SUMMARY
Fusarium graminearum is a significant pathogen of many cereal crops. With its genetic tractability, ease of culture, genome sequence availability and economic significance, F. graminearum has become the subject of intensive molecular research. Although molecular tools have been developed to enhance research into virulence determinants of F. graminearum, simple assays for infection‐related development are lacking. As such, the objective of this study was to develop an in vitro protocol for the analysis of infection‐related morphogenesis in F. graminearum. We demonstrate that two morphologically distinct hyphal structures are produced by F. graminearum during the invasion of detached wheat glumes: subcuticular hyphae and bulbous infection hyphae. Specialized wheat epidermal cells (papillae) appear to act as sites of invasion by F. graminearum on the adaxial side of detached wheat glumes. In addition, the development of bulbous infection hyphae is dependent on the pathogenicity mitogen‐activated protein kinase Gpmk1, further supporting the infection‐related nature of these structures. This relatively simple assay will contribute to the tractability of the F. graminearum system and help to uncover molecular requirements for infection‐related development.
INTRODUCTION
Fusarium graminearum (teleomorph Gibberella zeae) is a significant pathogen to a number of plant species, but it is most notorious as the main causal agent of Fusarium head blight (FHB) of wheat and barley (Goswami and Kistler, 2004; McMullen et al., 1997). Heads infected by F. graminearum produce less grain, and the grain that is produced usually harbours the mycotoxin deoxynivalenol (DON). This mycotoxin causes adverse effects in humans and animals, and contaminated grain is often unmarketable (Dexter and Nowicki, 2003). The relatively recent re‐emergence of this disease in the USA has prompted a renewed interest in the epidemiology and molecular biology of F. graminearum (McMullen et al., 1997).
Unlike many plant pathogenic fungi, F. graminearum invades host tissue without generating an appressorium (dome‐shaped infection structure used to penetrate host tissues). Instead, this pathogen tends to rely on natural openings (mainly stomata) and direct penetration for access to internal tissues. The abaxial side of glumes contains stomatal rows that are exploited by F. graminearum and closely related F. culmorum (Kang and Buchenauer, 2000; Pritsch et al., 2000). Interestingly, the fungus also produces subcuticular coral‐like hyphal mats on both glumes and ovarian tissue, although the role and significance of these structures are unknown (Jansen et al., 2005; Kang and Buchenauer, 2000; Pritsch et al., 2000). Perhaps most importantly, F. graminearum can readily invade young anthers and ramify through the filament to the ovary. The ovary is then invaded and provides access, through vascular tissue, to the rachis (Pugh et al., 1933). From the rachis, F. graminearum then spreads to other florets, thereby exacerbating the disease. Once the fungus gains access to internal tissue, it appears to exploit pits for intracellular travel (Guenther and Trail, 2005; Jansen et al., 2005). At these junctions, the hyphae of F. graminearum become uncharacteristically narrow in order to achieve passage. Despite the eloquent microscopy studies that have been conducted, there are still some unanswered questions regarding host invasion by F. graminearum. For example, over 20 genes have been implicated in the virulence/pathogenicity of F. graminearum; however, with a few exceptions (Ding et al., 2009; Jansen et al., 2005), microscopic observations of such mutants are often absent.
As part of the renewed interest in F. graminearum, several genomic tools have recently become available to investigate virulence on a molecular scale. The genome has been sequenced and is publicly available (http://www.broad.mit.edu; Cuomo et al., 2007). Also, an Affymetrix chip has been generated and used to study gene expression under several growth conditions (Ding et al., 2009; Guldener et al., 2006; Hallen et al., 2007; Seong et al., 2008). However, an in vitro protocol for the induction of infectious‐related development is lacking. Such methods have been used to determine the molecular requirements of pathogenicity in several other plant pathogenic fungi, particularly in the rice blast fungus Magnaporthe oryzae (Takano et al., 2003). As such, the objective of this study was to generate a protocol that induces F. graminearum infectious‐related development in vitro. We describe a relatively quick and easy method for the induction of both subcuticular and bulbous infection hyphae on detached wheat glumes. This method will complement many of the molecular tools that are currently available, and help to advance our knowledge of F. graminearum infection.
RESULTS
Initially, several plant tissues were assessed for F. graminearum infection, including onion epidermis, wheat lemmas, paleas and glumes. Each tissue was inoculated with F. graminearum macroconidia and incubated for a given amount of time. Samples were then stained with various compounds to highlight fungal hyphae. Of the tissues tested, macroconidia inoculated on wheat glumes consistently developed morphologically distinct structures (see below). Accordingly, subsequent analyses focused on inoculated glumes.
Morphologically distinct hyphal structures
At the earliest time points (24 h), wide, coral‐shaped hyphae were observed, particularly when glumes were inoculated on the adaxial side (Fig. 1b,c). On the abaxial side, stomata were regularly invaded by hyphae, but subcuticular hyphae were not as prevalent compared with glumes inoculated on the adaxial side (Fig. 1a). At later time points (48 and 72 h), hyphae were observed on focal planes lower than those of surface hyphae (Fig. 1d–f). Interestingly, these hyphae appeared to be morphologically distinct from surface hyphae, as they were wider and more bulbous in appearance (Fig. 1e,f). These wide hyphae probably represent the dikaryotic state described by Guenther and Trail (2005) in wheat stem tissue. In addition, these hyphae appeared to modify their bulbous morphology to travel through pits between epidermal cells, which has previously been documented in planta (Fig. 1g; Guenther and Trail, 2005; Jansen et al., 2005). In order to further confirm the in planta relevance of these wide hyphae, we inoculated wheat plants and observed the hyphae within the rachis (Fig. 1h). These data suggest that F. graminearum produces morphologically distinct hyphal structures when invading detached wheat glumes, and that these hyphal structures resemble those previously reported in planta.
