SUMMARY
In addition to the important role of abscisic acid (ABA) in abiotic stress signalling, basal and high ABA levels appear to have a negative effect on disease resistance. Using the ABA‐deficient sitiens tomato (Solanum lycopersicum) mutant and different application methods of exogenous ABA, we demonstrated the influence of this plant hormone on disease progression of Erwinia chrysanthemi. This necrotrophic plant pathogenic bacterium is responsible for soft rot disease on many plant species, causing maceration symptoms mainly due to the production and secretion of pectinolytic enzymes. On wild‐type (WT) tomato cv. Moneymaker E. chrysanthemi leaf inoculation resulted in maceration both within and beyond the infiltrated zone of the leaf, but sitiens showed a very low occurrence of tissue maceration, which never extended the infiltrated zone. A single ABA treatment prior to infection eliminated the effect of pathogen restriction in sitiens, while repeated ABA spraying during plant development rendered both WT and sitiens very susceptible. Quantification of E. chrysanthemi populations inside the leaf did not reveal differences in bacterial growth between sitiens and WT. Sitiens was not more resistant to pectinolytic cell‐wall degradation, but upon infection it showed a faster and stronger activation of defence responses than WT, such as hydrogen peroxide accumulation, peroxidase activation and cell‐wall fortifications. Moreover, the rapid activation of sitiens peroxidases was also observed after application of bacteria‐free culture filtrate containing E. chrysanthemi cell‐wall‐degrading enzymes and was absent during infection with an out E. chrysanthemi mutant impaired in secretion of these extracellular enzymes.
INTRODUCTION
The plant hormone abscisic acid (ABA) not only plays an important role in plant development and responses to abiotic stresses (Fujita et al., 2006), but is also involved in biotic stress signalling (Mauch‐Mani and Mauch, 2005). In general, ABA appears to suppress basal plant resistance to both biotrophic and necrotrophic fungi and bacteria. For example, the ABA‐deficient aba2‐1 mutant of Arabidopsis was more resistant to Fusarium oxysporum than the wild‐type plant (WT) (Anderson et al., 2004), while the aba1‐1 mutant was less susceptible to Hyaloperonospora (formally Peronospora) parasitica (Mohr and Cahill, 2003). The ABA‐deficient sitiens mutant of tomato (Solanum lycopersicum) was more resistant than the WT Moneymaker to Botrytis cinerea (Audenaert et al., 2002), Oidium neolycopersici (Achuo et al., 2006) and Pseudomonas syringae (Thaler and Bostock, 2004). Thus, in various plant pathosystems, low ABA levels seem to lower disease susceptibility, while exogenous ABA application enhances pathogen susceptibility. However, positive effects of ABA on pathogen defence have also been observed (for review see Mauch‐Mani and Mauch, 2005). The question arises as to how leaf ABA content influences the plant's susceptibility to pathogens.
During plant–pathogen interactions, ABA interacts in various ways with other plant hormone signalling pathways, mediated by salicylic acid (SA), jasmonate and ethylene. There is increasing evidence of important overlap between ABA regulatory networks and the pathways of plant response to pathogens, with ABA interfering at different levels of biotic stress signalling, depending on the plant pathosystem (for review see Mauch‐Mani and Mauch, 2005). Very recent studies in the Arabidopsis–Pseudomonas syringae pv. tomato interaction revealed that ABA induces susceptibility through suppression of SA accumulation and cell‐wall fortification (Mohr and Cahill, 2007). Moreover, bacterial type III‐secreted effectors were shown to manipulate ABA biosynthesis and ABA signalling as a virulence strategy to suppress defence responses (de Torres‐Zabala et al., 2007).
During abiotic stress, ABA‐derived signal transduction often involves accumulation of hydrogen peroxide (H2O2) (Apel and Hirt, 2004; Pei et al., 2000). On the other hand, in physiological processes such as seed germination, the release of H2O2 during seed germination was inhibited by ABA (Schopfer et al., 2001). The production of high amounts of H2O2 and other reactive oxygen species (ROS) during pathogen attack, referred to as the oxidative burst, is an essential and well‐studied element in plant defence that can have a direct antimicrobial function or can have a function in defence signalling, the hypersensitive response and cell‐wall strengthening (Apel and Hirt, 2004; Lamb and Dixon, 1997). However, whether ABA can influence ROS accumulation during pathogen defence remains unknown.
The necrotrophic bacterium Erwinia chrysanthemi (now reclassified in Dickeya species by Samson et al., 2005) causes soft rot on storage organs, succulent stems and leaf tissue of a wide range of greenhouse and open field‐grown plant species of diverse botanical families, including several Solanaceae species (Dickey, 1979; Pérombelon and Kelman, 1980; Schaad and Brenner, 1977). On greenhouse‐cultivated tomato, E. chrysanthemi causes stem rot by systemic infection from the roots and rapidly induces wilt and eventual collapse of the whole plant (Aysan et al., 2003). The virulence of E. chrysanthemi is largely attributed to its ability to secrete plant cell‐wall‐degrading enzymes (CWDEs) and particularly pectinases (Collmer and Keen, 1986; Kotoujansky, 1987). Most E. chrysanthemi CWDEs are secreted to the external medium via a common type II secretion system, the Out machinery, with Out− mutants being unable to degrade plant cell walls efficiently (Andro et al., 1984). Induction of the secretion machinery‐encoding genes and the production of exo‐enzymes and other virulence factors is tightly regulated and mediated by a number of global regulatory circuits, such as the KdgR regulon and the PecS–PecM regulatory couple. Environmental stimuli that were identified to regulate the synthesis of virulence factors are pectin‐degradation intermediates and other inducers from plant extracts, but also the plant's redox status (reviewed in Barras et al., 1994; Expert, 1999; Hugouvieux‐Cotte‐Pattat et al., 1996).
In the present study, we analysed the role of ABA in the tomato–E. chrysanthemi interaction using the ABA‐deficient sitiens mutant and exogenous ABA feeding. Sitiens appeared to be very resistant to E. chrysanthemi and the nature of this resistance was studied more in detail by testing the following hypotheses: (1) sitiens resistance is due to morphological differences; (2) sitiens resistance is due to inhibition of bacterial growth; (3) E. chrysanthemi CWDEs are ineffective in degrading sitiens cell walls; (4) active defence responses are triggered earlier/more efficiently in sitiens.
RESULTS
Disease symptoms in the E. chrysanthemi–tomato interaction
When E. chrysanthemi was infiltrated into leaf tissue of tomato cv. Moneymaker, the first symptoms could already be seen within 8 h post‐inoculation (hpi) and complete maceration of the infiltrated zone, consisting of wet and degraded leaf tissue, occurred within 24 hpi (Fig. 1a). In some cases, maceration of the infiltrated tissue aborted and a necrosis developed (Fig. 1b). A spreading maceration usually progressed into non‐infiltrated parts of the inoculated leaflet (Fig. 1c), often extending into non‐inoculated leaflets on the same leaf (Fig. 1d), and eventually through the primary petiole into the stem, causing lodging of the plant (Fig. 1e).
Figure 1.
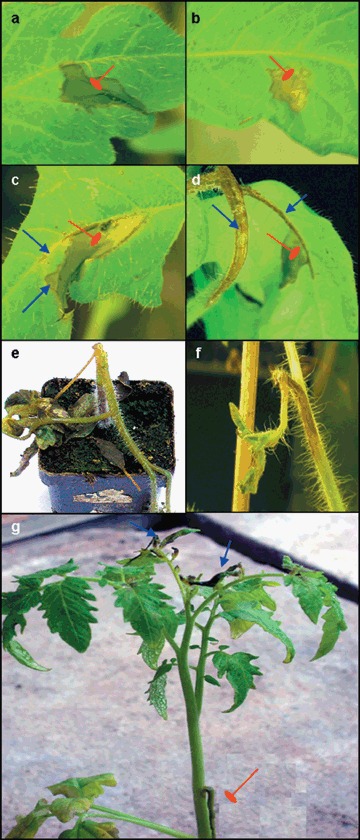
Disease symptoms on tomato cv. Moneymaker caused by syringe infiltration of 107 CFU E. chrysanthemi/mL. (a) Maceration contained in the infiltrated zone (24 hpi); (b) necrosis of the infiltrated zone (24 hpi); (c) maceration spreading beyond the infiltrated zone (sharp arrows) (48 hpi); (d) maceration spreading to petiolule and petiole of inoculated leaf (sharp arrows) (72 hpi); (e) complete collapse of plant due to stem maceration (7 dpi); (f) death of shoot apex due to systemic infection (7 dpi); (g) spread of local maceration to base of petiole of inoculated leaf (blunt arrow) and maceration of systemic leaves (sharp arrows) (7 dpi). Blunt arrows in (a), (b), (c) and (d) indicate the infiltrated zone.
