SUMMARY
Accumulation of viruses in vegetatively propagated plants causes heavy yield losses. Therefore, supply of virus‐free planting materials is pivotal to sustainable crop production. In previous studies, Raspberry bushy dwarf virus (RBDV) was difficult to eradicate from raspberry (Rubus idaeus) using the conventional means of meristem tip culture. As shown in the present study, it was probably because this pollen‐transmitted virus efficiently invades leaf primordia and all meristematic tissues except the least differentiated cells of the apical dome. Subjecting plants to thermotherapy prior to meristem tip culture heavily reduced viral RNA2, RNA3 and the coat protein in the shoot tips, but no virus‐free plants were obtained. Therefore, a novel method including thermotherapy followed by cryotherapy was developed for efficient virus eradication. Heat treatment caused subcellular alterations such as enlargement of vacuoles in the more developed, virus‐infected cells, which were largely eliminated following subsequent cryotherapy. Using this protocol, 20–36% of the treated shoot tips survived, 30–40% regenerated and up to 35% of the regenerated plants were virus‐free, as tested by ELISA and reverse transcription loop‐mediated isothermal amplification. Novel cellular and molecular insights into RBDV–host interactions and the factors influencing virus eradication were obtained, including invasion of shoot tips and meristematic tissues by RBDV, enhanced viral RNA degradation and increased sensitivity to freezing caused by thermotherapy, and subcellular changes and subsequent death of cells caused by cryotherapy. This novel procedure should be helpful with many virus–host combinations in which virus eradication by conventional means has proven difficult.
INTRODUCTION
Viral diseases constitute a major constraint to production of high yields with good quality in all major crops worldwide. Viruses are particularly problematic in vegetatively propagated crops in which they are transmitted from generation to generation in the planting materials (Agrios, 2005; Loebenstein and Thottapilly, 2003; Waterworth and Hadidi, 1998). Therefore, production and maintenance of virus‐free planting materials is pivotal for the control of viral diseases (Bhojwani and Razdan, 1996; Faccioli and Marani, 1998; Mink et al., 1998).
The main stages of method development for healthy plant production in the past can be briefly summarized as follows. Therapeutic treatments were tested since the 1920s to cure valuable cultivars from virus‐like diseases. Heat treatment (thermotherapy) at elevated temperatures (34–42 °C) was used to produce healthy plants from individuals suffering from diseases such as potato witches’ broom and aster yellows caused by phytoplasma (Kunkel, 1943). Kassanis (1950) applied thermotherapy on potato tubers and obtained plants free of Potato leaf roll virus (genus Polerovirus, Luteoviridae) but not Potato virus X (PVX; genus Potexvirus, Flexiviridae). Holmes (1948) observed that viruses were unevenly distributed in dahlia plants that could be ‘freed of virus by radical removal of diseased tissues, i.e, old roots, stems and lower leaves’. This was accomplished by taking tip cuttings from shoots and rooting them. Subsequently, ‘meristem‐tip culture’ with or without preceding thermotherapy of plants was adopted as a means to produce virus‐free plants because it was anticipated that meristems would be virus‐free owing to the uneven distribution of viruses in plants (reviewed by Quak, 1987). According to Quak (1987), meristem is a dome of actively dividing cells, on average c. 0.1 mm in diameter and c. 0.25 mm long. Excising and regenerating such small meristems is difficult in vitro and larger ‘meristem tips’ of c. 1.0 mm were usually excised, although this practice was found to reduce the chances of obtaining virus‐free plants. Indeed, meristems were found to contain particles of many viruses (Appiano and Pennazio, 1972; Roberts et al., 1970; Walkey and Webb, 1968) and, again, the procedure was revised. Infected plants were introduced to tissue culture, heat‐treated in vitro, and subjected to ‘meristem‐tip culture’, which resulted in markedly enhanced virus eradication (Cooper and Walkey, 1978; Walkey and Cooper, 1975). These conventional methods of virus eradication are widely used. Recently, cryotherapy of shoot tips, i.e. freezing in liquid nitrogen and subsequent thawing and regeneration to shoots, was found to result in virus‐free plants with a higher efficiency than meristem tip culture (Brison et al., 1997; Helliot et al., 2002; Wang et al., 2003, 2006).
Raspberry bushy dwarf virus (RBDV) is one of the viruses commonly infecting raspberries (Rubus idaeus L.) (Barbara et al., 2001). It is the only member of the genus Idaeovirus with no assigned family (Fauquet et al., 2005). The viral genome consists of two single‐stranded RNA molecules (RNA1 and RNA2). A third RNA (RNA3) corresponding to the 3′‐proximal part of RNA2 is a subgenomic RNA expressed and encapsidated but not replicated in infected cells (Mayo et al., 1991; Natsuaki et al., 1991; Ziegler et al., 1992). RNA1 encodes a 188‐kDa protein that contains motifs characteristic of helicases and polymerases (Ziegler et al., 1992), whereas RNA2 and RNA3 encode the coat protein (Mayo et al., 1991; Natsuaki et al., 1991). RBDV is efficiently transmitted via seed and pollen. No other modes of natural transmission are known. Infected, wild and cultivated raspberries act as virus reservoirs for re‐infection of the virus‐free plants in the field (Murant et al., 1974).
The aim of the present study was to develop a new virus eradication procedure for production of RBDV‐free raspberry plants. It was needed because the conventional methods of thermotherapy and meristem tip culture have not provided satisfactory, predictable and reproducible results. Only a few RBDV‐free plants have been occasionally obtained after repeated meristem tip culture (Lankes, 1995; Karesova et al., 2002; Theiler‐Hedtrich & Baumann, 1989). Furthermore, commercialization of a recently developed Finnish raspberry breeding line Z13 has not been possible because no RBDV‐free plants could be obtained (J. Laamanen and M. Uosukainen, personal communication). Therefore the aim was to test whether combined thermotherapy and cryotherapy could be advantageous for obtaining virus‐free plants, as compared with the traditional methods. The aim was also to understand better the mechanisms behind virus eradication. Therefore, studies were carried out to relate virus distribution in the shoot tips, subcellular changes caused by thermotherapy and cryotherapy, effects of thermotherapy on virus titres at protein and RNA levels, and survival of cells following cryotherapy. As a result, a novel procedure for efficient virus eradication and healthy plant production was developed, which also allows simultaneous preparation of the materials for long‐term preservation.
RESULTS
No RBDV‐free plants with meristem culture and thermotherapy
Initially, different sizes of shoot tips were excised and cultured in an attempt to obtain RBDV‐free plants of the raspberry clone Z13, which is an elite breeding line meant for commercial production. The smallest shoot tip that we were able to excise mechanically was 0.1 mm in length and included the first (youngest) leaf primordium (LP1). Larger shoot tips of 0.2 and 0.3 mm with two leaf primordia (LP1 and LP2) are commonly used for virus elimination in various plant species (Faccioli and Marani, 1998) and were included for comparison. Testing a total of 180 (0.1 and 0.2 mm each) or 90 (0.3 mm) shoot tips in three experiments revealed a positive correlation between the shoot tip size and plant regeneration. Nearly all (95%) of the 0.3‐mm shoot tips survived and regenerated to shoots, whereas survival was 40% and regrowth of the survived shoot tips was 65% with the 0.2‐mm shoot tips, and 25 and 40% with the 0.1‐mm shoot tips, respectively. Differences in survival and regrowth between the size classes were significant (P < 0.05, Student's t‐test). However, all regenerated shoots were RBDV‐infected, as shown by double‐antibody sandwich enzyme‐liked immunosorbent assay (DAS‐ELISA).
Thermotherapy of stock plants prior to meristem culture is often used to enhance virus elimination (Mink et al., 1998). Raspberry shoot cultures grown in vitro were subjected to a heat treatment under a regime of 16 h at 38 °C in the light and 8 h at 26 °C in the dark for 21, 28, 35 or 42 days, each treatment period including 40 shoots. In three experiments, survival of the shoot cultures was greatly decreased from 62 to 6% by extension of heat treatment from 21 to 42 days, respectively. Following thermotherapy, shoot tips (0.2 mm, with LP1 and LP2) were excised from the surviving shoots and cultured in vitro for survival and regeneration. Results showed that survival of the shoot tips was reduced by the extended periods of heat treatment received by the shoots. While 95% of the shoot tips survived and 90% regenerated following heat treatment for 21 days, only 32% survived and 38% of these regenerated following heat treatment for 42 days. The differences in survival and regrowth between the heat treatment periods were significant (P < 0.05, Student's t‐test). However, all regenerated shoots were RBDV‐infected, as tested by DAS‐ELISA at the age of 4 weeks.
Distribution of RBDV in the shoot tip
In order to understand why the conventional means of virus elimination described above failed to produce RBDV‐free plants, localization of RBDV in shoot tips was carried out. Immunostaining of sections with antibodies to RBDV coat protein (CP) revealed strong signals for the virus in almost all tissues, including leaf primordia (LP1 and LP2) and the basal part of the meristem in the non‐heat‐treated shoot tips (Fig. 1A). Only the least differentiated cells in the apical dome (AD) of the meristem contained no visible virus signals (Fig. 1A), indicating that these cells contained virus titres below the detection threshold or were virus‐free.
