Abstract
Objectives
To identify the pharmacokinetic (PK) and toxicodynamic (TD) relationship for vancomycin-induced kidney injury.
Methods
Male Sprague–Dawley rats received intravenous (iv) vancomycin. Doses ranging from 150 mg/kg/day to 400 mg/kg/day were administered as a single or twice-daily injection over 24 h (total protocol duration). Controls received iv saline. Plasma was sampled with up to eight samples in 24 h per rat. Twenty-four hour urine was collected and assayed for kidney injury molecule 1 (KIM-1), osteopontin and clusterin. Vancomycin in plasma was quantified via LC-MS/MS. PK analyses were conducted using Pmetrics for R. PK exposures during the first 24 h (i.e. AUC0–24h, Cmax 0–24h and Cmin 0–24h) were calculated. PK/TD relationships were assessed with Spearman’s rank coefficient (rs) and the best-fit mathematical model.
Results
PK/TD data were generated from 45 vancomycin-treated and 5 control rats. A two-compartment model fit the data well (Bayesian: observed versus predicted R2 = 0.97). Exposure–response relationships were found between AUC0–24h versus KIM-1 and osteopontin (R2 = 0.61 and 0.66) and Cmax 0–24h versus KIM-1 and osteopontin (R2 = 0.50 and 0.56) using a four-parameter Hill fit. Conversely, Cmin 0–24h was less predictive of KIM-1 and osteopontin (R2 = 0.46 and 0.53). A vancomycin AUC0–24h of 482.2 corresponded to a 90% of maximal rise in KIM-1.
Conclusions
Vancomycin-induced kidney injury as defined by urinary biomarkers is driven by vancomycin AUC or Cmax rather than Cmin. Further, an identified PK/TD target AUC0–24h of 482.2 mg·h/L may have direct relevance to human outcomes.
Introduction
Many critically ill patients receive vancomycin empirically or in a directed manner for their infection, making vancomycin the single most commonly prescribed antibiotic in the hospital setting.1–4 Based on 36.5 million hospital stays in the USA annually5 and a vancomycin prevalence of ∼100 days of therapy/1000 patient-days,3,4 >3 million people receive vancomycin annually. Clinical reports suggest that vancomycin-induced kidney injury (VIKI) rates may vary from 5% to 43%,6–12 and a prospective study of patients with hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) treated with vancomycin demonstrated an acute kidney injury (AKI) rate of 18.8%.13
Given vancomycin’s frequent use, it is imperative to identify the safest delivery strategy. However, before dosing regimens can be recommended, identifying vancomycin exposures that cause AKI is crucial for implementing strategies to minimize the probability of toxicity while maintaining vancomycin efficacy. Retrospective studies and meta-analyses have identified several risk factors for VIKI such as higher daily dose, elevated troughs and an increased duration of therapy.7,8,14,15 However, it is difficult to attribute causality of AKI in retrospective clinical studies. Non-modifiable patient factors such as severity of illness, obesity and concomitant nephrotoxic medications compound VIKI and are notoriously difficult to adjust/control.16 In retrospective studies, it is also difficult to determine if vancomycin exposures caused AKI or if AKI from another origin caused vancomycin exposures to be elevated (as a function of decreased clearance). In contrast, animal models allow an environment that is free of these confounding factors. Furthermore, a directed experimental design can allow data collection during an early observational window to assess pharmacokinetic–toxicodynamic (PK/TD) drivers before the onset of AKI confounds PK variables.
We recently demonstrated that sensitive urinary biomarkers for kidney injury such as kidney injury molecule-1 (KIM-1), osteopontin (OPN) and clusterin were highly correlated with vancomycin AUC0–24h and maximum concentration (Cmax 0–24h) in rats receiving vancomycin via intraperitoneal administration.17,18 Notably, these biomarkers identified AKI earlier than traditional markers such as serum creatinine (SCr), and were correlated with histopathological damage.19 Further, KIM-1 can increase within hours of proximal tubule injury, plateau at 24 h and stay elevated through 120 h from the time of renal injury.19 While our previous studies were able to identify the PK/TD parameters most associated with VIKI, discerning the exact quantitative relationship between vancomycin exposure and AKI was impeded because of variability of intraperitoneal absorption and confounded by the potential for instillation of vancomycin into the abdominal cavity (i.e. instillation into the retroperitoneal space). Thus, in this study, we employed intravenous (iv) dosing.