In addition to local symptoms, E. chrysanthemi caused systemic symptoms, visible within 7 days post‐inoculation (dpi). These systemic symptoms include maceration of leaves above the inoculated leaf without obvious stem maceration (Fig. 1g), shoot apex death (Fig. 1f) or total collapse of the plant.
ABA deficiency in the sitiens mutant of tomato results in resistance to E. chrysanthemi
To investigate the effect of endogenous ABA on tomato susceptibility to E. chrysanthemi infection, the ABA‐deficient sitiens mutant was compared with its WT cv. Moneymaker. Inoculation of greenhouse‐grown plants with a concentration of 107 CFU/mL resulted in maceration of more than half of WT inoculation sites within 24 hpi, of which about 20% exhibited spreading maceration during the following days. In contrast, only 10% of inoculated sitiens leaves showed maceration of the infiltrated tissue, and spreading of maceration outside the infiltrated zone was never observed (data not shown).
As ABA affects numerous plant developmental and physiological processes, including water balance and flux, the sitiens tomato mutant exhibits a different morphology compared with the WT (Nagel et al., 1994). To determine if these structural changes in the plant phenotype could be completely or partly responsible for sitiens loss of susceptibility to E. chrysanthemi, an infection was done on plants raised in a growth chamber at 28 °C and 100% relative humidity (RH), conditions that eliminate visible morphological differences between the two genotypes.
Two different bacterial inoculum concentrations were tested. Leaf infiltration with 106 CFU/mL caused local maceration in more than 50% of WT leaves within 48 hpi, while only a few sitiens leaves exhibited local maceration that aborted within the next 24 h (Fig. 2a). Such aborted maceration was also observed in a few cases on WT leaves at 72 hpi, but more often, maceration was spreading, indicating an overall disease progression in WT plants. Leaf infiltration with a more aggressive inoculum (107 CFU/mL) resulted in a relatively higher number of macerated leaves in general, but sitiens plants were still remarkably healthy compared with WT plants and no maceration was observed beyond the infiltration site (Fig. 2a). Furthermore, at 7 dpi the aggressive inoculum did not cause systemic infection of sitiens whereas in the WT, systemic infection occurred in more than half of the plants, and resulted in complete collapse of some plants (data not shown).
Figure 2.
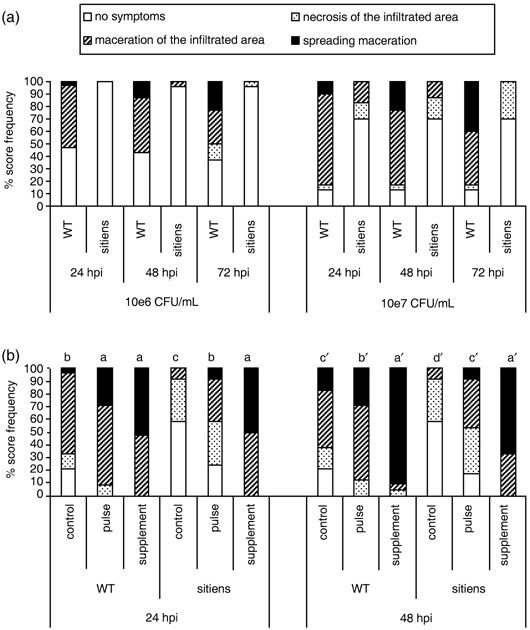
Effect of abscisic acid (ABA) on E. chrysanthemi disease symptoms on tomato. (a) Disease progression at 24, 48 and 72 hpi on sitiens and WT tomato, grown under conditions of high temperature (28 °C) and high relative humidity (100%), and infiltrated with 106 or 107 CFU E. chrysanthemi/mL. At all time points and for both inoculum concentrations, disease development was significantly lower in sitiens than in the WT parent at P = 0.05. (b) Effect of different exogenous ABA treatments on sitiens and WT susceptibility to E. chrysanthemi. Plants were sprayed with 100 µm ABA at 3‐ to 4‐day intervals for 2 weeks (physiological supplementation—‘supplement’ treatment) or 4 h before inoculation (‘pulse’ treatment) and infiltrated with 107 CFU/mL. Bars with different letters indicate a significant difference between the treatments at P = 0.05. Disease development was evaluated and data analysed by the Kruskal–Wallis/Mann–Whitney non‐parametric test. At least eight plants per genotype–treatment combination were used and on each plant, disease was evaluated on three infiltrated leaflets of the same leaf. For (a) and for (b), similar results were obtained in at least three independent experiments. Data of one representative experiment are presented.
Exogenous ABA increases susceptibility of tomato to E. chrysanthemi
To confirm further the role of ABA in the susceptibility of tomato to E. chrysanthemi, both WT and sitiens plants were supplied with exogenous ABA by supplementary feeding during plant growth or by pulse feeding 4 h before challenge with the pathogen. Whereas supplementation in sitiens increases ABA content and restores the WT leaf morphology (Achuo et al., 2006), pulse treatment is expected to increase leaf ABA content without changing the sitiens phenotype. To investigate the effect of leaf structural modification in sitiens, these experiments were conducted on plants grown in a greenhouse at relatively low temperatures (21 ± 1/18 ± 1 °C day/night) and RH (± 65%), to permit expression of the typical sitiens leaf morphology and of the ABA supplementation effect on structural modification. Incubation after bacterial inoculation was done at high RH (± 100%), as in previous experiments. Both ABA application by pulse treatment and by supplementation resulted in a significant loss of sitiens resistance to E. chrysanthemi infection (Fig. 2b). Pulse treatment with ABA restored the WT level of susceptibility in sitiens, while supplementation resulted in significantly higher susceptibility than untreated WT plants. Exogenous ABA also increased the susceptibility of WT plants, causing maceration in all inoculation sites compared with about two‐thirds of inoculation sites in the control at 24 hpi. At 48 hpi, nearly all inoculation sites of ABA‐supplemented WT plants exhibited spreading maceration symptoms, compared with about 30% of spreading maceration that was observed in pulse‐treated inoculation sites (Fig. 2b).
E. chrysanthemi in planta multiplication and effect of CWDEs on sitiens and WT leaf tissue
To check if the resistance of sitiens could be due to inhibition of bacterial growth, inoculated leaf material was sampled at different time points and plated out on LB medium to monitor local bacterial growth in the infiltrated tissue. Since signs of maceration became macroscopically visible at 24 hpi, samples at this time point were taken separately from macerating and non‐macerating infiltrated tissue of sitiens and WT leaves. The same amount of bacteria was retrieved in both WT and sitiens leaves just after inoculation (0 hpi), showing that equal amounts of bacteria per square millimetre were infiltrated in both genotypes (Fig. 3). Within 12 h after infiltration the bacteria grew at the same rate and to the same extent in WT and in sitiens leaves. Bacterial populations stabilized in non‐macerated tissue of both sitiens and WT at 12 hpi, while the bacteria continued to grow in macerated tissue of both genotypes (Fig. 3). While sampling at 48 hpi was not possible for macerated leaves due to advanced disintegration of the infiltrated tissue, the bacteria population in non‐macerated infiltrated tissue at 48 hpi was similar to that at 24 hpi (data not shown).
Figure 3.
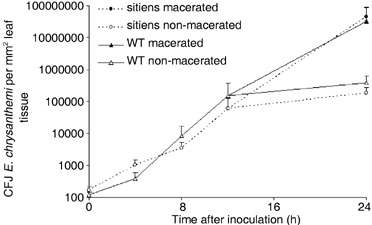
Survival and multiplication of E. chrysanthemi in sitiens and WT tomato leaf tissue. Leaf discs were taken from infiltrated tissue at the indicated time points, crushed in 50 mm KCl, plated out on LB medium and incubated at 28 °C for 24 h. Colonies of Erwinia chrysanthemi gfp9 strain were identified under UV light by their green fluorescence and counted. Bacterial counts at each time point were compared statistically by the t‐test and no significant differences were detected between sitiens and WT before 24 hpi in three independent experiments. Each data point represents the means + the standard error from one experiment using five or six plants per treatment. At 24 hpi, five macerated and five non‐macerated samples from WT and from sitiens were selected, without representing the frequency of maceration in both genotypes.
To test whether sitiens cell walls are more resistant to CWDEs than WT cell walls, various dilutions of a bacterial culture filtrate (CF) containing CWDEs were infiltrated in sitiens and WT leaves. When undiluted CF was infiltrated, cell‐wall degradation could already be observed within 1 hpi on both genotypes. This was seen macroscopically as a soft and transparent appearance of the infiltrated tissue, resembling maceration symptoms caused by the pathogen, and confirmed microscopically by the absence of cell walls and a protoplast‐like appearance of mesophyll cells (data not shown). The kinetics of occurrence of maceration symptoms on WT and sitiens was followed after infiltration of CF serial dilutions in detached leaves. The time at which one half of the leaves presented signs of maceration was extrapolated for each CF dilution and plotted against the dilution factor. The kinetics of macroscopically visible cell‐wall degradation occurrence was similar in both genotypes, being delayed with dilution of the CF (see supplementary Fig. S1).
