Abstract
Objectives
Efficacy is determined not only by size, but also by shape, of drug exposure. Here the critical importance of the temporal pattern of drug concentrations (pharmacokinetic profile) is examined for antitrypanosomals in vitro.
Methods
An in vitro hollow-fibre cartridge system was used to study contrasting drug profiles with four clinically used agents and two experimental candidates against the deadly parasite Trypanosoma brucei. Artificial kinetics were employed intentionally to favour either high peak concentration or sustained duration of drug.
Results
Changing the shape of drug exposure significantly impacted drug efficacy. Suramin, melarsoprol and pentamidine were concentration-driven and therefore more efficacious when applied as short-lived high peaks. In contrast, difluoromethylornithine (DFMO) was time-driven, and therefore maximally effective as a constant infusion. Kinetic preference was robust over a wide range of drug exposures. Promising clinical candidates SCYX-7158 (acoziborole) and fexinidazole (parent and sulfone) were concentration-driven, suggesting optimal clinical regimens would involve relatively high but intermittent dosing.
Conclusions
Antitrypanosomals have an intrinsic pharmacokinetic driver for optimal efficacy, with important implications for clinical management and future candidate development.
Introduction
Clinical studies remain a costly and rate-limiting step in developing and improving drugs against pathogens. A critical determinant of drug outcome is exposure: the pharmacokinetic/pharmacodynamic (PK/PD) relationship (reviewed in Drusano,1 Martinez et al.,2 Schmidt et al.3 and Mouton et al.4). PK describe the temporal pattern of drug concentrations in vivo: as an oral dose is absorbed blood concentration rises to a peak, then declines as the drug is distributed, metabolized and eliminated. The area under this curve (AUC), the integral of concentration across time, constitutes total drug exposure. The efficacy of a drug may be impacted by the shape of the concentration–time profile. Perhaps most notably, the antibacterial penicillins are time-driven and require sustained exposures to exert maximal clinical effect,5 whereas aminoglycosides are driven by the total AUC and peak concentration and are not enhanced by sustained drug exposures, which worsen their nephrotoxicity.6,7 The kinetic driver of drug activity is determined empirically and is not predicted by mechanism of action or time–kill studies, although the preference for concentration or time of exposure is usually class wide.2In vitro studies of dynamic PK/PD can greatly aid clinical work, allowing rational choice of dosing regimens and saving valuable time, resources, and risk to human subjects.8–10 We adapted a hollow-fibre cartridge for studying the in vitro PK/PD of agents against the protozoan pathogen Trypanosoma brucei.11 Clinical work with T. brucei is especially challenging given invasive diagnostic procedures and the resource-limited remote settings of this infection.
T. brucei is a flagellated and highly motile single-cell eukaryotic parasite. It is spread by the tsetse fly vector in sub-Saharan Africa, with subspecies causing human African trypanosomiasis (sleeping sickness) and nagana in cattle.12 In humans these extracellular parasites initially infect the periphery then subsequently invade the CNS and lead to fatality if untreated.13T. b. rhodesiense, found in east Africa, causes an acute aggressive disease progressing to death within a few months and sometimes just weeks. T. b. gambiense develops more slowly, with serious symptoms emerging months or years after infection. In the early 20th century trypanosomiasis was a public health crisis, and epidemics killed an estimated 800000 people before pioneering drug discovery efforts produced several antitrypanosomals, among the very first of the anti-infective drugs.12
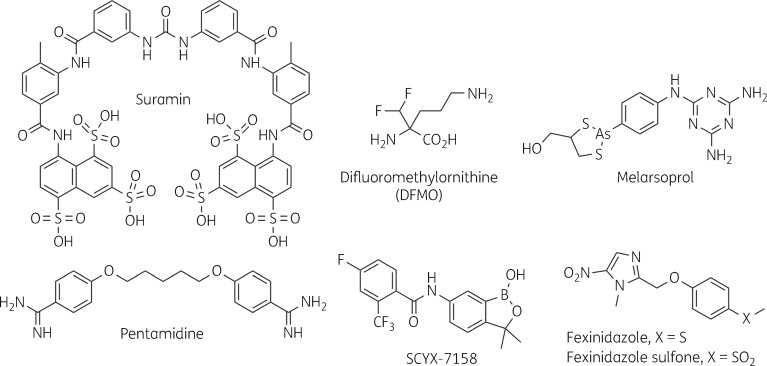
Initial therapy relied on arsenicals such as atoxyl, and later tryparsamide, somewhat effective but deadly in their own right and causing frequent irreversible optic nerve damage.14 Suramin, introduced in the early 1920s as Bayer 205, had fewer toxicities and excellent results in early-stage patients but was ineffective in CNS disease. Pentamidine followed in the 1940s, but was also useful only against early-stage infection. Ongoing work on the arsenicals eventually produced melarsoprol, which was more effective and less toxic for late-stage disease, despite the fatal encephalopathy it caused in 2%–10% of recipients. Melarsoprol remains the only available therapy for CNS T. b. rhodesiense, but was replaced in the 1980s by difluoromethylornithine (DFMO) for treatment of late-stage T. b. gambiense. DFMO, a rationally devised irreversible suicide substrate,15 was repurposed from the cancer field and is now given in combination with nifurtimox. Suramin, pentamidine, melarsoprol and DFMO (Figure 1) are all traditionally given in lengthy and cumbersome repeated injection regimens. Recent efforts to develop new treatments have resulted in two orally administered compounds. The nitro-heterocycle fexinidazole recently completed Phase II/III evaluation,16,17 benzoxaborole SCYX-7158 (acoziborole) is in Phase II/III studies (Figure 1), and research to produce new antitrypanosomal agents continues.18
Figure 1.
