopy, conidia were examined using a Leitz Laborlux phase contrast microscope (Leitz, Wetzlar, Germany).
Supporting information
Fig. S1 (a) Schematic illustration and comparison of functional domains between three mitogen‐activated protein (MAP) kinases, AaFUS3, AaSLT2 and AaHOG1, identified in the citrus pathogen Alternaria alternata. (b) Alignment of AaFUS3, AaSLT2 and AaHOG1 MAP kinases. Identical (asterisks), closely similar (colons) and more distant similar (full points) amino acid residues are indicated.
Fig. S2 Cladogram showing relationships between AaSLT2 and SLT2 mitogen‐activated protein (MAP) kinase orthologues found in other fungal species as indicated.
Supporting info item
ACKNOWLEDGEMENTS
We thank S. L. Yang for technical assistance. J. I. Yago was sponsored by the Fulbright‐Philippine Agriculture Scholarship Program. C.‐H. Lin was supported by the Hunt Brothers Fellowship. This research was supported by the Florida Agricultural Experiment Station.
REFERENCES
- Babitskaya, V.G. , Shcherba, V.V. , Filimonova, T.V. and Grigorchuk, E.A. (2000) Melanin pigments from the fungi Paecilomyces variotii and Aspergillus carbonarius . Appl. Biochem. Microbiol. 36, 128–133. [PubMed] [Google Scholar]
- Bardwell, L. (2006) Mechanisms of MAPK signalling specificity. Biochem. Soc. Trans. 34, 837–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, R.E. and Thorner, J. (2007) Function and regulation in MAPK signaling pathways: lessons learned from the yeast Saccharomyces cerevisiae . Biochem. Biophys. Acta, 1773, 1311–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, Y. , Cramer, R.A. Jr , Kim, K.‐H. , Davis, J. , Mitchell, T.K. , Figuli, P. , Pryor, B.M. , Lemasters, E. and Lawrence, C.B. (2007) The Fus3/Kss1 MAP kinase homolog Amk1 regulates the expression of genes encoding hydrolytic enzymes in Alternaria brassicicola . Fungal Genet. Biol. 44, 543–553. [DOI] [PubMed] [Google Scholar]
- Chung, K.‐R. (2003) Involvement of calcium/calmodulin signaling in cercosporin toxin biosynthesis by Cercospora nicotianae . Appl. Environ. Microbiol. 69, 1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, K.‐R. , Shilts, T. , Li, W. and Timmer, L.W. (2002) Engineering a genetic transformation system for Colletotrichum acutatum, the causal fungus of lime anthracnose and postbloom fruit drop. FEMS Microbiol. Lett. 213, 33–39. [DOI] [PubMed] [Google Scholar]
- Coleman, J.J. and Mylonakis, E. (2009) Efflux in fungi: La Pièce de Résistance. PLoS Pathogens. 5, e1000486. Doi: 10.1371/journal.ppat.1000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliahu, N. , Igbaria, A. , Rose, M.S. , Horwitz, B.A. and Lev, S. (2007) Melanin biosynthesis in the maize pathogen Cochliobolus heterostrophus depends on two mitogen‐activated protein kinases, CHk1 and Mps1, and the transcription factor Cmr1. Eukaryot. Cell, 6, 421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka, T. , Mizutani, O. , Furukawa, K. , Sato, N. , Yoshimi, A. , Yamagata, Y. , Nakajima, T. and Abe, K. (2007) MpkA‐dependent and ‐independent cell wall integrity signaling in Aspergillus nidulans . Eukaryot. Cell, 6, 1497–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulshan, K. and Moye‐Rowley, W.S. (2007) Multidrug resistance in fungi. Eukaryot. Cell, 6, 1933–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin, M.C. , Albertyn, J. , Alexander, M. and Davenport, K. (1998) MAP kinase pathways in the yeast Saccharomyces cerevisiae . Microbiol. Mol. Biol. Rev. 62, 1264–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, Z. , Xue, C. , Peng, Y. , Katan, T. , Kistler, H.C. and Xu, J.‐R. (2002) A mitogen‐activated protein kinase gene (MGV1) in Fusarium graminearum is required for female fertility, heterokaryon formation, and plant infection. Mol. Plant–Microbe Interact. 15, 1119–1127. [DOI] [PubMed] [Google Scholar]