Figure 1.
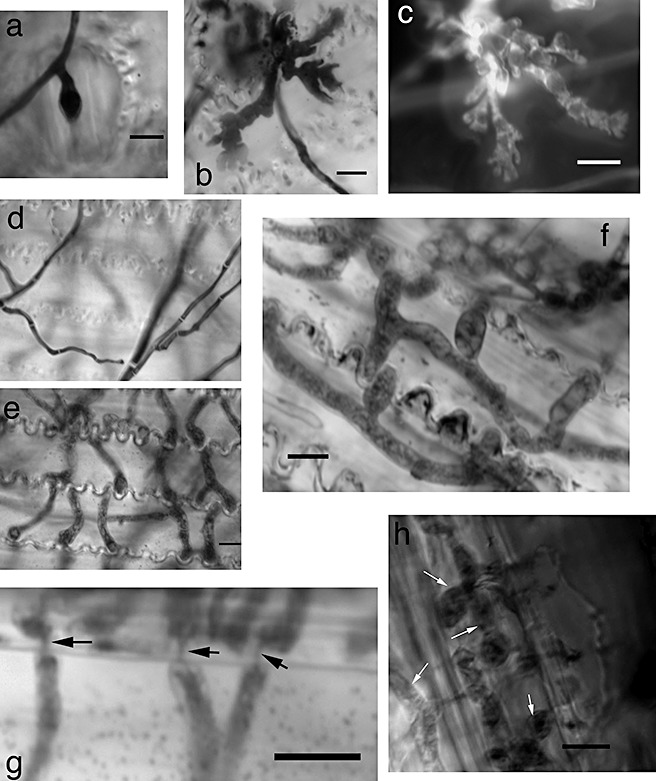
Morphologically distinct hyphal structures produced in vitro on wheat glumes. (a) Hyphae entering through stomata when inoculated on the abaxial side of a glume; 24 h post‐inoculation (hpi). (b, c) Coral‐like hyphae stained with lactophenol blue (b) or calcofluor white (c). Inoculated on adaxial side; 24 hpi. (d) Hyphae on the adaxial surface of glumes. (e) Same field and scale as (d) but lower focal plane showing bulbous infection hyphae that appear morphologically distinct from surface hyphae; 72 hpi. (f) Wide hyphae growing within epidermal cells. Inoculated on abaxial side; 48 hpi. (g) Narrowing of hyphae travelling between cells through pits (arrows); 72 hpi. (h) Wide hyphae growing within rachis tissue, 2 weeks after inoculation with wild‐type strain PH‐1. Scale bar, 10 µm in all panels.
Subcuticular hyphae
The coral‐like hyphal structures observed (Fig. 1b,c) have been reported as subcuticular hyphae (Pritsch et al., 2000). In order to assess whether or not these structures are growing in the subcuticular space (i.e. in the apoplastic space above the epidermal cell wall), cross‐sections were taken and stained with calcofluor white. In addition, transmission electron microscopy (TEM) was used to assess subcuticular growth. Both methods provided evidence that subcuticular growth occurs on detached wheat glumes (Fig. 2a,b). At later time points [48 and 72 h post‐inoculation (hpi)], the integrity of many subcuticular hyphae appeared to be compromised (Fig. 2c). Accordingly, deteriorating subcuticular hyphae were observed in 0/10, 6/10 and 10/10 inoculated glumes at 24, 48 and 72 hpi, respectively.
Figure 2.
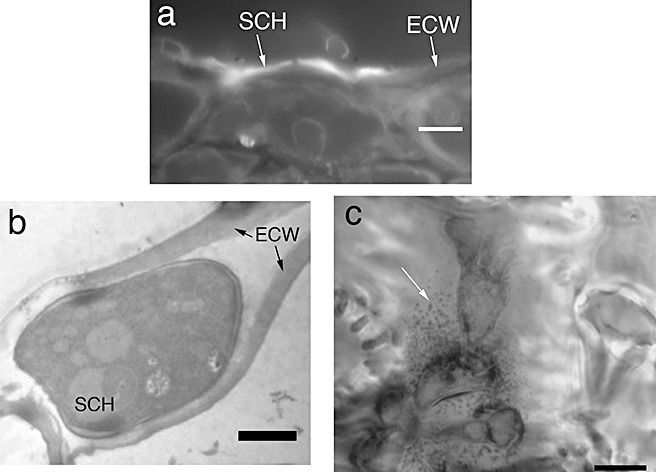
Subcuticular location of hyphae. (a) Cross‐section of glume on adaxial side at 24 h post‐inoculation (hpi). Hyphae stained with calcofluor white. (b) Transmission electron microscopy image of subcuticular hyphae. Scale bar, 2 µm. (c) Deteriorating subcuticular hyphae at 48 hpi. Note the heavy vacuolization and cell wall fragmentation (white arrow). Hyphae in (c) stained with trypan blue. Scale bar, 10 µm for (a, c). ECW, epidermal cell wall; SCH, subcuticular hyphae.
