SUMMARY
Harpins are extracellular glycine‐rich proteins eliciting a hypersensitive response (HR). In this study, we identified a new harpin, PopW, from Ralstonia solanacearum strain ZJ3721. This 380‐amino‐acid protein is acidic, rich in glycine and serine, and lacks cysteine. When infiltrated into the leaves of tobacco (non‐host), PopW induced a rapid tissue collapse via a heat‐stable but protease‐sensitive HR‐eliciting activity. PopW has an N‐terminal harpin domain (residues 1–159) and a C‐terminal pectate lyase (PL) domain (residues 160–366); its HR‐eliciting activity depends on its N‐terminal domain. Analyses of subcellular localization and plasmolysis demonstrated that PopW targeted the onion cell wall. This was further confirmed by its ability to specifically bind to calcium pectate, a major component of the plant cell wall. However, PopW had no detectable PL activity. Western blotting revealed that PopW was secreted by the type III secretion system in an hrpB‐dependent manner. Gene sequencing indicated that popW is conserved among 20 diverse strains of R. solanacearum. A popW‐deficient mutant retained the ability of wild‐type strain ZJ3721 to elicit HR in tobacco and to cause wilt disease in tomato (a host). We conclude that PopW is a new cell wall‐associated, hrpB‐dependent, two‐domain harpin that is conserved across the R. solanacearum species complex.
INTRODUCTION
hrp genes are present in most of the major families of Gram‐negative phytopathogenic bacteria. Together with secretory proteins and regulators, they encode harpins, a large family of proteins secreted by the type III secretion system (TTSS). Harpins are heat stable and can trigger the hypersensitive response (HR) in incompatible host plants (Kim and Beer, 1998). Although different harpins have little or no homology in their primary sequences, they contain abundant glycine (Gly) and/or serine (Ser), but no cysteine (Cys) residues (Arlat et al., 1994; Van Gijsegem et al., 1993; Willis et al., 1991). After the first harpin, HrpN, had been identified from Erwinia amylovora (Wei et al., 1992), many proteins with similar characteristics were detected, such as HrpW of Erwinia amylovora and HrpZ of Pseudomonas syringae (He et al., 1993; Kim and Beer, 1998).
Ralstonia (previously named Pseudomonas) solanacearum (Yabuuchi et al., 1995) is generally regarded as one of the most destructive plant pathogenic bacteria, as it causes great economic losses worldwide (Hayward, 1991). A wide range of genes involved in its pathogenicity and virulence have been recognized (Denny, 2000; Genin and Boucher, 2004; González et al., 2007). Its TTSS transports the Pseudomonas out proteins (Pops): PopA (Arlat et al., 1994), PopB, PopC, PopF1, PopF2, PopP1 and PopP2 (Racapéet al., 2005). Cultured R. solanacearum cells can secrete these proteins into the medium, but studies of PopP2 (Deslandes et al., 2003), PopB and PopC (Guéneron et al., 2000) suggest that these proteins normally function inside the host cell as the pathogen interacts with the plant. Amongst these extracellular proteins, PopA was the first harpin isolated from R. solanacearum GMI1000. PopA contains high proportions of Gly and alanine (Ala), but no Cys; it elicits an HR‐like response when infiltrated into tobacco leaves (Arlat et al., 1994). Further research has shown that popA is part of an operon also encoding PopB and PopC, which are also secreted through TTSS. Expression of popABC is regulated by hrpB (Guéneron et al., 2000).
In this study, we identified a new harpin named PopW from R. solanacearum strain ZJ3721. It is structurally different from PopA and composed of two domains; its C‐terminal domain is homologous to pectate lyases (PLs). We further investigated its gene regulation and conservation, subcellular localization and ability to bind to calcium pectate—a major component of the plant cell wall—and role in the virulence of R. solanacearum ZJ3721. Some of the results in this study have been reported previously in an abstract (Li et al., 2008).
RESULTS
Identification of PopW, a Gly‐rich protein
The genome of R. solanacearum GMI1000 contains the popW gene (accession no. AL646052) (Salanoubat et al., 2002). We isolated the full‐length popW open reading frame (ORF) (1143 bp) from ZJ3721 by polymerase chain reaction (PCR) with a pair of primers designed using the sequence of the GMI1000 popW gene. The encoded protein has a deduced molecular weight of 39.79 kDa. It is acidic [isoelectric point (pI) of 6.17], hydrophilic, rich in Gly, Ser, Ala and asparagine (Asn), low in glutamic acid (Glu), methionine (Met), arginine (Arg), tryptophan (Trp) and tyrosine (Tyr) and contains no Cys in its 380 amino acids. Thus, PopW has physical characteristics similar to those of harpins, although the amino acid sequence does not resemble any other harpins (Alfano and Collmer, 1996). Sequence analysis revealed that PopW is composed of two domains: an N‐terminal harpin‐like domain (amino acids 1–159) and a C‐terminal PL‐like domain (amino acids 160–366). The N‐terminal domain is rich in Gly (11.9%) and Ser (10.1%), and lacks Cys; the C‐terminal domain is homologous to PLs, as revealed by the conserved domain database (CDD) on the National Center for Biotechnology Information (NCBI) website (Marchler‐Bauer et al., 2007) (Fig. 1A). Using the PSIPRED Protein Structure Prediction Server (Jones, 1999; McGuffin et al., 2000), we predicted that the subregion (residues 28–54) of the N‐terminal domain of PopW might form an α‐helix (Fig. 1B).
Figure 1.
