Abstract
Objectives
To evaluate the proportion of children with lopinavir Cmin ≥1 mg/L when receiving a novel 8-hourly lopinavir/ritonavir dosing strategy during rifampicin co-treatment.
Methods
HIV-infected children on lopinavir/ritonavir and rifampicin were enrolled in a prospective pharmacokinetic study. Children were switched from standard-of-care lopinavir/ritonavir-4:1 with additional ritonavir (1:1 ratio) twice daily to 8-hourly lopinavir/ritonavir-4:1 using weight-banded dosing. Rifampicin was dosed at 10–20 mg/kg/day. After 2 weeks, plasma samples were collected ∼2, 4, 6, 8 and 10 h after the morning lopinavir/ritonavir-4:1 dose, ALT was obtained to assess safety and treatment was switched back to standard of care. ClinicalTrials.gov registration number: NCT01637558.
Results
We recruited 11 children in two weight bands: 5 (45%) were 10–13.9 kg and received 20–24 mg/kg/dose of lopinavir and 6 (55%) children weighed 6–9.9 kg and received 20–23 mg/kg/dose of lopinavir. The median age was 15 months (IQR = 12.6–28.8 months). The median (IQR) lopinavir Cmin was 3.0 (0.1–5.5) mg/L. Seven (63.6%) of the 11 children had Cmin values ≥1 mg/L. Children with a lopinavir mg/kg dose below the median 21.5 were more likely to have Cmin <1 mg/L (P = 0.02). There was a strong positive correlation between lopinavir and ritonavir concentrations. No associations were found between lopinavir AUC2–10 and age, sex, weight, nutritional status or mg/kg/dose of lopinavir.
Conclusions
These data do not support the use of 8-hourly lopinavir/ritonavir at studied doses. Evaluation of higher doses is needed to optimize treatment outcomes of TB and HIV in young children.
Introduction
Lopinavir co-formulated with ritonavir in a 4:1 ratio (lopinavir/ritonavir-4:1) in combination with two NRTIs is recommended by the WHO for children younger than 3 years initiating ART and is also a second-line option for children who fail initial therapy with an NNRTI-based regimen.1 Concomitant rifampicin reduces lopinavir concentrations by 90% in adults on standard doses of lopinavir/ritonavir-4:1.2 Doubling the lopinavir/ritonavir-4:1 dose achieves adequate lopinavir concentrations in adults.2,3 In children, doubling the dose fails in 60% of cases to achieve lopinavir Cmin ≥1 mg/L,4 the lower limit of the recommended range for lopinavir trough concentrations.5 Super-boosting with additional ritonavir to a 1:1 ratio (lopinavir/ritonavir-1:1) using weight-banded dosing achieves lopinavir exposures comparable with those in children not receiving rifampicin,6 but this strategy is logistically complex and formulations of ritonavir alone are not widely available. Pharmacokinetic modelling predicted that 8-hourly doses of lopinavir/ritonavir-4:1 at lopinavir doses of 27, 21, 20 and 18 mg/kg, respectively, for children in the 3–5.9, 6–9.9, 10–13.9 and 14–19.9 kg weight bands should achieve a Cmin of ≥1 mg/L in >95% of children receiving rifampicin.7 A dosing chart based on these data to approximate the predicted mg/kg dose for each weight band was developed and we carried out a prospective pharmacokinetic study to evaluate this novel 8-hourly dosing strategy for lopinavir/ritonavir-4:1.
Methods
Study design and participants
We performed a prospective pharmacokinetic study to evaluate lopinavir concentrations. Children at Tygerberg Hospital and Red Cross Children’s Hospital in Cape Town, South Africa, were eligible if they required a lopinavir/ritonavir-containing ART regimen along with rifampicin-containing TB treatment, were between 14 days and 12 years of age, weighed 3–19.9 kg and had an ALT <5 times the upper limit of normal.