These findings contradict the hypotheses that sitiens resistance is caused by preventing E. chrysanthemi to multiply, or by ineffectiveness of E. chrysantthemi CWDEs to degrade sitiens cell walls.
Hydrogen peroxide accumulation, peroxidase activity and cell‐wall fortification in WT and sitiens after infection with E. chrysanthemi
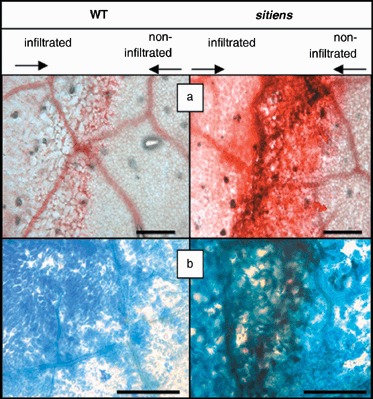
As early and strong H2O2 accumulation is often observed in active defence responses, we studied this reaction in WT and sitiens tomato leaves infiltrated with E. chrysanthemi by using the DAB staining technique described by Thordal‐Christensen et al. (1997). Accumulation of H2O2, visualized as brown precipitations formed by oxidation of DAB, was detectable in leaves infiltrated with E. chrysanthemi, but not in buffer‐infiltrated (mock) leaves. In WT leaf tissue, H2O2 accumulation was first detected in the infiltrated zone at 24 hpi. If maceration was spreading outside the infiltrated area at 48 hpi, H2O2 accumulation was located here as well. In sitiens, H2O2 already accumulated weakly at 12 hpi, followed by a more intense staining at 24 and 48 hpi (see supplementary Fig. S2). A remarkable difference in H2O2 accumulation between sitiens and WT was found at the border of the infiltrated zone: while WT displayed a more diffuse and gradual decline of DAB staining towards the outside of the lesion, the borders of sitiens lesions were sharp and intensely stained. This observation was confirmed by microscopical examination of the lesion borders on intact leaf discs (Fig. 4a) and on sections of leaf tissue that were embedded after DAB staining (Fig. 4b–d). Within the E. chrysanthemi infiltrated zone of both WT and sitiens, H2O2 was predominantly situated in the chloroplasts of mesophyll cells. WT lesion margins showed a gradual decrease in H2O2 staining intensity towards the outer part of the lesion, while there was an abrupt stop of DAB‐positive mesophyll cells in sitiens (Fig. 4a,b). Interestingly, in all samples that were examined, in addition to the H2O2 accumulation in chloroplasts, the lesion borders in sitiens were located at sites of intense extracellular H2O2 staining, originating from cells close to minor veins. In these regions with high cellular density and without large intercellular spaces, DAB accumulated in the walls of mesophyll, epidermal and bundle sheath cells (Fig. 4b,c). Moreover, by toluidine blue staining after sectioning of bacteria, chloroplasts and cell walls, we could observe that in sitiens the regions with apoplastic H2O2 delineate restriction of bacterial progression (Fig. 4d). In infected WT leaves no extracellular H2O2 accumulation was present and tissue regions with vascular bundles did not form barriers for bacterial progression (Fig. 4d). At 48 hpi, extracellular H2O2 accumulation was further amplified at the borders of sitiens lesions, leading to several layers of intensely stained cells adjacent to the infiltrated zone, which was by now severely necrotized or macerated (data not shown).
Figure 4.
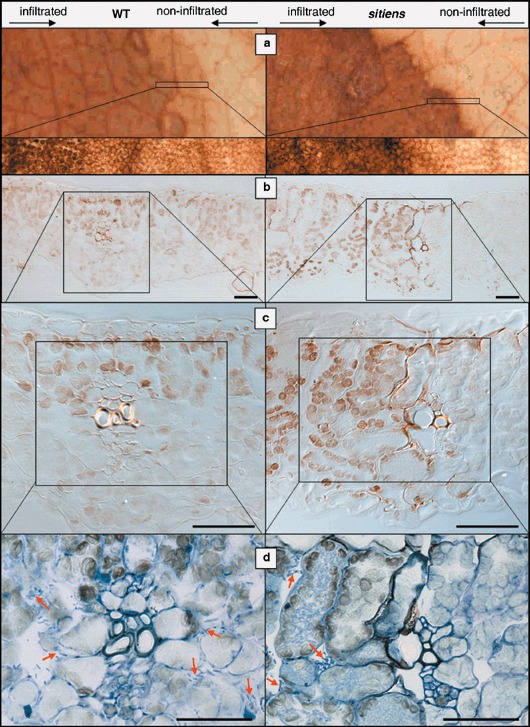
H2O2 accumulation at the border of E. chrysanthemi lesions in WT and sitiens leaf tissue at 24 hpi. DAB accumulation was evaluated on intact leaf discs (a), and on cross‐sections without (b and c) and with (d) toluidine blue staining. Lesions in WT are characterized by a gradual decrease in chloroplastic H2O2 accumulation towards the outside of the lesion, while sitiens lesions have a clear border between mesophyll cells that contain or lack chloroplastic H2O2. This border in sitiens is located at the site of minor veins and in addition, cells in the vicinity of vascular tissues of the border accumulate extracellular H2O2. Toluidine blue staining (d) reveals bacteria on both sides of the minor vein in WT leaves, while in sitiens no bacteria are present beyond the zone of extracellular H2O2 accumulation. Some bacterial microcolonies are marked with arrows. Scale bar = 50 µm.
We further examined the activity of peroxidases, enzymes important in many H2O2‐dependent pathogen defence processes. Leaf discs containing infiltrated zones were sampled and peroxidase activity was measured with the tetramethylbenzoidine (TMB) assay described by Ros Barceló (1998). Elevated levels of peroxidases were detected in sitiens leaf discs as early as 8 hpi, but this was delayed in WT until 24 hpi and the levels remained significantly lower than for sitiens (Fig. 5a). Buffer‐infiltrated and non‐infiltrated control samples also showed a limited increase in peroxidase activity, probably due to the effect of syringe infiltration and exposure to inoculation conditions, respectively. Interestingly, activities in control samples of sitiens increased earlier and reached slightly higher levels than WT controls, as was the case in E. chrysanthemi‐infiltrated samples. Nevertheless, activity values in the respective controls of both WT and sitiens always remained significantly lower than in infected samples (Fig. 5a). Pulse application of exogenous ABA did not alter the early peroxidase activation at 8 hpi in sitiens, but significantly reduced peroxidase activity during the later stages of inoculation (Fig. 5b). A concentration of 100 µm ABA was sufficient to decrease peroxidase activity to WT levels. Exogenous ABA had no significant effect on peroxidase activity in WT.
Figure 5.
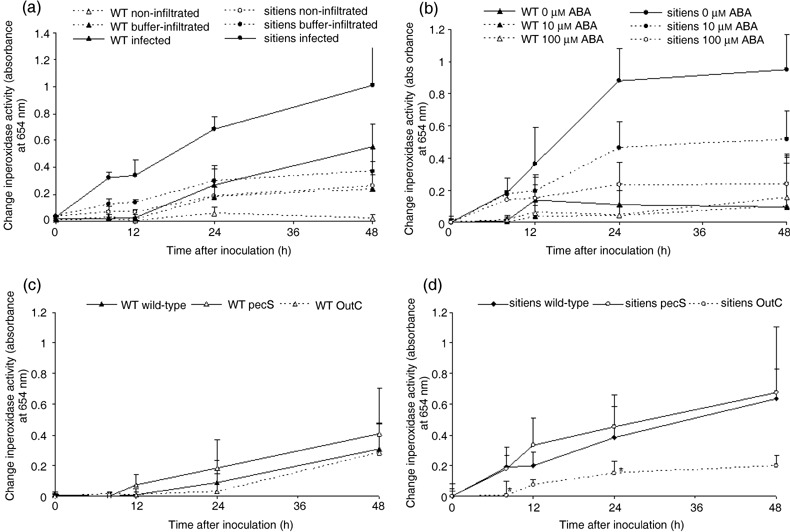
Extracellular peroxidase activity in WT and sitiens inoculated with E. chrysanthemi. (a) Leaf discs were sampled from leaves infiltrated with 106 CFU/mL E. chrysanthemi (‘infected’) or 50 mm KCl (‘buffer‐infiltrated’). Leaf discs without infiltration zone were also sampled (‘non‐infiltrated’). (b) Leaf discs were sampled from WT and sitiens infiltrated with 106 CFU/mL E. chrysanthemi 4 h after a single spray with 0, 10 or 100 µm ABA. All spray solutions contained 0.05% ethanol. (c,d) Leaf discs were sampled from WT (c) and sitiens (d) leaves infiltrated with 106 CFU/mL of E. chrysanthemi wild‐type strain, the pathogenicity factor overproducer pecS strain and the type II secretion‐deficient outC strain. In (b), (c) and (d), the presented change in peroxidase activity is the absolute activity value after subtraction of the activity level of buffer‐infiltrated samples for each time point. During sampling, discs were fixed in ethanol and peroxidase activity was measured at 654 nm after addition of TMB and 0.03% H2O2. The mean + standard error of the absorbance of the incubation solution from four discs of different plants are presented. In (c) and (d), significant differences at P = 0.05 between wild‐type and the two mutant treatments were determined by using one‐way anova and Duncan post‐hoc tests for each time point and are indicated with ‘*’. All experiments were repeated with similar results.