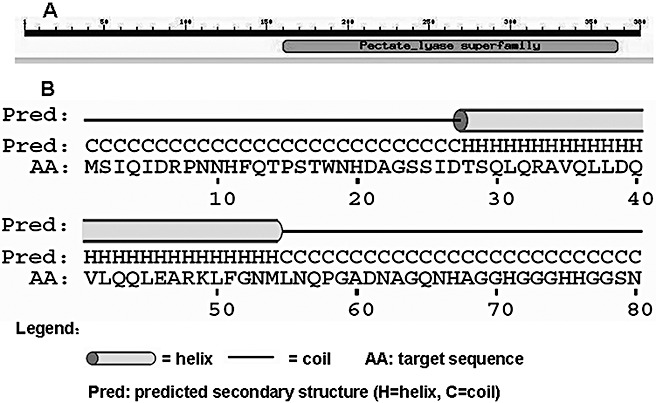
PopW protein structure. (A) A sketch of PopW including the N‐terminal harpin domain (residues 1–159) and C‐terminal pectate lyase domain (residues 160–366) according to the Conserved Domain Database (CDD) at the National Center for Biotechnology Information (NCBI) website. (B) The predicted secondary structure of the N‐terminal domain of PopW (residues 1–80) containing an α‐helix (residues 28–54). This result was analysed using the PSIPRED Protein Structure Prediction Server.
Both PopW and its harpin domain elicit HR in tobacco leaves
Because of its predicted characteristics, we postulated that PopW might be an HR elicitor. To test this hypothesis, we constructed Escherichia coli BL159 and BL366 by transforming E. coli BL21 (DE3) with plasmids expressing PopW(1–159) (N‐terminal 159 amino acids) and PopW(160–366) (C‐terminal 207 amino acids), respectively. Both proteins were tagged with 6 × histidine (6 × His) at their C‐terminals. The purified PopW and concentrated cell‐free lysate preparations (CLPs) of BL159 and BL366 (refer to Experimental Procedures for details) were infiltrated into tobacco leaves. Purified PopW at ≥25 µg/mL elicited HR necrosis in tobacco leaves. The necrosis induced by PopW developed less than 24 h after inoculation, which is faster than that induced by HrpN (24–36 h after inoculation) (Wei et al., 1992). Furthermore, heat‐treated (100 °C for 10 min) PopW, but not proteinase K‐treated PopW, elicited necrosis in tobacco leaves (Fig. 2A), which suggests that this protein's HR‐eliciting activity is heat stable, but sensitive to proteinase K degradation. An HR‐like rapid necrosis was also observed in tobacco leaves treated with the CLP of E. coli strain BL159 overexpressing the N‐terminus of PopW, but not by the CLPs of strain BL366 or the vector control (Fig. 2A), which implies that only the N‐terminal harpin domain of PopW has HR‐inducing ability.
Figure 2.
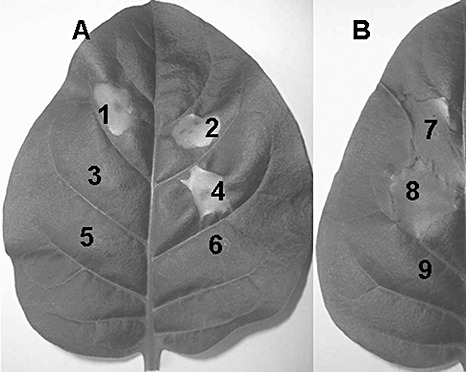
Elicitation of the hypersensitive response (HR) in tobacco leaves by a variety of treatments. (A) Treatments of purified PopW and cell‐free lysate preparations (CLPs) of Escherichia coli BL21 (DE3): 1, PopW (0.1 mg/mL); 2, heat‐treated PopW (0.1 mg/mL); 3, proteinase K‐treated PopW (0.1 mg/mL); 4, CLP of E. coli BL21(DE3) harbouring harpin domain of PopW; 5, CLP of E. coli BL21(DE3) harbouring PL domain of PopW; 6, CLP of E. coli BL21(DE3) harbouring control vector. (B) Suspensions of popW mutant ZJ3722 and wild‐type Ralstonia solanacearum ZJ3721: 7, bacterial suspension of wild‐type R. solanacearum ZJ3721 (107 colony‐forming units (cfu)/mL); 8, bacterial suspension of popW mutant ZJ3722 (107 cfu/mL); 9, sterile water control. (A) and (B) were taken 24 and 48 h after infiltration, respectively.
PopW is an inactive homologue of PL
Our comparison search in the database (Swiss‐Prot + TrEMBL) revealed that there is some similarity between the 207‐amino‐acid C‐terminal domain of PopW and PLs from several other bacteria and one fungus (Fig. 3). PopW has an identity of 79% with a putative PL from R. solanacearum UW551, 36% with the PL of Bacillus sp. BP‐23, and 39% with the PLs from Rhizobium etli and Saccharophagus degradans 2–40. In addition, PopW has an identity of 39% with HrpW from Erwinia amylovora, which is also a harpin containing a C‐terminal domain homologous to PLs. Among these proteins with sequence identity, most of the fully conserved amino acids are Gly residues (Fig. 3).
Figure 3.
