SUMMARY
Mechanisms leading to nonhost resistance of plants against nonadapted pathogens are thought to have great potential for the future management of agriculturally important diseases. In this article, we report an investigation of nonhost resistance motivated by the advantages of studying an interaction between two model organisms, namely Arabidopsis thaliana and Magnaporthe oryzae. During the course of our studies, however, we discovered an unexpected plasticity in the responses of Arabidopsis against this ostensibly nonhost pathogen. Thus, we elucidated that certain experimental conditions, such as the growth of plants under long days at constantly high humidity and the use of high inoculum concentrations of M. oryzae conidia, forced the interaction in leaves of some Arabidopsis ecotypes towards increased compatibility. However, sporulation was never observed. Furthermore, we observed that roots were generally susceptible to M. oryzae, whereas leaves, stems and hypocotyls were not infected. It must be concluded, therefore, that Arabidopsis roots lack an effective defence repertoire against M. oryzae, whereas its leaves possess such nonhost defence mechanisms. In summary, our findings point to organ‐specific determinants and environmental conditions influencing the effectiveness of nonhost resistance in plants.
Arabidopsis thaliana and Magnaporthe oryzae are both considered as model organisms for plants and phytopathogenic fungi, respectively (Ebbole, 2007; Meinke et al., 1998). Therefore, it was logical that researchers interested in plant–fungus interactions would combine the two organisms to take advantage of the molecular tool‐boxes available for both interaction partners. Being interested in nonhost resistance and assuming that Arabidopsis might not be a host for M. oryzae, we also followed this approach. Nonhost resistance is defined as the capacity of a plant species to resist pathogens from other hosts, e.g. pathogens adapted to wheat are often unable to colonize rice plants to which they have not been successively adapted during evolution (Heath, 1980). Essentially, it seems easy to classify a plant–pathogen combination to be of the host or nonhost type simply by looking for disease symptoms. However, it is not that easy to prove the definition to be true. This is because of the impossibility of testing all genotypes of a given plant species against all genotypes of a pathogen species, and because particular experimental conditions may favour the establishment of disease over resistance, or vice versa. The latter scenario is evidenced by two recent publications on the interaction between Arabidopsis and the hemibiotrophic fungus M. oryzae, which reported host and nonhost types of interaction, respectively (Maeda et al., 2009; Park et al., 2009). For clarification, we therefore tested whether the different experimental conditions in the two studies might have influenced the outcome of the interactions and, for comparability, we used the M. oryzae isolate (70‐15) employed in the study of Park et al. (2009). Both of the M. oryzae isolates used in the work reported here showed a nonhost interaction phenotype in Arabidopsis leaves, but were able to colonize roots. We investigated the responses of Arabidopsis ecotypes Est‐0 and Gre‐0 to inoculation with the M. oryzae isolate 70‐15. Both combinations were rated as susceptible by Park et al. (2009). Using the growing conditions reported by Park et al. (2009) (16‐h light period, 22 °C, 80% relative humidity) and drop inoculation with a spore concentration of 5 × 105 conidia/mL, macroscopic evaluation revealed chlorotic spots and necrotic lesions at 6 days post‐inoculation (dpi) on the leaves of both ecotypes, although ecotype Est‐0 showed more pronounced necrosis than Gre‐0 (Fig. S1, see Supporting Information). Necrosis on leaves was reduced significantly when both ecotypes were grown under short‐day conditions or at 65% relative humidity (Fig. S1). Similarly, spray rather than drop inoculation reduced chlorosis and necrosis symptoms on inoculated leaves (Fig. S1). Almost no disease symptoms were found on leaves of Arabidopsis ecotypes grown under short‐day conditions (8‐h light period, 22 °C) at 65% relative humidity (Fig. S1). We concluded, therefore, that prolongation of the illumination period, which generally induces flowering in Arabidopsis, in combination with elevated relative humidity and the application of high‐density inoculum in small droplets, cause Arabidopsis plants to become more susceptible to M. oryzae. Interestingly, in our experience, Gre‐0 plants did not flower under long‐day conditions up to the time of inoculation, and showed less leaf necrosis relative to Est‐0 plants, which, by contrast, had formed flowers by the same time point. Therefore, it may be that Arabidopsis plants lose the ability for adequate defence after induction of the reproductive stage. A similar phenomenon has been reported for the induction of systemic acquired resistance, which did not function in cucumber after the onset of flowering (Guedes et al., 1980). An influence of environmental conditions on the infection of wheat with nonadapted M. grisea isolates has been reported by Nga et al. (2009), who showed that incubation at 26 °C after inoculation can break this nonhost resistance. However, even when applying the most disease‐promoting experimental conditions which allowed fungal growth, in contrast with Park et al. (2009), we did not observe sporulation. This suggests additional, as yet unknown, factors that also affect the capability of Arabidopsis to resist infection by M. oryzae.
