SUMMARY
The soil‐borne, asexual fungus Fusarium oxysporum f.sp. melonis (FOM) is a causal agent of muskmelon wilt disease. The current study focused on the most virulent race of FOM—race 1,2. The tagged mutant D122, generated by Agrobacterium tumefaciens‐mediated transformation, caused the delayed appearance of initial wilt disease symptoms, as well as a 75% reduction in pathogenicity. D122 was impaired in the gene product homologous to the Snt2‐like transcription factor of Schizosaccharomyces pombe. Involvement of snt2 in the early stage of FOM pathogenesis and its requirement for host colonization were confirmed by targeted disruption followed by quantitative reverse transcription‐polymerase chain reaction analysis of snt2 expression in planta. Δsnt2 mutants of FOM and Neurospora crassa exhibited similar morphological abnormalities, including a reduction in conidia production and biomass accumulation, slower vegetative growth and frequent hyphal septation. In N. crassa, snt‐2 is required for sexual development, as Δsnt‐2 mutants were unable to produce mature perithecia. Suppressive subtraction hybridization analysis of the D122 mutant versus wild‐type isolate detected four genes (idi4, pdc, msf1, eEF1G) that were found previously in association with the target of rapamycin (TOR) kinase pathway. Expression of the autophagy‐related idi4 and pdc genes was found to be up‐regulated in the Δsnt2 FOM mutant. In N. crassa, disruption of snt‐2 also conferred a significant over‐expression of idi4.
INTRODUCTION
The soil‐borne fungus Fusarium oxysporum f.sp. melonis Snyder & Hansen (FOM) (Nelson et al., 1981) causes vascular wilt disease of melon (Cucumis melo L.); race 1,2 is the most virulent of four known races affecting most commercial cultivars (Cohen et al., 1989). Targeted or random mutagenesis has been instrumental in the identification of the fungal genes involved in pathogenesis by the efficient utilization of Agrobacterium tumefaciens‐mediated transformation (ATMT) in F. oxysporum (Mullins et al., 2001). Over the last two decades, 29 pathogenicity‐related genes of F. oxysporum have been detected (Michielse and Rep, 2009). Interaction between plant hosts and fungal pathogens has evolved in a polyphyletic manner (Ma et al., 2010), involving multiple fungal and host genes, including different biosynthetic and transduction pathways. Plant colonization is a critical stage for the success of the disease cycle and several genes have been found to play an important role in this process. At least six pathogenicity‐related genes of F. oxysporum involved in root penetration and colonization have been discovered to date: fow1, encoding a mitochondrial carrier protein, is required specifically for colonization (Inoue et al., 2002); a Zn(ii)2Cys6 transcription factor (TF) encoding fow2 is required for the invasive growth of FOM (Imazaki et al., 2007); frp1, encoding an F‐box protein, is involved in root penetration and colonization (Duyvesteijn et al., 2005); fmk1, a mitogen‐activated protein kinase (MAPK)‐encoding gene, is required for root attachment and host‐invasive growth (Di Pietro et al., 2001); a six1 gene encoding a small, cysteine‐rich protein that is secreted by F. oxysporum during colonization of the xylem of its host, tomato (Rep et al., 2005), and a recently identified transcriptional regulator‐encoding gene sge1, are required for parasitic growth (Michielse et al., 2009).
As TFs control biological processes by regulating the expression of multiple genes, their disruption may reveal novel regulatory pathways, especially in cases in which functional redundancy is present in downstream effectors. In particular, the study of TFs may also assist in the discovery of novel regulatory pathways of pathogenesis. Recently, several genes encoding TFs involved in the pathogenicity of F. oxysporum have been studied. Among them, the virulence factor ftf1 has been found to be expressed only in planta (Ramos et al., 2007), the pH‐responsive TF PacC has been demonstrated to be a negative pathogenicity regulator of F. oxysporum (Caracuel et al., 2003) and XlnR has been reported to be a regulator of three xylanase genes involved in the pathogenicity of this fungus (Calero‐Nieto et al., 2007).
Throughout their life cycle, fungi experience a vast variety of external signals which affect their development. Nutrient availability is one of the major forces of the host environment that needs to be overcome by fungi during infection. The requirement for the functional genes of FOM involved in the fundamental biosynthetic pathways of pathogenicity has been established over the last decade (Denisov et al., 2005; Namiki et al., 2001). Significant attention has also been given to the genes involved in nitrogen assimilation, with an emphasis on the involvement of GATA‐type TFs in nitrogen regulation and plant pathogenesis (Bolton and Thomma, 2008). The GATA‐type TF AreA is a global nitrogen regulator which, in F. verticillioides, has been shown to be involved in fumonisin production and the colonization of corn kernels (Kim and Woloshuk, 2008); in F. oxysporum, targeted disruption of the AreA homologue‐encoding gene (fnr1) causes impaired nitrogen utilization and affects pathogenicity (Divon et al., 2006). One of the factors involved in the regulation of AreA is the target of rapamycin (TOR) kinase, which has been shown to activate the AreA cascade during nitrogen‐limiting conditions in Fusarium fujikuroi (Teichert et al., 2006). The TOR kinase pathway is a well‐conserved mechanism among eukaryotes; it mediates cell growth in response to nutrient availability (Rohde et al., 2001). In fungi, following nutrient‐limiting conditions, the activation of heterokaryon incompatibility (het) genes or treatment with rapamycin, TOR kinase is repressed, allowing the activation of a range of downstream processes, one of which is autophagy (Dementhon et al., 2003; Pinan‐Lucarre et al., 2003). Autophagy is a catabolic process involving the degradation of cell components by lysosomal machinery. Part of this process is characterized by the formation of double‐membrane vesicles, called autophagosomes, which deliver cytoplasmic material to lysosomes. Autophagy is usually triggered by a variety of stresses and during nutrient‐limiting conditions and supports cell survival (Levine and Klionsky, 2004), yet research on autophagy in filamentous fungi is in its infancy (Pollack et al., 2009). Autophagy has been shown to be involved in normal developmental processes, such as growth, differentiation and sexual development in Podospora anserina (Pinan‐Lucarre et al., 2003), Aspergillus oryzae (Kikuma et al., 2006) and Magnaporthe grisea (Liu et al., 2007). Autophagy also plays an essential role during pathogenesis in the rice pathogen M. grisea (Veneault‐Fourrey et al., 2006) and the bean pathogen Colletotrichum lindemuthianum (Dufresne et al., 1998). In P. anserina, over‐expression of the TF‐encoding gene idi4 causes increased production of autophagosomes and leads to fungal cell death (Dementhon et al., 2004). idi4 has also been identified recently among additional, less characterized, elements in the TOR/AreA‐controlled fungal gene network (Schonig et al., 2008; Teichert et al., 2006). Interestingly, several genes, including the autophagy regulator idi4, have been found to be both nitrogen independent and TOR/AreA dependent, suggesting the existence of other regulatory elements that control idi4 expression (Schonig et al., 2008).
In this study, we have characterized a novel TF‐encoding gene, designated ‘snt2’, and demonstrated its involvement in the pathogenicity of FOM, hyphal growth and conidiation in FOM and Neurospora crassa. snt2 has been found to share the regulation of at least two genes with the TOR kinase pathway, one of which is an autophagy TF encoded by idi4. We have also determined that snt2 is a positive regulator of rbs1, a novel gene unique to FOM, identified in this study.
RESULTS
The TF‐encoding gene snt2 is required for the pathogenicity of FOM
In order to identify novel pathogenicity‐related genes in FOM, we employed a tagged mutagenesis approach on the basis of ATMT. Screening of 2000 stable transformants harbouring an insert of the pBHt2 and pKHt plasmids resulted in the recovery of five reduced‐pathogenicity transformants. One of the transformants, designated ‘D122’, exhibited a 75% reduction in plant mortality when compared with the wild‐type isolate (Fig. 1).
Figure 1.
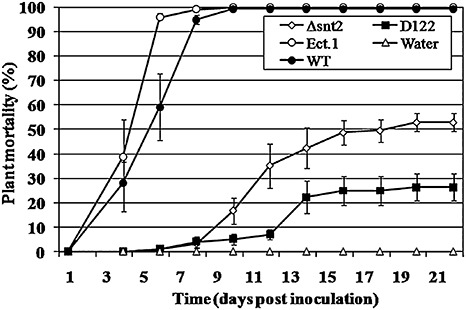
Disruption of snt2 impairs the pathogenicity of Fusarium oxysporum f.sp. melonis (FOM) on muskmelon plants. One‐week‐old muskmelon seedlings were inoculated by dipping the roots in a conidial suspension (5 × 105 conidia/mL) of FOM and plants were assessed for symptoms of vascular wilt disease over a 3‐week period. Means and standard errors were calculated from three independent experiments and compared using the Tukey–Kramer test (P < 0.05). Treatments: Δsnt2, targeted mutant; D122, tagged mutant; Ect.1, ectopic transformant; WT, wild‐type; Water, control.