Chemical structures of clinical and experimental antitrypanosomals.
In this study we used our in vitro PK/PD system11 to examine the impact of PK on efficacy for several classic antitrypanosomals and clinical candidates. We used artificial PK conditions, which were thus independent of in vivo tissue compartment or protein-binding concerns, to identify the intrinsic relationship between concentration or time of exposure and efficacy, and found that the kinetic preference is steadfast across a wide range of AUCs. Knowledge of PK/PD relationships provides a rational basis for assessing and improving dosing regimens, and provides a new criterion by which to evaluate drug leads.
Materials and methods
Cell culture and reagents
Unless otherwise indicated, all cartridge and microtitre plate assays were conducted with bloodstream-form T. brucei brucei (MiTat 1.2 strain 427, which has drug susceptibilities similar to those of human pathogenic subspecies19,20), maintained in phenol red-free HMI9,21 10% FBS, 10% Serum Plus (Sigma–Aldrich), at 37°C, 5% CO2, and for 24 h. Motile cells were counted by haemocytometer and light microscope. Drug stocks were stored aliquoted (−20°C): suramin (Mobay Chemical Corp.), pentamidine (American Pharmaceutical Partners), d,l-DFMO [National Cancer Institute (NCI) Developmental Therapeutics Program] in water; melarsoprol (US CDC) in 1,2-propanediol; and SCYX-7158, fexinidazole (and sulfone) in DMSO. Fexinidazole, fexinidazole sulfone and SCYX-7158 were synthesized using published methods (see Supplementary Methods and spectra in Figures S1 and S2, available as Supplementary data at JAC Online).22–25 Tracers were U[3H]suramin (42 Ci/mmol; Moravek) and [14C]pentamidine (32 mCi/mmol; NCI).
In vitro PK/PD for antitrypanosomals
Dynamic systems were assembled as described previously.11 Briefly, hollow-fibre cartridges (C2025, FiberCell Systems) were connected to autoclaved tubing and reservoirs in a biosafety cabinet, and filled with HMI9 medium. Non-specific drug binding to cartridges was assessed and subsequent cartridges were pre-incubated and central reservoir volumes adjusted to correct for this and to achieve desired PK profiles in the extra-fibre cartridge space (Table S1).11 Suramin and pentamidine PK were determined using radiolabelled tracers and the remaining drugs were followed by bioassay. Trypanosomes (105 cells/mL) were seeded in the extra-fibre space (3 mL), and medium was pumped unidirectionally into the extra-fibre space, through the walls of the fibres, then out via the fibre lumens to a waste compartment. Flow was 0.45 mL/min, to allow optimal growth of trypanosomes while ensuring rapid mixing of fluid to achieve faithful PK profiles.11 Every experiment included a no-drug cartridge, and the flask of seed culture used to inoculate cartridges was maintained alongside as a growth control. The entire system was incubated (37°C, 5% CO2, 24 h), then trypanosomes were harvested and counted. After each use cartridges were extensively rinsed with ethanol (70%) and water, then stored in HMI9. Reused cartridges were checked for reproducible trypanosome growth, ensuring any residual drug had been cleared from previous experiments.
Drug cytotoxicity and concentration bioassays
Cytotoxicity assays in 96-well plates were performed using an acid phosphatase-based method.26 For PK determinations, samples taken at intervals from the extra-fibre space of a cartridge were bioassayed, comparing cytotoxicity of unknowns with a standard curve of drug concentrations prepared in the same medium.
Time–kill and reversibility at 6 h
Cells (2 × 105/mL) in 24-well plates were exposed to 20×EC99 (except fexinidazole, 5 × EC99) for 6 h then split. One population remained in the drug; the other was washed three times with, then maintained in, drug-free medium (>4000× drug dilution). Cells were counted at intervals over 48 h.
Statistical analysis
Data are means ± SD; error bars are only shown if data were obtained from three or more independent experiments. Where indicated, an unpaired, two-tailed, equal-variance Student’s t-test assessed significance. Of 120 experiments, 8 were discarded because cells in the no-drug cartridge grew significantly less well than those in the parallel flask, by the modified Thompson τ test to identify outliers greater than two standard deviations from the mean. Sigmoidal curves were fitted with GraphPad Prism v7.01 using a sigmoidal dose–response (variable curve) equation; median effective activity (EA50; AUC0–24 that gives 50% response) values were calculated by interpolation.