Our next goal was to characterize the interaction between Arabidopsis and M. oryzae at the cellular level. Firstly, we analysed plants grown under short‐day conditions and spray inoculated with a spore concentration of 2.5 × 105 conidia/mL. Microscopic investigations at 2 dpi on Col‐0 wild‐type (wt) plants revealed the inability of M. oryzae to penetrate epidermal cells (Fig. 1A,C). The same holds true for other Arabidopsis wt accessions, such as Est‐0, Gre‐0 and Ws‐0 (data not shown). The lack of induced cellular defence reactions in wt plants, for example a hypersensitive response or papilla formation, might suggest a pre‐penetration resistance mechanism. Secondly, we examined plants grown under long‐day conditions, high humidity and spray inoculated with a high‐density conidial solution (5 × 105 conidia/mL). In this case, no substantial differences were found in the success of fungal penetration in comparison with the results described above (data not shown). However, it was noticed that the application of a high‐density inoculum resulted in the attack of individual epidermal cells by several appressoria, which was correlated with browning of the affected cells. The latter might be the reason for the macroscopic necrotic lesions observed at 6 dpi only on these plants (data not shown). Next, we asked whether Arabidopsis mutants, such as pen2, which are affected in penetration resistance against non‐adapted powdery mildew pathogens (Lipka et al., 2005), might also show an altered cellular defence against M. oryzae. Indeed, invasion of epidermal cells of pen2‐1 mutants was frequently observed, and this occurred together with the onset of cell death, as evidenced by trypan blue staining (Fig. 1B). A quantitative assessment of M. oryzae infection sites on Arabidopsis plants without pen mutation (Col‐0 and Col‐3gL1), in comparison with pen1‐1, pen2‐1, pen3‐1, pen1‐1pen2‐1 and pen2‐3pen3‐2 mutants, was achieved by assigning each infection site to one of four categories: (i) germination of conidia without formation of appressoria; (ii) germinated conidia with appressoria; (iii) formation of papillae beneath appressoria; and (iv) fungal hyphae in epidermal cells stained with trypan blue (Fig. 1C). In Col‐0 and Col‐3gL1 Arabidopsis genotypes, almost no cellular defence reactions were detected at sites of attempted fungal penetration. In contrast, papillae were found frequently beneath appressoria on the other genotypes, with the highest frequency on the pen2‐3pen3‐2 double mutant (Fig. 1C). Penetration of pen mutant epidermal cells by fungal invasive hyphae caused cell death, as evidenced by trypan blue staining (Keogh et al., 1980). Predominantly, the latter category was found on plants carrying a mutation in the PEN2 gene, although the overall frequency of this category was rather low (3%–6%) (Fig. 1C). Our results regarding the penetration frequency of M. oryzae on different pen mutant plants are in accordance with the data presented by Maeda et al. (2009). However, our data extended this study by investigating pen double mutants and by monitoring diverse cellular defence responses, such as the formation of papillae and the induction of cell death (Fig. 1C). Thus, we showed that papillae occurred with a frequency of 20%–30% in pen2‐1 and pen3‐1 single mutants and a frequency of 40% in pen2‐3pen3‐2 double mutants. On the basis of this observation, it could be hypothesized that PEN2 and PEN3 act synergistically in controlling penetration defence against M. oryzae. This would further support the idea of a concerted action of PEN2 and PEN3, whereby the PEN2 protein enzymatically activates a toxic compound that is brought to sites of infection by the PEN3 protein (Lipka et al., 2005; Stein et al., 2006).
Figure 1.
