Abstract
Objectives
To examine variations across general practices and factors associated with antibiotic prescribing for common infections in UK primary care to identify potential targets for improvement and optimization of prescribing.
Methods
Oral antibiotic prescribing for common infections was analysed using anonymized UK primary care electronic health records between 2000 and 2015 using the Clinical Practice Research Datalink (CPRD). The rate of prescribing for each condition was observed over time and mean change points were compared with national guideline updates. Any correlation between the rate of prescribing for each infectious condition was estimated within a practice. Predictors of prescribing were estimated using logistic regression in a matched patient cohort (1:1 by age, sex and calendar time).
Results
Over 8 million patient records were examined in 587 UK general practices. Practices varied considerably in their propensity to prescribe antibiotics and this variance increased over time. Change points in prescribing did not reflect updates to national guidelines. Prescribing levels within practices were not consistent for different infectious conditions. A history of antibiotic use significantly increased the risk of receiving a subsequent antibiotic (by 22%–48% for patients with three or more antibiotic prescriptions in the past 12 months), as did higher BMI, history of smoking and flu vaccinations. Other drivers for receiving an antibiotic varied considerably for each condition.
Conclusions
Large variability in antibiotic prescribing between practices and within practices was observed. Prescribing guidelines alone do not positively influence a change in prescribing, suggesting more targeted interventions are required to optimize antibiotic prescribing in the UK.
Introduction
Antibiotics are vital in medicine, not just for the treatment of infections, but for the prevention of infection-related complications, for example following surgery, Caesarean sections or chemotherapy.1 Antimicrobial resistance is of global concern as no new class of antibiotics has been discovered in the last 30 years. Prescribing of antibiotics is at an all-time global high. In the UK health system, >81% of antibiotics are prescribed in primary care.2 One recent study, based on The Health Improvement Network (THIN) database, observed variability in practice-level prescribing of antibiotics and that the most common indications for antibiotics were respiratory and urinary tract infections (RTIs and UTIs, respectively),3 whilst others have observed additional variability in high-risk prescribing and prescribing safety between practices4,5 and countries.6
Children, the elderly, women, smokers, more deprived populations and those with multiple comorbidities have a greater risk of infection-related complications and are prescribed antibiotics more often.7–10 However, these patient-related factors were generally identified in research on just one infectious condition (such as RTIs), whilst few studies have investigated whether these additional patient-level drivers have the same influence across all common infections.
To better understand antibiotic utilization in the UK and inform prescribing policies and future stewardship programmes, this study analysed electronic health records (EHRs) from the Clinical Practice Research Datalink (CPRD), including data from 587 practices over 15 years. The objectives of this study were to determine which factors are associated with the decision to prescribe antibiotics across a wide range of common infections and to evaluate the variability in antibiotic prescribing between general practices and whether this variability changed with updates to national prescribing guidelines.
Materials and methods
Data source
A retrospective observational study was conducted using CPRD. This data research service holds longitudinal, anonymized, patient-level EHRs from >500 general practices with >5 million active patient records in the UK, representing ∼8% of the UK population.11–13 The EHR includes the clinical diagnosis, medication prescribed, vaccination history, diagnostic testing, lifestyle information and clinical referrals, as well as the patient’s age, sex, ethnicity and BMI.13 On the basis of patients’ residential postcodes, we extracted Index of Multiple Deprivation (IMD) quintiles from the CPRD. The IMD measures area-level deprivation on the basis of several domains, including income, employment, health, education, barriers to services (including housing), crime and general living environment. It is derived for Lower-layer Super Output Areas (LSOAs), which contain 1000–3000 people, and are census derived.14 Prescriptions were classified using the BNF sections.
Study population
EHRs were obtained for patients who visited their general practice between January 2000 and June 2015 with a clinical Read code relating to a common infection. The selected Read codes were reviewed by two independent clinical epidemiologists and the most common infection-related conditions recorded were selected for investigation. These included upper and lower respiratory tract infections (URTIs and LRTIs, respectively), sinusitis, otitis externa, otitis media and UTIs. URTIs comprised unspecified URTIs, tracheitis, laryngitis, common cold, cough, sore throat and tonsillitis. LRTIs comprised unspecified LRTIs, unspecified chest infections and bronchitis, excluding COPD and pneumonia. The full code lists are available at clinicalcodes.org.
The study population consisted of patients who consulted their general practice for a common infection that did not have an infection-related Read code and/or an antibiotic prescription in their EHR in the 6 months before the consultation (to identify patients with an incidental case of infection). The index date was the date that the infection-related Read code was recorded in the EHR. Common infections that occurred within 12 months of registering with their practice were excluded (with the exception of those for newly born babies). If a patient had multiple consultations for each infectious condition over the study period, one single observation was randomly selected. This generated six unmatched study populations, one for each infectious condition. Matched study populations were derived from the unmatched populations. Cases were those patients who had received an antibiotic prescription. They were matched at random on a one-to-one basis by age, sex and calendar month and year of the consultation to a control patient who did not receive an antibiotic prescription for the same infectious condition. Matching by age was done stepwise by year up to a maximum difference of 5 years. Matching by time accounted for any change in national guidelines and seasonal changes in prescribing behaviours.
Statistical analysis
Descriptive statistics were used to investigate the rate of antibiotics being prescribed for each infection in the study population over the relevant period. The unmatched study population was used to determine the rate of antibiotic prescribing for each common infection by practice. This was followed by a change point analysis (CPA)15 of the mean annual antibiotic prescribing rates (for all practices) over time to evaluate changes in prescribing rates compared with the introduction of national guidelines, using the changepoint package in RStudio (R; version 3.3.3). To determine whether a practice’s prescribing rate was similar for each infectious condition, a correlation coefficient matrix was applied to practice-level antibiotic prescribing rates (a score of 1 indicated a perfect positive correlation and 0 indicated no correlation within a practice). Finally the unmatched population was used to estimate the effects of age and sex on the likelihood of receiving an antibiotic prescription. To account for non-linear associations, age was transformed using the multiple fractional polynomials (mfp) package in RStudio (R; version 3.3.3). Fractional polynomials are used to model the influence of non-linear continuous covariates on the outcome in regression models.16 The most appropriate transformation (selected using the mfp package) was then used in a crude logistic regression to estimate the risk of receiving an antibiotic for males and females by age for each indication. For this analysis, a cut-off was applied to patient age at the 1st and 99th centiles (Table 1), ensuring there were enough observations included in the analysis; this cut-off varied for each infectious condition.