An important consequence of the activation of peroxidases and the production of extracellular H2O2 upon pathogen attack is the fortification of the plant cell wall by peroxidative incorporation of phenolic compounds and cross‐linking of cell‐wall proteins (Bradley et al., 1992; Passardi et al., 2005). We stained phenolic compounds in fortified cell walls with safranin‐o, and cross‐linked proteins were visualized with Coomassie Blue staining after SDS denaturation (Fig. 6). In sitiens, cell‐wall fortification was present at the site of extracellular H2O2 accumulation, i.e. the border of the infiltrated zone, where both stains accumulated strongly. In WT tomato, cell‐wall fortification staining was absent or weak. Both genotypes showed an additional weak intracellular accumulation of the stains in the zones of the leaf infiltrated with E. chrysanthemi, representing dead cells stained after influx through damaged cell membranes and non‐specific binding of the staining molecules (Fig. 6).
Figure 6.
Cell‐wall fortification in WT and sitiens tomato 48 h after inoculation with E. chrysanthemi. Cell‐wall fortifications were visualized with safranin‐o (red–pink) to detect incorporation of phenolics (a) and Coomassie Blue staining after SDS denaturation to detect protein cross‐linking (dark blue) (b). In both genotypes, a weak accumulation of the stains is present inside dead cells of the infiltrated zone (left), while the non‐infiltrated zone (right) remains unstained. Cell‐wall fortifications at the border of the infiltrated zone in sitiens are strongly stained. Representative borders of sitiens and WT were selected after observation of at least ten inoculation sites. The experiment was repeated with similar results. Scale bar = 200 µm.
Effect of E. chrysanthemi pectinolytic cell‐wall degradation on peroxidase activity in WT and sitiens
To determine whether CWDEs play a role in activation of the observed active plant defence response in sitiens, we used mutant strains of E. chrysanthemi altered in the production of CWDEs to infiltrate WT and sitiens leaves. E. chrysanthemi pecS strain is mutated in the transcriptional repressor gene pecS, a mutation leading to derepressed synthesis of pectinases, cellulases and secretion‐machinery proteins and overproduction of indigoidine, a blue pigment involved in pathogenicity and in resistance to oxidative stress (Reverchon et al., 1994); the outC mutant strain is affected in type II secretion and is thereby incapable of secreting several proteins, mainly pectinolytic enzymes and cellulase (Andro et al., 1984; Kazemi‐Pour et al., 2004). As expected, infiltration of tomato leaves with outC did not cause tissue maceration, but on some plants, the infiltrated zone showed necrosis (Fig. 7). Symptom development after pecS infiltration was not significantly different from that after wild‐type E. chrysanthemi infiltration on both WT and sitiens. However, in sitiens at 24 hpi, pecS was able to cause maceration on a few plants while WT E. chrysanthemi could not. Nevertheless, by 48 hpi all disease progression had ceased in sitiens and no spreading maceration was observed (Fig. 7). Bacterial quantification in the infiltrated zone did not reveal differences between the mutant strains in the ability to grow inside WT and sitiens leaf tissue between 0 and 12 hpi (see supplementary Fig. S3). In WT leaves, no differences in peroxidase activity were detected after infection by the three E. chrysanthemi strains (Fig. 5c). In sitiens, derepression of pathogenicity factor production in the pecS strain did not cause a significant change in peroxidase activity either when compared with wild‐type E. chrysanthemi. However, the outC secretion mutant caused delayed and lower peroxidase activation than the wild‐type strain (Fig. 5d).
Figure 7.
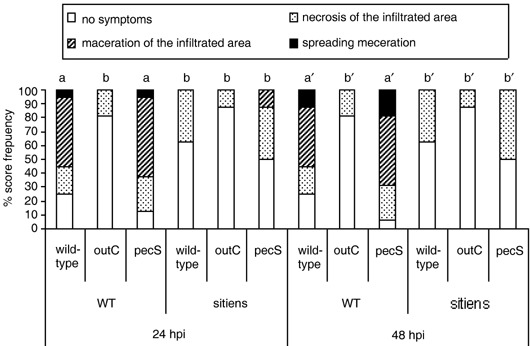
Disease progression of E. chrysanthemi strains with different pectinase production on sitiens and WT tomato at 24 and 48 hpi. The 2nd youngest leaf of plants at the three‐ to four‐leaf stage were infiltrated with 106 CFU/mL of E. chrysanthemi wild‐type strain, the pathogenicity factor overproducer pecS strain and the type II secretion‐deficient outC strain. Disease development was evaluated at 24 and 48 hpi and data analysed by the Kruskal–Wallis/Mann–Whitney non‐parametric test. Bars with different letters indicate a significant difference between the treatments at P = 0.05. Similar results were obtained from two independent experiments using at least eight plants per treatment. Data from one experiment are presented.
These results show that in WT tomato, the presence of E. chrysanthemi CWDEs does not significantly raise peroxidase activity levels, while in sitiens, type II secretion is necessary for the activation of peroxidases. To confirm a role for Out‐secreted proteins in sitiens defence activation we measured peroxidase activity after application of bacteria‐free E. chrysanthemi supernatants of bacterial cultures grown on a pectinase‐inducing medium. Pectinase production was triggered on a medium containing polygalacturonic acid as described above, and the CF dilution that was used (0.3) was sufficient to cause macroscopically visible tissue maceration within 5 hpi (see also supplementary Fig. S1). Peroxidase activity increased strongly in sitiens within 5 h after CF application (Fig. 8a). In WT leaves or sitiens leaves that were treated with ABA, CF infiltration did not result in an increase of peroxidase activity that was significantly different from the respective control treatments at any of the examined time points (Fig. 8a–c). If pectinase‐free outC CF was applied, the activity increase in sitiens was limited and did not exceed the levels of the control treatment containing pure medium (Fig. 8d). In addition, heat treatment of the CF (15 min at 80 °C) resulted in a loss of the CF's ability to cause a strong increase in sitiens peroxidase activity, which indicates that denaturation of type II secreted proteins in the CF results in a failure to trigger sitiens defence responses.
Figure 8.
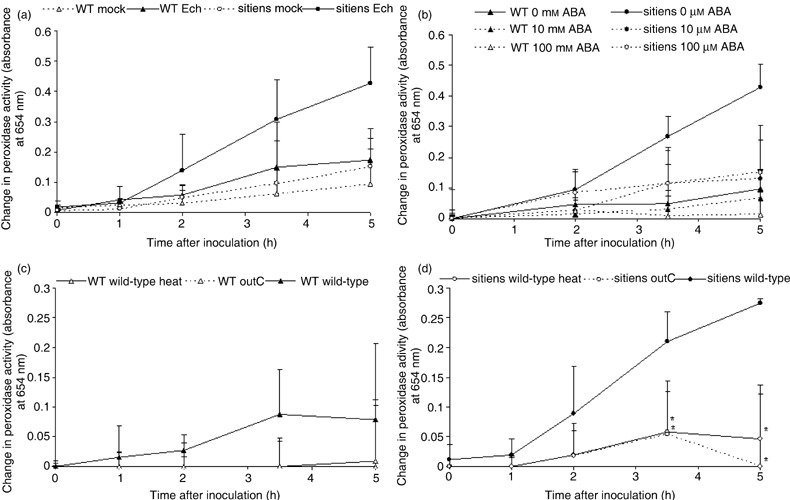
Extracellular peroxidase activity in WT and sitiens infiltrated with E. chrysanthemi pectinase‐containing culture filtrate. (a) Leaf discs were sampled from leaves infiltrated with the culture filtrate (CF) of E. chrysanthemi grown on a medium containing PGA to induce pectinase production (‘Ech’) or with pure medium (‘mock’). (b) Leaf discs were sampled from WT and sitiens infiltrated with CF 4 h after a single spray with 0, 10 or 100 µm ABA. All spray solutions contained 0.05% ethanol. (c,d) Leaf discs were sampled from WT (c) and sitiens (d) leaves infiltrated with CF of E. chrysanthemi strains with functional type II secretion (‘wild‐type’) and type II secretion deficiency (‘outC’). In addition, heat‐treated (80 °C for 15 min) pectinase‐containing CF (‘wild‐type heat’) was used. In (b), (c) and (d), the presented change in peroxidase activity is the absolute activity value after subtraction of the activity level of medium‐infiltrated samples for each time point. During sampling, discs were fixed in ethanol and peroxidase activity was measured at 654 nm after addition of TMB and 0.03% H2O2. The mean + standard error of the absorbance of the incubation solution from four discs of different plants are presented. In (c) and (d), significant differences at P = 0.05 between ‘wild‐type’ and the two other treatments were determined by using one‐way anova and Duncan post‐hoc tests for each time point and are indicated with ‘*’. All experiments were repeated with similar results.
