SUMMARY
Agrobacterium tumefaciens is a plant pathogenic bacterium that causes neoplastic growths, called ‘crown gall’, via the transfer and integration of transferred DNA (T‐DNA) from the bacterium into the plant genome. We characterized an acetosyringone (AS)‐induced tumour‐inducing (Ti) plasmid gene, tzs (trans‐zeatin synthesizing), that is responsible for the synthesis of the plant hormone cytokinin in nopaline‐type A. tumefaciens strains. The loss of Tzs protein expression and trans‐zeatin secretions by the tzs frameshift (tzs‐fs) mutant is associated with reduced tumorigenesis efficiency on white radish stems and reduced transformation efficiencies on Arabidopsis roots. Complementation of the tzs‐fs mutant with a wild‐type tzs gene restored wild‐type levels of trans‐zeatin secretions and transformation efficiencies. Exogenous application of cytokinin during infection increased the transient transformation efficiency of Arabidopsis roots infected by strains lacking Tzs, which suggests that the lower transformation efficiency resulted from the lack of Agrobacterium‐produced cytokinin. Interestingly, although the tzs‐fs mutant displayed reduced tumorigenesis efficiency on several tested plants, the loss of Tzs enhanced tumorigenesis efficiencies on green pepper and cowpea. These data strongly suggest that Tzs, by synthesizing trans‐zeatin at early stage(s) of the infection process, modulates plant transformation efficiency by A. tumefaciens.
INTRODUCTION
Agrobacterium tumefaciens genetically transforms the host cells of a wide range of plant species (DeCleene and DeLey, 1976). During infection, a defined piece of the tumour‐inducing (Ti) plasmid, the transferred DNA (T‐DNA), is transported from the bacterium into the nucleus of plant cells, where it becomes integrated into the plant genome (Citovsky et al., 2007; Gelvin, 2003; McCullen and Binns, 2006). In nature, wounded plants secrete a sap with a characteristic acidic pH (5.0–5.8) and high content of different phenolic compounds, some of which are recognized by A. tumefaciens and induce virulence (vir) gene expression. The best‐studied and most effective vir gene inducers are monocyclic phenolics, such as acetosyringone (AS; Stachel et al., 1985). Following vir gene induction, the T‐DNA is processed from the Ti plasmid and, together with several effector proteins, is transferred from the bacterium into the host plant cells via a VirB/D4‐encoded type IV secretion system (T4SS) (Christie et al., 2005). In plants, the expression of several oncogenes encoded by T‐DNA directs the production of the plant growth hormones auxin and cytokinin that are responsible for the proliferation of transformed plant cells (Akiyoshi et al., 1984; Montoya et al., 1977).
The products of the T‐DNA genes indoleacetamide hydrolase (iaaH) and tryptophan monooxygenase (iaaM), and isopentenyl transferase (ipt) (tmr), catalyse the production of auxin and cytokinin, respectively. The T‐DNA ipt gene encodes isopentenyl transferase (IPT) which converts dimethylallyl diphosphate (DMAPP) and adenosine monophosphate (AMP) to form the cytokinin isopentenyladenosine 5′‐monophosphate (iPMP) (1983, 1984; Barry et al., 1984; Sakakibara, 2005, 2006). The trans‐zeatin synthesizing (tzs) gene, localized in the vir region of nopaline‐type Ti plasmids, encodes a cytokinin biosynthetic prenyl transferase, which shares considerable homology with IPT encoded by T‐DNA (1985, 1987; Beaty et al., 1986; Powell et al., 1988). In addition to utilizing DMAPP and AMP to produce cytokinin iPMP, Tzs protein can metabolize 4‐hydroxy‐3‐methyl‐2‐(E)‐butenyl diphosphate (HMBDP) and directly produce zeatin riboside 5′‐phosphate (ZMP) (Krall et al., 2002). Plant‐encoded IPT predominantly utilizes DMAPP synthesized from the major isoprenoid pathway, the mevalonic acid (MVA) pathway; however, both the vir regulon‐encoded Tzs and the T‐DNA‐encoded IPT proteins probably use HMBDP as a substrate to synthesize trans‐zeatin (Krall et al., 2002; Sakakibara, 2005, 2006; Sugawara et al., 2008). HMBDP is a metabolic intermediate synthesized from the alternative methylerythritol phosphate (MEP) pathway, which occurs in bacteria and plastids of higher plants (Sakakibara, 2005, 2006). The recent resolved crystal structure of Tzs, in combination with site‐directed mutagenesis studies, identified the amino acid residues Asp‐173 and His‐214 as being responsible for the substrate specificity differences between plant (Arabidopsis) and bacterial (Agrobacterium) IPTs (Sugawara et al., 2008). A previous study has demonstrated that auxin and cytokinin pretreatment of petiole explants from transformation‐recalcitrant Arabidopsis ecotypes significantly enhances their susceptibility to transformation (Chateau et al., 2000). This study demonstrated that exogenous auxin and cytokinin treatments before Agrobacterium infections could have positive effects on the subsequent genetic transformations.
Unlike the ipt gene, the vir regulon‐encoded tzs gene is not incorporated into the host plant genome during infection. The tzs gene is constitutively expressed at low levels and is induced by AS in A. tumefaciens (Anand et al., 2008; Cho and Winans, 2005; John and Amasino, 1988; Lai et al., 2006; Powell et al., 1988; Yuan et al., 2007). In addition, virA and virG are necessary for phenolic‐induced Tzs protein expression and trans‐zeatin production (John and Amasino, 1988; Lai et al., 2006; Powell et al., 1988). The tzs gene may promote transformation, because root induction efficiency was higher when an Agrobacterium rhizogenes strain lacking the tzs gene was co‐inoculated on flax (Linum usitatissimum L.) cotyledon explants with a disarmed A. tumefaciens strain containing the tzs gene (Zhan et al., 1990). Interestingly, Tzs protein was detected in the membrane fraction and on the cell surface of A. tumefaciens (Aly et al., 2008; Lai et al., 2006) and interacted with VirB5, a component of T4SS and T‐pilus (Schmidt‐Eisenlohr et al., 1999), in yeast and in vitro (Aly et al., 2008). However, whether or how Tzs contributes to the virulence of A. tumefaciens remains unclear. In this study, we investigated the importance and possible role(s) of Tzs protein and cytokinin in Agrobacterium virulence. The data together suggest that Tzs and its catalysed product cytokinin modulate the transformation efficiency at early step(s) of Agrobacterium infection.
RESULTS
Tzs protein is responsible for trans‐zeatin secretion and promotes virulence on white radish
In order to investigate whether the tzs gene is involved in virulence, we generated a tzs mutant in the C58‐derived virulent strain NT1RE(pJK270). This tzs mutant, named tzs‐fs, contains a single base pair deletion in the tzs gene to create a frameshift mutation predicted to result in a 25‐kDa truncated protein retaining a short Tzs N‐terminus (17 amino acids). Although 27‐kDa wild‐type Tzs protein was highly expressed when the wild‐type strain NT1RE(pJK270) was grown in AB‐MES medium with AS at 19 °C for 40 h, no Tzs protein expression was detected in the tzs‐fs mutant from immunoblot analysis (Fig. 1A). Under the same induction conditions, NT1RE(pJK270) secreted 492.2 µg/L trans‐zeatin into the culture medium, as assayed by ultra‐performance liquid chromatography (UPLC) of AS‐induced culture medium (Fig. 1B). As expected, the loss of Tzs protein was correlated with the deficiency of trans‐zeatin secretion in the tzs‐fs mutant (Fig. 1B). To determine whether Tzs protein contributes to virulence, we performed tumour assays on stems of white radish plants. The tzs‐fs mutant remained virulent, but fewer plants formed tumours than with the wild‐type A. tumefaciens strain NT1RE(pJK270) (Fig. 1C; Table S1, see Supporting Information). We also included a Ti‐plasmidless A. tumefaciens strain, NT1RE, as a negative control, which did not cause tumour formations on white radish (data not shown).
Figure 1.