Figure 1.
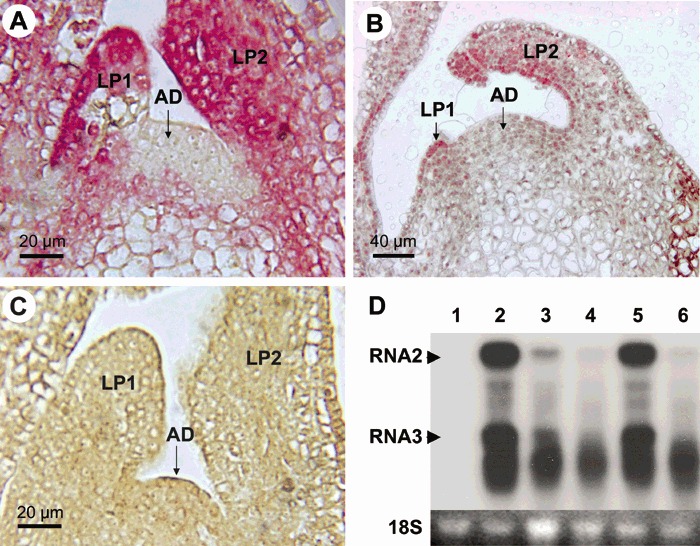
Immunolocalization of Raspberry bushy dwarf virus (RBDV) in raspberry shoot tips (Rubus idaeus, line Z13) (A) before and (B) 28 days after thermotherapy using antibodies to the viral coat protein (CP) antigen. Purple staining indicates cells and tissues containing the viral antigen. Note that the shoot tip and cells in B are approximately twice as large as those in A due to the heat treatment. (C) A shoot tip of a virus‐free in vitro plantlet of raspberry genotype TTA‐508. AD, apical dome; LP1, the first (youngest) leaf primordium; LP2, the second leaf primordium. (D) Detection of the viral RNA in shoot tips grown in vitro before and after thermotherapy by Northern blot analysis using the viral CP gene as a probe. The positions of viral RNA2 (2.2 kb) and RNA3 (0.9 kb) which corresponds to the 3′‐proximal part of RNA2 are indicated. RNA in lane 2 was extracted from shoot tips at the onset of thermotherapy and the RNA in lanes 3 and 4 after 5 and 8 days of thermotherapy, respectively. RNA in lane 5 was extracted from leaves before thermotherapy and in lane 6 after 5 days of thermotherapy. Lane 1, healthy control. The 18S RNA is shown at the bottom to control equal loading of RNA.
The suppressing effect of heat treatment on virus titres was readily observed in the heat‐treated shoot tips. Northern blot analysis using a probe to the CP gene showed that the amounts of viral genomic RNA2 were readily detectable in the non‐treated shoot tips (Fig. 1D, lane 2) but rapidly decreased and were barely detectable in the shoot tips (Fig. 1D, lane 3) and leaves (Fig. 1D, lane 6) following 5 days of heat treatment. Similarly, amounts of RNA3, a subgenomic RNA expressed from the 3′‐proximal part of RNA2 (Mayo et al., 1991; Natsuaki et al., 1991), were dramatically decreased and only degradation products of the viral RNA were detectable following 8 days of thermotherapy (Fig. 1D). However, the virus was not fully eliminated. Low titres of the viral CP antigen, as judged based on the weak signals, were still detected in most of the new tissues 28 days after thermotherapy (Fig. 1B). While signal intensities differed greatly, the distribution of virus signals was not significantly different between the non‐treated (Fig. 1A) and heat‐treated shoot tips (Fig. 1B). For example, LP1 and LP2 still contained an abundance of virus‐infected cells after 28 days of thermotherapy, as they did in the non‐treated shoot tips. As the smallest shoot tips (0.1 mm) that could be excised for meristem culture contained LP1, the results of virus localization largely explained why no RBDV‐free regenerants were obtained.
Combined thermotherapy and cryotherapy results in RBDV elimination
Cryotherapy of shoot tips can result in virus‐free plants at a high frequency (Brison et al., 1997; Helliot et al., 2002; Wang et al., 2003, 2006). In banana, only the cells in the AD of the meristem and the youngest cells of leaf primordia survive freezing in liquid nitrogen, which increases the chances for virus elimination (Helliot et al., 2002, 2003). The RBDV‐infected shoot tips were subjected to cryotherapy by the encapsulation‐vitrification method according to a protocol previously described for raspberry (Wang et al., 2005). A total of 120 shoot tips (1 mm) were excised and treated with cryotherapy. Most of them survived (85%) and regenerated (78%). However, all regenerants remained RBDV‐infected, as tested by DAS‐ELISA (Table 1).
Table 1.
Effects of different periods of thermotherapy applied on shoots in vitro on the survival, regrowth and virus‐free status of shoot tips excised from the treated shoots and subjected to cryotherapy.*
| Heat treatment of shoots (days) | Survival of cryo‐treated shoot tips (%) | Regrowth of survived shoot tips (%) | RBDV‐negative regenerants (%)† |
|---|---|---|---|
| 0 | 85a | 78a | 0 |
| 21 | 48b | 60b | 0 |
| 28 | 36c | 40c | 33 |
| 35 | 20d | 30d | 35 |
| 42 | 0 | 0 | 0 |
Means of two experiments, each including three replicates of 60 shoot tips. Survival and regrowth were recorded at 2 and 6 weeks of post‐culture, respectively. Figures marked with different letters in each column are significantly different (P < 0.05, Student's t‐test).
Leaf samples were taken from in vitro plantlets 2 months after regeneration and tested for Raspberry bushy dwarf virus (RBDV) using DAS‐ELISA and RT‐LAMP.
Subsequently, both thermotherapy followed by cryotherapy was used, which has not been reported in previous studies. In vitro shoot cultures of RBDV‐infected raspberry were heat‐treated for 21–42 days, and shoot tips (1 mm) were excised, subjected to cryotherapy and regenerated, as before. It was initially observed that following these combined therapies, the regenerated plantlets post‐cultured on basic medium (see Experimental procedures) suffered from chlorosis and many of them died following a few successive rounds of subculture (data not shown). This problem was circumvented by addition of 50 mg/L ferric ethylenediamino‐tetraacetate (Fe‐EDTA) to the post‐culture medium. Subsequently, experiments were continued to test virus elimination. It was found that prolonged duration of thermotherapy applied on the stock shoots significantly reduced the survival and regrowth of the cryo‐treated shoot tips (Table 1). After 21 days of heat treatment, 48% of shoot tips withstood freezing in liquid nitrogen, as compared with 36 and 20% following 28 and 35 days of heat treatment, respectively. The differences were significant (Table 1). Similarly, regrowth of the shoot tips after cryotherapy declined from 60 to 30% by the prolonged exposure of shoots to thermotherapy (Table 1). However, importantly, the prolonged thermotherapy followed by cryotherapy resulted in RBDV‐free regenerants with a frequency of 33–35% (Table 1). Thermotherapy could not be further extended to increase the proportion of RBDV‐free regenevants because no shoot tip survived cryotherapy if the stock shoots had been heat‐treated for up to 42 days.
Indexing of plants for RBDV using RT‐LAMP
Virus testing initially relied on DAS‐ELISA. RBDV was tested in the leaves of regenerated in vitro‐grown plantlets 2 months after cryotherapy. The virus‐negative plants were transferred to soil, grown in the greenhouse for 2 months and tested for RBDV again. The results confirmed that the plantlets which tested virus‐negative in vitro were indeed free of RBDV.
However, to obtain an efficient and sensitive diagnostic method that explicitly and specifically detects viral RNA, we employed the loop‐mediated isothermal amplification (LAMP) method (Mori et al., 2001; Nagamine et al., 2002) for detection of RBDV. LAMP is a recently described method for isothermal, linear DNA amplification that, in addition to other applications, can also be used for detection of plant viruses (Fukuta et al., 2003a,2003b, 2004; Nie, 2005). The method is advertized to possess many advantages, including higher target specificity than RT‐PCR due to the use of four primers that recognize six distinct regions in the template DNA (Fukuta et al., 2004). Furthermore, the method amplifies nucleic acids under isothermal conditions (65 °C) and, hence, no thermal cycler is needed. Finally, formation of turbidity due to a white precipitate of magnesium pyrophosphate indicates successful target amplification, which eliminates the need for detecting amplification products by gel electrophoresis.
To design primers for LAMP, the CP gene of the RBDV isolate from the raspberry line Z13 was amplified by RT‐PCR, cloned and sequenced. Two nearly identical clones were detected (deposited in the NCBI sequence database under accession numbers AY894678 and AY894679). They were 91–99% identical to the seven RBDV CP gene sequences available in the databases. The primers for LAMP were designed to detect the isolate of this study but also those described by Jones et al. (2000).