Table 1.
Characteristics of the study population for each common infection
| Characteristic | URTI | LRTI | Sinusitis | Otitis externa | Otitis media | UTI |
|---|---|---|---|---|---|---|
| Unmatched population (n) | 4249147 | 1250120 | 413138 | 702985 | 539571 | 758622 |
| Antibiotics prescribed, n (%) | 1786630 (42) | 1099459 (87.9) | 358647 (86.8) | 230056 (32.7) | 435930 (80.1) | 678236 (89.4) |
| Age, years | ||||||
| mean (SD) | 34.9 (25.2) | 45.5 (26.5) | 44.8 (16.9) | 37.3 (23.6) | 20.7 (21.6) | 48.3 (24.5) |
| 1st–99th centile | 0–88 | 0–93 | 10–83 | 1–87 | 0–80 | 2–94 |
| Matched population (n) | 3291304 | 301272 | 108928 | 459332 | 206806 | 160472 |
| Antibiotic prescribed, n (%) | 1645652 (50) | 150636 (50) | 54464 (50) | 229666 (50) | 103403 (50) | 80236 (50) |
| Age, years | ||||||
| mean (SD) | 37.04 (25.3) | 43.04 (31.6) | 44.72 (17.6) | 34.10 (23.3) | 26.87 (23.7) | 47.69 (28.7) |
| Sex, n (%) | ||||||
| male | 1458758 (44.3) | 143258 (47.6) | 41546 (38.1) | 208908 (45.5) | 95378 (46.1) | 44748 (27.9) |
| female | 1832546 (55.7) | 158014 (52.4) | 67382 (61.9) | 250424 (54.5) | 111428 (53.9) | 115724 (72.1) |
| BMI | ||||||
| mean (SD) | 25.9 (7.15) | 25.23 (7.58) | 26.79 (5.78) | 26.18 (7.33) | 24.56 (8) | 25.6 (6.5) |
| missing, n (%) | 1433930 (43.6) | 140022 (46.5) | 39024 (35.8) | 219029 (47.7) | 112157 (55.2) | 71869 (44.8) |
| Ethnicity, n (%) | ||||||
| White | 908264 (27.6) | 87843 (29.2) | 31043 (28.5) | 121569 (26.5) | 50899 (24.6) | 46411 (28.9) |
| Asian | 80897 ( 2.5) | 4688 (1.6) | 1732 (1.6) | 7336 (1.6) | 3375 (1.6) | 2684 (1.7) |
| Black | 39423 ( 1.2) | 1975 (0.7) | 842 (0.8) | 3182 (0.7) | 1247 (0.6) | 1145 (0.7) |
| Mixed | 21429 ( 0.7) | 1498 (0.5) | 399 (0.4) | 2205 (0.5) | 1147 (0.6) | 728 (0.5) |
| Unknown | 2241291 (68.1) | 205268 (68.1) | 74912 (68.8) | 325040 (70.8) | 150138 (72.6) | 109504 (68.2) |
| Smoking history, n (%) | ||||||
| non-smoker | 1134869 (34.5) | 83866 (27.8) | 47321 (43.4) | 140989 (30.7) | 50365 (24.4) | 62704 (39.0) |
| current smoker | 441827 (13.4) | 43788 (14.5) | 17992 (16.5) | 54057 (11.8) | 17280 (8.4) | 24995 (15.6) |
| past smoker | 519251 (15.8) | 47612 (15.8) | 21172 (19.4) | 75348 (16.4) | 24589 (11.9) | 23824 (14.8) |
| missing smoking information | 1195357 (36.3) | 126006 (41.8) | 22443 (20.6) | 188938 (41.1) | 114572 (55.4) | 48949 (30.5) |
| Region, n (%) | ||||||
| London | 350944 (10.7) | 22099 (7.3) | 10010 (9.2) | 42002 (9.1) | 16474 (8.0) | 14444 (9.0) |
| Scotland, Wales and Northern Ireland | 612872 (18.6) | 56453 (18.7) | 21318 (19.6) | 78311 (17.0) | 33806 (16.3) | 29784 (18.6) |
| North of England | 564625 (17.2) | 60418 (20.1) | 19496 (17.9) | 80982 (17.6) | 39353 (19.0) | 28753 (17.9) |
| Midlands of England | 767371 (23.3) | 76449 (25.4) | 25113 (23.1) | 116423 (25.3) | 53058 (25.7) | 40505 (25.2) |
| South of England | 995492 (30.2) | 85853 (28.5) | 32991 (30.3) | 141614 (30.8) | 64115 (31.0) | 46986 (29.3) |
| IMD quintiles, n (%) | ||||||
| 1 (least deprived) | 497366 (15.1) | 41349 (13.7) | 18367 (16.9) | 74203 (16.2) | 34750 (16.8) | 23261 (14.5) |
| 2 | 478904 (14.6) | 41398 (13.7) | 16743 (15.4) | 68760 (15.0) | 29794 (14.4) | 23526 (14.7) |
| 3 | 412858 (12.5) | 36995 (12.3) | 13152 (12.1) | 58785 (12.8) | 26004 (12.6) | 20485 (12.8) |
| 4 | 387332 (11.8) | 35143 (11.7) | 11109 (10.2) | 52826 (11.5) | 23463 (11.3) | 18522 (11.5) |
| 5 (most deprived) | 310386 ( 9.4) | 32173 (10.7) | 7918 (7.3) | 42033 (9.2) | 19160 (9.3) | 15581 (9.7) |
| missing IMD information | 1204458 (36.6) | 114214 (37.9) | 41639 (38.2) | 162725 (35.4) | 73635 (35.6) | 59097 (36.8) |
| Charlson index score, n (%) | ||||||
| 0 (no comorbidity) | 2354982 (71.6) | 188132 (62.4) | 77024 (70.7) | 346528 (75.4) | 163879 (79.2) | 99283 (61.9) |
| 1–4 (low) | 897586 (27.3) | 105025 (34.9) | 31132 (28.6) | 109419 (23.8) | 41931 (20.3) | 55875 (34.8) |
| ≥5 (high) | 38736 (1.1) | 8115 (2.7) | 772 (0.7) | 3385 (0.73) | 996 (0.5) | 5314 (3.3) |
| Consultation duration, min, median (IQR) | 7 (5–11) | 7 (4–11) | 8 (5–11) | 7 (4–10) | 7 (4–10) | 8 (4–12) |
| Entries to patient EHR, median (IQR)a,b | 13 (5–26) | 17 (7–34) | 14 (6–28) | 10 (4–23) | 9 (4–19) | 19 (7–38) |
| Flu vaccine, n (%)a | 704048 (21.4) | 95968 (31.9) | 21736 (20.0) | 74618 (16.2) | 27295 (13.2) | 50554 (31.5) |
| Same infectious disease, n (%)a | 303939 (9.2) | 22503 (7.5) | 6597 (6.1) | 32381 (7.0) | 12932 (6.3) | 16899 (10.5) |
| Non-antibiotic prescriptions, median (IQR)a | 4 (1–15) | 6 (1–34) | 5 (1–16) | 3 (0–11) | 2 (0–8) | 7 (1–36) |
| Antibiotic prescriptions, n (%)a | ||||||
| none | 2318726 (70.45) | 213291 (70.8) | 73665 (67.63) | 318111 (69.26) | 145050 (70.14) | 112610 (70.2) |
| 1–2 | 824594 (25.05) | 72098 (23.93) | 29875 (27.43) | 120279 (26.19) | 53200 (25.72) | 40454 (25.2) |
| >3 | 147984 (4.5) | 15883 (5.27) | 5388 (4.95) | 20942 (4.56) | 8556 (4.14) | 7408 (4.6) |
Original non-imputed data.