Materials and methods
This PK/TD study was conducted at Midwestern University in Downers Grove, IL, USA. All study methods were approved by the Institutional Animal Care and Use Committee (IACUC; Protocol #2295) and conducted in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, 8th edition.20
Experimental design and animals
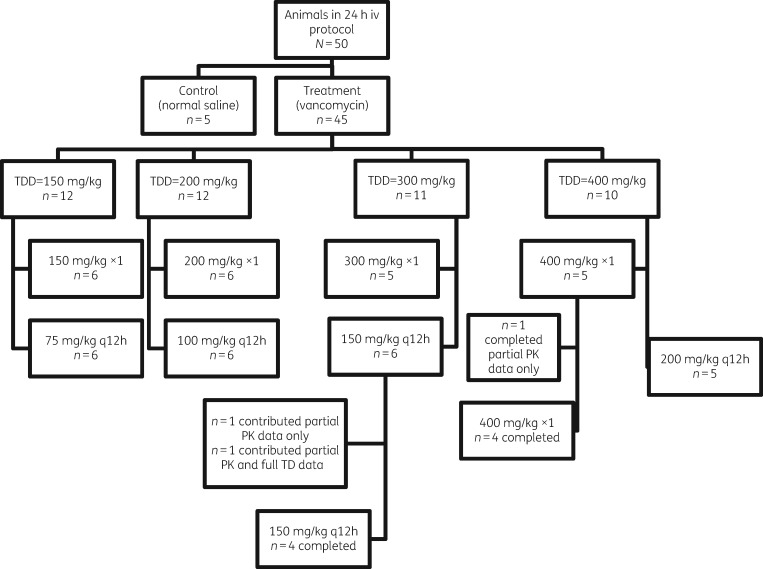
Experimental methods and design were similar to those previously described,17,18 with several notable differences. In brief, male Sprague–Dawley rats were randomized into either a treatment or control group [receiving vancomycin or normal saline (NS), respectively]. All dosages were administered through iv injection via an internal jugular vein catheter. Vancomycin-treated rats received total daily doses (dosing protocols) of 150, 200, 300 or 400 mg/kg as either a single or twice-daily divided dose over 24 h (e.g. 150 mg/kg was given as a single injection or as 75 mg/kg twice daily for a total of 24 h). Doses given twice daily were scheduled every 12 h. A complete dosing group disposition can be found in Figure 1. The dosing range was chosen based on previous studies17,19,21 and to span the clinical allometric range. For example, the clinical kidney injury threshold of ≥4 g/day in a 70 kg patient (i.e. 57 mg/kg/day in humans) scales allometrically to 350 mg/kg in the rat.14,22
Figure 1.
Randomization and animal dosing flowchart. TDD, total daily dose; ×1, once-daily dose.
Male Sprague–Dawley rats (n = 50, ∼8–10 weeks old, mean weight 310 g) were housed in a light- and temperature-controlled room for the duration of the study and allowed free access to water and food, except during the metabolic cage period (restricted). Rats (n = 5–6 per dosing protocol) were administered iv injections of vancomycin in NS or NS only (control). Data were analysed for all animals that initiated a protocol. When animals contributed incomplete data (i.e. early protocol termination), urinary biomarkers and urine output were treated as missing data.
Blood and urine sampling
Surgical catheters were implanted 24 h prior to protocol initiation. Blood samples were drawn from a single right-side internal jugular vein catheter, and dosing occurred via the left-side internal jugular vein catheter. A maximum of eight samples per animal were obtained and scheduled at 0, 15, 30, 60, 120, 240, 750 and 1440 min for the once-daily and twice-daily dosing treatment protocol. Each sample (0.25 mL aliquot) was replaced with an equivalent volume of NS to maintain euvolaemia. Blood samples from vancomycin-treated animals were immediately transferred to a disodium EDTA (Sigma–Aldrich Chemical Company, Milwaukee WI, USA) -treated microcentrifuge tube and centrifuged at 3000 g for 10 min. Plasma supernatant was collected and stored at −80°C for batch sample analysis.
Following the 2 h blood sample, animals were placed in metabolic cages for urine collection (Nalgene, catalogue # 650-0350, Rochester, NY, USA) for the remainder of the 24 h study (with the exception that they were briefly removed for scheduled blood samples). Urine volume was measured at 24 h. The urine was centrifuged at 400 g for 5 min, and the supernatant was stored at −80°C until batch analysis.
Chemicals and reagents
Animals were administered vancomycin hydrochloride (Lot#: 591655DD) for injection, obtained commercially (Hospira, Lake Forrest, IL, USA). All solvents were of LC-MS/MS grade. For LC-MS/MS, vancomycin hydrochloride, United States Pharmacopeia, was used (Enzo Life Science, Farmingdale, NY, USA) with a purity of 99.3%. Polymyxin B (Sigma–Aldrich, St Louis, MO, USA), acetonitrile and methanol were purchased from VWR International (Radnor, PA, USA). Formic acid was obtained from Fisher Scientific (Waltham, MA, USA). Frozen, non-medicated, non-immunized, pooled Sprague–Dawley rat plasma (anticoagulated with disodium EDTA) was used for calibration of standard curves (BioreclamationIVT, Westbury, NY, USA).
Determination of vancomycin concentrations in plasma
Plasma concentrations of vancomycin were quantified with LC and column conditions similar to our previous report,17,23 with the following notable changes to transfer the method to LC-MS/MS. A plasma sample volume of 40 µL was combined with 4 µL of the internal standard of polymyxin B at a concentration of 0.1 mg/mL. Protein precipitation was facilitated using 455 µL of methanol containing 1% formic acid. Following centrifugation for 10 min at 16000 g (Eppendorf Model: 5424), 75 µL of the supernatant was collected and reserved for analysis. A total of 2 µL was injected into an in an Agilent 1260 liquid chromatograph attached to an Agilent 6420 triple quadrupole mass spectrometer. MS was conducted with electrospray ionization in positive mode (ESI+). The MS source conditions were: gas temperature set to 350°C, gas flow set to 13 L/min, nebulizer set at 40 psi, fragmentor set at 140 V and cell accelerator voltage set at 4V. Vancomycin collision energy for the quantifier and qualifier was set at 15 and 34 eV, respectively. Polymyxin B1 collision energy was set at 22 eV. The following transitions (m/z) for vancomycin and polymyxin B1 were identified and utilized: 725.6 → 144.1 for vancomycin quantifier, 725.6 → 100.1 for vancomycin qualifier and 402.2 → 101.1 for polymyxin B1 quantifier. The assay was linear between concentrations of 0.5 and 100 mg/L (R2 = 0.998) with an applied weight of 1/x. The lower limit of quantification was 0.5 mg/L. Precision was <6.7% for all measurements, including intra- and interassay measurements. Greater than 92% accuracy was observed in all standards tested, with an overall mean assay accuracy of 100%. Any samples measuring above the upper limit of quantification were diluted.