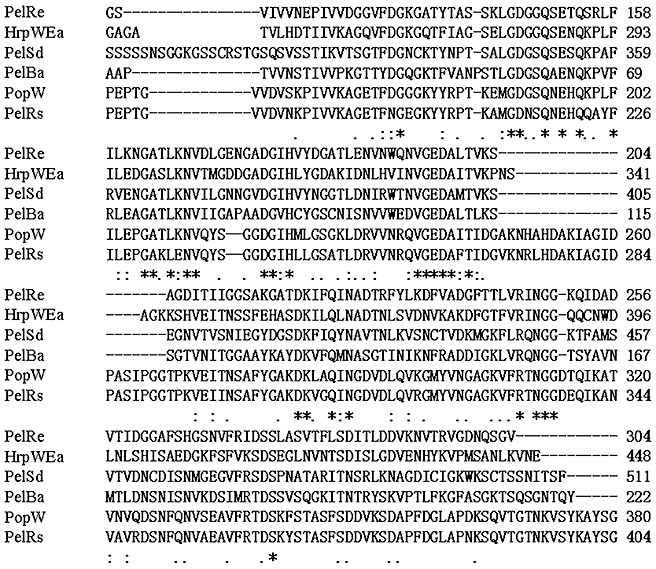
Alignment of PopW with pectate lyases of three bacterial and one fungal strain, and HrpW of Erwinia amylovora. The sequences were aligned by the program ClustalX (version 1.83) with default parameters. *, Positions having a single fully conserved residue; :, one of the ‘strong’ groups is fully conserved; ., one of the ‘weaker’ groups is fully conserved. Ba, Bacillus sp. BP‐23; Ea, E. amylovora Ea321; Pel, pectate lyase; Re, Rhizobium etli; Rs, Ralstonia solanacearum UW551; Sd, Saccharophagus degradans 2–40.
Neither the purified PopW nor the concentrated supernatant preparation (CSP) of an R. solanacearum culture had detectable PL activity under three different conditions of pH (6.5, 8.0 or 9.5), although the C‐terminal sequence of PopW is similar to the sequences of PLs.
PopW targets the plant cell wall and binds to calcium pectate
We examined the subcellular localization of PopW using green fluorescent protein (GFP) as a reporter protein. The full‐length PopW ORF (Met‐1 to Gly‐380) was fused to GFP, and the expression of the fusion protein was controlled by the cauliflower mosaic virus 35S promoter. The PopW:GFP cassette was introduced into onion epidermal cells using Agrobacterium tumefaciens, and the transfected cells were then incubated on Murashige and Skoog plates at 28 °C (Murashige and Skoog, 1962). Gene expression was examined under a fluorescence microscope after the cells had been incubated for 24 h. This revealed that the treated onion epidermal cells were surrounded by the PopW:GFP fusion proteins, indicating that PopW might target the onion cell wall. To eliminate the possibility of PopW targeting the cell membrane, the onion epidermal cells were plasmolysed by immersion in a 1.0 M sucrose solution for 10–30 min. After this treatment, the GFP signal in the plasmolysed cells was still concentrated in the cell wall, whereas the GFP signal in the cells transfected with the control vector was dispersed throughout the whole cytosol (Fig. 4). This result suggests that PopW targets the plant cell wall rather than the cell membrane.
Figure 4.
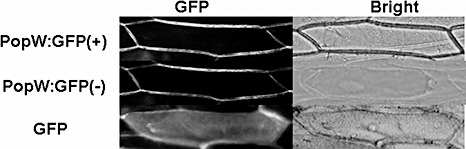
Subcellular localization of PopW in onion epidermal cells. The full‐length popW was transformed into onion epidermal cells via Agrobacterium tumefaciens LBA4404. The localization of fluorescent signals was examined 24 h after transfection under the fluorescence microscope. ‘PopW:GFP’ indicates cells expressing the PopW:GFP fusion protein; ‘GFP’ denotes cells expressing only GFP. (+) and (–) indicate normal cells and plasmolysed cells, respectively. The photographs are fluorescent image (left) and bright field (right).
In order to further confirm the localization of PopW in the plant cell wall and explore its potential function, we tested its ability to bind calcium pectate, a major plant cell wall component, by sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) using both the heat‐treated (100 °C for 10 min) and non‐heated PopW protein. The heat‐treated PopW bound to calcium pectate beads (Fig. 5A), but not to calcium alginate beads (Fig. 5B), and non‐heated PopW also exhibited the same binding specificity (data not shown). This result suggests that PopW can specifically bind to calcium pectate in the plant cell wall, and that this binding activity is heat stable. In addition, neither calcium chloride nor soluble pectate precipitated the heat‐treated PopW (Fig. 5C,D) or non‐heated PopW (data not shown), demonstrating that PopW did not bind to either of them. These experiments confirm the specificity of PopW binding to calcium pectate.
Figure 5.

Sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) showing PopW specifically binding to calcium pectate. Heat‐treated (100 °C for 10 min) PopW protein was used for the following assays: (A) PopW binding to calcium pectate beads; (B) PopW binding to calcium alginate beads; (C) PopW binding to calcium chloride; (D) PopW binding to soluble pectate. Lanes: 1, the original protein–bead mix; 2, the supernatant of the centrifuged protein–bead mix; 3, the washed beads treated with ethylenediaminetetraacetic acid (EDTA); 4, the final buffer wash; 5, PopW mixed with CaCl2 (C) or with soluble pectate (D); 6, the supernatant of the centrifuged mix; 7, the pellet of the centrifuged mix.
PopW is secreted via TTSS of R. solanacearum in an hrpB‐dependent manner
Most of the pathogenic factors of R. solanacearum are delivered through TTSS, whose expression is controlled by hrpB (Cunnac et al., 2004; Occhialini et al., 2005; Valls et al., 2006). The primary structures of PopW and HrpW from Erwinia amylovora described above suggest that the two proteins may have similar functions. HrpW is translocated through TTSS and regulated by hrpL (Fouts et al., 2002). We hypothesized that PopW might also be translocated through TTSS, but regulated by hrpB. In order to test this hypothesis, we used Western blotting to detect PopW in the CLPs and CSPs of wild‐type ZJ3721, hrpB mutant ZJ3723 and hrpV mutant ZJ3724 with an anti‐PopW polyclonal antibody. PopW was present in the CSP of the wild‐type strain, but not in those of the hrpV and hrpB mutants; PopW was also detected in the CLPs of the wild‐type strain and its hrpV mutant, but not in that of the hrpB mutant (Fig. 6). These results indicate that PopW is secreted through TTSS and that its expression is under the control of the hrpB gene.