Study intervention
Participants were switched from super-boosted lopinavir/ritonavir-1:1 twice daily dosing (South African standard of care for TB/HIV co-treatment in children) to 8-hourly lopinavir/ritonavir-4:1 dosed according to weight bands with children in the 3–5.9, 6–9.9, 10–13.9 and 14–19.9 kg weight bands receiving 24–33, 20–23, 20–24 and 18–20 mg/kg lopinavir per dose, respectively. Rifampicin (10–20 mg/kg) and isoniazid (7–15 mg/kg) were dosed according to WHO guidelines.8 Two weeks after starting the novel 8-hourly lopinavir/ritonavir-4:1, children underwent intensive sampling to evaluate plasma lopinavir and ritonavir concentrations. Parents provided the early morning lopinavir/ritonavir-4:1 dose at home and blood samples were collected at ∼2, 4, 6, 8 and 10 h after the morning dose; the next dose of lopinavir/ritonavir-4:1 was provided ∼8 h after the morning dose. ALT was obtained to assess safety. After the pharmacokinetic study, participants were switched back to standard super-boosted lopinavir/ritonavir-1:1 dosing for the remainder of their TB treatment course.
Plasma concentrations of lopinavir and ritonavir were measured using a validated protein precipitation extraction procedure followed by LC tandem MS analysis. An AB SCIEX 4000 mass spectrometer was operated at unit resolution in the multiple reaction monitoring mode, monitoring the precursor ions at m/z 629.5 and 721.5 for lopinavir and ritonavir, respectively, and the product ions at m/z 120.2 and 296.1 for lopinavir and ritonavir, respectively. Lopinavir-d8 and ritonavir-d6 stable labelled isotopes were used as internal standards. The assays were validated over the concentration ranges of 0.0195–20 mg/L for lopinavir and 0.005–5 mg/L for ritonavir.
Primary outcome
The primary outcome of this study was the proportion of children with lopinavir Cmin ≥1 mg/L when receiving the novel 8-hourly lopinavir/ritonavir-4:1 dosing during rifampicin co-treatment.
Statistical analyses
The AUC2–10 represents the 8 h AUC (from 2 h after the early morning dose to 2 h after the early afternoon dose) and was calculated via the linear trapezoidal rule. We elected to report the AUC over the measured 8 h interval because there may be diurnal variation and food effects. The Cmin values were determined by visual inspection of data. Concentrations below the lower limit of quantification (LLOQ; for lopinavir <0.02 mg/L and ritonavir <0.005 mg/L) were given a value of half LLOQ for the first occurrence and 0 mg/L for subsequent occurrences. For comparison of differences between children that achieved the lopinavir target and those that did not the Wilcoxon rank-sum test was used for non-normally distributed continuous data and Fisher’s exact test for categorical data. Spearman’s rank correlation was used to measure association between lopinavir and ritonavir concentrations. STATA version 13.1 (Stata Corporation, College Station, TX, USA) was used for all the analyses including the pharmacokinetic calculations.
Ethics
The study protocol was reviewed by the Data Safety and Monitoring Board (DSMB) and approved by the ethics committees of the Universities of Stellenbosch and Cape Town. The ClinicalTrials.gov registration number is NCT01637558. Parents or legal guardians provided written informed consent. Consent forms were available in English and local languages including Afrikaans and isiXhosa.
Results
We enrolled 11 co-treated children between 15 November 2013 and 23 May 2017. The children were young [median age = 15 months (IQR = 12.6–28.8 months)] and fairly well nourished [median weight-for-age z score = −1.5 (IQR = −2.6 to −0.6)]. Enrolled children fell into two of the four designated weight bands: 6 (55%) were 6–9.9 kg and 5 (45%) were 10–13.9 kg. Lopinavir dosage ranged from 20.3 to 22.4 (median = 21.5) mg/kg/dose every 8 h (Table 1).
Table 1.
