SUMMARY
Xanthomonas campestris pv. campestris, the causal agent of black rot disease, depends on its type III secretion system (TTSS) to infect cruciferous plants, including Brassica oleracea, B. napus and Arabidopsis. Previous studies on the Arabidopsis–Pseudomonas syringae model pathosystem have indicated that a major function of TTSS from virulent bacteria is to suppress host defences triggered by pathogen‐associated molecular patterns. Similar analyses have not been made for the Arabidopsis–X. campestris pv. campestris pathosystem. In this study, we report that X. campestris pv. campestris strain 8004, which is modestly pathogenic on Arabidopsis, induces strong defence responses in Arabidopsis in a TTSS‐dependent manner. Furthermore, the induction of defence responses and disease resistance to X. campestris pv. campestris strain 8004 requires NDR1 (NON‐RACE‐SPECIFIC DISEASE RESISTANCE1), RAR1 (required for Mla12 resistance) and SGT1b (suppressor of G2 allele of skp1), suggesting that effector‐triggered immunity plays a large role in resistance to this strain. Consistent with this notion, AvrXccC, an X. campestris pv. campestris TTSS effector protein, induces PR1 expression and confers resistance in Arabidopsis in a RAR1‐ and SGT1b‐dependent manner. In rar1 and sgt1b mutants, AvrXccC acts as a virulence factor, presumably because of impaired resistance gene function.
INTRODUCTION
Many plant pathogenic bacteria depend on the conserved type III secretion system (TTSS) to deliver effector proteins into plant cells and to promote parasitism (Buttner and Bonas, 2006; He et al., 2004). Some of the effector proteins were initially identified as products of avirulence (Avr) genes conditioning resistance (R) protein‐mediated defences, termed effector‐triggered innate immunity (ETI), in plants (Boller and He, 2009; Jones and Dangl, 2006; Zhou and Chai, 2008). ETI is often associated with the hypersensitive response (HR), a rapid, programmed host cell death at the infection site. However, some effector proteins elicit weak defence responses in the absence of HR, as exemplified by the recognition of the Pseudomonas syringae effector AvrB by the Arabidopsis R protein TAO1 (Eitas et al., 2008). In addition to ETI, plants are also equipped to perceive pathogen‐associated molecular patterns (PAMPs) and to mount immune responses (Bittel and Robatzek, 2007; Boller and He, 2009; Zhou and Chai, 2008). PAMP‐triggered innate immunity (PTI) is often accompanied by the activation of mitogen‐activated protein kinases (MAPKs), the production of reactive oxygen species, callose deposition and induced defence gene expression. ETI and PTI activate a common set of gene expression (Navarro et al., 2004). Likewise, callose deposition occurs during both PTI and ETI (Ham et al., 2007), suggesting that the two pathways converge before the activation of downstream defence responses. It should be noted, however, that the defence responses are not necessarily responsible for disease resistance. Instead, they often correlate with disease resistance in plants.
Plant–pathogen interactions can be divided into compatible, incompatible and nonhost interactions. Unlike incompatible interactions, which are typically dictated by one or a few R genes specifying strong ETI, compatible interactions do not display visible ETI. However, plants possess a low level of resistance in compatible interactions. This low level of resistance is referred to as basal resistance. In the literature, basal resistance has been used interchangeably with PTI without careful experimental data. It should be cautioned, however, that basal resistance, by definition, is a descriptive term, whereas PTI is a mechanistic term. Recent evidence indicates that weak ETI can play a large role in Arabidopsis basal resistance to the virulent P. syringae strain DC3000 (Zhang et al., 2010). Nonhost interaction refers to plant resistance to nonadapted pathogens, and PTI and ETI collectively contribute to nonhost resistance (Li et al., 2005; Wei et al., 2007; Zhang et al., 2010).
The largely overlapping defence responses hinder the distinction between PTI and ETI. However, because ETI is typically triggered by Gram‐negative bacterial TTSS effectors, TTSS mutants are useful tools in the dissection of PTI and ETI. In addition, several host proteins are known to play crucial roles in ETI. For example, NDR1 (NON‐RACE‐SPECIFIC DISEASE RESISTANCE1), a glycophosphatidyl‐inositol (GPI)‐anchored plasma membrane protein, is required for ETI resistance conditioned by many, but not all, R proteins in Arabidopsis (Day et al., 2006; Tornero et al., 2002). Likewise, EDS1 (enhanced disease susceptibility1) is also required for ETI specified by some R genes (Aarts et al., 1998). Furthermore, HSP90 co‐chaperones RAR1 (required for Mla12 resistance) and SGT1 (suppressor of G2 allele of skp1) are required to stabilize some R proteins, and rar1 and sgt1 mutants are often compromised in ETI (Azevedo et al., 2002; Hammond‐Kosack and Parker, 2003; 2003, 2009; Muskett et al., 2002; Takahashi et al., 2003). eds1, rar1 and sgt1 mutants are not affected in flg22‐induced disease resistance (Zipfel et al., 2004), suggesting that they can be used to differentiate between PTI and ETI.
It is well established that TTSS‐deficient strains of P. syringae induce strong defence responses because of the presence of a collection of PAMPs (Hauck et al., 2003; Li et al., 2005). These defences are largely suppressed by TTSS effectors from the virulent P. syringae strain DC3000 (Hauck et al., 2003; Li et al., 2005). Many P. syringae TTSS effectors are also capable of inhibiting ETI (Guo et al., 2009). Artificial inhibition of host defences by transgenic expression of effector proteins often enables P. syringae TTSS mutants to multiply to high levels in plants (Hauck et al., 2003; Kim et al., 2005; Li et al., 2005). The TTSS‐mediated suppression of callose deposition has also been reported for Xanthomonas campestris pv. vesicatoria (Brown et al., 1995). Therefore, suppression of host defences appears to be a major function of virulent P. syringae and X. campestris pv. vesicatoria TTSS effectors, at least in the early phase of infection. Whether this concept can be generally applied to other pathosystems remains to be determined.
Xanthomonas campestris pv. campestris (Xcc) is a xylem‐colonizing systemic pathogen and causes black rot disease on large numbers of crucifers worldwide (Williams, 1980). Xcc multiplies in vascular tissues after entry into the plant via hydathodes (Hugouvieux et al., 1998; Sun et al., 2006), which is in contrast with P. syringae pv. tomato, which enters the plant through stomata and causes disease in leaves primarily as a result of mesophyll colonization. Thus, the two pathogens assume completely different life styles which probably involve distinct host responses. Xcc strain 8004 can infect and cause disease symptoms on Arabidopsis plants, making it a useful model for the study of Xanthomonas pathogens. Both syringe infiltration and wound inoculation have been used to study the Arabidopsis–Xcc interaction (Meyer et al., 2005). Syringe infiltration is an unnatural route of Xcc infection and is generally used to study the bacterial growth of Xcc and defence responses in plants. Wound inoculation mimics natural Xcc infection and is sensitive for monitoring disease symptoms.