Figure 6.
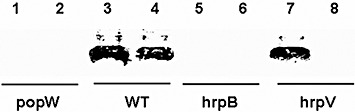
Western blotting showing the regulation and secretion of PopW in Ralstonia solanacearum ZJ3721. An anti‐PopW polyclonal antibody was used to identify the presence of PopW in preparations from different sources. The cell‐free lysate preparations (CLPs) and concentrated supernatant preparations (CSPs) correspond to the odd and even numbers, respectively. ‘popW,’‘WT,’‘hrpB’ and ‘hrpV’ denote CLPs and CSPs of popW mutant ZJ3722 (lanes 1 and 2), wild‐type strain ZJ3721 (lanes 3 and 4), hrpB mutant ZJ3723 (lanes 5 and 6) and hrpV mutant ZJ3724 (lanes 7 and 8), respectively.
popW is conserved amongst R. solanacearum strains
Using PCR, we detected popW in 19 genetically diverse strains of R. solanacearum (Table 3, see later). A DNA fragment was successfully amplified from each of these strains, although sequencing of these fragments revealed slight differences in length among the PCR products (1131–1155 bp) from the different strains (Table 3). A phylogenetic analysis demonstrated that the sequences of popW from 20 strains of R. solanacearum, including ZJ3721, were highly similar (>91%), which implies that popW is conserved in R. solanacearum strains (Fig. S1, see Supporting Information).
Table 3.
Ralstonial solanacearum strains used in the construction of the phylogenetic tree.
| Strains (source*) | Host plant | Geographical origin | Size† (bp) |
|---|---|---|---|
| FJ2003B4(E) | Ipomoea batatas | Fujian, China | 1143 |
| ICPM11119(B) | Zingiber officinale | Shandong, China | 1155 |
| GX526(A) | Arachis hypogaea | Guangxi, China | 1146 |
| HB512(A) | Lycopersicon esculentum | Hubei, China | 1146 |
| GD43(A) | Solanum melongena | Guangdong, China | 1155 |
| FJ1986Bd1(C) | Semen ricini | Fujian, China | 1143 |
| ZJ1993Bn1(A) | Boehmeria nivea | Zhejiang, China | 1143 |
| GX1993Pe1(C) | Capsicum annuum | Guangxi, China | 1146 |
| UW148(D) | Rapistrum rugosum | Australia | 1143 |
| JS526(A) | Lycopersicon esculentum | Jiangsu, China | 1143 |
| GX1993Ssp1(C) | Sesamum indicum | Guangxi, China | 1146 |
| GZ519(A) | Nicotiana tabacum | Guizhou, China | 1143 |
| UW360(D) | Morus alba | Guangdong, China | 1140 |
| GD1993C1(C) | Casuarina equisetifolia | Guangdong, China | 1155 |
| UW551(D) | Pelargonium capitatum | Wisconsin, USA | 1155 |
| UW76(D) | Capsicum annuum | Armuelles, Panama | 1134 |
| UW278(D) | Nicotiana tabacum | Mexico | 1134 |
| K60(D) | Lycopersicon esculentum | Wake Co., NC, USA | 1131 |
| GMI1000(D) | Lycopersicon esculentum | Guyana | 1143 |
Strains were contributed by: A, Department of Plant Pathology, Nanjing Agricultural University, China; B, International Collection of Micro‐Organisms from Plants (ICMP), Auckland, New Zealand; C, J. Feng, Chinese Academy of Agricultural Sciences, Beijing, China; D, C. Allen, University of Wisconsin, Madison, WI, USA; E, T. Lu, Fujian Academy of Agricultural Sciences, Fuzhou, China; F, K. Smalla, Julius Kühn‐Institut (JKI), Braunschweig, Germany.
Total length of the popW open reading frame from each strain.
popW is not essential for the virulence and HR‐eliciting ability of R. solanacearum
Ralstonia solanacearum ZJ3721 was isolated from an infected tomato in Zhejiang Province, China, and proven to be pathogenic to tomato according to Koch's postulates. In order to investigate the role of PopW in the interaction between the plant and the bacterium, we inoculated compatible tomato plants with wild‐type ZJ3721 and popW mutant ZJ3722, respectively. The two treatments caused the same wilt symptoms on the fifth day after inoculation. On the 20th day after inoculation, the disease severity caused by ZJ3721 and ZJ3722 reached 85.0% and 88.3%, respectively; they were not significantly different at P= 0.05 (least significant difference, LSD). These results indicate that PopW is not a major virulence determinant in R. solanacearum.
The leaves of tobacco (an incompatible plant) inoculated with ZJ3721 and ZJ3722, respectively, showed the same HR 24 h after inoculation. This result indicated that a strain lacking PopW retained normal HR‐inducing activity in tobacco (Fig. 2B).
DISCUSSION
In this study, we identified a new R. solanacearum harpin protein called PopW. PopW has two domains, but all other known harpins, except for HrpW, have only one domain. HrpW, which was isolated from Erwinia amylovora, was the first reported two‐domain harpin. Like PopW, it consists of a harpin‐like domain in its N‐terminus and a PL‐like domain in its C‐terminus (Kim and Beer, 1998). The protein sequence of PopW has only 39% identity with that of HrpW from Erwinia amylovora. Thus, we conclude that PopW is a distinct two‐domain harpin.
