Abstract
Objectives
To expand understanding of the virological potency of initial dolutegravir plus lamivudine dual therapy (dolutegravir/lamivudine), we compared the viral decay seen in the pilot ACTG A5353 study with the decay observed with dolutegravir plus two NRTIs in the SPRING-1 and SINGLE studies, while also exploring the impact of baseline viral load (VL).
Methods
Change in VL from baseline was calculated for timepoints shared by A5353 (n = 120, including 37 participants with pretreatment VL >100000 copies/mL), SPRING-1 (n = 51) and SINGLE (n = 417). The 95% CIs of change from baseline were determined for each observed week, using the mean log10-transformed VL, and compared between the dolutegravir/lamivudine and triple therapy groups using the Wilcoxon Rank Sum test for non-inferiority (δ = 0.5). To assess the impact of baseline VL on viral decay, we examined a bi-exponential non-linear mixed-effect model.
Results
The mean VL change from baseline to week 24 was −2.9 log10 copies/mL for dolutegravir/lamivudine versus −3.0 log10 copies/mL for dolutegravir-based three-drug therapy (P < 0.001). In the decay model, baseline VL >100000 copies/mL was associated with a slower initial decay rate (d1). A faster initial decay rate was seen with dolutegravir/lamivudine, which was partially offset when baseline VL was >100000 copies/mL as indicated by a significant interaction between baseline VL and drug therapy group. The secondary decay rate (d2) was not significantly different from zero, with no significant associations.
Conclusions
Viral decay with dolutegravir/lamivudine was comparable to viral decay with dolutegravir-based triple therapy, even in individuals with higher pretreatment VL (>100000 copies/mL).
Introduction
The virological efficacy of two-drug ART with lamivudine and dolutegravir in treatment-naive HIV-1-infected individuals was first explored in two single-arm pilot studies, PADDLE1,2 and A5353.3 These early successes with dual therapy were followed by the fully powered phase III GEMINI studies, which established the non-inferiority of dolutegravir/lamivudine versus a standard three-drug regimen in suppressing plasma HIV-1 RNA [viral load (VL)] below 50 copies/mL at week 48.4
The rapidity of viral decay after treatment initiation is another important piece of the virological profile of an antiretroviral regimen, and at least three distinct phases of viral decay have been identified.5 Phase 1 viral decay, which occurs during the first 10 days of ART, reflects turnover of short-lived infected cells,5,6 and correlates with subsequent virological response.7 Phases 2 and 3 are incrementally slower than phase 1 and are thought to reflect loss of longer-lived productively infected cells and decay of latently infected CD4+ T cells, respectively. Rapid viral decay (i.e. attainment of VL <50 copies/mL between week 2 and week 12) has been shown to be associated with virological response at 1 year.8 Furthermore, the rapidity of viral suppression below detection limits in plasma may provide some insight into when a regimen may be considered fully effective for HIV transmission prevention since evidence now exists that ‘undetectable = untransmittable’.9,10
The viral decay produced by dolutegravir plus lamivudine was evaluated in a PADDLE substudy11 and shown to be similar to that seen with the three-drug dolutegravir-based regimens used in the studies SPRING-1 (dolutegravir plus two NRTIs) and SINGLE (dolutegravir plus abacavir/lamivudine).12,13 Because the PADDLE study excluded participants with screening VL >100000 copies/mL, the early viral decay in this population remains largely unknown. The A5353 study included 120 participants, of whom 37 (31%) had a baseline VL >100000 copies/mL. To further characterize viral decay with dolutegravir plus lamivudine and determine the impact of baseline VL (≤100000 versus >100000 copies/mL), we compared the viral decay in A5353 with the viral decay observed in the SPRING-1 and SINGLE studies. We hypothesized that viral decay with dolutegravir plus lamivudine is comparable to dolutegravir-based triple therapy at both high and low baseline VLs.