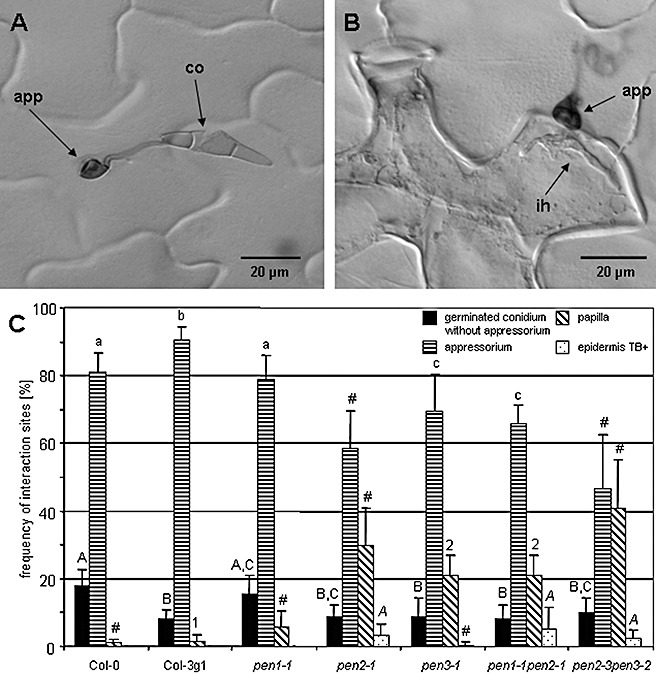
Microscopy of trypan blue‐stained infection sites between different Arabidopsis genotypes and Magnaporthe oryzae (isolate TH6772) at 2 days post‐inoculation. (A) M. oryzae conidium (co) germination and appressorium (app) formation on Arabidopsis Col‐0 plants did not induce cellular defence responses. (B) Penetration by an M. oryzae invasive hypha (ih) from an appressorium into an epidermal cell of a pen2‐1 mutant, which subsequently underwent cell death, as evidenced by trypan blue staining (Keogh et al., 1980). (C) Quantitative assessment of single‐cell interaction sites on Arabidopsis wild‐type and mutant plants. Plants were grown with an 8‐h light period, at 22 °C and 65% relative humidity, and spray inoculated with M. oryzae isolate TH6772 (2.5 × 105 conidia/mL, 5 weeks after sowing). Individual interaction sites were grouped into different classes (see text) and the frequency for each class is given as the mean plus standard deviation per leaf. At least eight leaves were analysed per genotype and 100 infection sites were inspected per leaf. Significant differences (P < 0.05) for each class on different genotypes were determined using one‐way analysis of variance and are indicated by different letters or numbers. Values excluded from statistical analysis because the normality test and/or equal variance test failed are indicated by #. The experiment was repeated once with a similar result.
So far, in accordance with Maeda et al. (2009), our data support the view that Arabidopsis is a nonhost for M. oryzae, although environmental conditions may compromise the effectiveness of resistance, as suggested by scrutinizing the data presented by Park et al. (2009). To gain further insight into the key question of which factors might render Arabidopsis more susceptible to M. oryzae, we investigated root inoculations. This work was mainly driven by our observation that resistance (R) gene‐mediated resistance, which effectively protects leaves of rice plants from being colonized by M. oryzae, operates less efficiently in roots (Jansen et al., 2006) and that, similarly in Arabidopsis, race‐specific resistance against Hyaloperonospora parasitica is not operative in roots (Hermanns et al., 2003). Therefore, we asked the question of whether nonhost resistance, which operates in Arabidopsis leaves against M. oryzae, might also fail in a similar fashion in Arabidopsis roots. It must be stated, however, that infections of M. oryzae on rice roots are discussed controversially and gene‐for‐gene resistance has been reported (Sesma and Osbourn, 2004). For the root inoculation assay, Arabidopsis Col‐0, pen2‐1pen3‐1 and pen2‐1pad4‐1 plants were grown under sterile conditions using a method established by Hermanns et al. (2003). Inoculations with M. oryzae isolate 70‐15 by the application of 3‐µL droplets of spore solutions [105 conidia/mL in 0.1% (w/v) gelatine] were carried out 18 days after sowing. In these experiments, we avoided spray inoculation because Arabidopsis seedlings were cultivated on a nutrient medium on microscope slides and droplets could be placed directly on the roots, whereas spraying would have dispersed conidia ineffectively. By using a low spore concentration during this drop inoculation, single root cells were, on