With regard to the functions of the two domains of PopW, our data demonstrate that its HR‐eliciting activity is dependent only on its harpin domain and does not require the PL domain. In addition, although there is some structural similarity between the PL domain of PopW and those of PLs, PopW showed no PL activity in the present study. Similarly, the PL domain in HrpW from Erwinia amylovora and from P. syringae pv. tomato showed no detectable pectinase activity (Charkowski et al., 1998; Kim and Beer, 1998). This might be ascribed to its pI (5.56), which is different from those of PLs (7.15–9.09) (Henrissat et al., 1995), or the absence of Cys residues. PLs are typically rich in Cys, which might play a crucial role in retaining the stability of their structures and their enzymatic activities. Kim and Beer (1998) inferred that HrpW might target the plant cell wall, and Charkowski et al. (1998) suggested that HrpW might bind to pectate. Our experiments provide evidence that the two‐domain harpin PopW targets the plant cell wall and specifically binds to calcium pectate, and this binding activity is heat stable. The association of PopW with the plant cell wall implies that this protein may play a role in the interaction between R. solanacearum and its hosts (Buttner and Bonas, 2002; Pühler et al., 2004) despite its lack of PL activity. Some one‐domain harpins, including HrpZPss from P. syringae pv. syringae, are also associated with the plant cell wall (Hoyos et al., 1996); however most, such as HrpNEa from Erwinia amylovora (Pike et al., 1998; Popham et al., 1995), PopA from R. solanacearum (Racapéet al., 2005) and HrpZPsph from P. syringae pv. phaseolicola (Lee et al., 2001), target the plant cell membrane.
PopW is not a major virulence factor as the popW mutant and the wild‐type strain caused indistinguishable disease severity in the host plant tomato in this study. This result was also observed for a mutant lacking PopA1, another harpin protein from R. solanacearum (Arlat et al., 1994). In contrast, HrpN and DspA from Erwinia amylovora play a major role in disease development; the virulence of both hrpN and dspA mutants was significantly lower than that of their wild‐type parent (Barny, 1995; Gaudriault et al., 1997). Furthermore, HrpN influences the translocation of DspA/E into Nicotiana tabacum‘Xanthi’, and HrpN and DspA/E are essential for the induction of cell death (Bocsanczy et al., 2008; Sinn et al., 2008). Our observation that the loss of PopW did not obviously affect the virulence of R. solanacearum ZJ3721 could be reflected by the genomic location of popW. It is present on the replicon chromosome that harbours most of the genes indispensable to the basic survival of this bacterium, but many of the genes located on the megaplasmid are involved in the virulence of R. solanacearum (Salanoubat et al., 2002). It is likely that the several harpin‐like proteins produced by R. solanacearum contribute to bacterial wilt virulence in an additive or redundant fashion, so that a single mutation does not noticeably reduce virulence.
The phenotypic and genotypic diversity of R. solanacearum is quite high; indeed, the group is considered to be a species complex (Guidot et al., 2007; Hayward, 1991). To determine whether popW is a highly conserved or core gene for bacterial wilt pathogens, we analysed 20 genetically diverse strains from 14 different host species and six countries, spanning five biovars (Heuer et al., 2007). The DNA sequences of popW from these 20 strains were more than 91% similar (Fig. S1, see Supporting Information). Indeed, this gene could be used as a diagnostic marker, like the ITS (16S to 23S internal transcribed spacer) region, mutS, hrpB and egl (2000a, 2000b), for the specific detection of this bacterium.
The fact that popW is well conserved among different strains of R. solanacearum suggests that PopW may have a function that is important for the survival of this pathogen. Further studies are needed to determine the precise mechanism(s) by which PopW triggers HR in non‐host plants, to understand why it is associated with the plant cell wall, and to determine how this protein contributes to the fitness of R. solanacearum.
EXPERIMENTAL PROCEDURES
Bacterial strains and plasmids
All bacterial strains and plasmids used in this study are listed in Table 1, except for R. solanacearum strains for the popW diversity study, which are listed in Table 3. Ralstonia solanacearum strains were incubated in MBG broth (0.5% bactopeptone, 0.1% casamino acids, 0.1% yeast extracts and 0.1% glucose) or on MBG agar (MBG broth + 1.6% agar) at 28 °C. Glucose minimal medium (MMG) with or without 1.6% agar (Clough et al., 1994) was used. Escherichia coli strains BL21 (DE3) and DH5α were grown in Luria–Bertani (LB) medium at 37 °C (Miller, 1972). Agrobacterium tumefaciens LBA4404 was cultured on yeast extract broth (YEB) medium (Sambrook et al., 1989). Tetracycline (Tc) (10 mg/L) and kanamycin (Km) (50 mg/L) were added to the media.
Table 1.