Results
Experiment design and set-up
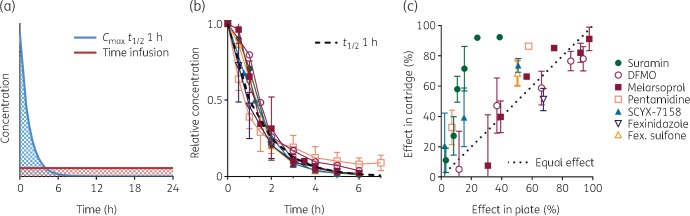
T. b. brucei was cultured in the extra-fibre space of a hollow-fibre cartridge and exposed to the desired drug PK profile (Figure 2a).11 PK/PD studies comprised three cartridges: a no-drug control and two drug-treated cartridges, with efficacy based on motile parasite counts at 24 h. Starting density of 105 cells/mL allowed log growth of control cells for the entire period. In no-drug control cartridges, trypanosome growth was 77 ± 13% (mean±SD of n = 112) of the parallel flask. Eight experiments were discarded for poor cartridge growth based on modified Thompson’s τ test (final τ·s value 25.7; δ for discarded experiments, 27–60). Rather than simulate known in vivo PK, we generated two artificial and sharply contrasting PK shapes of the same total drug exposure, in order to diagnose the preferred configuration for efficacy. The same AUC0–24 was given either as a steady constant infusion (time-intensive) or as a transient high peak with t½ = 1 h (concentration-intensive) (Figure 2a), and the antitrypanosomal efficacy of the two regimens was then compared in order to identify the driver as either time of exposure or concentration. A constant infusion maximizes the time component of an AUC, whereas a 1 h half-life bolus yields a peak, 16.8-fold higher than the infusion concentration, that falls by 98% over 6 h (the length of one T. brucei cell cycle). To achieve accurate PK, non-specific loss of drug to the cartridge was satisfied by pre-incubation with concentrations calculated to produce the desired t0 free-drug concentration (Table S1). For each drug the PK of dynamic Cmax regimens were confirmed in samples from the extra-fibre cartridge space (Figure 2b).
Figure 2.
Design and validation of in vitro PK/PD of antitrypanosomals. (a) In two cartridges, trypanosomes are exposed to the same AUC0–24 (shaded) but in contrasting (and artificial) regimens as either a constant infusion or as a high peak with t½ = 1 h. (b) Actual PK of tested drugs in samples obtained from the extra-fibre space of Cmax regimens were assayed in two or more experiments, and achieved t½ within 10% of 1 h (black dashed curve), except pentamidine, which achieved and was studied at t½ = 1.7 h. (c) Efficacy of constant infusion of drug in the cartridge system versus that of the same concentration in microtitre plate assays. Data are means; ±SD is shown where n ≥ 3. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
The initial AUC0–24 for study was chosen based on plate assays, such that the time-intensive infusion was expected to be ∼50% effective. This maximizes the chance of detecting better or worse efficacy of the contrasting concentration-intensive regimen. It also allows efficacy comparison of the dynamically deployed infusion with the same concentration in microtitre plates (Figure 2c). In 24 h exposures, suramin, pentamidine, SCYX-7158 and fexinidazole sulfone infusions in the cartridge were more effective than the same concentrations in plates, DFMO and melarsoprol had equivalent efficacies, and fexinidazole was less effective in the cartridge. Antitrypanosomal activity in the cartridge may be enhanced by constant provision of fresh drug (200-fold greater total mass of drug passes by cells in the cartridge versus in wells) and by pre-incubation to satisfy non-specific binding, or alternatively it may be hindered by fresh nutrients and removal of waste. In any case it is likely that cartridge conditions more closely mimic those in vivo.
Suramin efficacy is driven by peak concentration
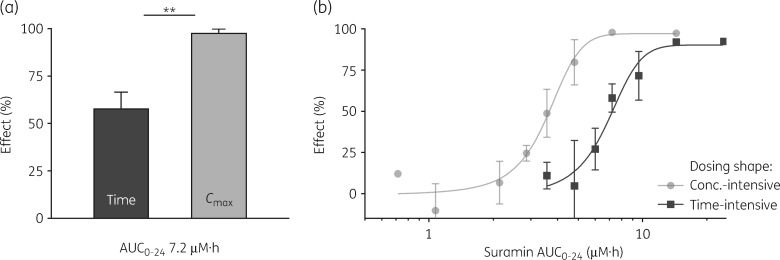
Suramin, an antitrypanosomal in continuous use since the 1920s, was examined in the dynamic in vitro PK/PD system. A time-intensive constant infusion of 0.3 μM over 24 h caused 58% reduction in final cell number compared with drug-free controls. The same total AUC0–24 applied as a 5.0 μM peak with t½ = 1 h resulted in 98% reduction (Figure 3a). Thus, for the same total dose and drug exposure, efficacy was nearly doubled by simply changing the shape of the AUC to favour peak concentration (Figure 3a). We conclude that suramin is concentration-driven and does not require sustained exposures for efficacy, a finding that, as expected, held with human pathogenic subspecies T. b. gambiense (Figure S3). To further validate this preference, we tested a wide AUC0–24 range, dosed either as an infusion or t½ = 1 h bolus, and generated an exposure–response curve for each regimen (Figure 3b). The linear response range of the curves was widely separated, systematically favouring Cmax- over time-intensive dosing. Vertically, for the same AUC0–24, a substantial gain was achieved by Cmax regimens: at 5 μM·h, 85% versus 15%. Horizontally, suramin was 2-fold more ‘potent’ if applied to favour Cmax: EA50 values were 3.6 μM·h for Cmax but 7.2 μM·h for time, and Cmax dosing caused maximal effect at half the total dose required for time-intensive dosing. Suramin efficacy is optimal when the shape of exposure is a short-lived high peak.
Figure 3.
