SUMMARY
Satellite RNAs (satRNAs) depend on cognate helper viruses for replication, encapsidation, movement and transmission. Many satRNAs with different symptom modulation effects have been reported. The pathogenicity of satRNAs is thought to be the result of a direct interaction among the satRNA, helper viruses and host factors by unknown mechanisms. To understand the effect of satRNA of Cucumber mosaic virus (a severe field ShanDong strain, SD‐CMV) on pathogenicity, and the possible involvement of host RNA silencing pathways in pathogenicity, we constructed biologically active CMV cDNA clones and a CMV‐Δ2b mutant lacking the open reading frame of 2b, a silencing suppressor protein, in order to infect Nicotiana benthamiana and Arabidopsis with or without SD‐satRNA. We found that SD‐satRNA reduced the accumulation of the 2b protein and its coding RNA4A and attenuated the yellowing caused by SD‐CMV infection. Small RNA analysis indicated that the 2b protein interfered with RNA silencing, specifically in the synthesis of CMV RNA3‐derived small interfering RNAs (R3‐siRNAs). The accumulation of R3‐siRNAs in CMV‐Δ2b infection was reduced in the presence of satRNA, for which greater accumulation of satRNA‐derived siRNAs (satsiRNAs) was detected. Our results suggest that abundant SD‐satRNA serving as target for RNA silencing may play a role in protecting helper CMV RNA, especially, subgenomic RNA4, from being targeted by RNA silencing. This compensates for the increase in RNA silencing resulting from the reduction in expression of the 2b suppressor in the presence of satRNA. Our data provide evidence that a plant silencing mechanism is involved in the pathogenicity of satRNA.
INTRODUCTION
Cucumber mosaic virus (CMV), the type species of the genus Cucumovirus in the family Bromoviridae, possesses a tripartite, positive‐sense RNA genome, which contains three genomic RNAs and two subgenomic RNAs that encode five proteins (Palukaitis and Garcia‐Arenal, 2003). The 1a and 2a proteins encoded by genomic RNA1 and RNA2, respectively, are RNA‐dependent RNA polymerases (RdRPs), which are involved in viral replication (Hayes and Buck, 1990). Genomic RNA3 encodes 3a protein, the movement protein (MP), which is required for cell‐to‐cell movement (Ding B et al., 1995). 2b and the coat protein (CP) are expressed from subgenomic RNAs, RNA4A and RNA4, which are transcribed from genomic RNA2 and RNA3, respectively (Ding et al., 1994; Schwinghamer and Symons, 1975). The 2b protein, a suppressor of post‐transcription gene silencing (PTGS), has been thoroughly investigated during the past decade (Brigneti et al., 1998; Gonzalez et al., 2010; Guo and Ding, 2002; Lewsey et al., 2007; Mlotshwa et al., 2002; Zhang et al., 2006). Previous reports have shown that 2b plays an important role in diverse processes, including symptom induction as a viral virulence determinant, host‐specific virus accumulation, inhibition of salicylic acid (SA)‐induced resistance and systemic spread of CMV (Brigneti et al., 1998; Ding B et al., 1995; Ji and Ding, 2001; Li et al., 1999; Shi et al., 2002; Soards et al., 2002).
Satellite RNA (satRNA), a type of noncoding RNA, depends on a helper virus for its replication, but is not required for replication of the virus. To date, over 100 CMV satRNA variants have been found to be associated with over 65 CMV isolates (Palukaitis and Garcia‐Arenal, 2003). CMV satRNA is a small 332–405‐nucleotide linear molecule. As with other plant virus satellites, CMV satRNA has little sequence homology with the helper CMV genomic RNAs (Roossinck et al., 1992).
CMV satRNA has been shown to be able to alter the virus titre in infected tissues and to modulate the symptoms induced by helper CMV. In most cases, CMV satRNAs attenuate symptoms induced by helper viruses, although some intensify them (Roossinck et al., 1992). The overwhelming view of satRNA protection is that satRNA replication overtakes viral replication, thus blocking the accumulation and symptom expression of the virus (Kaper, 1995; Roossinck et al., 1992). Interestingly, the same satRNA can ameliorate the symptoms induced by helper viruses in one host and intensify them in another (Palukaitis, 1988; Roossinck et al., 1992; Waterworth et al., 1979). Hence, the effect of satRNAs on helper virus‐induced symptoms varies with different strains of helper viruses and satRNAs, as well as with the host plant (Garcia‐Arenal et al., 2000; Roossinck et al., 1992). Specific sequences controlling chlorosis and necrosis induction have been mapped to specific nucleotides of satRNA (Devic et al., 1989; Hidaka and Hanada, 1994; Jaegle et al., 1990; Kuwata et al., 1991; Masuta and Takanami, 1989; Sleat and Palukaitis, 1990), and the formation of a secondary structure was also found to be involved in the induction of yellow symptoms (Masuta and Takanami, 1989). Competition for replication with the helper virus genome may be just one possible mechanism of satRNA in symptom attenuation and virus titre alteration. The involvement of host factors or pathways in pathogenicity remains to be illustrated.
In this study, we constructed biologically active cDNA clones of the severe field CMV isolate (ShanDong strain, SD‐CMV) to investigate the relationship between the 2b silencing suppressor and its satRNA variant (SD‐satRNA) and their effects on pathogenicity relevant to host RNA silencing mechanisms. Using this infectious clone and a mutation affecting the 2b function, we found that: (i) SD‐satRNA attenuates the yellowing phenotype induced by SD‐CMV in infected Nicotiana benthamiana and Arabidopsis; (ii) in the presence of SD‐satRNA, the accumulation level of 2b‐coding subgenomic RNA4A is greatly reduced; (iii) the 2b protein interferes with host RNA silencing in producing CMV genomic RNA3‐siRNAs; and (iv) satRNA serves as part of the host RNA silencing target to be degraded to small interfering RNA (siRNA), resulting in reduced CMV RNA‐derived siRNA production. Our data indicate that the effect of satRNAs on helper virus‐induced symptoms involves the host RNA silencing mechanism.
RESULTS
Infectivity of SD‐CMV cDNA clones
Infectious clones of CMV RNA1, RNA2 and RNA3 were constructed and named as pT‐R1, pT‐R2 and pT‐R3, respectively. Infectivity was first examined using in vitro transcription of pT‐R1, pT‐R2 and pT‐R3. A mixture of equal amounts of the three in vitro transcripts was inoculated onto N. benthamiana. At 12 days post‐inoculation (dpi), typical symptomatic phenotypes similar to SD‐CMV sap‐infected plants appeared systemically in noninoculated leaves (Fig. 1A), indicating that the DNA clones are biologically active in inducing CMV infection and symptom formation. To avoid in vitro transcription, the three CMV cDNAs were inserted into the binary pBI121 vector that contains the 35S promoter to produce 35S‐R1, 35S‐R2 and 35S‐R3, respectively (Fig. 1B). We infiltrated Agrobacterium carrying 35S‐R1, 35S‐R2 or 35S‐R3 alone or a mixture of the three Agrobacterium strains (designated as R1/R2/R3) into N. benthamiana leaves. Similar to SD‐CMV sap infection, symptoms such as leaf curling were initially observed at 5 dpi in newly growing leaves from the mixture‐infiltrated plants, but not from any single plasmid‐containing Agrobacterium‐infiltrated plants. At 12 dpi, systemically noninoculated leaves of the mixture‐infiltrated plants displayed severe developmental defects, such as mosaic symptoms, leaf curling and plant dwarfism, comparable with SD‐CMV sap‐infected plants (Fig. 1A). RNA gel blot analysis confirmed the expression of RNA1, RNA2 and RNA3 transcripts in local infiltrated leaves at 5 dpi (Fig. 1C). Consistent with the disease phenotype, mixture infiltration could sustain the replication of both subgenomic RNA4 and 4A detected by the 3′‐untranslated region (3′‐UTR)‐ and 2b‐specific probes (Fig. 1C). CMV genomic and subgenomic RNAs were also accumulated in systemically infected leaves. This result indicates that the primary transcripts derived from the 35S promoter are biologically active in supporting viral RNA replication and propagation in plants.