Using thermal asymmetric interlaced‐polymerase chain reaction (TAIL‐PCR), a 400‐bp region flanking the integrated vector was amplified. The sequence of the FOM gene amplicon showed similarity to the snt2 gene of Schizosaccharomyces pombe, and was therefore designated as ‘snt2’. The complete genomic DNA sequence of FOM snt2 was amplified using the genome walking procedure and found to be 5.2 kb long, containing five short introns (172, 67, 104, 105 and 53 bp), encoding a protein of 1567 amino acids. The FOM snt2 open reading frame was deduced by comparison with the sequence of Fusarium oxysporum f.sp. lycopersici (FOL) FOXG_01993.2 (http://www.broad.mit.edu) and with a 3.1‐kb partial cDNA sequence of FOM snt2, amplified by reverse transcription‐polymerase chain reaction (RT‐PCR) with primers BAHfom1F/2R. The FOM Snt2 protein contains five conserved domains: a bromo‐adjacent domain (BAH) located at amino acid position 205–324, three plant homeodomain Zn fingers (PHD) at positions 362–410, 752–793 and 1074–1144, and a GATA‐type Zn finger at position 925–971 (Fig. 2A). clustalw multiple alignment analysis of Snt2 showed that FOM Snt2 is 96%, 79% and 63% identical to proteins of FOL (FOXG_01993.2), F. graminearum (FGSG_06833.3) and F. verticillioides (FVEG_05152.3), respectively. The N and C termini of Snt2 displayed a lower percentage of similarity to the listed proteins. It was also found that FOXG_01993.2 contains a 60‐bp 5′‐untranslated region (5′‐UTR); however, in FOM snt2, this fragment was translated, similar to that observed in FGSG_06833.3 of F. graminearum. Another significant difference between FOM snt2 and its FOL homologue resides is the 84 bp shorter annotated third intron of the FOM gene compared with FOL, resulting in the translation of an additional PHD finger (Fig. 2A,B). The presence of the third PHD finger of Snt2 is common in different filamentous fungi (Fig. 2B); in Fusarium, it is found in the Snt2 proteins of FOM and F. verticillioides, but not in F. graminearum and FOL. The GATA‐type Zn finger was only found in F. oxysporum and in two necrotrophic phytopathogens Sclerotinia sclerotiorum and Botrytis cinerea, but not in the other annotated fungi (Fig. 2B).
Figure 2.
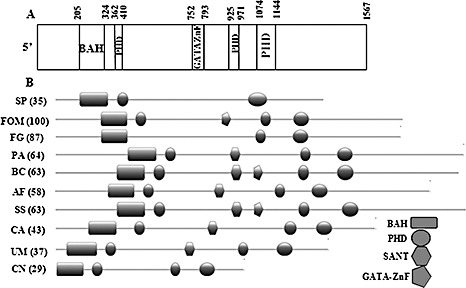
SNT2 protein structure of Fusarium oxysporum f.sp. melonis (FOM). (A) The FOM Snt2 protein carries five conserved domains: a bromo‐adjacent domain (BAH) at amino acid position 205–324; three plant homeodomain Zn fingers (PHD) at positions 362–410, 752–793 and 1074–1144; and a GATA‐type Zn finger at position 925–971. (B) Comparison of the structural domains of SNT2 proteins between different fungi (BAH, rectangle; PHD, circle; GATA Zn finger, pentagon; SANT, hexagon). Sequences shown: SNT2 (YGL131C), Schizosaccharomyces pombe (SP); Snt2, Fusarium oxysporum f.sp. melonis (FOM); FOXG_01993.2, Fusarium oxysporum f.sp. lycopersici (FOL); XP_387009, Fusarium graminearum (FG); XP_001908700, Podospora anserina (PA); XP_001549253, Botrytis cinerea (BC); XP_750273, Aspergillus fumigatus (AF); XP_001584831, Sclerotinia sclerotiorum (SS); XP_716567, Candida albicans (CA); XP_761724, Ustilago maydis (UM); AAN75722 (ZNF1), Cryptococcus neoformans (CN). Similarity (percentage, in parentheses) between SNT2 of FOM and other fungi was analysed by the MatGAT program (Campanella et al., 2003).
In order to determine the timing of snt2 expression in planta, transcript levels of snt2 in inoculated (infected) muskmelon plants were examined using quantitative RT‐PCR over a 6‐day post‐inoculation (dpi) period (Fig. 3). snt2 transcript levels became detectable at 2 dpi, most probably due to the small amount of fungal biomass within plant tissue at 1 dpi. At 3 dpi, the expression of snt2 was five‐fold higher (than at 2 dpi), whereas, at 5 dpi, snt2 expression was significantly lower and, by 6 dpi, its expression was undetectable (Fig. 3). The first significant wilt symptoms in wild‐type inoculated plants appeared at 4 dpi and, at 6 dpi, over 90% plant mortality was observed. In contrast, after inoculation with the D122 mutant, no wilt symptoms were detected until 6 dpi. Thereafter, wilt symptoms and mortality caused by D122 were evident (Fig. 1). In addition, the D122 mutant was impaired in its plant‐colonizing ability and exhibited significantly lower colonization of upper plant stems (38% ± 8%) when compared with the wild‐type isolate (100%). On the basis of these results, it was concluded that the expression of snt2 is important for invasive growth within the host and the characteristically rapid colonization of muskmelon plants.
Figure 3.
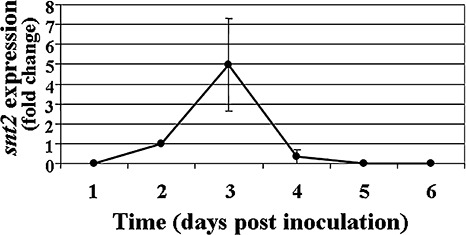
Expression of snt2 during pathogenesis of Fusarium oxysporum f.sp. melonis on muskmelon plants. cDNA was synthesized from 0.5 µg of mRNA isolated from infected plants. All samples were analysed in triplicate. Transcript levels from infected plants at 2‐days post‐inoculation were used as a reference. Averaged crossing point values were normalized to the endogenous control gene β‐tubulin. Expression levels of snt2 with standard error of two biological and three technical repeats were evaluated using the REST© program (Pfaffl et al., 2002).
Verification of snt2 function by targeted gene disruption
In order to verify the significance of snt2 in development and pathogenicity, and because, in strain D122, the mutating cassette was integrated downstream of the 5′‐coding region start site, targeted gene disruption of snt2 was performed. Approximately 200 stable transformants were obtained harbouring a 3.8‐kb snt2::hphR wild‐type gene fragment, carrying a hygromycin B resistance cassette, using ATMT (Fig. 4A). However, on the basis of the initial PCR analysis of transformant DNA, about 1% (two strains) of the isolates were confirmed to be knockout mutants. Southern blot analysis showed the presence of a single integration of the snt2::hphR construct at the snt2 locus, confirming insertional mutagenesis (Fig. 4B). The transcript of snt2 was undetectable in the Δsnt2 isolate (as well as in the D122 strain), as determined by RT‐PCR (Fig. 4C).
Figure 4.
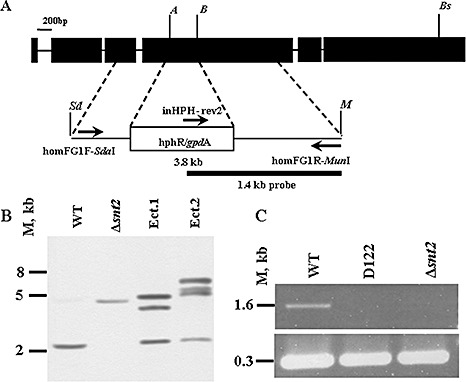
Targeted disruption of snt2 in Fusarium oxysporum f.sp. melonis (FOM). (A) The vector for targeted disruption was constructed by introducing a 2.13‐kb hygromycin B resistance cassette between the Acc65I (A) and BglII (B) sites of a 1.6‐kb fragment of FOM snt2, amplified with homFG1F/R primers and cloned into pGEM‐T Easy, creating a 3.8‐kb snt2::hphR cassette. The SdaI (Sd)/MunI (M) snt2::hphR cassette was transferred into the EcoRI/PstI binary vector pDHt in order to perform Agrobacterium‐mediated transformation. (B) Southern blot analysis using a 1.4‐kb fragment of snt2::hphR amplified with inHPH‐rev2/homFG1R, used as a probe (see A), was performed on Acc65I/Bsp1407I (Bs)‐digested genomic DNA, detecting a disruption of snt2 in one transformant (designated Δsnt2). Ectopic transformants (Ect.1 and Ect.2) with several insert copies and a wild‐type (WT) with an original snt2 copy are also shown. (C) Reverse transcription‐polymerase chain reaction (RT‐PCR) analysis of snt2 transcripts with the β‐tubulin gene used as a control. M denotes DNA size in kilobases.