Detection of RBDV in the plants via DAS‐ELISA, RT‐PCR (Fig. 2A) and LAMP (Fig. 2B) was fully consistent. Positive results of LAMP were revealed within 1 h by an apparent turbidity of the contents in the PCR tube (Fig. 2B). However, in samples with low viral RNA concentration, DNA amplification was not always sufficient to be detected by visual assessment of turbidity. To some extent, formation of the precipitate could be enhanced by incubating the tubes on ice after LAMP. In a few samples, the LAMP amplification products were also analysed by agarose gel electrophoresis to verify that turbidity correlated with the accumulation of amplification products (Fig. 2C).
Figure 2.
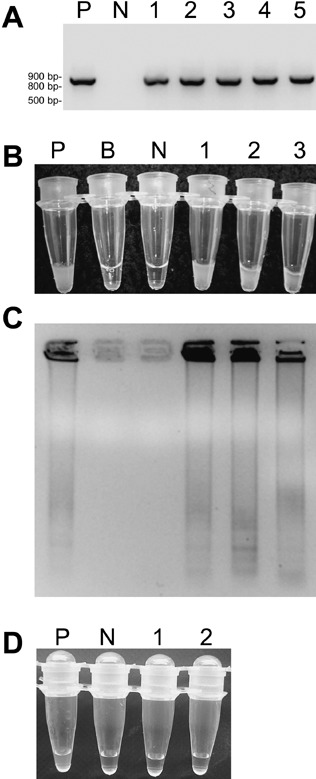
Detection of Raspberry bushy dwarf virus (RBDV) by (A) reverse transcription polymerase chain reaction (RT‐PCR) and (B–D) reverse transcription loop‐mediated isothermal amplification (RT‐LAMP). Primers were designed according to the coat protein (CP) gene of RBDV. (A) RT‐PCR: lane P, positive control (cloned RBDV CP gene); lane N, negative control (virus‐free raspberry clone TTA‐508); lanes 1–5, RBDV‐infected plants of raspberry line Z13. (B) Amplification of RBDV cDNA detected by turbidity caused by the magnesium pyrophosphate precipitate. P and N as above; B, reaction without template; 1–3, RBDV‐infected plantlets of line Z13. (C) The products of RT‐LAMP obtained in B analysed by agarose gel electrophoresis. The samples are in the same order as in the test tubes in B above. (D) Indexing of raspberry plants for RBDV by RT‐LAMP: P and N as above; 1 and 2, RBDV‐free plantlets of line Z13 obtained by thermotherapy followed by cryotherapy.
To enhance the virus detection procedure further, cDNA synthesis and LAMP were combined by adding the reverse transcriptase and DNA polymerase at the same time, omitting the primer used for cDNA synthesis. Hence, the time needed for testing the extracted RNA samples for RBDV was reduced from a total of 2.5 to 1.5 h. However, with this modified protocol, DNA amplification was not sufficient for visual detection of turbidity in some samples, probably due to low amounts of virus. Hence, the modified protocol was not used for routine testing.
Histological and subcellular changes in the heat‐treated tissues
As thermotherapy preceding cryotherapy significantly enhanced production of RBDV‐free plants, histological studies were carried out to compare the heat‐treated and non‐treated shoot tips for possible signs of changes associated with impaired stress tolerance and survival. It was hypothesized that heat stress may render the cells less tolerant to cryotherapy, which would restrict the number of surviving cells and thereby enhance elimination of virus‐infected cells.
The cells in non‐treated shoot tips were uniformly stained in meristem and leaf primordia (LP1 and LP2) (Fig. 3A). These well‐packed cells with well‐preserved cytoplasm contained nucleoli that were densely stained with safranin (Fig. 3A), which indicates that cells were in a good physiological condition (Chang and Reed, 1999; Helliot et al., 2002, 2003; Wilkinson et al., 2003). In contrast, staining of cells was weaker and the histological structure of tissues was looser in leaf primordia and the basal part of the meristem in the heat‐treated shoot tips (Fig. 3B). In the heat‐treated tissues, densely stained nucleoli were observed in the AD of meristem but only a few were seen in the cells of LP1 and LP2 (Fig. 3B). Hence, the heat‐treated tissues showed signs of stress and damage.
Figure 3.
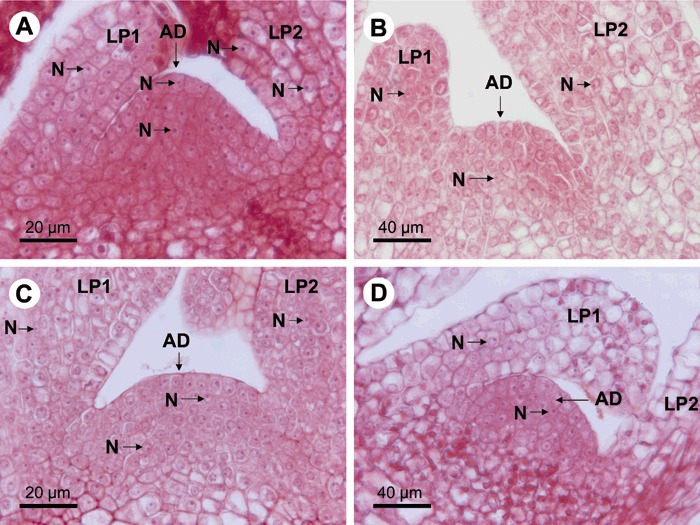
Longitudinal sections of shoot tips of raspberry (line Z13) stained with safranin and observed by light microscopy. Surviving cells were recognized by densely stained nucleoli (N, a few examples indicated) in nuclei located in well‐preserved and stained cytoplasm. (A) Untreated shoot tip. (B) Shoot tip following 28 days of thermotherapy. (C) Untreated shoot tip after cryotherapy and 1 day of post‐culture. (D) Heat‐treated (28 days) shoot tip after cryotherapy and 1 day of post‐culture. Note that the shoot tip and cells in B and D are approximately twice as large as those in A and C due to the heat treatment. Views including the meristem, the first (youngest) (LP1) and the second leaf primordium (LP2) are shown. AD, apical dome of the meristem; N, nucleolus.
Following cryotherapy, surviving cells were observed especially in the AD and also LP1 and LP2 (Fig. 3C) in shoot tips from non‐heat‐treated shoots. As compared with localization of RBDV in shoot tips (Fig. 1A), it was apparent that cryotherapy did not eliminate all virus‐infected cells. However, when the tips from heat‐treated shoots were subjected to cryotherapy, just a few densely stained nucleoli were observed in any other tissues but the AD of the meristem, which had maintained its normal histological structure (Fig. 2D). This part of the meristem (Fig. 2D) corresponded well to the area that contained no virus signals (Fig. 1A,B).
Transmission electron microscopy (TEM) was used to obtain a closer view of the subcellular changes associated with thermotherapy, especially in the AD, which survived the subsequent cryotherapy, and the basal part of LP1, which seemed to be at the border zone of surviving and dying cells. In non‐treated shoot tips, the cells of AD (Fig. 4A) and the basal part of LP1 (Fig. 4B) contained dense cytoplasm, a large nucleo‐cytoplasm ratio, small vacuoles and an abundance of mitochondria, which is characteristic of metabolically active cells. In heat‐treated shoot tips, cells in the AD (Fig. 4C) and especially in LP1 (Fig. 4D) were significantly enlarged (c. two‐fold; Table 2). As the nuclei were also enlarged, the nucleo‐cytoplasmic ratio was not altered significantly (Table 2). The cells of LP1 contained markedly enlarged vacuoles (Fig. 4D; Table 2). In the lower part of the shoot tip and older leaf primordia, the structures of the nucleus and nucleolus were disrupted and cells were affected by plasmolysis (data not shown). Hence, the cells of LP1 at the border zone of cell survival (as observed following cryotherapy) showed more pronounced alterations than those in the meristem that survived cryotherapy.
Figure 4.