DISCUSSION
Tomato defence responses upon E. chrysanthemi infection are controlled by ABA
This work shows that infiltration of tomato leaves with E. chrysanthemi resulted in both local and systemic infection of WT tomato plants, whereas there was little or no infection of ABA‐deficient sitiens mutant plants. These results are in agreement with the earlier observations that sitiens is more resistant to B. cinerea, O. neolycopersici (Audenaert et al., 2002; Achuo et al., 2006) and P. syringae pv. tomato (Thaler and Bostock, 2004). Furthermore, exogenous application of ABA suppressed the resistance to E. chrysanthemi in sitiens and significantly increased disease severity on WT. While exogenous ABA also restored susceptibility in sitiens to B. cinerea and O. neolycopersici to WT levels, it did not further increase disease severity in WT tomato (Achuo et al., 2006).
The sitiens tomato mutant differs from the WT in growth rate, plant development and water relations. The mutant exhibits a higher transpiration rate and a lower leaf water potential and turgor, resulting in reduced leaf expansion and a more compact leaf structure (Nagel et al., 1994). These morphological differences, however, do not account for the observed resistance in sitiens, as not only ABA supplementation during growth but also a pulse treatment with ABA prior to E. chrysanthemi infiltration completely restores WT susceptibility. In addition, sitiens plants grown at high temperature and RH (± 100%) (conditions in which morphological differences are no longer visible) are still highly resistant to E. chrysanthemi.
The possibilities that E. chrysanthemi resistance in sitiens is due to inhibition of bacterial growth or to an inability of E. chrysanthemi CWDEs to degrade sitiens cell walls were also excluded. However, this work demonstrates that E. chrysanthemi leaf infiltration triggers an active defence response in sitiens, involving accumulation of extracellular H2O2 and a faster and stronger increase in peroxidase activity compared with WT tomato.
Pathogen containment is a key event in the resistance of sitiens to E. chrysanthemi
It has been shown previously that H2O2 and peroxidase production are implicated in plant–Erwinia interactions. Wu et al. (1995) reported increased resistance to E. carotovora subsp. carotovora in transgenic potato plants expressing an H2O2‐generating glucose oxidase, and also peroxidase activity is known to increase after Erwinia infection (Wegener, 2002), although transgenic over‐expression of peroxidases does not always result in increased resistance to this pathogen (Ray et al., 1998). In addition, some sweetpotato peroxidase genes were expressed after E. chrysanthemi infection, while others were not, pointing to specificity of peroxidase isozymes in defence against E. chrysanthemi (Jang et al., 2004).
We did not find evidence for a direct antimicrobial role of H2O2, since E. chrysanthemi grew to a similar extent in WT and sitiens prior to maceration. In addition, Miguel et al. (2000), who studied the mechanisms involved in the protection of E. chrysanthemi against oxidative stress, proved that there is no direct antimicrobial effect of host‐produced H2O2 in potato and tobacco. Moreover, E. chrysanthemi pecS mutant, which is an overproducer of the antioxidant indigoidine and was shown to be more resistant to oxidative stress (Reverchon et al., 2002), was not able to cause sustained maceration symptoms on sitiens, which again opposes a direct effect of H2O2 on E. chrysanthemi survival in planta. Instead, extracellular H2O2 in sitiens specifically accumulated at the sites where bacterial progression was arrested, which points to a role of this accumulation in bacterial containment, because in WT tissue neither this reaction nor the halt of bacteria was observed.
During an oxidative burst, H2O2 is used as a substrate for oxidative polymerization of various cell‐wall components controlled by the peroxidative cycle of extracellular peroxidases. By this mechanism, plant peroxidases are essential to restrict pathogen progress because of their cell‐wall cross‐linking activity in the formation of lignin, extensin cross‐links and dityrosine bonds (Passardi et al., 2005). In the present work, accumulation of stains for protein cross‐linking and for incorporation of phenolic compounds in the cell wall was detected at the borders of E. chrysanthemi lesions in sitiens, again indicating that active containment of bacteria to the infiltrated zone might be an important mechanism for sitiens to limit E. chrysanthemi.
Source of sitiens H2O2 accumulation during E. crysanthemi infection
Besides their prominent role in peroxidative cell‐wall modification, plant cell‐wall peroxidases can have a function in regulating H2O2 levels and releasing ROS through a hydroxylic cycle (Passardi et al., 2005). Moreover, extracellular peroxidases are considered to function together with membrane‐bound NADPH oxidases as the two major mechanisms of ROS production during an oxidative burst (Bolwell and Wojtaszek, 1997). Time‐course experiments in sitiens revealed that the increase in peroxidase activity (at 8 hpi) occurs earlier than the first detection of H2O2 accumulating (at 12 hpi). However, our data do not allow appointing peroxidases as the main H2O2 source in sitiens during infection given that the TMB–peroxidase assay is much more sensitive than the DAB staining technique (Rye et al., 1984), which may explain the temporal shift in detection. On the other hand, in a recent model, Bindschedler et al. (2006) propose that apoplastic peroxidases are a general initial rapid source of ROS, and are essential for conferring at least partial resistance independently of any involvement of NADPH oxidases in a later stage. Other cellular compartments have been proposed for ROS generation during plant–pathogen interactions (Bolwell and Wojtaszek, 1997). Treatment of potato with the CWDE‐containing CF of E. carotovora resulted in down‐regulation of photosystem I and H2O2 accumulation in chloroplasts (Montesano et al., 2004). In addition to the extracellular H2O2 that was specifically present in sitiens lesion borders, H2O2 was also detected in chloroplasts within the infiltrated zone of both WT and sitiens tomato leaf tissue. Based on this work and others, however, we found no indications that H2O2 accumulation in chloroplasts is more than just a consequence of Erwinia symptom development, and it is doubtful if this reaction significantly contributes to enhanced defence.
Sitiens defence activation is triggered by type II secreted proteins
In most plant pathogenic bacteria, plant defence responses were mainly reported to be elicited by effector proteins injected in plant cells by the type III secretion system. Although E. chrysanthemi possesses a hypersensitive response and pathogenicity (Hrp) type III secretion system (Bauer et al., 1994), it was shown in the present work that defence responses in sitiens plants are activated by type II secreted proteins. Different proteins might be the effectors of these plant responses. First, analysis of the E. chrysanthemi secretome revealed that an Avr‐like protein is secreted by the Out system (Kazemi‐Pour et al., 2004). In addition to this protein, other type II secreted proteins produced during culture under conditions that induce pectinase production, as used in this work, are essentially pectinases (Kazemi‐Pour et al., 2004). These enzymes could interact with plants in two ways: either they could be directly recognized as elicitors, as it has been shown for the PelI protein that can cause a response on tobacco independently of its pectinolytic activity (Shevchik et al., 1998), or they could produce elicitors indirectly by enzymatic degradation of the plant pectin matrix. As heat degradation resulted in loss of the eliciting activity, the conformational integrity of the protein appears to be important for elicitation. Because protein elicitors such as harpins are heat‐stable for their activity (reviewed in Cornelis and Van Gijsegem, 2000), this points more to the involvement of pectinolytic activity to produce cell‐wall degradation products eliciting sitiens peroxidase activation. Indeed, recognition of oligogalacturonides (OGAs) by plants triggers defence responses within minutes, including an oxidative burst and cell‐wall strengthening by peroxidases (Ridley et al., 2001), and plant cell‐wall degradation by E. chrysanthemi pectinases results in the release of pectic oligomers of different size dependent on the acting enzyme (Roy et al., 1999).