Plant pathogenicity assays with the Δsnt2 mutant showed that the mutants exhibited impaired pathogenicity, as evident from the significant delay in initial symptom appearance, compared with the wild‐type and ectopic transformant (Fig. 1). Furthermore, inoculation of muskmelon plants by Δsnt2 caused a reduction by approximately 50% in plant mortality compared with the wild‐type isolate, which was also significantly higher than that of the mutant isolate D122. With regard to colonization ability, Δsnt2 demonstrated significantly decreased upper and lower stem colonization when compared with the wild‐type (27% ± 8% and 59% ± 11%, respectively). Similar results were observed with the second snt2 gene disruption strain, designated ‘3(15)’ (data not shown). Overall, these results confirm the requirement of snt2 for invasive pathogenic growth, and thus for full pathogenicity of FOM.
snt2 is required for fungal growth and development
In addition to the reduction in pathogenicity, cultures of both D122 and Δsnt2 mutants of FOM also exhibited defects in growth and development. The affected parameters examined included the linear growth rates, conidia production on solid medium and biomass accumulation in minimal liquid medium (Table 1). The phenotypic analysis of the second snt2 knockout mutant [isolate 3(15)] detected no significant differences in the parameters described above (Table 1).
Table 1.
Effect of snt2 disruption on developmental parameters of Fusarium oxysporum f.sp. melonis (FOM). Different letters (in parentheses) define significantly different groups.
| Isolate | Radial growth rate (mm/day)* | Conidia production (×104 conidia/cm2 colony) | Biomass accumulation (mg/25 mL) |
|---|---|---|---|
| Wild‐type | 7.2 ± 1.0 (a) | 12.7 ± 1.7 (a) | 94.6 ± 8.2 (a) |
| D122 | 4.3 ± 1.2 (b) | 6.2 ± 1.2 (b) | 65.7 ± 5.2 (b) |
| Δsnt2 | 3.5 ± 1.1 (b) | 2.0 ± 0.7 (b) | 62.8 ± 7.7 (b) |
| 3(15) | 3.4 ± 0.2 (b) | 0.6 ± 0.1 (b) | 58.7 ± 4.8 (b) |
Comparative analyses of the linear growth rate and conidial production of FOM snt2 mutants [D122 and the Δsnt2 mutant, as well as an additional confirmed snt2 disruptant, 3(15)] and the wild‐type isolate were performed on cultures grown on potato dextrose agar (PDA) medium. Biomass accumulation was examined after incubation in Fusarium minimal liquid medium (FMM). Standard errors and statistical differences [(P≤ 0.05) in parentheses] were calculated on the basis of three independent experiments by the Least Significant Difference (LSD) Tukey‐Kramer multiple comparison test.
In order to evaluate whether the involvement of snt2 in development is restricted to FOM, or can be observed in another fungal species, a partial characterization of the phenotypic consequences of snt2 disruption was assessed in the model fungus N. crassa. During growth on solid medium, the Δsnt‐2 strain (NCU07412.4) produced a thick, significantly slow‐growing (1.65 ± 0.38 mm/h) mat of hyphae with little aerial hyphae, when compared with the normal growth attributes (2.23 ± 0.14 mm/h) of the wild‐type isolate (Fig. S1, see Supporting Information). Macroconidia production of the Δsnt‐2 strain was only 10% of that of the wild‐type strain [(0.28 ± 0.04) × 109 and (2.19 ± 0.37) × 109 conidia/flask, respectively], which explains the less pronounced orange colour of its mycelium (Fig. S1). In this model organism, disruption of snt‐2 also affected perithecia formation, as the Δsnt‐2 mutant strains were unable to produce mature perithecia, indicating the requirement of snt‐2 for sexual development in N. crassa.
Disruption of snt2 causes increased hyphal septation in FOM and N. crassa
In light of the fact that snt2 mutants exhibited slower linear growth, cell wall morphology was examined microscopically. The cell wall‐binding stain Congo red was employed for this purpose, enabling the detection of irregularities in hyphal septation. Distances between septa along the hyphal cells of the Δsnt2 FOM and Δsnt‐2 N. crassa mutants were markedly shorter than in the respective wild‐type strains (Fig. 5).
Figure 5.
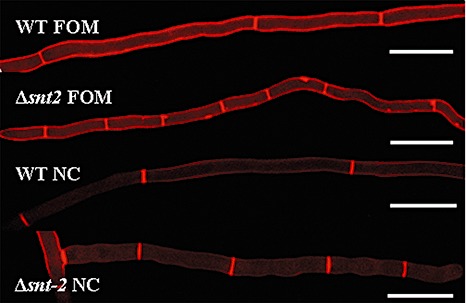
snt2 disruption leads to abnormalities of hyphal septation in Fusarium oxysporum f. sp. melonis (FOM) and Neurospora crassa (NC). Congo red staining detected irregularities in cell wall deposition and shorter hyphal segments between septa in the Δsnt2 mutant of FOM and the Δsnt2 mutant of NC, compared with their respective wild‐type strains. Bars indicate 50 µm.
In vivo viability staining detected increased cell death in Δsnt2 mutants of FOM and N. crassa
The first evidence of increased cell mortality caused by snt2 disruption was detected using Evans blue staining of FOM isolates (Fig. 6A–C), suggesting that disruption of snt2 leads to decreased cell viability. Furthermore, the ratios between stained and unstained cells in Δsnt2 of FOM and N. crassa were determined. Increased cell mortality was evident in the Δsnt2 mutants of both tested fungi, when compared with the wild‐type isolates (Fig. 7), thus confirming the requirement of snt2 for normal cell activities.
Figure 6.

Morphological abnormalities observed in snt2 mutants of Fusarium oxysporum f.sp. melonis (FOM). Comparative analysis between Evans blue‐stained mycelial cells of the wild‐type isolate (A) and snt2 mutant strains detected decreased viability of the cells in mutants D122 (B) and Δsnt2 (C) of FOM (areas marked by circles). Monodansylcadaverine (MDC) staining of autophagosomes detected significant differences between the wild‐type (D) and the mutant isolates D122 (E) and Δsnt2 (F). Formation of autophagosomes in the presence of phenylmethanesulphonylfluoride (PMSF) and stained by MDC in the wild‐type isolate (G), D122 (H) and Δsnt2 (I) mutant isolates. Elimination of MDC staining in PMSF‐treated wild‐type cells (J) after addition of the specific autophagosome inhibitor 3‐methyladenine (K). Bars in A–C indicate 50 µm, whereas those in D–K indicate 10 µm.
Figure 7.
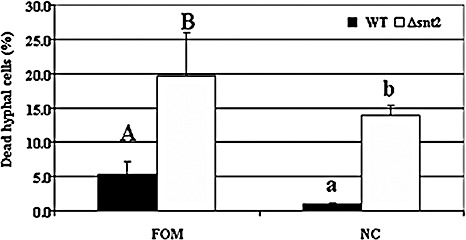
snt2 disruption leads to increased cell death in Fusarium oxysporum f.sp. melonis (FOM) and Neurospora crassa (NC). Percentage of dead cells in the Δsnt2 mutants of FOM and NC, compared with their respective wild‐type (WT) isolates. Results represent the mean of three independent experiments (each with 1000 cells observed), analysed separately for each fungal species by Least Significant Difference (LSD) Tukey‐Kramer multiple comparison test (P≤ 0.05); the levels of significance are marked with different letters.
Additional morphological abnormalities detected in F. oxysporum
To further explore the potential mechanism involved, cells were stained with the autophagosome‐detecting stain monodansylcadaverine (MDC), with and without the addition of the serine protease inhibitor phenylmethanesulphonylfluoride (PMSF), which has been shown to decrease the fast degradation of autophagosomes (Pollack et al., 2009). In the absence of PMSF, it was evident that mutant isolates D122 (Fig. 6E) and Δsnt2 (Fig. 6F) contained increased amounts of MDC‐stained organelles, presumably mature autophagosomes, when compared with the wild‐type isolate (Fig. 6D). The addition of PMSF enhanced the appearance of MDC‐stained organelles in all observed isolates (Fig. 6G–I). In order to verify the nature of these organelles, 3‐methyladenine (3‐MA), a specific inhibitor of autophagosome assembly (Pollack et al., 2009), was applied prior to MDC staining. As the presence of 3‐MA almost eliminated MDC staining (Fig. 6J), it is plausible to assume that the organelles observed were autophagosomes (Fig. 6K).