In the 12 months preceding the consultation.
The number of entries includes all entries in the patient’s EHR on the index date.
The matched study population was generated to control for the difference in risk of receiving an antibiotic for age and sex, as well as matching by calendar time to adjust for any seasonal effect or changes to guidelines on prescribing during the study period. A standard data-cleaning approach was applied; specifically, for any patient with a BMI <8, the BMI was amended to ‘missing’.17 Multiple imputation methods were implemented to impute missing BMIs, smoking information and IMDs, generating 15 datasets. Imputations were checked using density plots against the original data and subsequent models for predictors of antibiotic prescribing were adjusted using a missing indicator to account for the proportion of missing data in the original study population.
To identify patient predictors for antibiotic prescribing, conditional logistic regression was used to estimate ORs and 95% CIs for the outcome of receiving an antibiotic prescription, running the model on each imputed dataset and averaging the results from these runs. The variables included in the model were identified through a literature review and selected a priori. These variables included the patient’s BMI, smoking history, ethnicity, IMD quintile and health status using the Charlson comorbidity index.7,8,18,19 Other variables included the total number of all consultations, non-antibiotic prescriptions and antibiotic prescriptions received by the patient in the previous 12 months, the patient’s influenza vaccination history, whether a patient had presented with the same condition in the previous 12 months, the duration of the consultation and the practice region.18 Regions were grouped to include: (i) North of England (North West, North East and Yorkshire and The Humber), (ii) Midlands (West Midlands, East of England and East Midlands), (iii) South of England (South West, South Central and South East Coast), (iv) devolved administrations (Wales, Scotland and Northern Ireland) and (v) London. Reference categories included in the conditional logistic model are specified in the Results section. R version 3.3.3 was used for the statistical analysis.
Ethics
Data from CPRD were used for this work. In accordance with CPRD research approval, the study protocol was first approved by the Independent Scientific Advisory Committee (ISAC) for CPRD research (protocol number 16_153R3AMn2A).
Results
In total, 587 general practices were included in the analysis. The unmatched population generated >8 million patient records, with almost 4.9 million patient record observations in the age-, sex- and calendar time-matched study population. The demographics of the study populations are summarized in Table 1. On average, females received antibiotics more often than men, particularly for sinusitis and UTIs, where 61.9% and 72.1% of the matched study population were female, respectively. The mean patient age also varied by indication, ranging from 26.9 years (SD 23.67) for patients with otitis media up to 47.7 years (SD 28.7) for patients with UTIs. A large proportion of patients had no comorbidities (62%–79%). The most common infections identified in this study accounted for 72.2% of all oral antibiotics prescribed. URTIs accounted for 30.9% of oral antibiotic prescriptions, LRTIs 19%, sinusitis 6.2%, otitis externa 4%, otitis media 7.5% and UTIs 4.6%.
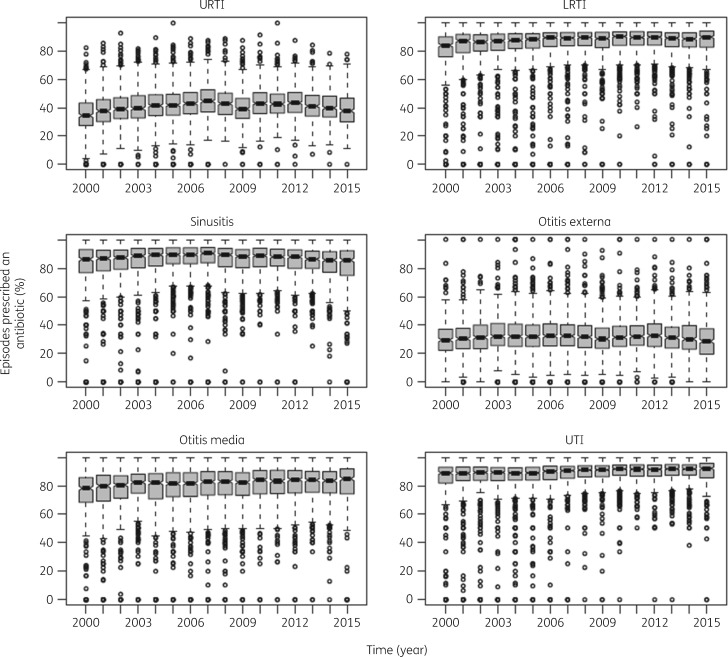
When observing prescribing rates, there were no major changes over time for each infectious condition between 2000 and 2015 (Figure 1). The propensity to prescribe antibiotics varied between practices considerably; for example, some practices prescribed just 10% of the time whilst other practice prescribed 70% of the time for cases of URTIs. This variability remained in 2015, but the number of lower-prescribing practices fell.
Figure 1.