Two hypotheses may explain the influence of ABA on sitiens defence responses. First, basal ABA levels in WT tomato plants may suppress the fast and more powerful reaction to the OGA signal or other putative Out‐related signals observed in sitiens. This view is supported by the limited increase in peroxidase activity in sitiens plants that were pulse‐treated with ABA before infection. However, ABA pulse treatment did not alter the initial sitiens peroxidase activation at 8 hpi, indicating that morphological changes resulting from ABA deficiency during growth can also have a significant effect on elicitation of defence responses. This is consistent with the stronger increase of disease after ABA supplementary feeding than after a single ABA spray. Therefore, as a second possibility, it can be postulated that CWDEs may release different types of OGAs in sitiens and WT. ABA is known to influence cell‐wall composition in various ways, including alteration of the pectin matrix (Lohani et al., 2004; Micheli, 2001). The biological activity of OGAs strongly depends on the degree of polymerization (PD) and esterification (Ridley et al., 2001). Specifically for E. chrysanthemi, it was suggested that the release of OGAs with a low PD stimulates induction of pectinase production, while OGAs with a higher PD (10–15 residues) activate plant defence systems, which indicates that the outcome of an interaction, i.e. resistance or susceptibility, might be influenced by the type of oligomers surrounding the bacterium (Barras et al., 1994). An alternative link between ABA and cell‐wall composition was recently provided by Hernández‐Blanco et al. (2007), who found an enhancement of ABA‐responsive defence‐related gene activation in Arabidopsis plants mutated in cellulose synthase genes required for secondary cell‐wall formation. We are currently studying the effect of ABA on cell‐wall modifications by comparing the cell‐wall composition of WT and sitiens tomato leaves.
EXPERIMENTAL PROCEDURES
Plant material and growing conditions
Seeds of tomato cv. Moneymaker and the ABA‐deficient sitiens mutant (Taylor et al., 1988), provided by Professor M. Koornneef (Wageningen University, the Netherlands), were sown directly in pots containing 150 g general‐purpose potting soil and seedlings were thinned to one plant per pot after sprouting. Two different experimental growing conditions were used: (1): growth chamber (day/night temperature of 28 ± 1 °C/26 ± 1 °C; 16‐h light; RH of ± 100%) and (2) greenhouse (day/night temperature of 21 ± 1 °C/18 ± 1 °C; 16‐h light; RH of ± 65%).
Bacterial inoculum and plant infection
Three different Erwinia chrysanthemi Burkholder et al. strains derived from wild‐type strain 3937 were used in this study: the gfp9 strain that was selected as a constitutive producer of the GFP protein by genomic insertion of the gfp mini‐transposon pAG408 (Suarez et al., 1997), a pecS strain carrying a mutation in the global transcriptional regulator pecS gene, which leads to derepressed synthesis of several pathogenicity factors (Reverchon et al., 1994), and an outC mutant strain, deficient in the type II secretion system, constructed by transduction of the outC::uidA‐Km fusion (Kazemi‐Pour et al., 2004) into the 3937 WT strain.
For all bacterial plant infiltrations, E. chrysanthemi was grown overnight on Luria–Bertani (LB) medium (Bertani, 1951) and bacterial cells were collected from the agar plates by washing with 50 mm KCl. Bacterial concentration was determined with a spectrophotometer at 600 nm and the inoculum solution prepared by dilution to 106 CFU/mL or 107 CFU/mL in 50 mm KCl. The second‐youngest leaves of plants at the three‐ to four‐leaf stage were inoculated by syringe infiltration on intact plants. Two to three leaflets of the same leaf were infiltrated per plant, using at least six plants per treatment. Control plants were inoculated with 50 mm KCl. Leaf infiltration was done by gently pushing the bacterial cell suspension on to the adaxial surface of the leaf using a 1 mL‐syringe without needle. Infiltrated plants were incubated in humid boxes at 28 °C.
Disease evaluation
A disease evaluation scheme was developed for E. chrysanthemi infection on tomato, based on the nature of symptoms produced locally on the inoculated leaf tissue and those produced on plant parts distant from the point of inoculation. Local disease symptoms were evaluated on the inoculated leaves at 24, 48 and 72 hpi on a 0–3 scale: 0, no disease symptoms; 1, necrosis of the infiltrated tissue (Fig. 1b); 2, maceration contained within the infiltrated tissue (Fig. 1a); and 3, spreading maceration beyond the infiltrated tissue (Fig. 1c,d). Data were analysed by the Kruskal–Wallis/Mann–Whitney non‐parametric tests in SPSS.
At 7 dpi plants were evaluated for appearance of symptoms on plant parts distant from the infiltrated leaf (systemic infection) (Fig. 1f,g).
Exogenous abscisic acid treatment
Two methods of exogenous ABA treatment were applied: (1) physiological supplementation whereby 2‐week‐old plants were sprayed with 100 µm ABA at 5‐day intervals for 25 days (six treatments in all) and (2) pulse application by spraying plants with 10 or 100 µm ABA, 4 h before bacterial inoculation. ABA solution was prepared from a stock of 10 mm in 5% ethanol and the final ethanol concentration in the treatment solution was 0.05%. Control plants were treated with water containing 0.05% ethanol. Plants were treated by foliar spray with a fine mist of the solutions to near run‐off. The physiological supplementation treatment was intended to restore the morphology of sitiens plants to the WT phenotype, while the pulse application was intended to raise ABA levels in the plants without any morphological changes.
Quantification of bacteria in plant tissue
To compare the survival and multiplication of the bacteria in infiltrated tomato leaf tissue, 5‐mm‐diameter leaf discs were cut out from the infiltrated zone using a cork borer. The samples were ground individually in 50 mm KCl and plated out on LB medium in a ten‐fold dilution series with the drop‐plate method (Herigstad et al., 2001). For each dilution in the series, three 10‐µL droplets were placed on LB plates and incubated at 28 °C for 24 h. E. chrysanthemi was identified on the basis of colony morphology and the presence of strain gfp9 was verified based on green fluorescence under UV light. CFUs were counted on plated droplets that contained between seven and 70 colonies and the number of CFU per mm2 of leaf area was calculated and compared statistically by the t‐test for pair‐wise comparisons of means. In the experiments in which WT E. chrysanthemi strain growth was compared for WT and sitiens, at least five leaf discs from different plants of each genotype were sampled at 0, 4, 8, 12, 24 and 48 hpi. At time points later than 12 hpi, samples were taken separately for macerated and non‐macerated leaf tissue. In the experiment in which growth of different E. chrysanthemi strains was compared, three leaf discs from different plants of each genotype were sampled at 0, 8 and 12 hpi.
Preparation and application of E. chrysanthemi CWDE‐containing culture filtrate
E. chrysanthemi was grown at 28 °C on liquid pectinase‐inducing medium [30 mm K2PO4, 8.3 mm NaH2PO4, 1.6 mm MgSO4, 50 mm (NH4)2SO4, 0.5% casamino acids (Sigma‐Aldrich, St. Louis, MO) supplemented with 0.5% polygalacturonic acid]. At the end of the exponential growth phase the cultures were centrifuged at 7000 g for 10 min. The supernatant containing the CWDEs was filter‐sterilized using a Millex®GV 0.22‐µm filter unit (Millipore Corp., Bedford, MA) and a five‐fold dilution series in the same medium without PGA was prepared.
Two different set‐ups were used to infiltrate the CF containing the CWDEs with a syringe into the second‐youngest leaves of tomato plants at the three‐ to four‐leaf stage. The first comprised using intact plants, which were incubated in humid boxes at 28 °C, as in plant infection trials. In a second set‐up, the CF was infiltrated into detached leaflets that were incubated on a moistened filter paper in sealed Petri dishes at 37 °C, using one leaflet per plant.
To compare cell‐wall degradation in sitiens and WT, the infiltration zones were evaluated for appearance of maceration at 2‐ to 4‐h intervals over a period of 24 h. For each CF concentration, the presence or absence of cell‐wall degradation was scored on six leaflets of each genotype at the various time points. From these data, the time required for half of the leaflets to show macroscopically visible cell‐wall degradation was extrapolated and plotted against the CF dilution factor.
Staining and microscopy procedures
For H2O2 accumulation, staining was performed according to the protocol of Thordal‐Christensen et al. (1997). Three hours before each sampling time point, leaf discs that contained part of the E. chrysanthemi infiltration zone were cut out of the leaf with a 1‐cm‐diameter cork bore and placed in standard 24‐well plates. Immediately therefater, the discs were floated in a solution of 1 mg/mL 3,3′‐diaminobenzidine (DAB)/HCl (pH 4) (Sigma). Polymerization of the DAB molecule at the site of H2O2 and peroxidase accumulation displays a brown reddish colour that is macroscopically visible and, because of the high spatial and temporal distribution of the oxidized DAB molecule, it can be visualized under bright‐field microscopy. After staining, the discs were fixed and cleared in 100% ethanol and mounted on microscopy slides for evaluation.
Representative parts of DAB‐stained E. chrysanthemi lesion borders were embedded in Technovit 7100 histo‐embedding medium (Heraeus Kulzer, Wehrheim, Germany) according to the manufacturer's descriptions and semithin sections (4 µm) were cut with a Leica RM2265 motorized rotary microtome (Leica Microsystems, Nussloch, Germany). A subset of the sections was stained with 1% toluidine blue for 1 min to visualize bacteria, chloroplasts and plant cell walls, while the remaining sections were evaluated without additional staining, allowing for ultrastructural detection of H2O2.