In this study, we show that, unlike TTSS‐deficient derivatives of P. syringae DC3000, Xcc strain 8004 TTSS mutants appear to induce only weak PTI defence responses in Arabidopsis. In contrast, wild‐type Xcc strain 8004 induces strong defence responses, but grows to significantly higher levels than do TTSS mutants. Xcc strain 8004‐induced defence responses require RAR1, SGT1b and NDR1, and the rar1, sgt1b and ndr1 mutants are compromised in their resistance to Xcc strain 8004. Furthermore, the Xcc strain 8004 effector AvrXccC confers resistance in WT Arabidopsis plants, but enhances bacterial virulence on rar1 and sgt1b mutant plants. These results collectively indicate that ETI plays an important role in Arabidopsis resistance to Xcc strain 8004, and that some Xcc strain 8004 TTSS effector proteins may contribute to virulence by modulating host processes independent of defence inhibition.
RESULTS
The TTSS of Xcc strain 8004 is required to induce defence responses in Arabidopsis
It is well established that PAMPs from P. syringae trigger strong defences in Arabidopsis, and these defences are largely suppressed by TTSS effectors from virulent P. syringae strains (Clay et al., 2009; DebRoy et al., 2004; Hauck et al., 2003). Some of the effectors from virulent P. syringae, however, appear to trigger weak ETI that contributes to basal resistance (Zhang et al., 2010). To determine the role of TTSS from Xcc in triggering Arabidopsis defences in response to this bacterium, wild‐type Xcc strain 8004 and its TTSS structural gene hrcV (ΔhrcV) mutant, which lacks a conserved inner membrane protein of the core TTSS (Wang et al., 2007), were inoculated into Arabidopsis Col‐0 leaves by syringe infiltration. Gene expression [FRK1(FLG22‐INDUCED RECEPTOR‐LIKE KINASE1), PR1 (PATHOGENESIS‐RELATED GENE1)] and callose deposition, which are defence responses activated during both PTI and ETI, were monitored. Wild‐type Xcc strain 8004 strongly triggered callose deposition in leaves at 24 h after inoculation (Fig. 1A). Surprisingly, the ΔhrcV mutant strain failed to induce callose deposition above the background level, suggesting that hrcV is required for callose induction. Indeed, when the ΔhrcV mutant strain was complemented with a plasmid containing the wild‐type hrcV gene, callose induction was restored (Fig. 1A). In addition, the expression of PR1 and FRK1 was induced more strongly by wild‐type Xcc strain 8004 than by the ΔhrcV mutant (Fig. 1B,C). These findings indicate that TTSS is required for Xcc‐induced defence responses in Arabidopsis and, in the absence of TTSS, Xcc PAMPs do not effectively trigger plant defences.
Figure 1.
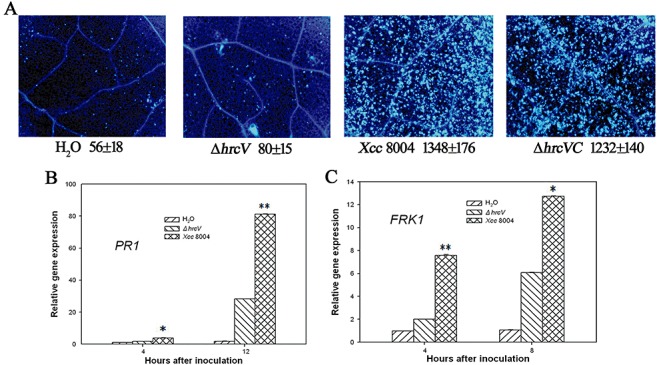
Xanthomonas campestris pv. campestris strain 8004 (Xcc 8004)‐induced defence responses in Arabidopsis are type III secretion system (TTSS) dependent. (A) Callose deposition in wild‐type (WT) Arabidopsis Col‐0 leaves. Callose deposition was performed on 5‐week‐old Arabidopsis leaves at 24 h after syringe infiltration with H2O or the indicated bacterial strains at 2 × 107 colony‐forming units (CFU)/mL. Average numbers of callose deposits per microscopic field of 0.1 mm2 are shown below each photograph. ‘±’ represents the standard deviations from three leaves of each plant. Similar results were obtained in three independent experiments. The mRNA abundance of PR1 (B) and FRK1 (C) in leaves treated with H2O, ΔhrcV and Xcc 8004 was determined at the indicated times by quantitative polymerase chain reaction (Q‐PCR). Gene expression was monitored by Q‐PCR after bacterial syringe infiltration at 2 × 107 CFU/mL at the indicated times. *(t‐test, P < 0.05) and **(t‐test, P < 0.01) indicate statistically significant differences between the hrcV mutant and WT Xcc 8004. Error bars indicate standard deviations. These experiments were repeated twice with similar results.
It has been reported that flagellin proteins isolated from different Xcc strains vary dramatically in their capability to elicit FLS2 (FLAGELLIN‐SENSITIVE2)‐mediated responses in Arabidopsis (Abramovitch et al., 2006; Schwessinger and Zipfel, 2008; Sun et al., 2006). We investigated whether FLS2 is involved in defence responses and disease resistance to Xcc strain 8004. When infiltrated with Xcc strain 8004, the wild‐type and fls2 mutant, Arabidopsis leaves displayed similar callose deposition (Fig. 2A) and chlorosis symptoms (Fig. 2B), and supported similar bacterial growth in leaves (Fig. 2C). These results suggest that FLS2 does not contribute to Arabidopsis resistance to Xcc strain 8004. It has been shown that the Xcc flagellin amino acid valine‐43 is essential for recognition by FLS2, and a Val43Asp substitution renders the flagellin noneliciting (Sun et al., 2006). We therefore aligned Xcc strain 8004, Xcc B186 (noneliciting) and Xcc B305 (eliciting) flagellin sequences corresponding to flg22. As expected, the Xcc 8004 flagellin 43 amino acid was identical to Xcc B186, but not Xcc B305 (Fig. 2D), suggesting that Xcc strain 8004 flagellin was not recognized by FLS2.
Figure 2.
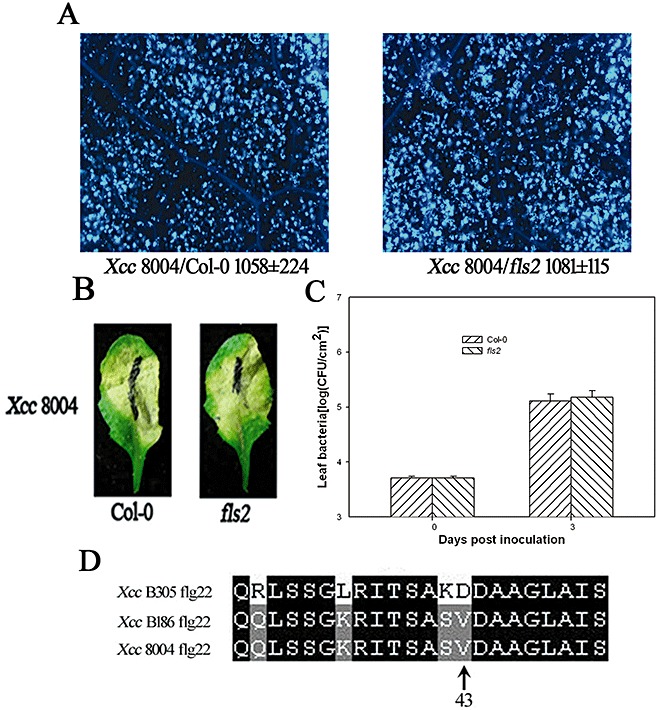
FLS2 is not required for Arabidopsis resistance to Xanthomonas campestris pv. campestris strain 8004 (Xcc 8004). (A) Callose deposition in wild‐type (WT) Col‐0 and fls2 mutant leaves was determined at 24 h following inoculation of Xcc 8004 at 2 × 107 colony‐forming units (CFU)/mL. Average numbers of callose deposits per microscopic field of 0.1 mm2 are shown below each photograph. ‘±’ represents the standard deviations from three leaves of each plant, and similar phenotypes were observed in three independent experiments. (B) Xcc 8004 induces chlorosis in both Col‐0 and fls2 mutant. Symptoms were photographed 5 days after inoculation of Xcc 8004 at 1 × 107 CFU/mL. (C) Bacterial growth in Col‐0 and fls2 mutant. Five‐week‐old Arabidopsis plants were inoculated by Xcc 8004 at 5 × 105 CFU/mL, and the bacterial population in the leaf was determined at the indicated times. Each data point represents three replicates. Error bars indicate standard deviations. The experiments were repeated twice with similar results. (D) Alignment of FliC sequences corresponding to flg22 in Xcc B305 (eliciting), Xcc B186 (noneliciting) and Xcc 8004.