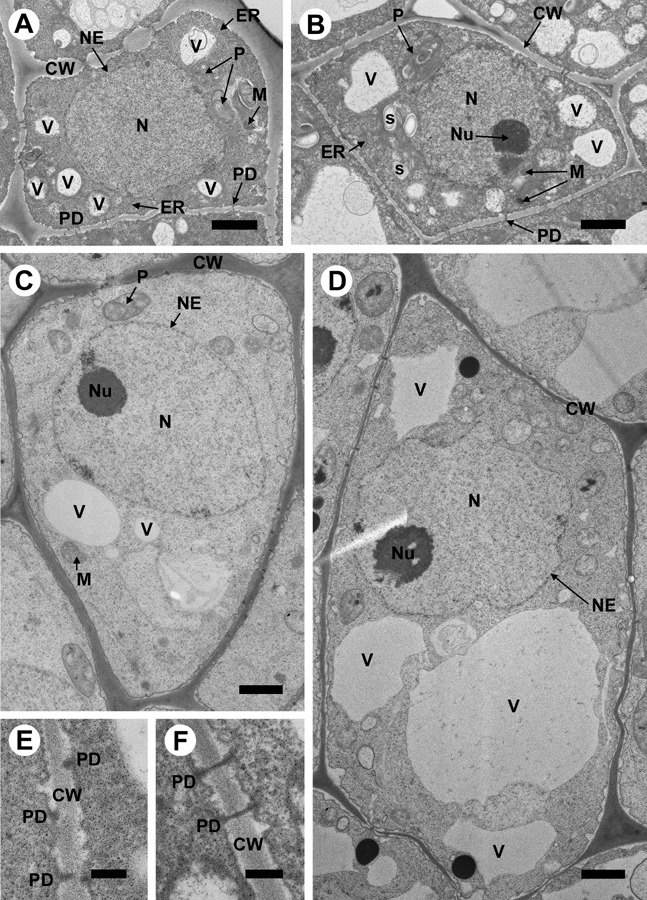
Observation by transmission electron microscopy of subcellular changes in the second layer of cells in the apical dome and the base of the first (youngest) leaf primordium (LP1) of raspberry (line Z13) following 28 days of thermotherapy. (A,B) Untreated cells in the apical dome of meristem and in LP1, respectively. (C,D) Enlarged cells in the apical dome of meristem and in LP1, respectively, following thermotherapy. Scale bars = 1 µm. The sizes of the cells shown are directly proportional to the true differences in their sizes before and after thermotherapy. The most pronounced subcellular alterations observed in the heat‐treated tissues included enlarged vacuoles and the proportion of the cell volume occupied by them, especially in LP1. (E,F) Close‐up of plasmodesmata detected in the cell walls in the apical dome. Scale bars = 200 nm. CW, cell wall; ER, endoplasmic reticulum; M, mitochondrion; N, nucleus; NE, nuclear envelope; Nu, nucleolus; P, plastid; PD, plasmodesm; S, starch grain; V, vacuole.
Table 2.
Size of the cells, nuclei and vacuoles in different parts of the youngest portion of the raspberry shoot tip before and 28 days after heat treatment (thermotherapy).
| Apical dome | Leaf primordium 1 | |||
|---|---|---|---|---|
| Non‐treated*, ‡ | Heat‐treated*, § | Non‐treated†, ‡ | Heat‐treated†, § | |
| Cell size (µm2) | 21.3a | 45.5b | 23.5a | 55.8c |
| Nucleus size (µm2) | 8.7a | 15.3b | 8.8a | 13.4b |
| Nucleo‐cytoplasmic ratio | 2.5a | 3.0a | 2.7a | 4.2a |
| Vacuole size (µm2): | ||||
| Range | 0.2–0.5 | 0.2–2.1 | 0.2–1.4 | 0.5–8.4 |
| Mean | 0.4a | 0.6a | 0.5a | 1.2b |
| Total area¶ | 1.9a | 2.7a | 2.0a | 12.7b |
Numbers are means from measurement of 20 cells in each treatment, unless otherwise stated. Means in the same row with different letters are significantly different (P < 0.05; Student's t‐test).
Cells in the uppermost and second top layers of the apical dome were measured.
Cells at the base of the first leaf primordium were measured.
Measurement was carried out before thermotherapy.
Measurement was carried out 28 days after thermotherapy.
Total area of the cell occupied by vacuoles.
Non‐branched plasmodesmata were typically observed to cross the cell walls in the basal part of LP1 (Fig. 4B,F) but there some were also seen in the AD of the meristem (Fig. 4A). In the AD close to tunica where cell walls were not yet fully developed (Fig. 4A,E) only a few non‐branched plasmodesmata reaching through the cell wall were observed (Fig. 4A,C). Indeed, these tissues of the AD contained mostly incomplete‐looking plasmodesmata (Fig. 4E). It is not known whether they were an artefact due to the angle at which the section was cut, but it is noteworthy that they were proportionally more frequent than the non‐branched, cell‐wall‐crossing plasmodesmata in the AD, whereas the reverse was observed in LP1. These differences were consistently found in independent samples.
DISCUSSION
The results of the present study represent a technical advance in the production of virus‐free plants. The procedure developed in this study and outlined in Fig. 5 allows efficient elimination of RBDV, a pollen‐transmitted and seed‐borne virus (Murant et al., 1974) that represents the type of virus that is considered the most challenging for eradication as they are presumed to infect meristems efficiently. Thermotherapy followed by cryotherapy resulted, reproducibly, in production of RBDV‐free plants from c. 5% of the treated raspberry shoot tips in this study. This is significant because the traditional methods, including thermotherapy, used in previous studies (Karesova et al., 2002; Lankes, 1995; Theiler‐Hedtrich and Baumann, 1989) did not result in any RBDV‐free plantlet after the initial meristem‐tip culture. Cryotherapy takes only a few days, which is a minor addition to the whole procedure of virus elimination, which occupies several months (Fig. 5). While demanding in terms of combining and optimizing the different tissue culture procedures, the general procedure of thermotherapy followed by cryotherapy will be applicable and should be helpful with many virus–host combinations in which virus eradication has proven difficult.
Figure 5.
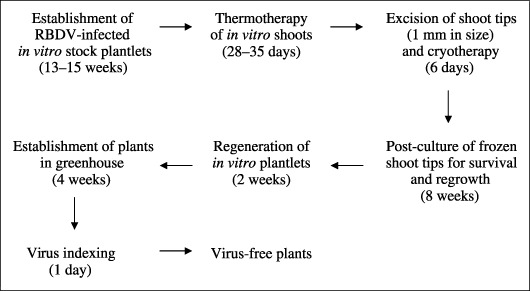
A flow chart of the production of RBDV‐free raspberry plants using thermotherapy followed by cryotherapy of shoot tips.
A few previous studies have shown that cryotherapy of shoot tips (Brison et al., 1997; Wang et al., 2003, 2006) or meristematic clumps (Helliot et al., 2002) results in virus‐free plants with a higher frequency than meristem tip culture. However, the viruses in these previous studies can also be eliminated with relative ease using the traditional meristem tip culture. There is little previous information about the mechanism by which cryotherapy enhances virus eradication from shoot tips. Helliot et al. (2002, 2003) carried out histological studies on cryo‐treated meristematic clumps of banana and found that only cells located in the meristematic dome and at the base of the leaf primordia survived cryotherapy by vitrification. It was presumed that cryotherapy was lethal to the more developed, virus‐infected cells, whereas the younger surviving cells were mostly not infected with Cucumber mosaic virus (CMV; genus Cucumovirus, Comoviridae) and Banana streak virus (genus Badnavirus, Caulimoviridae) because 30 and 90% of the shoots regenerated following cryotherapy were free of these viruses, respectively (Helliot et al., 2002). However, virus localization was not carried out in the banana shoot tips subjected to cryotherapy. In the present study, comparison of data from virus immunolocalization and histological analysis indicated that in the shoot tips subjected to cryotherapy, large areas of tissue infected with RBDV survived the cryo‐treatment. These results were consistent with all regenerants being RBDV‐infected.
Up to 47% of the seedlings grown from seeds of an RBDV‐infected raspberry plant can be RBDV‐infected and their proportion can increase to 77% if pollination is made with an RBDV‐infected raspberry (Murant et al., 1974). These data suggest that RBDV invades meristems efficiently, which is supported by the present data. While flower organs were not studied, immunolocalization of RBDV in raspberry shoot tips revealed the virus in nearly all parts of the meristem, including the lower part of the AD and the first leaf primordium. The data are significant because RBDV is the only member of the genus Idaeovirus (Fauquet et al., 2005) and this virus has not been previously localized in infected plant tissues. These data also contribute to the scarce information available on the overall distribution of virus‐infected cells in the meristematic tissues and first leaf primordia, as pollen‐transmitted and most other viruses are concerned. Recently, the seed‐borne and pollen‐transmitted Prunus necrotic ringspot virus (genus Ilarvirus, family Bromoviridae) was localized in the flower buds and anthers of apricot (Prunus armenica) (Amari et al., 2007). Particles of Cherry leaf roll virus (CLRV) (Walkey and Webb, 1968) and Tobacco ring spot virus (Roberts et al., 1970), both members of the genus Nepovirus (family Comoviridae), have been detected in meristematic cells of Nicotiana spp. by electron microscopy. The meristems are also a source of infectious CLRV (Walkey and Webb, 1968).
Only the non‐differentiated youngest cells of the AD contained no detectable amounts of RBDV. As seen in Fig. 1A, the group of cells free of virus signals was contrasted by the strong signals in the neighbouring, more developed cells. In those tissues where the virus was not detected, only few non‐branched plasmodesmata crossing the cell walls could be observed, whereas these types of plasmodesmata were frequently observed in the virus‐infected tissues such as at the base of LP1. These patterns suggest that virus distribution may be associated with the development of plasmodesmata and their ability to support virus movement. Plasmodesmata regulate cell‐to‐cell communication and, as widely accepted, serve for cell‐to‐cell movement of viruses (Ding, 1998; Lucas, 2006; Oparka et al., 1999). It is hypothesized that immunolocalization of RBDV in the meristem of raspberry pinpointed a cellular boundary across which RBDV cannot move, something that might resemble division of the embryo into four symplasmic subdomains with boundaries of distinct plasmodesmal size exclusion limit (Kim et al., 2005; Kim and Zambryski, 2005).