Papillae‐associated invasion
Whilst observing the adaxial side of inoculated glumes, most of the subcuticular and invasive bulbous hyphae appeared to be associated with circular structures surrounded by pits. On further investigation, these were identified as ‘papillae’, also referred to as short basal hair cells/prickle hairs (Bushnell et al., 2003; Tubb et al., 1993; Urban et al., 2003). Both light and electron microscopy showed that F. graminearum readily invaded these epidermal cells (Fig. 3). In addition, hyphae appeared to ramify through the pits at the bases of the papillae, providing an entryway to internal cells (Fig. 3b). Papillae‐associated invasion was also seen on the glumes of living wheat heads, although the occurrence was drastically reduced compared with detached wheat glumes (Fig. 3e; data not shown). These data suggest that papillae act as an infection court for F. graminearum on wheat glumes.
Figure 3.
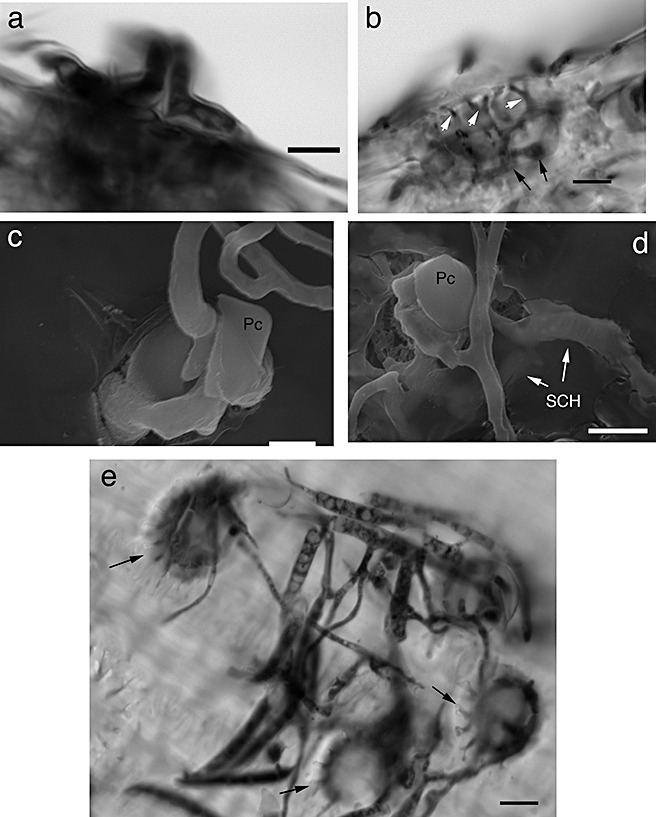
Hyphae invading through papillae. (a) Hyphae invading through a papilla cell. (b) Hyphae ramifying through pits at the base of a papilla (white arrows) and forming bulbous infection hyphae in epidermal cells (black arrows). (c, d) Scanning electron microscopy images of hyphae invading through papilla cells. (e) Papillae (arrows) of glumes on living wheat plants being invaded by Fusarium graminearum. Hyphae stained with trypan blue in (a, b, e). Pc, papilla cell; SCH, subcuticular hyphae. Scale bars: (a, b, d, e), 10 µm; (c), 5 µm.
Analysis of F. graminearum mutants for their ability to differentiate infectious hyphae
Different F. graminearum pathogenicity mutants were tested for their ability to form the morphological structures observed in this study. These included the mitogen‐activated protein kinase (MAPK) mutants Δgpmk1 (Jenczmionka et al., 2003; Urban et al., 2003) and Δmgv1 (Hou et al., 2002), and also the GTPase Δras2 (Bluhm et al., 2007). The Δmgv1 and Δras2 mutants were able to form both subcuticular hyphae and bulbous invasive hyphae that were indistinguishable from those of the wild‐type strain PH‐1 (Table 1; Fig. 4). This similarity in glume invasion was evident despite the fact that the growth of these two mutants on artificial media was greatly reduced (Bluhm et al., 2007; Hou et al., 2002).
Table 1.
Number of glumes with either subcuticular hyphae (SCH) or bulbous infection hyphae (BIH).*
| Strain | SCH† | BIH‡ | ||||
|---|---|---|---|---|---|---|
| 24 hpi | 48 hpi | 72 hpi | 24 hpi | 48 hpi | 72 hpi | |
| PH‐1 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 |
| Δras2 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 |
| Δmgv1 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 |
| Δgpmk1 | 9/10 | 10/10 | 7/10 | 0/10 | 0/10 | 0/10 |
Values show the number of glumes across two separate experiments (five glumes per experiment).
Number of glumes with coral‐like subcuticular hyphae at x hours post‐inoculation (hpi).
Number of glumes with bulbous infection hyphae at x hpi.
Figure 4.