Methods
A post-hoc analysis was conducted using VL data obtained from the timepoints shared by A5353 (n = 120), SPRING-1 (n = 51) and SINGLE (n = 417): baseline (pretreatment) and study weeks 2, 4, 8, 12, 16 and 24. From SPRING-1, a dose-ranging study exploring three different doses of dolutegravir, only the participants receiving the 50 mg dose were selected for this cross-study comparison. All three studies utilized the Abbott RealTime HIV-1 assay with a lower detection limit of 40 copies/mL, and observations below the lower limit of detection were assigned the lowest detection value of 39 copies/mL. Change in VL from baseline to each timepoint was calculated for each participant. The 95% CIs of change from baseline were examined for each observed week, using the mean log10-transformed VL, and compared across the two-drug (A5353) and three-drug (SPRING-1 and SINGLE) therapy groups using the Wilcoxon Rank Sum test for non-inferiority (δ = 0.5). Non-inferiority of these change estimates was tested using ±0.5 log10 VL as the maximum difference between the two groups.14 For the viral decay analysis, a bi-exponential non-linear mixed-effect model was examined. Three variables were added as covariates of the initial (d1) and secondary (d2) decay parameters: two-drug versus three-drug therapy, baseline VL (≤100000 versus >100000 copies/mL) and an interaction term of the drug therapy and baseline VL stratum. Simple slope decay rates were determined for the four groups. Using a maximum likelihood-based approach, all available observations were used in the estimation of model parameters. Therefore, for individual missing data at week 24, all other data from these individuals were utilized to estimate the slope and inform model parameter estimates.
Results
In the two-drug therapy group (A5353), 120 participants were included for analysis. One participant in the SINGLE study had missing data (baseline VL) and therefore was not included in the analysis, leaving 467 participants for inclusion in the three-drug therapy group (SPRING-1 and SINGLE). In this analytical sample of 587 participants, 183 (31%) participants had a baseline VL >100000 copies/mL, which included 37 (31%) participants from the two-drug therapy group and 146 (31%) participants from the three-drug therapy group.
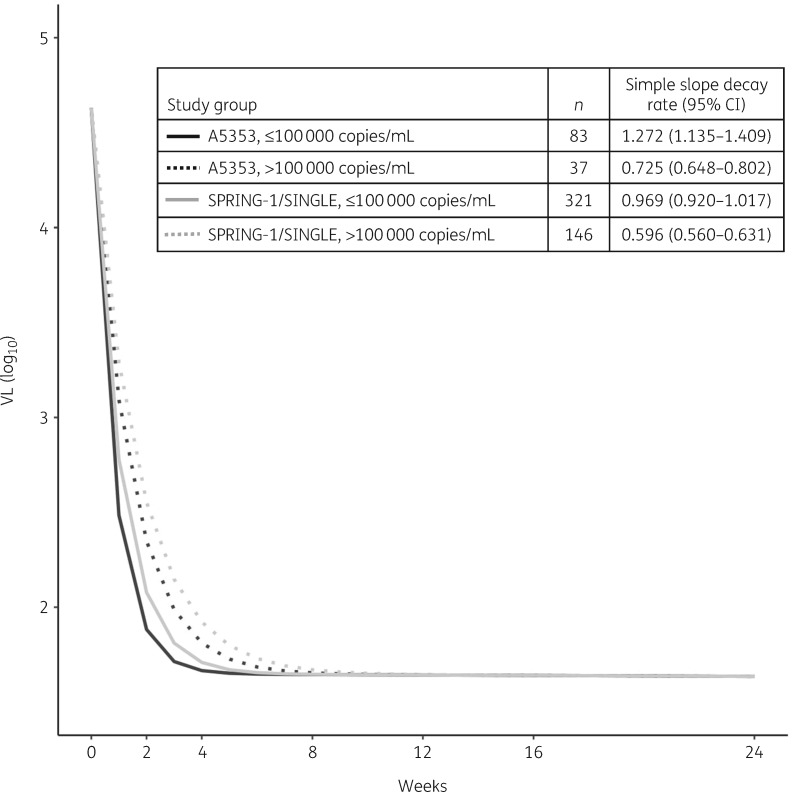
The VL change from baseline with two-drug therapy was non-inferior to that with three-drug therapy at all timepoints (P < 0.001; Tables 1 and 2). In bi-exponential effect modelling, the initial decay parameter (d1) was 0.969 (95% CI = 0.920–1.017; P < 0.001). The effect estimate for two-drug therapy for those with a baseline VL <100000 copies/mL was 0.303 (95% CI = 0.162–0.444), indicating faster initial decay with two-drug