Thermotherapy followed by cryotherapy, in contrast to cryotherapy alone, resulted in RBDV‐free regenerants from the raspberry line Z13. This finding raised the question of the mechanism by which thermotherapy enhanced eradication of RBDV. Early studies indicated that thermotherapy inhibits viral replication while virus degradation continues, which results in subsequent elimination of the virus from shoot tips (Cooper and Walkey, 1978; Kassanis, 1957). Consistent with this model, thermotherapy was not effective when applied on dormant, virus‐infected tissues (Cooper and Walkey, 1978). Since these studies, the mechanism of virus elimination from shoot tip cultures by thermotherapy has gained limited attention (Bhojwani and Razdan, 1996). In the present study, northern blot analysis revealed a drastic decrease in the amounts of RBDV RNA in shoot tips and leaves following 5 days of thermotherapy, and following 8 days the amounts of viral RNA were barely detectable. The plants did not have time to grow significantly in 8 days and the tissues sampled at different time points were essentially the same developmentally. Hence, the results indicated that viral RNA was degraded in leaves and shoot tips during thermotherapy. These data may be explained by enhanced RNA silencing, the cellular mechanism devoted to targeting and degradation of viral RNA in plants, at elevated temperatures. In previous studies, the efficiency of virus‐induced RNA silencing was compared at temperatures of 15–27 °C (Szittya et al., 2003), 21 or 27 °C (Qu et al., 2005) and 25–30 °C (Chellappan et al., 2005) and found to be significantly enhanced at the higher temperatures. The raspberry plants of this study were kept at higher temperatures (26–38 °C) than most plants of the aforementioned studies. Because the temperatures were still physiologically suitable for raspberry it seems conceivable that the conditions allowed efficient targeting of RBDV RNA by the silencing mechanism. RNA silencing has also been implicated in exclusion of viruses from meristems (Foster et al., 2002; Mochizuki and Ohki, 2004; Qu et al., 2005; Schwach et al., 2005). Isolation of small interfering RNA (siRNA) was attempted several times from shoot tips but the method yielded only small quantities of low‐molecular‐weight RNA which were insufficient for analysis. Hence, RNA silencing could not be confirmed by detection of siRNA but its involvement in the observed degradation of RBDV RNA during thermotherapy cannot be excluded. Despite the induced RNA degradation, which greatly reduced the overall amounts of the virus in the shoot tips, it seems that the observed lack of virus signals in the least differentiated cells of the AD was due to restricted entry of the virus rather than entry, replication and subsequent virus degradation. This model is suggested because recent data show that the systemic signal of silencing generated in other tissues where RNA silencing is actived may not enter the apical meristem (Kobayashi and Zambryski, 2007).
Thermotherapy did not result in complete elimination of RBDV from the treated tissues, as evidenced by immunolocalization of the viral CP antigen in newly developed leaf primordia after 28 days of thermotherapy. Indeed, RNA silencing does not necessarily remove all viral RNA (Chellappan et al., 2005; Qu et al., 2005; Szittya et al., 2003) and cannot degrade RNA that is encapsidated. Furthermore, thermotherapy followed by meristem tip culture did not result in any RBDV‐free regenerants. Therefore, thermotherapy had other effects which together with subsequent cryotherapy resulted in virus elimination. Cells of the meristem and LP1 in heat‐treated shoot tips were enlarged two‐fold, resulting in an enlarged meristem. However, the enlarged cells of the AD did not experience remarkable subcellular alterations, in contrast to those of LP1 which experienced a six‐fold increase in the portion of cell volume occupied by vacuoles. Histological analysis following cryotherapy indicated that these cells did not survive. The results were consistent with previous studies, which indicated that the proportion of cells that survive freezing decreases with increasing distance from the AD and is associated with the larger size of vacuoles in the cell (Helliot et al., 2002, 2003; Wang et al., 2005). Enlarged vacuoles may be the most harmful consequence of heat treatment because the water in them forms ice crystals during freezing (Hills and Nott, 1999), damages membranes and is lethal (Karlsson et al., 1993). Other morphological changes, such as those observed in membranes (Sward and Hallam, 1976), and physiological stress responses due to heat may also have reduced the tolerance of cells to freezing. Consequently, only few cells in leaf primordia and the top layer of the AD survived freezing in liquid nitrogen following thermotherapy. In conclusion, our results suggest a model in which thermotherapy enhanced degradation of RBDV in shoot tips and hence reduced the proportion of apical meristems heavily invaded by the virus. Thermotherapy also reduced the number of virus‐infected cells that survived the subsequent cryotherapy. These two effects together resulted in a situation in which >10% of the shoot tips that survived and were able to regenerate following cryotherapy escaped RBDV infection.
Since the first studies that aimed to produce virus‐free plants from infected individuals, it has been known that the efficiency of virus eradication in a given host species differs depending on the virus and the host genotype (variety) (Barnett et al., 1975; Kassanis, 1950; Walkey and Cooper, 1975). Furthermore, the examples above indicate that pollen‐transmissibility and meristem‐invasiveness are viral properties that implicate additional risk factors for failure in eradicating the virus with the conventional means of meristem tip culture and thermotherapy. These different risk factors are largely unknown for the various virus–host combinations. Therefore, the use of cryotherapy to augment the conventional virus eradication schemes should increase the success rate in healthy plant production and help to avoid loss of time and effort. An additional advantage is that the material can be simultaneously prepared for long‐term preservation.
EXPERIMENTAL PROCEDURES
Plant materials
In vitro RBDV‐infected stock cultures of the raspberry genotype Z13 (Wang et al., 2005) and virus‐free cultures of raspberry (line TTA‐508) were obtained from Laukaa Research and Elite Plant Station, MTT Agrifood Research Center, Finland, and maintained on a basic medium (BM) composed of MS medium (Murashige and Skoog, 1962) supplemented with (per litre) myoinositol (100 mg), sucrose (30 g), benzylaminopurine (BAP, 0.5 mg), indol‐3‐yl‐acetic acid (IBA, 0.05 mg), Bacto agar (3.5 g) (Difco Laboratories, Madison, WI) and Gelrite (1.2 g) (Merck & Co. Inc., Rahway, NJ). The pH of the medium was adjusted to 5.0 with NaOH prior to autoclaving at 121 °C for 16 min. Stock cultures were placed at a temperature of 22 ± 2 °C under a 16‐h photoperiod with a light intensity of 45 µE/s/m2 provided by cool‐white fluorescent tubes. Maintenance of the shoot cultures and subculture of plants (once in 4 weeks) were carried out in the aforementioned conditions in all experiments.
Meristem culture
Meristems of 0.1 mm (with one leaf primordium), and 0.2 and 0.3 mm (with two leaf primordia) were excised from in vitro shoot stocks, cultured for 3 days on solid BM supplemented with 2.5 g/L activated charcoal (AC) and then transferred to solid BM for survival and plant regeneration.
Cryotherapy
The protocol of Wang et al. (2005) was followed. Shoot tips (1 mm in size) excised from RBDV‐infected stock cultures (4 weeks old) were first stabilized by culturing for 2 days on BM containing 2.5 g AC/L. The stabilised shoot tips were encapsulated with 2.5% (w/v) Na‐alginate, 2 m glycerol and 0.4 m sucrose in 0.1 m CaCl2 solution containing 2 m glycerol and 0.4 m sucrose to form beads, each bead containing a single shoot tip. The beads were precultured stepwise on BM supplemented with increasing sucrose concentrations (0.25–0.75 m) for 3 days. The precultured beads were treated for 90 min with a loading solution of 2 m glycerol and 0.8 m sucrose, followed by dehydration with PVS2 (Sakai et al., 1990) at 24 °C for 180 min. The PVS2 solution contains 30% (w/v) glycerol, 15% (w/v) ethylene glycol, 15% (w/v) dimethylsulfoxide (DMSO) and 0.4 m sucrose in MS medium (pH 5.8). Dehydrated beads were quickly surface‐dried by blotting on cellulose tissue and transferred into a 2‐mL cryotube (ten beads per tube; Nunc CryoTube, Roskilde, Denmark). Cryotubes were directly immersed in liquid nitrogen for 1 h and then rapidly thawed in a water bath at 40 °C for 3 min. The beads were washed with MS medium containing 1 m sucrose for 20 min before post‐culture in a Petri dish (Ø 9 cm) containing 35 mL solidified BM. The cultures were maintained in the dark at 22 ± 2 °C for 3 days and transferred for survival and regrowth under the conditions described above.