To further confirm the function of Xcc TTSS in triggering host defence responses, we determined callose deposition in response to two additional TTSS mutants ΔhrpG and ΔhrpX (Wang et al., 2007). HrpG, a two‐component system response regulator belonging to the OmpR family, controls the expression of HrpX which is an AraC‐type transcriptional activator and regulates the expression of hrp and effector genes. Figure S1 (see Supporting Information) showed that the ΔhrpG and ΔhrpX mutants triggered weak callose deposition relative to Xcc strain 8004. Although the TTSS mutant strains induced less callose deposition, they grew substantially less than wild‐type bacteria at 3 days post‐inoculation (Fig. S2, see Supporting Information), indicating that the defence responses induced by Xcc TTSS were not sufficient to restrict Xcc bacterial growth in Arabidopsis.
NDR1, RAR1 and SGT1 are involved in Xcc‐induced defence responses in Arabidopsis, and the callose deposition induced by Xcc is dependent on salicylic acid (SA)
The TTSS‐dependent defence responses induced by Xcc suggest an involvement of R proteins that probably recognize some of the TTSS effectors. To further test this possibility, we determined whether the NDR1‐, RAR1‐ and SGT1‐dependent defences contributed to resistance to Xcc strain 8004. We inoculated ndr1, rar1 and sgt1b mutants with Xcc strain 8004 at 2 × 107 colony‐forming units (CFU)/mL. As expected, callose deposition in ndr1, rar1 and sgt1b mutants was substantially reduced relative to Col‐0 at 24 h after inoculation (Fig. 3A). In addition, the PR1 (Fig. 3B,C) and FRK1 (Fig. 3D) mRNA abundance in Col‐0 was significantly greater than that in the ndr1, rar1 and sgt1b mutants at 12, 24 and 8 h, respectively, after Xcc strain 8004 inoculation. The results indicate that NDR1, RAR1 and SGT1b are required for Xcc strain 8004‐induced defence responses.
Figure 3.
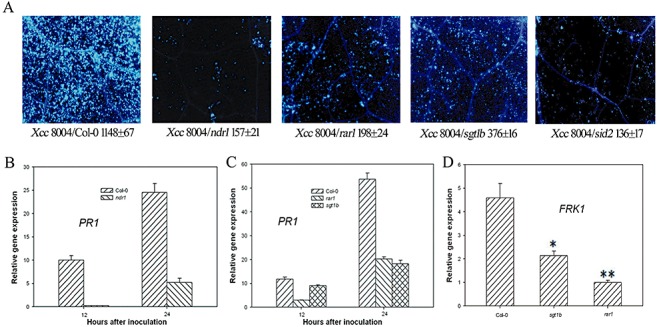
NDR1, RAR1, SGT1b and SID2 are required for Xanthomonas campestris pv. campestris strain 8004 (Xcc 8004)‐induced defence responses. (A) ndr1, rar1, sgt1b and sid2 mutants are compromised in callose deposition in response to Xcc 8004. Leaves of the indicated genotypes were inoculated with Xcc 8004 at 2 × 107 colony‐forming units (CFU)/mL, and callose deposits were determined 24 h later. Average numbers of callose deposits per microscopic field of 0.1 mm2 are shown below each photograph. ‘±’ represents the standard deviations from three leaves of each plant. Similar results were obtained in three independent experiments. (B) Xcc 8004‐induced PR1 expression requires NDR1. (C) Xcc 8004‐induced PR1 expression requires RAR1 and SGT1b. (D) Xcc 8004‐induced FRK1 expression requires RAR1 and SGT1b. *(t‐test, P < 0.05) and **(t‐test, P < 0.01) indicate statistically significant differences between rar1, sgt1b mutant and wild‐type (WT) Col‐0. Leaves of the indicated genotypes were infiltrated with Xcc 8004 at 2 × 107 CFU/mL, and RNA was extracted for quantitative reverse transcriptase‐polymerase chain reaction analyses.
Bacterial growth assays indicated that Xcc strain 8004 bacteria multiplied approximately eight‐fold more strongly in the ndr1 mutant (Fig. 4A) and four‐ to five‐fold more strongly in the rar1 and sgt1b mutants relative to Col‐0 at 3 days post‐inoculation (Fig. 4B). These data demonstrate that NDR1, RAR1 and SGT1 are required for Arabidopsis resistance to Xcc, and suggest that ETI contributes significantly to the resistance.
Figure 4.
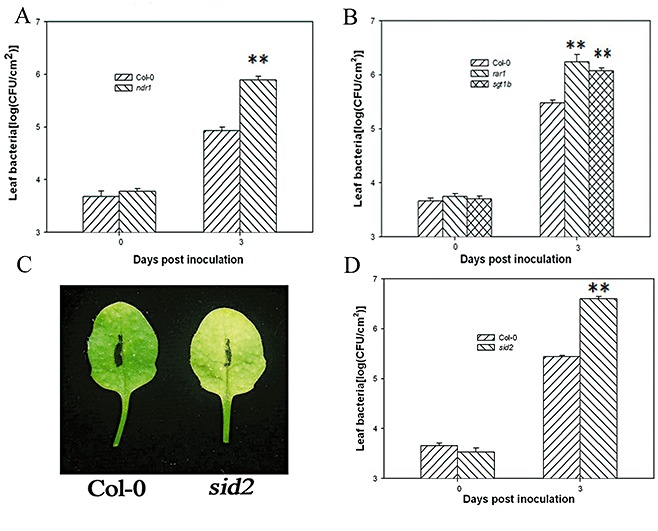
Arabidopsis resistance to Xanthomonas campestris pv. campestris strain 8004 (Xcc 8004) requires NDR1, RAR1, SGT1b and SID2. (A, B) ndr1, rar1 and sgt1b show enhanced susceptibility to Xcc 8004. (C) sid2 shows enhanced leaf chlorosis. (D) sid2 shows enhanced susceptibility. Leaves of the indicated genotypes were infiltrated with Xcc 8004 at 5 × 105 colony‐forming units (CFU)/mL for bacterial growth assay and 1 × 107 CFU/mL for leaf chlorosis assay. Each data point represents three replicates. Error bars indicate standard deviations. **(t‐test, P < 0.01) indicates statistically significant differences between ndr1, rar1, sgt1b, sid2 mutants and wild‐type (WT) Col‐0. The experiments were performed on 5‐week‐old Arabidopsis leaves and repeated twice with similar results.