Suramin is more efficacious when dosed to favour a transient high concentration. (a) Suramin AUC0–24 of 7.2 μM·h applied as a constant infusion of 0.30 μM (left) or as a high Cmax of 5.0 μM with t½ = 1 h (right). **P < 0.01. (b) Exposure–response curves. Suramin was applied as a constant infusion (squares, EA50 7.2 μM·h, R2 = 0.98) or as high Cmax with t½ = 1 h (circles, EA50 3.7 μM·h, R2 = 0.98), across a range of AUC0–24. The percentage effect is the reduction at 24 h in trypanosome count compared with the untreated control cartridge. Data are means; ±SD is shown where n ≥ 3.
DFMO efficacy is driven by time of exposure
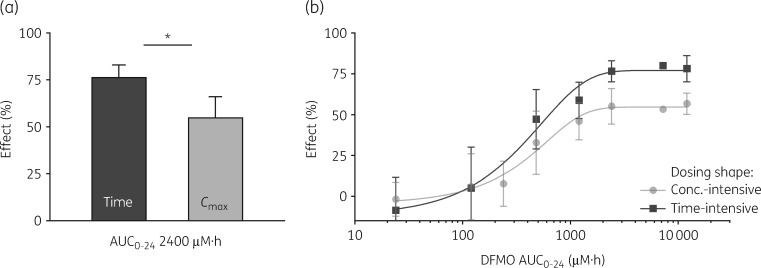
DFMO at AUC0–24 2400 μM·h was 76% effective as a time-intensive infusion, but only 55% effective as high Cmax with t½ = 1 h (Figure 4a). Thus, in contrast to suramin, antiparasitic activity of DFMO is driven by time of exposure rather than peak concentration. DFMO infusions had an EA50 of 600 μM·h versus 1500 for Cmax dosing (Figure 4b), a 2.5-fold ‘potency’ difference. Both exposure–response curves were shallow, and for the same AUC0–24 the greatest vertical difference in efficacies was 25 points. Interestingly, this difference was maintained at the maxima, such that Cmax dosing at best achieved only 55% reduction in parasite count, whereas infusion dosing reached 80%. By all measures DFMO is most advantageously applied to maintain concentrations over time rather than to favour peak concentration, and sustained exposure is necessary for maximum effect.
Figure 4.
DFMO efficacy is time-driven. (a) DFMO AUC0–24 of 2400 μM·h applied as a constant infusion of 100 μM (left) or as a high Cmax of 1700 μM with t½ = 1 h (right). *P < 0.05. (b) Across three logs of AUC0–24, the efficacy of a time-intensive constant infusion (squares, EA50 600 μM·h, R2 = 0.98) was compared with that of a short-lived high Cmax with t½ = 1 h (circles, EA50 1500 μM·h, R2 = 0.98). The percentage effect is cell count reduction versus controls. Data are means; ±SD is shown where n ≥ 3.
Melarsoprol, pentamidine, SCYX-7158 and fexinidazole are concentration-driven
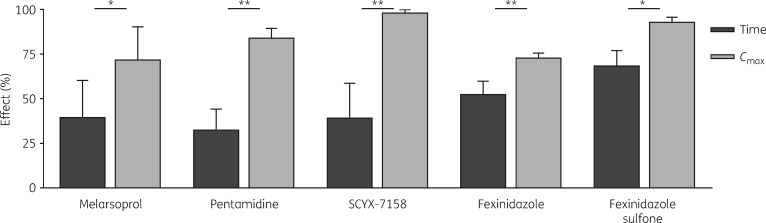
The remaining antitrypanosomals studied were all significantly more efficacious with Cmax-intensive dosing, over multiple AUCs (Figure 5; see Figure S4 for additional AUCs). Fexinidazole in vivo is metabolized to a sulfoxide and then sulfone, both active. We tested the PK driver of fexinidazole sulfone, which, like its parent, fexinidazole, was concentration-driven (Figure 5), consistent with the class-wide kinetic governance of other anti-infectives, including antitrypanosomals.2,11,27
Figure 5.
Melarsoprol, pentamidine, SCYX-7158, fexinidazole and fexinidazole sulfone are concentration-driven. A given AUC0–24 was applied as a constant infusion (Time) or as a transient high concentration (Cmax). Unless indicated otherwise, t½ = 1 h for Cmax regimens. Conditions were (AUC0–24; infusion concentration, Cmax): melarsoprol (310 nM·h; 13 nM, 220 nM); pentamidine (53 nM·h; 2.2 nM, 22 nM t½ = 1.7 h); SCYX-7158 (24 μM·h; 1 μM, 17 μM); fexinidazole (480 μM·h; 20 μM, 336 μM); and fexinidazole sulfone (110 μM·h; 4.8 μM, 80 μM). The percentage effect is cell count reduction versus controls. Data are means; ±SD is shown where n ≥ 3; *P < 0.05, **P < 0.01.
Time–kill and reversibility of drug action
As Cmax regimens have relatively transient exposures, concentration-driven compounds likely rapidly initiate cell-inhibitory activity, or have a significant post-antiparasitic effect. To assess some of these factors we exposed trypanosomes in plates to drug, with either wash-out at 6 h or maintained presence, and determined viability periodically over 48 h by counting motile cells (Figure 6). Exposure concentrations were high, 20-fold the microtitre plate EC99 (approximate peak concentration of an equivalent Cmax cartridge regimen), and reversibility was analysed after 6 h. (Fexinidazole was just 5-fold the EC99, reflecting solubility limit.) Viability and reversibility for a range of exposures were also investigated with ATP and phosphatase assays (Figure S5). These provided similar results, although as previously observed the ATP response lagged behind motile cell counts.28
Figure 6.
