SUMMARY
The genome of Musa balbisiana spp. contains several infectious endogenous sequences of Banana streak virus (eBSV). We have shown previously that in vitro micropropagation triggers the activation of infectious eBSOLV (endogenous sequences of Banana streak Obino l'Ewai virus) in the synthetic tetraploid interspecific hybrid FHIA21 (AAAB). In this work, we show that another synthetic tetraploid (AAAB) hybrid and two natural triploid (AAB) plantains are equally prone to the activation of infectious eBSOLV during tissue culture. These results are a strong indication that such activation is a general phenomenon in interspecific Musa cultivars, whether synthetic or natural. We also report the first in‐depth study of the correlation between the duration of tissue culture and the level of activation of infectious eBSOLV, and show that specific and common activation patterns exist in these banana plants. We hypothesize that these patterns result from the concomitant activation of infectious eBSOLV and a decrease in the virus titre in neoformed plantlets, resulting from cell multiplication outcompeting virus replication. We provide experimental data supporting this hypothesis. No activation of infectious eBSGFV (endogenous sequences of Banana streak Goldfinger virus) by tissue culture was observed in the two natural AAB plantain cultivars studied here, whereas such activation occurred in the AAAB synthetic hybrid studied. We demonstrate that this differential activation does not result from differences in the structure of eBSGFV, as all banana genomes harbour eaBSGFV‐7.
INTRODUCTION
The nuclear genome of numerous plants is invaded by a large number of viruses mainly belonging to the family Caulimoviridae. These integrations are named endogenous pararetrovirus sequences (EPRVs), and are thought to be relics of ancient infection events. Pararetroviruses neither integrate their hosts' genome nor encode an integrase function during viral replication (Harper et al., 2002; Hohn et al., 2008). Therefore, EPRVs probably integrated their hosts' genome by illegitimate recombination between host and viral DNA. EPRVs range from small, truncated fragments to larger rearranged sequences containing more than one copy of the viral genome. Some EPRVs are infectious because they have the potential to reconstitute the functional viral genome following activating stresses that contribute to viral infection. This is the case for sequences of Banana streak virus (BSV) integrated in the Musa balbisiana banana genome (denoted B) and named eBSV (endogenous BSV sequences) (Staginnus et al., 2009). Infectious eBSV can be activated by abiotic stresses, such as micropropagation by in vitro culture processes (Dallot et al., 2001), temperature differences (1998, 2000) or water stress, and after genetic hybridization (Lheureux et al., 2003), leading to infectious virions.
Banana streak viruses are plant bacilliform pararetroviruses containing a double‐stranded DNA genome of 7.4 kbp, belonging to the family Caulimoviridae and the genus Badnavirus, and are transmitted by mealybugs. Potentially infectious eBSV of four natural widespread BSV species have so far been identified in the B genome: Banana streak Obino l'Ewai virus (BSOLV), Banana streak Imové virus (BSImV), Banana streak Mysore virus and Banana streak Goldfinger virus (BSGFV) (Gayral et al., 2008; Geering et al., 2005; Harper et al., 1999; Iskra‐Caruana et al., 2003; Ndowora et al., 1999). It is hypothesized that the emergence of BSV infection in many micropropagated triploid (AAB) and tetraploid (AAAB) interspecific improved Musa hybrids, which has been observed in the last 15 years, very probably results from the triggering of eBSV (Fauquet et al., 2005; Hull et al., 2000).
The majority of cultivated bananas originate from inter‐ and intraspecific crosses between the two wild diploid species, Musa acuminata (denoted A genome) and M. balbisiana (denoted B genome). The B genome is the genome of many important cultivars, such as the famous plantain subgroup, which is a staple food for millions of people in West and Central Africa and in Latin America and the Caribbean (Jones, 2000). Nowadays, tissue culture remains the most appropriate method for supplying large quantities of clonal Musa planting material, the demand for which has been increasing steadily for the past 15 years, in particular for plantain. Unfortunately, the process of in vitro culture has been reported to systematically trigger the activation of infectious eBSV present in newly created interspecific hybrids. Indeed, Dallot et al. (2001) have shown that the proliferation stage of the in vitro micropropagation process triggers the expression of infectious eBSOLV in virus‐free suckers of the improved tetraploid interspecific hybrid FHIA21 (AAAB). This process greatly affects the plantlets, as 58% of micropropagated lines were affected by BSV after six in vitro subcultures.