Determination of urinary biomarkers of AKI
Urine samples were analysed in batch to determine concentrations of clusterin, KIM-1 and OPN. Microsphere-based Luminex X-MAP technology was used for the determination of all biomarker concentrations, as previously described.24,25 Urine samples were aliquoted into 96-well plates supplied with MILLIPLEX® MAP Rat Kidney Toxicity Magnetic Bead Panel 1 (EMD Millipore Corporation, Charles, MO, USA), prepared and analysed according to the manufacturer’s recommendations.
Vancomycin PK model and exposure determination
One- and two-compartment rate constant (Ke) models solved algebraically (minus the absorption constant in the stock model for the program)26 were considered as base models and were fit using the non-parametric adaptive grid (NPAG) algorithm within the Pmetrics package version 1.5.0 (Los Angeles, CA, USA) for R version 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria).27,28 Model performance was evaluated and compared utilizing a regression of observed versus predicted concentrations, visual plots of parameter estimates, Akaike information criterion (AIC) and the rule of parsimony. The initial estimate of parameter weighting was accomplished using the inverse of the assay variance. The observation variance was proportional, with a scalar (gamma) to assay variance.
Estimation of PK exposure profiles and statistical analysis
The best-fit model was utilized to obtain median maximum a posteriori probability (MAP) Bayesian vancomycin plasma concentration estimates at 12 min intervals over the 24 h study period, generated from each animal’s measured vancomycin concentrations, exact dose and dosing schedule. Bayesian posteriors for each animal were used to determine exposures over the 24 h time period (i.e. AUC0–24h, Cmax 0–24h and Cmin 0–24h). The PK values Cmax 0–24h and Cmin 0–24h were calculated using ‘makeNCA’ within Pmetrics.27,29 The highest predicted concentration was determined to be each individual animal’s Cmax 0–24h, and the concentration at hour 24 following the initial vancomycin dose was determined to be that subject’s Cmin 0–24h. Twenty-four hour exposure, as measured by AUC0–24h, was calculated using the trapezoidal rule under the Pmetrics command ‘makeAUC’.27,29 PK exposure measure variability was calculated as the percentage coefficient of variation (CV%). Relationships between PK exposure parameters were evaluated using Spearman’s rank coefficient (rs) as described below.
Association of PK measures with urinary AKI biomarkers
Exposure parameters were assessed for relationships with urinary biomarkers using GraphPad Prism version 7.02 (GraphPad Software Inc., La Jolla, CA, USA). PK/TD exposure–response relationships were evaluated using Spearman’s rs and Hill-type functions; log transformations of variables (i.e. log2 and log10) were employed to explore the relationship between PK parameters and urinary biomarkers. The 90th percentile effective concentration (EC90) for each PK exposure versus a specific biomarker was calculated from the best-fit Hill model. The EC90 was selected on the basis that (i) an antecedent value of KIM-1 >16 ng/mL was identified to predict AKI 2 days prior to the event (83% sensitivity and 95% specificity);30 (ii) KIM-1 values of 16.5 ng/mL corresponded to the 90th percentile for values in our model; and (iii) KIM-1 is the primary biomarker of interest as it is specific to the proximal tubule and associated with VIKI.17,31
Statistical analysis for between-treatment group comparisons
Urine output, body weight loss and PK exposure measures were compared across vancomycin total daily dose and dosing frequency groups. Log transformations were employed as needed to maintain parametric distributions. Differences were evaluated using either Student’s t-test or the Wilcoxon rank sum test, as appropriate. Regressions on biomarkers were completed with categories treated as independent variables and control animals set as the referent category. All tests were two-tailed, with an a priori level of statistical significance set at an α of 0.05.
Results
Characteristics of animal cohort
All 45 dosing protocol animals contributed PK model data. Mean weight loss and urine output were not significantly different between controls and vancomycin dosing protocol animals (4.8 g versus 8.6 g, P = 0.35, 16.86 mL versus 15.74 mL, P = 0.59; Table 1). Three animals (two animals in the 150 mg twice-daily cohort and one from the 400 mg once-daily cohort) did not complete the full protocol due to complications from either surgery or anaesthesia (Figure 1). Of these three animals, only one from 150 mg vancomycin twice daily contributed 12 h urinary biomarker data. Analyses were run including and excluding this animal. Since all interpretations were identical when excluding the animal with only 12 h urine (data not shown), the animal was included for all analyses. All animals contributed PK data and were thus included for PK study. All available/appropriate vancomycin plasma samples and urine biomarkers were utilized for model building and post-hoc Bayesian posterior generation.
Table 1.