Microbes can elicit callose formation in Arabidopsis leaves via the SA‐dependent pathway (DebRoy et al., 2004). To determine whether Xcc strain 8004‐induced callose formation was dependent on SA, we inoculated the leaves of the SA biosynthesis mutant SALICYLIC ACID INDUCTION DEFICIENT2 (sid2) and wild‐type Arabidopsis with Xcc strain 8004. The sid2 mutant showed increased chlorosis following wild‐type Xcc strain 8004 inoculation (Fig. 4C). Consistently, sid2 leaves supported significantly greater bacterial growth (Fig. 4D). These results are consistent with a previous report (O'Donnell et al., 2003). Correlating with the increased susceptibility, sid2 leaves showed a 10‐fold lower callose deposition compared with Col‐0 following Xcc strain 8004 inoculation (Fig. 3A).
RAR1 and SGT1 are required for Xcc effector protein AvrXccC‐induced defence responses in Arabidopsis
It has been reported that the Xcc strain 8004 TTSS effector protein AvrXccC triggers ETI in Brassica napiformis (Wang et al., 2007). We therefore tested whether AvrXccC also triggers ETI in Arabidopsis. AvrXccC is homologous to the P. syringae effector AvrB, which is recognized by the R protein RPM1 and elicits strong HR (Desveaux et al., 2007). However, AvrXccC is not recognized by RPM1 (Desveaux et al., 2007). Wild‐type Xcc strain 8004 and the ΔavrXccC mutant were similarly unable to induce electrolyte leakage in Col‐0 leaves (Fig. S3, see Supporting Information), indicating that AvrXccC does not trigger a strong ETI in these Arabidopsis plants. To investigate the function of AvrXccC in Arabidopsis, we inoculated the leaves of wild‐type Col‐0, rar1 and sgt1b mutants by infiltration with wild‐type Xcc strain 8004, the ΔavrXccC mutant and the avrXccC‐complemented (ΔavrXccCC) strain, respectively. The three strains showed indistinguishable disease symptoms and bacterial growth (data not shown). However, when inoculated by the piercing method, wild‐type Xcc strain 8004 induced typical black rot disease symptoms on rar1 and mild symptoms on sgt1b leaves, but was almost symptomless on wild‐type Col‐0 (Fig. 5A). The lack of symptoms in leaves pierced with wild‐type Xcc strain 8004 was consistent with a previous report (Xu et al., 2008). In contrast, ΔavrXccC induced typical symptoms in both Col‐0 and rar1, and mild symptoms in sgt1b, whereas the complemented strain was indistinguishable from the wild‐type strain (Fig. 5A). Consistent with the increased disease symptoms in rar1 and sgt1b leaves, the wild‐type Xcc strain 8004 grew to higher levels on rar1 and sgt1b leaves (Fig. 5B). These results are consistent with the notion that ETI contributes to Arabidopsis resistance to wild‐type Xcc strain 8004. Interestingly, rar1 and sgt1b leaves inoculated with wild‐type Xcc strain 8004 always showed more severe disease symptoms and a greater bacterial population than those inoculated with the ΔavrXccC strain (Fig. 5A,B), indicating that avrXccC conferred resistance in Col‐0 leaves, but virulence function in rar1 and sgt1b leaves. Consistent with a role of avrXccC in ETI, the ΔavrXccC mutation abolished Xcc strain 8004‐induced PR1 expression during piercing inoculation (Fig. 5C), indicating that avrXccC is required for PR1 induction. PR1 induction also required RAR1 and SGT1b in plants, further confirming that avrXccC‐induced PR1 expression is probably mediated by a single R gene or multiple R genes. However, Xcc strain 8004 failed to induce FRK1 expression and callose deposition when inoculated by the piercing method (data not shown), indicating that Xcc strain 8004 only triggers a subset of defence responses when multiplying within vascular tissue.
Figure 5.
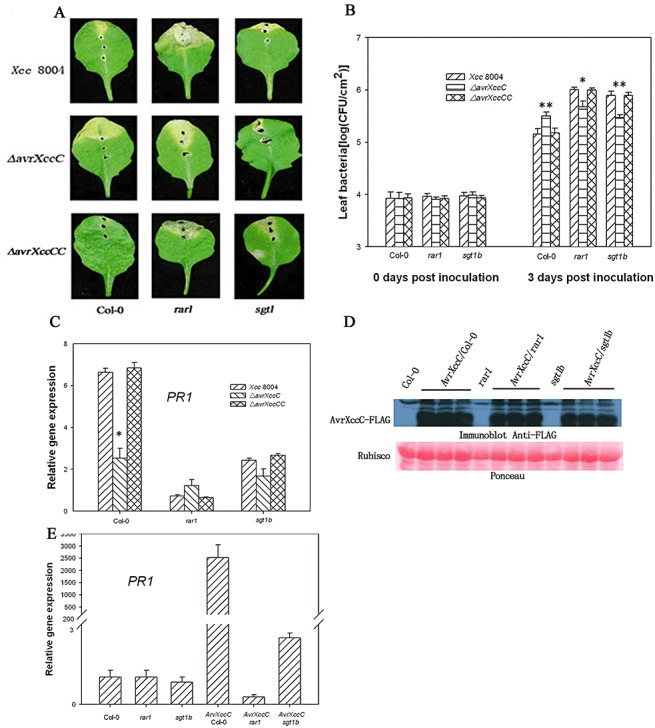
AvrXccC has both avirulence and virulence functions in Arabidopsis. Leaves of the indicated genotypes were inoculated with Xanthomonas campestris pv. campestris strain 8004 (Xcc 8004), the ΔavrXccC mutant and the avrXccC‐complemented (ΔavrXccCC) strain at 5 × 108 colony‐forming units (CFU)/mL using the piercing method. Disease symptoms (A) were photographed 5 days after inoculation. The bacterial population in leaves (B) was monitored at 0 and 3 days post‐inoculation. PR1 gene abundance (C) was determined at 24 h after inoculation. Five‐week‐old nontransgenic and AvrXccC‐transgenic lines in the indicated genetic background were induced by oestradiol for 12 h, and the accumulation of AvrXccC was determined by immunoblot using anti‐Flag antibody (D); PR1 gene expression was determined by quantitative polymerase chain reaction (E). *(t‐test, P < 0.05) and **(t‐test, P < 0.01) indicate statistically significant differences between the avrXccC mutant and wild‐type (WT) Xcc 8004. Each data point represents three replicates. Error bars indicate standard deviations. The experiments were repeated twice with similar results.
To further confirm whether AvrXccC can induce defence responses in Arabidopsis, an oestrogen‐inducible AvrXccC transgene was introduced into Col‐0, sgt1b and rar1. Homozygous T2 transgenic lines accumulating similar levels of AvrXccC following oestradiol treatment were selected for the experiments (Fig. 5D). We examined the FRK1 and PR1 mRNA levels and callose deposition in these lines, 12 h after oestradiol treatment without bacterial inoculation. Quantitative polymerase chain reaction (PCR) showed that PR1 gene expression was strongly induced in AvrXccC/Col‐0 and mildly in AvrXccC/sgt1b, but not in AvrXccC/rar1, transgenic lines (Fig. 5E). The findings further support a role for AvrXccC in ETI induction. However, the expression of AvrXccC failed to induce FRK1 expression and callose deposition in any genetic background tested (data not shown), suggesting that AvrXccC elicits only a subset of defence responses.