Nevertheless, the risk of activating eBSV following tissue culture has never been assessed in BSV species other than BSOLV and in natural interspecific cultivars containing the B genome. Several natural cultivars are regularly used to supply the increasing demand for diversification.
In this article, we report an extensive study of the spontaneous activation of eBSV of two distinct viral species (BSOLV and BSGFV) in natural plantain cultivars and synthetic interspecific hybrids by in vitro culture. We investigate the correlation between the duration of tissue culture and the level of activation of infectious eBSVs, and between the activation potential of infectious eBSVs and their molecular structure. We also establish the cleaning effect of in vitro culture in BSV multiplication from BSV‐infected plants.
RESULTS
Micropropagation by tissue culture triggers the expression of infectious eBSOLV in both natural and synthetic interspecific plantain cultivars
In this study, we used two natural triploid plantains Kelong Mekintu (AAB) and Black Penkelon (AAB), and one synthetic tetraploid plantain CRBP 39 (AAAB). The activation patterns of infectious eBSOLV, monitored by multiplex immunocapture polymerase chain reaction (M‐IC‐PCR), during the regeneration process are shown in Fig. 1A,C,E, respectively. The patterns were similar for the three cultivars studied and showed three distinct phases. During the first subculture cycles, there was a steep increase in the percentage of infected plants, until the number of total produced shoots (TPSs) reached values between 300 and 400. Then the percentage of infected plants increased more slowly until the number of TPSs reached values of 1200 (CRBP 39, Fig. 1E) and 2500/2000 [Kelong Mekintu (Fig. 1A)/Black Penkelon (Fig. 1C)]. The highest percentage of infected plants was reached at the end of this second phase and ranged from 9% to 20% depending on the cultivar. Immediately afterwards, there was a decrease in the percentage of infected plants for high TPS values. This decrease was slower in the cultivars Kelong Mekintu and Black Penkelon than in cultivar CRBP39, in which values of zero (i.e. below the sensitivity threshold of M‐IC‐PCR) were observed for TPS values of 4000 and above (Fig. 1E).
Figure 1.
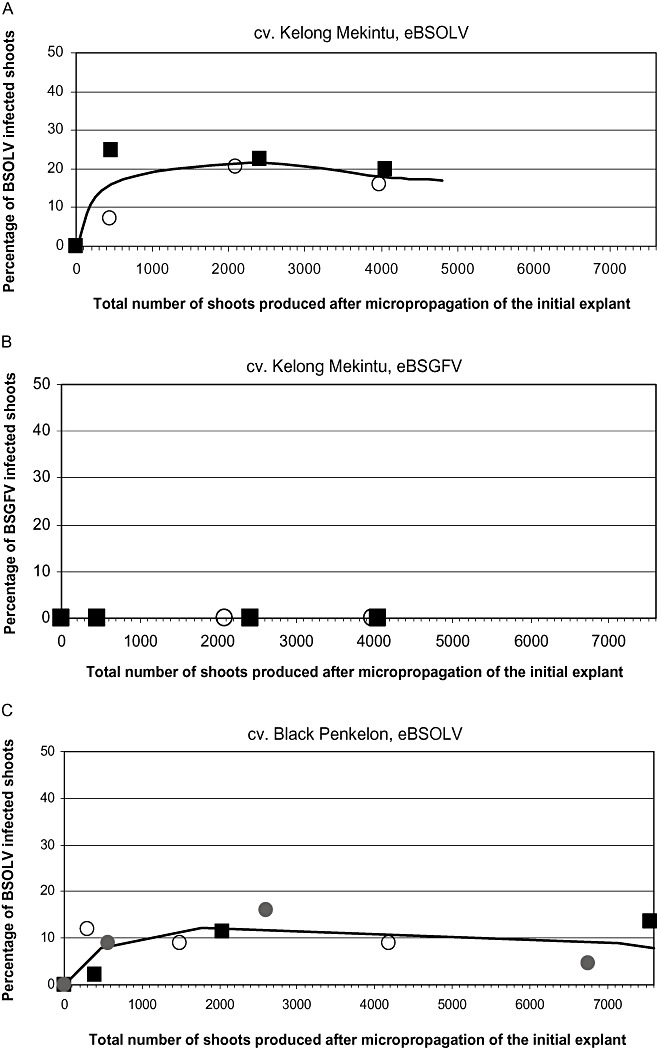

Activation patterns of infectious eBSOLV (endogenous sequences of Banana streak Obino l'Ewai virus) (A, C, E) and eBSGFV (endogenous sequences of Banana streak Goldfinger virus) (B, D, F) in cultivars Kelong Mekintu (A, B), Black Penkelon (C, D) and CRBP39 (E, F). For each cultivar, the results are shown for three (Black Penkelon, CRBP39) or two (Kelong Mekintu) distinct lines.