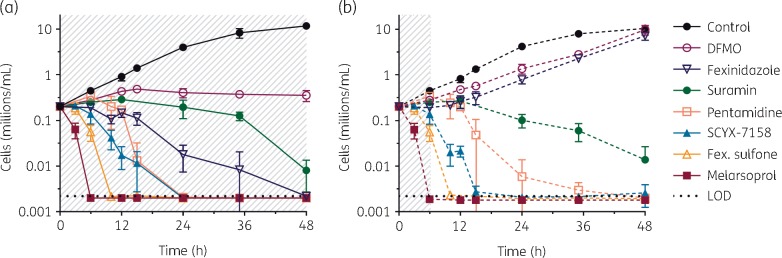
Time–kill and reversibility of antitrypanosomals. Trypanosomes were exposed to high concentrations of the indicated drugs (shaded areas), either (a) continuously (solid lines) or (b) for a 6 h period then washed (dashed lines). Motile cells were counted at indicated timepoints over 48 h. Drug concentrations were: DFMO, 10 mM; fexinidazole, 300 μM; suramin, 10 μM; pentamidine, 600 nM; SCYX-7158, 120 μM; fexinidazole sulfone, 300 μM; and melarsoprol, 2 μM. LOD, limit of detection, 0.0025 million cells/mL. Data are means; ±SD is shown where n ≥ 3. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Each drug had a distinctive time–effect profile, with different rates of growth inhibition, cell killing and post-drug effect (Figure 6). Melarsoprol, fexinidazole sulfone, SCYX-7158, pentamidine and suramin (in order of onset time and log kill rate) were all cytocidal (‘cidal) at these exposures, causing a progressive fall in cell number whether drug concentrations were sustained for 48 h or pulsed for 6 h then chased for 42 h. Both pentamidine and suramin exhibited delayed death effects. In contrast, fexinidazole and DFMO were cytostatic (‘static’): growth resumed after drug was washed away. In 10 mM DFMO onset of action was slow, with trypanosomes continuing to increase for 12 h, then stopping, but not declining. Fexinidazole caused immediate arrest and if maintained cell numbers slowly declined; on wash-out at 6 h there were several further hours of post-antiparasitic effect before growth resumed at 10 h.
Discussion
Dynamic in vitro PK/PD allowed us to assign intrinsic drivers of activity for both classic and proposed antitrypanosomals. We chose to use artificial kinetics so as to generate sharply contrasting shapes of exposure. Plotting AUC–response curves based on the shape of exposure (Figures 3b and 4b) captured information unobtainable through conventional in vitro assays, and not readily obtained in vivo. Across a wide range of AUCs the preferred driver remained consistent and clearly evident. Importantly, maximal efficacy is achieved with lower total AUC when the kinetic driver is favoured. Indeed, time-intensive dosing was necessary for DFMO to achieve maximal activity, perhaps reflecting its inability to eradicate parasites (Figures 4 and 6). Vertical separation of AUC–response curves highlights the efficacy gain from an optimally shaped exposure. Horizontal separation reflects differences in potency: the exposure required for equi-efficacy. The shape of the AUC determines both ‘potency’ and efficacy of a drug.
For all drugs studied we observed a statistically significant separation between the efficacies of Cmax- or time-intensive regimens (Figures 3–5); however, the extent of separation differed, and it is possible that for some drugs there would be insignificant separation. Suramin, melarsoprol, pentamidine, SCYX-7158 and fexinidazole all had greater activity when deployed as a concentration-intensive regimen (Figures 3 and 5). Though most of these drugs were cidal with a short 6 h exposure, they differed dramatically in temporal response (Figure 6). Also concentration-driven, fexinidazole was static, not cidal, in the time–kill studies, demonstrating that for antitrypanosomals, as for antibacterials,2 concentration- or time-driven efficacy does not equate with cidal or static activity. Interestingly, fexinidazole sulfone, like its parent, fexinidazole, was concentration-driven, but unlike its parent it was cidal on short exposures (Figures 5 and 6). Growth dynamics after exposure to fixed concentrations of drug do not readily predict a kinetic driver. Our experimental approach of testing a single AUC in two contrasting shapes allows efficient diagnosis of the preferred kinetic driver. Full exposure–response curves provide even greater insight.
Intrinsic kinetic drivers of antitrypanosomal activity indicate that for each agent there are ideal PK properties for optimal potency and efficacy. In vitro PK/PD is therefore a valuable tool for drug development. The intrinsic kinetic driver of a lead compound can be determined, and used either to optimize PK properties or alternatively to help inform a decision about its translational potential. The antitrypanosomals we studied are already in use or in clinical trials and their in vivo PK properties are fixed. However, knowledge of the optimal shape of exposure is also useful as a simple and powerful guide for dosing regimen design and improvement within the constraints of in vivo PK and clinically achievable AUCs. Intrinsically time-driven drugs should be dosed to sustain exposure. Concentration-driven drugs can be dosed intermittently, in large but tolerable amounts that achieve transient high peaks. In any optimization of dosing, the PK/PD relationship for efficacy must be balanced with those for toxicities, and when possible discrepant drivers should be exploited to favour efficacy and minimize side effects. For any anti-infective drug the emergence of resistance is another important consideration; however, the substantially longer replication time, lower attainable cell densities and lower mutation rate of eukaryote trypanosomes (versus bacteria, for example) make drug resistance less of a concern (and less readily studied) in relatively short-lived in vitro PK/PD studies of African trypanosomes.