therapy compared with three-drug therapy (P < 0.001). The effect estimate for baseline VL >100000 copies/mL for those on three-drug therapy was −0.373 (95% CI = −0.427 to −0.319), indicating slower initial decay at higher baseline VL (P < 0.001). The interaction term was −0.174 (95% CI = −0.336 to −0.011), demonstrating that the effects varied across groups (P = 0.036). The secondary decay parameter (d2) was non-significantly different from zero, with no significant associations. Simple slope decay rates are shown in Figure 1. As indicated in the figure, the two-drug group with a baseline VL <100000 copies/mL had the fastest decay rate (1.272, 95% CI = 1.135–1.409) followed by the three-drug group with a baseline VL <100000 copies/mL (0.969, 95% CI = 0.920–1.017), the two-drug group with baseline VL >100000 copies/mL (0.725, 95% CI = 0.648–0.802) and the three-drug group with a baseline VL >100000 copies/mL (0.596, 95% CI = 0.560–0.631).
Table 1.
VL change from baseline
| Mean change in VL, log10 copies/mL transformed (95% CI) |
Non-inferiority test (δ = 0.5) | ||
|---|---|---|---|
| A5353, n = 120 | SPRING-1 and SINGLE, n = 467 | ||
| Baseline–week 2 | −2.52 (−2.61 to −2.43) | −2.46 (−2.51 to −2.41) | P<0.001 |
| Baseline–week 4 | −2.80 (−2.91 to −2.69) | −2.86 (−2.91 to −2.81) | P<0.001 |
| Baseline–week 8 | −2.92 (−3.04 to −2.79) | −2.98 (−3.04 to −2.92) | P<0.001 |
| Baseline–week 12 | −2.91 (−3.04 to −2.78) | −3.00 (−3.07 to −2.94) | P<0.001 |
| Baseline–week 16 | −2.89 (−3.03 to −2.76) | −3.02 (−3.08 to −2.96) | P<0.001 |
| Baseline–week 24 | −2.89 (−3.03 to −2.74) | −3.02 (−3.09 to −2.95) | P<0.001 |
Table 2.
Mean values of log VL at each timepoint by drug category and baseline VL
| ≤100000 copies/mL, log10 copies/mL (95% CI) |
>100000 copies/mL, log10 copies/mL (95% CI) |
|||||
|---|---|---|---|---|---|---|
| A5353 (n = 83) | SPRING-1/SINGLE (n = 321) | non-inferiority test (δ = 0.5) | A5353 (n = 37) | SPRING-1/SINGLE (n = 146) | non-inferiority test (δ = 0.5) | |
| Baseline | 4.18 (4.06–4.30) | 4.30 (4.25–4.35) | P<0.001 | 5.31 (5.21–5.41) | 5.45 (5.40–5.50) | P<0.001 |
| Week 2 | 1.80 (1.72–1.87) | 1.95 (1.90–1.99) | P<0.001 | 2.50 (2.36–2.64) | 2.77 (2.67–2.88) | P<0.001 |
| Week 4 | 1.64 (1.61–1.67) | 1.67 (1.64–1.70) | P<0.001 | 1.96 (1.84–2.07) | 2.09 (2.02–2.16) | P<0.001 |
| Week 8 | 1.63 (1.57–1.69) | 1.61 (1.60–1.63) | P<0.001 | 1.70 (1.63–1.76) | 1.82 (1.75–1.89) | P<0.001 |
| Week 12 | 1.62 (1.58–1.67) | 1.62 (1.59–1.65) | P<0.001 | 1.62 (1.59–1.65) | 1.71 (1.67–1.75) | P<0.001 |
| Week 16 | 1.63 (1.58–1.67) | 1.60 (1.59–1.61) | P<0.001 | 1.63 (1.59–1.66) | 1.70 (1.65–1.75) | P<0.001 |
| Week 24 | 1.67 (1.56–1.77) | 1.63 (1.60–1.66) | P<0.001 | 1.66 (1.55–1.78) | 1.65 (1.62–1.68) | P<0.001 |
Figure 1.