Thermotherapy followed by meristem culture or cryotherapy
Well‐developed single shoots (> 2 cm in length) were excised from RBDV‐infected stock cultures (4 weeks old) and cultured in 15 × 2.5 cm test tubes containing 20 mL of solid BM, one shoot per tube. The test tubes were placed under the conditions described for maintenance of stock cultures. After 3 days, the tubes were sealed with Parafilm to maintain high humidity and transferred to a heat chamber that was programmed for a 16‐h photoperiod at 38 °C followed by an 8‐h dark period at 26 °C for 21–42 days. After thermotherapy, meristems of 0.2 mm with two leaf primordia were excised and cultured for 3 days on solid BM supplemented with 2.5 g AC/L and transferred to solid BM for survival and plant regeneration.
For cryotherapy, shoot tips (1 mm in size) were excised from the stock shoots that had been heat‐treated as described above. Shoot tips were stabilized for 2 days by culturing on BM that contained 2.5 g AC/L (Wang et al., 2005) and then subjected to encapsulation‐vitrification as described above. Thawed and washed beads were post‐cultured in a Petri dish (Ø 9 cm) containing 35 mL solidified BM supplemented with 50 mg/L Fe‐EDTA. The cultures were maintained in the dark at 22 ± 2 °C for 3 days and then under the conditions described above.
Plant regeneration
In all experiments, survival was defined as percentage of shoot tips or meristems showing green colour 2 weeks after post‐culture. Regrowth was determined as percentage of shoot tips or meristems that developed shoots 6 weeks after post‐culture. Elongated shoots (> 3 mm) were used for further in vitro multiplication. For rooting in vitro, shoots longer than 5 mm were grown in solid BM medium free of plant hormones for 4 weeks.
Double antibody sandwich enzyme‐linked immunosorbent assay (DAS‐ELISA)
DAS‐ELISA was applied for detection of RBDV as described (Finn and Martin, 1996). Commercially available polyclonal anti‐RBDV CP rabbit antibodies (diluted 1 : 200) and alkaline phosphatase‐conjugated anti‐RBDV CP monoclonal antibodies (1 : 1000) were used (LOEWE Biochemica GmbH, Sauerlach, Germany). p‐Nitrophenyl phosphate was used as the substrate. Absorbances were read at 405 nm (A405) using an ELISA plate reader (Benchmark Microplate Reader; BioRad Labs, Hercules, CA) after 1–3 h of incubation. The samples with an A405 value three‐fold higher than the mean of the values of healthy controls were considered to be RBDV‐positive.
Reverse transcription polymerase chain reaction (RT‐PCR), cloning and sequencing
Total RNA was extracted from leaves using Trizol Reagent (Invitrogen, Carlsbad, CA) following the manufacturer's recommendations, eluted in 50 µL of RNase‐free water and stored at –20 °C until used. The first‐strand cDNA synthesis on 5 µg total RNA was carried out in a total reaction volume of 20 µL using random hexamer primers (Sigma‐Genosys, Cambridge, UK) and Avian myeoblastosis virus reverse transcriptase (AMV‐RT; Promega, Madison, WI) according to the manufacturers’ instructions. The reaction was five‐fold diluted with RNase‐free water for use in PCR.
The forward (CP‐F: 5′‐TCATTGTTGAATTAATACTAAGTATTTAAG‐3′) and reverse (CP‐R: 5′‐CCCACTAGCAGGCAAATAGTC‐3′) primer were designed according to RBDV RNA2 sequences flanking the CP gene (825 nt) according to the sequence accession S55890 (Natsuaki et al., 1991) available in the EMBL database. The expected size of the amplification product was 886 bp. The PCR reaction mix (15 µL) contained 1.2 µL of 5 mm dNTPs, 1.5 µL of 25 mm MgCl2, 1.5 µL PCR buffer, 0.15 µL of DyNAzyme DNA polymerase (Finnzymes), 0.3 µL of each primer (10 µm), 2.05 µL of RNase‐free water and 8 µL of the diluted cDNA. For amplification in a thermal cycler the following programme was used: 94 °C for 4 min, followed by 40 cycles of 94 °C for 50 s, 52 °C for 1 min and 72 °C for 1 min, with a final extension at 72 °C for 5 min. PCR products were analysed by electrophoresis in a 1% agarose gel containing ethidium bromide (0.5 µg/mL). The PCR products of the expected size were purified from agarose (Qiagen Gel Purification kit, Germany), ligated to pGEM‐T vector (Promega) and cloned in Escherichia coli DH5α cells. Plasmids (five clones) were purified and the inserts sequenced on both strands with an ABI 3730xl DNA Analyzer at Biomedicum, Molecular Medicine Sequencing Laboratory, University of Helsinki. Multiple alignments of the RBDV CP gene sequences available from databases and those determined in this study were done with CLUSTAL X (Thompson et al., 1997).
For detection of RBDV, the previously published primers U2 and L3 specific to RBDV CP gene were used as described (Kokko et al., 1996).
Virus detection by loop‐mediated isothermal amplification (LAMP)
Detection of the RBDV CP gene by LAMP was carried out as described by Fukuta et al. (2003a) with the modifications described below. Four primers were designed based on the RBDV CP gene sequences using the LAMP primer design tool (Eiken Chemical Co., Tokyo, Japan):
-
1
F1c: 5′‐CGGGCTTCCGAGAAGGTAATCAA‐3′;
-
2
B1c: 5′‐CCATGACGGATGTGGTAGATTCCA‐3′;
-
3
FIP: 5′‐CGGGCTTCCGAGAAGGTAATCAACAAGGGTCGAGTGGCTGT‐3′; and
-
4
BIP: 5′‐CCATGACGGATGTGGTAGATTCCACTGGAAGACACACACCTTACG‐3′.
The reaction mix (25 µL) contained 0.5 µL each of the primers F1c and Blc (10 µm), 2.0 µL each of the primers FIP and BIP (10 µm), 1.0 µL of 10 mm dNTPs, 0.5 U VentR high‐fidelity DNA polymerase (New England Biolabs, MA), 16 U of Bst DNA polymerase (New England Biolabs), 2.5 µL Thermopol 10× buffer (New England Biolabs), 2 µL of 25 mm MgSO4, 5 µL of 5 m betaine, and 5 µL cDNA (diluted 1 : 10). Alternatively, cDNA synthesis and LAMP were carried out simultaneously. In that case 5 U of AMV‐RT reverse transcriptase (Promega) and 1.5 µg total RNA were added instead of cDNA. The LAMP reaction was incubated at 65 °C for 60 min and cooled on ice to enhance formation of the magnesium pyrophosphate precipitate that was visually observed.
RNA extraction from shoot tips and northern blot hybridization
Total RNA was extracted from shoot tips as described (Monte and Somerville, 2003), except that 6 mL of extraction buffer was used and RNA was precipitated with 1 volume of 4 m LiCl. Total RNA (3.5 µg) was separated in a 5.5% formaldehyde‐containing denaturing agarose gel and blotted onto a Hybond‐NX membrane (Amersham Biosciences) overnight by capillary transfer. RNA was fixed to the membrane by exposure to UV light for 1 min and pre‐hybridized in a solution containing 50% formamide (Sigma), 5× SSPE (sodium chloride/sodium phosphate/EDTA) buffer, 1% sodium dodecyl sulphate (SDS), 1× Denhardt's solution and 1 mg/mL herring sperm DNA (Sigma). Probes complementary to the RBDV CP gene sequence were prepared and labelled using P32‐UTP (Amersham) by in vitro transcription of the CP gene sequence cloned under the SP6 polymerase promoter. Hybridizations were done in a new batch of the pre‐hybridization solution containing 25 µL of the in vitro transcription reaction at 55 °C overnight. The following day, membranes were washed at 68 °C twice in 5× SSC (sodium chloride/sodium citrate buffer) + 0.5% SDS and twice in 0.2× SSC + 0.5% SDS. Membranes were exposed to RX‐films (Fuijfilm, TAMRO, Finland) for different periods of time (0.5, 12 and 24 h) before being developed using an X‐OMAT 1000 automated developer (Kodak, NY).
Histological studies and immunolocalization of RBDV
Shoot tips were excised, fixed in FAA [ethanol (50%) : formalin : acetic acid = 18 : 1 : 1] for 24 h, dehydrated through an incremental ethanol series (50, 70, 85 and 95%) and stored in 100% ethanol. After embedding in paraffin, 5‐µm sections were cut with a microtome and stained with 1% safranin (for nucleoli) (Chang and Reed, 1999) and 1% aniline (for cell walls) (Gurr, 1965). The sections were observed with a light microscope (Olympus CX 41).
Sections prepared as above were used for immunolocalization of RBDV essentially as described by Rajamäki and Valkonen (2002). Briefly, the sections were pre‐incubated with phosphate‐buffered saline (PBS) containing 4% bovine serum albumin (BSA) for 30 min, followed by incubation for 3 h at room temperature with the polyclonal antibodies (LOEWE) specific to RBDV CP (diluted 1 : 500 in PBS). After washing in PBS three times, the samples were incubated with mouse anti‐rabbit monoclonal antibodies conjugated with alkaline phosphatase (dilution 1 : 300) (Sigma) for 30 min at room temperature. After washing three times in PBS, the samples were stained using a freshly prepared fuchsin substrate solution. The sections were observed with a light microscope (Olympus CX 41).