DFMO was the only time-driven drug in our experiments, and reported results from in vivo studies reflect this property. Multiple-day dosing of DFMO in the drinking water is required for cure in a mouse model.29,30 The inopportune 3.5 h half-life of DFMO in humans requires frequent dosing to maintain concentrations,31 and early efforts with monotherapy to dose every 12 h instead of 6 h resulted in greater frequency of relapse.32 DFMO is now given every 12 h in combination with nifurtimox.33 In conjunction with in vitro findings that DFMO resistance can be selected with low concentrations and that nifurtimox and DFMO are antagonistic,34 our results suggest that more frequent dosing of the DFMO component may reduce the risk of efficacy failure or emergence of resistance.
The classic drugs suramin, pentamidine and melarsoprol were originally established with lengthy dosing regimens and little PK/PD knowledge.35 Concentration-driven efficacy provides a rationale for reassessing these regimens, suggesting that attention should be paid to the peak concentration required for activity, rather than trough levels. Suramin and pentamidine have rapid distribution phases to tissues and long terminal elimination half-lives.36,37 Our results of concentration-driven efficacy are consistent with the reported clearance of parasites from blood after just a single dose in vivo,38–40 and suggest activity in blood may stem from peak concentrations during the early distribution phases. However, the PK likely achieved in tissues are suboptimal for concentration-driven efficacy. Melarsoprol is used against CNS infection, and the original regimen has been improved to just 10 consecutive daily doses.41 Our findings suggest efficacy might be maintained by even fewer but larger doses; however, toxicity is a key concern and will have its own PK/PD. Intriguingly, doubling the starting dose of melarsoprol from 1.8 to 3.6 mg/kg decreased the incidence of toxic reactive encephalopathy in a trypanosomiasis clinical trial,42 implying there may be a margin for further dose increases.
The oxaborole SCYX-7158, which is in clinical development, was best dosed by Cmax-intensive regimens in the cartridge (Figure 5). This is seemingly inconsistent with previous time–kill results in microtitre plates, in which an AUC of 41 μM·h given as 1.7 μM for 24 h was irreversible (at 72 h), whereas as 6.8 μM for 6 h was reversible.20,43 However, higher concentrations of SCYX-7158 can be cidal on exposures of ≤6 h (Figure 6).20 In the cartridge an AUC0–24 of just 24 μM·h distributed as a 17 μM peak with t½ = 1 h caused maximal effect (Figure 5), highlighting the importance of dynamic AUC shapes. SCYX-7158 has a long in vivo half-life, 26 h in mice and 16 days in humans, making a short-lived peak impossible,18,20 and therefore entailing a greater total exposure than would be required if Cmax-intensive dosing were possible.
Fexinidazole has shown good efficacy in Phase II/III trials.16 We found that both parent and active sulfone metabolite are concentration driven (Figure 5). Consonant with this and despite the short half-lives (≤2 h) of fexinidazole and its metabolites in mouse plasma (and likely in brain), in the mouse chronic infection model once-daily dosing with 200 mg/kg achieves greater cures than does twice-daily dosing with 100 mg/kg.19,44 Plasma half-lives in humans are longer: ∼10 h for fexinidazole and sulfoxide, and 25 h for the subsequent sulfone.45 The Phase II/III regimen of once-daily doses leads to accumulation of, and sustained exposure to, the sulfone (simulated in Figure S6).45 Unfortunately, in mice the sulfone has the least brain penetration of the three moieties.44,46 Given our finding that high peaks are more effective than sustained exposures, the regimen may better be deployed as larger doses on intermittent and fewer days, if tolerability allows. This would optimize the shape of exposure of all three compounds (simulations shown in Figure S6).
Dynamic in vitro PK/PD studies, with trypanosome viability as an endpoint, can efficiently establish the intrinsic kinetic driver of efficacy. The system is versatile and could also be used to simulate animal or human PK, evaluate multiple dose regimens or explore different PD endpoints. Our findings with five clinical and two candidate antitrypanosomals are consistent with reported in vivo results and have implications for ideal drug management. Given the expense and challenge of clinical studies, dynamic in vitro PK/PD is a powerful tool that can inform all stages of drug development and clinical use.
Supplementary Material
Acknowledgements
We thank Rahul Bakshi, Elizabeth Nenortas and Emily Caton for technical support and manuscript reading, and Dennis Grab for T. b. gambiense. DFMO and [14C]pentamidine were generously provided by the Open Chemical Repository of the Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, NCI (http://dtp.cancer.gov). We gratefully acknowledge Dr Anne Moore's assistance in obtaining melarsoprol from the Centers for Disease Control and Prevention.
Funding
This work was supported by National Institutes of Health grant number R01 AI095453 (to T. A. S.), the Fulbright New Zealand and the Mustard Seed Foundation (to K. J. M.) and the Flight Attendant Medical Research Institute (to D. J. M.).
Transparency declarations
None to declare.
Author contributions
K. J. M. and T. A. S. planned experiments, analysed data and wrote the paper. K. J. M. performed experimental work. D. J. M. synthesized chemicals, wrote synthesis methods and edited the manuscript.