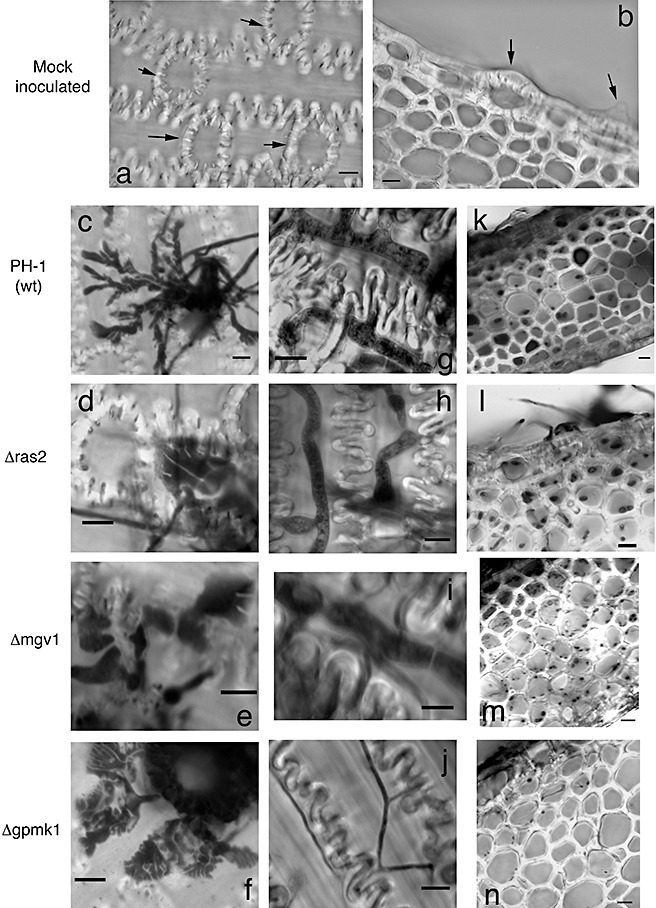
Mutants tested on detached wheat glumes for infectious‐related development. (a, b) Mock‐inoculated controls 72 h after inoculation. Black arrows indicate papillae. (c–f) Subcuticular hyphae produced after 24 h. (g–j) Intracellular hyphae observed after either 48 h (g–i) or 72 h (j). The thin hyphae illustrated in (j) were the only type of intracellular hyphae observed for the Δgpmk1 mutant. (k–n) Cross‐sectional view of inoculated glumes after either 48 h (k–m) or 72 h (n). Note the decreased hyphal ramification of the Δgpmk1 mutant (n). Hyphae in all panels stained with trypan blue. Scale bar, 10 µm for all panels.
In contrast with the Δras2 and Δmgv1 mutants, Δgpmk1 mutants formed large hyphal masses at the surface of the glume as opposed to invasive growth. The subcuticular hyphae were not as abundant on Δgpmk1‐inoculated glumes, although they were present (Table 1; Fig. 4f). None of the glumes inoculated with the Δgpmk1 mutant displayed bulbous invasive hyphae, even after 72 hpi (Table 1). However, at 72 hpi, Δgpmk1 grew internally as narrow hyphae, probably representing saprophytic growth (Fig. 4j). In addition, when observed in cross‐section, intracellular hyphae were scarce in glumes inoculated with the Δgpmk1 mutant (Fig. 4n). These data suggest that bulbous infection hyphae are morphologically distinct structures whose formation is genetically controlled by the pathogenicity MAPK Gpmk1.
DISCUSSION
The goal of this study was to develop an in vitro protocol that can be used to easily assess infection‐related morphogenesis in F. graminearum. Using detached wheat glumes, we were able to induce two morphologically distinct hyphal structures during glume invasion: subcuticular hyphae and bulbous infection hyphae. Furthermore, we demonstrated that the MAPK Gpmk1, previously implicated in the pathogenicity of F. graminearum, is essential for the induction of bulbous infection hyphae. Our results, coupled with previous studies, suggest that the bulbous infection hyphae represent a morphological state that may be specific to the infection of wheat (and possibly other plant) tissue (Guenther and Trail, 2005). Indeed, the bulbous infection hyphae seen in this study are similar in appearance to the dikaryotic hyphae observed in stem tissue (Guenther and Trail, 2005) and wide hyphae observed in ovarian tissue (Jansen et al., 2005). In addition, we developed a relatively quick and easy protocol that can be used to assay different strains/mutants of F. graminearum for their ability to differentiate infection‐related hyphal structures. Future work should focus on exploiting this protocol to monitor transcriptional changes during infectious development. Given that the host tissue was previously frozen, interference from host RNA may be less of an obstacle than in in planta studies. Transcriptional profiling of ‘artificially’ induced infection structures uncovered several virulence genes in other pathogenic fungi (Odenbach et al., 2007; Takano et al., 2003).
Members of the kss1/fus3/pmk1 MAPK family mediate changes in hyphal morphogenesis in many different fungi (reviewed by Zhao et al., 2007). In the rice blast fungus (M. oryzae), and the southern corn blight pathogen (Cochliobolus heterostrophus), pmk1 is absolutely required for the development of appressoria (Lev et al., 1999; Xu and Hamer, 1996). In addition, pmk1 homologues are needed for the pathogenicity of fungi that do not form appressoria, including F. graminearum and F. oxysporum (Di Pietro et al., 2001; Jenczmionka et al., 2003; Mey et al., 2002; Urban et al., 2003). However, infection‐related morphogenesis has not been studied in Δgpmk1 F. graminearum mutants. Our data illustrate that this MAPK is absolutely necessary for the development of bulbous infection hyphae, which is consistent with the role of Pmk1 homologues in mediating infection‐related morphogenesis. In the dimorphic corn pathogen Ustilago maydis, the Pmk1 orthologue Ubc3 is necessary for dikaryon formation and filamentous growth (Mayorga and Gold, 1999; Muller et al., 1999). Given that Guenther and Trail (2005) have demonstrated previously that the wide hyphae are dikaryotic, it is possible that Gpmk1 may serve a similar role in dikaryon formation in F. graminearum. Interestingly, Ras2 appears to be dispensable for the formation of bulbous infection hyphae, despite the fact that Ras2 contributes to the activation of Gpmk1 (Bluhm et al., 2007). However, Δras2 mutants still exhibit a basal level of Gpmk1 activation, which is apparently sufficient for the formation of bulbous infection hyphae.