Transmission electron microscopy
Samples were fixed overnight in 2% glutaraldehyde in 0.05 m sodium cacodylate buffer (pH 6.9), washed with the same buffer and post‐fixed in 2.5% aqueous osmium tetroxide for 2 h at room temperature. Subsequently, samples were dehydrated by passing through a graded series of ethanol (50, 70, 96 and 100%) and pure acetone and embedded in low‐viscosity embedding resin. Sections were cut with a Reicher‐Jung ultramicrotome, collected on pioloform‐coated copper grids (Stork VECO, Naarden, The Netherlands) and stained with uranyl acetate and lead citrate. The grids were observed under a JOEL JEM 1200 EX II transmission electron microscope. Micrographs were taken from different tissues, and sizes of the cells, nuclei and vacuoles were measured from at least 20 cells of the different tissues and treatments.
ACKNOWLEDGEMENTS
We thank Anna Nukari, Jaana Laamanen and Marjatta Uosukainen (Laukaa Research and Elite Plant Station, MTT AgriFood Research Center) for materials and collaboration, Eija Jokitalo and Mervi Lindman (Institute of Biotechnology, University of Helsinki) for technical assistance with TEM, and Motomu Akita (Kinki University, Japan) and Marjatta Raudaskoski (University of Helsinki) for helpful advice. Financial support from the Ministry of Agriculture and Forestry, Finland (grant 4782/501/2005) and University of Helsinki is gratefully acknowledged.
REFERENCES
- Agrios, G.N. (2005) Plant Pathology, 5th edn. Burlington: Elsevier Academic Press. [Google Scholar]
- Amari, K. , Burgos, L. , Pallas, V. and Sanchez‐Pina, M.A. (2007) Prunus necrotic ringspot virus early invasion and its effects on apricot pollen grain performance. Phytopathology, 97, 892–899. [DOI] [PubMed] [Google Scholar]
- Appiano, A. and Pennazio, S. (1972) Electron microscopy of potato meristem tips infected with potato virus X. J. Gen. Virol. 14, 273–276. [DOI] [PubMed] [Google Scholar]
- Barbara, D.J. , Morton, A. , Ramcharan, S. , Cole, I.W. , Phillips, A. and Knight, V.H. (2001) Occurrence and distribution of Raspberry bushy dwarf virus in commercial Rubus plantations in England and Wales. Plant Pathol. 50, 747–754. [Google Scholar]
- Barnett, O.W. , Gibson, P.B. and Seo, A. (1975) A comparison of heat treatment, cold treatment and meristem tip‐culture for obtaining virus‐free plants of Trifolium repens . Plant Dis. Rep. 59, 834–837. [Google Scholar]
- Bhojwani, S.S. and Razdan, M.K. (1996) Plant Tissue Culture: Theory and Practice, a Revised Edition. Amsterdam: Elsevier Science BV. [Google Scholar]
- Brison, M. , Boucaud, M.‐T. , Pierronnet, A. and Dosba, F. (1997) Effect of cryopreservation on the sanitary state of a cv Prunus rootstock experimentally contaminated with plum pox potyvirus. Plant Sci. 123, 189–196. [Google Scholar]
- Chang, Y. and Reed, B.M. (1999) Extended cold acclimation and recovery medium alteration improve regrowth of Rubus shoot tips following cryopreservation. Cryo Lett. 20, 371–376. [Google Scholar]
- Chellappan, P. , Vanitharani, R. , Ogbe, F. and Fauquet, C.M. (2005) Effect of temperature on geminivirus‐induced RNA silencing in plants. Plant Physiol. 138, 1828–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, V.C. and Walkey, D.G.A. (1978) Thermal inactivation of cherry leaf roll virus in tissue cultures of Nicotiana rustica raised from seeds and meristem tips. Ann. Appl. Biol. 88, 273–278. [Google Scholar]
- Ding, B. (1998) Intercellular protein trafficking through plasmodesmata. Plant Mol. Biol. 38, 279–310. [PubMed] [Google Scholar]
- Faccioli, G. and Marani, F. (1998) Virus elimination by meristem tip culture and tip micrografting In: Plant Virus Disease Control (Hadid A., Khetarpal R.K. and Koganezawa H., eds), pp. 346–380. St. Paul, MN: APS Press, The American Phytopathological Society. [Google Scholar]
- Fauquet, C.M. , Mayo, M.A. , Maniloff, J. , Desselberger, U. and Ball, L.A. (2005) Genus Idaeovirus In: Virus Taxonomy. Eighth Report of the International Committee on Taxonomy of Viruses, pp. 1063–1065. San Diego: Elsevier Academic Press. [Google Scholar]
- Finn, C.E. and Martin, R.R. (1996) Distribution of tobacco streak, tomato ringspot, and raspberry bushy dwarf viruses in Rubus ursinus and R. leucodermis collected from the Paciffic Northwest. Plant Dis. 80, 769–772. [Google Scholar]
- Foster, T.M. , Lough, T.J. , Emerson, S.J. , Lee, R.H. , Bowman, J.L. , Forster, R.L.S. and Lucas, W.J. (2002) A surveillance system regulates selective entry of RNA into the shoot apex. Plant Cell 14, 1497–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuta, S. , Iida, T. , Mizukami, Y. , Ishida, A. , Ueda, J. , Kanbe, M. and Ishimoto, Y. (2003a) Detection of Japanese yam mosaic virus by RT‐LAMP. Arch. Virol. 148, 1713–1720. [DOI] [PubMed] [Google Scholar]
- Fukuta, S. , Kato, S. , Yoshida, K. , Mizukami, Y. , Ishida, A. , Ueda, J. , Kanbe, M. and Ishimoto, Y. (2003b) Detection of tomato yellow leaf curl virus by loop‐mediated isothermal amplification reaction. J. Virol. Methods, 112, 35–40. [DOI] [PubMed] [Google Scholar]
- Fukuta, S. , Ohishi, K. , Yoshida, K. , Mizukami, Y. , Ishida, A. and Kanbe, M. (2004) Development of immunocapture reverse transcription loop‐mediated isothermal amplification for the detection of tomato spotted wilt virus from chrysanthemum. J. Virol. Methods, 121, 49–55. [DOI] [PubMed] [Google Scholar]
- Gurr, E. (1965) The Rational Use of Dyes in Biology and General Staining Methods. London: Leonard Hill. [Google Scholar]
- Helliot, B. , Panis, B. , Poumay, Y. , Swennen, R. , Lepoivre, P. and Frison, E. (2002) Cryopreservation for the elimination of cucumber mosaic and banana streak viruses from banana (Musa spp). Plant Cell Rep. 20, 1117–1122. [Google Scholar]
- Helliot, B. , Swennen, R. , Poumay, Y. , Frison, E. , Lepoivre, P. and Panis, B. (2003) Ultrastractural changes associated with cryopreservation of banana (Musa spp.) highly proliferating meristems. Plant Cell Rep. 21, 690–698. [DOI] [PubMed] [Google Scholar]
- Hills, B.P. and Nott, K.P. (1999) NMR studies of water compartmentation in carrot parenchyma tissue during drying and freezing. Appl. Magnetic Resonance 17, 521–535. [Google Scholar]
- Holmes, F.O. (1948) Elimination of spotted wilt from a stock of dahlia. In: Report and Abstracts of the Second Annual Meeting of the Northeastern Division of the American Phytopathological Society Phytopathology, 38, 314. [Google Scholar]
- Jones, A.T. , McGavin, W.J. , Mayo, M.A. , Angel‐Diaz, J.E. , Kokko H. and Karenlampi, S.O. (2000) Some properties of two laboratory variants of RBDV and their comparison with those of 3 previously characterised RBDV isolates. Eur. J. Plant Pathol. 106: 623–632. [Google Scholar]
- Karesova, R. , Janeckova, M. and Paprstein, F. (2002) Elimination of Raspberry bushy dwarf virus from raspberry cv. ‘Gatineau’. Acta Hortic. 585: 359–362. [Google Scholar]
- Karlsson, J.O.M. , Cravalho, E.G. , Rinkes, I.H.M.B. , Tompkins, R.G. , Yarmush, M.L. and Toner, M. (1993) Nucleation and growth of ice crystals inside cultured hepatocytes during freezing in the presence of dimethyl‐sulfoxide. Biophys. J. 65, 2524–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassanis, B. (1950) Heat inactivation of leaf‐roll virus in potato tubers. Ann. Appl. Biol. 37, 339–341. [Google Scholar]
- Kassanis, B. (1957) Effects of changing temperature on plant virus diseases. Adv. Virus Res. 4, 221–241. [DOI] [PubMed] [Google Scholar]
- Kim, I. and Zambryski, P.C. (2005) Cell‐to‐cell communication via plasmodesmata during Arabidopsis embryogenesis. Curr. Opin. Plant Biol. 8, 593–599. [DOI] [PubMed] [Google Scholar]