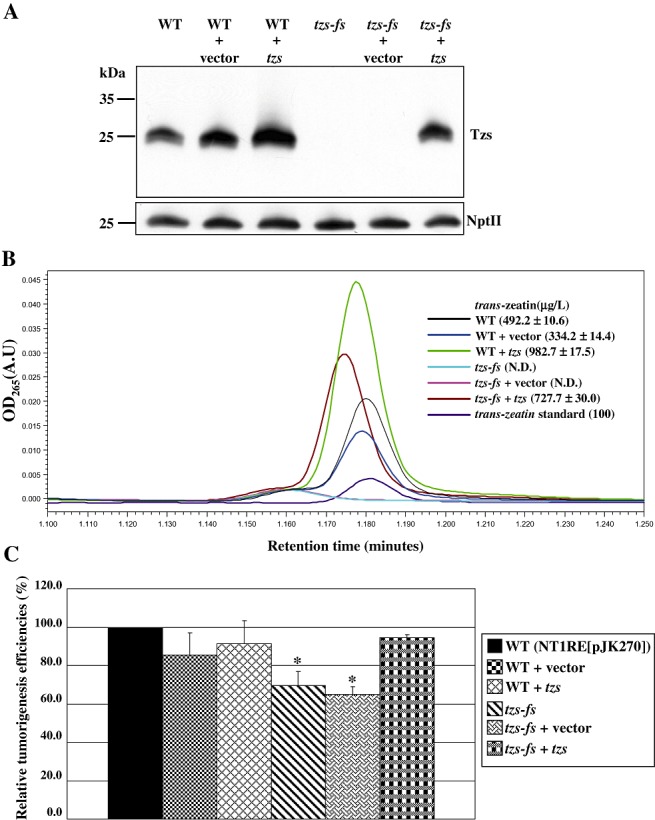
Tzs is responsible for trans‐zeatin secretion and promotes Agrobacterium tumefaciens virulence on white radish stems. (A) Immunoblot analyses of Tzs and neomycin phosphotransferase II (NptII) in the wild‐type (WT) (NT1RE[pJK270]), tzs‐fs mutant (tzs‐fs) and tzs‐complemented (tzs‐fs+tzs) strains grown in AB‐MES in the presence of 200 µm acetosyringone (AS) at 19 °C for 40 h. Equal amounts of protein were resolved by Tricine‐sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) and analysed by immunoblotting with antibodies against Tzs and NptII, which served as a loading control. The molecular weight markers are indicated on the left in kilodaltons. (B) Concentration of trans‐zeatin in the culture medium of the wild‐type, tzs‐fs mutant and tzs‐complemented strains by ultra‐performance liquid chromatography (UPLC). Culture medium from each bacterial strain grown in AB‐MES containing AS was subjected to UPLC analysis, and trans‐zeatin was eluted by methanol at a specific retention time (min, x‐axis) and detected by absorbance at 265 nm wavelength (y‐axis). The mean concentration of trans‐zeatin (µg/L) detected in each sample from three independent experiments is shown with standard deviations. N.D., not detected. The lowest concentration of trans‐zeatin detected with our UPLC system was 100 µg/L, whereas no signal could be detected when 10 µg/L of trans‐zeatin was analysed (data not shown). (C) Virulence of the wild‐type, tzs‐fs mutant and tzs‐complemented strains on white radish stems. The tumorigenesis efficiency of white radish stems infected with the wild‐type strain NT1RE(pJK270), with approximately 80%–100% of plants forming tumours in each experiment, was set at 100%, and that of the mutant is shown relative to that of the wild‐type strain. Mean values for relative tumorigenesis frequencies from at least three independent experiments are shown with standard errors. An asterisk above each mean represents a statistically significant difference between wild‐type (NTIRE[pJK270]) and tzs‐fs mutant strains based on analysis of variance (anova) and pairwise Student's t‐test. P < 0.05 for all significant differences between strain combinations.
To confirm that the loss of trans‐zeatin secretion and reduced virulence resulted from the loss of Tzs protein in the tzs‐fs mutant, we performed a genetic complementation test. We introduced the plasmid pIncP‐Tzs, with the tzs gene expressed under the control of its native promoter, into the wild‐type and tzs‐fs mutant strains. The expression of the wild‐type tzs gene in the mutant strain restored Tzs protein expression and trans‐zeatin secretion to levels slightly higher than those of the wild‐type (Fig. 1A,B), probably because of the increased copy number of the tzs gene when expressed in plasmid. Neither Tzs protein nor secreted trans‐zeatin could be detected in the tzs‐fs mutant alone or with the empty vector (Fig. 1A,B). The tzs‐complemented strain caused almost 100% of white radish plants to develop tumours (Fig. 1C; Table S1), which indicates that the reduced virulence phenotype of the tzs‐fs mutant resulted from the loss of a functional Tzs protein. These data show that Tzs protein and its synthesized product trans‐zeatin are not essential, but may play an important role in A. tumefaciens virulence.
Tzs promotes virulence on Arabidopsis roots at step(s) prior to T‐DNA integration
Because tumorigenesis is caused by the overproduction of auxin and cytokinin produced in T‐DNA‐transformed cells, we next investigated whether Tzs promotes Agrobacterium virulence at step(s) prior to T‐DNA integration or during the process of tumorigenesis after T‐DNA integration. We performed quantitative tumorigenesis and transient transformation assays using the T‐DNA binary vector pCAMBIA2201‐Gm harbouring a gusA‐intron reporter gene [the intron was utilized to prevent the expression of β‐glucuronidase (GUS) activity in A. tumefaciens] (1997, 1998, 1999; Zhu et al., 2003). We determined the tumorigenesis and transient transformation efficiency based on the percentage of Arabidopsis roots that had formed tumours 1 month after infection and that showed GUS activity 7 days after infection, respectively. In both assays, the tzs‐fs mutant was less than 50% as virulent as the wild‐type strain (Fig. 2A–C; Tables S2 and S3, see Supporting information). The tzs‐complemented strain restored the reduced tumorigenesis efficiency of the tzs‐fs mutant on Arabidopsis roots to 90% and 60% of the wild‐type level when inoculated at 108 and 107 colony‐forming units (cfu)/mL, respectively (Fig. 2A; Table S2). The representative results of Arabidopsis roots with tumorigenesis assays when infected with a bacterial concentration of 108 cfu/mL are shown in Fig. 2C. In transient transformation assays, the tzs‐complemented strain restored the reduced virulence of the tzs‐fs mutant to 80%–90% of the wild‐type level when inoculated at either 108 or 107 cfu/mL (Fig. 2B; Table S3). Because transient transformation does not require the integration of T‐DNA into the plant genome (Mysore et al., 1998), these data suggest the involvement of Tzs at the early step(s) of the transformation process prior to T‐DNA integration.
Figure 2.
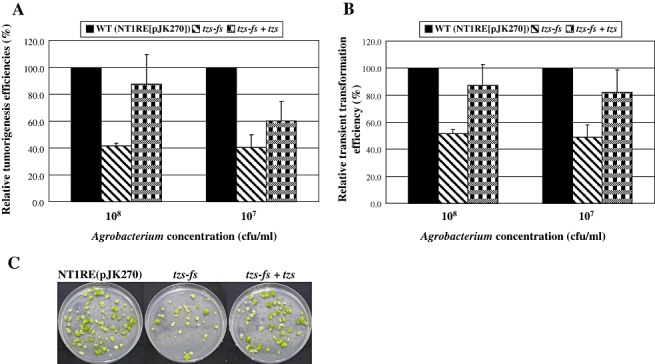
The tzs‐fs mutant shows lower tumorigenesis and transient transformation efficiency in Arabidopsis roots. Root segments from 3–4‐week‐old Arabidopsis plants were infected with the wild‐type NT1RE(pJK270), tzs‐fs mutant and tzs‐complemented Agrobacterium tumefaciens strains at two concentrations [108 and 107 colony‐forming units (cfu)/mL] for both tumorigenesis (A) and transient transformation (B) assays. The transformation efficiency of root segments infected with the wild‐type strain NT1RE(pJK270), with approximately 40%–70% of roots forming tumours or β‐glucuronidase (GUS) activity in each experiment, was set at 100% and that of the mutant is shown relative to that of the wild‐type strain. Mean values of virulence from at least three independent experiments are shown with standard errors. (C) Representative plates of Arabidopsis roots with tumours infected at a bacterial concentration of 108 cfu/mL.
The addition of cytokinin during infection enhances the transient transformation efficiency on Arabidopsis roots in strains lacking Tzs
To examine further whether the reduced virulence of the tzs‐fs mutant results from the lack of cytokinin production by A. tumefaciens, we determined whether the addition of cytokinin during infection could enhance virulence. Arabidopsis roots infected with 107 cfu/mL of bacteria were co‐cultivated on Murashige and Skoog (MS) medium containing various concentrations of a cytokinin, kinetin (0, 0.01, 0.3, 0.5, 1, 2.5, 5, 8, 10 and 15 mg/L). The transient transformation efficiencies of the tzs‐fs mutant with treatments of kinetin from 0.01 to 15 mg/L were higher than those without kinetin treatment (Fig. 3). In contrast, the transient transformation efficiency was not significantly increased when kinetin was added during co‐cultivation with the respective wild‐type strain NT1RE(pJK270) (Fig. 3). The results of the combination of genetic and chemical complementation experiments strongly suggest that the reduced virulence phenotype of the tzs‐fs mutant is caused by the absence of cytokinin secretion by A. tumefaciens during the early stage of infection.
Figure 3.