Identification of differential gene expression when FOM snt2 is impaired
Suppressive subtraction hybridization (SSH) was used to detect differentially expressed genes between the wild‐type and D122 isolates of FOM. Sequences of 200 random clones were analysed, identifying 14 putative genes. Over 15% of the transcript sequences analysed did not have matches in existing databases. Association between four of the SSH‐detected genes (idi4, induced during incompatibility; pdc, pyruvate decarboxylase; msf1, transporter of the major facilitator family; eEF1G, eukaryotic translation elongation factor 1‐γ) and the highly conserved TOR serine/threonine kinase pathway in the rice pathogen F. fujikuroi has been reported previously (Teichert et al., 2006). The TOR pathway controls numerous cellular processes, including the regulation of cell growth through nutrient sensing. Quantitative RT‐PCR was used to further investigate the expression of these genes. Two of the mentioned genes (idi4 and pdc) exhibited a significant change in transcript levels in the Δsnt2 mutant when compared with the wild‐type isolate (Table 2).
Table 2.
Expression profile of nitrogen metabolism‐related genes in the Δsnt2 mutant of Fusarium oxysporum f.sp. melonis (FOM).
| Gene | Accession number | Wild‐type/Δsnt2 expression ratio* | Description |
|---|---|---|---|
| idi4 † | FOXG_04081 | 24.5 ± 2.25+ | Induced during incompatibility |
| cpc1 | FOXG_05954 | 2.12 ± 0.09 | Cross‐pathway control |
| pdc † | BAE98181.1 | 20.78 ± 7.04+ | Pyruvate decarboxylase |
| fnr1 | DQ387858 | 1.2 ± 0.15 | Global nitrogen regulator |
| nit4 | FOXG_06396 | 0.95 ± 0.08 | Pathway‐specific nitrogen regulator |
| glnA | FOXG_05182 | 4.02 ± 0.50 | Glutamine synthetase |
| mfs1 † | FOXG_09760 | 1.60 ± 0.86 | Transporter of the major facilitator family |
| rbs1 † | FOXG_14252 | 0.15 ± 0.03‐ | Unknown functions |
| eEF1G † | FOXG_01492 | 2.72 ± 1.21 | Translation elongation factor |
cDNA was synthesized from 1 µg of total RNA. All samples were analysed in triplicate. Averaged crossing point values were normalized to the endogenous control gene β‐tubulin. Gene expression level and comparative analyses (*) of two biological and four technical repeats were evaluated using the REST© program (Pfaffl et al., 2002). Accession numbers were according to http://www.broad.mit.edu and National Center for Biotechnology Information (NCBI) databases. Statistically significant changes are marked by += up‐regulated and −= down‐regulated.
Identified by suppressive subtraction hybridization (SSH) screen.
The pdc gene encodes a key enzyme in pyruvate assimilation, and its expression was found to be negatively regulated by TOR kinase in F. fujikuroi. In this study, a 20.78 ± 7.04‐fold increase in pdc expression in Δsnt2 was identified, compared with the wild‐type isolate; however, in the D122 mutant, pdc expression did not differ significantly from that of the wild‐type (0.90 ± 0.13), even though this gene was detected in the suppression library of mutant isolate D122. We presume that this difference may be linked to the nature of the hphR cassette integrated site in the tagged D122 mutant. Another examined gene encoding a transporter of the major facilitator superfamily (mfs) is known to be negatively regulated by AreA (Schonig et al., 2008). However, its relative expression in the D122 and Δsnt2 mutant isolates was no different from that of the wild‐type isolate (1.32 ± 0.62‐ and 1.60 ± 0.86‐fold, respectively). Furthermore, expression of an additional SSH‐detected gene, eEF1G, encoding a translation elongation factor, which is negatively regulated by TOR kinase, was not significantly different in mutant isolate Δsnt2 (2.72 ± 1.21‐fold) when compared with the wild‐type isolate (Table 2).
In addition, a unique F. oxysporum gene (designated rbs1—regulated by snt2) was found. This gene encodes a 170‐amino‐acid protein with an as yet unknown function, and the only currently known homologue of this gene is a unique gene of FOL (FOXG_14252.2). rbs1 expression was significantly decreased in isolates D122 and Δsnt2 (−6.78 ± 0.37‐ and −8.47 ± 0.15‐fold, respectively) when compared with the wild‐type isolate.
Snt2 TF is involved in the regulation of the autophagy‐induced TF Idi4
On the basis of SSH analysis, disruption of snt2 led to over‐expression of the putative FOM idi4 gene. FOM Idi4 is similar (69%) to its P. anserina homologue IDI4, which has been shown to be involved in the regulation of autophagy (Dementhon et al., 2004). Using quantitative RT‐PCR analysis, significantly elevated idi4 expression was detected in D122 and Δsnt2 FOM mutants (20.53 ± 2.04‐ and 24.5 ± 2.25‐fold, respectively). This was also observed in N. crassa, where an increase in idi4 expression (8.36 ± 0.65) was detected in the Δsnt2 strain, suggesting a common regulatory pathway in the two fungal species.
Moreover, a significant increase in the presence of mature autophagosomes was detected in mutant D122 and Δsnt2 isolates by MDC staining, as well as increased amounts of nonvital cells in both D122 and Δsnt2, compared with the wild‐type isolate of FOM (Fig. 6D–K). To detect whether snt2 is involved in direct regulation of the autophagy process, the expression of a highly conserved gene, idi7/agt8, which regulates the initial steps of nonselective autophagy, was examined (Levine and Klionsky, 2004). Its relative expression in the Δsnt2 and D122 isolates (1.07 ± 0.55‐ and 0.59 ± 0.35‐fold, respectively) was not significantly different from that of the wild‐type; therefore, it is likely that snt2 is not directly involved in the regulation of nonselective autophagy.
To investigate a possible involvement of snt2 in nitrogen metabolism and to detect its possible connection with the TOR kinase pathway, the effect of the specific TOR inhibitor rapamycin on the utilization of different sole sources of nitrogen (nitrate, ammonia and glutamate) was examined. During growth on these three nitrogen sources, both snt2 mutants grew in an apparently indistinguishable manner compared with the wild‐type isolate. However, closer examination of radial growth showed that, initially, glutamate slightly delayed the vegetative growth of both snt2 mutants in the absence of rapamycin and, secondly, the addition of rapamycin did not delay nitrogen utilization of the Δsnt2 isolate, as opposed to the apparently normal growth of the tagged transformant D122 (data not shown). As demonstrated previously, both snt2 and areA appear to regulate the expression of at least two common genes (idi4 and pdc); therefore, we examined the transcript levels of three master nitrogen regulators [fnr1 (a homologue of areA in F. oxysporum), nit4 (the homologue of a pathway‐specific nitrogen regulator of N. crassa) and glnA (glutamine synthetase)] as well as the cpc1 (cross‐pathway control 1) gene, which regulates fungal amino acid biosynthesis and is negatively regulated by AreA (Schonig et al., 2008). Unexpectedly, the expression of these genes did not differ significantly in the Δsnt2 mutant when compared with the wild‐type isolate (Table 2). The growth of both mutant isolates was also not affected by 3‐aminotriazole (3‐AT), which induces the amino acid starvation response (data not shown) together with the expression of cpc1, supporting the results obtained by quantitative RT‐PCR. Moreover, all the examined isolates were unable to utilize chlorate (6%) and did not grow on hypoxanthine (0.05%), indicating the absence of defects in the nitrate reduction pathway. On the basis of these results, it is likely that snt2 is not necessary for nitrogen utilization.
DISCUSSION
In this study, we analysed the genetic and phenotypic nature of the FOM mutant (D122) isolated on the basis of its reduced pathogenicity.
The D122 mutant was found to be disrupted in the snt2 gene encoding a protein homologous to S. pombe Snt2p. Snt2p is a functional part of the Lid2p complex, which is only found in fission, but not budding, yeast, and is thought to be involved in the regulation of chromatin remodelling (Roguev et al., 2004). The annotation of snt2 identified several differences between the sequence in FOM and its closest relative FOL, expressed in changes in the Snt2 domain structures, probably as a result of errors in the computational annotation of the FOL gene (annotation problems may also have affected the F. graminearum snt2 homologue gene call; such discrepancies may be resolved once appropriate cDNAs are analysed in different Fusarium species). The Snt2 protein of FOM carries five conserved domains: BAH, three PHDs and a GATA‐type Zn finger. A multiple alignment analysis of Snt2 demonstrated a high degree of identity (63%–96%) among annotated Fusarium species, as well as between FOM and fungi from different genera (Fig. 2). A high degree of similarity in Snt2 architecture was observed between Snt2 proteins of FOM and the plant and human pathogens Ustilago maydis (XP_761724), Candida albicans (XP_716567) and ZNF1 (AAN75722) of the human pathogen Cryptococcus neoformans, involved in the pheromone response pathway (Lengeler et al., 2002). Most of the examined Snt2 proteins are characterized by the presence of the SANT domain, known to be an essential part of chromatin remodelling enzymes (Boyer et al., 2002). However, it is absent from the predicted proteins of Fusarium, C. neoformans and S. pombe. In addition, the presence of the GATA‐type Zn finger, which is involved in gene expression regulation by binding promoters of various target genes, including nitrogen catabolite repression, was detected in three annotated plant pathogenic fungi, namely F. oxysporum, Botrytis cinerea and Sclerotinia sclerotiorum. Interestingly, another PHD‐harbouring TF of F. oxysporum, FoCti6, has recently been found to be involved in fungal pathogenesis (Michielse et al., 2009). Similar to Snt2, Cti6 has also been found to participate in chromatin modifications.