Figure 1.
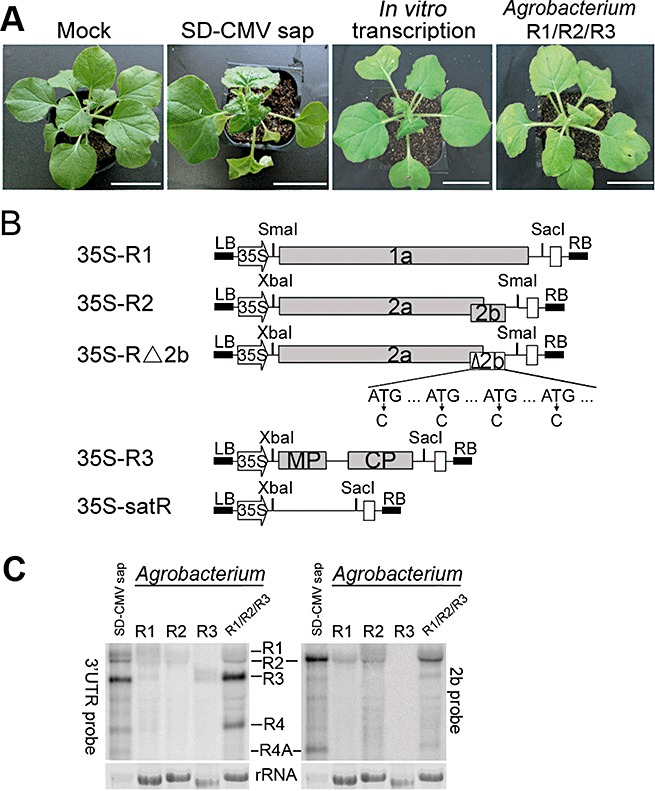
Construction and biological activities of Cucumber mosaic virus ShanDong strain (SD‐CMV) cDNA infectious clones. (A) Disease symptoms on Nicotiana benthamiana inoculated with sap extracted from natural SD‐CMV‐infected plants, a mixture of in vitro transcribed CMV RNAs or Agrobacterium‐mediated inoculation of SD‐CMV cDNA clones (designated as a mixture of R1/R2/R3). Photographs were taken at 12 days post‐inoculation (dpi). (B) Diagram of SD‐CMV cDNA infectious clone construction. Three full‐length clones of SD‐CMV genomic RNA1, RNA2, RNA3 and a chimeric RNA2‐Δ2b, which abolishes 2b protein expression by substituting ‘C’ for ‘T’ in the start codon ATG, as well as three other ATG codons present in the 2b coding sequence, and ShanDong satellite RNA (SD‐satRNA) were cloned downstream of the 35S promoter to generate 35S‐R1, 35S‐R2, 35S‐R3, 35S‐R2‐Δ2b and 35S‐satR, respectively. CMV‐encoded proteins and restrictive enzyme sites used in the construction are indicated. CP, coat protein; MP, movement protein. LB and RB denote the left and right borders of T‐DNA. White boxes represent the nopaline synthase (Nos) terminator. (C) RNA gel blot was performed to detect RNA expression derived from the 35S promoter and viral RNA replication in local Agrobacterium‐inoculated leaves in either single (R1, R2 or R3) or mixture (R1/R2/R3) infiltration assays at 5 dpi. 32P‐labelled SD‐CMV genomic RNA 3′‐UTR‐specific probes were used to detect the accumulation of viral RNAs (left panel). The membrane was stripped and re‐hybridized with 32P‐labelled 2b‐specific probe to confirm the accumulation of genomic RNA2 and subgenomic RNA4A (right panel); 1 µg and 6 µg of total RNAs extracted from sap inoculation or Agrobacterium inoculation, respectively, were loaded. Methylene blue‐stained ribosome rRNA was used as loading control.
SD‐satRNA greatly attenuates yellowing symptoms induced by SD‐CMV infection
To determine the effect of SD‐CMV‐associated satRNA (SD‐satRNA) on pathogenicity in SD‐CMV infection, a 334‐nucleotide SD‐satR cDNA (GenBank Accession No. D89673) was amplified by reverse transcription‐polymerase chain reaction (RT‐PCR) from SD‐CMV sap‐infected tobacco and cloned into the binary vector pCAMBIA‐1300221 to generate 35S‐SD‐satR (Fig. 1A). The mixture of Agrobacterium (R1/R2/R3) with either 35S‐SD‐satR‐containing Agrobacterium (designated as CMVwt/satR) or vector‐containing Agrobacterium (designated as CMVwt) was co‐infiltrated into N. benthamiana. Mosaic and leaf curling symptoms first appeared in the newly growing leaves of CMVwt‐infiltrated plants at 5 dpi, but, in CMVwt/satR‐infiltrated plants, at 6 dpi, suggesting that SD‐satRNA slightly delays the appearance of symptoms caused by the helper virus. At 20 dpi, CMVwt‐infected plants displayed severe developmental abnormalities with leaf deformation, mosaic symptoms and stunted phenotypes (Fig. 2, lane 2). CMVwt/satR‐infected plants also displayed typical disease symptoms with slightly less curling of systemically infected leaves (Fig. 2, lane 3). Notably, the CMVwt‐infected plants showed severe yellowing in systemically infected leaves compared with CMVwt/satR‐infected plants (Fig. 2, cf. lanes 2 and 3). The same results were obtained in three independent experiments with a total of 36 plants. These results demonstrate that SD‐satRNA attenuates the symptoms most obviously by decreasing the yellowing induced by helper CMV virus in N. benthamiana.
Figure 2.
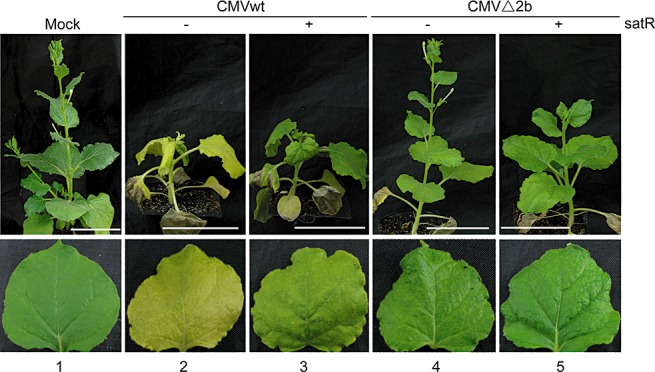
Symptom development of Agrobacterium‐mediated inoculation of Cucumber mosaic virus (CMV) in the absence or presence of SD‐satR on Nicotiana benthamiana. Nicotiana benthamiana leaves were co‐infiltrated with a mixture of Agrobacterium containing 35S‐R1, 35S‐R2 and 35S‐R3 (CMVwt) or 35S‐R1, 35S‐R2‐Δ2b and 35S‐R3 (CMVΔ2b) in the absence or presence of 35S‐SD‐satR as indicated at the top. Systemically infected, noninoculated leaves were photographed at 20 days post‐inoculation (dpi). Mock‐inoculated plant and leaf were used as control. Scale bar, 5 cm.
