SUMMARY
The fungus Venturia inaequalis infects members of the Maloideae, and causes the disease apple scab, the most important disease of apple worldwide. The early elucidation of the gene‐for‐gene relationship between V. inaequalis and its host Malus has intrigued plant pathologists ever since, with the identification of 17 resistance (R)–avirulence (Avr) gene pairings. The Avr gene products are presumably a subset of the total effector arsenal of V. inaequalis (predominantly proteins secreted in planta assumed to facilitate infection). The supposition that effectors from V. inaequalis act as suppressors of plant defence is supported by the ability of the pathogen to penetrate the cuticle and differentiate into large pseudoparenchymatous structures, termed stromata, in the subcuticular space, without the initiation of an effective plant defence response. If effectors can be identified that are essential for pathogenicity, the corresponding R genes will be durable and would add significant value to breeding programmes. An R gene cluster in Malus has been cloned, but no V. inaequalis effectors have been characterized at the molecular level. However, the identification of effectors is likely to be facilitated by the resolution of the whole genome sequence of V. inaequalis.
Taxonomy: Teleomorph: Venturia inaequalis Cooke (Wint.); Kingdom Fungi; Phylum Ascomycota; Subphylum Euascomycota; Class Dothideomycetes; Family Venturiaceae; genus Venturia; species inaequalis. Anamorph: Fusicladium pomi (Fr.) Lind or Spilocaea pomi (Fr.).
Life cycle: V. inaequalis is a hemibiotroph and overwinters as pseudothecia (sexual fruiting bodies) following a phase of saprobic growth in fallen leaf tissues. The primary inoculum consists of ascospores, which germinate and penetrate the cuticle. Stromata are formed above the epidermal cells but do not penetrate them. Cell wall‐degrading enzymes are only produced late in the infection cycle, raising the as yet unanswered question as to how V. inaequalis gains nutrients from the host. Conidia (secondary inoculum) arise from the upper surface of the stromata, and are produced throughout the growing season, initiating multiple rounds of infection.
Venturia inaequalis as a model pathogen of a woody host: V. inaequalis can be cultured and is amenable to crossing in vitro, enabling map‐based cloning strategies. It can be transformed readily, and functional analyses can be conducted by gene silencing. Expressed sequence tag collections are available to aid in gene identification. These will be complemented by the whole genome sequence, which, in turn, will contribute to the comparative analysis of different races of V. inaequalis and plant pathogens within the Dothideomycetes.
INTRODUCTION
Venturia inaequalis Cooke (Wint.) is a hemibiotrophic fungus that is the causal agent of the apple disease scab, more commonly known as black spot in Australasia (MacHardy, 1996). V. inaequalis has a wide geographical range and is found in almost all areas in which apples are grown commercially. It is the most important disease of apple worldwide in terms of economic cost of control (Carisse and Bernier, 2002). However, the disease is more severe in temperate countries with cool, moist climates during early spring (MacHardy, 1996; Manktelow et al., 1996). Apple scab is predominantly controlled by a combination of sanitation and cultivation measures, and judicious fungicide application, the correct timing of which is essential for effective control (Beresford and Manktelow, 1994). To date, the deployment of resistant cultivars has been very limited because of their relatively poor fruit quality. However, they are expected to play a major role in disease control, particularly in organic fruit production, once new cultivars with durable resistance to scab become available.
V. inaequalis has been researched continuously for over 100 years. The resultant body of knowledge is comprehensive in its scope, covering aspects of the basic biology and genetics of the fungus, and the epidemiology and control of the disease (reviewed extensively by MacHardy, 1996). This research has revealed the uncommon parasitic strategy adopted by V. inaequalis: no host cell penetration occurs, but fungal biomass accumulates in the subcuticular space prior to sporulation. This raises the question as to how the fungus remains undetected in the plant with the absence of a co‐ordinated host resistance reaction. The fungus, in all likelihood, produces effectors that suppress any resistance response. Effectors are proteins or secondary metabolites expressed in planta that are assumed to facilitate infection/suppress resistance (Chisholm et al., 2006; Dangl and Jones, 2001), some of which have been commandeered by resistance (R) genes for pathogen recognition and the initiation of defence. The putative production of effectors by V. inaequalis is supported by genetic data.
The fungus is heterothallic and controlled crosses can be performed readily in vitro. This led to in‐depth genetic studies of V. inaequalis in the 1940s and 1950s, when loci for colony colour (presumably involved in melanin biosynthesis), mating type, various nutritional markers and avirulence were identified, and genetic maps were produced (Boone, 1971; Boone and Keitt, 1957; Keitt and Langford, 1940; Shay and Hough, 1952; Williams and Shay, 1957). These studies led to the elucidation of the gene‐for‐gene nature of the relationship between V. inaequalis and Malus at the time at which Flor (1956) reported such interactions for the flax–Melampsora lini pathosystem. Thus, the V. inaequalis–Malus pathosystem appears to fit the effector paradigm.
In some pathosystems, effectors are secreted into the apoplast and exert their effect extracellularly, whereas, in others, they are targeted to the host cytoplasm. Generally, effectors that have been identified to date share little amino acid sequence similarity with each other or with other known proteins. Some have limited conserved sequence motifs, e.g. the ‘RXLR’ or crinkler motifs of many oomycete effectors (Haas et al., 2009; Schornack et al., 2009). V. inaequalis effectors remain elusive and have yet to be cloned (Bowen et al., 2009; Broggini et al., 2007, 2009a; Kucheryava et al., 2008; Win et al., 2003). Their identification, concomitant with the identification of R genes in the host (Belfanti et al., 2004; Boudichevskaia et al., 2009; Broggini et al., 2009b; Galli et al., 2010b; Malnoy et al., 2008; Szankowski et al., 2009; Vinatzer et al., 2001; Xu and Korban, 2002), is the focus of much of the current molecular research on apple scab disease. Tools for the dissection of the V. inaequalis–Malus interaction at the molecular level are available. The pathogen can be cultured easily and, as mentioned above, induced to sexually cross in vitro (Keitt and Langford, 1941; Keitt and Palmiter, 1938). Although slow growing, it can be transformed readily (Fitzgerald et al., 2003) and is amenable to functional analyses by gene silencing (Fitzgerald et al., 2004). V. inaequalis is therefore a tractable, model, hemibiotrophic pathogen of a woody host. Furthermore, assembly of the whole genome sequence (WGS), which is currently underway, will greatly accelerate the molecular dissection of this pathosystem.
TAXONOMY, PATHOGEN EVOLUTION AND HOST RANGE
Debate over the taxonomic classification of many fungal species is often fierce. The case of V. inaequalis proves to be no exception. The Venturiaceae, including V. inaequalis, has, until recently, been placed in the class Dothideomycetes, in the subclass Pleosporomycetidae, order Pleosporales, on the basis of both morphological and molecular criteria (Eriksson and Hawksworth, 1998; Goodwin, 2004; Lumbsch and Huhndorf, 2007). However, recent comprehensive molecular phylogenetic analyses of members of the Dothideomycetes, using data from both nuclear and mitochondrial genes, place the Venturiaceae outside the Pleosporales, without a subclass and order assignment (Kruys et al., 2006; Schoch et al., 2009).
The classification of the related anamorph (asexual) stage at the genus level has also proven to be controversial. Schubert et al. (2003), in the most comprehensive study to date, proposed that the anamorph be classified as Fusicladium pomi (Fr.) Lind, replacing the previous classification of Spilocaea pomi (Fr.). However, this has not been universally adopted, as the old name of S. pomi is still in use (Attrassi et al., 2007; Bini et al., 2008; Jha et al., 2009).
Members of the genus Venturia infect various fruit tree genera: V. inaequalis infects apple (Malus); V. pirina infects European pear (Pyrus); V. nashicola infects Asian pear (Pyrus); V. carpophila infects peach (Prunus); and V. cerasi infects cherry (Prunus). Venturia species can be differentiated into distinct clades on the basis of polymorphisms in ribosomal internal transcribed spacer (ITS) DNA sequences (Beck et al., 2005; Ishii and Yanase, 2000). The topology of the Venturia species phylogram aligns closely with that of the host genera, demonstrating a close co‐evolutionary relationship between the pathogenic Venturia species and their respective fruit tree hosts.
The boundaries between the Venturia species are highly distinct and matings between the species in the laboratory have not been achieved. There is evidence of significant variation within each fungal species, presumably as a consequence of annual, sexual reproduction and obligate outcrossing in these heterothallic species (Langford and Keitt, 1957). The species boundaries within a particular host genus, however, are less distinct, e.g. various Malus species are able to hybridize, permitting gene flow to occur between these species. It is likely that the resultant diversity within host genera also adds to the within‐species diversity of the Venturia species.
Simple sequence repeat (SSR) markers have been used to demonstrate population diversity and to understand the origin and spread of V. inaequalis with the domestication of apple worldwide. The domesticated apple is often referred to as Malus × domestica, with the ‘×’ in the species binomial denoting that this species resulted from various hybridizations between wild Malus species. Coalescence analysis of migration models suggests that V. inaequalis originated in Central Asia, the centre of origin of Malus spp. (2008, 2010). It is likely that the pathogen followed the dispersal of domesticated apple that accompanied Europeans as they migrated to new territories (2008, 2010). Consistent with this is the observation that there is greater genetic diversity amongst populations of V. inaequalis found on M. sierversii (the major progenitor of the domesticated apple) in Central Asia than in European populations from Malus × domestica and M. sylvestris (a secondary crabapple progenitor of the domesticated apple; Gladieux et al., 2010), and also in European subpopulations of V. inaequalis than in populations from areas in which apples have been introduced more recently (Gladieux et al., 2008; Tenzer and Gessler, 1997, 1999). In some cases, such as several states in the USA, Japan (Sawamura, 1988) and Australia, V. inaequalis introductions have been relatively recent, much later than the introduction of apple itself. Occasionally, in remote growing regions, the disease is absent, e.g. in Western Australia the disease has not become established to date, following continuous eradication efforts after rare and sporadic V. inaequalis incursions (McKirdy et al., 2001).
The lack of diversity in both the cultivated hosts and the associated V. inaequalis population in regions away from the centre of diversity has resulted in the wild Malus species of East Asia remaining as a substantial resource for disease resistance breeding. The majority of disease resistance genes currently being deployed in apple breeding programmes have been sourced from these wild Malus species, including those originating from East Asia, e.g. M. floribunda, M. micromalus, M. doumeri and M. hupehensis. This is consistent with Nikolai Vavilov's hypothesis that the best germplasm for resistance breeding can be found at the host's centre of origin and diversity, where host and pathogen have co‐evolved for the longest period of time (Zhukovsky, 1961).
Certain isolates that can be characterized as V. inaequalis on the basis of morphological criteria do not infect apple, but instead infect different hosts belonging to the subfamily Maloideae, such as Crataegus, Sorbus, Pyracantha and Eriobotrya species (Biggs, 1990; Le Cam et al., 2002). This indicates either a wide host range for V. inaequalis or the need to further subdivide the species into either formae speciales or distinct species with separate host specificities. The uncertainty over this issue is, in part, a result of a lack of a comprehensive host range survey. Le Cam et al. (2002) suggested that those isolates responsible for causing scab on Malus spp. and Pyracantha spp. should be considered as distinct formae speciales of V. inaequalis, rather than separate species, because they are able to mate with each other in vitro and they cannot be differentiated on the basis of polymorphisms in the ITS region of the ribosomal DNA (rDNA) sequences. In contrast, those isolates able to infect Eriobotrya should be considered as a separate species because of sequence differences in the ITS region and the gene encoding glyceraldehyde 3‐phosphate dehydrogenase (Sánchez‐Torres et al., 2009). However, a new study, looking at the variation of six nuclear loci, determined that isolates from the three different hosts did not resolve into three distinct phylogenetic species corresponding to host range (Gladieux et al., 2009), questioning the requirement for novel species delineation and perhaps even subspecific demarcation. Further studies, including WGS comparisons, should clarify the phylogenetic relationships of, and host specificity determinants between, these isolates.
LIFE CYCLE, EPIDEMIOLOGY AND CONTROL ON APPLE
V. inaequalis overwinters predominantly as pseudothecia (sexual fruiting bodies) that develop in apple leaf litter following a phase of saprobic growth after leaf abscission. The fungus is self‐sterile, and hence opposite mating types are required for mating to succeed (Keitt and Palmiter, 1937). Some overwintering may also occur as conidial pustules on shoots and bud scales without the involvement of the teleomorph (sexual stage; Becker et al., 1992; Holb et al., 2006). Infection is initiated in spring and early summer by ascospores (sexual spores) that are released by rainfall from pseudothecia. This release is timed to coincide with host budburst and leaf unfurling (Brook, 1976; MacHardy and Gadoury, 1986; Szkolnik, 1969). Infection risk is greatest early in the growing season when leaves and fruit are young and at their most susceptible (Schwabe, 1979; Schwabe et al., 1984; Xu and Robinson, 2005).