Summary of weight loss, urinary output and urinary biomarkers
| Control | Vancomycin | P value | |
|---|---|---|---|
| Animals (n) | 5 | 45 | – |
| Baseline weight (g), mean ± SD | 323.8 ± 22.42 | 312.87 ± 15.37 | 0.16 |
| Weight loss (g), mean ± SD | 4.8 ± 2.28 | 8.6 ± 8.95 (n = 40)a | 0.35 |
| Urine output (mL), mean ± SD | 16.86 ± 3.07 | 15.74 ± 4.48 (n = 41)a | 0.59 |
| KIM-1 (ng/mL), median (IQR) | 1.02 (0.88–1.05) | 12.3 (5.6–13.9) (n = 43)b | <0.001 |
| Clusterin (ng/mL), median (IQR) | 319.5 (308.9–332.2) | 879.4 (617.6–1362) (n = 43)b | 0.0043 |
| OPN (ng/mL), median (IQR) | 0.02 (0.01–0.03) | 0.218 (0.11–0.39) (n = 43)b | <0.001 |
Some values inadvertently not recorded.
For animals completing the urine endpoint.
Vancomycin PK models, parameter estimates and exposures
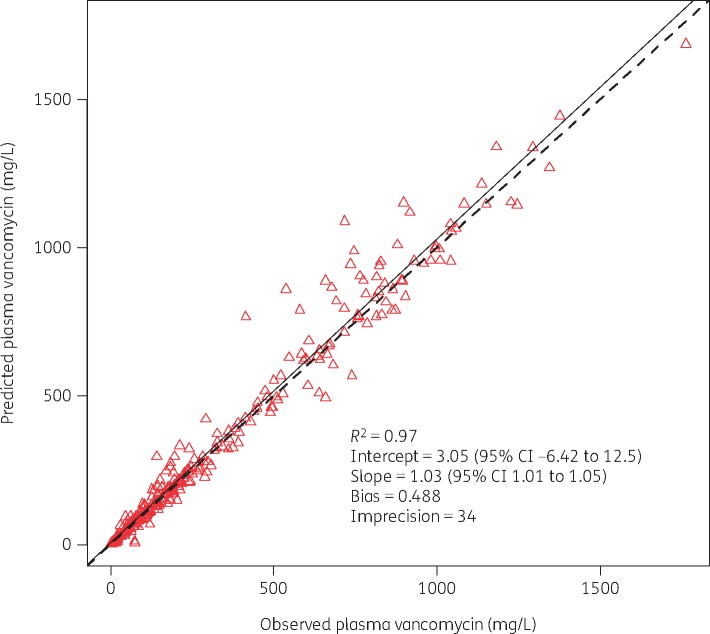
Models were successfully fit for one- and two-compartment models. A two-compartment model was chosen as the final model given that it was the most parsimonious with the least bias/imprecision and the lowest AIC compared with other models. The overall PK exposures for all rats are summarized in Table 2. The final model’s population mean parameter values (SD) for Ke, V, KCP and KPC were: 0.6 h−1 (0.38), 0.11 L (0.08), 5.33 h−1 (8.5) and 3.66 h−1 (6.56), respectively. Model predictive performance for observed versus Bayesian predicted concentrations, bias, imprecision (i.e. bias-adjusted mean weighted squared prediction error) and the coefficient of determination (R2) were 0.49 mg/L, 34 (mg/L)2 and 0.97, respectively (Figure 2). PK exposures (i.e. AUC0–24h, Cmax 0–24h and Cmin 0–24h) were variably correlated when assessed using Spearman’s rs correlation coefficient (AUC-Cmax, rs = 0.92; AUC-Cmin, rs = 0.76, Cmax–Cmin, rs = 0.62; P values < 0.01), and are displayed in Table 3.
Table 2.
PK exposure summary for vancomycin-treated animals
| PK exposure | Vancomycina (n = 45) |
|---|---|
| AUC0–24h (mg·h/L), median (IQR) | 643.1 (427.7–2769.4) |
| C max 0–24h (mg/L), median (IQR) | 350.9 (209.7–917.9) |
| C min 0–24h (mg/L), median (IQR) | 2.9 (0.8–36) |
Controls were excluded given that they did not receive vancomycin.
Figure 2.
Best-fit plot for Bayesian observed versus predicted plasma vancomycin concentrations utilizing the final two-compartment model. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Table 3.
Spearman correlation between untransformed PK exposure parameters
| PK exposure (n = 45)a | AUC0–24h (rs) | C max 0–24h (rs) | C min 0–24h (rs) |
|---|---|---|---|
| AUC0–24h | 1 | – | – |
| C max 0–24h | 0.92 (P < 0.001) | 1 | – |
| C min 0–24h | 0.76 (P < 0.001) | 0.62 (P < 0.001) | 1 |
Controls were excluded given that they did not receive vancomycin.
Urinary biomarkers
Urinary biomarkers (KIM-1, clusterin and OPN) for the vancomycin dosing group were all significantly different from control (P < 0.05), displayed in Table 1. For KIM-1, the median (IQR) for control versus vancomycin group was 1.02 ng/mL (0.88–1.05) and 12.3 ng/mL (5.6–13.9), respectively, P < 0.001. For clusterin, the median (IQR) for control versus vancomycin group was 319.5 ng/mL (308.9–332.2) and 879.4 ng/mL (617.6–1362), respectively P = 0.0043. For OPN, the median (IQR) for control versus vancomycin group was 0.02 ng/mL (0.01–0.03) and 0.218 ng/mL (0.11–0.39), respectively P < 0.001.