For protein cross‐linking, staining was performed as described by Mellersh et al. (2002). Ethanol‐fixed leaf discs containing part of the infiltrated zone were placed in 1% SDS at 80 °C for 24 h and stained with 0.1% Coomassie Blue in 40% ethanol/10% acetic acid for 15 min and subsequently washed in 40% ethanol/10% acetic acid. Cell‐wall fortification was visualized after safranin‐o staining according to Lucena et al. (2003). Leaf discs were incubated in 0.01% safranin‐o in 50% ethanol for 3 min.
Measurement of peroxidase activity
Extracellular peroxidase activity was measured with the TMB assay based on Ros Barceló (1998). One‐centimetre leaf discs containing part of the E. chrysanthemi infiltration zone were cut from the leaf as described above and fixed in pure ethanol. After subsequent washing in distilled water, the discs were incubated in 1.5 mL of a 50 mm Tris‐acetate buffer (pH 5.0) containing 0.1 mg/mL 3,5,3′,5′‐tetramethylbenzidine (TMB) (Sigma) and 0.03% H2O2 for 20 min. Peroxidase activity of the discs was determined by measuring the absorbance of the incubation solution at 654 nm and compared statistically by one‐way anova and Duncan's post‐hoc tests.
Supporting information
Fig. S1 Cell‐wall degradation in sitiens and WT tomato tissue after application of E. chrysanthemi pectinases.
Fig. S2 Temporal evolution of H2O2 accumulation in WT and sitiens tomato after infection with E. chrysanthemi.
Fig. S3 Survival and growth of E. chrysanthemi strains with different pectinase production in WT and sitiens leaf tissue.
This material is available as part of the online article from: http://www.blackwell‐synergy.com/doi/full/10.1111/j.1365‐2745.2007.01296.x
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
ACKNOWLEDGEMENTS
We thank Ilse Delaere for technical assistance, Igor Pecheu (in memoriam) for initiating B.A. and A.E.A. to microscopy techniques in Paris and Yvan Kraepiel for critical reading of the manuscript. The research was financed by the ‘Fonds voor Wetenschappelijk Onderzoek‐Vlaanderen’ (grant nos. 3G0061.03 and 3G052607) and supported in part by the bi‐national Vlaanderen‐France exchange TOURNESOL programme (‘Tournesol Project T2004.23’ of the ‘Ministerie van de Vlaamse gemeenschap—Departement Onderwijs’‐Ministère Français des Affaires Etrangères) and ‘Bijzonder Onderzoeksfonds’ of Ghent University.
REFERENCES
- Achuo, E.A. , Prinsen, E. and Höfte, M. (2006) Influence of drought, salt stress and abscisic acid on the resistance of tomato to Botrytis cinerea and Oidium neolycopersici . Plant Pathol. 55, 178–186. [Google Scholar]
- Anderson, J.P. , Badruzsaufari, E. , Schenk, P.M. , Manners, J.M. , Desmond, O.J. , Ehlert, C. , Maclean, D.J. , Ebert, P.R. and Kazan, K. (2004) Antagonistic interaction between abscisic acid and jasmonate‐ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell, 16, 3460–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andro, T. , Chambost, J.P. , Kotoujansky, A. , Cattaneo, J. , Bertheau, Y. , Barras, F. , Van Gijsegem, F. and Coleno, A. (1984) Mutants of Erwinia chrysanthemi defective in secretion of pectinase and cellulase. J. Bacteriol. 160, 1199–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel, K. and Hirt, H. (2004) Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Ann. Rev. Plant Biol. 55, 373–399. [DOI] [PubMed] [Google Scholar]
- Audenaert, K. , De Meyer, G.B. and Höfte, M. (2002) Abscisic acid determines basal susceptibility of tomato to Botrytis cinerea and suppresses salicylic acid‐dependent signaling mechanisms. Plant Physiol. 128, 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aysan, Y. , Karatas, A. and Cinar, O. (2003) Biological control of bacterial stem rot caused by Erwinia chrysanthemi on tomato. Crop Prot. 22, 807–811. [Google Scholar]
- Barras, F. , Van Gijsegem, F. and Chatterjee, A.K. (1994) Extracellular enzymes and pathogenesis of soft‐rot Erwinia . Ann. Rev. Phytopathol. 32, 201–234. [Google Scholar]
- Bauer, D.W. , Bogdanove, A.J. , Beer, S.V. and Collmer, A. (1994) Erwinia chrysanthemi hrp genes and their involvement in soft‐rot pathogenesis and elicitation of the hypersensitive response. Mol. Plant–Microbe Interact. 7, 573–581. [DOI] [PubMed] [Google Scholar]
- Bertani, G. (1951) Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli . J. Bacteriol. 62, 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindschedler, L.V. , Dewdney, J. , Blee, K.A. , Stone, J.M. , Asai, T. , Plotnikov, J. , Denoux, C. , Hayes, T. , Gerrish, C. , Davies, D.R. , Ausubel, F.M. and Bolwell, G.P. (2006) Peroxidase‐dependent apoplastic oxidative burst in Arabidopsis required for pathogen resistance. Plant J. 47, 851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell, G.P. and Wojtaszek, P. (1997) Mechanisms for the generation of reactive oxygen species in plant defence—a broad perspective. Physiol. Mol. Plant Pathol. 51, 347–366. [Google Scholar]
- Bradley, D.J. , Kjellbom, P. and Lamb, C.J. (1992) Elicitor‐induced and wound‐induced oxidative cross‐linking of a proline‐rich plant cell wall protein—a novel, rapid defense response. Cell, 70, 21–30. [DOI] [PubMed] [Google Scholar]
- Collmer, A. and Keen, N.T. (1986) The role of pectic enzymes in plant pathogenesis. Ann. Rev. Phytopathol. 24, 383–409. [Google Scholar]
- Cornelis, G.R. and Van Gijsegem, F. (2000) Assembly and function of type III secretory systems. Ann. Rev. Microbiol. 54, 735–774. [DOI] [PubMed] [Google Scholar]
- Dickey, R.S. (1979) Erwinia chrysanthemi—Comparative study of phenotypic properties of strains from several hosts and other Erwinia species. Phytopathology, 69, 324–329. [Google Scholar]
- Expert, D. (1999) Withholding and exchanging iron: interactions between Erwinia spp. & their plant hosts. Ann. Rev. Phytopathol. 37, 307–334. [DOI] [PubMed] [Google Scholar]
- Fujita, M. , Fujita, Y. , Noutoshi, Y. , Takahashi, F. , Narusaka, Y. , Yamaguchi‐Shinozaki, K. and Shinozaki, K. (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr. Opin. Plant Biol. 9, 436–442. [DOI] [PubMed] [Google Scholar]
- Herigstad, B. , Hamilton, M. and Heersink, J. (2001) How to optimize the drop plate method for enumerating bacteria. J. Microbiol. Meth. 44, 121–129. [DOI] [PubMed] [Google Scholar]
- Hernández‐Blanco, C. , Xin Feng, D. , Hu, J. , Sánchez‐Vallet, A. , Deslandes, L. , Llorente, F. , Berrocal‐Lobo, M. , Keller, H. , Barlet, X. , Sánchez‐Rodríguez, C. , Anderson, L.K. , Somerville, S. , Marco, Y. and Molina, A. (2007) Impairment of cellulose synthase required for Arabidopsis secondary cell wall formation enhances disease resistance. Plant Cell, 19, 890–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugouvieux‐Cotte‐Pattat, N. , Condemine, G. , Nasser, W. and Reverchon, S. (1996) Regulation of pectinolysis in Erwinia chrysanthemi . Ann. Rev. Microbiol. 50, 213–257. [DOI] [PubMed] [Google Scholar]
- Jang, I.C. , Park, S.Y. , Kim, K.Y. , Kwon, S.Y. , Kim, J.G. and Kwak, S.S. (2004) Differential expression of 10 sweetpotato peroxidase genes in response to bacterial pathogen, Pectobacterium chrysanthemi . Plant Physiol. Biochem. 42, 451–455. [DOI] [PubMed] [Google Scholar]
- Kazemi‐Pour, N. , Condemine, G. and Hugovieux‐Cotte‐Pattat, N. (2004) The secretome of the plant pathogenic bacterium Erwinia chrysanthemi . Proteomics, 4, 3177–3186. [DOI] [PubMed] [Google Scholar]
- Kotoujansky, A. (1987) Molecular genetics of pathogenesis by soft‐rot Erwinias . Ann. Rev. Phytopathol. 25, 405–430. [Google Scholar]
- Lamb, C. and Dixon, R.A. (1997) The oxidative burst in plant disease resistance. Ann. Rev. Plant Physiol. Plant Mol. Biol. 48, 251–275. [DOI] [PubMed] [Google Scholar]