The germ tubes arising from ascospores penetrate through the cuticle, either directly or via an appressorium (Keitt and Jones, 1926; Smereka et al., 1987), and develop into multilayered, pseudoparenchymatous structures, termed stromata, that are presumed to obtain nutrients from the subcuticular space (Fig. 1). The stromata, and the conidia that they produce, cause the characteristic leaf and fruit lesions that give the disease the name of scab or, in some countries, black spot (Fig. 2). Conidia are disseminated by wind and rain from lesions and allow secondary infection to occur within the orchard throughout the fruit development period. These spores, produced asexually, are responsible for an increase in disease if suitable weather conditions occur during the growing season (Fig. 3).
Figure 1.
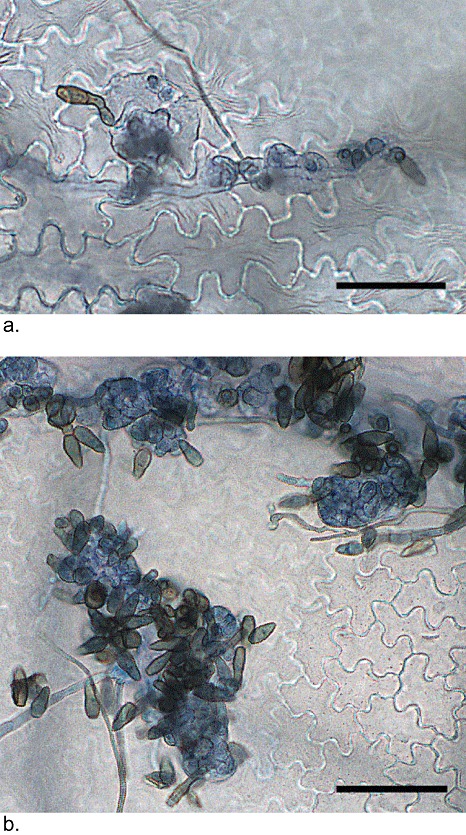
Formation of stromata during leaf infection by Venturia inaequalis: at 5 days post‐inoculation, with stromata beginning to form (a), and 10 days post‐inoculation, with sporulating stromata (b). Scale bar represents 50 µm. (Reprinted from Bowen et al., 2009. Copyright 2009 with permission from John Wiley & Sons.)
Figure 2.

Typical scab symptoms on apple fruit caused by Venturia inaequalis.
Figure 3.
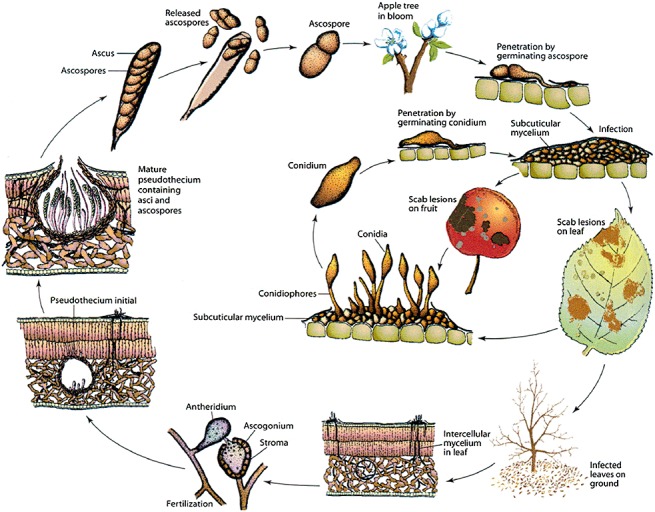
The life cycle of Venturia inaequalis. Subcuticular mycelium = stroma. (This diagram was published in Agrios, Plant Pathology, p. 506. Copyright Elsevier 2005.)
The severe impact of V. inaequalis on apple production (MacHardy, 1996) results from the very low level of scab that can render fruit unmarketable. Most commercial apple cultivars are susceptible to scab (Gessler et al., 2006; Holb, 2007). Consequently, a very high level of costly disease control is required to reduce infection in the orchard (Beresford and Manktelow, 1994; Manktelow et al., 1996). Fungicide spray programmes during spring and summer are required in most apple‐growing regions for conventional, integrated fruit production and organic production systems (Holb, 2006). V. inaequalis has successively developed resistance to dodine, benzimidazole, demethylation inhibitor (DMI; Köller, 1994) and quinone outside inhibitor (QoI; Köller et al., 2004) classes of fungicides. Strategies to delay the development of resistance to these fungicides in field populations of the pathogen rely on restricting the number of applications per season of fungicides in each class and mixing or alternating at‐risk fungicides with ones that are not at risk from resistance development (Brent and Holloman, 2007).
The molecular basis for resistance has been identified for these major classes of fungicides, except dodine. Resistance to benomyl (a benzimidazole) in the field is a result of specific mutations in the gene encoding the target protein β‐tubulin that modify amino acid 198 (from glutamate to alanine, lysine or glycine; Koenraadt et al., 1992). The mechanism of resistance to the DMI fungicides has not been as well characterized, but appears to involve an energy‐dependent efflux system (Palani and Lalithakumari, 1999). Although cross‐resistance to these fungicide classes is not observed, it has been noted that resistance to either benomyl or DMI occurs at a higher frequency in V. inaequalis isolates already resistant to dodine compared with sensitive isolates (Köller and Wilcox, 2001). The newer QoI class of fungicides target the Qo centre of cytochrome b. In the laboratory, resistance can occur either through the upregulation of an alternative oxidase pathway or a G143A mutation in the gene encoding cytochrome b itself (Zheng et al., 2000). Upregulation of the alternative oxidase pathway appears to be repressed in planta, rendering V. inaequalis sensitive; thus these types of mutants are not found in the field. Because cytochrome b is encoded by the mitochondrial genome, the G143A mutation must accumulate to a certain level before resistance develops. Thus, resistance development in the field can be delayed by limiting the number of applications of QoI fungicides each season (Köller et al., 2004).
Sanitation practices that reduce ascospore inoculum include autumn urea sprays, which suppress the pathogen's sexual stage and enhance leaf litter breakdown (Burchill et al., 1965), leaf shredding or mulching and the application of lime sulphur or synthetic fungicides in autumn (Holb et al., 2006).
Apple scab is most problematic in regions in which frequent rainfall during spring results in ascospore release and infection. Meteorological criteria defining the duration of wetness required for infection at different temperatures were first proposed by Mills and LaPlante (1954), and are known as Mills' periods. These criteria have become a standard tool, in conjunction with electronic weather monitoring and computer systems, for identifying when conditions suitable for infection occur, so that fungicide applications can be targeted effectively. Other predictive models to help manage fungicide applications against apple scab include ascospore maturation models (MacHardy and Gadoury, 1986) and orchard leaf canopy development models (Beresford et al., 2004).
Although our in‐depth understanding of the epidemiology of apple scab has reduced fungicide usage, scab control in most apple‐growing regions is still dependent on the significant input of agrochemicals. This is likely to continue until effective and durable scab resistance is incorporated into new apple cultivars.
THE SUSCEPTIBLE INTERACTION
Nusbaum and Keitt (1938) were the first to report the cytology of the interaction between V. inaequalis and apple. They recorded that conidia and ascospores germinate, most commonly from the apical end of the spore, when free moisture is available on the leaf surface. The spore and germ tube adhere to the cuticle, effected by the formation of adhesive mucilage, composed of proteins and carbohydrates (β‐galactose and N‐acetylglucosaminyl residues; Schumacher et al., 2008; Smereka et al., 1988). Usually, a relatively simple appressorium differentiates, but is not essential for penetration (Fig. 4). The appressorium wall is not highly melanized, although a melanin ring is located at the base of the penetration peg (Steiner and Oerke, 2007). Indeed, mechanical pressure is not required (Smereka et al., 1988), but localized enzymatic hydrolysis of the cuticle is proposed to effect penetration. Extracellular cutinase activity has been implicated, as this enzyme is produced by germinating conidia, and a cutinase inhibitor can prevent penetration (Köller and Parker, 1989; Köller et al., 1991). In addition, esterase‐like activity has been reported during the germination of conidia and in appressoria (Nicholson et al., 1972). Interestingly, in infected areas, the cuticle appears to expand significantly, up to 10 times thicker than normal (Kucheryava et al., 2008). Whether this is a result of enzyme action or the secretion of mucilage is not known (Fig. 5).
Figure 4.
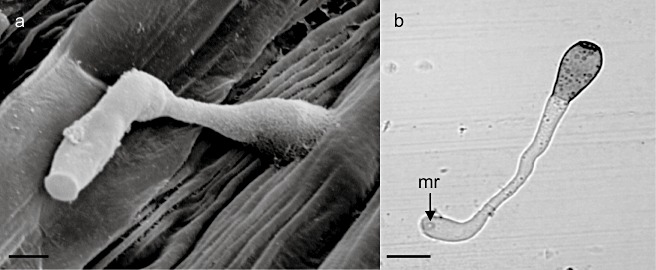
(a) A spore of Venturia inaequalis 9 h post‐inoculation, with germination from the apical end of the conidium and formation of an appressorium adhering to the leaf surface. Scale bar represents 5 µm. (b) A germinated conidium and appressorium of V. inaequalis adhering to a slide coated in apple wax, 24 h post‐inoculation; mr, melanized ring at the base of the penetration peg beneath the appressorium. Scale bar represents 10 µm.
Figure 5.
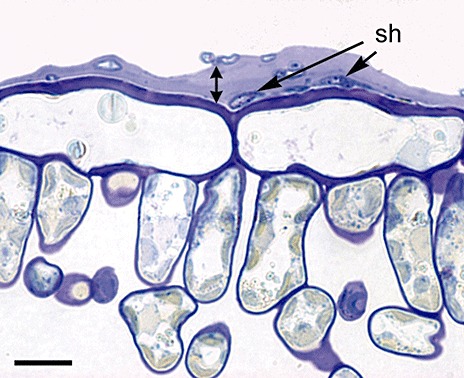
Cross‐section of an infected leaf at 14 days post‐ioculation, stained with aniline blue, showing the increased thickness of the cuticle (double arrow). Scale bar represents 10 µm; sh, subcuticular hyphae. (Reprinted from Kucheryava et al., 2008. Copyright 2008 with permission from Elsevier.)
Once the cuticle is breached, the infection peg differentiates to form subcuticular runner hyphae, which are much wider in diameter than surface mycelia. From these subcuticular hyphae, stromata differentiate at regular intervals. Stromata are made up of laterally dividing cells with diameters of up to 10 µm, markedly different from the tubular hyphae found on the plant surface, agar medium or in liquid culture (Kucheryava et al., 2008). Conidia are formed following enlargement of the uppermost cells of the stroma. V. inaequalis appears to have adapted to colonizing the subcuticular apoplastic space during the biotrophic phase of growth. This might explain why the observed induction of callose papillae in the compatible interaction, although implying recognition of the fungus, does not play a role in restricting colonization (Kucheryava et al., 2008). However, it raises the question of why other potential defence mechanisms, such as phytoalexin biosynthesis, appear not to be induced, suggesting that they may be suppressed by effectors that function in the host cytoplasm.
There is no obvious damage to the host throughout stroma development, apart from the breaching of the cuticle. Only on sporulation do the epidermal cells underlying the stroma undergo a progressive depletion of plastids and cytoplasm, accompanied by increasing vacuolation, leading ultimately to necrosis, which is possibly effected by partial cell wall degradation late in the infection cycle (Nusbaum and Keitt, 1938; Valsangiacomo and Gessler, 1988). Cellulase, β‐glucosidase, pectinase, and endo‐ and exo‐polygalacturonase activities have been detected in V. inaequalis growing in vitro. However, if these enzymes are only expressed late in infection, when host cell wall degradation is seen, they are unlikely to play a major role in nutrient acquisition during the establishment of infection (Kollar, 1998; MacHardy, 1996; Valsangiacomo and Gessler, 1992). This raises the question as to how the fungus gains nutrients from the host. There is evidence that, like other biotrophic phytopathogens, V. inaequalis influences hormone levels at infection sites, specifically cytokinins, to assist with its biotrophic nutrition (Cooper and Ashby, 1998; Walters and McRoberts, 2006). Under this scenario, β‐glucosidase activity releases active cytokinin from cytokinin‐O‐glucoside stored in the plant or fungus, in turn creating a cytokinin sink at the site of invasion. Cytokinin accumulation then results in the translocation of nutrients to the fungus at this site (Cooper and Ashby, 1998).