References
- 1. Drusano GL. Pharmacokinetics and pharmacodynamics of antimicrobials. Clin Infect Dis 2007; 45 Suppl 1: 89–95. [DOI] [PubMed] [Google Scholar]
- 2. Martinez MN, Papich MG, Drusano GL.. Dosing regimen matters: the importance of early intervention and rapid attainment of the pharmacokinetic/pharmacodynamic target. Antimicrob Agents Chemother 2012; 56: 2795–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schmidt S, Barbour A, Sahre M. et al. PK/PD: new insights for antibacterial and antiviral applications. Curr Opin Pharmacol 2008; 8: 549–56. [DOI] [PubMed] [Google Scholar]
- 4. Mouton JW, Ambrose PG, Canton R. et al. Conserving antibiotics for the future: new ways to use old and new drugs from a pharmacokinetic and pharmacodynamic perspective. Drug Resist Updat 2011; 14: 107–17. [DOI] [PubMed] [Google Scholar]
- 5. Eagle H, Fleischman R, Musselman AD.. Effect of schedule of administration on the therapeutic efficacy of penicillin; importance of the aggregate time penicillin remains at effectively bactericidal levels. Am J Med 1950; 9: 280–99. [DOI] [PubMed] [Google Scholar]
- 6. Moore RD, Lietman PS, Smith CR.. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis 1987; 155: 93–9. [DOI] [PubMed] [Google Scholar]
- 7. Rybak MJ, Abate BJ, Kang SL. et al. Prospective evaluation of the effect of an aminoglycoside dosing regimen on rates of observed nephrotoxicity and ototoxicity. Antimicrob Agents Chemother 1999; 43: 1549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gloede J, Scheerans C, Derendorf H. et al. In vitro pharmacodynamic models to determine the effect of antibacterial drugs. J Antimicrob Chemother 2010; 65: 186–201. [DOI] [PubMed] [Google Scholar]
- 9. Drusano GL. Pre-clinical in vitro infection models. Curr Opin Pharmacol 2017; 36: 100–6. [DOI] [PubMed] [Google Scholar]
- 10. Hope W, Drusano GL, Rex JH.. Pharmacodynamics for antifungal drug development: an approach for acceleration, risk minimization and demonstration of causality. J Antimicrob Chemother 2016; 71: 3008–19. [DOI] [PubMed] [Google Scholar]
- 11. Meyer KJ, Caton E, Shapiro TA.. Model system identifies kinetic driver of Hsp90 inhibitor activity against African trypanosomes and Plasmodium falciparum. Antimicrob Agents Chemother 2018; 62: e00056-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brun R, Blum J, Chappuis F. et al. Human African trypanosomiasis. Lancet 2010; 375: 148–59. [DOI] [PubMed] [Google Scholar]
- 13. Kennedy PG. Clinical features, diagnosis, and treatment of human African trypanosomiasis (sleeping sickness). Lancet Neurol 2013; 12: 186–94. [DOI] [PubMed] [Google Scholar]
- 14. Steverding D. The development of drugs for treatment of sleeping sickness: a historical review. Parasit Vectors 2010; 3: 15.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bey P, Gerhart F, Van Dorsselaer V. et al. alpha-(Fluoromethyl)dehydroornithine and alpha-(fluoromethyl)dehydroputrescine analogues as irreversible inhibitors of ornithine decarboxylase. J Med Chem 1983; 26: 1551–6. [DOI] [PubMed] [Google Scholar]
- 16. Mesu V, Kalonji WM, Bardonneau C. et al. Oral fexinidazole for late-stage African Trypanosoma brucei gambiense trypanosomiasis: a pivotal multicentre, randomised, non-inferiority trial. Lancet 2017; 391: 144–54. [DOI] [PubMed] [Google Scholar]
- 17. Pollastri MP. Fexinidazole: a new drug for African sleeping sickness on the horizon. Trends Parasitol 2018; 34: 178–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baker CH, Welburn SC.. The long wait for a new drug for human African trypanosomiasis. Trends Parasitol 2018; 34: 818–27. [DOI] [PubMed] [Google Scholar]
- 19. Kaiser M, Bray MA, Cal M. et al. Antitrypanosomal activity of fexinidazole, a new oral nitroimidazole drug candidate for treatment of sleeping sickness. Antimicrob Agents Chemother 2011; 55: 5602–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jacobs RT, Nare B, Wring SA. et al. SCYX-7158, an orally-active benzoxaborole for the treatment of stage 2 human African trypanosomiasis. PLoS Negl Trop Dis 2011; 5: e1151.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hirumi H, Hirumi K.. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J Parasitol 1989; 75: 985–9. [PubMed] [Google Scholar]
- 22. Samant BS, Sukhthankar MG.. Compounds containing 2-substituted imidazole ring for treatment against human African trypanosomiasis. Bioorg Med Chem Lett 2011; 21: 1015–8. [DOI] [PubMed] [Google Scholar]
- 23. Morrow N, Ramsden CA, Sargent BJ. et al. Oxidative decarbonylation of β-arylpyruvic acids using sodium perborate. Tetrahedron 1998; 54: 9603–12. [Google Scholar]
- 24. Fontana E, Pignatti A, Venegoni S. et al. Synthesis of 2H- and 14C-labeled fexinidazole and its primary metabolites labeled with 2H. J Label Compd Radiopharm 2011; 54: 714–9. [Google Scholar]