Percentage of episodes in which an antibiotic was prescribed from 2000 to 2015 in UK primary care settings stratified by time (year) and infectious condition. Each data point represents practice-level prescribing. Data are median (IQR).
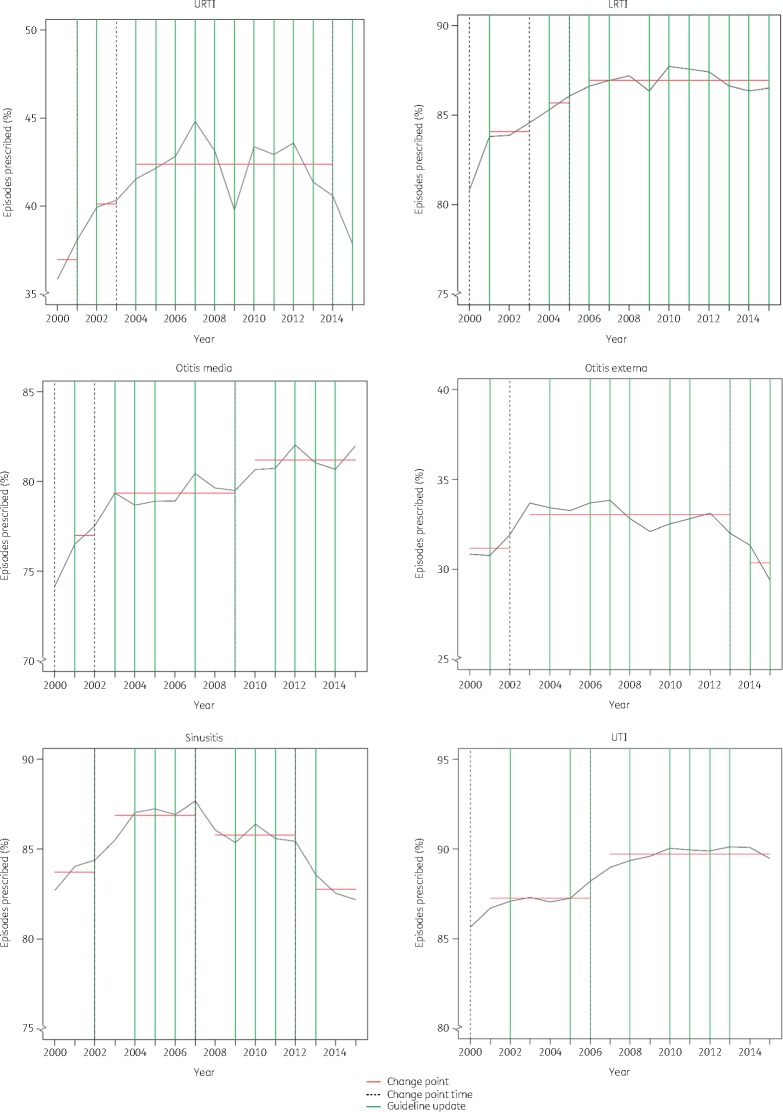
CPA of antibiotic prescribing rate identified between two and five change points for each infectious condition over the 15 years. These prescribing change points were not always consistent with regular NICE guideline updates or the launch of ‘Managing common infections: a guidance for primary care’, suggesting that changes in guidelines did not have a consistent positive influence on antibiotic prescribing rates (Figure 2).
Figure 2.
CPA for prescribing of antibiotics in the UK over time. To identify any change in the mean prescribing rate for each general practice over time, the optimal number of change points was estimated (red line) for each infectious condition. The optimal change point by year is highlighted with a vertical black dotted line. These optimal changes in mean prescribing were compared with updates to national guidelines published by NICE (green lines). The results were also compared with the PHE launch of ‘Managing common infections: a guidance for primary care’ in 2010. See Table S1 (available as Supplementary data at JAC online) for the guideline-related resources. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
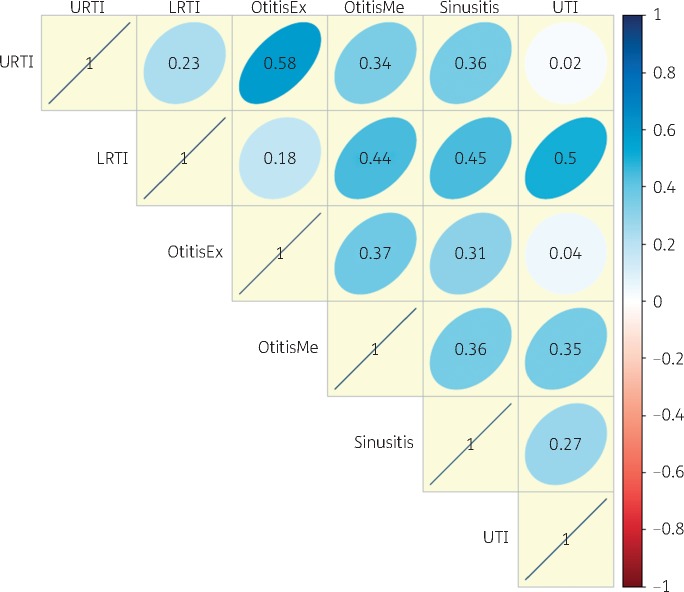
Additionally, practices that were high prescribers for some conditions were not necessarily high prescribers for other conditions, shown by little correlation for prescribing rates by infection within a practice (Figure 3).
Figure 3.