Baseline patient demographics and pharmacokinetic outcomes of 8-hourly lopinavir/ritonavir-4:1
| All patients (N = 11) | Characteristics by adequate lopinavir Cmin ≥1 mg/L |
|||
|---|---|---|---|---|
| adequate trough achieved (N = 7; 64%) | adequate trough not achieved (N = 4; 36%) | P | ||
| Patient characteristics | ||||
| age (months), median (IQR) | 15.0 (12.6–28.8) | 19.5 (12.4–32.6) | 14.9 (13.7–21.9) | 0.85 |
| male, n (%) | 7 (64) | 5 (71) | 2 (50) | 0.47 |
| weight (kg), median (IQR) | 9.8 (8.2–10.7) | 10.2 (8.2–10.7) | 9.2 (8.2–10.9) | 0.57 |
| height (m), median (IQR) | 0.71 (0.70–0.81) | 0.71 (0.70–0.86) | 0.73 (0.70–0.81) | 0.85 |
| weight-for-age z score, median (IQR) | −1.5 (−2.6 to −0.6) | −1.5 (−2.7 to −0.6) | −1.4 (−2.2 to −0.7) | 0.78 |
| weight-for-height z score, median (IQR) | 0.1 (−0.6 to 0.8) | −0.1 (−0.6 to 0.8) | 0.4 (−0.6 to 0.8) | 0.71 |
| body surface area (m2), median (IQR) | 0.42 (0.39–0.50) | 0.42 (0.39–0.51) | 0.41 (0.39–0.47) | 0.57 |
| median CD4 (cells/mm3) (n = 9), median (IQR) | 1234 (813–1749) | 910 (434–1234) | 1764 (1192–2002) | 0.14 |
| median CD4% (%) (n = 9), median (IQR) | 13.7 (10.4–24.9) | 13.7 (13.6–24.5) | 19.1 (10.0–31.5) | 0.62 |
| viral load (log copies) (n = 9), median (IQR) | 4.2 (3.7–5.5) | 3.7 (3.3–4.0) | 5.7 (5.3–6.1) | 0.01 |
| rifampicin dose (mg/kg), median (IQR) | 13.5 (11.8–14.2) | 13.9 (11.8–14.8) | 12.9 (11.8–13.8) | 0.45 |
| lopinavir dose (mg/kg), median (IQR) | 21.5 (20.3–22.4) | 22.2 (21.5–22.6) | 20.3 (20.2–20.8) | 0.06 |
| lopinavir weight bands (kg), n (%) | ||||
| 6–9.9 | 6 (55) | 3 (43) | 3 (75) | 0.55 |
| 10–13.9 | 5 (45) | 4 (57) | 1 (25) | |
| NRTI backbone of ABC+3TC, n (%) | 11 (100) | |||
| Pharmacokinetic outcomes | ||||
| lopinavir pharmacokinetic parameters | ||||
| AUC2–10 (mg·h/L), median (IQR) | 48.7 (4.6–72.7) | 67.6 (48.7–119.1) | 2.7 (0.5–5.6) | 0.008 |
| Tmax (h), median (IQR) | 8 (2–10) | 4 (2–10) | 10 (6–10) | 0.32 |
| Cmax (mg/L), median (IQR) | 7.3 (2.0–13.4) | 10.9 (7.3- 14.5) | 1.2 (0.3–2.2) | 0.008 |
| Cmin (mg/L), median (IQR) | 3 (0.1–5.5) | 4.8 (3.0–10.6) | 0.03 (0–0.05) | 0.008 |
| Cmin <1 mg/L, n (%) | 4 (36) | |||
| ritonavir pharmacokinetic parameters | ||||
| AUC2–10 (mg·h/L) | 2.09 (0.41–3.34) | 3.01 (2.09–3.82) | 0.26 (0.06–0.47) | 0.008 |
| Tmax (h) | 10 (4–10) | 10 (4–10) | 7 (3–10) | 0.53 |
| Cmax (mg/L) | 0.39 (0.07–0.81) | 0.51 (0.39–0.81) | 0.05 (0.01–0.16) | 0.008 |
| Cmin (mg/L) | 0.11 (0.02–0.22) | 0.20 (0.11–0.23) | 0.01 (0.001–0.02) | 0.008 |