Simple slope decay rates.
Discussion
Dual therapy with dolutegravir plus lamivudine offers a compelling option for the initial treatment of HIV-1 infection due to its potential for lower adverse events and cost when compared with some three-drug regimens.15 In the GEMINI studies, 91% of treatment-naive individuals receiving this two-drug regimen achieved VL <50 copies/mL at week 48, demonstrating non-inferiority to a three-drug regimen of tenofovir disoproxil fumarate/emtricitabine and dolutegravir.4 There were no significant differences between those with baseline VL ≤100000 versus >100000 copies/mL. Dolutegravir plus lamivudine was recently added to the US Department of Health and Human Services (DHHS) and European AIDS Clinical Society (EACS) treatment guidelines as an alternative option for initial HIV treatment16,17 and a single tablet formulation of this regimen has been approved by the FDA. Our study extends understanding of the virological profile of initial dolutegravir plus lamivudine dual therapy by showing that viral decay with this regimen is comparable to the decay with three-drug dolutegravir-based regimens.
While baseline VL >100000 copies/mL was associated with an overall slower decay rate in our model, viral decay with two-drug therapy in this subgroup was comparable to viral decay with three-drug therapy. This is consistent with the efficacy of dolutegravir plus lamivudine in this subgroup of the GEMINI studies. The finding of a faster decay rate with two-drug therapy was unexpected and should be interpreted with caution. A5353, unlike SPRING-1 and SINGLE, excluded participants with a screening VL ≥500000 copies/mL, who would be predicted to have the slowest decay rates and may have contributed to the slower decay seen with the three-drug regimens. Nevertheless, there were four participants in A5353 who, despite having VL <500000 copies/mL at screening, actually had VL ≥500000 copies/mL at study entry. SPRING-1 included 3 participants with VL ≥500000 copies/mL at study entry and SINGLE included 27 participants with VL ≥500000 copies/mL at study entry.
To explore the potential impact of the differing inclusion criteria on our results, we performed a sensitivity analysis examining the same bi-exponential model when excluding all participants with VL >500000 copies/mL at study entry. The results of this model indicated that the statistically significant faster decay with two-drug therapy remained. The interaction term, however, was no longer significant, indicating that the faster decay in the two-drug therapy group did not differ according to baseline VL. Therefore, the results of the sensitivity analysis suggest that our findings are robust to the difference in baseline VL inclusion criteria. Nonetheless, we continue to urge caution in interpreting this difference given the possibility of further unmeasured confounders in any non-randomized post-hoc analysis.