Strains, plasmids and gene targets used in this study.
| Strain, plasmid | Relevant information* | Reference or source |
|---|---|---|
| Strain | ||
| Escherichia coli DH5α | F‐Φ80dlacZΔM15 recA1 endA1 gyrA96 thi‐1 hsdR17 (rK‐ mK +)supE44 relA1 deoRΔ(lacZYA‐argF)U169 | Gibco‐BRL |
| BL21(DE3) | F‐ ompT hsdSB (rB ‐ mB ‐) gal dcm (DE3) | Novagen |
| BL159 | BL21(DE3) express protein of PopW(1–159) | This study |
| BL366 | BL21(DE3) express protein of PopW(160–366) | This study |
| Agrobacterium tumefaciens LBA4404 | Wild‐type, Rifr, Smr | Hoekema et al., 1983 |
| Ralstonia solanacearum ZJ3721 | Wild‐type isolated from Ipomoea batatas, Zhejiang, China | This study |
| R. solanacearum ZJ3722 | popW‐deficient mutant of ZJ3721 | This study |
| R. solanacearum ZJ3723 | hrpB‐deficient mutant of ZJ3721 | This study |
| R. solanacearum ZJ3724 | hrpV‐deficient mutant of ZJ3721 | This study |
| Plasmid | ||
| pMD19‐T Simple | T‐A cloning vector for polymerase chain reaction (PCR) fragments, Apr | TaKaRa |
| pET30a(+) | T7 promoter‐based expression vector; Kmr | Novagen |
| pETpopW | 1140 bp NdeI and HindIII fragment of popW in pET30a(+) | This study |
| pETpopW(1–159) | popW(1–159) in pET30a(+) | This study |
| pETpopW(160–366) | popW(160–366) in pET30a(+) | This study |
| pTOK2 | Narrow host‐range cloning vector, Tcr | Kitten and Willis, 1996 |
| pTOKpopW′ | 449 bp HindIII and BamHI fragment of interal popW in pTOK2 | This study |
| pTOKhrpB′ | 489 bp HindIII and BamHI fragment of interal hrpB in pTOK2 | This study |
| pTOKhrpV′ | 599 bp HindIII and BamHI fragment of interal hrpV in pTOK2 | This study |
| pBI121‐GFP | Plant expression binary vector, Kmr | Andreeva and Kutuzov, 2001 |
Apr, Kmr, Tcr, Rmr and Smr indicate ampicillin‐, kanamycin‐, tetracycline‐, rifampicin‐ and streptomycin‐resistant, respectively.
Only the host and origin of Ralstonia solanacearum strains were provided.
DNA manipulation, transformation and sequence analysis
The cloning, preparation of competent cells, electroporation and heat‐shock transformation of E. coli strains followed standard protocols (Ausubel et al., 1989). PCR primers (Table 2) were synthesized by Shanghai SBS Genetech Co., Ltd. (Shanghai, China). Genomic and plasmid DNAs from R. solanacearum were isolated using an Axyprep Bacterial Genomic DNA Kit and Axyprep Plasmid Minikit Kit, respectively (Hangzhou, China). Restriction enzymes and T4 DNA ligase were purchased from TaKaRa (Dalian, China). DNA sequencing was performed in the Unite Gene Biotechnology Laboratory (Shanghai, China).
Table 2.
Primers used in this study.
| Gene target | Primer sequence (5′ → 3′)* | Restriction site added |
|---|---|---|
| popW | popW F: CGCATATGTCCATCCAGATTGATCGC | NdeI |
| popW R: GCAAGCTTGCCCGAGTAGGCCTTGTAG | HindIII | |
| popW (1–159) | popW(1–159) F: ATAAGCTTGGTCGGCTCGGGCGGCTT | HindIII |
| popW(1–159) R: GCCATATGTCCATCCAGATTGATCGCCCG | NdeI | |
| popW (160–366) | popW(160–366) F: GCAAGCTTCAGCCCATCGAAGGGCGCATC | HindIII |
| popW(160–366) R: GCCATATGGGCGTGGTCGACGTCAGCAAG | NdeI | |
| popW′ | popW′ F: GCGGATCCCCACGGCGGCGGTCATCAT | BamHI |
| popW′ R: CGAAGCTTCGTCGCCGCCGG A ATACTGC | HindIII | |
| hrpB′ | hrpB′ F: GCGGATCCATTTCGACCGTGCGTATCAGC | BamHI |
| hrpB′ R: GCAAGCTTTCGAGCAGGCGGTTGGAGCAG | HindIII | |
| hrpV′ | hrpV′ F: GCAAGCTTGCGGACGAGCCGACCACCGACAG | HindIII |
| hrpV′ R: GCGGATCCGCGCTGAGCAGCATCAAGGGCAGCA | BamHI | |
| popW″ | popW″ F: TCTAGAATGTCCATCCAGATTGATCGC | XbaI |
| popW″ R: GGATCCGCCCGAGTAGGCCTTGTAGCT | BamHI |
Italic letters show restriction endonuclease recognition sequences. ‘F’ and ‘R’ denote forward and reverse primers of the genes, respectively.
Mutagenesis of popW, hrpB and hrpV
To inactivate popW, hrpB and hrpV, internal fragments of 449 bp of popW (from nucleotide +210 to +658), 489 bp of hrpB (from nucleotide +89 to +577) and 599 bp of hrpV (from nucleotide +262 to +860) were amplified by PCR with the primer pairs popW′F/popW′R, hrpB′F/hrpB′R and hrpV′F/hrpV′R, respectively (Table 2). Each DNA fragment was cloned into the TA cloning vector pMD19‐T Simple (TaKaRa). These fragments were digested by BamHI and HindIII from the recombinant plasmids and were subcloned into the suicide plasmid pTOK2 (Kitten and Willis, 1996) to create pTOKpopW′, pTOKhrpB′ and pTOKhrpV′, respectively. Then, the three plasmids derived from pTOK2 were electroporated into the competent cells of R. solanacearum. This created the mutant strains ZJ3722 (popW), ZJ3723 (hrpB) and ZJ3724 (hrpV). The transformants were selected following PCR, as described by Liu et al. (2005), and confirmed by Southern blotting using popW as the probe, according to the manufacturer's instructions of the DIG high prime DNA labelling and detection starter kit I (Roche, Germany).