Micropropagation by tissue culture triggers the expression of infectious eBSGFV in the synthetic hybrid CRBP39 only
The activation pattern of infectious eBSGFV was assessed in the three cultivars studied using the same plantain lines, experimental approaches and leaf extracts as above. No activation was observed in cultivars Black Penkelon and Kelong Mekintu (Fig. 1B,D). In contrast, activation of infectious eBSGFV was observed in the three lines of CRBP39. Figure 1F shows similar activation patterns as that described above for eBSOLV, whatever the cultivar. However, the maximum percentage of infected plants was lower for BSGFV than for BSOLV (5%–7% vs. 7%–15%; Fig. 1E,F).
Origin of the decrease in the percentage of BSV‐infected plants observed for high TPS values
In order to explain the decrease in infected plants observed at high TPS values, four distinct BSOLV‐infected CRBP39 proliferation clumps were selected and used as starting material to produce vitroplants. The percentage of BSOLV‐infected vitroplants regenerated from each infected clump was monitored by M‐IC‐PCR. Figure 2 shows that the percentage decreased steadily over time in the four regenerated lines and reached zero values in all four lines for TPS values between 100 and 400.
Figure 2.
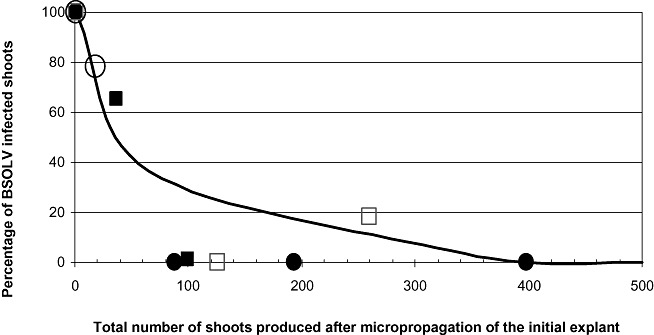
Infection patterns of BSOLV (Banana streak Obino l'Ewai virus) during micropropagation of CRBP39 plants infected by BSOLV. Symbols in the figure are the results for four distinct lines. The curve represents the mean curve of these lines.
eBSGFV signature in both natural and synthetic interspecific plantain species
The eBSGFV signature was established for Kelong Mekintu, Black Penkelon and CRBP39 using sets of eight PCR markers (Gayral, 2008). These PCR markers are specific to the allelic eBSGFV of the natural diploid M. balbisiana Pisang Klutuk Wulung—PKW (BB), named eaBSGFV‐7 and eBSGFV‐9 (Gayral et al., 2008; Staginnus et al., 2009). One set of primers (VV5F/R) was specific to eBSGFV‐9. All natural cultivars and the synthetic hybrid, including FHIA21 as control, displayed the same patterns characteristic of an eaBSGFV‐7 signature (Fig. 3). No PCR was recorded with either VV5F/R or VV5bisF/R primers.
Figure 3.