Exposure–response relationships and EC90
Four parameter Hill models best described the exposure–biomarker relationships. Exposure–biomarker relationships were found between AUC0–24h versus KIM-1 and OPN (R2 = 0.61 and R2 = 0.66) and Cmax 0–24h versus KIM-1 and OPN (R2 = 0.50 and R2 = 0.56). Conversely, Cmin 0–24h and mg/kg/day were less predictive of KIM-1 (R2 = 0.46 and R2 = 0.47) and OPN (R2 = 0.53 and R2 = 0.50). All exposure–biomarker relationships are shown in Figure 3. The EC90 (95% CI) for PK/TD pairs were as follows: AUC0–24h versus KIM-1 (482.2 mg·h/L, 95% CI 320.2–1030) and OPN (939.4 mg·h/L, 95% CI 539.9–3171); Cmax 0–24h versus KIM-1 (323.1 mg/L, 95% CI unable to estimate) and OPN (695.8 mg/L, 95% CI unable to estimate); Cmin 0–24h versus KIM-1(95% CI unable to estimate) and OPN (17.45 mg/L 95% CI unable to estimate).
Figure 3.
Exposure [AUC0–24h (mg·h/L), Cmax 0–24h (mg/L) and Cmin 0–24h (mg/L)] versus urinary biomarkers KIM-1a (a, b, c) and OPNa (d, e, f) relationship by a four-parameter Hill model fit TD: four-parameter Hill model equation: Y = Bottom + (Top–Bottom)/{1 + 10[(logEC50–X)×Hill Slope]}. Data for clusterin are not shown as they did not show a strong relationship or correlation. aUnits for biomarker in ng/mL. Biomarker values were log2 transformed and exposure values were log10 transformed. EC50, concentration at 50% of total biomarker increase. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
PK/TD correlations
Correlations of urinary biomarkers and vancomycin exposure metrics (i.e. AUC0–24h, Cmax 0–24h and Cmin 0–24h) are shown in Table 4. KIM-1 was highly correlated with AUC0–24h (rs = 0.53, P < 0.01). OPN demonstrated the highest correlation with AUC0–24h (rs = 0.75, P < 0.01), followed by Cmax 0–24h and Cmin 0–24h (both rs = 0.68, P < 0.01). Correlations between Cmin 0–24h and urinary biomarkers were less pronounced, and clusterin was less correlated with all biomarkers (data not shown).
Table 4.
Spearman correlation for untransformed PK exposure parameters and urinary biomarkers
| Biomarker | AUC0–24h (rs) | C max 0–24h (rs) | C min 0–24h (rs) |
|---|---|---|---|
| KIM-1 | 0.53 (P < 0.001) | 0.40 (P < 0.001) | 0.49 (P < 0.001) |
| Clusterin | 0.27 (P = 0.06) | 0.18 (P = 0.22) | 0.19 (P = 0.19) |
| OPN | 0.75 (P < 0.001) | 0.68 (P < 0.001) | 0.68 (P < 0.001) |
Discussion
In this study, we found that AUC0–24h and Cmax 0–24h were highly correlated with increased urinary concentrations of KIM-1 and OPN. Cmin 0–24h was less correlated with AKI measures. Here, we identified that an AUC0–24h of 482.2 mg·h/L and a Cmax 0–24h of 323.1 mg/L were associated with a 90% maximal increase in KIM-1 within a 24 h period. We believe KIM-1 to be the most relevant urinary biomarker as it is specific for proximal tubule injury.17,32,33 OPN, on the other hand, is more generically indicative of non-specific tubule or glomerular damage.21,34 Further studies looking into this are warranted. Our previous work indicates that KIM-1 is more specific to the proximal tubule in VIKI as it was correlated with the proximal tubule injury score,17 and this is consistent with the known mechanistic effect.32 Further, the threshold that we used (KIM-1 >16 ng/mL) has demonstrated predictive ability for AKI in humans as defined by AKIN criteria (urinary output <0.5 mL/kg/h during a 6 h period or when the serum creatinine increased >0.3 mg/dL or a 1.5- to 2-fold increase from the baseline value).30 It is notable that our identified AUC target is in agreement with a prospective multicentre clinical study of vancomycin concentrations at day 2 that caused AKI (defined as a ≥1.5-fold increase in serum creatinine for patients with a baseline creatinine <2.0 mg/dL) during vancomycin therapy.35 The results of the clinical study demonstrated that the first risk stratification by classification and regression tree (CART) analysis occurred between 343 and 793 mg·h/L,35 which agrees with the threshold of 482 mg·h/L that we found. We believe that our results can be viewed as similar to MIC testing for bacterial resistance classification. While the testing conditions are different from the clinical environment (i.e. the MIC testing environment does not mimic a standard physiological environment), the numerical agreement of the surrogate (e.g. MIC to clinical efficacy) is important for predicting downstream patient outcomes. Hence, we believe that the 24 h iv rat model and biomarker thresholds will be translatable for early identification of meaningful kidney injury. Notably, our results are also qualitatively similar to our previous rat studies when we utilized doses between 150 and 400 mg/kg given as either single or twice-daily intraperitoneal injections.17,18 In those studies, we identified a correlation between the PK parameters vancomycin AUC0–24 and Cmax 0–24h and urinary biomarkers, demonstrating that histopathological damage correlates with an increase in KIM-1.17,18 Other laboratories19,21 conducting vancomycin dose–response studies have similarly identified KIM-1 and OPN as sensitive and specific biomarkers predictive of kidney damage in rat models. Our results and concordance amongst the studies provides further evidence to support that vancomycin Cmin is not likely to be the parameter that mediates VIKI. Notably, the current vancomycin treatment guidelines focus solely on the measurement of trough concentrations.36
The present study focuses on iv-treated rats utilizing a range of dosages to allometrically scale low to high clinical exposures. Using iv dosing offers several advantages. First, in contrast to intraperitoneal (ip) dosing, there is a more predictable and reproducible exposure profile with iv dosing. This is important because erratic absorption from ip studies requires that multiple samples are drawn after every dose (in order to characterize each exposure curve). In small animal studies, the number of blood samples possible is limited. Second, with ip dosing, vancomycin may inadvertently be injected into the retroperitoneal space and serve as a direct nephrotoxin via external contact. Additionally, there is a remote possibility for the injection to physically damage the kidney. Thus, iv dosing in rats represents a biodistribution profile more reflective of that seen in typical clinical use in humans, and additionally avoids unnecessary variability.