THE RESISTANCE REACTION
Susceptibility is usually associated with unbridled growth of a pathogen on a host. Large spreading lesions caused by V. inaequalis have been observed on fruit and leaves in the field, but whether these originate from one or multiple infection foci has not been established (R. Beresford and K. Plummer, personal observation). Typically, this is not the case, however, with V. inaequalis lesions on apple leaves appearing to reach a maximum radius of 1 cm from the point of infection, then ceasing to expand (MacHardy, 1996). This phenomenon has been attributed to ontogenic resistance that develops as leaves and fruit mature, and is complete by the time leaves are fully expanded (Gessler and Stumm, 1984; Schwabe, 1979). Infections become established only on unfolding leaves (Valsangiacomo and Gessler, 1988) and the internodes in between (Jeger and Alston, 1986), with ontogenic resistance developing faster on the ventral than on the dorsal sides of the leaves during their expansion phase (Keitt and Jones, 1926). Ontogenic resistance is slower to develop under glasshouse conditions than in the field, presumably reflecting differential plant tissue maturity rates under different environmental conditions (Szkolnik, 1978). The rates of conidial germination and appressorial differentiation of V. inaequalis are not affected by leaf age, but stroma formation and sporulation are reduced in both rate and amount with increasing age of host tissue (Gessler and Stumm, 1984). The basis of this resistance is unknown, but it has been shown that it becomes inactive again late in the growing season when leaves start to senesce and dormant lesions renew their development, even on the oldest leaves (Kollar, 1996). This senescence may compromise the ability of the plant to induce a resistance response. Ontogenic resistance is observable in all apple cultivars and appears never to have been overcome by the pathogen; if the pathogen can build sufficient biomass to enable successful sexual crossing during winter, despite ontogenic resistance operating, it has no selective drivers to overcome this resistance (MacHardy et al., 2001).
Although ontogenic resistance provides protection against scab on mature tissues, growing leaves and fruit remain vulnerable, requiring additional resistance that is predominantly provided by quantitative trait loci (QTLs) and R genes. A number of scab resistance QTLs have been identified to date in both glasshouse (Calenge et al., 2004) and field (Liebhard et al., 2003) experiments. Some of these map to the same regions in which major scab R genes have been located, e.g. on linkage groups (LGs) 1 and 2 (Calenge et al., 2004; Dunemann and Egerer, 2010; Durel et al., 2003; Soriano et al., 2009). Other QTLs, such as those on LG11 and LG17, are not associated with major genes, with LG17 being a particular ‘hot‐spot’ for broad‐spectrum scab resistance QTLs.
QTLs generally confer low levels of partial resistance to a broad spectrum of the pathogen population. In apple, it is increasingly becoming evident, however, that some QTLs are only active against a narrow spectrum of the V. inaequalis population. For example, Calenge et al. (2004) detected three QTLs with only a single V. inaequalis isolate of the eight that were tested and, although some QTLs had a broad spectrum, it was never complete; none of the 24 QTLs were identified with all isolates tested. An isolate‐specific QTL was also identified by Durel et al. (2003) in close proximity to the Rvi6 gene. The individual effects of QTLs are variable, ranging from marked (Calenge et al., 2004) to weak. However, when combined, complementation results in an overall strong resistance. A good example of this is the cultivar ‘Antonovka’, in which quantitative resistance is the result of a combination of narrow‐spectrum genes that together provide a strong and seemingly durable resistance to the pathogen population at large (Gessler et al., 2006).
Despite the durability of ontogenic and QTL‐controlled resistance, it is the R genes which control the host's participation in, and contribution to, the gene‐for‐gene relationship between Malus and V. inaequalis that have attracted the attention of many researchers. This is a result of the simplicity of their genetic analysis and manipulation in relation to breeding material, combined with the relative ineffectiveness of ontogenic resistance for protection of the plant throughout the growing season. To date, 17 R–avirulence (Avr) gene pairings have been defined (Table 1), the outcome of the interactions of their products determining whether a susceptible or resistant reaction occurs between host and pathogen.
Table 1.
Nomenclature of the differential host–pathogen interactions of Venturia inaequalis and Malus. Research is in progress to identify F1 progeny in current differential hosts that carry multiple resistance genes.
| Malus | Venturia inaequalis | |||||
|---|---|---|---|---|---|---|
| Differential host | Resistance locus | Avirulence locus | Race | |||
| No. | Accession | Old | New | New | Old | |
| h(0) | (Royal) Gala | — | — | 0 | ||
| h(1) | Golden Delicious | Vg | Rvi1 | AvrRvi1 | (1) | |
| h(2) | TSR34T15 | Vh2 = Vr − A | Rvi2 | AvrRvi2 | p‐9 | (2) |
| h(3) | (F1 of) Geneva | Vh3 | Rvi3 | AvrRvi3 | p‐10 | (3) |
| h(4) | TSR33T239 | Vh4 = Vx = Vr1 | Rvi4 | AvrRvi4 | (4) | |
| h(5) | 9‐AR2T196 | Vm | Rvi5 | AvrRvi5 | (5) | |
| h(6) | Priscilla | Vf | Rvi6 | AvrRvi6 | (6) | |
| h(7) | (F1 of) M. × floribunda 821 | Vfh | Rvi7 | AvrRvi7 | (7) | |
| h(8) | GMAL3631‐W193B | Vh8 | Rvi8 | AvrRvi8 | (8) | |
| h(9) | (F1 of) Dolgo | Vdolgo | Rvi9 | AvrRvi9 | p‐8 | (9) |
| h(10) | A723‐6 | Va | Rvi10 | AvrRvi10 | (10) | |
| h(11) | A722‐7 | Vbj | Rvi11 | AvrRvi11 | (11) | |
| h(12) | Hansen's baccata #2 | Vb | Rvi12 | AvrRvi12 | (12) | |
| h(13) | Durello di Forlì | Vd | Rvi13 | AvrRvi13 | (13) | |
| h(14) | (F1 of) Dülmener Rosen | Vdr1 | Rvi14 | AvrRvi14 | (14) | |
| h(15) | GMAL2473 | Vr2 | Rvi15 | AvrRvi15 | (15) | |
| h(16) | MIS op 93.051 G07‐098 | Vmis | Rvi16 | AvrRvi16 | (16) | |
| h(17) | (F1 of) Antonovka APF22 | Va1 | Rvi17 | AvrRvi17 | (17) | |
Based on foliar resistance reactions, apple R genes can be grouped into three predominant resistance classes exhibiting distinctive resistance responses: the classical hypersensitive response (HR) in which fungal growth is normally terminated very rapidly on penetration, e.g. conditioned by Rvi4 and Rvi5 (Win et al., 2003) and Rvi15 (Galli et al., 2010a); responses involving limited subcuticular growth inducing stellate necrosis, e.g. conditioned by Rvi2 and Rvi8 (Bus et al., 2005); and chlorosis, often accompanied by limited sporulation, hence providing only partial resistance, e.g. conditioned by Rvi6 and Rvi12 (Fig. 6).
Figure 6.

Characteristic scab resistance reactions on apple leaves: pin‐point pit, hypersensitive response conditioned by the Rvi4 gene (left); stellate necrosis by the Rvi2 gene (middle); and chlorosis with limited sporulation conditioned by the Rvi6 gene (right) under glasshouse conditions.
When an HR is involved, the response is often induced immediately after the cuticle is penetrated. For example, in the case of the Rvi5 gene, the pin‐point pit resistance reactions (visible macroscopically as small depressions in the leaf surface) typical of an HR are visible 2–3 days after infection. However, when other R genes are involved, e.g. Rvi15, it may take 10–11 days for the reaction to be obvious (Galli et al., 2010a; Shay and Hough, 1952). At the epicentre of the response zone, the epidermal cells collapse, creating the small pit. The response goes beyond the initially affected cell into the palisade mesophyll and neighbouring epidermal cells, often affecting a large number of cells, presumably involving cell‐to‐cell signalling. The response is readily visualized as these cells autofluoresce under ultraviolet (UV) light (Fig. 7). The cells also undergo ultrastructural modifications, such as cell wall apposition and changes in the cellular organelles; the extent of the ultrastructural changes of the epidermal and palisade mesophyll cells varies with the R gene involved (Chevalier and Lespinasse, 1991; Chevalier et al., 1991). On resistant hosts that exhibit stellate necrosis, the fungus grows very fine mycelial structures that often show autofluorescence under UV light (2005, 2010). The chlorotic resistance reaction shows less autofluorescence under UV light compared with HR and stellate necrosis reactions, and encompasses a wide range of intensity of response.
Figure 7.
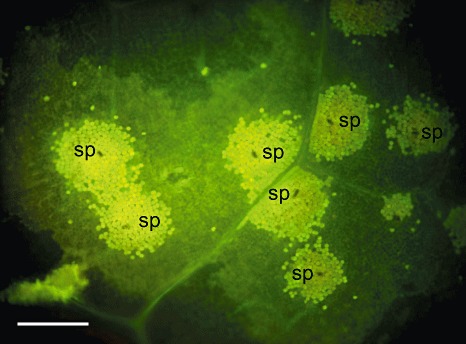
Microscopic interference blue autofluorescence observations, demonstrating the hypersensitive response involving the epidermis and the palisade mesophyll conditioned by the Rvi5 gene. Several hypersensitive responses are shown, each initiated by a Venturia inaequalis spore (sp) at its centre. Scale bar represents 200 µm.
These differences in phenotypic expression of resistance (HR, stellate necrosis and chlorosis) may reflect differences in the defence signalling cascades and resistance reactions induced as a consequence of different R gene‐mediated recognition events. In addition, they may also reflect the virulence role of their cognate effectors, perhaps involving suppression of components of the resistance reaction, or differences in the temporal expression of these effectors.
In the past, some interactions have been classified as compatible as a result of the production of spores by the pathogen. However, in some cases, this sporulation is still preceded by a resistance reaction that is unable to contain the infection, but does slow down pathogen spread and sporulation rate. This was demonstrated with two putative race (4) isolates of V. inaequalis, where the resistance conditioned by Rvi4 is commonly expressed as immunity but, in the case of isolates 1638 and US4, this was reduced to partial resistance (Bus, 2006). Such isolate‐dependent variation could indicate the presence of an additional effector in these isolates, able to suppress the defence reaction.
MOLECULAR DISSECTION OF R GENES
Despite the knowledge of their existence for over half a century, and extensive classical genetic studies on many of the currently identified R genes (reviewed by Gessler et al., 2006; MacHardy, 1996), molecular analysis of only one locus has resulted in the cloning and demonstration of the proof of function of an R gene active against V. inaequalis. The Rvi6 locus derived from the crabapple species Malus floribunda 821 was dissected independently by two groups (Vinatzer et al., 2001; Xu and Korban, 2002), attracting significant interest as it is widely deployed in commercial cultivars (Janick et al., 1996; Korban, 1998). The map‐based cloning of the locus was facilitated by the development of a comprehensive array of molecular markers throughout the 1990s (reviewed by Tartarini and Sansavini, 2004). Both groups identified four R gene paralogues at the locus (termed HcrVf1–4 and Vfa1–4, respectively; Vinatzer et al., 2001; Xu and Korban, 2002), one of which (HcrVf3) appeared to be a truncated pseudogene. The HcrVf genes exhibit high sequence similarity to a class of R genes found in tomato, that are characterized by multiple N‐terminal leucine‐rich repeats (LRRs) and transmembrane domains (Dangl and Jones, 2001). They are predicted to encode extracytoplasmic glycoproteins, anchored to the cell membrane, with the LRRs comprising the majority of the extracytoplasmic portion (Vinatzer et al., 2001). The three putative functional genes at the Rvi6 locus are expressed constitutively, although differentially. HcrVf1 and HcrVf2 are highly expressed in immature leaves, but negligibly in mature leaves and, conversely, the expression of HcrVf4 is higher in mature than in immature leaves.
Early indications that HcrVf2 is an active R gene (Barbieri et al., 2003) were corroborated by Belfanti et al. (2004). Belfanti et al. (2004) transformed susceptible ‘Gala’ with HcrVf2, utilizing the strong constitutive Cauliflower mosaic virus (CaMV) 35S promoter. The transgenic lines were only resistant to isolates of V. inaequalis that expressed the cognate AvrRvi6 effector gene. In contrast, when native promoters were used to control either HcrVf1 or HcrVf2 expression in susceptible cultivars ‘Gala’ and ‘McIntosh’, both genes conferred resistance, although the levels of resistance were lower than those found in conventionally bred cultivars carrying the Rvi6 gene (Malnoy et al., 2008). This was attributed to a dose effect equivalent to plants homozygous for Rvi6 having a higher resistance response compared with plants heterozygous for the gene (Gessler et al., 1997; Tartarini et al., 2000), or a requirement for both HcrVf1 and HcrVf2 for effective resistance. However, subsequent optimization of the native promoter length resulted in resistance equivalent to that exhibited by cultivars with Rvi6 introgressed via classical breeding being achieved with HcrVf2 in a ‘Gala’ or ‘Elstar’ background (Silfverberg‐Dilworth et al., 2005; Szankowski et al., 2009). LRR receptor‐like protein kinases may also be involved in pathogen perception, downstream of the Rvi6 gene product, as the expression of two genes belonging to the LRPKm family is upregulated during a resistance response in the presence of HcrVf2 (Cova et al., 2010; Komjanc et al., 1999).