Overexpression and purification of PopW, PopW(1–159) and PopW(160–366)
The DNA fragments encoding PopW, PopW(1–159) (the N‐terminal 159 amino acids of PopW) and PopW(160–366) (the C‐terminal 207 amino acids of PopW) were amplified by PCR using primers popWF/popWR, popW(1–159)F/popW(1–159)R and popW(160–366)F/popW(160–366)R, respectively. Their PCR products were ligated into pET30a(+) digested with NdeI and HindIII, and the resulting recombinant plasmids were introduced into E. coli strain BL21 (DE3) for protein overexpression. The expected clones in the kanamycin‐resistant colonies were confirmed by PCR amplification and digestion. Selected transformants harbouring popW or its subfragments were grown overnight in LB containing Km; the cultures were diluted 100‐fold with LB and incubated at 37 °C at 180 r.p.m. When the optical density at 600 nm (OD600) of the cultures reached 0.8, isopropyl‐β‐D‐thiogalactopyranoside (IPTG) was added at a final concentration of 1 mm, and the cultures were grown at 37 °C for an additional 3 h to induce gene expression. Subsequently, the cells were harvested by centrifugation at 1.0 × 104 g for 10 min at 4 °C, resuspended in 20 mm Tris‐HCl (pH 8.0), sonicated in the presence of 1 mm phenylmethylsulphonyl fluoride (PMSF) and centrifuged at 1.0 × 104 g for 10 min at 4 °C. Supernatant proteins were quantified with bovine serum albumin as the standard (Bradford, 1976). These protein samples were checked by 10% SDS‐PAGE and were also used for the analysis of HR‐eliciting activity in tobacco leaves. PopW, PopW(1–159) and PopW(160–366), with 6 × His tags at their C‐terminals, were purified using High‐Affinity Ni‐IDA Resin, as described by Kim et al. (2004).
Heat treatment and proteinase K digestion of PopW
PopW was heated at 100 °C for 10 min in a boiling water bath. To determine the proteinaceous nature of its HR‐eliciting activity, 100 µg of protein were incubated in 10 mm Tris (pH 8.0) containing 1 mm CaCl2 with 30 µg proteinase K (TaKaRa, China) at 37 °C for 2 h. Subsequently, the reaction was boiled for 10 min to inactivate the enzyme. Proteinase K‐treated samples were monitored by SDS‐PAGE and tested for their HR‐eliciting activity in tobacco.
Virulence assay and HR test
Tomato plants (cv. Shanghai 903) were cultivated in 5‐cm pots filled with a soil–peat mixture. Plants (height, 10–15 cm) were selected for inoculation. Fifty millilitres of an aqueous suspension containing 5 × 108 colony‐forming units (cfu)/mL of R. solanacearum were poured on the soil near the roots of the tomato plant in the pot. Inoculated plants were then moved into a growth chamber (30 °C, 16 h light, 1.5 × 104 lx, 70% humidity). Both wild‐type ZJ3721 and ZJ3722 with mutated popW were tested for virulence in three biological replicates of 24 plants for each treatment, and disease development was scored daily using a disease index in the range 0–4 (0, symptomless plants; 1, 1–25% of leaves wilted; 2, 26–50% of leaves wilted; 3, 51–75% of leaves wilted; 4, 76–100% of leaves wilted or dead) (Roberts et al., 1988). Disease severity was calculated according to the following formula:
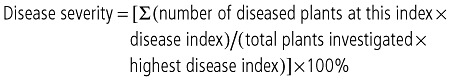 |
HR‐eliciting ability was tested by infiltrating a protein preparation or bacterial suspension into the intercellular space of tobacco leaves (Nicotiana tabacum cv. Samsun NN) (Kim et al., 1997). Plants were incubated in a growth chamber (30 °C, 16 h light, 1.5 × 104 lx, 70% humidity). The response of the plants was observed 12–48 h after inoculation.
Subcellular localization of PopW
The DNA sequence of popW without its stop codon was amplified with primers popW″ F/popW″ R (Table 2) and cloned into plasmid pBI121‐GFP digested with BamHI and XbaI. The resulting PopW:GFP fusion plasmid pBI121‐PopW:GFP and the control plasmid pBI121‐GFP were introduced into A. tumefaciens LBA4404. Onion epidermal cells were inoculated separately with the strains harbouring these two plasmids, as described by Niu et al. (2005). GFP was visualized and photographed under a fluorescence microscope (Leica, Wetzlar, Germany) equipped with a 450–490‐nm excitation filter and 505‐nm barrier filter.
Pectate binding assay
The ability of PopW to bind pectate was determined according to the method described by Charkowski et al. (1998) with minor modifications. CaCl2 (100 mm) was added dropwise to vigorously stirred 0.2% (w/v) sodium pectate (Sigma, St. Louis, MO, USA) dissolved in 100 µm Tris (pH 8.0) to prepare calcium pectate beads. The beads were pelleted by low‐speed centrifugation and resuspended in two volumes of the buffer. For the pectate binding assay, 500 µg of purified PopW protein was mixed with 500 µL of the resuspended calcium pectate beads, and the protein–bead mix was incubated at room temperature for 12 h. The beads were then pelleted by centrifugation, washed 10 times with 500 µL of buffer each time, and resuspended in 500 µL of the same buffer; ethylenediaminetetraacetic acid (EDTA) was added to the washed beads to solubilize any attached PopW protein. Samples were collected of the original protein–bead mix prior to centrifugation, the supernatant after centrifugation, the wash buffer at each step and the washed beads treated with EDTA. Aliquots of 20 µL of the original protein–bead mix, supernatant, final wash buffer and washed beads treated with EDTA were subjected to SDS‐PAGE. Calcium alginate beads were prepared from medium‐viscosity sodium alginate (Genetime, Nanjing, China), and an alginate binding assay was conducted according to the protocol described above. The possibility of PopW binding to either calcium chloride (100 mm) or 0.2% (w/v) soluble pectate was assessed with the addition of 0.2% (w/v) hydrated beads of agarose (Genetime).