Importantly, our data reinforce that the rat model is highly translational for understanding vancomycin-induced kidney damage. In the clinical setting, the median onset of vancomycin-induced AKI has been reported as 6–7 days into therapy, but these studies have relied on traditional and insensitive markers of AKI such as SCr and blood urea nitrogen (BUN).8,12,13 We previously analysed SCr in animals and found no relationship in the 24 h to 3 day studies, probably since SCr and BUN did not have enough time to become demonstrably elevated. As SCr requires 30%–50% parenchymal damage before it is detectable, kidney injury can be severe before changes in SCr can be detected.37 Using sensitive biomarkers for detection of early kidney damage may allow for early indication of renal injury and enable clinicians to change therapy prior to more substantial damage. Both the FDA and EMA have issued letters of support for KIM-1 and OPN.38,39 In addition, KIM-1, clusterin and cystatin C have already been qualified for pre-clinical toxicological evaluations by the EMEA and the Pharmaceutical and Medical Devices Agency Japan (PMDA).40,41 Further, urinary biomarkers (e.g. KIM-1 and clusterin) are already qualified for rat42 and human43 drug trials by the FDA (i.e. for drug-induced AKI). Thus, our results and agreement with robust clinical data indicate that KIM-1 can serve as an early surrogate for realized kidney injury.
We acknowledge several limitations in our study. First, our study was limited to 24 h dosing. However, as previously noted, elevations in biomarkers have already been linked to histopathological damage within this time period.19 Despite the 1 day nature of our study, we have demonstrated similar AUC thresholds to those identified in humans.35 Thus we suggest that this model is a surrogate for clinical human outcomes. Second, this study does not effectively separate Cmax and AUC, and additional studies will be needed to understand which is the primary driver of toxicity. These future studies will have implications for dosing administration time (e.g. continuous infusion versus intermittent infusion). Notably, this study employed allometric scaled doses which is different from parameter scaling (matching Cmax in the rat to the human Cmax).44 FDA guidance suggests allometrically scaled doses for toxicological analysis.45 Tethered animal models employing continuous infusion are needed for parameter scaling, and it is not clear if these results will be more translational than current studies. Third, when fitting the data to the TD model, some mathematical relationships were not sufficient for defining the 95% CI of the EC90. For Cmin, this is due to overall poor model fit. It will be necessary to design studies to formally compare AUC to Cmax to fully define the PK/TD link; however, for standard intermittent infusions, our AUC data are presently the most translational. Fourth, clinical studies have not identified single urinary biomarkers that are highly specific for drug-induced kidney injury where multifactorial processes are the rule rather than the exception.46 Clinical studies may benefit from employing blood biomarkers where dilution is less of a concern than with urinary markers,47 and one biomarker alone may not be sufficient to identify vancomycin-induced kidney damage. Despite these concerns, it is notable that KIM-1 in the rat is a homologue of KIM-1b in humans, thus further enhancing future translation.48 These quantitative relationships require further exploration and clinical trials.
In summary, these data demonstrate that VIKI is caused by either elevated peak plasma concentrations (Cmax 0–24h) or total plasma exposure (AUC0–24h) of vancomycin rather than with troughs (Cmin 0–24h). These findings may have clinical implications for vancomycin monitoring schemes. Further clarification of the drivers of VIKI are needed to improve dosing regimens that maximize efficacy while minimizing toxicity. Finally, our study demonstrates the utility of the rat model for understanding PK/TD for vancomycin.
Acknowledgements
We gratefully acknowledge the Core Facility at Midwestern University for access to the LC-MS/MS.
Funding
The research reported in this publication was supported in part by the National Institute of Allergy and Infectious Diseases under award number R15-AI105742.