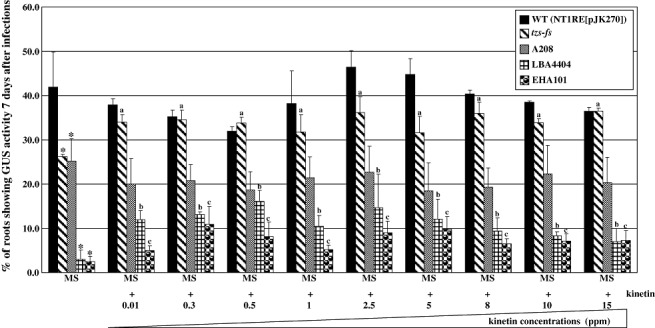
Cytokinin supplementation during infection increases the transient transformation efficiencies of strains lacking Tzs. Arabidopsis root segments were infected with a bacterial concentration of 107 colony‐forming units (cfu)/mL of various Agrobacterium tumefaciens strains, the wild‐type (NT1RE[pJK270]), the tzs‐fs mutant, A208, LBA4404 and EHA101, on Murashige and Skoog (MS) medium with or without different kinetin concentrations for 2 days. Seven days after infection, root segments were stained with 5‐bromo‐4‐chloro‐3‐indolyl‐β‐d‐glucuronic acid (X‐gluc) staining solution. Mean values of the transformation frequencies from at least three independent experiments are shown with standard errors. An asterisk above each mean represents a statistically significant difference between the transformation frequencies of the wild‐type and various tested strains in the absence of exogenous cytokinins during infection based on analysis of variance (anova) and pairwise Student's t‐test. P < 0.05 for all significant differences between strains. The letters ‘a’, ‘b’ and ‘c’ above each mean represent statistically significant differences with and without exogenous cytokinins using the same strain during infection based on anova and pairwise Student's t‐test. GUS, β‐glucuronidase.
We further examined whether cytokinin supplementation during co‐cultivation could enhance the virulence of A. tumefaciens LBA4404 and EHA101, two strains lacking the tzs gene. As shown in Fig. 3, the transient transformation efficiencies were below 5% for LBA4404 and EHA101 when no cytokinins were added during infection. With kinetin treatment of 0.01–15 mg/L during infection, the transformation efficiencies of LBA4404 and EHA101 increased two‐ to five fold (Fig. 3). These data suggest that the exogenous addition of cytokinin during infection can enhance the transformation efficiencies of Arabidopsis roots infected by A. tumefaciens strains deficient in cytokinin secretion.
The addition of cytokinin enhances the transient transformation efficiencies on Arabidopsis roots infected with NT1RE(pJK270) at lower bacterial concentrations
The transient transformation efficiencies of Arabidopsis roots infected by the wild‐type nopaline strain NT1RE(pJK270) were not increased significantly with kinetin treatments with a bacterial concentration of 107 cfu/mL used in these assays (Fig. 3). Similar results were observed with another nopaline‐type A. tumefaciens strain, A208 (Fig. 3). It is possible that sufficient amounts of cytokinin were secreted by the wild‐type nopaline A. tumefaciens strains when roots were infected at 107 cfu/mL. We therefore performed transient transformation assays with different concentrations, from 105 to 108 cfu/mL, of the wild‐type nopaline A. tumefaciens strain NTIRE(pJK270). Figure 4 shows no significant increase in transient transformation efficiency in cytokinin‐treated roots infected with 107–108 cfu/mL bacteria. However, when lower bacterial concentrations (106 and 105 cfu/mL) were used, the transient transformation efficiencies increased at least two fold when 8 and 10 mg/L of cytokinin were added to the medium during infection (Fig. 4). Thus, the addition of cytokinin when Arabidopsis roots were infected by lower bacterial concentrations of A. tumefaciens, which presumably secrete lower levels of trans‐zeatin, may provide adequate amounts of cytokinin to assist Agrobacterium‐mediated transformation efficiency.
Figure 4.
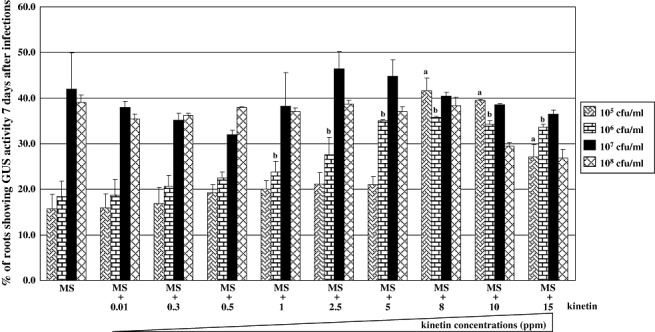
Addition of cytokinin enhances transient transformation efficiencies on Arabidopsis roots infected with NT1RE(pJK270) at lower bacterial concentrations. Arabidopsis root segments were infected with different concentrations [105, 106, 107 and 108 colony‐forming units (cfu)/mL] of the wild‐type Agrobacterium tumefaciens strain NT1RE(pJK270) on Murashige and Skoog (MS) medium with or without kinetin for 2 days. Root segments were stained with 5‐bromo‐4‐chloro‐3‐indolyl‐β‐d‐glucuronic acid (X‐gluc) staining solution 7 days after infection. The mean values of the transformation frequencies from at least three independent experiments are shown with standard errors. Letters ‘a’ or ‘b’ above each mean represent statistically significant differences between the presence and absence of exogenous cytokinins with the same wild‐type bacterial concentration during infection based on analysis of variance (anova) and pairwise Student's t‐test. GUS, β‐glucuronidase.
Tzs affects virulence to different extents on different plant species
The Ti plasmid determines, in part, the host range of A. tumefaciens (Kao et al., 1982; Loper and Kado, 1979). Octopine and nopaline strains of A. tumefaciens differ in their ability to induce tumours on Nicotiana glauca (Jarchow et al., 1991; Melchers et al., 1990; Otten et al., 1985). To determine whether Tzs affects virulence on various plant species, we performed tumour assays on 30 plant species (Table 1). The tzs‐fs mutant reduced virulence by approximately 20%–40% on potato (Solanum tuberosum) tuber discs, stems of pai‐tsai (Brassica rapa L. R. Chinensis Group), loose leaf lettuce (Lactuca sativa L. var. crispa) and carnation (Dianthus caryophyllus L.) when compared with the wild‐type strain NTIRE(pJK270) (Fig. 5A,B; Tables S4 and S5, see Supporting Information). In contrast, the tzs‐fs mutant resulted in higher virulence on the stems of cowpea (Vigna unguiculata cv. Green Pod Kaoshiung) and green pepper (Capsicum annuum cv. Vega) (Fig. 5C; Table S5). No significant differences in virulence between the wild‐type A. tumefaciens and the tzs‐fs mutant were observed with other plant species tested (Tables 1 and S5). These data suggest that Tzs and/or its cytokinin product may play different roles when A. tumefaciens infects different host plants.
Table 1.
Summary of tumour assay results on various plant species.
| Effects of tzs‐fs mutation on virulence | Family name | Test host |
|---|---|---|
| Reduced transformation efficiency | Brassicaceae | Arabidopsis thaliana |
| White radish (Raphanus sativus cv. Rosy) | ||
| Pai‐tsai (Brassica rapa L. R. Chinensis Group) | ||
| Asteraceae | Loose leaf lettuce (Lactuca sativa L. var. crispa) | |
| Solanaceae | Potato (Solanum tuberosum) | |
| Caryophyllaceae | Carnation (Dianthus caryophyllus L.) | |
| Enhanced transformation efficiency | Solanaceae | Green pepper (Capsicum annuum cv. Vega) |
| Leguminosae | Cowpea (Vigna unguiculata cv. Green Pod Kaoshiung) | |
| No difference | Brassicaceae | Ching chiang pai‐tsai (Brassica chinensis) |
| Broccoli (Brassica oleracea L. var. Italica Group) | ||
| Kale (Brassica oleracea) | ||
| Head mustard (Brassica juncea var. capitata Hort.) | ||
| Chinese cabbage (Brassica campestris L. Pekinensis Group) | ||
| Matthiola (Matthinola incana L. R. Br.) | ||
| Asteraceae | Sunflower (Helianthus annuus) | |
| Gazania (Gazania splendens) | ||
| Pointed leaf lettuce (Lactuca indica L. var. idivisa (Mak.) Hara) | ||
| Romaine lettuce (Lactuca sativa L. var. romana) | ||
| Chicory (Cichorium intybus L.) | ||
| Solanaceae | Tomato (Lycopersicon esculentum cv. microtone) | |
| Tobacco (Nicotiana tobacum cv. W38) | ||
| Egg plant (Solanum melongena L. var. esculentum) | ||
| Petunia (Petunia hybrida hort.) | ||
| Apiaceae | Carrot (Daucus carota cv. Red Sky) | |
| Cilantro (Coriadium sativum) | ||
| Celery (Apium graveolens L.) | ||
| Leguminosae | Kidney bean (Phaseolus vulgaris) | |
| Amaranthaceae | Amaranth (Amaranthus mangostanus L.) | |
| Spinach (Spinacia oleracea) | ||
| Cucurbitaceae | Cucumber (Cucumis sativus cv. Merry Swallow) | |
| Apocynaceae | Vinca (Cathranthus roseus) |
Figure 5.