Our results show that dolutegravir plus lamivudine achieves viral suppression in a similar time frame to dolutegravir-based three-drug therapy, which is important since suppression of plasma viraemia is effective in preventing viral transmission.10 There was also no evidence from our analysis that dolutegravir plus lamivudine is likely to increase the risk of incomplete viral suppression, which has been associated with resistance emergence in some settings.18,19 In fact, the initial decay in viraemia with both two-drug therapy and three-drug therapy was so rapid that subsequent decay was modest. This suggests a dominant effect of dolutegravir in the different regimens, consistent with an early dose-ranging study that showed a VL reduction of up to 2.46 log10 copies/mL following 10 days of dolutegravir monotherapy.20
Limitations of our study include the fact that it was a post-hoc analysis. Furthermore, we compared studies that had different exclusion and inclusion criteria, hence the populations could have differed in ways that we have not identified or accounted for. In the three-drug therapy group, 93% of participants (433/467) were on a nucleoside backbone of abacavir and lamivudine, therefore the results may not be fully generalizable to individuals on a nucleoside backbone of tenofovir disoproxil fumarate and emtricitabine. Given the absence of VL measurements between week 0 and week 2, we were unable to undertake a comprehensive viral dynamics evaluation as reported in other studies.21 As such, the initial and secondary decay rates reported in our analysis are not the same as the phase 1 and phase 2 viral decays characterized with more intense sampling.5 Finally, the covariates included in our modelling did not include CD4+ T cell count because the substantial overlap in VL and CD4 would lead to extremely low cell counts in observations. Median CD4+ T cell counts were similar between the three trials: 335 cells/mm3 in the dolutegravir arm of SINGLE, 305 cells/mm3 in the dolutegravir 50 mg arm of SPRING-1 and 387 cells/mm3 in A5353.3,13,22 In the GEMINI studies, FDA snapshot analysis showed that among participants with CD4+ counts of ≤200 cells/mm3, 79% in the two-drug regimen group achieved HIV-1 RNA values of <50 copies/mL compared with 93% in the three-drug regimen group; however, most of the reasons for snapshot failures in this subgroup were unrelated to virological efficacy or treatment failure.4
Despite the limitations, we have shown that viral decay with initial dolutegravir plus lamivudine is comparable to that with dolutegravir-based three-drug therapy, even in individuals with pretreatment VL >100000 copies/mL.
Acknowledgements
We acknowledge participants in A5353, SPRING-1 and SINGLE, and the different study teams.
Funding
A5353 was supported by the Institute of Allergy and Infectious Diseases of the National Institutes of Health (grant numbers UM1 AI068634, UM1 AI068636 and UM1 AI106701), while SPRING-1 and SINGLE were funded by ViiV.
Transparency declarations
J. G. has received funds for speaking at symposia organized by Gilead and AbbVie. C. L. W. consults or has consulted for the International Partnership for Microbicides (IPM) and Celera Diagnostics, and has received funds for conducting training in HIV drug resistance from Janssen, MSD and Right-To-Care. R. B. has received funds for research from ViiV and Merck, and has served as a scientific advisor to ViiV and Merck. K. S. and M. A. are employed by ViiV. B. T. has served as a consultant to ViiV, GSK, Merck, Gilead and Janssen, and has received research funding through Northwestern University from ViiV/GSK. All other authors: none to declare.