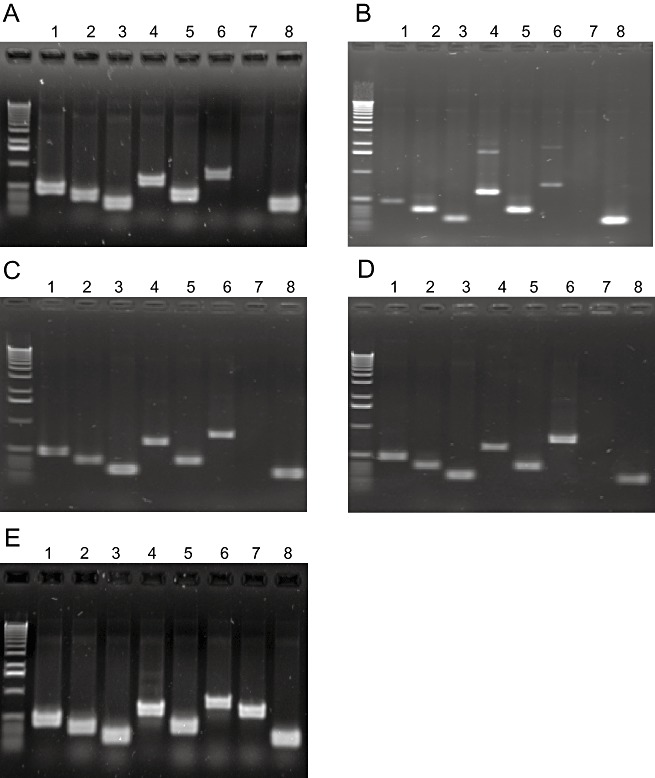
Molecular signature obtained by polymerase chain reaction (PCR) analysis of infectious eBSGFV (endogenous sequences of Banana streak Goldfinger virus) in cultivars CRBP39 (A), FHIA21 (B), Kelong Mekintu (C), Black Penkelon (D) and Pisang Klutuk Wulung (E). Primer pair numbers refer to Table 1; 1–8 refer to primer pairs VM1F/R, VM2F/R, VV1F/R, VV2F/R, VV3F/R, VV4F/R, VV5F/R and VV6F/R.
DISCUSSION
Among the growing number of EPRVs characterized so far in the genomes of crops, only endogenous sequences of Petunia vein clearing virus (ePVCV), Tobacco vein clearing virus (eTVCV) and Banana streak virus (eBSV) have the potential to generate infectious viral particles on activation by abiotic stresses (Staginnus and Richert‐Pöggeler, 2006). Although substantial progress has been made in the genomic characterization of eBSV (Gayral et al., 2008) and the molecular regulation of the expression of ePVCV (Noreen et al., 2007), little is known about the exact mechanisms underlying the expression of functional viral genomes from eBSV.
The pioneering work of Dallot et al. (2001) demonstrated that shoot tip micropropagation triggers the expression of infectious eBSOLV in the synthetic tetraploid interspecific hybrid FHIA21 (AAAB). It has since been shown that the genome of M. balbisiana hosts distinct infectious eBSV originating from at least four BSV species, including BSOLV (Iskra‐Caruana et al., 2003; Ndowora et al., 1999). Whether the activation of eBSOLV occurs in other synthetic interspecific hybrids and in natural interspecific cultivars, and whether all infectious eBSVs are equally triggered during tissue culture, is still unknown. The work reported here provides answers to some of these questions.
Our results showed that infectious eBSOLV was expressed during tissue culture in CRBP39, a synthetic tetraploid interspecific hybrid (AAAB) other than FHIA21, as well as in Kelong Mekintu and Black Penkelon, two natural triploid plantains (AAB). The maximum percentages of BSOLV‐infected plantlets monitored in our assays ranged from 5% to 20% depending on the cultivar, but were consistently below 30%. These values are lower than those reported by Dallot et al. (2001) for the FHIA21 hybrid, but are in agreement with those reported by Meyer et al. (2008) for the same hybrid. The activation of infectious eBSOLV took place in a limited number of cells and did not occur in the entire shoot tip.
Dallot et al. (2001) studied the activation of infectious eBSOLV over six proliferation cycles. Our results confirmed that a strong increase in the percentage of infected plants occurred during the first subcultures. Extending the time frame of our study to 9–12 proliferation cycles revealed a decrease in the percentage of BSOLV‐positive plantlets for high TPS values in all of the lines of the three cultivars used in this study. The TPS values at which this decrease occurred varied among the cultivars. We hypothesize that this decrease results from the conjunction of two distinct biological phenomena: (i) the activation of infectious eBSOLV, resulting in an increase in BSOLV‐infected plants; and (ii) a concomitant decrease in the episomal particle titre in neoformed plantlets, resulting from cell multiplication outcompeting virus replication. Our experimental data support this hypothesis. They showed that the proportion of infected vitroplants regenerated by tissue culture from a BSOLV‐infected CRBP39 plant decreased steadily over time, and that no infected plantlet could be detected from TPS values between 100 and 400 (see Fig. 2). Thus micropropagation by tissue culture results in a cleaning of BSV in banana, usually referred to as the dilution effect. This phenomenon is often exploited to regenerate healthy plantlets from virus‐infected mother plants, e.g. to regenerate healthy banana plants from M. acuminata mother plants (Helliot et al., 2002). However, it is important to note that all Musa genotypes harbouring infectious eBSV still retain their capacity to exhibit virus infection during in vitro culture. The virus‐free regenerated CRBP39 plants displayed activation patterns of both infectious eBSOLV and eBSGFV, similar to those shown in Fig. 1E,F (data not shown).