In this study, we performed the first targeted disruption of an snt2 TF‐encoding gene in a filamentous fungus, which was not analysed in a previous high‐throughput gene knockout of N. crassa TF‐encoding genes (Colot et al., 2006). An unexpectedly low gene targeting efficiency (1%) was encountered in this study, even though the use of ATMT for targeted disruption has been reported to result in higher gene targeting efficiencies, ranging from 14% to 75% in different fungi, including F. oxysporum (Michielse et al., 2005). These observations could be the result of either a general difficulty in targeted integration at the snt2 locus or a significantly slower growth rate of mutant isolates compared with ectopic transformants. Remarkably, targeted disruption of snt2 was possible only with the GV3101 strain of A. tumefaciens, but not with the EHA105 strain. These data are consistent with previously reported research demonstrating that, in general, the EHA105 Agrobacterium strain is less suitable for targeted disruption in F. oxysporum, because of a relatively high percentage of false positive transformants (Khang et al., 2005).
snt2 was only expressed during the primary invasive growth and colonization stages of Fusarium wilt disease, supporting the observed delay in the appearance of the first symptoms in both snt2 impaired mutants. At a later stage, disease progress differed between the D122 and Δsnt2 mutants, whereby D122 caused significantly less plant mortality. The success of fungal pathogen attack frequently depends on the colonization rate of the inoculated host. In the case of FOM, the wild‐type strain caused nearly 100% mortality in inoculated plants within 1 week. However, both of the snt2 mutants did not colonize the lower and upper stem sections, in contrast with the wild‐type, and rarely conferred plant mortality.
A comparative phenotypic characterization of Δsnt2 mutants of the two Ascomycota fungi, FOM and N. crassa, indicated an association of the snt2 gene with numerous developmental aspects in the tested fungi. Interestingly, in both fungi, the disruption of snt2 caused similar sets of morphological abnormalities, including reduction in conidial production, slower vegetative growth and reduced biomass accumulation. Moreover, abnormalities were also observed in hyphal septation and in Congo red staining patterns in both FOM and N. crassa. Similar morphological abnormalities were observed in Podospora anserina during processes such as vegetative incompatibility, starvation or following treatment with a specific inhibitor of TOR kinase, rapamycin (Pinan‐Lucarréet al., 2007). An additional trait, linking snt2 to sexual development, was observed in Δsnt‐2 N. crassa mutants, which produced only protoperithecia that were unable to mature. On the basis of these results, it is suggested that snt2 functions are conserved among Ascomycota and are essential for normal fungal development. As the involvement of the F. graminearum mgv1gene in female fertility and pathogenesis has been demonstrated (Hou et al., 2002), and in the light of our data, it would be interesting to study the effect of snt2 disruption on the pathogenicity of the head blight fungus.
In order to detect potential target genes of the Snt2 TF and to elucidate its mode of action, we combined an SSH technique with subsequent quantitative RT‐PCR analysis of selected genes in both the random (D122) and targeted (Δsnt2) mutants, compared with the wild‐type isolate. Recovery of idi4, homologous to an autophagy regulator gene of P. anserina, in the suppression library was of interest, as one of the striking traits of the FOM snt2 mutants was the increased presence of mature autophagosomes (as detected by MDC staining). As expected, the expression of idi4 was significantly higher in both the D122 and Δsnt2 mutants compared with the wild‐type isolate. Moreover, a significant increase in expression of the idi4 homologue of N. crassa (NCU08055.4) was also detected in the respective Δsnt2 strain, suggesting a common regulatory pathway in the two fungal species.
Furthermore, it was demonstrated that the over‐expression of idi4 led to irregularity in cell wall deposition and a reduced growth rate in P. anserina (Dementhon et al., 2004).
In this study, it was shown that, in both FOM and N. crassa, disruption of the snt2 gene led to similar phenotypical abnormalities, accompanied by idi4 over‐expression. Likewise, it was demonstrated in P. anserina that the expression of idi4 was up‐regulated as a response to a variety of stress signals, such as oxidative, osmotic stresses, starvation and autophagy (Dementhon et al., 2004). Therefore, it is conceivable that the disruption of snt2 generates a stressful intracellular environment which triggers idi4 expression.
The autophagy process is a survival mechanism, which enables the recycling of damaged organelles or cells and supports the delicate balance between the life and death of an organism. However, autophagy has been studied extensively mainly in yeasts and mammals. In filamentous fungi, the involvement of autophagy in pathogenesis and, in particular, its regulation remain unclear (Pollack et al., 2009). Therefore, it is interesting that snt2 disruptants of FOM displayed increased cell death (evident by Evans blue staining), indicating a shift in this equilibrium, implying that snt2, although not essential, is important for fungal survival.
In this study, four genes (i.e. idi4, pdc, mfs and eEF1G) were detected using the SSH method, and were found to be regulated by TOR kinase in the rice pathogen F. fujikuroi (Teichert et al., 2006). Furthermore, in F. fujikuroi, control of the AreA global nitrogen regulator via the TOR pathway may inhibit the expression of idi4 (Schonig et al., 2008). TOR kinase plays an important and key role in autophagy regulation by linking nutrient sensing to cellular growth through histone acetylation (Rohde and Cardenas, 2003). An additional gene, pdc, involved in alcoholic fermentation, was found to be redundant in the suppression library (14%). When analysed by quantitative RT‐PCR, its expression was found to be increased in the targeted Δsnt2 mutant, compared with the wild‐type isolate. In F. fujikuroi, pdc was also shown to be down‐regulated by TOR kinase (Teichert et al., 2006). In Aspergillus nidulans, PDC is required for anaerobic survival of the fungus and its expression is negatively regulated by AreA (Lockington et al., 1997). Nevertheless, the roles of genes involved in anaerobic pyruvate assimilation and associated with pathogenicity and nitrogen metabolism are not yet clear (Bolton and Thomma, 2008). In this study, we have provided additional data supporting the probable association of pyruvate assimilation with fungal pathogenesis. These data led us to explore the possibility of Snt2 involvement in nitrogen utilization. However, no visual phenotypic difference was found when the FOM D122 and Δsnt2 mutants were cultured on different sole nitrogen sources. Nevertheless, a significant difference in biomass accumulation, during nutrient‐limiting growth conditions, may indicate a general nutrient‐sensing problem. This hypothesis is supported by the evaluation of the expression of several genes involved in nitrogen metabolism (e.g. nit4, glnA, fnr1) in the D122 and Δsnt2 mutants, which showed no difference from the wild‐type isolate.
Hence, we conclude that Snt2 is a novel fungal TF, required for invasive growth, initial symptom development and complete pathogenicity of FOM. Snt2 is also required for the maintenance of normal morphology of at least two fungal species—FOM and N. crassa. Overall, the up‐regulation of the autophagy‐related TF‐coding idi4 gene in both FOM and N. crassa links snt2 to a variety of cellular processes and is indicative of the existence of a novel, probably conserved, regulatory mechanism. Nonetheless, at this stage, more components of the Snt2 regulatory network need to be identified. In this study, we detected a unique F. oxysporum gene (rbs1), with unknown function, which appears to be up‐regulated by snt2. Furthermore, our SSH screen results indicate that the expression of additional genes, yet to be analysed, is altered in association with this network. Finally, it is tempting to speculate that the mode of action of Snt2 in F. oxysporum may also involve gene expression regulation at an epigenetic level, similar to its homologue in the Lid2p complex of S. pombe (Li et al., 2008).
Nonselective autophagy and nitrogen metabolism have been shown to be connected and tightly regulated by TOR kinase (Klionsky and Emr, 2000; Teichert et al., 2006). snt2 function appears to be linked to autophagosome abundance, but not to nitrogen metabolism. The fact that SNT2 and TOR kinase share common pathway components (idi4 and pdc) supports the presence of an overlapping regulatory mechanism in maintaining cell survival modules.