Although R genes have been cloned, many questions still remain as to how they and related genes operate; many genes homologous to HcrVf genes have been found in resistant hosts in addition to M. floribunda 821 (Boudichevskaia et al., 2009) and its derivatives (Broggini et al., 2009b). The HcrVf2 gene, and its homologues at the Rvi6 locus, is most closely related to the Cf genes of tomato for resistance to Cladosporium fulvum, particularly those belonging to the Hcr9 (homologues of C. fulvum resistance gene Cf‐9) gene family (Cf‐4, Cf‐4E, Cf‐9B, Cf‐9; Jones et al., 1994; Kruijt et al., 2005; Laugéet al., 1998; Parniske et al., 1997; Takken et al., 1998; Thomas et al., 1997). This similarity in R gene may reflect the similarity in pathogenic strategy adopted by the two pathogens and the effectors produced; they both colonize the plant extracellularly, occupying either the leaf apoplast, in the case of C. fulvum, or the subcuticular space, in the case of V. inaequalis. The proteins predicted to be encoded by HcrVf and the Hcr9 gene family show the same overall structure with similar domains, with the same domains exhibiting the most variability [the cysteine‐rich B domain and the N‐terminus of the LRR domain (C); Kruijt et al. 2005]. The HcrVf and Hcr9 gene clusters share the same structure with tandemly repeated genes residing at a complex locus. They may also share a similar mode of evolution involving duplication events and positive selection for diversification in the highly variable domains (Kruijt et al., 2005; Xu and Korban, 2004). However, inferences as to whether the HcrVf gene products operate by a direct interaction with their cognate effector or are a component of a guard system are harder to draw, as members of the Hcr9 gene family may operate by either method. In the Cf‐9–Avr9 system, there is a high‐affinity Avr9‐binding site that suggests that this may be a guarded system (Kooman‐Gersmann et al., 1996). Similarly, Cf‐9B may also be a ‘guarding’R gene as its cognate effector interacts with a necrosis‐inducing factor (Chakrabarti et al., 2009). In contrast, Cf‐4 is thought to interact directly with Avr4, but no binding experiments have demonstrated this categorically (van Esse et al., 2007; Wulff et al., 2009).
Recently, three clustered genes were identified as candidates for the R gene Rvi15 in accession GMAL 2473. All three genes were found to be transcribed in young leaf tissue and to share a similar structure: an N‐terminus homologous to the Toll and mammalian interleukin‐1 receptor proteins (TIR), a central nucleotide‐binding site (NBS) and an LRR domain at the C‐terminus (Galli et al., 2010b). Whether these genes are responsible for the Rvi15 resistance phenotype remains to be established. However, if the Rvi15 status is confirmed, this too raises questions regarding V. inaequalis effector biology and the recognition events leading to resistance. TIR‐NBS‐LRR (TNL) R proteins were originally thought to be localized exclusively to the cytoplasm, but have been shown recently to be found in a variety of intracellular compartments, often associated with membrane systems, with their putative recognition domains exposed to the cytoplasm (Rafiqi et al., 2009). Galli et al. (2010b) postulated that the Rvi15 protein may recognize cuticle or cell wall degradation products that pass into the cytoplasm, resulting from the subcuticular action of an effector from V. inaequalis. An alternative hypothesis is that V. inaequalis targets an effector to the host cytoplasm, where it exerts an effect as a suppressor of host defence, enabling V. inaequalis to remain subcuticularly without eliciting an effective resistance response. There are precedents for filamentous fungal effectors to be taken up intracellularly and to act as suppressors of host defence (2008, 2009); hence V. inaequalis may not merely be secreting effectors to the subcuticular space as previously thought. This supposition is supported by the downregulation of the expression of two LRR receptor‐like protein kinase genes following inoculation with V. inaequalis. These genes are putatively involved in plant basal defence, and their downregulation may be a result of the action of a suppressor from V. inaequalis (Cova et al., 2010).
Insights into the C. fulvum–tomato interaction may shed light on the nature of ontogenic resistance in Malus. Cf‐9B confers resistance to mature plants of tomato against C. fulvum, but not seedlings, although it is expressed in young plants (Panter et al., 2002). This suggests that the cognate effector of Cf‐9B, Avr9B, may target a protein that is guarded by Cf‐9B, the expression of which is only upregulated in mature plants (Wulff et al., 2009). A similar system could be operating to confer, or contribute to, ontogenic resistance in Malus, albeit not mediated via an HR, which, because of its ubiquitous nature, has not been genetically dissected. HcrVf4 at the Rvi6 locus is highly expressed in mature leaves, although it is still expressed in immature leaves (Xu and Korban, 2002). Such a gene could be guarding a host target that is expressed at increasing levels throughout maturation, a target for an effector from V. inaequalis.
WGS for apple (cv. ‘Golden Delicious’) is now available [Istituto Agrario di San Michele all'Adige (IASMA), unpublished results]. As other Malus spp. also become sequenced, additional R genes will become available. However, it is not possible to ascertain a priori which R genes recognize particular pathogens or to predict their durability from sequence alone. The durability of an R gene is a function of its cognate effector. R genes that match effectors that are redundant or dispensable are unlikely to be durable, whereas R genes that recognize essential effectors are likely to be so. Therefore, the key to durable resistance is the pyramiding of R genes that recognize effectors with significant roles in pathogenicity. The identification and functional characterization of the effector complement from V. inaequalis are crucial for this goal.
MOLECULAR APPROACHES FOR THE IDENTIFICATION OF EFFECTOR GENES OF V. INAEQUALIS
Several molecular approaches have been employed to identify and clone effector genes of V. inaequalis. These include the forward genetic techniques of expressed sequence tag (EST) database mining, as well as map‐based cloning (Broggini et al., 2007) and a reverse genetics proteomics approach (Gau et al., 2004; Win, 2003; Win et al., 2003).
EST database mining
The first large‐scale analysis of ESTs derived from the V. inaequalis–Malus pathosystem resulted in the identification of 4215 ESTs (clustered into 2090 nonredundant sequences) from five distinct cDNA libraries (Bowen et al., 2009). Two of the five were in planta libraries: one prepared from infected leaf material of a compatible interaction between V. inaequalis and apple, and the other prepared by probing the former with genomic DNA of V. inaequalis to enrich for fungal sequences (Newcomb et al., 2006). The remaining three were in vitro libraries: two generated from mycelia grown in potato dextrose broth shake cultures, and one, a suppression subtractive hybridization library, prepared from growth on cellophane overlying potato dextrose agar, subtracted against growth on potato dextrose agar alone, to enrich for cellophane‐induced genes (Bowen et al., 2009). Combined, a total of 937 ESTs were classified as putatively fungal in origin, and could be assembled into 633 nonredundant sequences (Bowen et al., 2009). Mining of this EST database resulted in the identification of 16 candidate effector genes (Vice1–Vice16; Venturia inaequalis candidate effector) based on features common to the characterized effector genes from filamentous fungi, i.e. they encode small (<400 amino acids in length), novel, cysteine‐rich proteins, with a putative signal peptide (Rep, 2005; Stergiopoulos and de Wit, 2009; Templeton et al., 1994; de Wit et al., 2009), although candidates were not required to meet all of these criteria. Three of the 16 candidates, specifically Vice4, Vice12 and Vice16, were shown to be significantly upregulated during growth in planta compared with liquid growth in vitro (Bowen et al., 2009).
Growth of V. inaequalis within cellophane can be viewed as a potential in vitro model for subcuticular growth and morphogenetic differentiation in planta (Kucheryava et al., 2008). This is corroborated by the expression of two cellophane‐induced genes, cin1 and cin3, which are upregulated over 1000‐fold in planta, compared with liquid growth in vitro (Kucheryava et al., 2008). For cin1, mRNA expression is strongly upregulated during the early stages of infection [5 days post‐inoculation (dpi)], whereas, for cin3, expression is strongly upregulated at both 5 and 10 dpi. Both genes are novel, and encode secreted proteins with an imperfect repeat domain structure, in which the repeat number and diversity vary in an isolate‐specific manner (Kucheryava et al., 2008). The repetitive nature of Cin1 and Cin3 is of particular interest, given the role played by some secreted phytopathogenic fungal proteins with tandem repeats in the pathogenesis of a host plant (Müller et al., 2008). To simplify future structural determination of the Cin1 protein using nuclear magnetic resonance spectroscopy, a two‐domain truncated version has been purified to homogeneity (Jones et al., 2009). Functional analysis of the Vice candidates, cin1 and cin3, is currently being performed using RNA interference (RNAi).
RNAi has been used to silence the trihydroxynaphthalene reductase (THN) gene involved in the melanin biosynthesis pathway (Fitzgerald et al., 2004). Melanin is required for the pathogenesis of several phytopathogenic fungi that penetrate their host through the production of a highly melanized appressorium (Henson et al., 1999). Under in vitro conditions, THN‐silenced transformants of V. inaequalis exhibited a distinctively modified light‐brown phenotype, but retained their ability to infect apple, although the degree of infection was not quantified (Fitzgerald et al., 2004).
Comprehensive EST collections have also been produced from the host plant (M. × domestica, M. baccata and M. sieboldii). These are derived from several tissues, stress treatments and genotypes (Gasic et al., 2009; Newcomb et al., 2006), and include a subset of V. inaequalis‐modulated apple genes (Paris et al., 2009). Some of the tissues for these libraries were collected from orchard‐grown trees; therefore, it is likely that they contain genes corresponding to pathogen sequences as a consequence of pathogen contamination of host material. Taken together, these EST libraries provide a valuable genomic resource for V. inaequalis and Malus spp., particularly M. × domestica, and provide important information for understanding the molecular basis of the V. inaequalis–Malus interaction.
Map‐based cloning
Maps generated from phenotypic markers have been expanded upon recently by the incorporation of molecular markers: amplified fragment length polymorphisms and SSRs (Guérin et al., 2004). These maps are now up to 950 and 1053 cM in length and span 11–14 LGs (Xu et al., 2009; G. A. L. Broggini and V. G. M. Bus, unpublished results). In contrast, seven chromosomes were identified by cytology (Day et al., 1956), although other earlier cytological estimates range from four to eight (reviewed in Boone, 1971). Accurate determination of chromosome number will be supported by the resolution of the complete WGS, whole chromosome optical maps of V. inaequalis and the physical resolution of chromosomes by pulsed‐field gel electrophoresis.
Despite the ease of accomplishing in vitro mating in V. inaequalis, a map‐based approach for cloning effector genes has not yet borne fruit. Combining sequence characterized amplified region and SSR markers with a bacterial artificial chromosome library enabled Broggini et al. (2007) to isolate a 330‐kb region that encompasses the AvrRvi1 locus. A bioinformatics search of this contiguous sequence resulted in the identification of 40 open reading frames coding for proteins that were predicted to be secreted via either a signal peptide or a nonclassical secretion pathway (Broggini et al., 2009a). Expression and functional analyses of these candidates are ongoing.
Proteomics
A proteomics approach has proven to be effective for the identification and cloning of effector genes from phytopathogenic fungi. For example, all the effector genes of C. fulvum and Fusarium oxysporum f. sp. lycopersici identified to date have been cloned using a reverse genetics proteomics approach (van der Does et al., 2008; 2007, 2008; Houterman et al., 2007; van Kan et al., 1991). In these studies, effector proteins were characterized from fluids extracted from infected host tissues. A similar approach was conducted for scab‐infected apple leaves, but no fungal proteins were identified, although the regulation of some pathogenesis‐related proteins in response to scab infection was elucidated (Gau et al., 2004).
In an early study, Raa and Sijpesteijn (1968) reported ‘toxins’ in liquid cultures of V. inaequalis capable of eliciting rapid leaf wilt in resistant, but not susceptible, seedlings of apple derived from an ‘Antonovka 34‐20’ (resistant) and ‘Golden Delicious’ (susceptible) cross. These ‘toxins’ are potentially effector proteins, with the leaf wilt reminiscent of a rapid, severe HR. The differential action of these putative effectors was abolished by boiling the culture filtrate and, following gel filtration‐mediated fractionation of the culture filtrate, paper chromatography yielded spots positive for ninhydrin, suggesting a proteinaceous nature. Unfortunately, the putative effectors and the cognate host R genes used in this study were not identified (Raa and Sijpesteijn, 1968).
This approach was pursued further with the aim of cloning the AvrRvi5 effector gene of V. inaequalis (Win et al., 2003). Compounds secreted by V. inaequalis into liquid culture were investigated for their ability to elicit cell death, reminiscent of an HR, in a host plant carrying the cognate Rvi5 R gene. At least three major and three minor candidates for the AvrRvi5 effector protein were identified in the purest fraction of the cell death‐inducing cell‐free culture filtrate. This fraction also elicited cell death in a host plant carrying Rvi4, suggesting that it may also contain AvrRvi4, or an effector with dual host specificities to both R genes. Full‐length cDNA transcripts for three of the AvrRvi4,5 candidate proteins were then identified from EST libraries. Sequence similarity was found to an allergen protein, a protein involved in cell adhesion and a β‐1,3‐endoglucanase enzyme (Win, 2003). The allergen‐like protein and putative β‐1,3‐endoglucanase were also identified as major components of proteins isolated from scab‐infected leaves of a compatible interaction (Fitzgerald, 2004). In this study, proteins were isolated from V. inaequalis race (1)‐infected apple seedling leaves (grown from seeds of open‐pollinated apple cv. ‘Royal Gala’). Various methods, similar to those used for the isolation of proteins from tomato–C. fulvum interactions (van Kan et al., 1991), including the extraction of total proteins and the assay of extracts from vacuum‐infiltrated leaves, were trialled, but were unsuccessful for the isolation of fungal proteins from infected apple leaves. The subcuticular location of V. inaequalis led to the adoption of a different approach, in which the surface of infected leaves was rubbed gently with carborundum grit to disrupt the cuticle and release the fungal proteins. Using this method, novel fungal proteins were identified and are under further investigation. In addition, nucleotide sequences of the allergen‐like genes from avirulent and virulent isolates were identical, suggesting that the bona fide effectors of the cell death‐inducing fraction were not represented in the EST libraries, although these candidates have yet to be ruled out.