We observed that all CRBP39 plantlets generated after TPS = 4000 were BSOLV free. This was not the case in the two other cultivars. The percentage of BSOLV‐infected shoots during the first phase of activation was slower in CRBP39 than in Kelong Mekintu and Black Penkelon (Fig. 1A,C,E). Whether such differential activation of eBSOLV results from structural differences, such as the number of infectious eBSV activated or the localization in the B genome, or differences in the regulation of expression, remains to be investigated.
Nevertheless, taken together, these results are a strong indication that the activation of infectious eBSOLV by tissue culture is a general phenomenon in interspecific Musa cultivars, whether synthetic or natural.
Conversely, our work showed that infectious eBSGFV was activated in all CRBP39 lines, but not in Kelong Mekintu and Black Penkelon. This indicates that differential activation does not result from structural differences in infectious eBSGFV insertions among the cultivars studied, as they all harboured the infectious eaBSGFV‐7 allele at the same locus in the B genome. The observed differential activation pattern might result from differences in the regulation of expression of eaBSGFV‐7, as infectious eBSOLV is activated in Kelong Mekintu and Black Penkelon. Whether such differences are related to the ploidy of the cultivars—triploid vs. tetraploid—or to their respective M. acuminata background remains to be investigated.
In CRBP39, infectious eBSOLV and eBSGFV displayed different maximum activation levels, indicating that the activated cells could express BSOLV or BSGFV, or both BSOLV and BSGFV. This indicates an independent regulation of the expression of each infectious eBSV in CRBP39.
These differences indicate that the activation potential differs between cultivars for a given infectious eBSV and between infectious eBSV within given interspecific Musa genotypes.
Our results have profound implications for the management of eBSV in interspecific Musa cultivars. They show that the mass micropropagation technique cannot be considered to be safe because of the risk of activating infectious eBSV. This work highlights the risk of promoting outbreaks of BSV by large‐scale distribution of risky or infected vitroplants of interspecific banana and plantain genotypes. Considering the increasing demand for Musa germplasm worldwide, there is a need for more research efforts to identify alternative mass propagation of interspecific Musa germplasm and to further investigate the activation mechanisms of infectious eBSV in order to develop and implement inactivating strategies.
EXPERIMENTAL PROCEDURES
Plant material
Two natural triploid (AAB) plantain varieties, ‘Kelong Mekintu’ and ‘Black Penkelon’, and one synthetic tetraploid (AAAB) hybrid plantain, CRBP39, were used for this study. These varieties are part of the ex situ Musa collection of the Centre Régional de Recherche sur Bananier et Plantain, Cameroon (CARBAP), and were kindly provided by CARBAP.
Virus indexing
Leaf samples from all suckers used for tissue culture and from their respective mother plants were symptomless and, prior to tissue culture, were subjected to full indexing for all known viruses that infect Musa spp. using the appropriate enzyme‐linked immunosorbent assay (ELISA) or PCR‐based detection techniques. Indexing for BSV was performed on leaf tissues or shoots of proliferation clumps by M‐IC‐PCR using primers specific for each BSV species, analysed as described by Le Provost et al. (2006). The absence of viral particles in the suckers used for tissue culture was further investigated by observations of semipurified extracts from all leaf samples by electron microscopy.