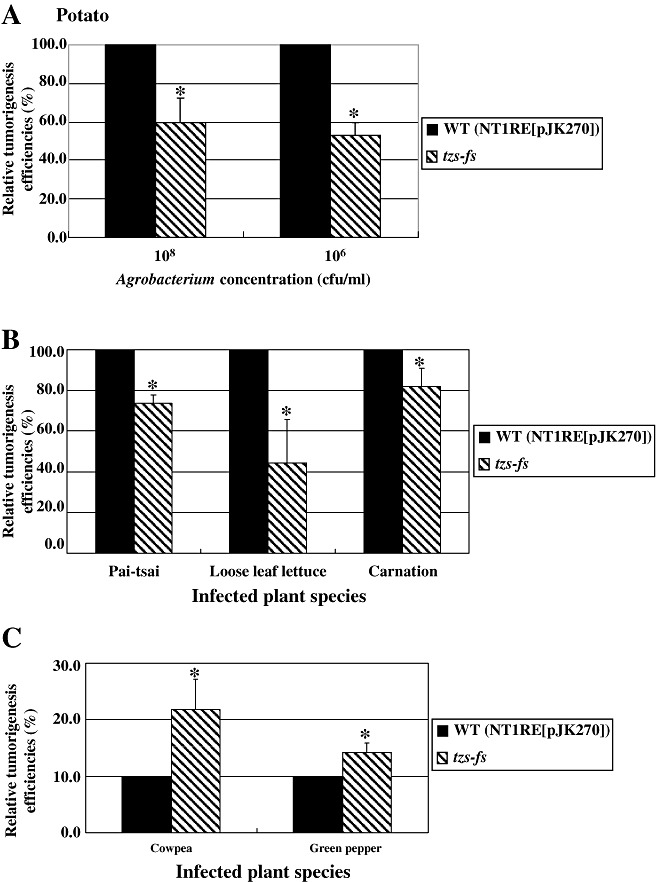
The tzs‐fs mutant decreased virulence on potato tubers, pai‐tsai, loose leaf lettuce and carnation and caused more plants to produce tumours on cowpea and green pepper. Potato tuber discs (A), stems of pai‐tsai, loose leaf lettuce and carnation (B), stems of cowpea and green pepper (C) were infected with the wild‐type NT1RE(pJK270) and tzs‐fs mutant as indicated. Tumours were scored 5 weeks after infection. The tumorigenesis efficiency of potato tuber discs (A) infected with the wild type was approximately 20–30 tumours per disc, and those of pai‐tsai, loose leaf lettuce and carnation (B) were approximately 40%–90% of stems forming tumours in each experiment. The tumorigenesis efficiency of the wild‐type shown in (A) and (B) was set at 100% and that of the mutant is shown relative to that of the wild‐type strain. The transformation efficiency of stems of cowpea and green pepper (C) infected with the wild‐type Agrobacterium tumefaciens strain NT1RE(pJK270), with approximately 10%–30% of stems forming tumours in each experiment, was set at 10% and that of the mutant is shown relative to that of the wild‐type strain. Mean values of the tumorigenesis frequencies from at least three independent experiments are shown with standard errors. An asterisk above each mean represents a statistically significant difference between the wild‐type and various tested strains based on analysis of variance (anova) and pairwise Student's t‐test. P < 0.05 for all significant differences between strains.
DISCUSSION
Many pathogenic fungi and bacteria that are intimately associated with plants, including Pseudomonas, Agrobacterium, Rhizobium and Azospirillum species, can produce and secrete substantial amounts of cytokinins and/or cause plant cells to synthesize plant hormones (Costacurta and Vanderleyden, 1995; Goethals et al., 2001; Morris, 1986; Panopoulos and Peet, 1985; Walters and McRoberts, 2006). These plant hormones contribute to pathogenesis by some microorganisms and, in other cases, may be involved in root growth stimulation by beneficial bacteria and associative symbiosis (Costacurta and Vanderleyden, 1995; Walters and McRoberts, 2006). In this study, we characterized the effect of the tzs gene on A. tumefaciens virulence. The reduced transformation efficiency caused by the tzs‐fs mutant compared with its wild‐type counterpart NT1RE(pJK270) on several plant species tested (1, 2, 3, 5) indicates a role for Tzs in Agrobacterium virulence.
The vir regulon‐encoded tzs gene is constitutively expressed at low levels and is induced by AS in A. tumefaciens (Anand et al., 2008; Cho and Winans, 2005; John and Amasino, 1988; Lai et al., 2006; Powell et al., 1988; Yuan et al., 2007). AS‐induced Tzs protein expression and trans‐zeatin secretion (Fig. 1A,B; Lai et al., 2006; Powell et al., 1988) suggest a possible role for Tzs in the promotion of Agrobacterium virulence. From our finding that the tzs‐fs mutant caused the reduction of both tumorigenesis and transient transformation on Arabidopsis roots (Fig. 2A,B), we suggest that Tzs is involved in the early step(s) prior to T‐DNA integration. Because Tzs functions as an IPT for trans‐zeatin biosynthesis, the role of Tzs in Agrobacterium virulence could be contributed by trans‐zeatin. This hypothesis was supported by results of our chemical complementation assays with the cytokinin kinetin. Exogenous application of kinetin increased the ability of the tzs‐fs mutant bacteria to transiently transform Arabidopsis roots (Fig. 3). In addition, the presence of exogenous cytokinin during infection enhanced the transient transformation efficiencies of two tzs‐lacking strains, LBA4404 and EHA101 (Fig. 3). The levels of increased transient transformation efficiencies in the tzs‐fs mutant treated with kinetin were independent of the kinetin concentrations used in our experiments. These results could indicate that the lowest kinetin concentration, 0.01 mg/L, used in the chemical complementation tests may be comparable with that secreted from its wild‐type counterpart NT1RE(pJK270) in these infection conditions. A previous study has shown that 5 µm (1.096 mg/L) to 35 µm (8 mg/L) of zeatin is secreted from A. tumefaciens C58 when the bacteria are grown at 28 °C for 24 h in the presence of 100–1000 µm AS (Powell et al., 1988). The levels of trans‐zeatin secreted from NT1RE(pJK270) when the bacteria were induced by 200 µm AS at 19 °C for 40 h (Fig. 1B) were slightly lower than those expected if the strains were induced at 28 °C. Although different growth conditions indeed affect the amounts of cytokinin secretion from A. tumefaciens, the levels of trans‐zeatin secretion during the Agrobacterium infection process in planta could not be estimated on the basis of the levels detected in AS‐induced culture. Nevertheless, our data suggest that Tzs contributes to Agrobacterium virulence by promoting transformation efficiency during the early stages of infection. Future work will investigate an association of cytokinin concentration and transformation efficiency during Agrobacterium plant cultivation.
The discovery that Tzs interacts with VirB5 and its localization on the Agrobacterium cell surface (Aly et al., 2008) and membrane (Lai et al., 2006) suggests that Tzs may participate in the contact between host plant cells and bacterial cells. Tzs protein, an IPT, is generally believed to use substrates (i.e. HMBDP or DMAPP and AMP) in bacterial cytoplasm to synthesize trans‐zeatin, which is then secreted into the culture medium (Krall et al., 2002; Sakakibara, 2005, 2006; Sugawara et al., 2008; Takei et al., 2001). However, we cannot yet rule out that surface‐localized Tzs may access substrates secreted from agrobacteria or infected plant cells to synthesize trans‐zeatin directly in the extracellular space.
How do Tzs and its cytokinin product contribute to Agrobacterium virulence? One possible mechanism is that trans‐zeatin secretion may stimulate plant cell division and growth at the infection sites at which plant cells become more susceptible to Agrobacterium infection. Alternatively, the accumulation of cytokinin at the infection sites by A. tumefaciens may create nutrient sinks at the wounded sites (Letham and Palni, 1983; Sakakibara, 2005, 2006; Walters and McRoberts, 2006), which may result in the suppression of host cell death and possibly facilitate bacterial establishment and growth.