THE WGS AND FUTURE DIRECTIONS
The identification of genes coding for effectors from V. inaequalis has been challenging. Reverse genetics and proteomics approaches have been hampered by the low volume of apoplastic fluid that can be extracted from infected apple leaves. ESTs have been useful, but are limited by the low percentage of transcripts that can be confirmed as being of fungal origin in infected leaves. Both of these approaches will be advanced by the application of new sequencing technologies, which will provide WGS and transcriptomes with deeper sequence penetration. WGS will enable the identification of effectors by similarity to known effectors from related fungi and by selecting for small secreted novel proteins, especially those that are cysteine rich. Furthermore, WGS will greatly facilitate the identification of proteins using proteomics.
Three laboratories, including a consortium led by Genoscope (http://www.genoscope.cns.fr/spip/), are currently generating V. inaequalis WGS. It is likely that a complete genome will be available in 2010. Preliminary data from our laboratory suggest that there are approximately 36 Mb of nonrepeated DNA. Approximately 95% of genes can be found in these sequences, as 94 candidate effectors, house‐keeping genes and other ESTs characterized in previous work were found to have strong blast hits to the draft WGS, as did 33 of 37 SSR markers. Orthologues of several fungal effector genes were identified, including Ecp6, AvrLm6 and Avr‐Pita (Bolton et al., 2008; Fudal et al., 2007; Orbach et al., 2000; Table 2). Moreover, amino acid sequence information from a candidate AvrRvi4,5 effector, not present in EST libraries, that elicited a cell death response on hosts 4 and 5 (Win, 2003), exactly matched part of a novel 154‐amino‐acid protein, with a signal peptide and eight cysteines, that was predicted from the WGS. Functional analysis of the corresponding gene is currently underway by gene silencing. Functional analysis utilizing a disruption strategy, requiring ready access to flanking DNA, will now be more easily attempted with the increased availability of sequence data, although the feasibility of gene disruption has yet to be tested in V. inaequalis. Further sequencing, including transcriptome analysis and paired‐end reads, will be carried out to reduce the number of scaffolds in the assembly and to aid annotation.
Table 2.
Effector genes from filamentous fungal pathogens with orthologues in the Venturia inaequalis whole genome sequence (WGS).
| Gene | Similarity score against Venturia inaequalis WGS | Organism | Host | Biological activity | Involvement in virulence/pathogenicity | Corresponding R gene | No. cysteine residues |
|---|---|---|---|---|---|---|---|
| Ecp6 | 6e‐43 | Cladosporium fulvum | Tomato | LysM domains: chitin binding to scavenge chitin to prevent recognition by plant | Required for full virulence | 8 | |
| AvrLm6 | 3e‐08 | Leptosphaeria maculans | Oil seed rape | Induces an HR | Not required for full virulence | Rlm6 | 6 |
| Avr‐Pita | 3e‐11 | Magnaporthe oryzae | Rice | Putative zinc metalloprotease | Not required for pathogenicity | Pi‐ta | 8 |
HR, hypersensitive reaction.
Many Dothideomycete genomes are currently being sequenced, mainly by the Broad Institute (http://www.broadinstitute.org/), the Joint Genome Institute ( http://www.jgi.doe.gov/) and Genoscope. A few genomes have been completed (e.g. Mycosphaerella graminicola, Mycosphaerella fijiensis, Stagonospora nodorum and Pyrenophora tritici‐repentis) and many are nearing completion (e.g. Cochliobolus heterostrophus, Dothistroma septosporum, Leptosphaeria maculans). Plant pathogens are well represented in the Dothideomycetes, and thus comparative genomics may well prove to be invaluable in the identification of effectors in this fungal class. A low rate of sequence conservation of filamentous fungal effectors has, until now, hampered their discovery. Although this may be attributable to a high rate of evolution of effector genes, it may merely be a result of the, so far, sparse genome coverage of many closely related plant pathogens. Recently, orthologues of C. fulvum effector genes Avr4 and Ecp6 have been found in many fungal species (Bolton et al., 2008); indeed, there are functional orthologues of Avr4 and Ecp2 in M. fijiensis and their encoded effectors are able to interact with the respective tomato R gene products (Stergiopoulos et al., 2010), and a functional homologue of the ToxA peptide gene from S. nodorum was found in the P. tritici‐repentis genome (2006, 2008). This trend is repeated in the V. inaequalis WGS, with the presence of orthologues of Ecp6, AvrLm6 and Avr‐Pita. This conservation of effector sequence may be an indication of the effectiveness of function of the orthologues across a wide host range, or a measure of the essential nature of the effectors with the fitness cost of loss or mutation too great. If the effectors are essential, their cognate R genes may well be durable; thus, with the advent of comparative genomics using the WGS of V. inaequalis, the identification of key effectors and durable cognate R genes is arguably a step closer.
ACKNOWLEDGEMENTS
This work was funded by the New Zealand Foundation for Research, Science and Technology contracts C06X0812 and C06X0810. The authors wish to thank Lorraine Berry and the staff at the Allan Wilson Centre Genome Service, New Zealand, for carrying out the whole genome sequencing, Ross Crowhurst for assembly of the WGS, and Erik Rikkerink and Kerry Everett for critically reviewing the manuscript.
REFERENCES
- Agrios, G.N. (2005) Plant Pathology. San Diego, CA: Elsevier. [Google Scholar]
- Attrassi, K. , Benkirane, R. , Attrassi, B. and Douira, A. (2007) Effect of the association of certain fungicides with calcium chloride on the development of several fungi responsible for apple rot in conservation. Phytoprotection, 88, 17–26. [Google Scholar]
- Barbieri, M. , Belfanti, E. , Tartarini, S. , Vinatzer, B.A. , Sansavini, S. , Silfverberg‐Dilworth, E. , Gianfranceschi, L. , Hermann, D. , Patocchi, A. and Gessler, C. (2003) Progress of map‐based cloning of the Vf‐resistance gene and functional verfication: preliminary results from expression studies in transformed apple. HortScience, 38, 329–331. [Google Scholar]
- Beck, A. , Ritschel, A. , Schubert, K. , Braun, U. and Triebel, D. (2005) Phylogenetic relationships of the anamorphic genus Fusicladium s. lat. as inferred by ITS nrDNA data. Mycol. Prog. 4, 111–116. [Google Scholar]
- Becker, C.M. , Burr, T.J. and Smith, C.A. (1992) Overwintering of conidia of Venturia inaequalis in apple buds in New York orchards. Plant Dis. 76, 121–126. [Google Scholar]
- Belfanti, E. , Silfverberg‐Dilworth, E. , Tartarini, S. , Patocchi, A. , Barbieri, M. , Zhu, J. , Vinatzer, B.A. , Gianfranceschi, L. , Gessler, C. and Sansavini, S. (2004) The HcrVf2 gene from a wild apple confers scab resistance to a transgenic cultivated variety. Proc. Natl. Acad. Sci. USA, 101, 886–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beresford, R.M. and Manktelow, D.W.L. (1994) Economics of reducing fungicide use by weather‐based disease forecasts for control of Venturia inaequalis in apples. N. Z. J. Crop Hortic. Sci. 22, 113–120. [Google Scholar]
- Beresford, R.M. , Henshall, W.R. and Palmer, J.W. (2004) A new apple scab risk model that integrates ascospore release, infection risk and susceptible leaf area. N. Z. Plant Prot. 57, 20–24. [Google Scholar]
- Biggs, A.R. (1990) Apple scab In: Compendium of Apple and Pear Diseases (Jones A.L. and Aldwinckle H.S., eds), pp. 6–9. St. Paul, MN: APS Press. [Google Scholar]
- Bini, F. , Ragaini, A. and Bazzi, C. (2008) Resistance responses induced by the plant growth retardant prohexadione‐Ca in apple against scab infections. Ann. Appl. Biol. 152, 19–27. [Google Scholar]
- Bolton, M.D. , van Esse, H.P. , Vossen, J.H. , de Jonge, R. , Stergiopoulos, I. , Stulemeijer, I.J.E. , Berg, G.C.M. , Borrás‐Hidalgo, O. , Dekker, H.L. , de Koster, C.G. , de Wit, P.J.G.M. , Joosten, M.H.A.J. and Thomma, B.P.H.J. (2008) The novel Cladosporium fulvum lysin motif effector Ecp6 is a virulence factor with orthologues in other fungal species. Mol. Microbiol. 69, 119–136. [DOI] [PubMed] [Google Scholar]
- Boone, D.M. (1971) Genetics of Venturia inaequalis . Annu. Rev. Phytopathol. 9, 297–318. [Google Scholar]
- Boone, D.M. and Keitt, G.W. (1957) Venturia inaequalis (Cke.) Wint. XII. Genes controlling pathogenicity of wild‐type lines. Phytopathology, 47, 403–409. [Google Scholar]
- Boudichevskaia, A. , Flachowsky, H. and Dunemann, F. (2009) Identification and molecular analysis of candidate genes homologous to HcrVf genes for scab resistance in apple. Plant Breed. 128, 84–91. [Google Scholar]
- Bowen, J.K. , Mesarich, C.H. , Rees‐George, J. , Cui, W. , Win, J. , Fitzgerald, A. , Plummer, K.M. and Templeton, M.D. (2009) Candidate effector gene identification in the ascomycete fungal phytopathogen Venturia inaequalis by expressed sequence tag analysis. Mol. Plant Pathol. 10, 431–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent, K.J. and Holloman, D.W. (2007) Fungicide resistance in crop pathogens: how can it be managed? FRAC Monograph No. 1 (second, revised edition).Brussels: Fungicide Resistance Action Committee. [Google Scholar]
- Broggini, G.A.L. , Le Cam, B. , Parisi, L. , Wu, C. , Zhang, H.‐B. , Gessler, C. and Patocchi, A. (2007) Construction of a contig of BAC clones spanning the region of the apple scab avirulence gene AvrVg . Fungal Genet. Biol. 44, 44–51. [DOI] [PubMed] [Google Scholar]
- Broggini, G.A.L. , Di Gennaro, F. , Le Cam, B. , Parisi, L. , Gessler, C. and Patocchi, A. (2009a) Expression of candidate AvrVg genes during apple scab infection. Acta Hortic. 814, 777–780. [Google Scholar]
- Broggini, G.A.L. , Galli, P. , Parravicini, G. , Gianfranceschi, L. , Gessler, C. and Patocchi, A. (2009b) HcrVf paralogs are present on linkage groups 1 and 6 of Malus . Genome, 52, 129–138. [DOI] [PubMed] [Google Scholar]
- Brook, P.J. (1976) Seasonal pattern of maturation of Venturia inaequalis ascospores in New Zealand. N. Z. J. Agric. Res. 19, 103–109. [Google Scholar]
- Burchill, R.T. , Hutton, K.E. , Crosse, J.E. and Garrett, C.M.E. (1965) Inhibition of the perfect stage of Venturia inaequalis (Cooke) Wint., by urea. Nature, 205, 520–521. 14265313 [Google Scholar]
- Bus, V.G.M. (2006) Differential host–pathogen interactions of Venturia inaequalis and Malus . PhD Thesis. University of Auckland, Auckland. [DOI] [PubMed]
- Bus, V.G.M. , Laurens, F.N.D. , van de Weg, W.E. , Rusholme, R.L. , Rikkerink, E.H.A. , Gardiner, S.E. , Bassett, H.C.M. , Kodde, L.P. and Plummer, K.M. (2005) The Vh8 locus of a new gene‐for‐gene interaction between Venturia inaequalis and the wild apple Malus sieversii is closely linked to the Vh2 locus in Malus pumila R12740‐7A. New Phytol. 166, 1035–1049. [DOI] [PubMed] [Google Scholar]