- 25. Jacobs RT, Plattner JJ, Nare B. et al. Benzoxaboroles: a new class of potential drugs for human African trypanosomiasis. Future Med Chem 2011; 3: 1259–78. [DOI] [PubMed] [Google Scholar]
- 26. Bodley AL, McGarry MW, Shapiro TA.. Drug cytotoxicity assay for African trypanosomes and Leishmania species. J Infect Dis 1995; 172: 1157–9. [DOI] [PubMed] [Google Scholar]
- 27. Bakshi RP, Nenortas E, Tripathi AK. et al. Model system to define pharmacokinetic requirements for antimalarial drug efficacy. Sci Transl Med 2013; 5: 205ra135.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Worthen C, Jensen BC, Parsons M.. Diverse effects on mitochondrial and nuclear functions elicited by drugs and genetic knockdowns in bloodstream stage Trypanosoma brucei. PLoS Negl Trop Dis 2010; 4: e678.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bacchi CJ, Nathan HC, Hutner SH. et al. Polyamine metabolism: a potential therapeutic target in trypanosomes. Science 1980; 210: 332–4. [DOI] [PubMed] [Google Scholar]
- 30. Jennings FW. Chemotherapy of trypanosomiasis: the potentiation of melarsoprol by concurrent difluoromethylornithine (DFMO) treatment. Trans R Soc Trop Med Hyg 1988; 82: 572–3. [DOI] [PubMed] [Google Scholar]
- 31. Abeloff MD, Slavik M, Luk GD. et al. Phase I trial and pharmacokinetic studies of alpha-difluoromethylornithine—an inhibitor of polyamine biosynthesis. J Clin Oncol 1984; 2: 124–30. [DOI] [PubMed] [Google Scholar]
- 32. Milord F, Pepin J, Loko L. et al. Efficacy and toxicity of eflornithine for treatment of Trypanosoma brucei gambiense sleeping sickness. Lancet 1992; 340: 652–5. [DOI] [PubMed] [Google Scholar]
- 33. Priotto G, Kasparian S, Mutombo W. et al. Nifurtimox-eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: a multicentre, randomised, phase III, non-inferiority trial. Lancet 2009; 374: 56–64. [DOI] [PubMed] [Google Scholar]
- 34. Vincent IM, Creek D, Watson DG. et al. A molecular mechanism for eflornithine resistance in African trypanosomes. PLoS Pathog 2010; 6: e1001204.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Burri C. Chemotherapy against human African trypanosomiasis: is there a road to success? Parasitology 2010; 137: 1987–94. [DOI] [PubMed] [Google Scholar]
- 36. Jodrell DI, Reyno LM, Sridhara R. et al. Suramin: development of a population pharmacokinetic model and its use with intermittent short infusions to control plasma drug concentration in patients with prostate cancer. J Clin Oncol 1994; 12: 166–75. [DOI] [PubMed] [Google Scholar]
- 37. Conte JE., Jr. Pharmacokinetics of intravenous pentamidine in patients with normal renal function or receiving hemodialysis. J Infect Dis 1991; 163: 169–75. [DOI] [PubMed] [Google Scholar]
- 38. Wenyon CM. The action of “Bayer 205” on Trypanosoma equiperdum in experimentally infected mice. Br Med J 1921; 2: 746.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Van Hoof L, Henrard C, Peel E.. Pentamidine in the prevention and treatment of trypanosomiasis. Trans R Soc Trop Med Hyg 1944; 37: 271–80. [Google Scholar]
- 40. Findlay GM. Recent Advances in Chemotherapy. Philadelphia, PA, USA: P. Blakiston's Sons & Co. Inc, 1930. [Google Scholar]
- 41. Burri C, Nkunku S, Merolle A. et al. Efficacy of new, concise schedule for melarsoprol in treatment of sleeping sickness caused by Trypanosoma brucei gambiense: a randomised trial. Lancet 2000; 355: 1419–25. [DOI] [PubMed] [Google Scholar]
- 42. Pepin J, Mpia B.. Randomized controlled trial of three regimens of melarsoprol in the treatment of Trypanosoma brucei gambiense trypanosomiasis. Trans R Soc Trop Med Hyg 2006; 100: 437–41. [DOI] [PubMed] [Google Scholar]
- 43. Wring S, Gaukel E, Nare B. et al. Pharmacokinetics and pharmacodynamics utilizing unbound target tissue exposure as part of a disposition-based rationale for lead optimization of benzoxaboroles in the treatment of Stage 2 human African trypanosomiasis. Parasitology 2014; 141: 104–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Torreele E, Bourdin Trunz B, Tweats D. et al. Fexinidazole—a new oral nitroimidazole drug candidate entering clinical development for the treatment of sleeping sickness. PLoS Negl Trop Dis 2010; 4: e923.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tarral A, Blesson S, Mordt OV. et al. Determination of an optimal dosing regimen for fexinidazole, a novel oral drug for the treatment of human African trypanosomiasis: first-in-human studies. Clin Pharmacokinet 2014; 53: 565–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Burrell-Saward H, Harris AJ, de LaFlor R. et al. Dose-dependent effect and pharmacokinetics of fexinidazole and its metabolites in a mouse model of human African trypanosomiasis. Int J Antimicrob Agents 2017; 50: 203–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.