average, attacked by a single penetration event. Microscopic inspection of the inoculated roots revealed that M. oryzae formed regularly shaped appressoria at the root surface of different Arabidopsis wt Col‐0 or mutant plants, which resulted in penetration of the fungus into root cells (Fig. 2A). The formation of melanized appressoria is a new observation not reported to date from root infection assays with M. oryzae on different plant species (Guimil et al., 2005; Jansen et al., 2006; Sesma and Osbourn, 2004). However, far more frequently, and in accordance with observations reported by the laboratory of Sesma and Osbourn (Sesma and Osbourn, 2004; Tucker et al., 2010), non‐melanized hyphopodia‐like structures were observed on contact of M. oryzae germ tubes with Arabidopsis roots. Hyphopodia similarly enabled entry of the fungus into root cells. Hyphopodia were found regularly on Arabidopsis wt and mutant plants, such as pen2‐1pad4‐1, which is phytoalexin deficient (Fig. 2B). Recently, Heupel et al. (2009) reported that ERL1, an Era (Escherichia coli Ras)‐like GTPase, is required for full root virulence of M. oryzae on rice. The virulence defect of a Δerl1 strain could be complemented by the orthologous protein from the mutualistic fungus Glomus intraradices, referred to as Gin1 (GTPase, intein), which is presumed to play a role in establishing compatibility with plant roots. Taken together, these data support the hypothesis that symbiotic and pathogenic fungi use conserved strategies for root infection (Heupel et al., 2009). Thinking ahead, it might be that today's leaf pathogens have retained the capability to invade plant roots since prehistoric times, although this feature is no longer required. Experimental evidence for this hypothesis comes from recently published results suggesting that root‐infecting hyphopodia may represent a primitive form of appressoria which were acquired by pathogens later in the evolution during leaf colonization (Tucker et al., 2010). In turn, it might be concluded that roots, which are not generally exposed to adapted leaf pathogens, have not had the need to develop resistance against them, as has been necessary for leaves.
Figure 2.
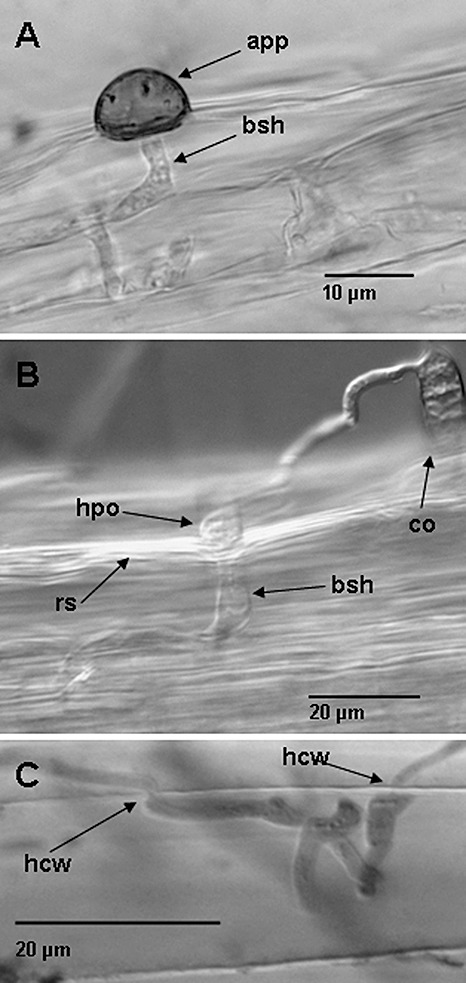
Magnaporthe oryzae (isolate TH6772) infection sites on roots of different Arabidopsis genotypes at 4 days post‐inoculation. (A) A melanized appressorium (app) can be seen on the surface of a pen2‐1pen3‐1 root and the fungus has invaded the root tissue producing bulbous‐shaped hyphae (bsh). (B) Conidium (co) of M. oryzae germinating on roots of Arabidopsis pen2‐1pad4‐1 mutant plants and forming a hyphopodium‐like (hpo) structure on contact with the root surface (rs). Bulbous invasive hyphae can be seen penetrating the underlying cell. The photograph was taken with a microscope using differential interference contrast at 4 days post‐inoculation. (C) Intracellular hyphae of M. oryzae cross cell walls (hcw) between Col‐0 root cells in a fashion similar to that observed at pit fields of leaf cells. Samples recorded in (A) and (C) were trypan blue stained before microscopic investigation. The photographs shown are representative examples of infection sites found on the respective genotypes. All infection experiments were repeated at least in triplicate with a similar outcome.