- Bus, V.G.M. , Bassett, H.C.M. , Bowatte, D. , Chagné, D. , Ranatunga, C.A. , Ulluwishewa, D. , Wiedow, C. and Gardiner, S.E. (2010) Genome mapping of an apple scab, a powdery mildew and a woolly apple aphid resistance gene from open‐pollinated Mildew Immune Selection. Tree Genet. Genomes, 6, 477–487. [Google Scholar]
- Calenge, F. , Faure, A. , Goerre, M. , Gebhardt, C. , van de Weg, W.E. , Parisi, L. and Durel, C.‐E. (2004) Quantitative trait loci (QTL) analysis reveals both broad‐spectrum and isolate‐specific QTL for scab resistance in an apple progeny challenged with eight isolates of Venturia inaequalis . Phytopathology, 94, 370–379. [DOI] [PubMed] [Google Scholar]
- Carisse, O. and Bernier, J. (2002) Effect of environmental factors on growth, pycnidial production and spore germination of Microsphaeropsis isolates with biocontrol potential against apple scab. Mycol. Res. 106, 1455–1462. [Google Scholar]
- Chakrabarti, A. , Panter, S.N. , Harrison, K. , Jones, J.D.G. and Jones, D.A. (2009) Regions of the Cf‐9B disease resistance protein able to cause spontaneous necrosis in Nicotiana benthamiana lie within the region controlling pathogen recognition in tomato. Mol. Plant–Microbe Interact. 22, 1214–1226. [DOI] [PubMed] [Google Scholar]
- Chevalier, M. and Lespinasse, Y. (1991) Resistance of the apple tree (Malus x domestica Borkh.) to Venturia inaequalis (Cke.) Wint. Histological and ultrastructural study of the pin‐point symptom. C. R. Acad. Sci. Ser. III, 312, 117–124. [Google Scholar]
- Chevalier, M. , Lespinasse, Y. and Renaudin, S. (1991) A microscopic study of the different classes of symptoms coded by the Vf gene in apple for resistance to scab (Venturia inaequalis). Plant Pathol. 40, 249–256. [Google Scholar]
- Chisholm, S.T. , Coaker, G. , Day, B. and Staskawicz, B.J. (2006) Host–microbe interactions: shaping the evolution of the plant immune response. Cell, 124, 803–814. [DOI] [PubMed] [Google Scholar]
- Cooper, S.J. and Ashby, A.M. (1998) Comparison of cytokinin and cytokinin‐O‐glucoside cleaving β‐glucosidase production in vitro by Venturia inaequalis and other phytopathogenic fungi with differing modes of nutrition in planta . Physiol. Mol. Plant Pathol. 53, 61–72. [Google Scholar]
- Cova, V. , Paris, R. , Passerotti, S. , Zini, E. , Gessler, C. , Pertot, I. , Loi, N. , Musetti, R. and Komjanc, M. (2010) Mapping and functional analysis of four apple receptor‐like protein kinases related to LRPKm1 in HcrVf2‐transgenic and wild‐type apple plants. Tree Genet. Genomes, 6, 389–403. [Google Scholar]
- Dangl, J.L. and Jones, J.D.G. (2001) Plant pathogens and integrated defence responses to infection. Nature, 411, 826–833. [DOI] [PubMed] [Google Scholar]
- Day, P.R. , Boone, D.M. and Keitt, G.W. (1956) Venturia inaequalis (Cke.) Wint. XI. The chromosome number. Am. J. Bot. 43, 835–838. [Google Scholar]
- van der Does, H.C. , Duyvesteijn, R.G.E. , Goltstein, P.M. , van Schie, C.C.N. , Manders, E.M.M. , Cornelissen, B.J.C. and Rep, M. (2008) Expression of effector gene SIX1 of Fusarium oxysporum requires living plant cells. Fungal Genet. Biol. 45, 1257–1264. [DOI] [PubMed] [Google Scholar]
- Dunemann, F. and Egerer, J. (2010) A major resistance gene from Russian apple ‘Antonovka’ conferring field immunity against apple scab is closely linked to the Vf locus. Tree Genet. Genomes, DOI: 10.1007/s11295‐010‐0278‐x [Google Scholar]
- Durel, C.E. , Parisi, L. , Laurens, F. , van de Weg, W.E. , Liebhard, R. and Jourjon, M.F. (2003) Genetic dissection of partial resistance to race 6 of Venturia inaequalis in apple. Genome, 46, 224–234. [DOI] [PubMed] [Google Scholar]
- Eriksson, O.E. and Hawksworth, D.L. (1998) Outline of the Ascomycetes—1998. Systema Ascomycetum, 16, 83–296. [Google Scholar]
- van Esse, H.P. , Bolton, M.D. , Stergiopoulos, I. , de Wit, P.J.G.M. and Thomma, B.P.H.J. (2007) The chitin‐binding Cladosporium fulvum effector protein Avr4 is a virulence factor. Mol. Plant–Microbe Interact. 20, 1092–1101. [DOI] [PubMed] [Google Scholar]
- van Esse, H.P. , van't Klooster, J.W. , Bolton, M.D. , Yadeta, K.A. , van Baarlen, P. , Boeren, S. , Vervoort, J. , de Wit, P.J.G.M. and Thomma, B.P.H.J. (2008) The Cladosporium fulvum virulence protein Avr2 inhibits host proteases required for basal defense. Plant Cell, 20, 1948–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald, A. (2004) Investigation of proteins at the apple scab interface. PhD Thesis. University of Auckland, Auckland.
- Fitzgerald, A.M. , Mudge, A.M. , Gleave, A.P. and Plummer, K.M. (2003) Agrobacterium and PEG‐mediated transformation of the phytopathogen Venturia inaequalis . Mycol. Res. 107, 803–810. [DOI] [PubMed] [Google Scholar]
- Fitzgerald, A.M. , van Kan, J.A.L. and Plummer, K.M. (2004) Simultaneous silencing of multiple genes in the apple scab fungus, Venturia inaequalis, by expression of RNA with chimeric inverted repeats. Fungal Genet. Biol. 41, 963–971. [DOI] [PubMed] [Google Scholar]
- Flor, H.H. (1956) The complementary genic systems in flax and flax rust. Adv. Genet. 8, 29–54. [Google Scholar]
- Friesen, T.L. , Stukenbrock, E.H. , Liu, Z. , Meinhardt, S. , Ling, H. , Faris, J.D. , Rasmussen, J.B. , Solomon, P.S. , McDonald, B.A. and Oliver, R.P. (2006) Emergence of a new disease as a result of interspecific virulence gene transfer. Nat. Genet. 38, 953–956. [DOI] [PubMed] [Google Scholar]
- Friesen, T.L. , Faris, J.D. , Solomon, P.S. and Oliver, R.P. (2008) Host‐specific toxins: effectors of necrotrophic pathogenicity. Cell. Microbiol. 10, 1421–1428. [DOI] [PubMed] [Google Scholar]
- Fudal, I. , Ross, S. , Gout, L. , Blaise, F. , Kuhn, M.L. , Eckert, M.R. , Cattolico, L. , Bernard‐Samain, S. , Balesdent, M.H. and Rouxel, T. (2007) Heterochromatin‐like regions as ecological niches for avirulence genes in the Leptosphaeria maculans genome: map‐based cloning of AvrLm6 . Mol. Plant–Microbe Interact. 20, 459–470. [DOI] [PubMed] [Google Scholar]
- Galli, P. , Broggini, G.A.L. , Gessler, C. and Patocchi, A. (2010a) Phenotypic characterization of the Rvi15 (Vr2) apple scab resistance. J. Plant Pathol. 92, 219–226. [DOI] [PubMed] [Google Scholar]
- Galli, P. , Patocchi, A. , Broggini, G.A.L. and Gessler, C. (2010b) The Rvi15 (Vr2) apple scab resistance locus contains three TIR‐NBS‐LRR genes. Mol. Plant–Microbe Interact. 23, 608–617. [DOI] [PubMed] [Google Scholar]
- Gasic, K. , Han, Y. , Kertbundit, S. , Shulaev, V. , Iezzoni, A.F. , Stover, E.W. , Bell, R.L. , Wisniewski, M.E. and Korban, S.S. (2009) Characteristics and transferability of new apple EST‐derived SSRs to other Rosaceae species. Mol. Breed. 23, 397–411. [Google Scholar]
- Gau, A.E. , Koutb, M. , Piotrowski, M. and Kloppstech, K. (2004) Accumulation of pathogenesis‐related proteins in the apoplast of a susceptible cultivar of apple (Malus domestica cv. Elstar) after infection by Venturia inaequalis and constitutive expression of PR genes in the resistant cultivar Remo. Eur. J. Plant Pathol. 110, 703–711. [Google Scholar]
- Gessler, C. and Stumm, D. (1984) Infection and stroma formation by Venturia inaequalis on apple leaves with different degrees of susceptibility to scab. J. Phytopathol. 110, 119–126. [Google Scholar]
- Gessler, C. , Patocchi, A. , Kellerhals, M. and Gianfranceschi, L. (1997) Molecular marker applied to apple breeding and map‐based cloning of resistance genes. OILB-WPRS Bull. 20, 105–109. [Google Scholar]
- Gessler, C. , Patocchi, A. , Sansavini, S. , Tartarini, S. and Gianfranceschi, L. (2006) Venturia inaequalis resistance in apple. CRC Crit. Rev. Plant Sci. 25, 473–503. [Google Scholar]
- Gladieux, P. , Zhang, X.‐G. , Afoufa‐Bastien, D. , Sanhueza, R.‐M.V. , Sbaghi, M. and Le Cam, B. (2008) On the origin and spread of the scab disease of apple: out of central Asia. PLoS ONE, 3, e1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladieux, P. , Caffier, V. , Devaux, M. and Le Cam, B. (2009) Host‐specific differentiation among populations of Venturia inaequalis causing scab on apple, pyracantha and loquat. Fungal Genet. Biol. 47, 511–521. [DOI] [PubMed] [Google Scholar]
- Gladieux, P. , Zhang, X.‐G. , Róldan‐Ruiz, I. , Caffier, V. , Leroy, T. , Devaux, M. , van Glabeke, S. , Coart, E. and Le Cam, B. (2010) Evolution of the population structure of Venturia inaequalis, the apple scab fungus, associated with the domestication of its host. Mol. Ecol. 19, 658–674. [DOI] [PubMed] [Google Scholar]
- Goodwin, S.B. (2004) Minimum phylogenetic coverage: an additional criterion to guide the selection of microbial pathogens for initial genomic sequencing efforts. Phytopathology, 94, 800–804. [DOI] [PubMed] [Google Scholar]
- Guérin, F. , Franck, P. , Loiseau, A. , Devaux, M. and Le Cam, B. (2004) Isolation of 21 new polymorphic microsatellite loci in the phytopathogenic fungus Venturia inaequalis . Mol. Ecol. Notes, 4, 268–270. [Google Scholar]
- Haas, B.J. , Kamoun, S. , Zody, M.C. , Jiang, R.H.Y. , Handsaker, R.E. , Cano, L.M. , Grabherr, M. , Kodira, C.D. , Raffaele, S. , Torto‐Alalibo, T. , Bozkurt, T.O. , Ah‐Fong, A.M.V. , Alvarado, L. , Anderson, V.L. , Armstrong, M.R. , Avrova, A. , Baxter, L. , Beynon, J. , Boevink, P.C. , Bollmann, S.R. , Bos, J.I.B. , Bulone, V. , Cai, G. , Cakir, C. , Carrington, J.C. , Chawner, M. , Conti, L. , Costanzo, S. , Ewan, R. , Fahlgren, N. , Fischbach, M.A. , Fugelstad, J. , Gilroy, E.M. , Gnerre, S. , Green, P.J. , Grenville‐Briggs, L.J. , Griffith, J. , Grünwald, N.J. , Horn, K. , Horner, N.R. , Hu, C.‐H. , Huitema, E. , Jeong, D.‐H. , Jones, A.M.E. , Jones, J.D.G. , Jones, R.W. , Karlsson, E.K. , Kunjeti, S.G. , Lamour, K. , Liu, Z. , Ma, L. , MacLean, D. , Chibucos, M.C. , McDonald, H. , McWalters, J. , Meijer, H.J.G. , Morgan, W. , Morris, P.F. , Munro, C.A. , O'Neill, K. , Ospina‐Giraldo, M. , Pinzón, A. , Pritchard, L. , Ramsahoye, B. , Ren, Q. , Restrepo, S. , Roy, S. , Sadanandom, A. , Savidor, A. , Schornack, S. , Schwartz, D.C. , Schumann, U.D. , Schwessinger, B. , Seyer, L. , Sharpe, T. , Silvar, C. , Song, J. , Studholme, D.J. , Sykes, S. , Thines, M. , van de Vondervoort, P.J.I. , Phuntumart, V. , Wawra, S. , Weide, R. , Win, J. , Young, C. , Zhou, S. , Fry, W. , Meyers, B.C. , van West, P. , Ristaino, J. , Govers, F. , Birch, P.R.J. , Whisson, S.C. , Judelson, H.S. and Nusbaum, C. (2009) Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans . Nature, 461, 393–398. [DOI] [PubMed] [Google Scholar]
- Henson, J.M. , Butler, M.J. and Day, A.W. (1999) The dark side of the mycelium: melanins of phytopathogenic fungi. Annu. Rev. Phytopathol. 37, 447–471. [DOI] [PubMed] [Google Scholar]
- Holb, I. (2006) Effect of six sanitation treatments on leaf litter density, ascospore production of Venturia inaequalis and scab incidence in integrated and organic apple orchards. Eur. J. Plant Pathol. 115, 293–307. [Google Scholar]