DISCUSSION
TTSS is critical for Xcc pathogenicity, and Xcc mutants lacking TTSS are unable to multiply or spread in plant tissues (Qian et al., 2005). In this study, we showed that, unlike P. syringae TTSS mutants which induce strong defences, the Xcc strain 8004 TTSS ΔhrcV mutant induced only weak defence responses when FRK1 and PR1 gene expression and callose deposition were examined. Surprisingly, wild‐type Xcc strain 8004 strongly induced these defence responses in a RAR1‐ and SGT1‐dependent manner, suggesting that ETI is responsible for Xcc‐induced defence responses in Arabidopsis.
It has been reported that the Xcc strain 8004 TTSS effector protein AvrAC has an avirulence function in Arabidopsis ecotype Col‐0 by piercing inoculation (Xu et al., 2008). This is consistent with our findings that Xcc strain 8004‐induced defence responses require TTSS in the bacterium and NDR1, RAR1 and SGT1b in the plant. We further showed that the Xcc strain 8004 effector protein AvrXccC, when delivered from the bacterium or expressed as a transgene, induces PR1 expression and confers disease resistance in Arabidopsis. Wild‐type Xcc strain 8004 and the ΔavrXccC strain were indistinguishable in the induction of FRK1 expression and callose deposition, indicating that avrXccC is not required for these responses. It remains to be determined whether other effectors are responsible for the induction of these responses. Nonetheless, these results reinforce the notion that ETI plays an important role in Arabidopsis resistance to Xcc strain 8004.
It is interesting to note that Xcc strain 8004 bacteria induce callose deposition and FRK1 expression only when inoculated through infiltration, but not through the piercing method. One explanation is that endogenous elicitors or damage‐associated molecular patterns (DAMPs) released from affected cells (Boller and Felix, 2009) could act together with effectors to trigger defence responses. Alternatively, microscopic cell death may occur in infiltrated mesophyll cells, and this may indirectly induce callose deposition and FRK1 expression.
Xcc strain 8004 appears to carry an inactive flagellin sequence for FLS2 recognition. This is consistent with our findings that the fls2 mutant was not affected in terms of defence responses, disease symptoms and bacterial growth when inoculated with Xcc strain 8004. However, other PAMPs derived from Xcc, including lipopolysaccharides, harpins, cold shock proteins and flagellin, could induce defence responses in host and nonhost plants (Felix and Boller, 2003; Newman et al., 1995; Silipo et al., 2005; Xu et al., 2008). The Xcc strain 8004 ΔhrcV mutant, which carries a collection of PAMPs, induced only weak defence responses. This contrasts with the strong defence responses induced by P. syringae TTSS mutants and X. campestris pv. vesicatoria TTSS mutants. Xcc may have evolved multiple strategies to evade PTI in plants. For example, Xcc cyclic glucan and extracellular polysaccharide xanthan act as suppressors of host defences to promote bacterial growth (Silipo et al., 2009; Yun et al., 2006). Xanthan appears to be a major virulence determinant which acts by chelating extracellular calcium, thereby inhibiting PTI defences (Aslam et al., 2008).
Pathogen effectors are generally believed to assist pathogen infection or propagation in plants; it is often difficult to demonstrate a virulence function to many effectors. Our analyses using rar1 and sgt1b plants revealed a previously unknown virulence function for AvrXccC in Arabidopsis. As other effectors may similarly possess both avirulence and virulence functions, the use of plant mutants compromised in ETI will allow a better assessment of effector virulence functions.
In conclusion, we have shown that ETI is primarily responsible for defence responses and disease resistance in the Arabidopsis–Xcc strain 8004 interaction. Although the Xcc TTSS mutants elicit much smaller defence responses than the wild‐type bacterium, they do not grow or cause disease in Arabidopsis leaves. In contrast, wild‐type Xcc strain 8004 grew to a significantly higher level in the presence of strong defence responses. A major role of virulent P. syringae TTSS effector proteins is to inhibit host defences. It remains to be determined to what extent Xcc strain 8004 effectors assist parasitism by inhibiting host defences. Because Xcc is a vascular pathogen which has a different life style, it is possible that some Xcc strain 8004 effector proteins may promote parasitism through mechanisms other than defence suppression.
EXPERIMENTAL PROCEDURES
Bacterial strains and culture conditions
The strains used in this study included wild‐type Xcc strain 8004 (Turner et al., 1984), ΔhrcV (Tn5 insertion mutant), ΔhrcVC (hrcV mutant containing pHMVC), ΔavrXccC and ΔavrXccCC (Wang et al., 2007). Xcc strains were grown in peptone sucrose agar plates at 28 °C. Antibiotics were used at the following concentrations: 50 µg/mL kanamycin and 50 µg/mL spectinomycin for Escherichia coli; 100 µg/mL spectinomycin and 50 µg/mL rifampicin for Xcc.
Arabidopsis and mutants
Arabidopsis plants used in this study included the wild‐type (Col‐0) and the following mutants: ndr1 (Zhang et al., 2010), sid2‐2 (Dewdney et al., 2000), sgt1b (formerly described as edm1‐1; Tor et al., 2002), rar1‐20 (Tornero et al., 2002 ) and fls2, salk_141277 (Xiang et al., 2008).
Callose deposition assay
Five‐week‐old Arabidopsis leaves were hand infiltrated with an Xcc bacterial suspension at 2 × 107 CFU/mL. Leaves were harvested 24 h after infiltration, cleared, stained with aniline blue (Hauck et al., 2003) and mounted in 50% glycerol. The leaves were examined with a fluorescence microscope under ultraviolet light. The number of callose deposits per microscopic field of 0.1 mm2 was calculated from six leaves using Image J software (http://www.uhnresearch.ca/wcif).
RNA isolation and real‐time reverse transcriptase (RT)‐PCR
Arabidopsis leaves were infiltrated with an Xcc strain 8004 bacterial suspension at 2 × 107 CFU/mL for the indicated times before RNA was isolated with Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Five micrograms of RNA were used for cDNA synthesis. The gene expression level was determined by real‐time RT‐PCR using a SYBR Premix Ex Taq™ kit (TaKaRa, Changping, Beijing, China). Actin was used as a reference gene. Primers 5′‐TACGCAGAACAACTAAGAGG‐3′ and 5′‐TCGTTCACATAATTCCCACG‐3′ were used for PR1, and primers 5′‐TGGTGGAAGCACAGAAGTTG‐3′ and 5′‐GATCCATGTTTGGCTCCTTC‐3′ were used for actin. Primers 5′‐TCTGAAGAATCAGCTCAAGGC‐3′ and 5′‐TGTTGGCTTCACATCTCTGTG‐3′ were used for FRK1. The RT‐PCR conditions were as follows: 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, 55 °C for 15 s and 72 °C for 25 s. The expression level was normalized to the actin control, and relative expression values were determined against buffer or wild‐type Col‐0 using the comparative C t method.
Bacterial growth assay
Five‐week‐old Arabidopsis leaves were hand infiltrated with an Xcc strain 8004 bacterial suspension (5 × 105 CFU/mL), and the bacterial population in the leaves was counted at the indicated times. Alternatively, 5‐week‐old Arabidopsis leaves were inoculated by piercing three holes in the central vein with a needle dipped in a bacterial suspension (5 × 108 CFU/mL), and the tips of the inoculated leaves were selected for the bacterial growth assay. At least four leaves were inoculated for each strain tested. Each data point consisted of at least six replicates.