Recent studies showing that plant hormones can affect both agrobacterial growth and vir gene expression (Anand et al., 2008; Liu and Nester, 2006; Yuan et al., 2007) also suggest a possible role of Tzs and cytokinin in agrobacterial physiology. Indoleacetic acid (IAA), the plant hormone auxin overproduced on T‐DNA integration, inhibits bacterial growth and vir gene induction in vitro by competing with AS, the vir gene inducer (Liu and Nester, 2006). Exogenous application of the plant defence molecule salicylic acid (SA) to Agrobacterium cells inhibited the expression of vir genes including tzs, bacterial growth, bacterial attachment to plant cells and virulence (Anand et al., 2008; Yuan et al., 2007). On the basis of these studies, we examined whether the absence of Tzs protein and trans‐zeatin secretion in A. tumefaciens may affect bacterial growth and vir gene expression under the AS‐induced condition. The tzs‐fs mutation caused a modest bacterial growth defect when induced by 200 µm AS at 19 °C up to 64 h (data not shown). However, growth of the tzs‐fs mutant and wild‐type bacteria was similar in medium lacking AS (data not shown). In contrast with the effects of auxin and SA, which simultaneously inhibit agrobacterial growth and vir gene expression (Anand et al., 2008; Liu and Nester, 2006; Yuan et al., 2007), several Vir proteins indeed accumulated at higher levels in the tzs‐fs mutant when grown in the same AS‐induced condition as used for growth assays (data not shown). These results imply that Tzs and trans‐zeatin may play a positive role in agrobacterial growth, but may negatively regulate vir gene expression in the AS‐induced condition. The tzs mutant is still virulent, but whether this is a result of the higher expression of Vir proteins is unknown. Comprehensive and systematic analyses comparing the tzs‐fs mutant and the wild‐type in terms of bacterial growth, vir gene expression, bacterial attachment, cytokinin secretion and host cell responses during the infection process are required to elucidate the mechanistic role of Tzs in Agrobacterium virulence.
Intriguingly, our host range study revealed that the tzs‐fs mutant enhanced tumorigenesis on green pepper and cowpea, whereas the same mutant caused reduced tumorigenesis on several plant species including Arabidopsis, white radish, potato, pai‐tsai, loose leaf lettuce and carnation (1, 2, 3, 5; Table 1). These results suggest that Tzs may have additional roles that contribute to virulence on certain plant species. Further elucidation of the role of Tzs in the specificity of Agrobacterium–plant interactions might provide useful information for plant biotechnology applications.
EXPERIMENTAL PROCEDURES
Plasmid constructions
The bacterial strains and plasmids used in this study are listed in Table 2. The A. tumefaciens tzs frameshift mutant strain was generated by a double‐crossover recombination using the suicide plasmid pJQ200KS (Quandt and Hynes, 1993). The plasmid pJQ200KS‐tzs‐fs, used to generate tzs frameshift mutants, was constructed by ligating XmaI/BamHI‐digested Tzs‐fs1 polymerase chain reaction (PCR) product (927‐bp DNA fragment containing the 3′ end and sequences downstream of tzs) and the BamHI/XbaI‐digested Tzs‐fs2 PCR product (703‐bp DNA fragment containing sequences upstream of and the 5′ region of tzs) into the XmaI/XbaI sites of the suicide vector pJQ200KS. The Tzs‐fs1 PCR product was amplified with primers Tzs1‐XmaI‐F (5′‐CCCCCCGGG TAGGTGCCCGAGCCGTTCTT‐3′) and Tzs‐fs‐BamHI‐R (5′‐GGACAT GGGATCC AAATCGCAC‐3′). The Tzs‐fs2 PCR product was amplified with primers Tzs‐fs‐BamHI‐F (5′‐GTGCGATTT GGATCC CATGTCC‐3′) and Tzs2‐XbaI‐R (5′‐GCTCTAGA AATGAAACACAGCCTCGGCA‐3′). The resulting plasmid pJQ200KS‐tzs‐fs contained a single base‐pair deletion in tzs obtained by introduction of a BamHI site to create a frameshift mutation. The primer sequences of the designed restriction enzyme sites are shown in bold and the primer sequences complementary to the specific region in the A. tumefaciens genome are shown in italics.
Table 2.
Bacterial strains and plasmids.
| Strains/plasmids | Relevant characteristics | References/sources |
|---|---|---|
| Strains Agrobacterium tumefaciens | ||
| C58 | Wild‐type virulent strain containing nopaline‐type Ti plasmid pTiC58 | Lin and Kado, 1977 |
| A136 | C58 cured of its Ti plasmid pTiC58 | Watson et al., 1975 |
| A208 | A136 containing nopaline‐type Ti plasmid pTiT37 | Sciaky et al., 1978 |
| LBA4404 | Ach5 strain containing a disarmed octopine‐type Ti plasmid pAL4404 | Hoekema et al., 1983 |
| EHA101 | A136 strain containing a disarmed agropine‐type Ti plasmid pEHA105 | Hood et al., 1986 |
| NT1RE | RmR EmR, C58 cured of its pTiC58 | Watson et al., 1975 |
| NT1RE(pJK270) | RmR EmR, KmR/NmR, pJK270 is pTiC58TraC with Tn5 insertion in T‐DNA region without affecting virulence | Kao et al., 1982 |
| NT1RE(pJK270tzs‐fs) | RmR EmR, KmR/NmR, pJK270 containing tzs frameshift mutation | This study |
| Escherichia coli | ||
| DH5α | Host for DNA cloning | Hanahan, 1983 |
| S‐17 | Host for conjugation | Simon et al., 1983 |
| Plasmids | ||
| pGEMT‐Easy | ApR, TA cloning vector | Promega, Madison, WI, USA |
| pUC19 | ApR, cloning vector | US Biochemical, Cleveland, OH, USA |
| pBluescript SK+ | ApR, cloning vector for blue/white screening | Stratagene, Cedar Creek, TX, USA |
| pCAMBIA 2201 | CmR, a binary vector for plant transformation | Hajdukiewicz et al., 1994, Hiei et al., 1994 |
| pCAMBIA 2201‐Gm | CmR, GmR, a binary vector for plant transformation | This study |
| pJQ200KS | Plasmid containing GmR and sacB gene for selection of double crossover | Quandt and Hynes, 1993 |
| pJQ200KS‐tzs‐fs | GmR, pJQ200KS carrying tzs frameshift mutation | This study |
| pRU1064 | ApR, TcR, a stable broad host range vector with IncP replicon | Karunakaran et al., 2005 |
| pIncP‐Tzs | ApR, TcR, a pRU1064‐derived IncP plasmid carrying the promoter region and full length of tzs gene | This study |
| pEML771 | ApR, TcR, an IncP plasmid with deletion of tzs open reading frame from pIncP‐Tzs | This study |
ApR, CmR, EmR, GmR, KmR, NmR, RmR, SpR and TcR, resistant to ampicillin, chloramphenicol, erythromycin, gentamycin, kanamycin, neomycin, rifampicin, spectinomycin and tetracycline, respectively.
To generate the A. tumefaciens tzs‐complemented strain, a 2.4‐kbp genomic fragment of the tzs region (encompassing 927 bp upstream to 703 bp downstream of the tzs coding region) was amplified by PCR with the primers Tzs1‐XmaI‐F and Tzs2‐XbaI‐R. The XmaI/XbaI‐digested PCR product was subsequently cloned into the same sites of pBluescript SK+, resulting in the plasmid pE453. The XbaI/KpnI‐digested 2.4‐kbp tzs fragment from pE453 was cloned into the same sites of pUC19, thus generating the plasmid pE541. The 1.8‐kbp tzs fragment was digested by HindIII from pE541 and was inserted at the same sites of an IncP plasmid pEML652 (Liu et al., 2008) derived from the IncP broad host range vector pRU1064 (Karunakaran et al., 2005; Prell et al., 2002), thus resulting in the tzs‐complemented construct pIncP‐Tzs. The plasmid pIncP‐Tzs was further digested by SacI/PstI to delete the tzs open reading frame (ORF), thus resulting in the plasmid pEML771 which served as a vector control.
For PCR, A. tumefaciens NT1RE(pJK270) cells grown overnight in 523 medium (10 g/L sucrose, 8 g/L casein enzymatic hydrolysate, 4 g/L yeast extract, 3 g/L K2HPO4, 0.3 g/L MgSO4.7H2O, pH 7.0), supplemented with the appropriate antibiotics, were used to isolate genomic DNA via a Qiagen genomic DNA kit (Qiagen Inc., Valencia, CA, USA). The PCR product was amplified using the Advantage 2 PCR Enzyme System (BD Biosciences Clontech, Palo Alto, CA, USA) with 1 µg of genomic DNA as template. The PCR amplification cycles were performed as follows, unless indicated: 95 °C for 2 min (one cycle); 95 °C for 1 min, 58 °C for 1 min and 72 °C for 1 min (35 cycles); and 72 °C for 10 min (one cycle). The plasmid constructs were confirmed by restriction mapping and DNA sequencing.