- Holb, I.J. (2007) Classification of apple cultivar reactions to scab in integrated and organic production systems. Can. J. Plant Pathol. 29, 251–260. [Google Scholar]
- Holb, I.J. , Heijne, B. and Jeger, M.J. (2006) Effects of integrated control measures on earthworms, leaf litter and Venturia inaequalis infection in two European apple orchards. Agric. Ecosyst. Environ. 114, 287–295. [Google Scholar]
- Houterman, P.M. , Speijer, D. , Dekker, H.L. , de Koster, C.G. , Cornelissen, B.J.C. and Rep, M. (2007) The mixed xylem sap proteome of Fusarium oxysporum‐infected tomato plants. Mol. Plant Pathol. 8, 215–221. [DOI] [PubMed] [Google Scholar]
- Houterman, P.M. , Cornelissen, B.J.C. and Rep, M. (2008) Suppression of plant resistance gene‐based immunity by a fungal effector. PLoS Pathog. 4, e1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houterman, P.M. , Lisong, M. , van Ooijen, G. , de Vroomen, M.J. , Cornelissen, B.J.C. , Takken, F.L.W. and Rep, M. (2009) The effector protein Avr2 of the xylem colonizing fungus Fusarium oxysporum activates the tomato resistance protein I‐2 intracellularly. Plant J. 58, 970–978. [DOI] [PubMed] [Google Scholar]
- Ishii, H. and Yanase, H. (2000) Venturia nashicola, the scab fungus of Japanese and Chinese pears: a species distinct from V. pirina . Mycol. Res. 104, 755–759. [Google Scholar]
- Janick, J. , Cummins, J.N. , Brown, S.K. and Hemmat, M. (1996) Apples. In: Fruit Breeding: Tree and Tropical Fruits (Janick J. and Moore J.N., eds), pp. 1–77. New York: Wiley. [Google Scholar]
- Jeger, M.J. and Alston, F.H. (1986) Resistance in apple to shoot infection by Venturia inaequalis . Ann. Appl. Biol. 108, 387–394. [Google Scholar]
- Jha, G. , Thakur, K. and Thakur, P. (2009) The Venturia–apple pathosystem: pathogenicity mechanisms and plant defense responses. J. Biomed. Biotechnol. 2009, 2009: 680160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, D.A. , Thomas, C.M. , Hammond‐Kosack, K.E. , Balint‐Kurti, P.J. and Jones, J.D. (1994) Isolation of the tomato Cf‐9 gene for resistance to Cladosporium fulvum by transposon tagging. Science, 266, 789–793. [DOI] [PubMed] [Google Scholar]
- Jones, W.T. , Harvey, D. , Sun, X. , Greenwood, D.R. , Al‐Sammarai, T.H. , Mesarich, C.H. , Lowry, J. and Templeton, M.D. (2009) Heterologous expression, isotopic‐labeling and immuno‐characterisation of Cin1, a novel protein secreted by the phytopathogenic fungus Venturia inaequalis . Protein Expr. Purif. 65, 140–147. [DOI] [PubMed] [Google Scholar]
- van Kan, J.A.L. , Ackerveken, G.F.J.M. and de Wit, P.J.G.M. (1991) Cloning and characterization of cDNA of avirulence gene avr9 of the fungal pathogen Cladosporium fulvum, causal agent of tomato leaf mold. Mol. Plant–Microbe Interact. 4, 52–59. [DOI] [PubMed] [Google Scholar]
- Keitt, G.W. and Jones, I.K. (1926) Studies of the epidemiology and control of apple scab. Wis. Agric. Exp. Stn. Res. Bull. 73, 1–104. [Google Scholar]
- Keitt, G.W. and Langford, M.H. (1940) A preliminary report on variability and inheritance in Venturia inaequalis . Phytopathology, 30, 452–453. [Google Scholar]
- Keitt, G.W. and Langford, M.H. (1941) Venturia inaequalis (Cke.) Wint. I. A groundwork for genetic studies. Am. J. Bot. 28, 805–820. [Google Scholar]
- Keitt, G.W. and Palmiter, D.H. (1937) Heterothallism in Venturia inaequalis . Science, 85, 498. [DOI] [PubMed] [Google Scholar]
- Keitt, G.W. and Palmiter, D.H. (1938) Heterothallism and variability in Venturia inaequalis . Am. J. Bot. 25, 338–345. [Google Scholar]
- Koenraadt, H. , Somerville, S.C. and Jones, A.L. (1992) Characterization of mutations in the beta‐tubulin gene of benomyl‐resistant field strains of Venturia inaequalis and other plant pathogenic fungi. Phytopathology, 82, 1348–1354. [Google Scholar]
- Kollar, A. (1996) Evidence for loss of ontogenetic resistance of apple leaves against Venturia inaequalis . Eur. J. Plant Pathol. 102, 773–778. [Google Scholar]
- Kollar, A. (1998) Characterization of an endopolygalacturonase produced by the apple scab fungus, Venturia inaequalis . Mycol. Res. 102, 313–319. [Google Scholar]
- Köller, W. (1994) Chemical control of apple scab—status quo and future. Norweg. J. Agric. Sci. Suppl. 17, 149–170. [Google Scholar]
- Köller, W. and Parker, D.M. (1989) Purification and characterization of cutinase from Venturia inaequalis . Phytopathology, 79, 278–283. [Google Scholar]
- Köller, W. and Wilcox, W.F. (2001) Evidence for the predisposition of fungicide‐resistant isolates of Venturia inaequalis to a preferential selection for resistance to other fungicides. Phytopathology, 91, 776–781. [DOI] [PubMed] [Google Scholar]
- Köller, W. , Parker, D.M. and Becker, C.M. (1991) Role of cutinase in the penetration of apple leaves by Venturia inaequalis . Phytopathology, 81, 1375–1379. [Google Scholar]
- Köller, W. , Parker, D.M. , Turechek, W.W. , Avila‐Adame, C. and Cronshaw, K. (2004) A two‐phase resistance response of Venturia inaequalis populations to the QoI fungicides kresoxim‐methyl and trifloxystrobin. Plant Dis. 88, 537–544. [DOI] [PubMed] [Google Scholar]
- Komjanc, M. , Festi, S. , Rizzotti, L. , Cattivelli, L. , Cervone, F. and de Lorenzo, G. (1999) A leucine‐rich repeat receptor‐like protein kinase (LRPKm1) gene is induced in Malus × domestica by Venturia inaequalis infection and salicylic acid treatment. Plant Mol. Biol. 40, 945–957. [DOI] [PubMed] [Google Scholar]
- Kooman‐Gersmann, M. , Honée, G. , Bonnema, G. and de Wit, P.J.G. (1996) A high‐affinity binding site for the AVR9 peptide elicitor of Cladopsorium fulvum is present on plasma membranes of tomato and other solanaceous plants. Plant Cell, 8, 929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korban, S.S. (1998) What's new with disease‐resistant apple cultivars. Trans. Ill. State Hortic. Soc. 131, 74–76. [Google Scholar]
- Kruijt, M. , de Kock, M.J.D. and de Wit, P. (2005) Receptor‐like proteins involved in plant disease resistance. Mol. Plant Pathol. 6, 85–97. [DOI] [PubMed] [Google Scholar]
- Kruys, Å. , Eriksson, O.E. and Wedin, M. (2006) Phylogenetic relationships of coprophilous Pleosporales (Dothideomycetes, Ascomycota), and the classification of some bitunicate taxa of unknown position. Mycol. Res. 110, 527–536. [DOI] [PubMed] [Google Scholar]
- Kucheryava, N. , Bowen, J.K. , Sutherland, P.W. , Conolly, J.J. , Mesarich, C.H. , Rikkerink, E.H.A. , Kemen, E. , Plummer, K.M. , Hahn, M. and Templeton, M.D. (2008) Two novel Venturia inaequalis genes induced upon morphogenetic differentiation during infection and in vitro growth on cellophane. Fungal Genet. Biol. 45, 1329–1339. [DOI] [PubMed] [Google Scholar]
- Langford, M.H. and Keitt, G.W. (1957) Heterothallism and variability in Venturia pirina . Phytopathology, 32, 357–369. [Google Scholar]
- Laugé, R. , Dmitriev, A.P. , Joosten, M.H.A. and de Wit, P.J.G.M. (1998) Additional resistance gene(s) against Cladosporium fulvum present on the Cf‐9 introgression segment are associated with strong PR protein accumulation. Mol. Plant–Microbe Interact. 11, 301–308. [Google Scholar]
- Le Cam, B. , Parisi, L. and Arene, L. (2002) Evidence of two formae speciales in Venturia inaequalis, responsible for apple and Pyracantha scab. Phytopathology, 92, 314–320. [DOI] [PubMed] [Google Scholar]
- Liebhard, R. , Koller, B. , Patocchi, A. , Kellerhals, M. , Pfammatter, W. , Jermini, M. and Gessler, C. (2003) Mapping quantitative field resistance against apple scab in a ‘Fiesta’ × ‘Discovery’ progeny. Phytopathology, 93, 493–501. [DOI] [PubMed] [Google Scholar]
- Lumbsch, H.T. and Huhndorf, S.M. (2007) Notes on ascomycete systematics. Nos. 4408‐4750. Myconet, 13, 59–99. [Google Scholar]
- MacHardy, W.E. (1996) Apple Scab: Biology, Epidemiology, and Management.St. Paul, MN: The American Phytopathological Society Press. [Google Scholar]
- MacHardy, W.E. and Gadoury, D.M. (1986) Patterns of ascospore discharge by Venturia inaequalis in commercial apple orchards. Phytopathology, 76, 985–990. [Google Scholar]
- MacHardy, W.E. , Gadoury, D.M. and Gessler, C. (2001) Parasitic and biological fitness of Venturia inaequalis: relationship to disease management strategies. Plant Dis. 85, 1036–1051. [DOI] [PubMed] [Google Scholar]
- Malnoy, M. , Xu, M. , Borejsza‐Wysocka, E. , Korban, S.S. and Aldwinckle, H.S. (2008) Two recepror‐like genes, Vfa1 and Vfa2, confer resistance to the fungal pathogen Venturia inaequalis inciting apple scab disease. Mol. Plant–Microbe Interact. 21, 448–458. [DOI] [PubMed] [Google Scholar]
- Manktelow, D.W.L. , Beresford, R.M. , Batchelor, T.A. and Walker, J.T.S. (1996) Use patterns and economics of fungicides for disease control in New Zealand apples. Acta Hortic. 422, 187–192. [Google Scholar]
- McKirdy, S.J. , Mackie, A.E. and Kumar, S. (2001) Apple scab successfully eradicated in Western Australia. Australas. Plant Pathol. 30, 371. [Google Scholar]
- Mills, W.D. and LaPlante, A.A. (1954) Apple scab. In: Disease and insects in the orchard. Cornell Ext. Bull. 711, 20–28. [Google Scholar]
- Müller, O. , Schreier, P.H. and Uhrig, J.F. (2008) Identification and characterization of secreted and pathogenesis‐related proteins in Ustilago maydis . Mol. Genet. Genomics, 279, 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb, R.D. , Crowhurst, R.N. , Gleave, A.P. , Rikkerink, E.H.A. , Allan, A.C. , Beuning, L.L. , Bowen, J.H. , Gera, E. , Jamieson, K.R. , Janssen, B.J. , Laing, W.A. , McArtney, S. , Nain, B. , Ross, G.S. , Snowden, K.C. , Souleyre, E.J.F. , Walton, E.F. and Yauk, Y.K. (2006) Analyses of expressed sequence tags from apple. Plant Physiol. 141, 147–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson, R.L. , Kuc, J. and Williams, E.B. (1972) Histochemical demonstration of transitory esterase activity in Venturia inaequalis . Phytopathology, 62, 1242–1247. [Google Scholar]
- Nusbaum, C.J. and Keitt, G.W. (1938) A cytological study of host–parasite relations of Venturia inaequalis on apple leaves. J. Agric. Res. 56, 595–618. [Google Scholar]
- Orbach, M.J. , Farrall, L. , Sweigard, J.A. , Chumley, F.G. and Valent, B. (2000) A telomeric avirulence gene determines efficacy for the rice blast resistance gene Pi‐ta . Plant Cell, 12, 2019–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palani, P.V. and Lalithakumari, D. (1999) Resistance of Venturia inaequalis to the sterol biosynthesis‐inhibiting fungicide, penconazole [1‐(2‐(2,4‐dichlorophenyl)pentyl)‐1H‐1,2,4‐triazole]. Mycol. Res. 103, 1157–1164. [Google Scholar]
- Panter, S.N. , Hammond‐Kosack, K.E. , Harrison, K. , Jones, J.D.G. and Jones, D.A. (2002) Developmental control of promoter activity is not responsible for mature onset of Cf‐9B‐mediated resistance to leaf mold in tomato. Mol. Plant–Microbe Interact. 15, 1099–1107. [DOI] [PubMed] [Google Scholar]