SD‐satRNA reduces the accumulation level of 2b‐coding subgenomic RNA4A
To examine whether the symptom attenuation by SD‐satRNA is associated with an alteration in the accumulation of CMV RNAs, we extracted total RNA from systemically infected leaves of CMVwt‐ and CMVwt/satR‐infected plants at 10 and 20 dpi, respectively, and performed RNA gel blot analysis. RNAs extracted from systemically infected leaves of SD‐CMV sap or mock inoculation were used as positive and negative controls, respectively. As shown in Fig. 3, the difference in the accumulation levels of the three CMV genomic RNAs and subgenomic RNA4 in the absence or presence of SD‐satRNA was not distinct (Fig. 3A, cf. lanes 3 and 4, and lanes 9 and 10, top panel). However, in comparison with CMVwt‐infected samples, the accumulation level of subgenomic RNA4A, which encodes the 2b suppressor protein, was clearly reduced in CMVwt/satR‐infected systemically noninoculated leaves at both time points in which satRNA was also detected (Fig. 3A, cf. lanes 3 and 4, and lanes 9 and 10). This result strongly suggests that SD‐satRNA specifically depresses the accumulation of the 2b coding sequence. Protein hybridization further confirmed that the 2b protein accumulated at lower levels in CMVwt/satR‐infected systemically noninoculated leaves at both time points (Fig. 3A, cf. lanes 3 and 4, and lanes 9 and 10). Together with the symptom attenuation in CMV infection in the presence of SD‐satRNA (Fig. 2), and a previous report that 2b interferes with host RNA silencing and abnormal development of 2b‐expressing plants (Zhang et al., 2006), our result suggests that decreased yellowing in the presence of SD‐satRNA is a result of reduced 2b suppressor protein accumulation by SD‐satRNA.
Figure 3.
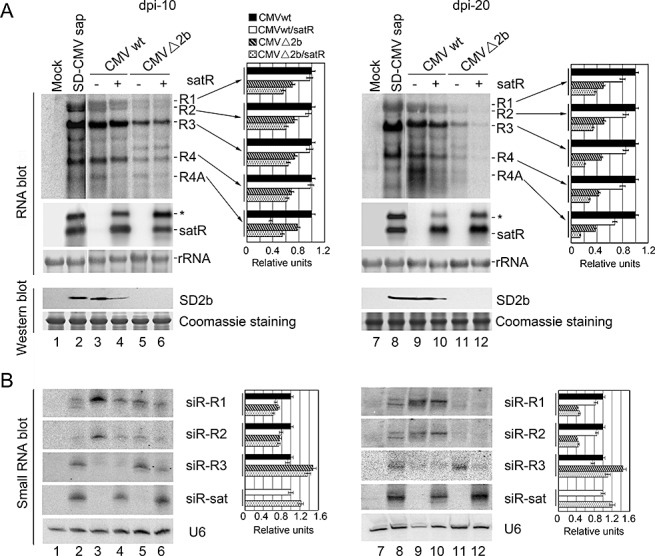
RNA gel blot analysis of viral RNAs, satellite RNA (satRNA) and pathogen‐derived small interfering RNA (siRNA) accumulations in systemically infected leaves of Agrobacterium‐inoculated Nicotiana benthamiana. (A) Cucumber mosaic virus (CMV) RNAs and satRNA accumulation were examined in similar layers of systemically infected leaves of Agrobacterium‐inoculated N. benthamiana, as described in Fig. 2, collected at 10 days post‐inoculation (dpi) (left panel) and 20 dpi (right panel). Pools of seven plants were analysed for each inoculation. Hybridization was performed with 32P‐labelled SD‐CMV genomic RNA 3′‐UTR‐ and satRNA‐specific probes, respectively. Six micrograms of total RNAs were loaded. Methylene blue‐stained ribosome rRNA was used as loading control. Accumulation of the 2b protein was also detected by protein immunoblot with anti‐2b polyclonal antiserum. ‘Coomassie’ staining was used as loading control. (B) CMV RNAs‐ and satRNA‐derived siRNAs were examined in the leaf samples, as described in (A). 32P‐labelled in vitro transcripts from viral RNA1, RNA2, RNA3 and satRNA were used as probes. U6 RNA hybridizations are shown as loading control. Quantifications of each CMV RNA and siRNA relative to loading control RNA are shown on the right of each panel using ImageQuant TL (GE Healthcare Life Sciences). RNAs obtained from CMVwt infection were arbitrarily assigned a value of 1.0. An asterisk denotes possible double‐stranded satRNA.
Effect of SD‐satRNA on pathogenicity in the absence of 2b protein
To investigate the effects of SD‐satRNA on pathogenicity, other than reduced accumulation of 2b‐coding RNA4A, we constructed a chimeric RNA2Δ2b infectious clone, named CMVΔ2b, in which 2b protein expression was abolished, but 2a protein expression was unaffected (Fig. 1B). The mixture of Agrobacterium (R1/R2Δ2b/R3) with vector‐containing Agrobacterium (designated as CMVΔ2b) was co‐infiltrated into N. benthamiana. Mosaic and leaf curling symptoms were greatly reduced in plants inoculated with CMVΔ2b relative to those in CMVwt‐infected plants (Fig. 2, lanes 4 and 2). Moreover, no plant infected with CMVΔ2b displayed the stunted phenotype that was observed in CMVwt‐infiltrated plants (Fig. 2, lane 2). The reduced virulence was correlated with reduced accumulation levels of viral RNAs detected by RNA gel blot analysis at 10 and 20 dpi (Fig. 3A, cf. lanes 3 and 5, and lanes 9 and 11), which is consistent with the previous report that 2b protein has a role in the systemic spreading of CMV in N. benthamiana and functions as a symptom determinant (Ding SW et al., 1995), because of its suppression of host antiviral silencing. Notably, the yellowing phenotype was not observed in CMVΔ2b‐infected plants (Fig. 2, lane 4), further supporting the idea that the reduced yellowing is a result of SD‐satRNA acting on the accumulation of 2b‐coding RNA.