Generation of a tzs frameshift mutant
pJQ200KS‐tzs‐fs was used to generate a tzs frameshift mutation in A. tumefaciens NT1RE(pJK270) as described in Wu et al. (2008). To screen the tzs frameshift mutant, genomic colony PCR products amplified with primers Tzs‐HindIII‐R (5′‐AAGCTT CCGAATTCGCGTCAGCGTGA‐3′) and Tzs2‐XbaI‐R were digested by BamHI to confirm that the BamHI site was created in the mutant, which was further confirmed by DNA sequencing.
Immunoblot and trans‐zeatin analyses
Agrobacterium tumefaciens cells grown overnight in 523 medium (10 g/L sucrose, 8 g/L casein enzymatic hydrolysate, 4 g/L yeast extract, 3 g/L K2HPO4 and 0.3 g/L MgSO4.7H2O, pH 7.0) with the appropriate antibiotics were harvested (6000 g, 10 min) and resuspended in 20 mL of AB‐MES medium [20 g/L glucose, 3 g/L K2HPO4, 0.3 g/L MgSO4.7H2O, 1 g/L NaH2PO4, 1 g/L NH4Cl, 0.15 g/L KCl, 10 mg/L CaCl2, 2.5 mg/L FeSO4.7H2O and 9.76 g/L 2‐(N‐morpholino)ethanesulphonic acid (MES), pH 5.5] without antibiotics at an optical density at 600 nm (OD600) of 0.1. After growth at 28 °C to mid‐logarithmic phase for 6 h, the bacterial cultures were further cultivated at 19 °C with or without 200 µm AS (Sigma‐Aldrich, St. Louis, MO, USA), and the cells were collected at 40 h for further analysis after AS addition.
For immunoblot analysis, bacterial cells were harvested by centrifugation at 10 000 g, 4 °C for 10 min, resuspended in TE buffer [10 mm Tris‐HCl, 1 mm ethylenediaminetetraacetic acid (EDTA)] to OD600= 10, and subsequently disrupted by boiling for 10 min with sample loading buffer [0.0625 m Tris‐HCl, 2.25% sodium dodecylsulphate (SDS) and 7.5% glycerol]. The total cell lysate was centrifuged at 10 000 g for 10 min to remove cell debris. The final protein concentrations were measured by a BCA protein assay (Pierce Biotechnology, Rockford, IL, USA) with a Power Wave X 340 Microplate Reader (Bio‐Tek Instruments Inc., Winooski, VT, USA). Equal amounts (15 µg) of total protein extract were resolved by electrophoresis through 16.5% Tricine SDS‐polyacrylamide gels (Schagger and von Jagow, 1987) and transferred to Hybond ECL nitrocellulose membranes (Amersham Biosciences, Uppsala, Sweden). Immunoblot analyses were performed with a Tzs antibody (1 : 5000 or 1 : 10 000 dilution) (Aly et al., 2008; Krall et al., 2002) or with neomycin phosphotransferase II (NptII, encoded by Tn5 inserted in pJK270) antibody (1 : 50 000 dilution) (Sigma‐Aldrich) as an internal control, followed by incubation with goat anti‐rabbit immunoglobulin G (IgG) conjugated with horseradish peroxidase (HRP) (1 : 50 000 dilution) (Chemicon International, Inc., Temecula, CA, USA). The membranes were developed by a chemiluminescent detection method (PerkinElmer Life and Analytical Sciences, Inc., Boston, MA, USA).
To detect trans‐zeatin by UPLC, AS‐induced bacterial cultures grown at 19 °C for 40 h in AB‐MES (pH 5.5) were harvested by centrifugation at 13 000 g for 15 min. In total, 1 mL of the supernatant fluid was filtered through a 0.2‐µm membrane to remove contaminating bacterial cells. For each analysis, 5 µL of trans‐zeatin (Sigma‐Aldrich) standard (0.01, 0.1, 1 and 10 mg/L) or filtered supernatant was subjected to a Waters Acquity UPLC system (Milford, MA, USA) by fractionation through an Acquity UPLC BEH C18 column (1.7 µm, 2.1 × 50 mm; Waters). The column was equilibrated in 0.2 m acetic acid (pH 3.2) and eluted with a linear gradient of methanol (1%–99% over 3 min, 99% for 0.5 min, 99%–1% over 0.1 min, 1% for 0.4 min). Samples were detected with a UV–visible photodiode array detector at 265 nm. The trans‐zeatin concentration was determined on the basis of the peak area and calculated according to the standard curve obtained from the standards.
Tumorigenesis and transient transformation assays
The Arabidopsis root transformation assays (Hwang and Gelvin, 2004; 1997, 1998, 1999; Zhu et al., 2003) and the quantitative tumorigenesis assay on potato tuber discs (Shurvinton and Ream, 1991; Wu et al., 2008) were performed as described previously. All A. tumefaciens strains were grown in 523 medium supplemented with appropriate antibiotics (erythromycin, 50 µg/mL; rifampicin, 50 µg/mL; gentamycin, 50 µg/mL; kanamycin, 20 µg/mL) at 28 °C and to OD600= 0.8–1.0. The bacterial cells were washed with 0.9% sodium chloride and resuspended in 0.9% sodium chloride at the desired bacterial concentrations for infection. For transient transformation assays on Arabidopsis roots, root segments were infected with each A. tumefaciens strain containing the binary vector pCAMBIA2201‐Gm. A gentamycin‐resistance gene cassette was cloned as a BamHI‐EcoRI fragment into the same sites of pCAMBIA2201 (Hajdukiewicz et al., 1994; Hiei et al., 1994), resulting in the plasmid pCAMBIA2201‐Gm. For both stable and transient transformation of Arabidopsis roots, at least 10 different 3–4‐week‐old Arabidopsis thaliana plants (ecotype: Ws) were infected with 100 µL of each A. tumefaciens strain and co‐cultivated on MS medium with or without cytokinin in the medium at 22–24 °C for 2 days. More than 60 root segments were examined for each plant for each independent transformation assay. For transient transformation assays, 7 days after infection, the Arabidopsis root segments were stained with 5‐bromo‐4‐chloro‐3‐indolyl‐β‐d‐glucuronic acid (X‐gluc) staining solution (50 mm sodium phosphate buffer, pH 7.0; 0.1% Tween 20, 3% sucrose and 1–2 mm X‐gluc) for 1 day at 37 °C. Roots were then examined using a stereoscopic microscope to obtain transient transformation efficiencies. For tumour assays on potato tuber discs, the discs were infected with 10 µL of bacterial cultures and co‐incubated for 2 days. The tumours were scored after 5 weeks, and at least 60 potato discs were infected with each A. tumefaciens strain for each independent transformation assay. The tumour assays on other plant species were performed as described previously (Palumbo et al., 1998) with minor modification. Stems of 3–4‐week‐old plants were injected with 100 µL of 109 cfu/mL bacterial cultures using a syringe. After inoculation, the plants were grown in a glasshouse for 5–7 weeks before scoring the tumours. At least 15 different plants were infected with each A. tumefaciens strain for each independent transformation assay. The strain NT1RE (no Ti plasmid) was tested as a negative control in each tumour assay experiment.
Supporting information
Table S1 Summary of tumour assay results for various Agrobacterium strains on white radish stems.
Table S2 Tumour assay results of the wild‐type Agrobacterium strain NT1RE(pJK270), tzs‐fs mutant and tzs‐complemented strain on Arabidopsis roots.
Table S3 Transient transformation assay results of the wild‐type Agrobacterium strain NT1RE(pJK270), the tzs‐fs mutant and the tzs‐complemented strain on Arabidopsis roots.
Table S4 Tumour assay results of the wild‐type Agrobacterium strain NT1RE(pJK270) and the tzs‐fs mutant on potato tuber discs.
Table S5 Tumour assay results of the wild‐type Agrobacterium strain NT1RE(pJK270) and the tzs‐fs mutant on various plant species.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
ACKNOWLEDGEMENTS
The authors thank Dr Clarence Kado for providing various wild‐type A. tumefaciens strains, Dr Christian Baron for providing the Tzs antibody and Yi‐Chun Chen for technical assistance. They also thank Dr Barbara Hohn and Dr Stanton B. Gelvin for critical reading of the manuscript, and Dr Shu‐Hsing Wu, Dr Kuo‐Chen Yeh and the Lai laboratory members for discussion. Acknowledgement also goes to Yu‐Ching Wu and the IPMB Metabolomics Core Laboratory for technical assistance in UPLC analysis. This research was funded by a grant from Academia Sinica to E. M. Lai and a National Science Council grant NSC97‐2311‐B‐005‐002‐MY3 to H.‐H. Hwang.