Tissue culture and kinetics of expression of infectious eBSOLV during micropropagation
Vitroplants were produced from selected virus‐free suckers according to the in vitro budding method described by Côte et al. (1990). Distinct suckers were used to establish at least two independent lines for each banana cultivar. The kinetics of activation of infectious eBSOLV and eBSGFV were studied in each line during the multiplication (proliferation) process. Forty‐four shoots were randomly selected from proliferation clumps for virus indexing at each subculture step. After grinding, each shoot was tested for both BSOLV and BSGFV expression by M‐IC‐PCR using primers specific for each BSV species, analysed as described by Le Provost et al. (2006). The shoots not selected for virus indexing were divided into two parts: one‐half was removed and the other half was transferred to fresh culture medium for multiplication.
Considering that Musa accessions and even suckers of a given accession display different proliferation rates, resulting in different numbers of plantlets being produced after a given number of subcultures, the results were expressed as the percentage of BSV‐infected plantlets per number of TPSs. This facilitated the comparison of the percentages of infected plantlets. TPS is defined here as TPS =pn, where p is the proliferation rate and n is the number of subcultures. TPS is therefore directly proportional to the number of subcultures. The percentage of BSV‐infected plantlets was measured for TPS values between 200 and 8000, which correspond to 6–12 proliferation subcultures, depending on the cultivar and on the sucker from which the different regenerated lines originated.
Micropropagation of BSOLV‐infected vitroplants
To assess and quantify the sanitation effect of tissue culture, a selection of BSOLV‐infected proliferation clumps was used to generate plantlets as described above. The progeny of four lines regenerated from these infected clumps were indexed for BSOLV throughout the regeneration process as described above.
Genotyping of eBSGFV
Total genomic DNA was extracted from banana leaf tissue by the method of Gawel and Jarret, (1991). The quality of DNA was estimated visually after migration of 5 µL of DNA in a 0.8% agarose gel, stained with ethidium bromide, and visualized under UV light and by PCR using housekeeping primers of the Musa actin gene.
PCR was performed on plant DNA extracts using specific primers (Gayral, 2008) which amplified the eight characterized junctions of eaBSGFV‐7 and eBSGFV‐9 in PKW shown in Table 1 (Gayral et al., 2008). To ensure that the absence of amplification did not result from single nucleotide polymorphisms, PCRs were performed with a set of primers designed as external to the first set on the same DNA fragment (labelled ‘bis’ in Table 1).
Table 1.
Primers used in the polymerase chain reaction (PCR) screen of endogenous Banana streak Goldfinger virus (eBSGFV).
| Primer name | Primer sequence (5′–3′)* | Expected product size (bp) |
|---|---|---|
| VM1‐F† | TTGTCCAAAATCTGCTCGTG | 481 |
| VM1‐R | TGTAATTCCTGCTCCTGCAA | |
| VM2‐F† | TTCTCCCTTTTCGATCCGTA | 374 |
| VM2‐R | TTTTGATGCATCTCCAGCAG | |
| VV1‐F | ACAGCTCCAGGAGATTGGAA | 268 |
| VV1‐R | CTGAAGTGTGCCTGTGGAGA | |
| VV2‐F | TCTGAGATCTCCAGCCAGGT | 639 |
| VV2‐R | GACAGTTCCAGCACAGCAGA | |
| VV3‐F | TTGCCAAGAATTCCTCCAAG | 376 |
| VV3‐R | AAGTTCTTGTCGGCAAGGTG | |
| VV4‐F | GAGCAACACGAGTCAACGAA | 784 |
| VV4‐R | TCTCCACAGGCACACTTCAG | |
| VV5‐F‡ | CCATGGAGGTTGACCTGTCT | 588 |
| VV5‐R | ACCCCTCTGTCTTCCCAACT | |
| VV5bis‐F§ | CGCACCTTCATCACAGAAGA | 628 |
| VV5bis‐R | TACCAGATGGGGAGAAATCG | |
| VV6‐F | GCATGAAGCATGACTGGAGA | 264 |
| VV6‐R | AATGCATAAGGGCCTCGAAT |
Primer annealing temperature: 60 °C.
Primer pairs ‘VM’ amplify the junction between the Musa genome and endogenous Banana streak virus (eBSV); primer pairs ‘VV’ amplify the internal fragment junctions within eBSGFV.
Primer pairs characterize eBSGFV‐9.
Primer pairs external to primer ‡ on the same DNA fragment.