- Ito, K. , Tanaka, T. , Hatta, R. , Yamamoto, M. , Akimitsu, K. and Tsuge, T. (2004) Dissection of the host range of the fungal plant pathogen Alternaria alternata by modification of secondary metabolism. Mol. Microbiol. 52, 399–411. [DOI] [PubMed] [Google Scholar]
- Joubert, A. , Bataille‐Simoneau, N. , Campion, C. , Guillemette, T. , Hudhomme, P. , Lcomi‐Vasilescu, B. , Leroy, T. , Pochon, S. , Poupard, P. and Simoneau, P. (2011) Cell wall integrity and high osmolarity glycerol pathways are required for adaptation of Alternaria brassicicola to cell wall stress caused by brassicaceous indolic phytoalexins. Cell. Microbiol. 13, 62–80. doi:10.1111/j.1462‐5822.2010.01520.x. [DOI] [PubMed] [Google Scholar]
- Kema, G.H.J. , van der Lee, T.A.J. , Mendes, O. , Verstappen, E.C.P. , Lankhorst, R.K. , Sandbrink, H. , van der Burgt, A. , Zwiers, L.‐H. , Csukai, M. and Waalwijk, C. (2008) Large‐scale gene discovery in the Septoria tritici blotch fungus Mycosphaerella graminicola with a focus on in planta expression. Mol. Plant–Microbe Interact. 21, 1249–1260. [DOI] [PubMed] [Google Scholar]
- Kohmoto, K. , Akimitsu, K. and Otani, H. (1991) Correlation of resistance and susceptibility of citrus to Alternaria alternata with sensitivity to host‐specific toxins. Phytopathology, 81, 719–722. [Google Scholar]
- Kohmoto, K. , Itoh, Y. , Shimomura, N. , Kondoh, Y. , Otani, H. , Nishimura, S. and Nakatsuka, S. (1993) Isolation and biological activities of two host‐specific toxins from tangerine pathotype of Alternaria alternata . Phytopathology, 83, 495–502. [Google Scholar]
- Kojima, K. , Kikuchi, T. , Takano, Y. , Oshiro, E. and Okuno, T. (2002) The mitogen‐activated protein kinase gene MAF1 is essential for early differentiation phase of appressorium formation in Colletotrichum lagenarium . Mol. Plant–Microbe Interact. 15, 1268–1276. [DOI] [PubMed] [Google Scholar]
- Kraus, P.R. , Fox, D.S. , Cox, G.M. and Heitman, J. (2003) The Cryptococcus neoformans MAP kinase Mpk1 regulates cell integrity in response to antifungal drugs and loss of calcineurin function. Mol. Microbiol. 48, 1377–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kültz, D. (1998) Phylogenetic and functional classification of mitogen‐ and stress‐activated protein kinases. J. Mol. Evol. 46, 571–588. [DOI] [PubMed] [Google Scholar]
- Lin, C.‐H. and Chung, K.‐R. (2010) Specialized and shared functions of the histidine kinase‐ and HOG1 MAP kinase‐mediated signaling pathways in Alternaria alternata, a filamentous fungal pathogen of citrus. Fungal Genet. Biol. 47, 818–827. [DOI] [PubMed] [Google Scholar]
- Lin, C.‐H. , Yang, S.L. and Chung, K.‐R. (2009) The YAP1 homolog‐mediated oxidative stress tolerance is crucial for pathogenicity of the necrotrophic fungus Alternaria alternata in citrus. Mol. Plant–Microbe Interact. 22, 942–952. [DOI] [PubMed] [Google Scholar]
- Lin, C.‐H. , Yang, S.L. , Wang, N.‐Y. and Chung, K.‐R. (2010) The FUS3 MAPK signaling pathway of the citrus pathogen Alternaria alternata functions independently or cooperatively with the fungal redox‐responsive AP1 regulator for diverse developmental, physiological and pathogenic processes. Fungal Genet. Biol. 47, 381–391. [DOI] [PubMed] [Google Scholar]