REFERENCES
- Akiyoshi, D.E. , Morris, R.O. , Hinz, R. , Mischke, B.S. , Kosuge, T. , Garfinkel, D.J. , Gordon, M.P. and Nester, E.W. (1983) Cytokinin/auxin balance in crown gall tumors is regulated by specific loci in the T‐DNA. Proc. Natl. Acad. Sci. USA, 80, 407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyoshi, D.E. , Klee, H. , Amasino, R.M. , Nester, E.W. and Gordon, M.P. (1984) T‐DNA of Agrobacterium tumefaciens encodes an enzyme of cytokinin biosynthesis. Proc. Natl. Acad. Sci. USA, 81, 5994–5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyoshi, D.E. , Regier, D.A. , Jen, G. and Gordon, M.P. (1985) Cloning and nucleotide sequence of the tzs gene from Agrobacterium tumefaciens strain T37. Nucleic Acids Res. 13, 2773–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyoshi, D.E. , Regier, D.A. and Gordon, M.P. (1987) Cytokinin production by Agrobacterium and Pseudomonas spp. J. Bacteriol. 169, 4242–4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly, K.A. , Krall, L. , Lottspeich, F. and Baron, C. (2008) The type IV secretion system component VirB5 binds to the trans‐zeatin biosynthetic enzyme Tzs and enables its translocation to the cell surface of Agrobacterium tumefaciens . J. Bacteriol. 190, 1595–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand, A. , Uppalapati, S.R. , Ryu, C.M. , Allen, S.N. , Kang, L. , Tang, Y. and Mysore, K.S. (2008) Salicylic acid and systemic acquired resistance play a role in attenuating crown gall disease caused by Agrobacterium tumefaciens . Plant Physiol. 146, 703–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry, G.F. , Rogers, S.G. , Fraley, R.T. and Brand, L. (1984) Identification of a cloned cytokinin biosynthetic gene. Proc. Natl. Acad. Sci. USA, 81, 4776–4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty, J.S. , Powell, G.K. , Lica, L. , Regier, D.A. , MacDonald, E.M.S. , Hommes, N.G. and Morris, R.O. (1986) Tzs, a nopaline Ti plasmid gene from Agrobacterium tumefaciens associated with trans‐zeatin biosynthesis. Mol. Gen. Genet. 203, 274–280. [Google Scholar]
- Chateau, S. , Sangwan, R.S. and Sangwan‐Norreel, B.S. (2000) Competence of Arabidopsis thaliana genotypes and mutants for Agrobacterium tumefaciens‐mediated gene transfer: role of phytohormones. J. Exp. Bot. 51, 1961–1968. [DOI] [PubMed] [Google Scholar]
- Cho, H. and Winans, S.C. (2005) VirA and VirG activate the Ti plasmid repABC operon, elevating plasmid copy number in response to wound‐released chemical signals. Proc. Natl. Acad. Sci. USA, 102, 14 843–14 848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie, P.J. , Atmakuri, K. , Krishnamoorthy, V. , Jakubowski, S. and Cascales, E. (2005) Biogenesis, architecture, and function of bacterial type IV secretion systems. Annu. Rev. Microbiol. 59, 451–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citovsky, V. , Kozlovsky, S.V. , Lacroix, B. , Zaltsman, A. , Dafny‐Yelin, M. , Vyas, S. , Tovkach, A. and Tzfira, T. (2007) Biological systems of the host cell involved in Agrobacterium infection. Cell Microbiol. 9, 9–20. [DOI] [PubMed] [Google Scholar]
- Costacurta, A. and Vanderleyden, J. (1995) Synthesis of phytohormones by plant‐associated bacteria. Crit. Rev. Microbiol. 21, 1–18. [DOI] [PubMed] [Google Scholar]
- DeCleene, M. and DeLey, J. (1976) The host range of crown gall. Bot. Rev. 42, 389–466. [Google Scholar]
- Gelvin, S.B. (2003) Agrobacterium‐mediated plant transformation: the biology behind the ‘gene‐jockeying’ tool. Microbiol. Mol. Biol. Rev. 67, 16–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goethals, K. , Vereecke, D. , Jaziri, M. , Van Montagu, M. and Holsters, M. (2001) Leafy gall formation by Rhodococcus fascians . Annu. Rev. Phytopathol. 39, 27–52. [DOI] [PubMed] [Google Scholar]
- Hajdukiewicz, P. , Svab, Z. and Maliga, P. (1994) The small versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol. Biol. 25, 989–994. [DOI] [PubMed] [Google Scholar]
- Hanahan, D. (1983) Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166, 557–580. [DOI] [PubMed] [Google Scholar]
- Hiei, Y. , Ohta, S. , Komari, T. and Kumashiro, T. (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T‐DNA. Plant J. 6, 271–282. [DOI] [PubMed] [Google Scholar]
- Hoekema, A. , Hirsch, P.R. , Hooykaas, P.J.J. and Schilperoort, R.A. (1983) A binary plant vector strategy based on separation of vir and T‐region of the Agrobacterium tumefaciens Ti plasmid. Nature, 303, 179–180. [Google Scholar]
- Hood, E.E. , Chilton, W.S. , Chilton, M.D. and Fraley, R.T. (1986) T‐DNA and opine synthetic loci in tumors incited by Agrobacterium tumefaciens A281 on soybean and alfalfa plants. J. Bacteriol. 168, 1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, H.‐H. and Gelvin, S.B. (2004) Plant proteins that interact with VirB2, the Agrobacterium pilin protein, mediate plant transformation. Plant Cell, 16, 3148–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarchow, E. , Grimsley, N.H. and Hohn, B. (1991) virF, the host‐range‐determining virulence gene of Agrobacterium tumefaciens, affects T‐DNA transfer to Zea mays . Proc. Natl. Acad. Sci. USA, 102, 14 843–14 848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John, M.C. and Amasino, R.M. (1988) Expression of an Agrobacterium Ti plasmid gene involved in cytokinin biosynthesis is regulated by virulence loci and induced by plant phenolic compounds. J. Bacteriol. 170, 790–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao, J.C. , Perry, K.L. and Kado, C.I. (1982) Indoleacetic acid complementation and its relation to host range specifying genes on the Ti plasmid of Agrobacterium tumefaciens . Mol. Gen. Genet. 188, 425–432. [DOI] [PubMed] [Google Scholar]
- Karunakaran, R. , Mauchline, T.H. , Hosie, A.H.F. and Poole, P.S. (2005) A family of promoter probe vectors incorporating autofluorescent and chromogenic reporter proteins for studying gene expression in Gram‐negative bacteria. Microbiology, 151, 3249–3256. [DOI] [PubMed] [Google Scholar]
- Krall, L. , Raschke, M. , Zenk, M.H. and Baron, C. (2002) The Tzs protein from Agrobacterium tumefaciens C58 produces zeatin ribose 5′‐phosphate from 4‐hydroxy‐3‐methyl‐2‐(E)‐butenyl diphosphate and AMP. FEBS Lett. 527, 315–318. [DOI] [PubMed] [Google Scholar]
- Lai, E.M. , Shih, H.W. , Wen, S.R. , Cheng, M.W. , Hwang, H.‐H. and Chiu, S.H. (2006) Proteomic analysis of Agrobacterium tumefaciens response to the vir gene inducer acetosyringone. Proteomics, 6, 4130–4136. [DOI] [PubMed] [Google Scholar]
- Letham, D.S. and Palni, L.M.S. (1983) The biosynthesis and metabolism of cytokinins. Annu. Rev. Plant Physiol. 34, 163–197. [Google Scholar]
- Lin, B.C. and Kado, C.I. (1977) Studies on Agrobacterium tumefaciens. VIII. Avirulence induced by temperature and ethidium bromide. Can. J. Microbiol. 23, 1554–1561. [DOI] [PubMed] [Google Scholar]
- Liu, P. and Nester, E.W. (2006) Indoleacetic acid, a product of transferred DNA, inhibits vir gene expression and growth of Agrobacterium tumefaciens C58. Proc. Natl. Acad. Sci. USA, 103, 4658–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, A.C. , Shih, H.W. , Hsu, T. and Lai, E.M. (2008) A citrate‐inducible gene, encoding a putative tricarboxylate transporter, is downregulated by the organic solvent DMSO in Agrobacterium tumefaciens . J. Appl. Microbiol. 105, 1372–1383. [DOI] [PubMed] [Google Scholar]
- Loper, J.E. and Kado, C.I. (1979) Host range conferred by the virulence‐specifying plasmid of Agrobacterium tumefaciens . J. Bacteriol. 139, 591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullen, C.A. and Binns, A.N. (2006) Agrobacterium tumefaciens and plant cell interactions and activities required for interkingdom macromolecular transfer. Annu. Rev. Cell Dev. Biol. 22, 101–127. [DOI] [PubMed] [Google Scholar]