Generation of AvrXccC transgenic plants
The AvrXccC fragment was PCR amplified from Xcc strain 8004 genome DNA using the following primers: 5′‐CCGCTCGAGATGGGTCTATGCGCTTCA‐3′ and 5′‐CCCATCGATAATTGGGGGGCGCTCAAA‐3′. The AvrXccC fragment was ligated into pER8 (Shang et al., 2006) that had been digested with XhoI and Csp45I. The resulting clone containing AvrXccC under the control of the oestrogen‐inducible promoter was transformed into Arabidopsis (wild‐type Col‐0 and mutants rar1, sgt1b) by Agrobacterium‐mediated transformation. Transgenic plants were selected on Murashige and Skoog plates containing hygromycin. For AvrXccC induction, plants were sprayed with 50 µm oestradiol containing 0.01% silwet L‐77. Three independent homozygous T2 transgenic lines were selected for the experiments.
Immunoblot analysis
Protein was extracted with a buffer containing 50 mm N‐2‐hydroxyethylpiperazine‐N'‐2‐ethanesulphonic acid (HEPES), pH 7.5, 150 mm NaCl, 5 mm ethylenediaminetetraacetic acid (EDTA), 0.1% Triton, 1 mm dithiothreitol (DTT), 2 mm NaF and protease inhibitor cocktail (Roche, Indianapolis, IN, USA). Protein samples were electrophoresed through a 12% sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) gel, and protein was electrotransferred to an Immobilon P membrane (Millipore, Bedford, MA, USA). Immunodetection was performed with a 1 : 5000 dilution of an anti‐FLAG monoclonal antibody (Sigma, Louis, MA, USA). The blot was then hybridized with horseradish peroxidase (HRP)‐conjugated secondary antibodies and visualized with ECL Western blotting detection reagents (GE Healthcare, Amersham™, Buckinghamshire, UK).
Electrolyte leakage measurement
Five‐week‐old Arabidopsis leaves were inoculated with bacteria in water. Immediately after inoculation, 0.7‐cm‐diameter leaf discs were taken from injected leaves and washed with double‐distilled H2O three times for 15 min each time. Electrolyte leakage was measured using a Seveneasy S30 conductivity meter (Mettler Toledo, Schwerzenbach, Switzerland) according to Torres et al. (2005). Each treatment consisted of three replicates with four leaf discs per replicate.
Supporting information
Fig. S1 The Xanthomonas campestris pv. campestris type III secretion system (Xcc TTSS) is required for callose induction. Arabidopsis leaves were infiltrated with the indicated bacterial strains at 2 × 107 colony‐forming units (CFU)/mL, and callose deposition was examined 24 h later. Average numbers of callose deposits per microscopic field of 0.1 mm2 are shown below each photograph. ‘±’ represents the standard deviations from three replicates. Similar results were obtained in three independent experiments.
Fig. S2 Type III secretion system (TTSS) mutants ΔhrcV, ΔhrpG and ΔhrpX are nonpathogenic on Arabidopsis. Five‐week‐old Col‐0 plants were inoculated with wild‐type (WT) Xanthomonas campestris pv. campestris strain 8004 (Xcc 8004), ΔhrcV, ΔhrpG and ΔhrpX mutants [bacterial suspension at a concentration of 5 × 105 colony‐forming units (CFU)/mL]. The bacterial populations were determined at 0 and 3 days post‐inoculation. Each data point represents three replicates. Error bars indicate standard deviations. The experiments were repeated twice with similar results.
Fig. S3 Electrolyte leakage assay on leaves infiltrated with wild‐type (WT) Xanthomonas campestris pv. campestris strain 8004 (Xcc 8004) or ΔhrcV mutant. Five‐week‐old Arabidopsis leaves were inoculated with DC3000 (avrB), WT Xcc 8004 and ΔhrcV mutant [bacterial suspension at a concentration of 2 × 107 colony‐forming units (CFU)/mL]. The electrolyte leakage was measured at the indicated times. Each data point represents three replicates. Error bars indicate standard deviations. The experiments were repeated twice with similar results.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item
ACKNOWLEDGEMENTS
This work was supported by the Programme for State Key Laboratories, Ministry of Science and Technology of China.
REFERENCES
- Aarts, N. , Metz, M. , Holub, E. , Staskawicz, B.J. , Daniels, M.J. and Parker, J.E. (1998) Different requirements for EDS1 and NDR1 by disease resistance genes define at least two R gene‐mediated signaling pathways in Arabidopsis . Proc. Natl. Acad. Sci. USA, 95, 10306–10311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramovitch, R.B. , Anderson, J.C. and Martin, G.B. (2006) Bacterial elicitation and evasion of plant innate immunity. Nat. Rev. Mol. Cell Biol. 7, 601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam, S.N. , Newman, M.A. , Erbs, G. , Morrissey, K.L. , Chinchilla, D. , Boller, T. , Jensen, T.T. , De Castro, C. , Ierano, T. , Molinaro, A. , Jackson, R.W. , Knight, M.R. and Cooper, R.M. (2008) Bacterial polysaccharides suppress induced innate immunity by calcium chelation. Curr. Biol. 18, 1078–1083. [DOI] [PubMed] [Google Scholar]
- Azevedo, C. , Sadanandom, A. , Kitagawa, K. , Freialdenhoven, A. , Shirasu, K. and Schulze‐Lefert, P. (2002) The RAR1 interactor SGT1, an essential component of R gene‐triggered disease resistance. Science, 295, 2073–2076. [DOI] [PubMed] [Google Scholar]
- Bittel, P. and Robatzek, S. (2007) Microbe‐associated molecular patterns (MAMPs) probe plant immunity. Curr. Opin. Plant Biol. 10, 335–341. [DOI] [PubMed] [Google Scholar]
- Boller, T. and Felix, G. (2009) A renaissance of elicitors: perception of microbe‐associated molecular patterns and danger signals by pattern‐recognition receptors. Annu. Rev. Plant Biol. 60, 379–406. [DOI] [PubMed] [Google Scholar]
- Boller, T. and He, S.Y. (2009) Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science, 324, 742–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, I. , Mansfield, J. and Bonas, U. (1995) hrp Genes in Xanthomonas campestris pv. vesicatoria determine ability to suppress papilla deposition in pepper mesophyll cells. Mol. Plant Microbe Interact. 8, 825–836. [Google Scholar]
- Buttner, D. and Bonas, U. (2006) Who comes first? How plant pathogenic bacteria orchestrate type III secretion. Curr. Opin. Microbiol. 9, 193–200. [DOI] [PubMed] [Google Scholar]