Correlation coefficient matrix for antibiotic prescribing between infections within UK general practices; to determine if a practice’s prescribing rate was similar for each infectious condition, a correlation coefficient matrix was applied. A score of 1 indicates a perfect positive correlation (that antibiotic prescribing rates are the same between the two infectious conditions within a practice); a score of 0 indicates no correlation (antibiotic prescribing rates between the two infectious conditions are not correlated). OtitisEx, otitis externa; OtitisMe, otitis media. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
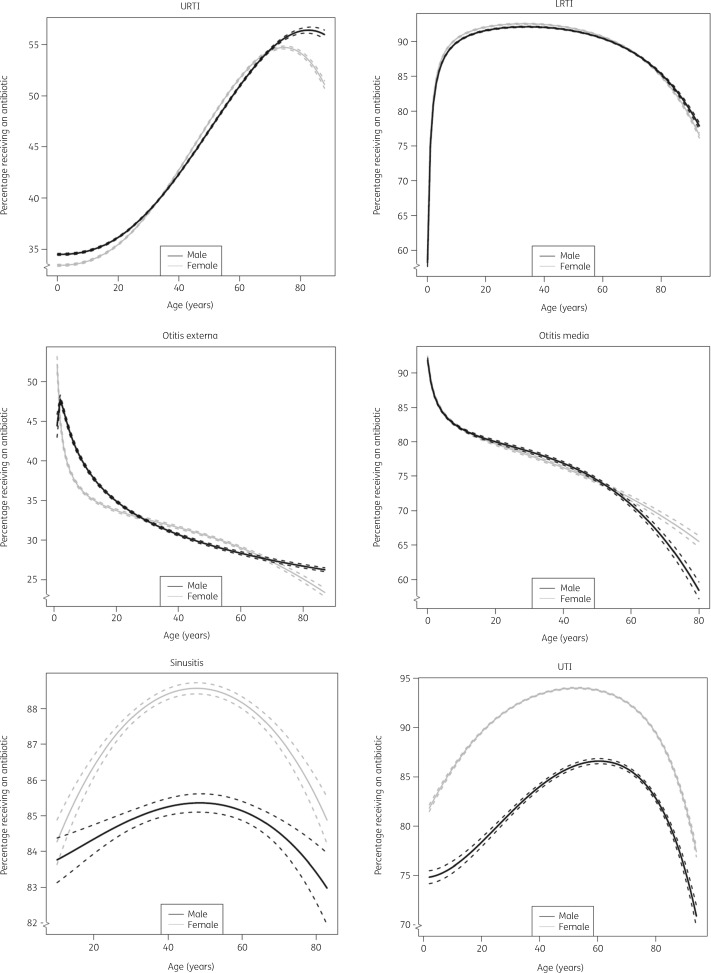
Differences are known to exist for prescribing rates by age and gender. In the current cohort, the probability of receiving an antibiotic for each indication varied considerably by age (Figure 4). Elderly patients were more likely to receive an antibiotic for URTI compared with children while the reverse was found for otitis externa and otitis media. Males and females had comparable probabilities of getting an antibiotic for most indications, except sinusitis and UTI, where females were more likely to receive an antibiotic across all ages.
Figure 4.
Percentage of patients receiving an antibiotic for each common infection stratified by age and sex (based on fractional polynomials in logistic models). Dashed lines represent 95% CI.
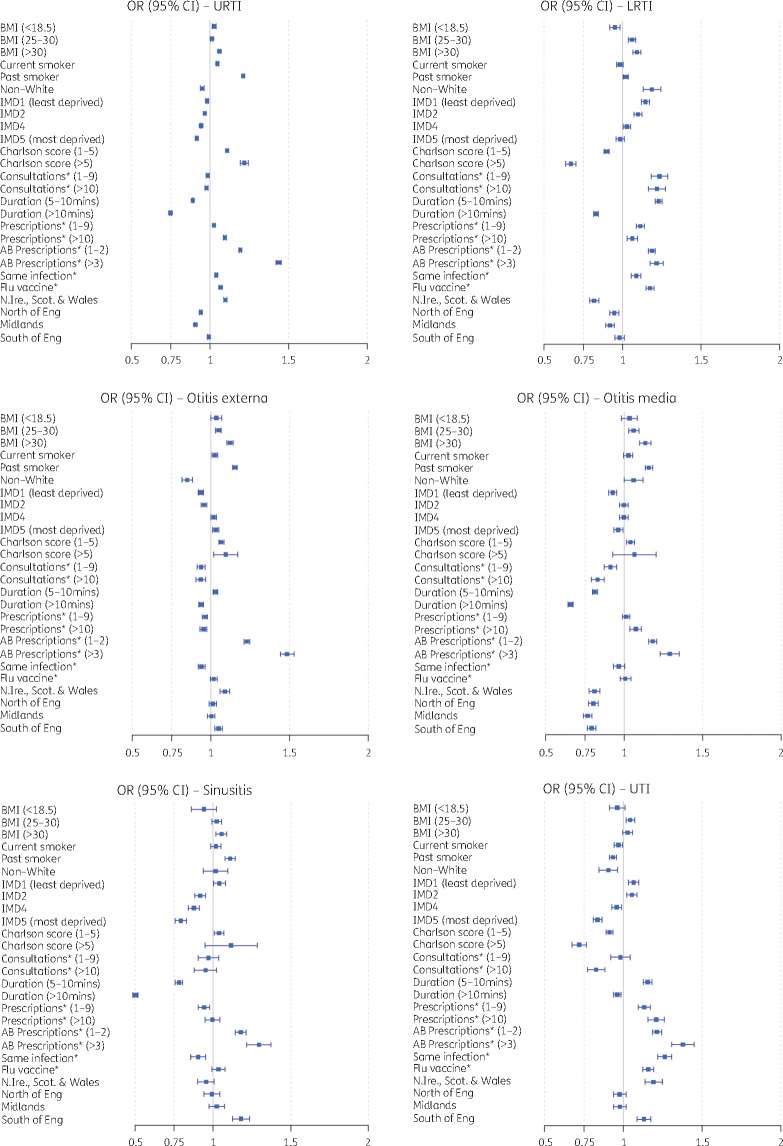
As differences in age and sex were observed, a matched cohort was used to identify predictors of antibiotic prescribing. Consistencies and inconsistencies were observed for each infectious condition when examining the predictors of antibiotic prescribing (Figure 5). The greatest increase in odds of receiving an antibiotic prescription was associated with patients that had received one or more antibiotic prescriptions in the previous 12 months. Patients that had one or two antibiotic prescriptions were 9%–23% more likely to be prescribed an antibiotic compared with patients that received no antibiotic prescriptions during the previous year. These odds increased with the number of antibiotics a patient received. Patients with three or more antibiotic prescriptions had 22%–48% increased odds of receiving an antibiotic for all infectious conditions examined.
Figure 5.