References
- 1. Cahn P, Rolon MJ, Figueroa MI. et al. Dolutegravir–lamivudine as initial therapy in HIV-1 infected, ARV-naive patients, 48-week results of the PADDLE (Pilot Antiretroviral Design with Dolutegravir LamivudinE) study. J Int AIDS Soc 2017; 20: 21678.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Figueroa MI, Rolon MJ, Patterson P. et al. Dolutegravir–lamivudine as initial therapy in HIV-infected, ARV naive patients: 96 week results of the PADDLE trial In: Abstracts of the Ninth IAS Conference on HIV Science, Paris, France, 2017. Abstract MOPEB0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taiwo BO, Zheng L, Stefanescu A. et al. ACTG A5353: a pilot study of dolutegravir plus lamivudine for initial treatment of human immunodeficiency virus-1 (HIV-1)-infected participants with HIV-1 RNA <500000 copies/mL. Clin Infect Dis 2018; 66: 1689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cahn P, Madero JS, Arribas JR. et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet 2019; 393: 143–55. [DOI] [PubMed] [Google Scholar]
- 5. Andrade A, Rosenkranz SL, Cillo AR. et al. Three distinct phases of HIV-1 RNA decay in treatment-naive patients receiving raltegravir-based antiretroviral therapy: ACTG A5248. J Infect Dis 2013; 208: 884–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dahl V, Josefsson L, Palmer S.. HIV reservoirs, latency, and reactivation: prospects for eradication. Antiviral Res 2010; 85: 286–94. [DOI] [PubMed] [Google Scholar]
- 7. Polis MA, Sidorov IA, Yoder C. et al. Correlation between reduction in plasma HIV-1 RNA concentration 1 week after start of antiretroviral treatment and longer-term efficacy. Lancet 2001; 358: 1760–5. [DOI] [PubMed] [Google Scholar]
- 8. Eron JJ, Cooper DA, Steigbigel RT. et al. Association between first-year virological response to raltegravir and long-term outcomes in treatment-experienced patients with HIV-1 infection. Antivir Ther 2015; 20: 307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rodger AJ, Cambiano V, Bruun T. et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA 2016; 316: 171–81. [DOI] [PubMed] [Google Scholar]
- 10.The Lancet HIV. U=U taking off in 2017. Lancet HIV 2017; 4: e475. [DOI] [PubMed] [Google Scholar]
- 11. Sued O, Figueroa MI, Rolon MJ. et al. Comparable viral decay in dual and triple dolutegravir-based antiretroviral therapy. In: Abstracts of the Conference on Retroviruses and Opportunistic Infections, Boston, MA, USA, 2016. Abstract 947. Foundation for Retrovirology and Human Health, Alexandria, VA, USA.
- 12. Stellbrink HJ, Reynes J, Lazzarin A. et al. Dolutegravir in antiretroviral-naive adults with HIV-1: 96-week results from a randomized dose-ranging study. AIDS 2013; 27: 1771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walmsley SL, Antela A, Clumeck N. et al. Dolutegravir plus abacavir–lamivudine for the treatment of HIV-1 infection. N Engl J Med 2013; 369: 1807–18. [DOI] [PubMed] [Google Scholar]
- 14. Murray JS, Elashoff MR, Iacono-Connors LC. et al. The use of plasma HIV RNA as a study endpoint in efficacy trials of antiretroviral drugs. AIDS 1999; 13: 797–804. [DOI] [PubMed] [Google Scholar]
- 15. Girouard MP, Sax PE, Parker RA. et al. The cost-effectiveness and budget impact of 2-drug dolutegravir–lamivudine regimens for the treatment of HIV infection in the United States. Clin Infect Dis 2016; 62: 784–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV Department of Health and Human Services. http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf.
- 17.European AIDS Clinical Society. EACS Treatment Guidelines Version 9.1 http://www.eacsociety.org/files/2018_guidelines-9.1-english.pdf.
- 18. Ryscavage P, Kelly S, Li JZ. et al. Significance and clinical management of persistent low-level viremia and very-low-level viremia in HIV-1-infected patients. Antimicrob Agents Chemother 2014; 58: 3585–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Taiwo B, Zheng L, Gallien S. et al. Efficacy of a nucleoside-sparing regimen of darunavir/ritonavir plus raltegravir in treatment-naive HIV-1-infected patients (ACTG A5262). AIDS 2011; 25: 2113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Min S, Sloan L, DeJesus E. et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of dolutegravir as 10-day monotherapy in HIV-1-infected adults. AIDS 2011; 25: 1737–45. [DOI] [PubMed] [Google Scholar]
- 21. Imaz A, Martinez-Picado J, Niubo J. et al. HIV-1-RNA decay and dolutegravir concentrations in semen of patients starting a first antiretroviral regimen. J Infect Dis 2016; 214: 1512–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Lunzen J, Maggiolo F, Arribas JR. et al. Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naive adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomised, phase 2b trial. Lancet Infect Dis 2012; 12: 111–8. [DOI] [PubMed] [Google Scholar]