- Lin, C.‐H. , Yang, S.L. and Chung, K.‐R. (2011) Cellular responses required for oxidative stress tolerance, colonization and lesion formation by the necrotrophic fungus Alternaria alternata in citrus. Curr. Microbiol. (in press). DOI: 10.1007/s00284‐010‐9795‐y. [DOI] [PubMed] [Google Scholar]
- Mehrabi, R. , van der Lee, T. , Waalwijk, C. and Kema, G.H.J. (2006) MgSlt2, a cellular integrity MAP kinase gene of the fungal wheat pathogen Mycosphaerella graminicola, is dispensable for penetration but essential for invasive growth. Mol. Plant–Microbe Interact. 19, 389–398. [DOI] [PubMed] [Google Scholar]
- Mey, G. , Held, K. , Scheffer, J. , Tenberge, K.B. and Tudzynski, P. (2002) CPMK2, an SLT2‐homologous mitogen‐activated protein (MAP) kinase, is essential for pathogenesis of Claviceps purpurea on rye: evidence for a second conserved pathogenesis‐related MAP kinase cascade in phytopathogenic fungi. Mol. Microbiol. 46, 305–318. [DOI] [PubMed] [Google Scholar]
- Miyamoto, Y. , Masunaka, A. , Tsuge, T. , Yamamoto, M. , Ohtani, K. , Fukumoto, T. , Gomi, K. , Peever, T.L. and Akimitsu, K. (2009) Functional analysis of a multicopy host‐selective ACT‐toxin biosynthesis gene in the tangerine pathotype of Alternaria alternata using RNA silencing. Mol. Plant–Microbe Interact. 21, 1591–1599. [DOI] [PubMed] [Google Scholar]
- Monge, R.A. , Román, E. , Nombela, C. and Pla, J. (2006) The MAP kinase signal transduction network in Candida albicans . Microbiology, 152, 905–912. [DOI] [PubMed] [Google Scholar]
- Moye‐Rowley, W.S. (2003) Regulation of the transcriptional response to oxidative stress in fungi: similarities and differences. Eukaryot. Cell, 2, 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerud, F. , Baldrian, P. , Gabriel, J. and Ogbeifun, D. (2001) Decolorization of synthetic dyes by the Fenton reagent and the Cu/pyridine/H2O2 system. Chemosphere, 44, 957–961. [DOI] [PubMed] [Google Scholar]
- Park, G. , Pan, S. and Borkovich, K.A. (2008) Mitogen‐activated protein kinase cascade required for regulation of development and secondary metabolism in Neurospora crassa . Eukaryot. Cell, 7, 2113–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelech, S.J. and Sanghera, J.S. (1992) MAP kinases: charting the regulatory pathways. Science, 257, 1355–1356. [DOI] [PubMed] [Google Scholar]
- Robinson, M.J. and Cobb, M.H. (1997) Mitogen‐activated protein kinase pathways. Curr. Opin. Cell Biol. 9, 180–186. [DOI] [PubMed] [Google Scholar]
- Rui, O. and Hahn, M. (2007) The Slt2‐type MAP kinase Bmp3 of Botrytis cinerea is required for normal saprotrophic growth, conidiation, plant surface sensing and host tissue colonization. Mol. Plant Pathol. 8, 173–184. [DOI] [PubMed] [Google Scholar]
- Schwartz, M.A. and Madhani, H.D. (2004) Principles of MAP kinase signaling specificity in Saccharomyces cerevisiae . Annu. Rev. Genet. 38, 725–748. [DOI] [PubMed] [Google Scholar]
- Scott, D.C. (2009) The cell wall integrity‐associated MAP kinase homolog, AbSlt2 in the necrotrophic fungus Alternaria brassicicola is required for pathogenicity of Brassicas . Masters Thesis, Virginia Polytechnic Institute and State University. Available at: http://scholar.lib.vt.edu/thesis/available/etd‐02102009‐234303
- Selvaggini, S. , Munro, C.A. , Paschoud, S. , Sanglard, D. and Gow, N.A.R. (2004) Independent regulation of chitin synthase and chitinase activity in Candida albicans and Saccharomyces cerevisiae . Microbiology, 150, 921–928. [DOI] [PubMed] [Google Scholar]