EXPERIMENTAL PROCEDURES
Fungal strains and culture conditions
A wild‐type strain of FOM (race 1,2) (Cohen et al., 1989) was used throughout this study. Neurospora crassa mutant strains were obtained from the Fungal Genetics Stock Center (Kansas, MO, USA) (McCluskey, 2003). Routinely, FOM isolates were grown in Petri dishes containing potato dextrose agar (PDA) (Difco, Detroit, MI, USA) amended with chloramphenicol (Sigma, St. Louis, MO, USA) (0.25 mg/L). Transformants were maintained on PDA medium containing 50 mg/mL hygromycin B (Cayla, Toulouse, France). N. crassa isolates were maintained on Vogel's minimal medium (Davis and de Serres, 1970) and crosses were performed on cornmeal agar (CMA). All fungal isolates were purified as single spore cultures and stored at −80 °C in a 30% (v/v) glycerol solution. For Fusarium conidia production or DNA extraction, plugs of mycelia were cultured on Fusarium liquid culture (FLC) medium (Freeman et al., 2001) in 250‐mL flasks with or without shaking (at 110 rpm), respectively. For growth rate analyses, radial growth was measured on Fusarium minimal medium (FMM) (Correll et al., 1987) with required amendments. For nitrogen utilization assessment, synthetic medium (SM) (Di Pietro and Roncero, 1996) was amended with different sole nitrogen sources at a concentration of 10 mm. The TOR kinase inhibitor, rapamycin (LC Laboratories, Woburn, MA, USA), was used at a concentration of 500 ng/mL.
Plant material and inoculation
A cultivar of muskmelon (cv. Ein Dor), susceptible to race 1,2 of FOM, which was used throughout this study, was a gift from Zeraim Gedera (Gedera, Israel). Plant inoculation assays were conducted in a glasshouse under controlled conditions at 25 ± 1°C and a 16‐h light photoperiod (Freeman et al., 2001). Two‐week‐old muskmelon plants were inoculated by dipping roots for 30 min in conidial suspensions (1.5 × 106 or 5 × 105 conidia/mL), followed by transfer to water or soil medium.
Statistical analysis
All experiments were conducted at least three times. Data (apart from the results of real‐time PCR) were analysed using JMP software (version 3.2.6; SAS Institute, Inc., Cary, NC, USA). All values were subjected to analysis of variance (anova), and then mean comparisons of calculated values were performed and analysed using least‐significant difference (LSD) analysis according to the Tukey–Kramer multiple comparison test (P≤ 0.05).
Nucleic acid manipulations
Genomic DNA was extracted from mycelia as described previously (Moller et al., 1992). For Southern hybridization, DNA was treated with appropriate restriction enzymes and analysed following standard protocols (Sambrook et al., 1989) using a digoxigenin‐based labelling kit (Roche, Mannheim, Germany). The complete sequence of the snt2 gene was obtained using the Genome Walking Kit (Clontech Laboratories, Mountain View, CA, USA) and wild‐type DNA as a template, followed by manual annotation. Prior to total RNA extraction, harvested samples were treated with RNAlater® solution (Ambion Inc., Austin, TX, USA) and stored at −80 °C. Total RNA was extracted using the SV RNA Isolation System Kit (Promega, Madison, WI, USA). mRNA was purified using DynaBeads® (Invitrogen, Carlsbad, CA, USA). Details on the primers used throughout this research are provided in Table S1 (see Supporting Information).
Tagged and targeted mutagenesis of FOM and construction of the snt2 disruption vector
Tagged mutagenesis of FOM was performed by ATMT using plasmids pBHt2 and pKHt kindly provided by S. Kang (Department of Plant Pathology, Pennsylvania State University, University Park, PA, USA). GV3101 and EHA105 strains of A. tumefaciens were used throughout this research. Transformation of FOM was carried out as described previously (Mullins et al., 2001) with the following modifications: 200 µL of a mixture of bacterial cells [optical density at 600 nm (OD600) = 0.3] and conidia (105 conidia/mL) were plated directly on 12 mL of co‐cultivation medium, followed by incubation at 25 °C for 48 h. Twelve millilitres of selection medium amended with 100 µg/mL hygromycin B and cefotaxime (Sanofi Aventis Laboratory, Paris, France) were overlaid on each plate and incubated at room temperature for 5–7 days. Transformants with resistance to hygromycin were maintained on PDA plates amended with hygromycin B (75 µg/mL). A collection of 2000 mitotically stable transformants was screened for pathogenicity on muskmelon plants in order to detect reduced‐pathogenicity isolates. Detection of inserts in ATMT‐generated transformants was performed using TAIL‐PCR, following standard protocols (Mullins et al., 2001).
The vector for snt2‐targeted disruption (pDΔsnt2) was constructed as follows. The fungal transformation vector ks:hph (Horowitz et al., 2006), containing the Escherichia coli hygromycin phosphotransferase (hph) gene regulated by the promoter from the trpC gene of Aspergillus nidulans, was used as the source of the HygB resistance cassette. A 1.7‐kb fragment of snt2 was amplified with primers homFGF1‐SdaI/homFGR1‐MunI, introducing SdaI and MunI cloning sites. This segment was cloned into pGEM‐T Easy (Promega) and its 335‐bp inner fragment was replaced with a 2.13‐kb hphB/trpC cassette from the vector ks:hph, using Acc65I/BglII restriction. The resulting 3.8‐kb snt2::hphR SdaI/MunI‐digested construct was transferred into EcoRI/PstI‐digested pDHt (Mullins et al., 2001). The targeted gene disruption event was confirmed by Southern blot analysis on Acc65I/Bsp1407I‐digested genomic DNA using an inHPH‐rev2/homFG1R (Table S1) PCR‐amplified 1.4‐kb fragment as a probe.
SSH
SSH (Diatchenko et al., 1996) was carried out using the Clontech PCR Select‐Subtraction cDNA Kit (Clontech). For subtracted cDNA library construction, 500‐mL flasks containing 25 mL of FMM were inoculated with the wild‐type and D122 strains at concentrations of 106 conidia/mL and incubated for 14 h on an orbital shaker at 25 °C and 100 rpm. Germinated conidia were harvested by centrifugation at 12 000 g for 15 min. The ‘tester’ sample cDNA isolated from germinated wild‐type conidia, and the ‘driver’ sample cDNA isolated from germinated D122 strain conidia, were cloned into pGEM‐T Easy (Promega) and sequenced (Macrogen Inc., Seoul, South Korea). The sequences obtained were used to search the Fusarium group genome database (http://www.broad.mit.edu/annotation/fgi/) and the National Center for Biotechnology Information (NCBI) database.
Gene expression analysis by quantitative RT‐PCR and RT‐PCR
Total RNA was extracted from cultured mycelia, germinated conidia of FOM and/or infected muskmelon seedlings. Reverse transcription was carried out on 1 µg of total RNA treated by Turbo DNase (Ambion Inc.) using Verso™ RTase (ABgene, Epsom, UK) with an anchored oligo‐dT primer. cDNA was stored at −20 °C. For RT‐PCR experiments, amplification was performed in 25‐µL reactions with the following components: 5 µL cDNA (from the reaction mentioned above), 2.5 U Taq DNA polymerase, X1 Taq polymerase buffer, 0.2 mm deoxynucleoside triphosphates (dNTPs) and sense and anti‐sense primers at final concentrations of 0.5 µm. All RNA samples were treated by DNase prior to further manipulations. Transcript levels of the selected fungal genes were analysed by quantitative RT‐PCR using cDNA dilutions as templates for calibration curves. snt2 expression in planta was evaluated by quantitative RT‐PCR analyses using mRNA extracted from inoculated and noninoculated muskmelon seedlings at 24‐h intervals from 1 to 5 dpi. cDNA obtained at 2 dpi was used as a reference (at 1 dpi no expression of the reference fungal ‘housekeeping’ gene was detected). Quantitative RT‐PCRs were carried out in 15‐µL volumes using 4 µL of cDNA, sense and anti‐sense primers at final concentrations of 0.3 µm and X1 DyNamo Flash Master SYBR Green Mix (FinnEnzymes, Espoo, Finland). Quantitative RT‐PCR amplification was performed using a Rotor‐Gene 3000 machine (Corbett Research, Sydney, Australia). Relative quantification of a target transcript was analysed using the REST‐2005© program, which is based on the mean crossing point (CP) deviation of the control and sample group, normalized by a reference (β‐tubulin) transcript (Pfaffl et al., 2002). A Pair Wise Fixed Reallocation Randomization Test© was used to analyse the mean of at least two biological and four technical repeats with standard error, detecting the relative quantification as well as the level of significance.
Microscopy
For microscopic analyses, mycelium was grown on FMM medium for 48 h. The mycelium was examined using an inverted laser scanning confocal microscope (Olympus IX 81, Tokyo, Japan). Specific staining of mature autophagosomes was performed with 0.05 mm monodansylcadaverine (MDC) (Biederbick et al., 1995) with or without the addition of 1 µm of the serine protease inhibitor PMSF (Pollack et al., 2009). The specific autophagosome inhibitor 3‐MA (Pollack et al., 2009) was applied at a concentration of 1 mm. For vitality staining, 0.1% Evans blue dye was applied (Dementhon et al., 2003). Cell wall abnormalities were detected by Congo red staining. The mycelium was overlaid with 0.1% (w/v) Congo red solution in 150 mm NaCl for 2 min and rinsed with 150 mm NaCl.