When intracellular hyphae were present in glumes inoculated with Δgpmk1, they were thin in appearance and restricted to the top two to three layers of cells in wheat glumes (Fig. 4n). This is in contrast with glumes inoculated with wild‐type strain PH‐1, in which bulbous hyphae proliferated to lower cell layers of the glume, even after 48 h (Fig. 4k). As the deletion of gpmk1 has little to no effect on saprophytic growth in culture (Jenczmionka et al., 2003; Urban et al., 2003), the defect in hyphal proliferation could be caused by the decreased expression of certain virulence factors. Given that over 300 genes are differentially regulated in the Δgpmk1 mutant (Ding et al., 2007), the identification of which genes contribute specifically to infectious‐related development in F. graminearum may prove to be a daunting task. However, this protocol can be used as a tool to help unravel the genetic requirements for the formation of bulbous infection hyphae.
Despite its role in the development of bulbous hyphae, Gpmk1 was not absolutely required for the formation of subcuticular hyphae. However, the subcuticular hyphae produced by the Δgpmk1 strain were not as prolific as those in the wild‐type. As subcuticular hyphae grow in the apoplastic space above epidermal cells, one could predict that pectinases, xylanases and other hydrolytic enzymes would be crucial for their growth. Unfortunately, each class of hydrolytic enzyme contains multiple representatives in the genome of F. graminearum, making gene redundancy a barrier when considering single‐gene deletions. Gpmk1 simultaneously regulates the expression of several hydrolytic enzymes, including cellulases and xylanases, but not pectinase (Jenczmionka and Shafer, 2005). The decreased ramification of Δgpmk1 subcuticular hyphae may represent a defect in the degradation of host cell wall materials. In order to test the role of cell wall‐degrading enzymes in subcuticular growth, other mutants that affect the overall expression of such genes should be explored. For example, Gzsnf1 is a putative protein kinase that controls the expression of genes encoding cell wall‐degrading enzymes and represents an excellent candidate for further study (Lee et al., 2009).
Subcuticular hyphae were readily observed after 24 h when glumes were inoculated on the adaxial side. Although the significance of this hyphal morphology is unknown, it has been postulated that it might provide a suitable niche for plant pathogenic fungi during the early stages of host invasion (Bushnell et al., 2003). Considering this hypothesis, once host infection is firmly established, subcuticular hyphae would presumably become obsolete. Consistent with this notion, many subcuticular hyphae appeared to be degraded after bulbous infection hyphae proliferated within host cells. Other plant pathogenic fungi undergo programmed cell death in older hyphal structures and transport the recycled cellular reserves to the invasion front (Asakura et al., 2009; Veneault‐Fourry et al., 2006). Further investigation is needed to determine the cause of degradation of subcuticular hyphae and the role it may play in plant infection. In addition, the genetic requirements of subcuticular growth remain unknown. A recent study using the apple scab pathogen Venturia inaequalis demonstrated that two genes, cin1 and cin3, are significantly up‐regulated during subcuticular growth on cellophane sheets (Kucheryava et al., 2008). However, there are no cin1 or cin3 orthologues present in the F. graminearum genome. Our protocol allows for similar transcriptional studies in F. graminearum and represents the first step to the identification of the genetic contributions to subcuticular growth.
When inoculated onto the adaxial side of glumes, F. graminearum appeared to exploit papillae as invasion sites. It is possible that the elevated topography of papilla cells induces invasive development, but this is unlikely as hyphae often grow over the top of papilla cells without invading them (data not shown). A more likely possibility is that the junction between papillae and neighbouring epidermal cells contains ‘weak spots’ where lacerations occur. Given that the glumes were stored at −20 °C, the freezing process may have exacerbated existing weak spots and caused these lacerations. Fusarium graminearum also invaded through papillae on living wheat plants (Fig. 4e), although this was not as readily observed as on detached wheat glumes. Nevertheless, the inherent features of papillae make them interesting candidates for infection courts. Papillae are dome‐shaped epidermal cells whose precise function is unknown, but they are surrounded by 9–13 pits that adjoin them to nearby cells (Tubb et al., 1993). As F. graminearum often invades ‘passively’ through stomata and ramifies internally through pits (Jansen et al., 2005; Kang and Buchenauer, 2000; Pritsch et al., 2000), invasion through papillae would be advantageous in that it allows simultaneous access to several nearby cells. Indeed, this appears to be the case on detached glumes (Fig. 3b).
Although anthers and ovaries are considered to be the primary sites of infection by F. graminearum, the adaxial sides of glumes represent more easily accessible infection sites that could act as inoculum reservoirs for further spread. Several studies have demonstrated that F. graminearum readily invades the stomata on the abaxial side of wheat glumes (Kang and Buchenauer, 2000; Pritsch et al., 2000). However, the dry microenvironment on the abaxial side is not conducive to fungal growth or survival. Nevertheless, the cavity on the adaxial side, which is partially covered by the lemma, is more humid and favourable for infection. Consistent with this notion, macroconidia inoculated onto the abaxial side often grow to the edge of the glume and gain access to the adaxial side (Kang and Buchenauer, 2000). Kang and Buchenauer (2000)postulated several routes for F. graminearum to reach the vascular tissue of the rachis, one of which is via glume invasion. However, direct evidence of this route is lacking. Further microscopic and epidemiological investigations are necessary to determine the significance of glume invasion on both scales. Regardless of their importance in planta, we have demonstrated that they can be used to ‘artificially’ induce infectious‐related development of F. graminearum.