- Paris, R. , Cova, V. , Pagliarani, G. , Tartarini, S. , Komjanc, M. and Sansavini, S. (2009) Expression profiling in HcrVf2‐transformed apple plants in response to Venturia inaequalis . Tree Genet. Genomes, 5, 81–91. [Google Scholar]
- Parniske, M. , Hammond‐Kosack, K.E. , Golstein, C. , Thomas, C.M. , Jones, D.A. , Harrison, K. , Wulff, B.B.H. and Jones, J.D.G. (1997) Novel disease resistance specificities result from sequence exchange between tandemly repeated genes at the Cf‐4/9 locus of tomato. Cell, 91, 821–832. [DOI] [PubMed] [Google Scholar]
- Raa, J. and Sijpesteijn, A.K. (1968) A biochemical mechanism of natural resistance of apple to Venturia inaequalis . Neth. J. Plant Pathol. 74, 229–231. [Google Scholar]
- Rafiqi, M. , Bernoux, M. , Ellis, J.G. and Dodds, P.N. (2009) In the trenches of plant pathogen recognition: role of NB‐LRR proteins. Semin. Cell Dev. Biol. 20, 1017–1024. [DOI] [PubMed] [Google Scholar]
- Rep, M. (2005) Small proteins of plant‐pathogenic fungi secreted during host colonization. FEMS Microbiol. Lett. 253, 19–27. [DOI] [PubMed] [Google Scholar]
- Sánchez‐Torres, P. , Hinarejos, R. and Tuset, J.J. (2009) Characterization and pathogenicity of Fusicladium eriobotryae, the fungal pathogen responsible for loquat scab. Plant Dis. 93, 1151–1157. [DOI] [PubMed] [Google Scholar]
- Sawamura, K. (1988) Apple scab in Japan In: Proceedings of the Deciduous Tree Fruit (Apple) Disease Workshop (Sawamura K. and Mink G.I., eds), pp. 32–38. Hirosaki: Hirosaki University. [Google Scholar]
- Schoch, C.L. , Crous, P.W. , Groenewald, J.Z. , Boehm, E.W.A. , Burgess, T.I. , de Gruyter, J. , de Hoog, G.S. , Dixon, L.J. , Grube, M. , Gueidan, C. , Harada, Y. , Hatakeyama, S. , Hirayama, K. , Hosoya, T. , Huhndorf, S.M. , Hyde, K.D. , Jones, E.B.G. , Kohlmeyer, J. , Kruys, Å. , Li, Y.M. , Lücking, R. , Lumbsch, H.T. , Maranová, L. , Mbatchou, J.S. , McVay, A.H. , Miller, A.N. , Mugambi, G.K. , Muggia, L. , Nelsen, M.P. , Nelson, P. , Owensby, C.A. , Phillips, A.J.L. , Phongpaichit, S. , Pointing, S.B. , Pujade‐Renaud, V. , Raja, H.A. , Rivas Plata, E. , Robbertse, B. , Ruibal, C. , Sakayaroj, J. , Sano, T. , Selbmann, L. , Shearer, C.A. , Shirouzu, T. , Slippers, B. , Suetrong, S. , Tanaka, K. , Volkmann‐Kohlmeyer, B. , Wingfield, M.J. , Wood, A.R. , Woudenberg, J.H.C. , Yonezawa, H. , Zhang, Y. and Spatafora, J.W. (2009) A class‐wide phylogenetic assessment of Dothideomycetes . Stud. Mycol. 64, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schornack, S. , Huitema, E. , Cano, L.M. , Bozkurt, T.O. , Oliva, R. , van Damme, M. , Schwizer, S. , Raffaele, S. , Chaparro‐Garcia, A. , Farrer, R. , Segretin, M.E. , Bos, J. , Hass, B.J. , Zody, M.C. , Nusbaum, C. , Win, J. , Thines, M. and Kamoun, S. (2009) Ten things to know about oomycete effectors. Mol. Plant Pathol. 10, 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert, K. , Ritschel, A. and Braun, U. (2003) A monograph of Fusicladium s. lat. (Hyphomycetes). Schlechtendalia, 9, 1–132. [Google Scholar]
- Schumacher, C.F.A. , Steiner, U. , Dehne, H.‐W. and Oerke, E.‐C. (2008) Localized adhesion of nongerminated Venturia inaequalis conidia to leaves and artificial surfaces. Phytopathology, 98, 760–768. [DOI] [PubMed] [Google Scholar]
- Schwabe, W.F.S. (1979) Changes in scab susceptibility of apple leaves as influenced by age. Phytophylactica, 11, 53–56. [Google Scholar]
- Schwabe, W.F.S. , Jones, A.L. and Jonker, J.P. (1984) Changes in the susceptibility of developing apple fruit to Venturia inaequalis . Phytopathology, 74, 118–121. [Google Scholar]
- Shay, J.R. and Hough, L.F. (1952) Evaluation of apple scab resistance in selections of Malus . Am. J. Bot. 39, 288–297. [Google Scholar]
- Silfverberg‐Dilworth, E. , Besse, S. , Paris, R. , Belfanti, E. , Tartarini, S. , Sansavini, S. , Patocchi, A. and Gessler, C. (2005) Identification of functional apple scab resistance gene promoters. Theor. Appl. Genet. 110, 1119–1126. [DOI] [PubMed] [Google Scholar]
- Smereka, K.J. , MacHardy, W.E. and Kausch, A.P. (1987) Cellular differentiation in Venturia inaequalis ascospores during germination and penetration of apple leaves. Can. J. Bot. 65, 2549–2561. [Google Scholar]
- Smereka, K.J. , Kausch, A.P. and MacHardy, W.E. (1988) Intracellular junctional structures in germinating ascopores of Venturia inaequalis . Protoplasma, 142, 1–4. [Google Scholar]
- Soriano, J.M. , Joshi, S.G. , van Kaauwen, M. , Noordijk, Y. , Groenwold, R. , Henken, B. , van de Weg, W.E. and Schouten, H.J. (2009) Identification and mapping of the novel apple scab resistance gene Vd3 . Tree Genet. Genomes, 5, 475–482. [Google Scholar]
- Steiner, U. and Oerke, E.‐C. (2007) Localized melanization of appressoria is required for pathogenicity of Venturia inaequalis . Phytopathology, 97, 1222–1230. [DOI] [PubMed] [Google Scholar]
- Stergiopoulos, I. and de Wit, P.J.G.M. (2009) Fungal effector proteins. Annu. Rev. Phytopathol. 47, 233–263. [DOI] [PubMed] [Google Scholar]
- Stergiopoulos, I. , van den Burg, H.A. , Ökmen, B. , Beenen, H.G. , van Liere, S. , Kema, G.H.J. and de Wit, P.J.G.M. (2010) Tomato Cf resistance proteins mediate recognition of cognate homologous effectors from fungi pathogenic on dicots and monocots. Proc. Natl. Acad. Sci. USA, 107, 7610–7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szankowski, I. , Waidmann, S. , Degenhardt, J. , Patocchi, A. , Paris, R. , Silfverberg‐Dilworth, E. , Broggini, G.A.L. and Gessler, C. (2009) Highly scab‐resistant transgenic apple lines achieved by introgression of HcrVf2 controlled by different native promoter lengths. Tree Genet. Genomes, 5, 349–358. [Google Scholar]
- Szkolnik, M. (1969) Maturation and discharge of ascospores of Venturia inaequalis . Plant Dis. Rep. 53, 534–537. [Google Scholar]
- Szkolnik, M. (1978) Relative susceptibility to scab and production of conidia among 30 apple varieties Proceedings of the Apple and Pear Scab Workshop (Jones A.L. and Gilpatrick J.D., eds), 11–14. Kansas City, MO: New York Agricultural Experimental Station Special Report. [Google Scholar]
- Takken, F.L.W. , Schipper, D. , Nijkamp, H.J.J. and Hille, J. (1998) Identification and Ds‐tagged isolation of a new gene at the Cf‐4 locus of tomato involved in disease resistance to Cladosporium fulvum race 5. Plant J. 14, 401–411. [DOI] [PubMed] [Google Scholar]
- Tartarini, S. and Sansavini, S. (2004) The use of molecular markers in pome fruit breeding. Acta Hortic. 622, 129–140. [Google Scholar]
- Tartarini, S. , Sansavini, S. , Vinatzer, B. , Gennari, F. and Domizi, C. (2000) Efficiency of marker assisted selection (MAS) for the Vf scab resistance gene. Acta Hortic. 538, 549–552. [Google Scholar]
- Templeton, M.D. , Rikkerink, E.H.A. and Beever, R.E. (1994) Small, cysteine‐rich proteins and recognition in fungal–plant interactions. Mol. Plant–Microbe Interact. 7, 320–325. [Google Scholar]
- Tenzer, I. and Gessler, C. (1997) Subdivision and genetic structure of four populations of Venturia inaequalis in Switzerland. Eur. J. Plant Pathol. 103, 565–571. [Google Scholar]
- Tenzer, I. and Gessler, C. (1999) Genetic diversity of Venturia inaequalis across Europe. Eur. J. Plant Pathol. 105, 545–552. [Google Scholar]
- Thomas, C.M. , Jones, D.A. , Parniske, M. , Harrison, K. , Balint‐Kurti, P.J. , Hatzixanthis, K. and Jones, J.D.G. (1997) Characterization of the tomato Cf‐4 gene for resistance to Cladosporium fulvum identifies sequences that determine recognitional specificity in Cf‐4 and Cf‐9. Plant Cell, 9, 2209–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsangiacomo, C. and Gessler, C. (1988) Role of the cuticular membrane in ontogenic and Vf‐resistance of apple leaves against Venturia inaequalis . Phytopathology, 78, 1066–1069. [Google Scholar]
- Valsangiacomo, C. and Gessler, C. (1992) Purification and characterization of an exo‐polygalacturonase produced by Venturia inaequalis, the causal agent of apple scab. Physiol. Mol. Plant Pathol. 40, 63–77. [Google Scholar]
- Vinatzer, B.A. , Patocchi, A. , Gianfranceschi, L. , Tartarini, S. , Zhang, H.‐B. , Gessler, C. and Sansavini, S. (2001) Apple contains receptor‐like genes homologous to the Cladosporium fulvum resistance gene family of tomato with a cluster of genes co‐segregating with Vf apple scab resistance. Mol. Plant–Microbe Interact. 14, 508–515. [DOI] [PubMed] [Google Scholar]
- Walters, D.R. and McRoberts, N. (2006) Plants and biotrophs: a pivotal role for cytokinins? Trends Plant Sci. 11, 581–586. [DOI] [PubMed] [Google Scholar]
- Williams, E.B. and Shay, J.R. (1957) The relationship of genes for pathogenicity and certain other characters in Venturia inaequalis (Cke.) Wint. Genetics, 42, 704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win, J. (2003) Molecular quest for avirulence factors in Venturia inaequalis. PhD Thesis. University of Auckland, Auckland.
- Win, J. , Greenwood, D.R. and Plummer, K.M. (2003) Characterisation of a protein from Venturia inaequalis that induces necrosis in Malus carrying the Vm resistance gene. Physiol. Mol. Plant Pathol. 62, 193–202. [Google Scholar]
- de Wit, P.J.G.M. , Mehrabi, R. , Burg, H.A. and Stergiopoulos, I. (2009) Fungal effector proteins: past, present and future. Mol. Plant Pathol. 10, 735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff, B.B.H. , Chakrabarti, A. and Jones, D.A. (2009) Recognitional specificity and evolution in the tomato–Cladosporium fulvum pathosystem. Mol. Plant–Microbe Interact. 22, 1191–1202. [DOI] [PubMed] [Google Scholar]
- Xu, M. and Korban, S.S. (2002) A cluster of four receptor‐like genes resides in the Vf locus that confers resistance to apple scab disease. Genetics, 162, 1995–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, M.L. and Korban, S.S. (2004) Somatic variation plays a key role in the evolution of the Vf gene family residing in the Vf locus that confers resistance to apple scab disease. Mol. Phylogenet. Evol. 32, 57–65. [DOI] [PubMed] [Google Scholar]
- Xu, X. , Roberts, T. , Barbara, D. , Harvey, N.G. , Gao, L. and Sargent, D.J. (2009) A genetic linkage map of Venturia inaequalis, the causal agent of apple scab. BMC Res. Tes. 2, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, X.M. and Robinson, J. (2005) Modelling the effects of wetness duration and fruit maturity on infection of apple fruits of Cox's Orange Pippin and two clones of Gala by Venturia inaequalis . Plant Pathol. 54, 347–356. [Google Scholar]
- Zheng, D. , Olaya, G. and Köller, W. (2000) Characterization of laboratory mutants of Venturia inaequalis resistant to the strobilurin‐related fungicide kresoxim‐methyl. Curr. Genet. 38, 148–155. [DOI] [PubMed] [Google Scholar]
- Zhukovsky, P.M. (1961) Grundlagen der introduktion der pflanzen auf resistenz gegen krankheiten. Der Züchter, 31, 248–253. [Google Scholar]