Intriguingly, plants infected with the mixture of Agrobacterium (R1/R2Δ2b/R3) and 35S‐SD‐satR‐containing Agrobacterium (designated as CMVΔ2b/satR) grew more strongly (Fig. 2, lane 5). RNA gel blot analysis showed similar accumulation levels of helper viral RNA in systemically infected leaves at 10 dpi relative to CMVΔ2b infection (Fig. 3A, lanes 5 and 6). The reduction in RNA4A on infection with CMVΔ2b/satRNA was not as great as on infection with CMV/sat (Fig. 3A, cf. lanes 5 and 6 with lanes 3 and 4); the lack of the coding 2b open reading frame of RNA4A in CMVΔ2b might not draw out the entire effects of satRNA at this time point. However, greatly reduced accumulation levels of viral RNAs were observed in systemically infected leaves at 20 dpi (Fig. 3A, lanes 11 and 12). Replication of SD‐satRNA was almost the same at both 10 and 20 dpi in the absence of 2b protein (Fig. 3A, lanes 6 and 12). These results clearly suggest that SD‐satRNA has an effect on restraining helper virus movement or replication in the absence of 2b protein, in addition to reducing the accumulation of the 2b coding sequence.
Roles of SD‐satRNA and 2b protein in viral siRNA (vsiRNA) production
To examine whether the restraint of CMV propagation by SD‐satRNA was mediated by the enhancement of host silencing to degrade helper viral RNAs in the absence of 2b protein, we analysed vsiRNA in systemically infected leaves at both 10 and 20 dpi. Figure 3B shows that infection with SD‐satRNA resulted in a reduction in helper CMV‐derived vsiRNA in both the presence and absence of 2b protein relative to infection without SD‐satRNA (Fig. 3B, cf. lanes 3 and 4, and lanes 5 and 6). SD‐satRNA‐derived siRNA was also detected by the SD‐satRNA‐specific probe (Fig. 3B). These results indicate that host silencing‐triggered degradation of helper CMV RNAs was not increased in the presence of SD‐satRNA. In contrast, abundant SD‐satRNA served as a target for the host silencing machinery, and protected helper viral RNAs from degradation to a certain extent. Moreover, CMV RNA2‐ and subgenomic RNA4A‐derived vsiRNAs, detected by the CMV RNA2 probe, were not increased at either time point in the presence of SD‐satRNA (Fig. 3B, lanes 4, 6, 10 and 12), indicating that reduced accumulation levels of 2b‐coding RNA4A in the presence of SD‐satRNA (Fig. 3A) were not a result of an increase in its degradation.
Very low levels of CMV RNA3‐derived vsiRNA were detected in Agrobacterium‐mediated infection in the presence of 2b protein (Fig. 3B, cf. lanes 3 and 4 with lanes 5 and 6). Together with the observation of higher levels of genomic RNA3 and its subgenomic RNA4 in the presence of 2b protein (Fig. 3A), our results indicate that the 2b‐mediated suppression of silencing suppresses the host RNA silencing factors that specifically target CMV RNA3/RNA4 for degradation. CMV RNA3‐derived vsiRNA was detected in SD‐CMV sap‐infected plants (Fig. 3B, lane 2). One possibility is that, prior to 2b protein synthesis, infection with CMV virions might carry some RNA3 and/or its subgenomic RNA4, which encode MP and CP, respectively, and are detected at higher accumulation levels than other genomic and subgenomic RNAs (Fig. 3A), and the suppression function of 2b might act on newly synthesized RNA3 as observed in Agrobacterium‐mediated infection. We cannot rule out that this divergence between sap‐ and Agrobacterium‐mediated infections may have an effect on the development of the infected plants; however, the appearances of the disease symptoms were not significantly different (Fig. 1A).
Effect of SD‐satRNA on pathogenicity in Arabidopsis
To investigate whether SD‐satRNA has similar effects on Arabidopsis as observed in N. benthamiana, we generated SD‐satR‐transgenic Arabidopsis plants, because protocols for Agrobacterium infection of Arabidopsis are not well established. Expression of the SD‐satRNA transcript was confirmed by RT‐PCR analysis. SD‐satR plants showed a similar developmental phenotype to wild‐type plants (Col‐0, Fig. 4A), suggesting that SD‐satRNA expression alone (i.e. without helper virus to support its replication) does not induce symptoms or affect normal plant development. SD‐satR transgenic and wild‐type Arabidopsis were inoculated with CMVwt sap extracted from N. benthamiana leaves infected with CMVwt via Agrobacterium infection. Mosaic symptoms appeared in all infected plants at 5 dpi. At 12 dpi, inoculated leaves in the infected wild‐type plants became light yellow, whereas those in infected SD‐satR plants maintained a normal green phenotype (Fig. 4B,C), a result consistent with that obtained from infection in N. benthamiana (Fig. 2). Moreover, at this time point, systemically infected leaves of CMVwt‐infected wild‐type plants displayed severe leaf curling and whole plant development was arrested (Fig. 4B). However, with obvious but mild disease symptoms, CMVwt‐infected SD‐satR plants maintained a green colour and continued to develop (Fig. 4B). This result suggests that SD‐satRNA also attenuates the CMV‐induced disease symptoms and yellowing phenotype in Arabidopsis. A previous study has shown that symptom development of CMV infection in tobacco plants transformed with a DNA copy of CMV satRNA is largely suppressed; the replication of CMV in these satRNA‐transgenic tobacco plants was also greatly decreased (Harrison et al., 1987). We carried out RNA gel blot analysis. It was shown that similar levels of CMV genomic RNAs and subgenomic RNA4 were accumulated in both infected plants (Fig. 4D). Our results show that SD‐satR‐transgenic Arabidopsis supports efficient CMV infection, although the disease symptoms were attenuated (Fig. 4B). Together with symptom suppression, but with little or no decrease in Tomato aspermy virus (TAV) genome synthesis in TAV‐infected satRNA‐transgenic tobacco (Harrison et al., 1987), the results suggest that suppression or support of helper virus replication by the expression of a DNA copy of satRNA is both plant host species and virus strand specific. Our results also support the idea that symptom suppression does not necessarily depend on a decrease in virus replication (Harrison et al., 1987). However, a lower level of subgenomic RNA4A was clearly observed in infected SD‐satR‐transgenic Arabidopsis relative to infected wild‐type plants (Fig. 4D). This result further supports the conclusion that SD‐satRNA reduces the accumulation of subgenomic RNA4A, resulting in the attenuation of SD‐CMV infection disease symptoms.
Figure 4.
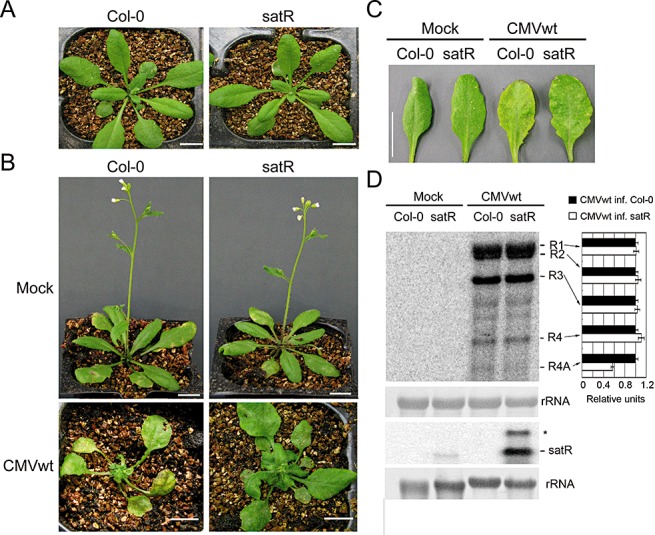
Analysis of effect of ShanDong satellite RNA (SD‐satRNA) on pathogenicity in Arabidopsis. (A) The morphological phenotype of 35S‐satR‐transgenic plants (satR) was comparable with that of wild‐type (WT) Arabidopsis (Col‐0). (B, C) Col‐0 and SD‐satR plants were inoculated with CMVwt sap, extracted from Nicotiana benthamiana leaves infected with CMVwt via Agrobacterium infection. Mock inoculation was used as a control. The disease symptoms of whole plants (B) and early systemically infected leaves (C) were photographed at 12 days post‐inoculation (dpi). Scale bar, 1 cm. (D) RNA gel blot analysis was performed to detect viral RNAs and satRNA accumulation in systemically noninoculated leaves at 12 dpi. Methylene blue‐stained rRNA served as loading control. Quantifications of each CMV RNA relative to loading control RNA are shown on the right of the panel using ImageQuant TL (GE Healthcare Life Sciences). RNAs obtained from CMVwt‐infected Col‐0 were arbitrarily assigned a value of 1.0. An asterisk denotes possible double‐stranded satRNA.