EXPERIMENTAL PROCEDURES
Strains and culture conditions
Fusarium graminearum strain PH‐1 (NRRL 31084) was used as the wild‐type strain for glume inoculations. Strains Δras2, Δmgv1 and Δgpmk1 were kindly provided by Dr Jin‐Rong Xu (Purdue University, West Lafayette, IN, USA). All strains were stored as mycelial debris in 30% glycerol at −80 °C. Strains were revived on V8 agar and transferred to yeast malt agar (YMA) (Harris, 2005) for conidiation. Carboxymethyl cellulose (CMC) medium (Cappellini and Peterson, 1965) was used for macroconidia production of Δmgv1, as it did not conidiate well on YMA. Macroconidia were harvested in sterile distilled water from YMA or CMC plates with a bent glass rod and filtered through one layer of miracloth (Calbiochem, San Diego, CA, USA) to remove mycelial debris. Macroconidia were resuspended in 0.05% Tween‐20 to a concentration of 5 × 104 macroconidia/mL for inoculation.
Plant material and inoculation
Wheat heads (variety ‘Norm’) were removed from plants at anthesis and stored at −20 °C before inoculation. Heads were removed 1 h prior to inoculation and allowed to thaw at room temperature. After thawing, glumes were removed and placed on 3% water agar in Petri dishes. Glumes were then irradiated for 30 s with ultraviolet light for sanitation. The cavities of glumes (adaxial side) were filled with approximately 20 µL of a suspension of 5 × 104 macroconidia/mL in 0.05% Tween‐20. Suspensions were allowed to stand for 1 min before being removed. For inoculation of the abaxial side of glumes, a 100‐µL droplet of macroconidia was placed on a polystyrene Petri dish, and the abaxial side of the glume was held in this droplet for 1 min. The glume was then removed and placed on 3% water agar, abaxial side up. Glumes were then incubated at room temperature until processed for microscopy.
For the inoculation of living wheat plants, 20 mL of a suspension of 5 × 104 macroconidia/mL in 0.05% Tween‐20 were placed on the adaxial side of the glume. The droplet was removed 1 min later. The wheat heads were then covered with a plastic bag to promote a humid environment. Glumes were removed and processed for microscopy as described above. A total of 12 glumes was observed from two experiments.
Processing glumes for microscopy
For light microscopy, inoculated glumes were placed in clearing/fixing solution (3 : 1 ethanol–acetic acid) and incubated at room temperature on a rotary shaker at 75 r.p.m. for 16 h. Glumes were further fixed in a 5 : 1 : 1 solution (ethanol–acetic acid–glycerol) for at least 4 h under the same conditions as above. The glumes were then stained in a lactophenol blue solution (33% lactic acid, 33% phenol, 0.01% trypan blue) for at least 16 h under the conditions described above. After staining, glumes were washed in 60% glycerol for at least 3 h. Glumes were then sectioned under a dissecting scope, mounted in 60% glycerol, and observed in a bright field with an Olympus (Hitschfel Instruments, St. Louis, MO, USA) BX51 microscope. For the quantitative analysis of mutants, five glumes were inoculated for each time point (15 glumes in total). This experiment was performed twice for a total of 10 glumes per time point per strain.
In addition, calcofluor white (fluorescent brightener 28; Sigma, St. Louis, MO, USA) was used to stain some samples. For calcofluor staining, glumes were cleared/fixed in the solutions described above, and then washed in water before incubating in 0.3% calcofluor white–30% glycerol solution for 5 min in the dark. The samples were then washed twice in water, and once in 30% glycerol. Samples were then mounted in 60% glycerol and observed with fluorescent settings on an Olympus BX51 microscope.
For scanning electron microscopy, glumes were removed from Petri dishes at the given time points and fixed in 2.5% glutaraldehyde overnight at 4 °C prior to processing. The samples were then fixed for 1 h in 1% OsO4 at room temperature. The samples were then washed with water and serially dehydrated with ethanol (25%–100%) and dried with CO2. The samples were then affixed to aluminium stubs and coated with gold–palladium alloy before observation. The samples were then observed with a Hitachi (Pleasanton, CA, USA) S4700 field‐emission scanning electron microscope. Glumes from three replicates were observed.
For transmission electron microscopy, the glumes were sectioned and fixed as described above for scanning electron microscopy. The samples were then embedded in LR‐white and polymerized for 10 days. Ultrathin sections were cut and embedded on 200‐mesh copper grids. Samples were stained with uranyl acetate and lead citrate and observed with a Hitachi H7500 microscope. Glumes from three replicates were observed.
ACKNOWLEDGEMENTS
We thank Dr Jin‐Rong Xu (Purdue University, West Layfeyette, IN, USA) for kindly providing the ras2, mgv1 and gpmk1 deletion mutants. We would also like to thank Dr Han Chen of the University of Nebraska‐Lincoln Microscopy Core Facility (NE, USA) for his assistance with electron microscopy. This work was supported by the Nebraska Research Foundation.