- Kim, I. , Kobayashi, K. , Cho, E. and Zambryski, P.C. (2005) Sub‐domains for transport via plasmodesmata corresponding to the apical‐basal axis are established during Arabidopsis embryogenesis. Proc. Natl Acad. Sci. USA, 102, 11945–11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, K. and Zambryski, P. (2007) RNA silencing and its cell‐to‐cell spread during Arabidopsis embryogenesis. Plant J. 50, 597–604. [DOI] [PubMed] [Google Scholar]
- Kokko, H. , Kivineva, M. and Kärenlampi, S.O. (1996) Single‐step immunocapture RT‐PCR in the detection of raspberry bushy dwarf virus. BioTech. 20, 842–846. [DOI] [PubMed] [Google Scholar]
- Kunkel, L.O. (1943) Potato witches’‐broom transmission by dodder and cure by heat. Proc. Am. Phil. Soc. 86, 470–475. [Google Scholar]
- Lankes, C. (1995). Elimination of raspberry bushy dwarf virus. Act. Hort. 385, 70–75. [Google Scholar]
- Loebenstein, G. and Thottapilly, G. (2003) (eds) Virus and Virus‐like Diseases of Major Crops in Developing Countries. Dordrecht: Kluwer Academic Press. [Google Scholar]
- Lucas, W.J. (2006) Plant viral proteins: agents for cell‐to‐cell trafficking of viral genomes. Virology 344, 169–184. [DOI] [PubMed] [Google Scholar]
- Mayo, M.A. , Jolly, C.A. , Murant, A.F. and Raschke, J.H. (1991) Nucleotide sequence of raspberry bushy dwarf virus RNA‐3. J. Gen. Virol. 72, 469–472. [DOI] [PubMed] [Google Scholar]
- Mink, G.I. , Wample, R. and Howell, W.E. (1998) Heat treatment of perennial plants to eliminate phytoplasms, viruses, and viroids while maintaining plant survival In: Plant Virus Disease Control (Hadid A., Khetarpal R.K. and Koganezawa H., eds), pp. 332–345. St. Paul, MN: APS Press, The American Phytopathological Society. [Google Scholar]
- Mochizuki, T. and Ohki, S.T. (2004) Shoot meristem tissue of tobacco inoculated with Cucumber mosaic virus is infected with the virus and subsequently recovers from infection by RNA silencing. J. Gen. Plant Pathol. 70, 363–366. [Google Scholar]
- Monte, D. and Somerville, S. (2003) Pine tree method for isolation of plant RNA In: DNA Microarrays, A Molecular Cloning Manual (Bowtell D. and Sambrook J., eds), pp. 124–126. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Mori, Y. , Nagamine, K. , Tomita, N. and Notomi, T. (2001) Detection of loop‐mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem. Biophys. Res. Commun. 289, 150–154. [DOI] [PubMed] [Google Scholar]
- Murant, A.J. , Chambers, J. and Jones, A.T. (1974) Spread of raspberry bushy dwarf virus by pollination, its association with crumbly fruit, and problems of control. Ann. Appl. Biol. 77, 271–281. [Google Scholar]
- Murashige, T. and Skoog, F. (1962) A revised medium for rapid growth and bioassays with tobacco cell cultures. Physiol. Plant. 15, 473–497. [Google Scholar]
- Nagamine, K. , Hase, T. and Notomi, T. (2002) Accelerated reaction by loop‐mediated isothermal amplification using loop primers. Mol. Cell. Probes, 16, 223–229. [DOI] [PubMed] [Google Scholar]
- Natsuaki, T. , Mayo, M.A. , Jolly, C.A. and Murant, A.F. (1991) Nucleotide sequence of raspberry bushy dwarf virus RNA‐2: a bicistronic component of a bipartite genome. J. Gen. Virol. 73, 2183–2189. [DOI] [PubMed] [Google Scholar]
- Nie, X. (2005) Reverse transcription loop‐mediated isothermal amplification of DNA for detection of Potato virus Y . Plant Dis. 89, 605–610. [DOI] [PubMed] [Google Scholar]
- Oparka, K.J. , Roberts, A.G. , Boevink, P. , Santa Cruz, S. , Roberts, I. , Pradel, K.S. , Imlau, A. , Kotlizky, G. , Sauer, N. and Epel, B. (1999) Simple, but not branched, plasmodesmata allow the non‐specific trafficking of proteins in developing tobacco leaves. Cell 97, 743–754. [DOI] [PubMed] [Google Scholar]
- Qu, F. , Ye, X.H. , Hou, G.C. , Sato, S. , Clemente, T.E. and Morris, T.J. (2005) RDR6 has a broad‐spectrum but temperature‐dependent antiviral defense role in Nicotiana benthamiana . J. Virol. 79, 15209–15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quak, F. (1987) Therapy of individual plants In: Viruses of Potatoes and Seed‐Potato Production (De Bokx J.A. and Van Der Want J.P.H., eds), pp. 151–161. Wageningen: Pudoc. [Google Scholar]
- Rajamäki, M.‐L. and Valkonen, J.P.T. (2002) Viral genome‐linked protein (VPg) controls accumulation and phloem‐loading of a potyvirus in inoculated potato leaves. Mol. Plant–Microbe Interact. 12, 1074–1081. [DOI] [PubMed] [Google Scholar]
- Roberts, D.A. , Christie, R.G. and Archer, M.C. (1970) Infection of apical initials in tobacco shoot meristems by tobacco ringspot virus. Virology 42, 217–220. [DOI] [PubMed] [Google Scholar]
- Sakai, A. , Kobayashi, S. and Oiyama, I. (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep. 9, 30–33. [DOI] [PubMed] [Google Scholar]
- Schwach, F. , Vaistij, F.E. , Jones, L. and Baulcombe D.C. (2005) An RNA‐dependent RNA polymerase prevents meristem invasion by potato virus X and is required for the activity but not the production of a systemic silencing signal. Plant Physiol. 138, 1842–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sward, R.J. and Hallam, N.D. (1976) Changes in fine structure of the potato meristem following heat treatment for virus eradication. Aust. J. Bot. 24, 597–605. [Google Scholar]
- Szittya, G. , Silhavy, D. , Molnar, A. , Havelda, Z. , Lovas, A. , Lakatos, L. , Banfalvi, Z. and Burgyan, J. (2003) Low temperature inhibits RNA silencing‐mediated defence by the control of siRNA generation. EMBO J. 22, 633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiler‐Hedtrich, R. and Baumann, G. (1989) Elimination of apple mosaic virus and raspberry bushy dwarf virus from infected red raspberry (Rubus idaeus L.) by tissue culture. J Phytopath. 127, 193–199. [Google Scholar]
- Thompson, J.D. , Gibson, T.J. , Plewniak, F. , Jeanmougin, F. and Higgins, D.C. (1997) The Clustal X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25, 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkey, D.G.A. and Cooper, V.C. (1975) Effect of temperature on virus eradication and growth of infected tissue cultures. Ann. Appl. Biol. 80, 185–190. [DOI] [PubMed] [Google Scholar]
- Walkey, D.G.A. and Webb, M.J.W. (1968) Virus in plant apical meristem. J. Gen. Virol. 3, 311–313. [Google Scholar]
- Wang, Q.C. , Laamanen, J. , Uosukainen, M. and Valkonen, J.P.T. (2005) Cryopreservation of in vitro‐grown shoot tips of raspberry (Rubus idaeus L.) by encapsulation‐vitrification and encapsulation‐dehydration. Plant Cell Rep. 24, 280–288. [DOI] [PubMed] [Google Scholar]
- Wang, Q.C. , Mawassi, M. , Li, P. , Gafny, R. , Sela, I. and Tanne, E. (2003) Elimination of grapevine virus A (GVA) by cryopreservation of in vitro‐grown shoot tips of Vitis vinifera L. Plant Sci. 165, 321–327. [Google Scholar]
- Wang, Q.C. , Liu, Y. , He, W. , Xie, Y.H. and You, M.S. (2006) Cryotherapy of potato shoot tips for efficient elimination of Potato leaf roll virus (PLRV) and Potato virus Y (PVY). Potato Res. 49, 119–129. [Google Scholar]
- Waterworth, H.E. and Hadidi, A. (1998) Economic losses due to plant viruses In: Plant Virus Disease Control (Hadidi A., Khetarpal R.K. and Koganezawa H., eds), pp. 1–13. St. Paul, MN: APS Press, The American Phytopathological Society. [Google Scholar]
- Wilkinson, T. , Wetten, A. , Prychid, C. and Fay, M. (2003) Suitability of cryopreservation for the long‐term storage of rare and endangered plant species: a case history for Cosmos atrosanguineus . Ann. Bot. 91, 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler, A. , Natsuaki, T. , Mayo, M.A. , Jolly, C.A. and Murant, A.F. (1992) Nucleotide sequence of raspberry bushy dwarf virus RNA‐1. J. Gen. Virol. 73, 3213–3218. [DOI] [PubMed] [Google Scholar]