DISCUSSION
In this study, we report trilateral interactions among host plant, helper virus SD‐CMV and SD‐satRNA relevant to RNA silencing. We found that SD‐satRNA mainly reduced the accumulation of the helper virus subgenomic RNA4A, which encodes 2b protein that functions as a viral silencing suppressor, and attenuated the yellowing phenotype induced by SD‐CMV in the presence of the 2b protein in infected N. benthamiana and Arabidopsis.
CMV 2b has been identified as a nuclear viral protein (Lucy et al., 2000) that interferes with local and systemic silencing (Guo and Ding, 2002), presumably by interacting directly with host silencing effector protein AGO1 to inhibit its slicer activity in the cytoplasm (Zhang et al., 2006). Recently, a CMV 2b protein from subgroup IA Fny strain has been shown to interact with AGO4, a nuclear silencing pathway protein (Gonzalez et al., 2010), suggesting that 2b protein may, through interaction with different AGO proteins, interfere with various silencing pathways. A reduced accumulation level of 2b coding sequence by SD‐satRNA (3, 4) relieves normal host regulatory silencing processes influenced by the 2b protein, consequently rescuing plant normal development, such as the recovery of green development, as observed in plants infected with 2b‐containing CMV in the presence of SD‐satRNA (2, 4). Our infection system of SD‐satR‐transgenic Arabidopsis combined with CMV will help us to further investigate physiologically important host gene(s) or pathway(s) targeted by the 2b protein, and the molecular basis of interaction between the 2b suppressor and RNA silencing pathways.
The fact that subgenomic RNA4A‐derived vsiRNAs were not increased at either time point in the presence of SD‐satRNA (Fig. 3B) indicates that reduced accumulation of 2b‐coding RNA4A by SD‐satRNA (Fig. 3A) is not a result of increased degradation of the 2b coding sequence by the RNA silencing machinery. satRNA depends on a helper virus for its replication; whether there is specific competition for replication with the SD‐CMV subgenomic RNA4A by SD‐satRNA requires further investigation.
In plants, RNA silencing involves an amplification process that requires RNA‐directed RNA polymerases (RDRs) for persistent silencing (Baulcombe, 2007). The RDR6‐dependent amplification and systemic spread of silencing is a crucial step towards the achievement of efficient antiviral silencing response in plants (Schwach et al., 2005; Ying et al., 2010). CMV 2b has been found to inhibit the production of RDR1‐dependent CMV‐derived siRNAs (Diaz‐Pendon et al., 2007). Recently, using mutations of RDRs in Arabidopsis (rdr mutants), Wang et al. (2010) found some target specificity for RDR1 and RDR6 in the amplification of CMV‐derived siRNAs in the absence of 2b protein (Wang et al., 2010). We found that CMV RNA3‐derived siRNAs were readily detected in N. benthamiana agro‐infected with CMVΔ2b, but not with CMVwt (Fig. 3B). As RDR1 in N. benthamiana is a natural loss‐of‐function variant (Yang et al., 2004; Ying et al., 2010), our data suggest that N. benthamiana RDR6 is probably involved in SD‐CMV RNA3‐specific vsiRNA production and amplification. However, the generation of the RDR6‐dependent RNA3‐derived vsiRNAs was inhibited with CMV infection in the presence of 2b protein.
Lower levels of helper CMV‐specific vsiRNAs were detected in infection with SD‐satRNA in either the presence or absence of 2b protein (Fig. 3B). These data indicate that the restraint of CMV movement or replication in newly grown leaves (Fig. 3A) and the lack of visual deficiencies in infected plants (Fig. 2) are not a result of enhanced degradation of helper viral RNAs by host silencing, which was strengthened because of reduced 2b protein in the presence of SD‐satRNA. In contrast, abundant SD‐satRNA‐derived vsiRNAs were detected (Fig. 3B), revealing that SD‐satRNA functions to interfere with silencing by recruiting host silencing components. SD‐satRNA‐derived vsiRNAs were detected in the presence and absence of 2b protein, suggesting that the RDR6‐dependent siRNA amplification process is not involved in SD‐satRNA‐derived siRNA production, which is consistent with the result that RDR6 is essential for sense transgene‐induced post‐transcriptional gene silencing (S‐PTGS), but not for inverted repeat dsRNA‐induced PTGS (IR‐PTGS) (Dalmay et al., 2000; Mourrain et al., 2000; Parizotto et al., 2004; Vaistij et al., 2002; Vance and Vaucheret, 2001). Our previous study has shown that DCL4, an RNase III enzyme for siRNA biogenesis, is the primary producer of SD‐satRNA‐derived siRNAs (Du et al., 2007), supporting the idea that the formation of a secondary structure of SD‐satRNA directly triggers host RNA silencing for the production of SD‐satRNA‐derived siRNA. We cannot rule out the possibility that SD‐satRNA might somehow compete with other RNAs of the helper CMV for replication in the absence of 2b protein. Nevertheless, on the one hand, by reducing the expression of the 2b suppressor protein, SD‐satRNA attenuates the damage to plant development caused by interference with the regulatory silencing process by the strong suppressor 2b protein; on the other, it protects helper viral RNAs from excessive targeting by antiviral silencing pathways, which should be enhanced as a result of reduced expression of the 2b protein, by recruiting silencing machinery to its own abundant and more highly structured RNA.
Our data provide evidence that the plant silencing mechanism is involved in the pathogenicity of satRNA. In other words, the pathogenicity of SD‐satRNA is a result of a complex interaction among SD‐satRNA, helper CMV and host silencing mechanisms. Together with the recent finding of the de novo emergence of a novel satRNA of CMV‐Fny following serial passages of CMV‐Fny in plants (Hajimorad et al., 2009), our finding that CMV‐infected plants grow better in the presence of satRNA (Fig. 2) suggests that satRNA is generated de novo after the activation of the host silencing mechanism to reduce expression of the viral suppressor for the host's own benefit by keeping the viral titre under control. However, the recruitment by satRNA of silencing components to its own abundant RNA for degradation protects the replication of the CMV helper virus and maintains satRNA under the control of functional host silencing mechanisms.