As yet, our microscopic investigations have not revealed the induction of any cellular defence reaction in the roots of Arabidopsis during invasion by M. oryzae, which confirms the general observation that defence pathways and resistance mechanisms described for leaf pathogens are only found to a minor degree or not at all in roots (Hermanns et al., 2003; Okubara and Paulitz, 2005). After the entrance of M. oryzae into Arabidopsis root tissue, the shape and bulbous growth habit of invasive hyphae closely resembled the morphology known from leaf infections on M. oryzae's usual host, rice (Talbot, 2003). Recently, it has been shown that common genetic requirements control the ability of M. oryzae to infect leaf or root tissue of rice (Tucker et al., 2010). The occurrence in roots of these bulbous hyphae, which have been discussed as analogous structures to haustoria of biotrophic fungi (Wilson and Talbot, 2009), indicates a remarkably high degree of compatibility established between a particular organ of the ostensible nonhost plant Arabidopsis and M. oryzae. After penetration into the first root cell, invasive hyphae ramify and start to colonize the surrounding tissue intracellularly (2, 3). Thereby, fungal movement from cell to cell occurs in a fashion similar to that described for the colonization of rice leaves by M. oryzae, and is suggestive that the fungus likewise crosses cell walls at pit fields (Kankanala et al., 2007). The latter observation further underpins the suggestion that M. oryzae colonizes Arabidopsis roots in a manner reminiscent of growth in the leaf tissue of host plants.
Figure 3.
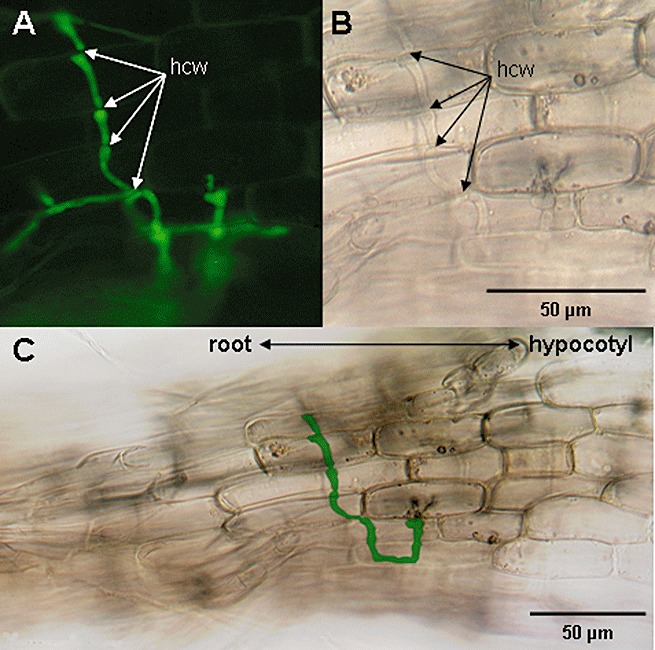
Magnaporthe oryzae is unable to grow from colonized root tissue into the hypocotyl of Arabidopsis plants. Fungal hyphae of a constitutively green fluorescent protein (GFP)‐expressing M. oryzae (70‐15‐GFP) isolate can be seen colonizing the root tissue of Arabidopsis pen2‐3pen3‐2 plants at 4 days post‐inoculation. Fungal hyphae cross root cell walls (hcw) in a fashion suggesting the utilization of pit fields. We observed that the growth of fungal hyphae was blocked at the border between roots and hypocotyl tissue. (A) and (B) show the same representative infection site using epifluorescence and bright‐field microscopy, respectively. For clarity, an overview of the region, showing the intersection between root and hypocotyl tissues, is given in (C), with fungal hyphae artificially coloured green by computer.