REFERENCES
- Asakura, M. , Ninomiya, S. , Sugimoto, M. , Oku, M. , Yamashita, S. , Okuno, T. , Sakai, Y. and Takano, Y. (2009) Atg26‐mediated pexophagy is required for host invasion by the plant pathogenic fungus Colletotrichum orbiculare . Plant Cell, 21, 1291–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm, B.H. , Zhao, X. , Flaherty, J.E. , Xu, J.‐R. and Dunkle, L.D. (2007) RAS2 regulates growth and pathogenesis in Fusarium graminearum . Mol. Plant–Microbe. Interact. 20, 627–636. [DOI] [PubMed] [Google Scholar]
- Bushnell, W.R. , Hazen, B.E. and Pritsch, C. (2003) Histology and physiology of Fusarium head blight In: Fusarium Head Blight of Wheat and Barley (Leonard K.J. and Bushnell W.R., eds), pp. 44–83. St. Paul, MN: The American Phytopathological Society. [Google Scholar]
- Cappellini, R.A. and Peterson, J.L. (1965) Macroconidium formation in submerged cultures by a non‐sporulating strain of Gibberella zeae . Mycologia, 57, 962–966. [Google Scholar]
- Cuomo, C.A. , Guldener, U. , Xu, J.‐R. , Trail, F. , Turgeon, B.G. , Di Pietro, A. , Walton, J.D. , Ma, L.‐J. , Baker, S.E. , Rep, M. , Adam, G. , Antoniw, J. , Baldwin, T. , Calvo, S. , Chang, Y.‐L. , DeCaprio, D. , Gale, L.R. , Gnerre, S. , Goswami, R.S. , Hammond‐Kosack, K. , Harris, L.J. , Hilburn, K. , Kennell, J.C. , Kroken, S. , Magnuson, J.K. , Mannhaupt, G. , Mauceli, E. , Mewes, H.‐W. , Mitterbauer, R. , Muehlbauer, G. , Munsterkotter, M. , Nelson, D. , O'Donnell, K. , Ouellet, T. , Qi, W. , Quesneville, H. , Roncero, M.I.G. , Seong, K.‐Y. , Tetko, I.V. , Urban, M. , Waalwijk, C. , Ward, T.J. , Yao, J. , Birren, B.W. and Kistler, H.C. (2007) The genome of a filamentous pathogenic fungus shows excess polymorphism in regions with high levels of recombination. Science, 317, 1400–1402. [DOI] [PubMed] [Google Scholar]
- Dexter, J.E. and Nowicki, T.W. (2003) Safety assurance and quality assurance issues associated with Fusarium head blight in wheat In: Fusarium Head Blight of Wheat and Barley (Leonard K.J. and Bushnell W.R., eds), pp. 420–460. St. Paul, MN: The American Phytopathological Society. [Google Scholar]
- Di Pietro, A. , Garcia‐Maceira, F.I. , Meglecz, E. and Roncero, M.I.G. (2001) A MAP kinase of the vascular wilt fungus Fusarium oxysporum is essential for root penetration and pathogenesis. Mol. Microbiol. 39, 1140–1152. [PubMed] [Google Scholar]
- Ding, S. , Shou, X. , Kistler, H.C. and Xu, J.‐R. (2007) FMK1 regulates the expression of 333 genes in Fusarium graminearum [abstract]. Natl. Fusarium Head Blight Forum Proc. 25.
- Ding, S. , Mehrabi, R. , Koten, C. , Kang, Z. , Wei, Y. , Seong, K. , Kistler, H.C. and Xu, J.‐R. (2009) Transducin beta‐like gene FTL1 is essential for pathogenesis in Fusarium graminearum . Eukaryot. Cell, 8, 867–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami, R.S. and Kistler, H.C. (2004) Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 5, 515–525. [DOI] [PubMed] [Google Scholar]
- Guenther, J.C. and Trail, F. (2005) The development and differentiation of Gibberella zeae (anamorph: Fusarium graminearum) during colonization of wheat. Mycologia, 97, 229–237. [DOI] [PubMed] [Google Scholar]
- Guldener, U. , Seong, K.‐Y. , Boddu, J. , Cho, S. , Trail, F. , Xu, J.‐R. , Adam, G. , Mewes, H.‐W. , Muehlbauer, G.J. and Kistler, H.C. (2006) Development of a Fusarium graminearum Affymetrix GeneChip for profiling fungal gene expression in vitro and in planta . Fungal Genet. Biol. 43, 316–325. [DOI] [PubMed] [Google Scholar]
- Hallen, H.E. , Huebner, M. , Shiu, S.‐H. , Guldener, U. and Trail, F. (2007) Gene expression shifts during perithecium development in Gibberella zeae (anamorph Fusarium graminearum), with particular emphasis on ion transport proteins. Fungal Genet. Biol. 44, 1146–1156. [DOI] [PubMed] [Google Scholar]
- Harris, S.D. (2005) Morphogenesis in germinating Fusarium graminearum macroconidia. Mycologia, 97, 880–887. [DOI] [PubMed] [Google Scholar]
- Hou, Z. , Xue, C. , Peng, Y. , Katan, T. , Kistler, H.C. and Xu, J.‐R. (2002) A mitogen‐activated protein kinase gene (mgv1) in Fusarium graminearum is required for female fertility, heterokaryon formation, and plant infection. Mol. Plant–Microbe. Interact. 15, 1119–1127. [DOI] [PubMed] [Google Scholar]
- Jansen, C. , Wettstein, D.V. , Shafer, W. , Kogel, K.‐H. , Felk, A. and Maier, F.J. (2005) Infection patterns in barley and wheat spikes inoculated with wild‐type and trichodiene synthase gene disrupted Fusarium graminearum . Proc. Natl. Acad. Sci. USA, 102, 16892–16897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenczmionka, N.J. and Shafer, W. (2005) The Gpmk1 MAP kinase of Fusarium graminearum regulates the induction of specific secreted enzymes. Curr. Genet. 47, 29–36. [DOI] [PubMed] [Google Scholar]