| Cmin <0.1 mg/L, n (%) | 5 (45) | 1 (14) | 4 (100) | 0.015 |
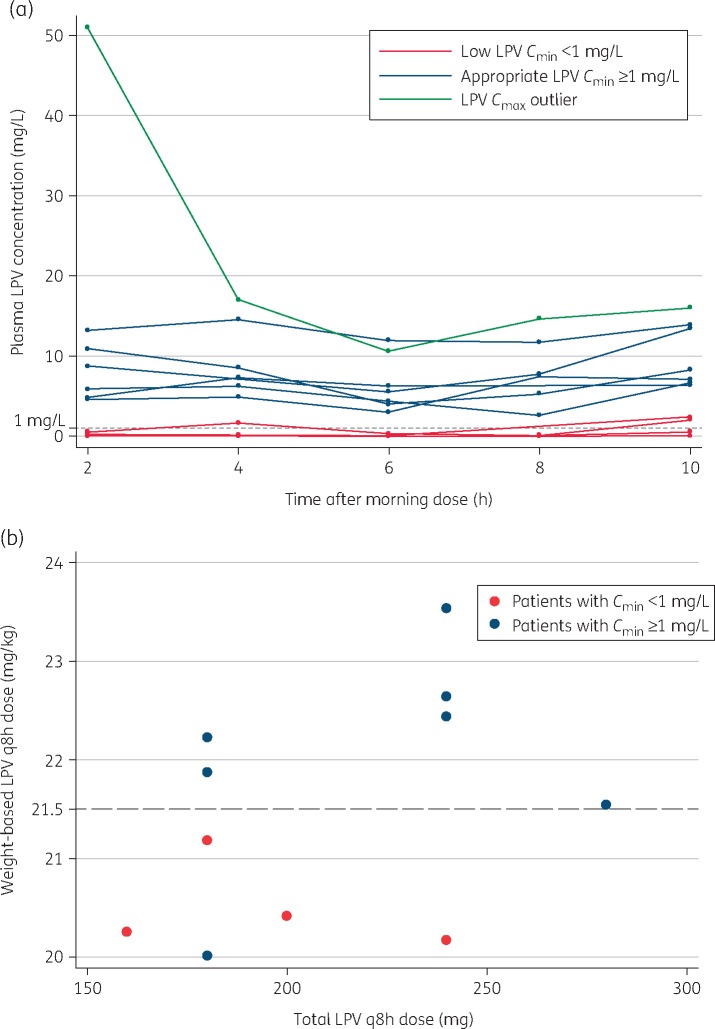
Lopinavir pharmacokinetic parameters are summarized in Table 1. The median (IQR) lopinavir Cmin was 3.0 (0.1–5.5) mg/L. Four (36%) of the 11 patients had Cmin values <1 mg/L (Figure 1a). Participants with a lopinavir mg/kg dose below the median 21.5 were more likely to have Cmin <1 mg/L (P = 0.02; Figure 1b). Three of the four participants with Cmin <1 mg/L were in the lower weight band of 6–9.9 kg, with a target lopinavir dose of 21 mg/kg/dose.
Figure 1.
(a) Individual lopinavir concentration–time curves. (b) Lopinavir mg/kg dose below median (21.5 mg/kg/dose) associated with low Cmin. LPV, lopinavir.
The median lopinavir 8 h AUC2–10 was 48.7 (4.6–72.7) mg·h/L. No associations were found between AUC2–10 and age, sex, weight, nutritional status or mg/kg/dose of lopinavir. No instances of elevated ALT developed among children receiving adjusted-dose 8-hourly lopinavir/ritonavir-4:1. Ritonavir concentrations showed a positive linear correlation with lopinavir concentrations (P < 0.0001) and patients with ritonavir concentrations <0.1 mg/L were significantly more likely to have lopinavir concentrations <1 mg/L (P < 0.001).
Discussion
In this study of a novel 8-hourly lopinavir/ritonavir-4:1 dosing strategy, 64% of children achieved adequate lopinavir concentrations ≥1 mg/L. While this is higher than what can be achieved by doubling the dose of lopinavir/ritonavir (adequate concentrations in only 40% of children),4 it is lower than the 95% of children expected to have an adequate dose as predicted by the pharmacokinetic model on which our study was based. The difference between what was predicted and observed may be due to the dose of rifampicin used in our cohort. The model utilized data from children treated with rifampicin dosed at 8–12 mg/kg/day, while children in our study received a higher rifampicin dose [median = 13.5 (IQR = 11.8–14.2) mg/kg/day] (Table 1) in accordance with updated WHO guidance, which recommends dosing rifampicin at 10–20 mg/kg/day.8 Data from in vitro studies suggest that CYP-3A4 and P-glycoprotein induction by rifampicin is concentration dependent.9 The higher rifampicin dosage used in our study compared with the model may thus have resulted in lower lopinavir concentrations in general, although children who failed to achieve the lopinavir target received a similar dose of rifampicin when compared with those that did achieve the target.