- Clay, N.K. , Adio, A.M. , Denoux, C. , Jander, G. and Ausubel, F.M. (2009) Glucosinolate metabolites required for an Arabidopsis innate immune response. Science, 323, 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day, B. , Dahlbeck, D. and Staskawicz, B.J. (2006) NDR1 interaction with RIN4 mediates the differential activation of multiple disease resistance pathways in Arabidopsis . Plant Cell, 18, 2782–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DebRoy, S. , Thilmony, R. , Kwack, Y.B. , Nomura, K. and He, S.Y. (2004) A family of conserved bacterial effectors inhibits salicylic acid‐mediated basal immunity and promotes disease necrosis in plants. Proc. Natl. Acad. Sci. USA, 101, 9927–9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desveaux, D. , Singer, A.U. , Wu, A.J. , McNulty, B.C. , Musselwhite, L. , Nimchuk, Z. , Sondek, J. and Dangl, J.L. (2007) Type III effector activation via nucleotide binding, phosphorylation, and host target interaction. PLoS Pathog. 3, e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewdney, J. , Reuber, T.L. , Wildermuth, M.C. , Devoto, A. , Cui, J. , Stutius, L.M. , Drummond, E.P. and Ausubel, F.M. (2000) Three unique mutants of Arabidopsis identify eds loci required for limiting growth of a biotrophic fungal pathogen. Plant J. 24, 205–218. [DOI] [PubMed] [Google Scholar]
- Eitas, T.K. , Nimchuk, Z.L. and Dangl, J.L. (2008) Arabidopsis TAO1 is a TIR‐NB‐LRR protein that contributes to disease resistance induced by the Pseudomonas syringae effector AvrB. Proc. Natl. Acad. Sci. USA, 105, 6475–6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix, G. and Boller, T. (2003) Molecular sensing of bacteria in plants. The highly conserved RNA‐binding motif RNP‐1 of bacterial cold shock proteins is recognized as an elicitor signal in tobacco. J. Biol. Chem. 278, 6201–6208. [DOI] [PubMed] [Google Scholar]
- Guo, M. , Tian, F. , Wamboldt, Y. and Alfano, J.R. (2009) The majority of the type III effector inventory of Pseudomonas syringae pv. tomato DC3000 can suppress plant immunity. Mol. Plant Microbe Interact. 22, 1069–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham, J.H. , Kim, M.G. , Lee, S.Y. and Mackey, D. (2007) Layered basal defenses underlie non‐host resistance of Arabidopsis to Pseudomonas syringae pv. phaseolicola . Plant J. 51, 604–616. [DOI] [PubMed] [Google Scholar]
- Hammond‐Kosack, K.E. and Parker, J.E. (2003) Deciphering plant–pathogen communication: fresh perspectives for molecular resistance breeding. Curr. Opin. Biotechnol. 14, 177–193. [DOI] [PubMed] [Google Scholar]
- Hauck, P. , Thilmony, R. and He, S.Y. (2003) A Pseudomonas syringae type III effector suppresses cell wall‐based extracellular defense in susceptible Arabidopsis plants. Proc. Natl. Acad. Sci. USA, 100, 8577–8582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, S.Y. , Nomura, K. and Whittam, T.S. (2004) Type III protein secretion mechanism in mammalian and plant pathogens. Biochim. Biophys. Acta, 1694, 181–206. [DOI] [PubMed] [Google Scholar]
- Hubert, D.A. , Tornero, P. , Belkhadir, Y. , Krishna, P. , Takahashi, A. , Shirasu, K. and Dangl, J.L. (2003) Cytosolic HSP90 associates with and modulates the Arabidopsis RPM1 disease resistance protein. EMBO J. 22, 5679–5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert, D.A. , He, Y. , McNulty, B.C. , Tornero, P. and Dangl, J.L. (2009) Specific Arabidopsis HSP90.2 alleles recapitulate RAR1 cochaperone function in plant NB‐LRR disease resistance protein regulation. Proc. Natl. Acad. Sci. USA, 106, 9556–9563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugouvieux, V. , Barber, C.E. and Daniels, M.J. (1998) Entry of Xanthomonas campestris pv. campestris into hydathodes of Arabidopsis thaliana leaves: a system for studying early infection events in bacterial pathogenesis. Mol. Plant Microbe Interact. 11, 537–543. [DOI] [PubMed] [Google Scholar]
- Jones, J.D. and Dangl, J.L. (2006) The plant immune system. Nature, 444, 323–329. [DOI] [PubMed] [Google Scholar]
- Kim, M.G. , Cunha, L. , McFall, A.J. , Belkhadir, Y. , DebRoy, S. , Dangl, J.L. and Mackey, D. (2005) Two Pseudomonas syringae type III effectors inhibit RIN4‐regulated basal defense in Arabidopsis . Cell, 121, 749–759. [DOI] [PubMed] [Google Scholar]
- Li, X.Y. , Lin, H.Q. , Zhang, W.G. , Zou, Y. , Zhang, J. , Tang, X.Y. and Zhou, J.M. (2005) Flagellin induces innate immunity in nonhost interactions that is suppressed by Pseudomonas syringae effectors. Proc. Natl. Acad. Sci. USA, 102, 12990–12995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, D. , Lauber, E. , Roby, D. , Arlat, M. and Kroj, T. (2005) Optimization of pathogenicity assays to study the Arabidopsis thaliana–Xanthomonas campestris pv. campestris pathosystem. Mol. Plant Pathol. 6, 327–333. [DOI] [PubMed] [Google Scholar]
- Muskett, P.R. , Kahn, K. , Austin, M.J. , Moisan, L.J. , Sadanandom, A. , Shirasu, K. , Jones, J.D. and Parker, J.E. (2002) Arabidopsis RAR1 exerts rate‐limiting control of R gene‐mediated defenses against multiple pathogens. Plant Cell, 14, 979–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro, L. , Zipfel, C. , Rowland, O. , Keller, I. , Robatzek, S. , Boller, T. and Jones, J.D. (2004) The transcriptional innate immune response to flg22. Interplay and overlap with Avr gene‐dependent defense responses and bacterial pathogenesis. Plant Physiol. 135, 1113–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman, M.A. , Daniels, M.J. and Dow, J.M. (1995) Lipopolysaccharide from Xanthomonas campestris induces defense‐related gene expression in Brassica campestris . Mol. Plant Microbe Interact. 8, 778–780. [DOI] [PubMed] [Google Scholar]
- O'Donnell, P.J. , Schmelz, E.A. , Moussatche, P. , Lund, S.T. , Jones, J.B. and Klee, H.J. (2003) Susceptible to intolerance—a range of hormonal actions in a susceptible Arabidopsis pathogen response. Plant J. 33, 245–257. [DOI] [PubMed] [Google Scholar]
- Qian, W. , Jia, Y. , Ren, S.X. , He, Y.Q. , Feng, J.X. , Lu, L.F. , Sun, Q. , Ying, G. , Tang, D.J. , Tang, H. , Wu, W. , Hao, P. , Wang, L. , Jiang, B.L. , Zeng, S. , Gu, W.Y. , Lu, G. , Rong, L. , Tian, Y. , Yao, Z. , Fu, G. , Chen, B. , Fang, R. , Qiang, B. , Chen, Z. , Zhao, G.P. , Tang, J.L. and He, C. (2005) Comparative and functional genomic analyses of the pathogenicity of phytopathogen Xanthomonas campestris pv. campestris . Genome Res. 15, 757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwessinger, B. and Zipfel, C. (2008) News from the frontline: recent insights into PAMP‐triggered immunity in plants. Curr. Opin. Plant Biol. 11, 389–395. [DOI] [PubMed] [Google Scholar]