Transparency declarations
M. H. S. reports having a research grant from Nevakar. All other authors: none to declare.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1. Pakyz AL, MacDougall C, Oinonen M. et al. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch Intern Med 2008; 168: 2254–60. [DOI] [PubMed] [Google Scholar]
- 2. Polk RE, Hohmann SF, Medvedev S. et al. Benchmarking risk-adjusted adult antibacterial drug use in 70 US academic medical center hospitals. Clin Infect Dis 2011; 53: 1100–10. [DOI] [PubMed] [Google Scholar]
- 3. Kelesidis T, Braykov N, Uslan DZ. et al. Indications and types of antibiotic agents used in 6 acute care hospitals, 2009–2010: a pragmatic retrospective observational study. Infect Control Hosp Epidemiol 2016; 37: 70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baggs J, Fridkin SK, Pollack LA. et al. Estimating national trends in inpatient antibiotic use among US hospitals from 2006 to 2012. JAMA Intern Med 2016; 176: 1639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weiss AJ, Elixhauser A.. Overview of Hospital Stays in the United States, 2012: Statistical Brief #180 Healthcare Cost and Utilization Project (HCUP) Statistical Briefs Rockville, MD, USA, 2006. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf.
- 6. Gelfand MS, Cleveland KO.. Vancomycin-induced nephrotoxicity. Antimicrob Agents Chemother 2013; 57: 2435.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elyasi S, Khalili H, Dashti-Khavidaki S. et al. Vancomycin-induced nephrotoxicity: mechanism, incidence, risk factors and special populations: a literature review. Eur J Clin Pharmacol 2012; 68: 1243–55. [DOI] [PubMed] [Google Scholar]
- 8. Lodise TP, Patel N, Lomaestro BM. et al. Relationship between initial vancomycin concentration–time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis 2009; 49: 507–14. [DOI] [PubMed] [Google Scholar]
- 9. Bosso JA, Nappi J, Rudisill C. et al. Relationship between vancomycin trough concentrations and nephrotoxicity: a prospective multicenter trial. Antimicrob Agents Chemother 2011; 55: 5475–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rybak MJ, Lomaestro BM, Rotschafer JC. et al. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis 2009; 49: 325–7. [DOI] [PubMed] [Google Scholar]
- 11. Cano EL, Haque NZ, Welch VL. et al. Incidence of nephrotoxicity and association with vancomycin use in intensive care unit patients with pneumonia: retrospective analysis of the IMPACT-HAP Database. Clin Ther 2012; 34: 149–57. [DOI] [PubMed] [Google Scholar]
- 12. Minejima E, Choi J, Beringer P. et al. Applying new diagnostic criteria for acute kidney injury to facilitate early identification of nephrotoxicity in vancomycin-treated patients. Antimicrob Agents Chemother 2011; 55: 3278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wunderink RG, Niederman MS, Kollef MH. et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 2012; 54: 621–9. [DOI] [PubMed] [Google Scholar]
- 14. Lodise TP, Lomaestro B, Graves J. et al. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother 2008; 52: 1330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bosch K, McLaughlin MM, Esterly JS. et al. Impact of vancomycin treatment duration and dose on kidney injury. Int J Antimicrob Agents 2014; 43: 297–8. [DOI] [PubMed] [Google Scholar]
- 16. Gupta A, Biyani M, Khaira A.. Vancomycin nephrotoxicity: myths and facts. Neth J Med 2011; 69: 379–83. [PubMed] [Google Scholar]
- 17. O'Donnell JN, Rhodes NJ, Lodise TP. et al. 24-hour pharmacokinetic relationships for vancomycin and novel urinary biomarkers of acute kidney injury. Antimicrob Agents Chemother 2017; 61: e00416-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rhodes NJ, Prozialeck WC, Lodise TP. et al. Evaluation of vancomycin exposures associated with elevations in novel urinary biomarkers of acute kidney injury in vancomycin-treated rats. Antimicrob Agents Chemother 2016; 60: 5742–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vaidya VS, Ozer JS, Dieterle F. et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol 2010; 28: 478–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Academy of Science. Guide for the Care and Use of Laboratory Animals, 8th edn. Washington, DC, USA: National Academies Press, 2011. https://www.ncbi.nlm.nih.gov/books/NBK54050/. [Google Scholar]
- 21. Fuchs TC, Frick K, Emde B. et al. Evaluation of novel acute urinary rat kidney toxicity biomarker for subacute toxicity studies in preclinical trials. Toxicol Pathol 2012; 40: 1031–48. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research (CDER). Guidance for Industry. Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. 2005. https://www.fda.gov/downloads/Drugs/Guidances/UCM078932.pdf%23search=%27guidekines+for+industry+sfe+starting%27.
- 23. Joshi MD, O'Donnell JN, Venkatesan N. et al. High-performance liquid chromatography method for rich pharmacokinetic sampling schemes in translational rat toxicity models with vancomycin. Clin Transl Sci 2017; 10: 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Prozialeck WC, Edwards JR, Lamar PC. et al. Expression of kidney injury molecule-1 (Kim-1) in relation to necrosis and apoptosis during the early stages of Cd-induced proximal tubule injury. Toxicol Appl Pharmacol 2009; 238: 306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prozialeck WC, Edwards JR, Vaidya VS. et al. Preclinical evaluation of novel urinary biomarkers of cadmium nephrotoxicity. Toxicol Appl Pharmacol 2009; 238: 301–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laboratory of Applied Pharmacokinetics and Bioinformatics. Optimizing Drug Therapy for Populations and Individuals Pmetrics Model Files. http://www.lapk.org/ModelLib.php.