Our data also support the notion that a Cmin of ritonavir may be necessary to support lopinavir levels. The EC50 of ritonavir has previously been suggested to be in the range of 0.05–0.1 mg/L.7,10 In our study ritonavir concentrations <0.1 mg/L were associated with inadequate (<1 mg/L) lopinavir concentrations.
Though the 2018 WHO guidelines on HIV treatment are moving towards the goal of a universal integrase inhibitor-based first-line regimen for all,11 lopinavir/ritonavir is currently still the preferred first-line treatment for young children (<3 years) in low- and middle-income countries (LMICs) and for older children failing initial treatment with nevirapine or efavirenz.12,13 Children starting or switching to lopinavir/ritonavir-4:1-based ART are often at highest risk of TB. A large study of South African co-treated children found that adding ritonavir to achieve a 1:1 ratio is effective and safe.6 However it is poorly tolerated due to bitter taste and poses logistical challenges. South Africa stands out as one of the few LMICs in which single-drug ritonavir is available, but stockouts are frequent.14 Triple NRTI is not suppressive and, though used in a number of older children, there are no data in infants and young children with severe disease.1 Data from an ongoing study of raltegravir and rifampicin suggest that doubling the raltegravir dose will overcome negative interactions with rifampicin,15 but raltegravir is not widely adopted in programmes at this stage. The dolutegravir dose and appropriate formulations are still lacking for infants and very young children.11
This study was limited by the narrow range of patient weights (7.9–13.0 kg) among recruited patients, so the full range of model-predicted dosages were not evaluated. Further, the model-predicted outcomes were based on lower rifampicin dosage than used in this study. The pharmacokinetics of 8-hourly dosing was not compared with that of lopinavir/ritonavir-4:1 without rifampicin or with lopinavir/ritonavir in a 1:1 ratio with rifampicin.
Nevertheless, our data represent an important step forward as this study is the first to evaluate a novel dosing strategy that uses the widely available lopinavir/ritonavir-4:1 formulation and rifampicin dose present in the standard fixed-dose anti-TB therapy. While this dosing strategy failed to achieve adequate concentrations in 36% of patients, the strategy was safe, with no episodes of increased ALT.
Conclusions
Although 8-hourly lopinavir/ritonavir-4:1 at the currently studied doses cannot be recommended in children on rifampicin, the evaluation of alternative dosing approaches, including higher doses of liquid formulation and the new solid formulations, remains critical.
Data sharing
Data sharing will be considered after submission of a request to the senior author.
Acknowledgements
These data were previously presented at the Annual Conference on Retroviruses and Opportunistic Infections, Boston, MA, USA, 2018 (Abstract 838).
We thank all the patients, caregivers and staff from the clinical sites involved in the DATiC lopinavir substudy for their participation and the members of the data safety and monitoring board for study oversight.
Funding
This research was sponsored by the National Institutes of Health [NIH (Bethesda, MD, USA)] and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD069175). H. M. is funded by the Wellcome Trust (206379/Z/17/Z). H. Rabie consults for Aid for Aids and is part of an NIH-funded Clinical Trails Unit. The University of Cape Town, Clinical PK Laboratory is supported in part via ACTG, by the NIAID of the NIH (UM1 AI068634, UM1 AI068636 and UM1 AI106701). Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the NIAID (U01 AI068632), the Eunice Kennedy Shriver National Institute of Child Health and Human Development and National Institute of Mental Health grant AI068632.
The funders of the study had no role in study design, data collection, data analysis, data interpretation or writing of this paper.
Transparency declarations
None to declare.
Author contributions
H. Rabie recruited patients, assisted with analysis and wrote the paper, H. Rawizza analysed the data and wrote the paper, P. Z. and J. W. recruited and managed patients and assisted with data collection, H. Z. and A. V. R. assisted with the protocol and paper writing, L. W. oversaw the laboratory process and H. M. wrote the protocol, analysed the data and wrote the paper.