Next, we addressed the very important question of whether M. oryzae might be able to overcome the nonhost resistance shown by Arabidopsis aerial tissues by growing from colonized roots through the hypocotyl into stems and leaves, a phenomenon observed after M. oryzae infection of rice roots (Sesma and Osbourn, 2004). Importantly, we observed that this was not the case. In contrast, we found that fungal hyphae which grow towards the hypocotyl are unable to cross the border between root and hypocotyl tissue, which remains free from colonization by the fungus (Fig. 3A–C). Furthermore, we elucidated that, on inoculation of hypocotyls from Arabidopsis wt plants, the fungus is unable to penetrate into this tissue (data not shown). Thus, nonhost resistance against M. oryzae seems to function in leaves, stems and hypocotyls of Arabidopsis, but is not active in roots. Consistently, we found that the fungus was able to penetrate hypocotyls of pen2‐3pen3‐2 double mutants similarly to leaves of the same genotype (data not shown).
Finally, it must be concluded that host–pathogen interactions show an unpredictable plasticity regarding compatibility or incompatibility in relation to experimental design, which may force host/nonhost interactions into either direction. Furthermore, organ identity may greatly influence the ability of a plant to establish a host or nonhost interaction with a given pathogen.
Supporting information
Fig. S1 Influence of different environmental and inoculation conditions on the interaction between four different Arabidopsis ecotypes (Col‐0, Est‐0, Gre‐0 and Ws‐0) and Magnaporthe oryzae (isolate 70‐15). All plants were inoculated with M. oryzae isolate 70‐15 (5 × 105 conidia/mL) and symptoms were documented at 6 days post‐inoculation (dpi). LD, long day (16 h light); SD, short day (8 h light); ↑ rH, high relative humidity (∼80%), ↓ rH, low relative humidity (∼65%); DS, disease severity; n.d., not determined.
Supporting info item
ACKNOWLEDGEMENTS
The authors are grateful to Dr Ralph Panstruga and Professor Volker Lipka for sharing Arabidopsis mutant plants. Dr Eckhard Thines is acknowledged for providing fungal isolates.
REFERENCES
- Ebbole, D.J. (2007) Magnaporthe as a model for understanding host–pathogen interactions. Annu. Rev. Phytopathol. 45, 437–456. [DOI] [PubMed] [Google Scholar]
- Guedes, M.E.M. , Richmond, S. and Kuc, J. (1980) Induced systemic resistance to anthracnose in cucumber is influenced by the location of the inducer inoculation with Colletotrichum lagenarium and the onset of flowering and fruiting. Physiol. Plant Pathol. 17, 229–233. [Google Scholar]
- Guimil, S. , Chang, H.S. , Zhu, T. , Sesma, A. , Osbourn, A. , Roux, C. , Ioannidis, V. , Oakeley, E.J. , Docquier, M. , Descombes, P. , Briggs, S.P. and Paszkowski, U. (2005) Comparative transcriptomics of rice reveals an ancient pattern of response to microbial colonization. Proc. Natl. Acad. Sci. USA, 102, 8066–8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath, M.C. (1980) Reactions of nonsuscepts to fungal pathogens. Annu. Rev. Phytopathol. 18, 211–236. [Google Scholar]
- Hermanns, M. , Slusarenko, A.J. and Schlaich, N.L. (2003) Organ‐specificity in a plant disease is determined independently of R gene signaling. Mol. Plant–Microbe Interact. 16, 752–759. [DOI] [PubMed] [Google Scholar]
- Heupel, S. , Roser, B. , Kuhn, H. , Lebrun, M.‐H. , Villalba, F. and Requena, N. (2009) Erl1, a novel Era‐lke GTPase from Magnaporthe oryzae, is required for full root virulence and is conserved in the mutualistic symbiont Glomus intraradices . Mol. Plant–Microbe Interact. 23, 67–81. [DOI] [PubMed] [Google Scholar]