PCRs were performed with 5–20 ng of DNA, 20 mm Tris–HCl (pH 8.4), 50 mm KCl, 100 mm of each deoxynucleoside triphosphate (dNTP), 1.5 mm MgCl2, 10 pmol of each primer and 1 U Taq DNA polymerase (Eurogentech, Seraing, Belgium) in a total reaction volume of 25 µL. PCRs were performed as follows: one cycle at 95 °C for 5 min, followed by 30 cycles at 95 °C for 30 s, 60 °C for 30 s and 72 °C for 30 s, and one elongation cycle at 72 °C for 10 min. PCR products were visualized under UV light after migration of 10 µL of PCR products on a 1.5% agarose gel in 0.5 × TBE [45 mm Tris‐borate, 1 mm ethylenediaminetetraacetic acid (EDTA), pH 8] stained with ethidium bromide.
ACKNOWLEDGEMENTS
This work was supported in part by the Banana and Plantain section of Bioversity International (Montpellier, France). The authors are grateful to N. Roux and R. Markham for their support, to Professor B.E.L. Lockhart for generously providing the anti‐BSV antiserum, to N. Laboureau for her technical support in establishing the eBSGFV signature of plantains and to Vitropic S.A. (Saint‐Mathieu‐de‐Tréviers, France) for their technical support with tissue culture.
REFERENCES
- Côte, F.X. , Domergue, R. , Folliot, M. , Navarro‐Mastache, L. and Teisson, C. (1990) In vitro propagation of banana. Fruits Special Issue, 112–119. [Google Scholar]
- Dahal, G. , Hughes, D'A.J. , Thottappilly, G. and Lockhart, B.E.L. (1998) Effect of temperature on symptom expression and expression and reliability of Banana streak badnavirus detection in naturally infected plantain and banana (Musa spp). Plant Dis. 82, 16–21. [DOI] [PubMed] [Google Scholar]
- Dahal, G. , Ortiz, R. , Tenkouano, A. , Hughes, D'A.J. , Thottappilly, G. , Vuylsteke, D. and Lockhart, B.E.L. (2000) Relationship between natural occurrence of Banana streak badnavirus and symptom expression, relative concentration of viral antigen, and yield characteristics of some micropropagated Musa spp. Plant Pathol. 49, 68–79. [Google Scholar]
- Dallot, S. , Acuna, P. , Rivera, C. , Ramirez, P. , Cote, F. , Lockhart, B.E.L. and Caruana, M.L. (2001) Evidence that the proliferation stage of micropropagation procedure is determinant in the expression of Banana streak virus integrated into the genome of the FHIA 21 hybrid (Musa AAAB). Arch. Virol. 146, 2179–2190. [DOI] [PubMed] [Google Scholar]
- Fauquet, C.M. , Mayo, M.A. , Maniloff, J. , Desselberger, U. and Ball, L.A. (2005) Virus taxonomy: 8th report of the International Committee of the Taxonomy of Viruses. Amsterdam: Elsevier Academic Press. [Google Scholar]
- Gawel, N.J. and Jarret, R.L. (1991) A modified CTAB DNA extraction procedure for Musa and Ipomoea. Plant Mol. Biol. Rep. 9, 262–266. [Google Scholar]
- Gayral, P. (2008) Evolution des pararétrovirus endogènes de plantes; le cas des séquences intégrées du Banana streak virus chez le bananier (Musa sp.). Section: Evolutionary History of infectious endogenous banana streak viruses and their host banana (Musa sp.) 163–2003.Thèse de Docteur de l'Université Montpellier II Sciences et Techniques du Languedoc Roussillon 12 novembre 2008, 250 p.