- Shang, Y. , Li, X. , Cui, H. , He, P. , Thilmony, R. , Chintamanani, S. , Zwiesler‐Vollick, J. , Gopalan, S. , Tang, X. and Zhou, J.M. (2006) RAR1, a central player in plant immunity, is targeted by Pseudomonas syringae effector AvrB. Proc. Natl. Acad. Sci. USA, 103, 19200–19205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silipo, A. , Molinaro, A. , Sturiale, L. , Dow, J.M. , Erbs, G. , Lanzetta, R. , Newman, M.A. and Parrilli, M. (2005) The elicitation of plant innate immunity by lipooligosaccharide of Xanthomonas campestris . J. Biol. Chem. 280, 33660–33668. [DOI] [PubMed] [Google Scholar]
- Silipo, A. , Erbs, G. , Shinya, T. , Dow, J.M. , Parrilli, M. , Lanzetta, R. , Shibuya, N. , Newman, M.A. and Molinaro, A. (2009) Glyco‐conjugates as elicitors or suppressors of plant innate immunity. Glycobiology, 20, 406–419. [DOI] [PubMed] [Google Scholar]
- Sun, W. , Dunning, F.M. , Pfund, C. , Weingarten, R. and Bent, A.F. (2006) Within‐species flagellin polymorphism in Xanthomonas campestris pv campestris and its impact on elicitation of Arabidopsis FLAGELLIN SENSING2‐dependent defenses. Plant Cell, 18, 764–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, A. , Casais, C. , Ichimura, K. and Shirasu, K. (2003) HSP90 interacts with RAR1 and SGT1 and is essential for RPS2‐mediated disease resistance in Arabidopsis . Proc. Natl. Acad. Sci. USA, 100, 11777–11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tor, M. , Gordon, P. , Cuzick, A. , Eulgem, T. , Sinapidou, E. , Mert‐Turk, F. , Can, C. , Dangl, J.L. and Holub, E.B. (2002) Arabidopsis SGT1b is required for defense signaling conferred by several downy mildew resistance genes. Plant Cell, 14, 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornero, P. , Merritt, P. , Sadanandom, A. , Shirasu, K. , Innes, R.W. and Dangl, J.L. (2002) RAR1 and NDR1 contribute quantitatively to disease resistance in Arabidopsis, and their relative contributions are dependent on the R gene assayed. Plant Cell, 14, 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres, M.A. , Jones, J.D. and Dangl, J.L. (2005) Pathogen‐induced, NADPH oxidase‐derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat. Genet. 37, 1130–1134. [DOI] [PubMed] [Google Scholar]
- Turner, P. , Barber, C. and Daniels, M. (1984) Behavior of the transposons Tn5 and Tn7 in Xanthomonas campestris pv. campestris . Mol. Gen. Genet. 195, 101–107. [Google Scholar]
- Wang, L.F. , Tang, X.Y. and He, C.Z. (2007) The bifunctional effector AvrXccC of Xanthomonas campestris pv. campestris requires plasma membrane‐anchoring for host recognition. Mol. Plant Pathol. 8, 491–501. [DOI] [PubMed] [Google Scholar]
- Wei, C.F. , Kvitko, B.H. , Shimizu, R. , Crabill, E. , Alfano, J.R. , Lin, N.C. , Martin, G.B. , Huang, H.C. and Collmer, A. (2007) A Pseudomonas syringae pv. tomato DC3000 mutant lacking the type III effector HopQ1‐1 is able to cause disease in the model plant Nicotiana benthamiana . Plant J. 51, 32–46. [DOI] [PubMed] [Google Scholar]
- Williams, P.H. (1980) Black rot: a continuing threat to world crucifers. Plant Dis. 64, 736–742. [Google Scholar]
- Xiang, T. , Zong, N. , Zou, Y. , Wu, Y. , Zhang, J. , Xing, W. , Li, Y. , Tang, X. , Zhu, L. , Chai, J. and Zhou, J.M. (2008) Pseudomonas syringae effector AvrPto blocks innate immunity by targeting receptor kinases. Curr. Biol. 18, 74–80. [DOI] [PubMed] [Google Scholar]
- Xu, R.Q. , Blanvillain, S. , Feng, J.X. , Jiang, B.L. , Li, X.Z. , Wei, H.Y. , Kroj, T. , Lauber, E. , Roby, D. , Chen, B. , He, Y.Q. , Lu, G.T. , Tang, D.J. , Vasse, J. , Arlat, M. and Tang, J.L. (2008) AvrAC(Xcc8004), a type III effector with a leucine‐rich repeat domain from Xanthomonas campestris pathovar campestris confers avirulence in vascular tissues of Arabidopsis thaliana ecotype Col‐0. J. Bacteriol. 190, 343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun, M.H. , Torres, P.S. , El Oirdi, M. , Rigano, L.A. , Gonzalez‐Lamothe, R. , Marano, M.R. , Castagnaro, A.P. , Dankert, M.A. , Bouarab, K. and Vojnov, A.A. (2006) Xanthan induces plant susceptibility by suppressing callose deposition. Plant Physiol. 141, 178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Lu, H.B. , Li, X.Y. , Li, Y. , Cui, H.T. , Wen, Q.G. , Tang, X.Y. , Su, Z. and Zhou, J.M. (2010) Effector‐triggered and PAMP‐triggered immunity differentially contribute to basal resistance to Pseudomonas syringae . Mol. Plant Microbe Interact. 23, 940–948. [DOI] [PubMed] [Google Scholar]
- Zhou, J.M. and Chai, J. (2008) Plant pathogenic bacterial type III effectors subdue host responses. Curr. Opin. Microbiol. 11, 179–185. [DOI] [PubMed] [Google Scholar]
- Zipfel, C. , Robatzek, S. , Navarro, L. , Oakeley, E.J. , Jones, J.D. , Felix, G. and Boller, T. (2004) Bacterial disease resistance in Arabidopsis through flagellin perception. Nature, 428, 764–767. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 The Xanthomonas campestris pv. campestris type III secretion system (Xcc TTSS) is required for callose induction. Arabidopsis leaves were infiltrated with the indicated bacterial strains at 2 × 107 colony‐forming units (CFU)/mL, and callose deposition was examined 24 h later. Average numbers of callose deposits per microscopic field of 0.1 mm2 are shown below each photograph. ‘±’ represents the standard deviations from three replicates. Similar results were obtained in three independent experiments.
Fig. S2 Type III secretion system (TTSS) mutants ΔhrcV, ΔhrpG and ΔhrpX are nonpathogenic on Arabidopsis. Five‐week‐old Col‐0 plants were inoculated with wild‐type (WT) Xanthomonas campestris pv. campestris strain 8004 (Xcc 8004), ΔhrcV, ΔhrpG and ΔhrpX mutants [bacterial suspension at a concentration of 5 × 105 colony‐forming units (CFU)/mL]. The bacterial populations were determined at 0 and 3 days post‐inoculation. Each data point represents three replicates. Error bars indicate standard deviations. The experiments were repeated twice with similar results.
Fig. S3 Electrolyte leakage assay on leaves infiltrated with wild‐type (WT) Xanthomonas campestris pv. campestris strain 8004 (Xcc 8004) or ΔhrcV mutant. Five‐week‐old Arabidopsis leaves were inoculated with DC3000 (avrB), WT Xcc 8004 and ΔhrcV mutant [bacterial suspension at a concentration of 2 × 107 colony‐forming units (CFU)/mL]. The electrolyte leakage was measured at the indicated times. Each data point represents three replicates. Error bars indicate standard deviations. The experiments were repeated twice with similar results.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item