- Jansen, M. , Slusarenko, A.J. and Schaffrath, U. (2006) Competence of roots for race‐specific resistance and the induction of acquired resistance against Magnaporthe oryzae . Mol. Plant Pathol. 7, 191–195. [DOI] [PubMed] [Google Scholar]
- Kankanala, P. , Czymmek, K. and Valent, B. (2007) Roles for rice membrane dynamics and plasmodesmata during biotrophic invasion by the blast fungus. Plant Cell, 19, 706–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh, R.C. , Deverall, B.J. and Mcleod, S. (1980) Comparison of histological and physiological responses to Phakopsora pachyrhizi in resistant and susceptible soybean. T. Brit. Mycol. Soc. 74, 329–333. [Google Scholar]
- Lipka, V. , Dittgen, J. , Bednarek, P. , Bhat, R. , Wiermer, M. , Stein, M. , Landtag, J. , Brandt, W. , Rosahl, S. , Scheel, D. , Llorente, F. , Molina, A. , Parker, J. , Somerville, S. and Schulze‐Lefert, P. (2005) Pre‐ and postinvasion defenses both contribute to nonhost resistance in Arabidopsis . Science, 310, 1180–1183. [DOI] [PubMed] [Google Scholar]
- Maeda, K. , Houjyou, Y. , Komatsu, T. , Hori, H. , Kodaira, T. and Ishikawa, A. (2009) AGB1 and PMR5 contribute to PEN2‐mediated preinvasion resistance to Magnaporthe oryzae in Arabidopsis thaliana . Mol. Plant–Microbe Interact. 22, 1331–1340. [DOI] [PubMed] [Google Scholar]
- Meinke, D.W. , Cherry, J.M. , Dean, C. , Rounsley, S.D. and Koornneef, M. (1998) Arabidopsis thaliana: a model plant for genome analysis. Science, 282, 662–682. [DOI] [PubMed] [Google Scholar]
- Nga, N.T.T. , Hau, V.T.B. and Tosa, Y. (2009) Identification of genes for resistance to a Digitaria isolate of Magnaporthe grisea in common wheat cultivars. Genome, 52, 801–809. [DOI] [PubMed] [Google Scholar]
- Okubara, P. and Paulitz, T. (2005) Root defense responses to fungal pathogens: a molecular perspective. Plant Soil, 274, 215–226. [Google Scholar]
- Park, J.‐Y. , Jin, J. , Lee, Y.‐W. , Kang, S. and Lee, Y.‐H. (2009) Rice blast fungus (Magnaporthe oryzae) infects Arabidopsis via a mechanism distinct from that required for the infection of rice. Plant Physiol. 149, 474–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesma, A. and Osbourn, A.E. (2004) The rice blast pathogen undergoes developmental processes typical of root‐infecting fungi. Nature, 431, 582–586. [DOI] [PubMed] [Google Scholar]
- Stein, M. , Dittgen, J. , Sanchez‐Rodriguez, C. , Hou, B.H. , Molina, A. , Schulze‐Lefert, P. , Lipka, V. and Somerville, S. (2006) Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to nonhost resistance to inappropriate pathogens that enter by direct penetration. Plant Cell, 18, 731–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot, N.J. (2003) On the trail of a cereal killer: investigating the biology of Magnaporthe grisea . Annu. Rev. Microbiol. 57, 177–202. [DOI] [PubMed] [Google Scholar]
- Tucker, S.L. , Besi, M.I. , Galhano, R. , Franceschetti, M. , Goetz, S. , Lenhert, S. , Osbourn, A. and Sesma, A. (2010) Common genetic pathways regulate organ‐specific infection‐related development in the rice blast fungus. Plant Cell, 22, 953–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, R.A. and Talbot, N.J. (2009) Under pressure: investigating the biology of plant infection by Magnaporthe oryzae . Nat. Rev. Microbiol. 7, 185–195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Influence of different environmental and inoculation conditions on the interaction between four different Arabidopsis ecotypes (Col‐0, Est‐0, Gre‐0 and Ws‐0) and Magnaporthe oryzae (isolate 70‐15). All plants were inoculated with M. oryzae isolate 70‐15 (5 × 105 conidia/mL) and symptoms were documented at 6 days post‐inoculation (dpi). LD, long day (16 h light); SD, short day (8 h light); ↑ rH, high relative humidity (∼80%), ↓ rH, low relative humidity (∼65%); DS, disease severity; n.d., not determined.
Supporting info item