- Shock, T.R. , Thompson, J. , Yates, J.R. III and Madhani, H.D. (2009) Hog1 mitogen‐activated protein kinase (MAPK) interrupts signal transduction between the Kss1 MAPK and the Tec1 transcription factor to maintain pathway specificity. Eukaryot. Cell, 8, 606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, F.D. and Scott, J.D. (2002) Signaling complexes: junctions on the intracellular information super highway. Curr. Biol. 12, R32–R40. [DOI] [PubMed] [Google Scholar]
- Sweigard, J.A. , Chumley, F.C. , Carroll, A.M. , Farrall, L. and Valent, B. (1997) A series of vectors for fungal transformation. Fungal Genet. Newsl. 44, 52–53. [Google Scholar]
- Thomason, P. and Kay, R. (2000) Eukaryotic signal transduction via histidine‐aspartate phosphorelay. J. Cell Sci. 113, 3141–3150. [DOI] [PubMed] [Google Scholar]
- Valiante, V. , Heinekamp, T. , Jain, R. , Härtl, A. and Brakhage, A.A. (2008) The mitogen‐activated protein kinase MpkA of Aspergillus fumigatus regulates cell wall signaling and oxidative stress response. Fungal Genet. Biol. 45, 618–627. [DOI] [PubMed] [Google Scholar]
- Wang, N.‐Y. , Lin, C.‐H. and Chung, K.‐R. (2010) A Gα subunit gene is essential for conidiation and potassium efflux but dispensable for pathogenicity of Alternaria alternata in citrus. Curr. Genet. 56, 43–51. [DOI] [PubMed] [Google Scholar]
- Watanabe, T. , Koller, K. and Messner, K. (1998) Copper‐dependent depolymerisation of lignin in the presence of fungal metabolite, pyridine. J. Biotechnol. 62, 221–230. [DOI] [PubMed] [Google Scholar]
- Xu, J.‐R. (2000) MAP kinases in fungal pathogens. Fungal Genet. Biol. 31, 137–152. [DOI] [PubMed] [Google Scholar]
- Xu, J.‐R. , Staiger, C.J. and Hamer, J.E. (1998) Inactivation of the mitogen‐activated protein kinase MPS1 in the rice blast fungus prevents penetration of host cells but allows activation of plant defense responses. Proc. Natl. Acad. Sci. USA, 95, 12 713–12 718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, S.L. and Chung, K.‐R. (2010) Transcriptional regulation of elsinochrome phytotoxin biosynthesis by an EfSTE12 activator in the citrus scab pathogen Elsinoë fawcettii . Fungal Biol. 114, 64–73. [DOI] [PubMed] [Google Scholar]
- Yang, S.L. , Lin, C.‐H. and Chung, K.‐R. (2009) Coordinate control of oxidative stress, vegetative growth and fungal pathogenicity via the AP1‐mediated pathway in the rough lemon pathotype of Alternaria alternata . Physiol. Mol. Plant Pathol. 74, 100–110. [Google Scholar]
- You, B.‐J. , Lee, M.‐H. and Chung, K.‐R. (2009) Gene‐specific disruption in the filamentous fungus Cercospora nicotianae using a split‐marker approach. Arch. Microbiol. 191, 615–622. [DOI] [PubMed] [Google Scholar]
- Zhou, X. , Mehrabi, R. and Xu, J.‐R. (2007) Mitogen‐activated protein kinase pathways and fungal pathogenesis. Eukaryot. Cell, 6, 1701–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 (a) Schematic illustration and comparison of functional domains between three mitogen‐activated protein (MAP) kinases, AaFUS3, AaSLT2 and AaHOG1, identified in the citrus pathogen Alternaria alternata. (b) Alignment of AaFUS3, AaSLT2 and AaHOG1 MAP kinases. Identical (asterisks), closely similar (colons) and more distant similar (full points) amino acid residues are indicated.
Fig. S2 Cladogram showing relationships between AaSLT2 and SLT2 mitogen‐activated protein (MAP) kinase orthologues found in other fungal species as indicated.
Supporting info item