EXPERIMENTAL PROCEDURES
Plasmid construction and site‐directed mutagenesis
A tobacco isolate of CMV subgroup IB, a China ShanDong strain (SD‐CMV), was used in this study. Full‐length cDNAs of SD‐CMV RNA1 (AF071551), RNA2 (D86330) and RNA3 (AB008777) were amplified by RT‐PCR using total RNAs extracted from SD‐CMV‐infected tobacco leaves as template, with the following forward and reverse primers: SDR1‐F, 5′‐CCCGGGGTTTTATTTACAAGAGCGTAC‐3′; SDR1‐R, 5′‐GAGCTCTGGTCTCCTTTAGGAGGCTC‐3′; SDR2‐F, 5′‐TCTAGAGTTTATTTACAAGAGCGTAC‐3′; SDR2‐R, 5′‐CCCGGGTGGTCTCCTATAGAGGACC‐3′; SDR3‐F, 5′‐TCTAGAGTAATCTTACCACTGTGTGTG‐3′; SDR3‐R, 5′‐CCCGGGTGGTCTCCTAAAGGAGGCTC‐3′. The nucleotides in italic indicate the introduced restrictive cleavage sites, as described in Fig. 1A. The PCR products were first cloned downstream of bacteriophage T7 promoters in pGEM‐T easy vector (Promega, Madison, WI, USA), and named as pT‐R1, pT‐R2 and pT‐R3, respectively. After DNA sequencing, correct RNA1, RNA2 and RNA3 cDNA clones were digested by SmaI/SacI, XbaI/SmaI and XbaI/SacI, respectively, inserted downstream of the cauliflower mosaic virus (CaMV) 35S promoter in the binary vector pBI121 (GenBank Accession No. AF485783) and digested by the corresponding restrictive enzymes to generate 35S‐R1, 35S‐R2 and 35S‐R3, respectively (Fig. 1A).
To construct the RNA2‐Δ2b cDNA clone which abolishes 2b protein expression and maintains the 2a protein sequence, site‐directed mutagenesis was performed by substituting ‘C’ for ‘T’ present in four start codons ‘ATG’ of the 2b coding sequence (Fig. 1A) with a site‐directed Takara (Tokyo, Japan) MutanBEST Kit according to the manufacturer's instructions. The mutated RNA2‐Δ2b cDNA clone was also cloned into binary vector pBI121 to generate 35S‐R2‐Δ2b as described above. The sequences of the primers used for Δ2b are as follows: Δ2b‐L1, 5′‐TGTCGTTGCGCCTTCGTTCAATTCCGTCTTTCTTCTTTCG‐3′; Δ2b‐L2, 5′‐TGGTGGTACGTCTCTATCAGTTCCG‐3′; Δ2b‐R1, 5′‐AACGTCGAACTCCAACTGGCTCGTACGGTGGAGGCGAAGA‐3′; Δ2b‐R2, 5′‐TGTGAACACGGTGGGATTGTCCGAG‐3′; Δ2b‐L, 5′‐ATCTTTCTTCTTTCGTTGCTTAGTG‐3′; Δ2b‐R, 5′‐TAATGAAACCTCCCCTTCCTAATCTCC‐3′.
To produce the SD‐satR cDNA clone, a 334‐nucleotide fragment was RT‐PCR‐amplified from native SD‐CMV sap as described above, which was finally cloned downstream of the 35S promoter in the binary vector pCAMBIA‐1300221 to give 35S‐SatR. The primers were as follows: satR‐F, 5′‐TCTAGAGTTTTGTTTGATGGAGAA‐3′; satR‐R, 5′‐GAGCTCGGGTCCTGCAGAGGAATGA‐3′; the nucleotides in italic indicate the introduced restrictive cleavage sites of XbaI and SacI.
Plant growth, in vitro transcription and virus inoculation
Nicotiana benthamiana plants were grown in a glasshouse at 25 °C with a 16‐h light/8‐h dark cycle. Five‐week‐old leaves were used for inoculation. For SD‐CMV sap infection, leaves from confirmed infected plants were ground in 5 mm sodium phosphate buffer (pH 7.2) and clarified by filtration. The sap was then applied to carborundum‐dusted leaves with mechanical rubbing. The virus sap‐inoculated plants were covered for 24 h and then grown under normal conditions. Buffer‐treated plants were used as a negative control.
For in vitro transcription‐mediated infection, infectious RNAs were transcribed from cDNA clones in linearized pGEM‐T easy vector (Promega, USA) using an mMESSAGE mMACHINE® T7 Kit (Ambion, Forster, CA, USA) according to the manufacturer's instructions. The combinations of infectious RNAs were then applied to carborundum‐dusted leaves as described above. The symptoms were monitored for 1 month, and plant photographs were taken at 10 and 20 dpi.
Agrobacterium tumefaciens‐mediated transient expression and plant transformation
Agrobacterium cells containing recombinant plasmids were grown overnight in Luria–Bertani medium containing antibiotic, 10 mm 2‐(N‐morpholino)ethanesulphonic acid (MES) (pH 5.6) and 0.04 mm acetosyringone at 28 °C. After centrifugation, Agrobacterium pellets were suspended in buffer containing10 mm MgCl2 and 0.2 mm acetosyringone, and the optical density at 600 nm (OD600) was adjusted to 1.0. The bacterial inoculum was left at room temperature for 3–5 h and then injected into the leaves of 5‐week‐old N. benthamiana plants with a 2‐mL syringe. For co‐infiltration of three CMV cDNA copies, equal volumes of Agrobacterium inocula containing different recombinant plasmids were mixed together before infiltration. Similarly, for the co‐infiltration of cDNA inocula with satRNA cDNA inocula, Agrobacterium inocula of cDNAs were mixed with those of satRNA cDNA or vector control in the ratio 1:1:1:1. The phenotypes of infiltrated, SD‐CMV sap‐inoculated and mock‐inoculated plants were observed for at least 3 weeks.
Plant transformation was carried out using an Agrobacterium‐mediated flower dip method to obtain SD‐satR‐transgenic Arabidopsis. Transgenic plants were screened on Murashige and Skoog medium containing 20 µg/L hygromycin.