- Lohani, S. , Trivedi, P.K. and Nath, P. (2004) Changes in activities of cell wall hydrolases during ethylene‐induced ripening in banana: effect of 1‐MCP, ABA and IAA. Postharvest Biol. Technol. 31, 119–126. [Google Scholar]
- Lucena, M.A. , Romero‐Aranda, R. , Mercado, J.A. , Cuartero, J. , Valpuesta, V. and Quesada, M.A. (2003) Structural and physiological changes in the roots of tomato plants over‐expressing a basic peroxidase. Physiol. Plant. 118, 422–429. [Google Scholar]
- Mauch‐Mani, B. and Mauch, F. (2005) The role of abscisic acid in plant–pathogen interactions. Curr. Opin. Plant Biol. 8, 409–414. [DOI] [PubMed] [Google Scholar]
- Mellersh, D.G. , Foulds, I.V. , Higgins, V.J. and Heath, M.C. (2002) H2O2 plays different roles in determining penetration failure in three diverse plant–fungal interactions. Plant J. 29, 257–268. [DOI] [PubMed] [Google Scholar]
- Micheli, F. (2001) Pectin methylesterases: cell wall enzymes with important roles in plant physiology. Trends Plant Sci. 6, 414–419. [DOI] [PubMed] [Google Scholar]
- Miguel, E. , Poza‐Carrion, C. , Lopez‐Solanilla, E. , Aguilar, I. , Llama‐Palacios, A. , Garcia‐Olmedo, F. and Rodriguez‐Palenzuela, P. (2000) Evidence against a direct antimicrobial role of H2O2 in the infection of plants by Erwinia chrysanthemi . Mol. Plant–Microbe Interact. 13, 421–429. [DOI] [PubMed] [Google Scholar]
- Mohr, P.G. and Cahill, D.M. (2003) Abscisic acid influences the susceptibility of Arabidopsis thaliana to Pseudomonas syringae pv. tomato and Peronospora parasitica . Funct. Plant Biol. 30, 461–469. [DOI] [PubMed] [Google Scholar]
- Mohr, P.G. and Cahill, D.M. (2007) Suppression by ABA of salicylic acid and lignin accumulation and the expression of multiple genes, in Arabidopsis infected with Pseudomonas syringae pv. tomato. Funct. Integr. Genomics, 7, 181–191. [DOI] [PubMed] [Google Scholar]
- Montesano, M. , Scheller, H.V. , Wettstein, R. and Palva, E.T. (2004) Down‐regulation of photosystem I by Erwinia carotovora‐derived elicitors correlates with H2O2 accumulation in chloroplasts of potato. Mol. Plant Pathol. 5, 115–123. [DOI] [PubMed] [Google Scholar]
- Nagel, O.W. , Konings, H. and Lambers, H. (1994) Growth‐rate, plant development and water relations of the ABA‐deficient tomato mutant sitiens. Physiol. Plant. 92, 102–108. [Google Scholar]
- Passardi, F. , Cosio, C. , Penel, C. and Dunand, C. (2005) Peroxidases have more functions than a Swiss army knife. Plant Cell Report, 24, 255–265. [DOI] [PubMed] [Google Scholar]
- Pei, Z.M. , Murata, Y. , Benning, G. , Thomine, S. , Klusener, B. , Allen, G.J. , Grill, E. and Schroeder, J.I. (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature, 406, 731–734. [DOI] [PubMed] [Google Scholar]
- Pérombelon, M.C.M. and Kelman, A. (1980) Ecology of the soft rot Erwinias . Ann. Rev. Phytopathol. 18, 361–387. [Google Scholar]
- Ray, H. , Douches, D.S. and Hammerschmidt, R. (1998) Transformation of potato with cucumber peroxidase: expression and disease response. Physiol. Mol. Plant Pathol. 53, 93–103. [Google Scholar]
- Reverchon, S. , Nasser, W. and Robert‐Baudouy, J. (1994) PecS—a locus controlling pectinase, cellulase and blue pigment production in Erwinia chrysanthemi . Mol. Microbiol. 11, 1127–1139. [DOI] [PubMed] [Google Scholar]
- Reverchon, S. , Rouanet, C. , Expert, D. and Nasser, W. (2002) Characterization of indigoidine biosynthetic genes in Erwinia chrysanthemi and role of this blue pigment in pathogenicity. J. Bacteriol. 184, 654–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley, B.L. , O’Neill, M.A. and Mohnen, D.A. (2001) Pectins: structure, biosynthesis, and oligogalacturonide‐related signaling. Phytochemistry, 57, 929–967. [DOI] [PubMed] [Google Scholar]
- Ros Barceló, A. (1998) Hydrogen peroxide production is a general property of the lignifying xylem from vascular plants. Ann. Bot. 82, 97–103. [Google Scholar]
- Roy, C. , Kester, H. , Visser, J. , Shevchik, V. , Hugouvieux‐Cotte‐Pattat, N. , Robert‐Baudouy, J. and Benen, J. (1999) Modes of action of five different endopectate lyases from Erwinia chrysanthemi 3937. J. Bacteriol. 181, 3705–3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rye, D.B. , Saper, C.B. and Wainer, B.H. (1984) Stabilization of the tetramethylbenzidine (TMB) reaction product—application for retrograde and anterograde tracing, and combination with immunohistochemistry. J. Histochem. Cytochem. 32, 1145–1153. [DOI] [PubMed] [Google Scholar]
- Samson, R. , Legendre, J.B. , Christen, R. , Fischer‐Le Saux, M. , Achouak, W. , Gardan, L. (2005) Transfer of Pectobacterium chrysanthemi (Burkholder et al. 1953) Brenner et al. 1973 and Brenneria paradisiaca to the genus Dickeya gen. nov. as Dickeya chrysanthemi comb. nov. and Dickeya paradisiaca comb. nov and delineation of four novel species, Dickeya dadantii sp. nov., Dickeya dianthicola sp. nov., Dickeya dieffenbachiae sp. nov. and Dickeya zeae sp. nov. Int. J. Syst. Evol. Microbiol. 55, 1415–1427. [DOI] [PubMed] [Google Scholar]
- Schaad, N.W. and Brenner, D. (1977) Bacterial wilt and root rot of sweet potato caused by Erwinia chrysanthemi . Phytopathology, 67, 302–308. [Google Scholar]
- Schopfer, P. , Plachy, C. and Frahry, G. (2001) Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant Physiol. 125, 1591–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchik, V.E. , Boccara, M. , Vedel, R. and Hugovieux‐Cotte‐Pattat, N. (1998) Processing of the pectate lysae PelI by extracellular proteases of Erwinia chrysanthemi 3937. Mol. Microbiol. 29, 1459–1469. [DOI] [PubMed] [Google Scholar]
- Suarez, A. , Guttler, A. , Stratz, M. , Staendner, L.H. , Timmis, K.N. and Guzman, C.A. (1997) Green fluorescent protein‐based reporter systems for genetic analysis of bacteria including monocopy applications. Gene, 196, 69–74. [DOI] [PubMed] [Google Scholar]
- Taylor, I.B. , Linforth, R.S.T. , Alnaieb, R.J. , Bowman, W.R. and Marples, B.A. (1988) The wilty tomato mutants flacca and sitiens are impaired in the oxidation of ABA aldehyde to ABA. Plant Cell Environ. 11, 739–745. [Google Scholar]
- Thaler, J.S. and Bostock, R.M. (2004) Interactions between abscisic acid‐mediated responses and plant resistance to pathogens and insects. Ecology, 85, 48–58. [Google Scholar]
- Thordal‐Christensen, H. , Zhang, Z.G. , Wei, Y.D. and Collinge, D.B. (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley–powdery mildew interaction. Plant J. 11, 1187–1194. [Google Scholar]
- De Torres‐Zabala, M. , Truman, W. , Bennett, M.H. , Lafforgue, G. , Mansfield, J.W. , Egea, P.R. , Bogre, L. and Grant, M. (2007) Pseudomonas syringae pv. tomato hijacks the Arabidopsis abscisic acid signalling pathway to cause disease. EMBO J. 26, 1434–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener, C.B. (2002) Induction of defence responses against Erwinia soft rot by an endogenous pectate lyase in potatoes. Physiol. Mol. Plant Pathol. 60, 91–100. [Google Scholar]
- Wu, G.S. , Shortt, B.J. , Lawrence, E.B. , Levine, E.B. , Fitzsimmons, K.C. and Shah, D.M. (1995) Disease resistance conferred by expression of a gene encoding H2O2 generating glucose oxidase in transgenic potato plants. Plant Cell, 7, 1357–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Cell‐wall degradation in sitiens and WT tomato tissue after application of E. chrysanthemi pectinases.
Fig. S2 Temporal evolution of H2O2 accumulation in WT and sitiens tomato after infection with E. chrysanthemi.
Fig. S3 Survival and growth of E. chrysanthemi strains with different pectinase production in WT and sitiens leaf tissue.
This material is available as part of the online article from: http://www.blackwell‐synergy.com/doi/full/10.1111/j.1365‐2745.2007.01296.x
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item