Supporting information
Fig. S1 The snt2 strain of Neurospora crassa is impaired in aerial hyphae production and conidiation. On solid medium, the Δsnt2 strain (B) developed thicker, slower growing mycelium, with few aerial hyphae, reduced numbers of conidia and therefore a less pronounced orange‐coloured culture, compared with the corresponding wild‐type isolate (A).
Table S1 Primers used in this study.
Supporting info item
Supporting info item
ACKNOWLEDGEMENTS
We thank Eduard Belausov for assistance with microscopy, Seogchan Kang for providing the pDHt, pBHt2 and pKHt plasmids, and Zeraim Gedera (Israel) for cv. Ein Dor muskmelon seeds. YD is a recipient of a fellowship from the Israeli Phytopathological Society.
Nucleotide sequence data reported here are available in the GenBank database under accession numbers HM246662 (snt2) and HM246663 (rbs1).
REFERENCES
- Biederbick, A. , Kern, H.F. and Elsässer, H.P. (1995) Monodansylcadaverine (MDC) is a specific in vivo marker for autophagic vacuoles. Eur. J. Cell Biol. 66, 3–14. [PubMed] [Google Scholar]
- Bolton, M.D. and Thomma, B.P.H.J. (2008) The complexity of nitrogen metabolism and nitrogen‐regulated gene expression in plant pathogenic fungi. Physiol. Mol. Plant Pathol. 72, 101–110. [Google Scholar]
- Boyer, L.A. , Langer, M.R. , Crowley, K.A. , Tan, S. , Denu, J.M. and Peterson, C.L. (2002) Essential role for the SANT domain in the functioning of multiple chromatin remodeling enzymes. Mol. Cell, 10, 935–942. [DOI] [PubMed] [Google Scholar]
- Calero‐Nieto, F. , Di Pietro, A. , Roncero, M.I.G. and Hera, C. (2007) Role of the transcriptional activator XlnR of Fusarium oxysporum in regulation of xylanase genes and virulence. Mol. Plant–Microbe Interact. 20, 977–985. [DOI] [PubMed] [Google Scholar]
- Campanella, J.J. , Bitincka, L. and Smalley, J. (2003) MatGAT: an application that generates similarity/identity matrices using protein or DNA sequences. BMC Bioinformatics, 4, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caracuel, Z. , Roncero, M.I.G. , Espeso, E.A. , Gonzalez‐Verdejo, C.I. , Garcia‐Maceira, F.I. and Di Pietro, A. (2003) The pH response transcription factor PacC controls virulence in the plant pathogen Fusarium oxysporum . Mol. Microbiol. 48, 765–779. [DOI] [PubMed] [Google Scholar]
- Cohen, R. , Katan, T. , Katan, J. and Cohn, R. (1989) Occurrence of Fusarium oxysporum f. sp. melonis race 1,2 on muskmelon in Israel. Phytoparasitica, 17, 319–322. [Google Scholar]
- Colot, H.V. , Park, G. , Turner, G.E. , Ringelberg, C. , Crew, C.M. , Litvinkova, L. , Weiss, R.L. , Borkovich, K.A. and Dunlap, J.C. (2006) A high‐throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc. Natl. Acad. Sci. USA, 103, 10 352–11 057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll, J.C. , Klittich, C.J.R. and Leslie, J.F. (1987) Nitrate non‐utilizing mutants of Fusarium oxysporum and their use in vegetative compatibility tests. Phytopathology, 77, 1640–1646. [Google Scholar]
- Davis, R.H. and de Serres, F.J. (1970) Genetic and microbiological research techniques for Neurospora crassa. Methods Enzymol. 27, 79–143. [Google Scholar]
- Dementhon, K. , Paoletti, M. , Pinan‐Lucarre, B. , Loubradou‐Bourges, N. , Sabourin, M. , Saupe, S.J. and Clave, C. (2003) Rapamycin mimics the incompatibility reaction in the fungus Podospora anserina . Eukaryot. Cell, 2, 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dementhon, K. , Saupe, S.S. and Clave, C. (2004) Characterization of IDI‐4, a b‐ZIP transcription factor inducing autophagy and cell death in the fungus Podospora anserina . Mol. Microbiol. 53, 1625–1640. [DOI] [PubMed] [Google Scholar]
- Denisov, Y. , Yarden, O. and Freeman, S. (2005) Impaired purine biosynthesis affects pathogenicity of Fusarium oxysporum f. sp. melonis . Eur. J. Plant Pathol. 112, 293–297. [Google Scholar]
- Di Pietro, A. and Roncero, M.I.G. (1996) Endopolygalacturonase from Fusarium oxysporum f.sp. lycopersici: purification, characterization, and production during infection of tomato plants. Phytopathology, 86, 1324–1330. [Google Scholar]
- Di Pietro, A. , Garcia‐Maceira, F.I. , Meglecz, E. and Roncero, M.I.G. (2001) A MAP kinase of the vascular wilt fungus Fusarium oxysporum is essential for root penetration and pathogenesis. Mol. Microbiol. 39, 1140–1152. [PubMed] [Google Scholar]
- Diatchenko, L. , Lau, Y.‐F.C. , Campbell, A.P. , Chenchik, A. , Moqadam, F. , Huang, B. , Lukyanov, S. , Lukyanov, K. , Gurskaya, N. , Sverdlov, E.D. and Siebert, P.D. (1996) Suppression subtractive hybridization: a method for generating differentially regulated or tissue‐specific cDNA probes and libraries. Proc. Natl. Acad. Sci. USA, 93, 6025–6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divon, H.H. , Ziv, C. , Davydov, O. , Yarden, O. and Fluhr, R. (2006) The global nitrogen regulator, FNR1, regulates nitrogen‐genes and fitness during Fusarium oxysporum pathogenesis. Mol. Plant–Microbe Interact. 7, 485–497. [DOI] [PubMed] [Google Scholar]
- Dufresne, M. , Bailey, J.A. , Dron, M. and Langin, T. (1998) Clk1, a serine/threonine protein kinase‐encoding gene, is involved in pathogenicity of Colletotrichum lindemuthianum on common bean. Mol. Plant–Microbe Interact. 11, 99–108. [DOI] [PubMed] [Google Scholar]
- Duyvesteijn, R.G.E. , Wijk, R. , van Boer, Y. , Rep, M. , Cornelissen, B.J.C. and Haring, M.A. (2005) Frp1 is a Fusarium oxysporum F‐box protein required for pathogenicity on tomato. Mol. Microbiol. 57, 1051–1063. [DOI] [PubMed] [Google Scholar]
- Freeman, S. , Zveibil, A. , Vintal, H. and Maymon, M. (2001) Isolation of nonpathogenic mutants of Fusarium oxysporum f. sp. melonis for biocontrol of Fusarium wilt in cucurbits. Phytopathology, 92, 164–168. [DOI] [PubMed] [Google Scholar]
- Horowitz, S. , Freeman, S. , Zveibil, A. and Yarden, O. (2006) A defect in nir1, a nirA‐like transcription factor, confers morphological abnormalities and loss of pathogenicity in Colletotrichum acutatum . Mol. Plant Pathol. 7, 341–354. [DOI] [PubMed] [Google Scholar]
- Hou, Z. , Xue, C. , Peng, Y. , Katan, T. , Kistler, H.C. and Xu, J.R. (2002) A mitogen‐activated protein kinase gene (MGV1) in Fusarium graminearum is required for female fertility, heterokaryon formation, and plant infection. Mol. Plant–Microbe Interact. 15, 1119–1127. [DOI] [PubMed] [Google Scholar]
- Imazaki, I. , Kurahashi, M. , Iida, Y. and Tsuge, T. (2007) Fow2, a Zn(II)2Cys6‐type transcription regulator, controls plant infection of the vascular wilt fungus Fusarium oxysporum . Mol. Microbiol. 63, 737–753. [DOI] [PubMed] [Google Scholar]
- Inoue, I. , Namiki, F. and Tsuge, T. (2002) Plant colonization by the vascular wilt fungus Fusarium oxysporum requires FOW1, a gene encoding a mitochondrial protein. Plant Cell, 14, 1869–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khang, C.H. , Park, S.Y. , Lee, Y.H. and Kang, S.C. (2005) A dual selection based, targeted gene replacement tool for Magnaporthe grisea and Fusarium oxysporum . Fungal Genet. Biol. 42, 483–492. [DOI] [PubMed] [Google Scholar]
- Kikuma, T. , Ohneda, M. , Arioka, M. and Kitamoto, K. (2006) Functional analysis of the ATG8 homologue Aoatg8 and role of autophagy in differentiation and germination in Aspergillus oryzae . Eukaryot. Cell 8, 1328–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. and Woloshuk, C.P. (2008) Role of AREA, a regulator of nitrogen metabolism, during colonization of maize kernels and fumonisin biosynthesis in Fusarium verticillioides . Fungal Genet. Biol. 45, 947–953. [DOI] [PubMed] [Google Scholar]