RNA isolation and RNA gel blot analysis
Total RNAs were isolated with Trizol reagent (Invitrogen, CA, USA) according to the instruction manual. For viral RNAs and satRNA gel blot hybridization, total RNAs were separated in a 1.2% agarose denaturing gel containing 0.1% formaldehyde and then blotted onto a nylon membrane (Hybond‐N+, Amersham Pharmacia Biotech, Piscataway, NJ, USA). The membrane was prehybridized in hybridization buffer [7% sodium dodecylsulphate (SDS), 1% bovine serum albumin (BSA), 20 mm ethylenediaminetetraacetic acid (EDTA) (pH 8.0) and 43 mm sodium phosphate buffer (pH 7.2)] for 1–2 h. The cDNA probes specific for viral RNAs or satRNA were radioactively labelled by [α‐32P]‐dCTP using a Rediprime™ II Random Prime Labelling System (Amersham Pharmacia Biotech). For genomic and subgenomic RNA hybridization, three 1‐kb fragments at the 3′‐terminus of each cDNA clone were amplified, which were then labelled and mixed as probes. The satRNA and SD2b full‐length coding sequences were amplified and radioactively labelled as probes to hybridize satRNA and RNA4A. Hybridization was performed in hybridization buffer containing 50% formamide, 7% SDS, 50 mm sodium phosphate buffer (pH 7.2), 5 × Denhardt's solution (0.1% polyvinylpyrrolidone, 0.1% Ficoll 400, 0.1% BSA V) and 0.3 m NaCl at 65 °C overnight. After two washes with 2 × standard saline citrate (SSC)/0.2% SDS, the blots were immediately exposed to a storage phosphor screen for 2–4 h. For detection of vsiRNAs, 30 µg of total RNA was separated on 17% polyacrylamide–8 m urea gels. After electrophoresis, the RNA was electroblotted onto Hybond N+ membranes with a semidry transfer cell with 1 × TBE (90 mM Tris base, 90 mM Boric acid and 2 mM EDTA at pH of 8.0) and fixed by UV crosslinking. The [α‐32P]‐UTP‐labelled T7 RNA transcripts from cDNA clones in pGEM‐T easy vector using the MAXIscript kit (Ambion), digested by carbonate buffer (120 mm Na2CO3 and 80 mm NaHCO3) at 60 °C, were used as probes. The blots were hybridized in hybridization buffer containing 50% formamide, 7% SDS, 50 mm sodium phosphate buffer (pH 7.2), 5 × Denhardt's solution and 0.3 m NaCl at 40 °C overnight. For U6 hybridization, synthesized DNA oligo was 5′‐end‐labelled with [γ‐32P]‐ATP using T4 polynucleotide kinase (NEB), and then added to hybridization buffer [5 × SSC, 7% SDS, 2 × Denhardt's solution and 20 mm sodium phosphate buffer (pH 7.2)] to perform overnight hybridization at 37 °C. Similar washing as described above was performed, and the membrane was then subjected to exposure to a phosphor screen.
Western blotting
Total protein was extracted from N. benthamiana leaves as described above using extraction buffer containing 50 mm sodium phosphate (pH 7.0), 200 mm NaCl, 10 mm MgCl2, 10% glycerol, 5 mm dithiothreitol and protease inhibitor cocktail (EDTA‐free, Roche, Basel, Switzerland). Total protein was separated on 12% SDS‐polyacrylamide gel and transferred to poly(vinylidene difluoride) (PVDF) membrane. The membrane was blotted with anti‐SD2b polyclonal rabbit antiserum and detected using ECL detection reagent (Pierce, Waltham, MA, USA). Polyclonal anti‐SD2b anti‐serum was generated in rabbits immunized with the recombinant GST (Glutathione S‐transferase)‐tagged SD2b fusion protein, which was purified by Glutathione Sepharose™ 4B affinity columns (GE Healthcare, Waukesha, WI, USA).
ACKNOWLEDGEMENTS
This research was supported by grants from the Ministry of Science and Technology (Grant 2011CB100700) and the National Science Foundation of China (Grants 30900057 and 31030009).
REFERENCES
- Baulcombe, D.C. (2007) Molecular biology. Amplified silencing. Science, 315, 199–200. [DOI] [PubMed] [Google Scholar]
- Brigneti, G. , Voinnet, O. , Li, W.X. , Ji, L.H. , Ding, S.W. and Baulcombe, D.C. (1998) Viral pathogenicity determinants are suppressors of transgene silencing in Nicotiana benthamiana . EMBO J. 17, 6739–6746. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dalmay, T. , Hamilton, A. , Rudd, S. , Angell, S. and Baulcombe, D.C. (2000) An RNA‐dependent RNA polymerase gene in Arabidopsis is required for posttranscriptional gene silencing mediated by a transgene but not by a virus. Cell, 101, 543–553. [DOI] [PubMed] [Google Scholar]
- Devic, M. , Jaegle, M. and Baulcombe, D. (1989) Symptom production on tobacco and tomato is determined by two distinct domains of the satellite RNA of cucumber mosaic virus (strain Y). J. Gen. Virol. 70 (Pt 10), 2765–2774. [DOI] [PubMed] [Google Scholar]
- Diaz‐Pendon, J.A. , Li, F. , Li, W.X. and Ding, S.W. (2007) Suppression of antiviral silencing by cucumber mosaic virus 2b protein in Arabidopsis is associated with drastically reduced accumulation of three classes of viral small interfering RNAs. Plant Cell, 19, 2053–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, B. , Li, Q. , Nguyen, L. , Palukaitis, P. and Lucas, W.J. (1995) Cucumber mosaic virus 3a protein potentiates cell‐to‐cell trafficking of CMV RNA in tobacco plants. Virology, 207, 345–353. [DOI] [PubMed] [Google Scholar]
- Ding, S.W. , Anderson, B.J. , Haase, H.R. and Symons, R.H. (1994) New overlapping gene encoded by the cucumber mosaic virus genome. Virology, 198, 593–601. [DOI] [PubMed] [Google Scholar]
- Ding, S.W. , Li, W.X. and Symons, R.H. (1995) A novel naturally occurring hybrid gene encoded by a plant RNA virus facilitates long distance virus movement. EMBO J. 14, 5762–5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, Q.S. , Duan, C.G. , Zhang, Z.H. , Fang, Y.Y. , Fang, R.X. , Xie, Q. and Guo, H.S. (2007) DCL4 targets Cucumber mosaic virus satellite RNA at novel secondary structures. J. Virol. 81, 9142–9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Arenal, F. , Escriu, F. , Aranda, M.A. , Alonso‐Prados, J.L. , Malpica, J.M. and Fraile, A. (2000) Molecular epidemiology of Cucumber mosaic virus and its satellite RNA. Virus Res. 71, 1–8. [DOI] [PubMed] [Google Scholar]
- Gonzalez, I. , Martinez, L. , Rakitina, D.V. , Lewsey, M.G. , Atencio, F.A. , Llave, C. , Kalidina, N.O. , Carr, J.P. , Palukaitis, P. and Canto, T. (2010) Cucumber mosaic virus 2b protein subcellular targets and interactions: their significance to RNA silencing suppressor activity. Mol. Plant–Microbe Interact. 23, 294–303. [DOI] [PubMed] [Google Scholar]
- Guo, H.S. and Ding, S.W. (2002) A viral protein inhibits the long range signaling activity of the gene silencing signal. EMBO J. 21, 398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajimorad, M.R. , Ghabrial, S.A. and Roossinck, M.J. (2009) De novo emergence of a novel satellite RNA of cucumber mosaic virus following serial passages of the virus derived from RNA transcripts. Arch. Virol. 154, 137–140. [DOI] [PubMed] [Google Scholar]
- Harrison, B.D. , Mayo, M.A. and Baulcombe, D.C. (1987) Virus resistance in transgenic plants that express cucumber mosaic virus satellite RNA. Nature, 328, 799–802. [Google Scholar]