- Gayral, P. , Noa‐Carrazana, J.C. , Lescot, M. , Lheureux, F. , Lockhart, B.E.L. , Matsumoto, T. , Piffanelli, P. and Iskra‐Caruana, M.‐L. (2008) A single Banana streak virus integration event in the banana genome as the origin of infectious endogenous pararetrovirus. J. Virol. 82, 6697–6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geering, A.D.W. , Olszewski, N.E. , Harper, G. , Lockhart, B.E.L. , Hull, R. and Thomas, J.E. (2005) Banana contains a diverse array of endogenous badnaviruses. J. Gen. Virol. 8, 511–520. [DOI] [PubMed] [Google Scholar]
- Harper, G. , Osuji, J.O. , Heslop‐Harrison, J.S. and Hull, R. (1999) Integration of Banana streak badnavirus into the Musa genome: molecular and cytogenetic evidence. Virology, 255, 207–213. [DOI] [PubMed] [Google Scholar]
- Harper, G. , Hull, R. , Lockhart, B.E.L. , Olszewski, N. and Harper, G. (2002) Viral sequences integrated into plant genomes. Annu. Rev. Phytopathol. 40, 119–136. [DOI] [PubMed] [Google Scholar]
- Helliot, B. , Panis, B. , Poumay, Y. , Swennen, R. , Lepoivre, P. and Frison, E. (2002) Cryopreservation for the elimination of cucumber mosaic and banana streak viruses from banana (Musa spp.). Plant Cell Rep. 20, 1117–1122. [Google Scholar]
- Hohn, T. , Richert‐Pöggeler, K. , Staginnus, C. , Harper, G. , Schwartzacher, T. , Teo, C.‐H. , Teycheney, P.‐Y. , Iskra‐Caruana, M.L. and Hull, R. (2008) Evolution of integrated plant viruses In: Plant Virus Evolution (Roossinck M. ed.), pp. 53–58. Heidelberg: Springer. [Google Scholar]
- Hull, R. , Harper, G. and Lockhart, B. (2000) Viral sequences integrated into plant genomes. Trends Plant Sci. 5, 362–365. [DOI] [PubMed] [Google Scholar]
- Iskra‐Caruana, M.‐L. , Lheureux, F. , Noa‐Carrazana, J.C. , Piffanelli, P. , Carreel, F. , Jenny, C. , Laboureau, N. and Lockhart, B.E.L. (2003) Unstable balance of relation between pararetrovirus and its host plant: the BSV‐EPRV banana pathosystem [Abstract]. In: EMBO Workshop on Genomic Approaches in Plant Virology, May 28–31. EMBO, Heidelberg (DEU): Keszthely (HUN).
- Jones, D.R. (2000) Diseases of Banana, Abacá, and Enset. Wallingford, Oxon; New York: CABI Publishing. [Google Scholar]
- Le Provost, G. , Iskra‐Caruana, M.‐L. , Acina, I. and Teycheney, P.‐Y. (2006) Improved detection of episomal Banana streak viruses by multiplex immunocapture PCR. J. Virol. Methods, 137, 7–13. [DOI] [PubMed] [Google Scholar]
- Lheureux, F. , Carreel, F. , Jenny, C. , Lockhart, B.E.L. and Iskra‐Caruana, M.‐L. (2003) Identification of genetic markers linked to banana streak disease expression in inter‐specific Musa hybrids. Theor. Appl. Genet. 106, 594–598. [DOI] [PubMed] [Google Scholar]
- Meyer, J.B. , Kasdorf, G.G.F. , Nel, L.H. and Pietersen, G. (2008) Transmission of activated‐episomal Banana streak OL (badna)virus (BSOLV) to cv. Williams Banana (Musa sp.) by three mealybug species. Plant Dis. 92, 1158–1163. [DOI] [PubMed] [Google Scholar]
- Ndowora, T. , Dahal, G. , LaFleur, D. , Harper, G. , Hull, R. , Olszewski, N.E. and Lockhart, B.E.L. (1999) Evidence that badnavirus infection in Musa can originate from integrated pararetroviral sequences. Virology, 255, 214–220. [DOI] [PubMed] [Google Scholar]
- Noreen, F. , Abkergenov, R. , Hohn, T. and Richert‐Pöggeler, K. (2007) Distinct expression of endogenous Petuniva vein clearing virus and the DANN transposon dTph1 in two Petunia hybrida lines is correlated with differences in histone modification and siRNA production. Plant J. 50, 219–229. [DOI] [PubMed] [Google Scholar]
- Staginnus, C. and Richert‐Pöggeler, K.R. (2006) Endogenous pararetroviruses: two‐faced travellers in the plant genome. Trends Plant Sci. 11, 485–449. [DOI] [PubMed] [Google Scholar]
- Staginnus, C. , Iskra‐Caruana, M.‐L. , Lockhart, B.E.L. , Hohn, T. and Richert‐Pöggeler, K.R. (2009) Suggestions for a nomenclature of endogenous pararetroviral (EPRV) sequences in plants. Arch. Virol. 154 (7), 1189–1193. [DOI] [PubMed] [Google Scholar]