- 27. Neely MN, van Guilder MG, Yamada WM. et al. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit 2012; 34: 467–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tatarinova T, Neely M, Bartroff J. et al. Two general methods for population pharmacokinetic modeling: non-parametric adaptive grid and non-parametric Bayesian. J Pharmacokinet Pharmacodyn 2013; 40: 189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O'Donnell JN, O'Donnell EP, Kumar EJ. et al. Pharmacokinetics of centhaquin citrate in a dog model. J Pharm Pharmacol 2016; 68: 803–9. [DOI] [PubMed] [Google Scholar]
- 30. Morales-Buenrostro LE, Salas-Nolasco OI, Barrera-Chimal J. et al. Hsp72 is a novel biomarker to predict acute kidney injury in critically ill patients. PLoS One 2014; 9: e109407.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wong-Beringer A, Joo J, Tse E. et al. Vancomycin-associated nephrotoxicity: a critical appraisal of risk with high-dose therapy. Int J Antimicrob Agents 2011; 37: 95–101. [DOI] [PubMed] [Google Scholar]
- 32. King DW, Smith MA.. Proliferative responses observed following vancomycin treatment in renal proximal tubule epithelial cells. Toxicol In Vitro 2004; 18: 797–803. [DOI] [PubMed] [Google Scholar]
- 33. Han WK, Bailly V, Abichandani R. et al. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int 2002; 62: 237–44. [DOI] [PubMed] [Google Scholar]
- 34. Bonventre JV, Vaidya VS, Schmouder R. et al. Next-generation biomarkers for detecting kidney toxicity. Nat Biotechnol 2010; 28: 436–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lodise TP, Rosencranz SL, Finnemeyer M. et al. The emperor’s new clothes: prospective observational evaluation of the association between the day 2 vancomycin exposure and failure rates among adult hospitalized patients with MRSA bloodstream infections (PROVIDE). In: Abstract of ID Week 2017, San Diego, CA, USA. Oral Presentation 985. [DOI] [PMC free article] [PubMed]
- 36. Rybak M, Lomaestro B, Rotschafer JC. et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2009; 66: 82–98. [DOI] [PubMed] [Google Scholar]
- 37. Duarte CG, Preuss HG.. Assessment of renal function—glomerular and tubular. Clin Lab Med 1993; 13: 33–52. [PubMed] [Google Scholar]
- 38. Dieterle F, Sistare F, Goodsaid F. et al. Renal biomarker qualification submission: a dialog between the FDA–EMEA and Predictive Safety Testing Consortium. Nat Biotechnol 2010; 28: 455–62. [DOI] [PubMed] [Google Scholar]
- 39. Weiss AJ, Elixhauser A, Steiner C.. Readmissions to U.S. Hospitals by Procedure, 2010: Statistical Brief #154 Healthcare Cost and Utilization Project (HCUP) Statistical Briefs Rockville, MD, USA, 2006. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb154.pdf.
- 40. Weiss AJ, Elixhauser A.. Obesity-Related Hospitalizations, 2004 Versus 2009: Statistical Brief #137 Healthcare Cost and Utilization Project (HCUP) Statistical Briefs Rockville, MD, USA, 2006. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb137.pdf. [PubMed]
- 41. Prozialeck WC, Lamar PC, Lynch SM.. Cadmium alters the localization of N-cadherin, E-cadherin, and β-catenin in the proximal tubule epithelium. Toxicol Appl Pharmacol 2003; 189: 180–95. [DOI] [PubMed] [Google Scholar]
- 42.US Food and Drug Administration. Safety Biomarker to be Used with Traditional Indicators to Indicate Renal Injury in Rat Biomarker Qualification Program: List of Qualified Biomarkers.https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugDevelopmentToolsQualificationProgram/BiomarkerQualificationProgram/ucm535383.htm.
- 43.US Food and Drug Administration. Safety Biomarker Panel to Aid in the Detection of Kidney Tubular Injury in Phase 1 Trials in Healthy Volunteers. Biomarker Qualification Program: List of Qualified Biomarkers https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugDevelopmentToolsQualificationProgram/BiomarkerQualificationProgram/ucm535383.htm.
- 44. Reisfeld B, Mayeno AN.. Computational Toxicology. New York, NY, USA: Humana Press, Springer, 2012. [Google Scholar]
- 45. Mellen CK, Ryba JE, Rindone JP.. Does piperacillin–tazobactam increase the risk of nephrotoxicity when used with vancomycin: a meta-analysis of observational trials. Curr Drug Saf 2017; 12: 62–6. [DOI] [PubMed] [Google Scholar]
- 46. Ostermann M, Chang RW.. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med 2007; 35: 1837–43; quiz 52. [DOI] [PubMed] [Google Scholar]
- 47. Schley G, Koberle C, Manuilova E. et al. Comparison of plasma and urine biomarker performance in acute kidney injury. PLoS One 2015; 10: e0145042.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang Z, Humphreys BD, Bonventre JV.. Shedding of the urinary biomarker kidney injury molecule-1 (KIM-1) is regulated by MAP kinases and juxtamembrane region. J Am Soc Nephrol 2007; 18: 2704–14. [DOI] [PubMed] [Google Scholar]