References
- 1.WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach—Second Edition 2016; 97. http://www.who.int/hiv/pub/arv/arv-2016/en/. [PubMed]
- 2. La Porte CJ, Colbers EP, Bertz R. et al. Pharmacokinetics of adjusted-dose lopinavir-ritonavir combined with rifampin in healthy volunteers. Antimicrob Agents Chemother 2004; 48: 1553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Decloedt EH, Maartens G, Smith P. et al. The safety, effectiveness and concentrations of adjusted lopinavir/ritonavir in HIV-infected adults on rifampicin-based antitubercular therapy. PLoS One 2012; 7: e32173.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McIlleron H, Ren Y, Nuttall J. et al. Lopinavir exposure is insufficient in children given double doses of lopinavir/ritonavir during rifampicin-based treatment for tuberculosis. Antivir Ther 2011; 16: 417–21. [DOI] [PubMed] [Google Scholar]
- 5. Higgins N, Tseng A, Sheehan NL. et al. Antiretroviral therapeutic drug monitoring in Canada: current status and recommendations for clinical practice. Can J Hosp Pharm 2009; 62: 500–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rabie H, Denti P, Lee J. et al. Lopinavir-ritonavir super-boosting in young HIV-infected children on rifampicin-based tuberculosis therapy compared with lopinavir-ritonavir without rifampicin: a pharmacokinetic modelling and clinical study. Lancet HIV 2018; doi:10.1016/S2352-3018(18)30293-5. [DOI] [PubMed] [Google Scholar]
- 7. Zhang C, McIlleron H, Ren Y. et al. Population pharmacokinetics of lopinavir and ritonavir in combination with rifampicin-based antitubercular treatment in HIV-infected children. Antivir Ther 2012; 17: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. Guidance for National Tuberculosis Programmes on the Management of Tuberculosis in Children—Second Edition 2014. http://apps.who.int/medicinedocs/documents/s21535en/s21535en.pdf. [PubMed]
- 9. Williamson B, Dooley KE, Zhang Y. et al. Induction of influx and efflux transporters and cytochrome P450 3A4 in primary human hepatocytes by rifampin, rifabutin, and rifapentine. Antimicrob Agents Chemother 2013; 57: 6366–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van der Laan LE, Garcia-Prats AJ, Schaaf HS. et al. Pharmacokinetics and drug-drug interactions of lopinavir-ritonavir administered with first- and second-line antituberculosis drugs in HIV-infected children treated for multidrug-resistant tuberculosis. Antimicrob Agents Chemother 2018; 62: e00420–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. Updated Recommendations on First-Line and Second-Line Antiretroviral Regimens and Post-Exposure Prophylaxis and Recommendations on Early Infant Diagnosis of HIV—Interim Guidance https://www.who.int/hiv/pub/guidelines/ARV2018update/en/.
- 12.WHO. Combined Global Demand Forecasts for Antiretroviral Medicines and HIV Diagnostics in Low- and Middle-Income Countries From 2015 to 2020 2016. http://www.who.int/hiv/pub/amds/arv-diagnostics-forecast-2015-2020/en/.
- 13.Clinton Health Access Initiative. ARV Market Report: The State of the Antiretroviral Drug Market in Low- and Middle-Income Countries, 2016–2021 Issue 8, 2017. https://clintonhealthaccess.org/content/uploads/2017/09/2017-ARV-Market-Report_Final.pdf.
- 14.Doctors Without Borders (MSF), the Rural Doctors Association of Southern Africa (RuDASA), the Rural Health Advocacy Project (RHAP), the Treatment Action Campaign (TAC), SECTION27 and the Southern African HIV Clinicians Society (SAHIVSoc). STOP Stockouts SSP Stockouts National Survey 2016. https://www.groundup.org.za/media/uploads/documents/StopStockoutsSurvey2016.pdf.
- 15. Meyers T, Krogstad P, Samson P. et al. P1101: phase I/II study of raltegravir containing regimen in HIV-TB cotreated children. In: Abstracts of the Annual Conference on Retroviruses and Opportunistic Infections, Boston, MA, USA,2018. Abstract 845. Foundation for Retrovirology and Human Health, Alexandria, VA, USA. http://www.croiconference.org/sessions/p1101-phaseiii-study-raltegravir-containing-regimen-hiv-tb-cotreated-children.