- Hayes, R.J. and Buck, K.W. (1990) Complete replication of a eukaryotic virus RNA in vitro by a purified RNA‐dependent RNA polymerase. Cell, 63, 363–368. [DOI] [PubMed] [Google Scholar]
- Hidaka, S. and Hanada, K. (1994) Structural features unique to a new 405‐nucleotide satellite RNA of cucumber mosaic virus inducing tomato necrosis. Virology, 200, 806–808. [DOI] [PubMed] [Google Scholar]
- Jaegle, M. , Devic, M. , Longstaff, M. and Baulcombe, D. (1990) Cucumber mosaic virus satellite RNA (Y strain): analysis of sequences which affect yellow mosaic symptoms on tobacco. J. Gen. Virol. 71 (Pt 9), 1905–1912. [DOI] [PubMed] [Google Scholar]
- Ji, L.H. and Ding, S.W. (2001) The suppressor of transgene RNA silencing encoded by Cucumber mosaic virus interferes with salicylic acid‐mediated virus resistance. Mol. Plant–Microbe Interact. 14, 715–724. [DOI] [PubMed] [Google Scholar]
- Kaper, J.M. (1995) Role of satellites in viral pathogenesis: nested parasitic nucleic acids competing for expression. Viruses Viroids, III, 373–392. [Google Scholar]
- Kuwata, S. , Masuta, C. and Takanami, Y. (1991) Reciprocal phenotype alterations between two satellite RNAs of cucumber mosaic virus. J. Gen. Virol. 72 (Pt 10), 2385–2389. [DOI] [PubMed] [Google Scholar]
- Lewsey, M. , Robertson, F.C. , Canto, T. , Palukaitis, P. and Carr, J.P. (2007) Selective targeting of miRNA‐regulated plant development by a viral counter‐silencing protein. Plant J. 50, 240–252. [DOI] [PubMed] [Google Scholar]
- Li, H.W. , Lucy, A.P. , Guo, H.S. , Li, W.X. , Ji, L.H. , Wong, S.M. and Ding, S.W. (1999) Strong host resistance targeted against a viral suppressor of the plant gene silencing defence mechanism. EMBO J. 18, 2683–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucy, A.P. , Guo, H.S. , Li, W.X. and Ding, S.W. (2000) Suppression of post‐transcriptional gene silencing by a plant viral protein localized in the nucleus. EMBO J. 19, 1672–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuta, C. and Takanami, Y. (1989) Determination of sequence and structural requirements for pathogenicity of a cucumber mosaic virus satellite RNA (Y‐satRNA). Plant Cell, 1, 1165–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlotshwa, S. , Voinnet, O. , Mette, M.F. , Matzke, M. , Vaucheret, H. , Ding, S.W. , Pruss, G. and Vance, V.B. (2002) RNA silencing and the mobile silencing signal. Plant Cell, 14 (Suppl), S289–S301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourrain, P. , Beclin, C. , Elmayan, T. , Feuerbach, F. , Godon, C. , Morel, J.B. , Jouette, D. , Lacombe, A. , Nikic, S. , Picault, N. , Remoue, K. , Sanial, M. , Vo, T. and Vaucheret, H. (2000) Arabidopsis SGS2 and SGS3 genes are required for posttranscriptional gene silencing and natural virus resistance. Cell, 101, 533–542. [DOI] [PubMed] [Google Scholar]
- Palukaitis, P. (1988) Pathogenicity regulation by satellite RNAs of cucumber mosaic virus: minor nucleotide sequence changes alter host responses. Mol. Plant–Microbe Interact. 1, 175–181. [DOI] [PubMed] [Google Scholar]
- Palukaitis, P. and Garcia‐Arenal, F. (2003) Cucumoviruses. Adv. Virus Res. 62, 241–323. [DOI] [PubMed] [Google Scholar]
- Parizotto, E.A. , Dunoyer, P. , Rahm, N. , Himber, C. and Voinnet, O. (2004) In vivo investigation of the transcription, processing, endonucleolytic activity, and functional relevance of the spatial distribution of a plant miRNA. Genes Dev. 18, 2237–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roossinck, M.J. , Sleat, D. and Palukaitis, P. (1992) Satellite RNAs of plant viruses: structures and biological effects. Microbiol. Rev. 56, 265–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwach, F. , Vaistij, F.E. , Jones, L. and Baulcombe, D.C. (2005) An RNA‐dependent RNA polymerase prevents meristem invasion by potato virus X and is required for the activity but not the production of a systemic silencing signal. Plant Physiol. 138, 1842–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwinghamer, M.W. and Symons, R.H. (1975) Fractionation of cucumber mosaic virus RNA and its translation in a wheat embryo cell‐free system. Virology, 63, 252–262. [DOI] [PubMed] [Google Scholar]
- Shi, B.J. , Palukaitis, P. and Symons, R.H. (2002) Differential virulence by strains of Cucumber mosaic virus is mediated by the 2b gene. Mol. Plant–Microbe Interact. 15, 947–955. [DOI] [PubMed] [Google Scholar]
- Sleat, D.E. and Palukaitis, P. (1990) Site‐directed mutagenesis of a plant viral satellite RNA changes its phenotype from ameliorative to necrogenic. Proc. Natl. Acad. Sci. USA, 87, 2946–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soards, A.J. , Murphy, A.M. , Palukaitis, P. and Carr, J.P. (2002) Virulence and differential local and systemic spread of cucumber mosaic virus in tobacco are affected by the CMV 2b protein. Mol. Plant–Microbe Interact. 15, 647–653. [DOI] [PubMed] [Google Scholar]
- Vaistij, F.E. , Jones, L. and Baulcombe, D.C. (2002) Spreading of RNA targeting and DNA methylation in RNA silencing requires transcription of the target gene and a putative RNA‐dependent RNA polymerase. Plant Cell, 14, 857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance, V. and Vaucheret, H. (2001) RNA silencing in plants—defense and counterdefense. Science, 292, 2277–2280. [DOI] [PubMed] [Google Scholar]
- Wang, X.B. , Wu, Q. , Ito, T. , Cillo, F. , Li, W.X. , Chen, X. , Yu, J.L. and Ding, S.W. (2010) RNAi‐mediated viral immunity requires amplification of virus‐derived siRNAs in Arabidopsis thaliana . Proc. Natl. Acad. Sci. USA, 107, 484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterworth, H.E. , Kaper, J.M. and Tousignant, M.E. (1979) CARNA 5, the small cucumber mosaic virus‐dependent replicating RNA, regulates disease expression. Science, 204, 845–847. [DOI] [PubMed] [Google Scholar]
- Yang, S.J. , Carter, S.A. , Cole, A.B. , Cheng, N.H. and Nelson, R.S. (2004) A natural variant of a host RNA‐dependent RNA polymerase is associated with increased susceptibility to viruses by Nicotiana benthamiana . Proc. Natl. Acad. Sci. USA, 101, 6297–6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying, X.B. , Dong, L. , Zhu, H. , Duan, C.G. , Du, Q.S. , Lv, D.Q. , Fang, Y.Y. , Garcia, J.A. , Fang, R.X. and Guo, H.S. (2010) RNA‐dependent RNA polymerase 1 from Nicotiana tabacum suppresses RNA silencing and enhances viral infection in Nicotiana benthamiana . Plant Cell, 22, 1358–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Yuan, Y.R. , Pei, Y. , Lin, S.S. , Tuschl, T. , Patel, D.J. and Chua, N.H. (2006) Cucumber mosaic virus‐encoded 2b suppressor inhibits Arabidopsis Argonaute1 cleavage activity to counter plant defense. Genes Dev. 20, 3255–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]