- Jenczmionka, N.J. , Maier, F.J. , Losch, A.P. and Shafer, W. (2003) Mating, conidiation, and pathogenicity of Fusarium graminearum, the main causal agent of the head‐blight disease of wheat, are regulated by the MAP kinase gpmk1. Curr. Genet. 43, 87–95. [DOI] [PubMed] [Google Scholar]
- Kang, Z. and Buchenauer, H. (2000) Cytology and ultrastructure of the infection of wheat spikes by Fusarium culmorum . Mycol. Res. 9, 1083–1093. [Google Scholar]
- Kucheryava, N. , Bowen, J.K. , Sutherland, P.W. , Conolly, J.J. , Mesarich, C.H. , Rikkerink, E.H.A. , Kemen, E. , Plummer, K.M. , Hahn, M. and Templeton, M.D. (2008) Two novel Venturia inaequalis genes induced upon morphogenetic differentiation during infection and in vitro growth on cellophane. Fungal Genet. Biol. 45, 1329–1339. [DOI] [PubMed] [Google Scholar]
- Lee, S.‐H. , Lee, J. , Lee, S. , Park, E.‐H. , Kim, K.‐W. , Kim, M.‐D. , Yun, S.‐H. and Lee, Y.‐H. (2009) GzSNF1 is required for normal sexual and asexual development in the ascomycete Gibberella zeae . Eukaryot. Cell, 8, 116–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev, S. , Sharon, A. , Hadar, R. , Ma, H. and Horwitz, B.A. (1999) A mitogen‐activated protein kinase of the corn leaf pathogen Cochliobolus heterostrophus is involved in conidiation, appressorium formation, and pathogenicity: diverse roles for mitogen‐activated protein kinase homologs in foliar pathogens. Proc. Natl. Acad. Sci. USA, 96, 13542–13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayorga, M.E. and Gold, S.E. (1999) A MAP kinase encoded by the ubc3 gene of Ustilago maydis is required for filamentous growth and full virulence. Mol. Microbiol. 34, 485–497. [DOI] [PubMed] [Google Scholar]
- McMullen, M. , Jones, R. and Gallenberg, D. (1997) Scab of wheat and barley: a re‐emerging disease of devastating impact. Plant Dis. 81, 1340–1348. [DOI] [PubMed] [Google Scholar]
- Mey, G. , Oeser, B. , Lebrun, M.H. and Tudzynski, P. (2002) The biotrophic, non‐appresorium‐forming grass pathogen Claviceps purpurea needs a Fus3/Pmk1 homologous mitogen‐activated protein kinase for colonization of rye ovarian tissue. Mol. Plant–Microbe. Interact. 15, 303–312. [DOI] [PubMed] [Google Scholar]
- Muller, P. , Aichinger, C. , Feldbrugge, M. and Kahmann, R. (1999) The MAP kinase Kpp2 regulates mating and pathogenic development in Ustilago maydis . Mol. Microbiol. 34, 1007–1017. [DOI] [PubMed] [Google Scholar]
- Odenbach, D. , Breth, B. , Thines, E. , Weber, R.W.S. , Heidrun, A. and Foster, A. (2007) The transcription factor Con7p is a central regulator of infection‐related morphogenesis in the rice blast fungus Magnaporthe grisea . Mol. Microbiol. 64, 293–307. [DOI] [PubMed] [Google Scholar]
- Pritsch, C. , Muehlbauer, G.J. , Bushness, W.R. , Somers, D.A. and Vance, C.P. (2000) Fungal development and induction of defense response genes during early infection of wheat spikes by Fusarium graminearum . Mol. Plant–Microbe. Interact. 13, 159–169. [DOI] [PubMed] [Google Scholar]
- Pugh, G.W. , Johann, H. and Dickson, J.G. (1933) Factors affecting infection of wheat heads by Gibberella saubinetii . J. Agric. Res. 46, 771–797. [Google Scholar]
- Seong, K.‐Y. , Zhao, X. , Xu, J.‐R. , Guldener, U. and Kistler, H.C. (2008) Conidial germination in the filamentous fungus Fusarium graminearum . Fungal Genet. Biol. 45, 389–399. [DOI] [PubMed] [Google Scholar]
- Takano, Y. , Choi, W. , Mitchell, T.K. , Okuno, T. and Dean, R.A. (2003) Large scale parallel analysis of gene expression during infection‐related morphogenesis of Magnaporthe grisea . Mol. Plant Pathol. 4, 337–346. [DOI] [PubMed] [Google Scholar]
- Tubb, H.J. , Hodson, M.J. and Hodson, G.C. (1993) The influorescence papillae of the Triticeae: a new tool for taxonomic and archaeological research. Ann. Bot. 72, 537–545. [Google Scholar]
- Urban, M. , Mott, E. , Farley, T. and Hammond‐Kosack, K. (2003) The Fusarium graminearum MAP1 gene is essential for pathogenicity and development of perithecia. Mol. Plant Pathol. 4, 347–359. [DOI] [PubMed] [Google Scholar]
- Veneault‐Fourry, C. , Barooah, M. , Egan, M. , Wakley, G. and Talbot, N.J. (2006) Autophagic fungal cell death is necessary for infection by the rice blast fungus. Science, 312, 580–583. [DOI] [PubMed] [Google Scholar]
- Xu, J.‐R. and Hamer, J.E. (1996) MAP kinase and cAMP signaling regulate infection structure formation and pathogenic growth in the rice blast fungus Magnaporthe grisea . Genes Dev. 10, 2696–2706. [DOI] [PubMed] [Google Scholar]
- Zhao, X. , Mehrabi, R. and Xu, J.‐R. (2007) Mitogen‐activated protein kinase pathways and fungal pathogenesis. Eukaryot. Cell, 6, 1701–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]