- Melchers, L.S. , Maroney, M.J. , Den Dulk‐Ras, A. , Thompson, D.V. , Van Vuuren, H.A.J. , Schilperoort, R.A. and Hooykaas, P.J.J. (1990) Octopine and nopaline strains of Agrobacterium tumefaciens differ in virulence; molecular characterization of the virF locus. Plant Mol. Biol. 14, 249–259. [DOI] [PubMed] [Google Scholar]
- Montoya, A.L. , Chilton, M.D. , Gordon, M.P. , Sciaky, D. and Nester, E.W. (1977) Octopine and nopaline metabolism in Agrobacterium tumefaciens and crown gall tumor cells: role of plasmid genes. J. Bacteriol. 129, 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, R.O. (1986) Genes specifying auxin and cytokinin biosynthesis in phytopathogens. Annu. Rev. Plant Physiol. 37, 509–538. [Google Scholar]
- Mysore, K.S. , Bassuner, B. , Deng, X.‐B. , Darbinian, N.S. , Motchoulski, A. , Ream, W. and Gelvin, S.B. (1998) Role of the Agrobacterium tumefaciens VirD2 protein in T‐DNA transfer and integration. Mol. Plant–Microbe Interact. 11, 668–683. [DOI] [PubMed] [Google Scholar]
- Nam, J. , Matthysse, A.G. and Gelvin, S.B. (1997) Differences in susceptibility of Arabidopsis ecotypes to crown gall disease may result from a deficiency in T‐DNA integration. Plant Cell, 9, 317–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam, J. , Mysore, K.S. and Gelvin, S.B. (1998) Agrobacterium tumefaciens transformation of the radiation hypersensitive Arabidopsis thaliana mutants uvh1 and rad5 . Mol. Plant–Microbe Interact. 11, 1136–1141. [DOI] [PubMed] [Google Scholar]
- Nam, J. , Mysore, K.S. , Zheng, C. , Knue, M.K. , Matthysse, A.G. and Gelvin, S.B. (1999) Identification of T‐DNA tagged Arabidopsis mutants that are resistant to transformation by Agrobacterium . Mol. Gen. Genet. 261, 429–438. [DOI] [PubMed] [Google Scholar]
- Otten, L. , Piotrowiak, G. , Hooykaas, P. , Dubois, M. , Szegedi, E. and Schell, J. (1985) Identification of an Agrobacterium tumefaciens pTiB6S3 vir region fragment that enhances the virulence of pTiC58. Mol. Gen. Genet. 199, 189–193. [Google Scholar]
- Palumbo, J.D. , Phillips, D.A. and Kado, C.I. (1998) Characterization of a new Agrobacterium tumefaciens strain from alfalfa. Arch. Microbiol. 169, 381–386. [DOI] [PubMed] [Google Scholar]
- Panopoulos, N.J. and Peet, R.C. (1985) The molecular genetics of plant pathogenic bacteria and their plasmid. Annu. Rev. Phytopathol. 23, 381–419. [Google Scholar]
- Powell, G.K. , Hommes, N.G. , Kuo, J. , Castle, L.A. and Morris, R.O. (1988) Inducible expression of cytokinin biosynthesis in Agrobacterium tumefaciens by plant phenolics. Mol. Plant–Microbe Interact. 1, 235–242. [DOI] [PubMed] [Google Scholar]
- Prell, J. , Boesten, B. , Poole, P. and Priefer, U.B. (2002) The Rhizobium leguminosarum bv. viciae VF39 γ‐aminobutyrate (GABA) aminotransferase gene (gabT) is induced by GABA and highly expressed in bacteroids. Microbiology, 148, 615–623. [DOI] [PubMed] [Google Scholar]
- Quandt, J. and Hynes, M.F. (1993) Versatile suicide vectors which allow direct selection for gene replacement in Gram‐negative bacteria. Gene, 127, 15–21. [DOI] [PubMed] [Google Scholar]
- Sakakibara, H. (2005) Cytokinin biosynthesis and regulation. Vitam. Horm. 72, 271–287. [DOI] [PubMed] [Google Scholar]
- Sakakibara, H. (2006) Cytokinins: activity, biosynthesis, and translocation. Annu. Rev. Plant Biol. 57, 431–449. [DOI] [PubMed] [Google Scholar]
- Schagger, H. and Von Jagow, G. (1987) Tricine‐sodium dodecyl sulfate‐polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166, 368–379. [DOI] [PubMed] [Google Scholar]
- Schmidt‐Eisenlohr, H. , Domke, N. , Angerer, C. , Wanner, G. , Zambryski, P.C. and Baron, C. (1999) Vir proteins stabilize VirB5 and mediate its association with the T pilus of Agrobacterium tumefaciens . J. Bacteriol. 181, 7485–7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciaky, D. , Montoya, A.L. and Chilton, M.‐D. (1978) Fingerprints of Agrobacterium Ti plasmids. Plasmid, 1, 238–253. [DOI] [PubMed] [Google Scholar]
- Shurvinton, C.E. and Ream, W. (1991) Stimulation of Agrobacterium tumefaciens T‐DNA transfer by overdrive depends on a flanking sequence but not on helical position with respect to the border repeat. J. Bacteriol. 173, 5558–5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, R. , Priefer, U. and Puhler, A. (1983) A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Biotechnology, 1, 784–791. [Google Scholar]
- Stachel, S.E. , Messens, E. , Van Montagu, M. and Zambryski, P. (1985) Identification of the signal molecules produced by wounded plant cells that activate T‐DNA transfer in Agrobacterium tumefaciens . Nature, 318, 624–629. [Google Scholar]
- Sugawara, H. , Ueda, N. , Kojima, M. , Makita, N. , Yamaya, T. and Sakakibara, H. (2008) Structural insight into the reaction mechanism and evolution of cytokinin biosynthesis. Proc. Natl. Acad. Sci. USA, 105, 2734–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei, K. , Sakakibara, H. and Sugiyama, T. (2001) Identification of genes encoding adenylate isopentenyltransferase, a cytokinin biosynthesis enzyme, in Arabidopsis thaliana . J. Biol. Chem. 276, 26 405–26 410. [DOI] [PubMed] [Google Scholar]
- Walters, D.R. and McRoberts, N. (2006) Plants and biotrophs: a pivotal role for cytokinins? Trends Plant Sci. 11, 581–586. [DOI] [PubMed] [Google Scholar]
- Watson, B. , Currier, T.C. , Gordon, M.P. , Chilton, M.D. and Nester, E.W. (1975) Plasmid required for virulence of Agrobacterium tumefaciens . J. Bacteriol. 123, 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, H.‐Y. , Chung, P.‐C. , Shih, H.–.W. , Wen, S.–.R. and Lai, E.M. (2008) Secretome analysis uncovers an Hcp‐family protein secreted via a type VI secretion system in Agrobacterium tumefaciens . J. Bacteriol. 190, 2841–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, Z.C. , Edlind, M.P. , Liu, P. , Saenkham, P. , Banta, L.M. , Wise, A.A. , Ronzone, E. , Binns, A.N. , Kerr, K. and Nester, E.W. (2007) The plant signal salicylic acid shuts down expression of the vir regulon and activates quormone‐quenching genes in Agrobacterium . Proc. Natl. Acad. Sci. USA, 104, 11 790–11 795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan, X.C. , Jones, D.A. and Kerr, A. (1990) The pTiC58 tzs gene promotes high‐efficiency root induction by agropine strain 1855 of Agrobacterium rhizogenes . Plant Mol. Biol. 14, 785–792. [DOI] [PubMed] [Google Scholar]
- Zhu, Y. , Nam, J. , Humara, J.M. , Mysore, K.S. , Lee, L.Y. , Cao, H. , Valentine, L. , Li, J. , Kaiser, A.D. , Kopecky, A.L. , Hwang, H.‐H. , Bhattacharjee, S. , Rao, P.K. , Tzfira, T. , Rajagopal, J. , Yi, H. , Veena, Yadav, B.S. , Crane, Y.M. , Lin, K. , Larcher, Y. , Gelvin, M.J. , Knue, M. , Ramos, C. , Zhao, X. , Davis, S.J. , Kim, S.I. , Ranjith‐Kumar, C.T. , Choi, Y.J. , Hallan, V.K. , Chattopadhyay, S. , Sui, X. , Ziemienowicz, A. , Matthysse, A.G. , Citovsky, V. , Hohn, B. and Gelvin, S.B. (2003) Identification of Arabidopsis rat mutants. Plant Physiol. 132, 494–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Summary of tumour assay results for various Agrobacterium strains on white radish stems.
Table S2 Tumour assay results of the wild‐type Agrobacterium strain NT1RE(pJK270), tzs‐fs mutant and tzs‐complemented strain on Arabidopsis roots.
Table S3 Transient transformation assay results of the wild‐type Agrobacterium strain NT1RE(pJK270), the tzs‐fs mutant and the tzs‐complemented strain on Arabidopsis roots.
Table S4 Tumour assay results of the wild‐type Agrobacterium strain NT1RE(pJK270) and the tzs‐fs mutant on potato tuber discs.
Table S5 Tumour assay results of the wild‐type Agrobacterium strain NT1RE(pJK270) and the tzs‐fs mutant on various plant species.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item