Adjusted ORs (filled squares) with 95% CIs (lines) for drivers of antibiotic prescribing for each common infection. Reference categories were as follows: BMI, 20–25 mg/kg2; smoking history, non-smoker; ethnicity, white; IMD, 3; Charlson comorbidity score, 0; number of consultations*, 0; duration of consultations*, 0–5 min; number of non-antibiotic prescriptions*, 0; number of antibiotic (AB) prescriptions*, 0; same infection*, no; influenza (flu) vaccine*, no; region of practice, London. Asterisks indicate data 12 months prior to the index date. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
The odds of receiving an antibiotic also increased across all infections (except for UTI) for patients with an overweight or obese BMI (by 1%–13%) and past smokers (by 1%–21%). Influenza vaccines in the previous 12 months increased the likelihood of receiving an antibiotic prescription by 3% (sinusitis) to 17% (LRTIs), but had no effect for ear-related infections.
Other predictors of receiving an antibiotic varied considerably for each infection (Figure 5). Higher comorbidity score increased the odds of receiving an antibiotic for URTIs (11%–22%), but the opposite was observed for LRTIs and UTIs, with lower odds of receiving an antibiotic with greater comorbidity score (10%–33% and 9%–29% less likely, respectively). Similarly, a previous history of the same infection in the year before increased patient odds of receiving an antibiotic for both RTIs and UTIs (9% and 20%, respectively), but reduced the odds for antibiotic prescribing for sinusitis and ear-related infections up to 10%. Other predictors of prescribing that demonstrated variability across all infectious conditions included ethnicity, socioeconomic status, practice location, the duration of the consultation, the number of non-antibiotic prescriptions and the number of consultations in the previous 12 months.
Discussion
This is the first study to simultaneously investigate multiple common infectious conditions to determine which factors drive the decision to prescribe an antibiotic. Antibiotic prescribing rates were not influenced by updates to prescribing guidelines. A lack of association was observed in prescribing rates for each infectious condition at the practice level. Considerable variability was observed with respect to which patient characteristics influence antibiotic prescribing. Particularly high prescribing was observed for cases of RTIs, UTIs and ear-related infections, which included a large proportion of middle-aged, otherwise healthy patients that would probably recover if left untreated. This is consistent with previous studies, where there were significant levels of antibiotic prescribing for likely viral infections.3,10,20,21
The number of antibiotics a patient received in the previous year significantly increased the odds of receiving subsequent antibiotic treatment, similar to findings by Shallcross et al. (2017),22 where heavy users of antibiotics also received at least five antibiotic prescriptions in the previous 3 years. Part of this may be appropriate and risk/need-based due to underlying medical conditions. However, a patient who received an antibiotic in a previous consultation may have an increased expectation of receiving an antibiotic in subsequent similar consultations.23 This may be an important target for antimicrobial stewardship programmes.
Antibiotic prescribing has been reported to be higher in socio-economically deprived populations8,18,24,25 because GPs perceive these patients to have an increased risk of complications.26 A Scottish study observed increased antibiotic prescribing for less-affluent patients across all age categories in 2010–12.8 However, in our study, in a longer but contiguous timeframe, the association between a patient’s deprivation status and the probability of getting an antibiotic was inconsistent. Patients with a lower socio-economic status had greater odds of receiving an antibiotic for outer ear infections, but lower odds for URTIs, LRTIs, UTIs, inner ear infections and sinusitis. There may be several reasons for these inconsistent findings. We evaluated the effects of socioeconomic status by each infectious condition in patients who had no other recent prescription for antibiotics (incidental users), while published studies evaluated the effect of patient deprivation on overall rates of prescribing irrespective of indication and do not stratify the population with respect to incidental use. Also, studies often investigated the effect of patient deprivation on prescribing in selected populations, such as children27 or the elderly,24 but generalize their findings to all patients with a lower socioeconomic status.
A higher incidence of deprivation and health inequality across regions in the north of England exists,18,28,29 indicating practices with more deprived communities and particularly practices located in the north of England prescribe antibiotics 2-fold more than practices in the south.18,25 This study found large variability in antibiotic prescribing by region for each infectious condition, supporting the findings of Dolk et al.3 However, in our study matching patients by age, sex and calendar time, the north–south divide was reversed for some conditions and completely removed for others. The underlying reasons for this variation between prescribing are only partly understood.
A study of paediatric centres found that prescribing variability could not be explained by patient-specific factors alone.30 Prescriber behaviour also varies significantly.31 Older GPs may prescribe antibiotics more often than newly qualified GPs with UK training18 and increased prescribing is associated with higher GP workload.9 Prescriber gender may influence prescribing32,33 and prescribers are influenced by peer social norms.34 National guidelines for the treatment of common infections alone are important but not sufficient to optimize the prescribing of antibiotics,35 but more research is needed to understand inter-practice dynamics and how these influence prescribing.
The focus of current guidelines is largely symptom-based, for example FeverPAIN for acute sore throat.36 Patient factors (including age and comorbidity) are also important influences on the decision to prescribe antibiotics.22,37 However, this study found high prescribing rates for young and middle-aged adults with no pre-existing health issues, suggesting prescribing may be more symptom-based or patient demand-based,38 which is associated with poorer prescribing quality39 than risk-related prescribing. This is supported by a study looking at drivers for the variability in the rate of antibiotic prescribing in UK general practices, which suggests that the influence of overall patient health in the decision to prescribe is limited,20 and in the current study, where patients with similar risk profiles (such as Charlson scores) were also treated differently between different common infections. This variability in antibiotic prescribing after adjustment for age, gender and calendar time confirms that more consistent prescribing and optimization in decision making, such as shared decision making, could impact prescribing40 and are essential if we are to reduce inappropriate overuse and thus avoidable resistance.
Antimicrobial stewardship programmes can reduce prescribing.41 However, the lack of association between prescribing rates for each condition within a practice, a novel finding, emphasizes the need for future stewardship interventions and guideline development to focus at practice and patient level to be effective. These interventions should also consider that a practice may perform well for one indicator but poorly for others.42
In non-infectious conditions, risk models are available to support prescribing decisions. As an example, statin prescribing is now mostly based on a patient’s overall risk of developing cardiovascular disease. To optimize antibiotic prescribing and reduce the variability, developing and implementing systematic approaches based on a patient’s risk of poor outcomes or infection-related complications (such as hospital admission for pneumonia) could be of value. These risks could be calculated based not only on the patient’s symptom score but also on their age, any underlying diseases and other risk factors, such as IMD.