- Klionsky, D.H. and Emr, S.D. (2000) Autophagy as a regulated pathway of cellular degradation. Science, 290, 1717–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler, K.B. , Fox, D.S. , Fraser, J.A. , Allen, A. , Forrester, K. , Dietrich, F.S. and Heitman, J. (2002) Mating‐type locus of Cryptococcus neoformans: a step in the evolution of sex chromosomes. Eukaryot. Cell, 1, 704–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, B. and Klionsky, D.J. (2004) Development by self‐digestion: molecular mechanisms and biological functions of autophagy. Dev. Cell, 6, 463–477. [DOI] [PubMed] [Google Scholar]
- Li, F. , Huarte, M. , Zaratiegui, M. , Vaughn, M.W. , Shi, Y. , Martienssen, R. and Cande, W.Z. (2008) Lid2 is required for coordinating H3K4 and H3K9 methylation of heterochromatin and euchromatin. Cell, 135, 272–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X.H. , Lu, J.P. and Lin, F.C. (2007) Autophagy during conidiation, conidial germination and turgor generation in Magnaporthe grisea . Autophagy, 3, 472–473. [DOI] [PubMed] [Google Scholar]
- Lockington, R.A. , Borlace, G.N. and Kelly, J.M. (1997) Pyruvate decarboxylase and anaerobic survival in Aspergillus nidulans . Gene, 191, 61–67. [DOI] [PubMed] [Google Scholar]
- Ma, L.J. , van der Does, H.C. , Borkovich, K.A. , Coleman, J.J. , Daboussi, M.J. , Di Pietro, A. , Dufresne, M. , Freitag, M. , Grabherr, M. , Henrissat, B. , Houterman, P.M. , Kang, S. , Shim, W.B. , Woloshuk, C. , Xie, X. , Xu, J.R. , Antoniw, J. , Baker, S.E. , Bluhm, B.H. , Breakspear, A. , Brown, D.W. , Butchko, R.A. , Chapman, S. , Coulson, R. , Coutinho, P.M. , Danchin, E.G. , Diener, A. , Gale, L.R. , Gardiner, D.M. , Goff, S. , Hammond‐Kosack, K.E. , Hilburn, K. , Hua‐Van, A. , Jonkers, W. , Kazan, K. , Kodira, C.D. , Koehrsen, M. , Kumar, L. , Lee, Y.H. , Li, L. , Manners, J.M. , Miranda‐Saavedra, D. , Mukherjee, M. , Park, G. , Park, J. , Park, S.Y. , Proctor, R.H. , Regev, A. , Ruiz‐Roldan, M.C. , Sain, D. , Sakthikumar, S. , Sykes, S. , Schwartz, D.C. , Turgeon, B.G. , Wapinski, I. , Yoder, O. , Young, S. , Zeng, Q. , Zhou, S. , Galagan, J. , Cuomo, C.A. , Kistler, H.C. and Rep, M. (2010) Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature, 464, 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluskey, K. (2003) The Fungal Genetics Stock Center: from molds to molecules. Adv. Appl. Microbiol. 52, 246–262. [DOI] [PubMed] [Google Scholar]
- Michielse, C.B. and Rep, M. (2009) Pathogen profile update: Fusarium oxysporum . Mol. Plant Pathol. 10, 311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michielse, C.B. , Hooykaas, P.J.J. , Hondel, C.A.M.J.J. and Ram, A.F. (2005) Agrobacterium‐mediated transformation as a tool for functional genomics in fungi. Curr. Genet. 48, 1–17. [DOI] [PubMed] [Google Scholar]
- Michielse, C.B. , van Wijk, R. , Reijnen, L. , Cornelissen, B.J.C. and Rep, M. (2009) Insight into the molecular requirements for pathogenicity of Fusarium oxysporum f. sp. lycopersici through large‐scale insertional mutagenesis. Genom. Biol. 10, R4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller, E.M. , Bahnweg, G. , Sanderman, H. and Geiger, H.H. (1992) A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies and infected plant tissues. Nucleic Acids Res. 20, 6115–6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins, E.D. , Chen, X. , Romanie, P. , Raina, R. , Geiser, D.M. and Kang, S. (2001) Agrobacterium‐mediated transformation of Fusarium oxysporum: an efficient tool for insertional mutagenesis and gene transfer. Phytopathology, 91, 173–180. [DOI] [PubMed] [Google Scholar]
- Namiki, F. , Matsunaga, M. , Okuda, M. , Inoue, I. , Fujita, Y. and Tsuge, T. (2001) Mutation of an arginine biosynthesis gene causes reduced pathogenicity in Fusarium oxysporum f. sp. melonis . Mol. Plant–Microbe Interact. 14, 580–584. [DOI] [PubMed] [Google Scholar]
- Nelson, P.E. , Toussoun, T.A. and Cook, R.J. eds. (1981) Fusarium: Disease, Biology and Taxonomy. University Park, Pennsylvania: The Pennsylvania State University Press. 457 pp. [Google Scholar]
- Pfaffl, M.W. , Horgan, G.W. and Demfie, L. (2002) Relative expression software tool (REST©) for group‐wise comparison and statistical analysis of relative expression results in real‐time PCR. Nucleic Acids Res. 30, 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinan‐Lucarre, B. , Paoletti, M. , Dementhon, K. , Coulary‐Salin, B. and Clave, C. (2003) Autophagy is induced during cell death by incompatibility and is essential for differentiation in the filamentous fungus Podospora anserina . Mol. Microbiol. 47, 321–333. [DOI] [PubMed] [Google Scholar]
- Pinan‐Lucarré, B. , Paoletti, M. and Clave, C. (2007) Cell death by incompatibility in the fungus Podospora . Semin. Cancer Biol. 17, 101–111. [DOI] [PubMed] [Google Scholar]
- Pollack, J.K. , Harris, S.D. and Marten, M.R. (2009) Autophagy in filamentous fungi. Fungal Genet. Biol. 46, 1–8. [DOI] [PubMed] [Google Scholar]
- Ramos, B. , Alves‐Santos, F.M. , García‐Sanchez, M.A. , Martín‐Rodrigues, N. , Eslava, A.P. and Díaz‐Mínguez, J.M. (2007) The gene coding for a new transcription factor (ftf1) of Fusarium oxysporum is only expressed during infection of common bean. Fungal Genet. Biol. 44, 864–876. [DOI] [PubMed] [Google Scholar]
- Rep, M. , Meijer, M. , Houterman, P.M. , van der Does, H.C. and Cornelissen, B.J.C. (2005) Fusarium oxysporum evades I‐3‐mediated resistance without altering the matching avirulence gene. Mol. Plant–Microbe Interact. 18, 15–23. [DOI] [PubMed] [Google Scholar]
- Roguev, A. , Shevchenko, A. , Schaft, D. , Thomas, H. , Stewart, A.F. and Shevchenko, A. (2004) A comparative analysis of an orthologous proteomic environment in the yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe . Mol. Cell. Proteom. 3, 125–132. [DOI] [PubMed] [Google Scholar]
- Rohde, J.R. and Cardenas, M.E. (2003) The Tor pathway regulates gene expression by linking nutrient sensing to histone acetylation. Mol. Cell Biol. 23, 629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde, J.R. , Heitman, J. and Cardenas, M.E. (2001) The TOR kinases link nutrient sensing to cell growth. J. Biol. Chem. 276, 9583–9586. [DOI] [PubMed] [Google Scholar]
- Sambrook, J. , Fritsch, E.F. and Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual, 2nd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory. [Google Scholar]
- Schonig, B. , Brown, D.W. , Oeser, B. and Tudzynski, B. (2008) Cross‐species hybridization with Fusarium verticillioides microarrays reveals new insights into Fusarium fujikuroi nitrogen regulation and the role of AreA and NMR. Eukaryot. Cell, 7, 1831–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichert, S. , Wottawa, M. , Schonig, B. and Tudzynski, B. (2006) Role of the Fusarium fujikuroi TOR kinase in nitrogen regulation and secondary metabolism. Eukaryot. Cell, 5, 1807–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veneault‐Fourrey, C. , Barooah, M. , Egan, M. , Wakley, G. and Talbot, N.J. (2006) Autophagic fungal cell death is necessary for infection by the rice blast fungus. Science, 312, 580–583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 The snt2 strain of Neurospora crassa is impaired in aerial hyphae production and conidiation. On solid medium, the Δsnt2 strain (B) developed thicker, slower growing mycelium, with few aerial hyphae, reduced numbers of conidia and therefore a less pronounced orange‐coloured culture, compared with the corresponding wild‐type isolate (A).
Table S1 Primers used in this study.
Supporting info item
Supporting info item