The strengths of this study included the study size: >5 million patient-level health records for up to 15 years, larger than previous studies.3,20,22 The analysis was matched and was able to look at the effect of multiple drivers across many common infectious conditions. Unlike most studies that observe antibiotic prescribing, this study selected a population of incident antibiotic users to determine the association of drivers of antibiotic prescribing for new incidences of infection, removing bias from repeat prescription and heavy users in the general population.
The main limitation was that we did not have data on the severity and type of symptoms as these are not well recorded in EHRs. However, it is unlikely that our finding of large variability in antibiotic prescribing can be explained by different types and severities of symptoms. It may be very useful if symptom scores of common infections could be captured more systematically and analysed for use in systematic decision support.43 We evaluated prescribed antibiotics rather than antibiotics dispensed or taken by patients. An estimated 13.3% of antibiotic prescriptions are for delayed antibiotics.44 This information was unavailable in the current study but future analysis could be stratified by prescribing strategy. Similarly, future investigation of prescribing variability over time should take into consideration the uptake of delayed prescribing, adjusting for the rate of change. Medication compliance has been reported to be 70%–80% for short-term therapy,45 so the majority of patients who collected their prescription are likely to have taken the full prescribed course of antibiotics. Read codes were grouped by infectious condition and used to select cases and controls with an overall infectious term. Some GPs code diagnoses differently according to whether or not they prescribe an antibiotic, which may have created some bias in the analysis; however, an additional matching by specific Read codes would dramatically reduce the sample size and therefore statistical power. Random sampling was conducted when patients had multiple consultations for the same infection, which may have introduced bias as the more consultations the patient had the greater the odds of receiving an antibiotic prescription. Sensitivity analysis (including interaction terms in a model between all the key predictors and the variable ‘same infection 12 months before’) did not show a consistent pattern for the interaction terms and lacked significance, suggesting that bias was not introduced by selecting one event at random. There was a large proportion of missing data for BMI, smoking history and patient IMD score. This was accounted for by applying a robust, well-established method to impute missing values,46 as well as adjusting the models with a missing indicator.
Important drivers for antibiotic prescribing were age, sex, region, level of comorbidities and prior antibiotic prescribing and deprivation. A change to antibiotic prescribing at practice level over time was not associated with updates to national prescribing guidelines. Antimicrobial stewardship programmes need to be tailored to the specific challenges in a general practice and interventions may be more effective at the practice level. Interventions are needed to support practitioners during a consultation, such as risk models and decision support tools with up-to-date information on local guidelines.
Supplementary Material
Acknowledgements
This study is based on data from the Clinical Practice Research Datalink obtained under licence from the UK Medicines and Healthcare Products Regulatory Agency (MHRA). The data are provided by patients and collected by the NHS as part of their care and support.
Funding
Connected Health Cities is a Northern Health Science Alliance (NHSA)-led programme funded by the Department of Health and delivered by a consortium of academic and NHS organizations across the north of England.
Transparency declarations
None to declare.
Disclaimer
The interpretation and conclusions contained in this study are those of the authors alone, and not necessarily those of the MHRA, NHSA, NHS or the Department of Health.
References
- 1. O'Neill J. Tackling Drug-resistant Infections Globally: Final Report and Recommendations. The Review on Antimicrobial Resistance 2016. https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf.
- 2.PHE. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR). London, 2018. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/759975/ESPAUR_2018_report.pdf.
- 3. Dolk FCK, Pouwels KB, Smith DRM. et al. Antibiotics in primary care in England: which antibiotics are prescribed and for which conditions? J Antimicrob Chemother 2018; 73 Suppl 2: ii2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guthrie B, Yu N, Murphy D. et al. Measuring Prevalence, Reliability and Variation in High-Risk Prescribing in General Practice Using Multilevel Modelling of Observational Data in a Population Database. Southampton, UK, 2015. https://www.ncbi.nlm.nih.gov/books/NBK322054. [PubMed] [Google Scholar]
- 5. Stocks SJ, Kontopantelis E, Akbarov A. et al. Examining variations in prescribing safety in UK general practice: cross sectional study using the Clinical Practice Research Datalink. BMJ 2015; 351. doi:10.1136/bmj.h5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tyrstrup M, Van der Velden A, Engstrom S. et al. Antibiotic prescribing in relation to diagnoses and consultation rates in Belgium, the Netherlands and Sweden: use of European quality indicators. Scand J Prim Health 2017; 35: 10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Steinberg MB, Akincigil A, Kim EJ. et al. Tobacco smoking as a risk factor for increased antibiotic prescription. Am J Prev Med 2016; 50: 692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Covvey JR, Johnson BF, Elliott V. et al. An association between socioeconomic deprivation and primary care antibiotic prescribing in Scotland. J Antimicrob Chemother 2014; 69: 835–41. [DOI] [PubMed] [Google Scholar]
- 9. Serna MC, Real J, Ribes E. et al. Factors determining antibiotic prescription in primary care. Enferm Infecc Microbiol Clin 2011; 29: 193–200. [DOI] [PubMed] [Google Scholar]
- 10. Brauer R, Ruigomez A, Downey G. et al. Prevalence of antibiotic use: a comparison across various European health care data sources. Pharmacoepidemiol Drug Saf 2016; 25: 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williams T, van Staa T, Puri S. et al. Recent advances in the utility and use of the General Practice Research Database as an example of a UK Primary Care Data resource. Ther Adv Drug Saf 2012; 3: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Campbell JD, Dedman DJ, Eaton SC. et al. Is the CPRD GOLD population comparable to the U.K. population? Pharmacoepidemiol Drug Saf 2013; 22: 280. [Google Scholar]
- 13. Herrett E, Gallagher AM, Bhaskaran K. et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015; 44: 827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Department for Communities and Local Government. The English Index of Multiple Deprivation (IMD) 2015—Guidance London, 2015. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/464430/English_Index_of_Multiple_Deprivation_2015_-_Guidance.pdf.
- 15. Killick R, Eckley IA.. changepoint: An R Package for Changepoint Analysis Journal of Statistical Software 2014. https://www.jstatsoft.org/article/view/v058i03/v58i03.pdf.
- 16. Royston P, Altman DG.. Regression using fractional polynomials of continuous covariates: parsimonious parametric modelling (with discussion). J R Stat Soc 1994; 43: 429–67. [Google Scholar]
- 17. Kontopantelis E, Parisi R, Springate DA. et al. Longitudinal multiple imputation approaches for body mass index or other variables with very low individual-level variability: the mibmi command in Stata. BMC Res Notes 2017; 10: 41.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang KY, Seed P, Schofield P. et al. Which practices are high antibiotic prescribers? A cross-sectional analysis. Br J Gen Pract 2009; 59: e315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Falagas ME, Karageorgopoulos DE.. Adjustment of dosing of antimicrobial agents for bodyweight in adults. Lancet 2010; 375: 248–51. [DOI] [PubMed] [Google Scholar]
- 20. Pouwels KB, Dolk FCK, Smith DRM. et al. Explaining variation in antibiotic prescribing between general practices in the UK. J Antimicrob Chemother 2018; 73 Suppl 2: ii27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gulliford MC, Dregan A, Moore MV. et al. Continued high rates of antibiotic prescribing to adults with respiratory tract infection: survey of 568 UK general practices. BMJ Open 2014; 4: e006245.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shallcross L, Beckley N, Rait G. et al. Antibiotic prescribing frequency amongst patients in primary care: a cohort study using electronic health records. J Antimicrob Chemother 2017; 72: 1818–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McNulty CA, Nichols T, French DP. et al. Expectations for consultations and antibiotics for respiratory tract infection in primary care: the RTI clinical iceberg. Br J Gen Pract 2013; 63: e429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Odubanjo E, Bennett K, Feely J.. Influence of socioeconomic status on the quality of prescribing in the elderly—a population based study. Br J Clin Pharmacol 2004; 58: 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilson RP, Hatcher J, Barton S. et al. The association of some practice characteristics with antibiotic prescribing. Pharmacoepidemiol Drug Saf 1999; 8: 15–21. [DOI] [PubMed] [Google Scholar]
- 26. Kumar S, Little P, Britten N.. Why do general practitioners prescribe antibiotics for sore throat? Grounded theory interview study. BMJ 2003; 326: 138.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Henricson K, Melander E, Molstad S. et al. Intra-urban variation of antibiotic utilization in children: influence of socio-economic factors. Eur J Clin Pharmacol 1998; 54: 653–7. [DOI] [PubMed] [Google Scholar]
- 28. Buchan IE, Kontopantelis E, Sperrin M. et al. North-South disparities in English mortality 1965-2015: longitudinal population study. J Epidemiol Community Health 2017; 71: 928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Millett ER, Quint JK, Smeeth L. et al. Incidence of community-acquired lower respiratory tract infections and pneumonia among older adults in the United Kingdom: a population-based study. PLoS One 2013; 8: e75131.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gerber JS, Prasad PA, Russell Localio A. et al. Variation in antibiotic prescribing across a pediatric primary care network. J Pediatric Infect Dis Soc 2015; 4: 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cordoba G, Siersma V, Lopez-Valcarcel B. et al. Prescribing style and variation in antibiotic prescriptions for sore throat: cross-sectional study across six countries. BMC Fam Pract 2015; 16: 7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lazkani A, Delespierre T, Benattar-Zibi L. et al. Do male and female general practitioners differently prescribe chronic pain drugs to older patients? Pain Med 2015; 16: 696–705. [DOI] [PubMed] [Google Scholar]
- 33. Hoffmann K, Paget J, Wojczewski S. et al. Influenza vaccination prevalence and demographic factors of patients and GPs in primary care in Austria and Croatia: a cross-sectional comparative study in the framework of the APRES project. Eur J Public Health 2016; 26: 395–401. [DOI] [PubMed] [Google Scholar]
- 34. Pedersen IK, Jepsen KS.. Prescribing antibiotics: general practitioners dealing with “non-medical issues”? P&P 2018; 8: e1983. [Google Scholar]
- 35. Hawker JI, Smith S, Smith GE. et al. Trends in antibiotic prescribing in primary care for clinical syndromes subject to national recommendations to reduce antibiotic resistance, UK 1995-2011: analysis of a large database of primary care consultations. J Antimicrob Chemother 2014; 69: 3423–30. [DOI] [PubMed] [Google Scholar]
- 36.Greater Manchester Shared Services. Greater Manchester Antimicrobial Guidelines Greater Manchester, 2018. http://gmmmg.nhs.uk/docs/guidance/GM-Antimicrobial-guidelines-Amended-March-2019-v3-0.pdf.
- 37.PHE. Managing common infections: guidance for primary care. PHE Publications: Public Health England, 2017. https://www.gov.uk/government/publications/managing-common-infections-guidance-for-primary-care. [Google Scholar]
- 38. Brabers AE, Van Esch TE, Groenewegen PP. et al. Is there a conflict between general practitioners applying guidelines for antibiotic prescribing and including their patients' preferences? Patient Prefer Adherence 2018; 12: 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grant A, Sullivan F, Dowell J.. An ethnographic exploration of influences on prescribing in general practice: why is there variation in prescribing practices? Implement Sci 2013; 8: 72.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Esch TEM, Brabers AEM, Hek K. et al. Does shared decision-making reduce antibiotic prescribing in primary care? J Antimicrob Chemother 2018; 73: 3199–205. [DOI] [PubMed] [Google Scholar]
- 41. McNulty C, Hawking M, Lecky D. et al. Effects of primary care antimicrobial stewardship outreach on antibiotic use by general practice staff: pragmatic randomized controlled trial of the TARGET antibiotics workshop. J Antimicrob Chemother 2018; 73: 1423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Roosmalen MS, Braspenning JCC, De Smet P. et al. Antibiotic prescribing in primary care: first choice and restrictive prescribing are two different traits. Qual Saf Health Care 2007; 16: 105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Delaney BC, Curcin V, Andreasson A. et al. Translational medicine and patient safety in Europe: TRANSFoRm–architecture for the learning health system in Europe. Biomed Res Int 2015; 2015: 961526.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Little P, Stuart B, Smith S. et al. Antibiotic prescription strategies and adverse outcome for uncomplicated lower respiratory tract infections: prospective cough complication cohort (3C) study. BMJ 2017; 357. doi:10.1136/bmj.j2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. DiMatteo MR. Patient adherence to pharmacotherapy: the importance of effective communication. Formulary 1995; 30: 596–8, 601–2, 5. [PubMed] [Google Scholar]
- 46. Donders AR, van der Heijden GJ, Stijnen T. et al. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol 2006; 59: 1087–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.