Antibody production and Western blotting
A rabbit was injected with 1 mL of purified PopW (1 mg/mL) three times at 2‐week intervals. The rabbit blood was collected 2 weeks after the final injection and an anti‐PopW polyclonal antibody was isolated as described by Ausubel et al. (1989).
Ralstonia solanacearum ZJ3721 and its derivatives (ZJ3722, ZJ3723 and ZJ3724) were incubated in MMG at 28 °C for 20 h. Cultures were centrifuged at 1.5 × 104 g for 15 min. The supernatant was dialysed (molecular mass cut off, 10 kDa) against MilliQ water and then concentrated to 0.01 × volume of the original culture by lyophilization, and the resulting solution was designated the CSP. The cell pellets resulting from centrifugation were resuspended in MilliQ water in 0.01 × volume of the original culture, and sonicated. The sonicated lysate was centrifuged at 1.5 × 104 g for 10 min to remove the cell debris, and the resulting supernatant was designated the CLP.
The CSPs and CLPs from all four strains were loaded onto two 10% SDS‐PAGE gels and subjected to electrophoresis. Subsequently, one gel was stained with Coomassie Brilliant Blue R, and the proteins on the other gel were transferred onto a nitrocellulose membrane. Immunoblotting was performed using 5 × 103‐fold diluted rabbit polyclonal antibody against PopW. Alkaline phosphatase‐conjugated goat anti‐rabbit (Sigma), the secondary antibody, was visualized by 5‐bromo‐4‐chloro‐3‐indolylphosphate (BCIP)‐nitroblue tetrazolium according to the instructions of the manufacturer (Sigma).
Pectic enzyme assay of PopW
The cells of E. coli BL21 (DE3) harbouring pETpopW were pelleted, resuspended in 10 mm Tris‐HCl (pH 8.5) in 0.1 × volume of original culture, sonicated on ice and centrifuged. The PopW proteins in the supernatant were purified with High‐Affinity Ni‐IDA Resin (Jinsite, Shanghai, China), and the PL activity of the purified PopW was determined. The 100 × concentrated culture supernatant of R. solanacearum ZJ3721 was tested for PL activity using the method described by Kim and Beer (1998).
Distribution of popW among diverse R. solanacearum strains
To examine the presence of popW in heterogeneous strains of R. solanacearum, we chose 19 strains with a wide genetic diversity (Table 3). These strains were isolated from 14 different host species from six countries and spanning five biovars (Heuer et al., 2007). The popW gene was detected by PCR amplification with the same pair of primers as used to clone popW from ZJ3721. Multiple sequence alignment was performed using the program ClustalX 1.83. The phylogenetic tree was generated using the software package mega 3.1 by neighbour joining.
Supporting information
Fig. S1 Phylogenetic tree of the popW sequences of 20 Ralstonia solanacearum strains. The numbers at the nodes represent the bootstrap values.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
ACKNOWLEDGEMENTS
This research was supported by Chinese 863 High‐Tech Program (2006AA10Z431), the National Natural Science Foundations of China (30800714, 30971956), the Specialized Research Fund for the Doctoral Program of Higher Education of China (200803071032) and the Program for New Century Excellent Talents in University (NCET‐06‐0492). We thank Dr David K. Willis (University of Wisconsin, Madison, WI, USA) for the kind gift of suicide plasmid pTOK2, and J. Feng (Chinese Academy of Agricultural Sciences, Beijing, China), T. Lu (Fujian Academy of Agricultural Sciences, Fuzhou, China) and K. Smalla [Julius Kühn‐Institut (JKI), Braunschweig, Germany] for the gift of strains of R. solanacearum.
REFERENCES
- Alfano, J.R. and Collmer, A. (1996) Bacterial pathogens in plants: life up against the wall. Plant Cell, 8, 1683–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreeva, A.V. and Kutuzov, M.A. (2001) Nuclear localization of plant protein Ser/Thr phosphatase PP7. Mol. Cell Biol. Res. Commun. 4, 345–352. [DOI] [PubMed] [Google Scholar]
- Arlat, M. , Van, G.F. , Huet, J.C. , Pernollet, J.C. and Boucher, C.A. (1994) PopA l, a protein which induced a hypersensitivity‐like response on specific Petunia genotypes, is secreted via the Hrp pathway of Pseudomonas solanecearum . EMBO J. 13, 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel, F.M. , Brent, R. , Kingston, R.E. , More, D.D. , Seidman, J.G. , Smith, J.A. and Struhl, K. (1989) Short Protocols in Molecular Biology. New York: Green Publishing Association and Wiley‐Interscience. [Google Scholar]
- Barny, M.A. (1995) Erwinia amylovora hrpN mutants, blocked in harpin synthesis, express a reduced virulence on host plants and elicit variable hypersensitive reactions on tobacco. Eur. J. Plant Pathol. 101, 333–340. [Google Scholar]
- Bocsanczy, A.M. , Nissinen, R.M. , Oh, C.S. and Beer, S.V. (2008) HrpN of Erwinia amylovora functions in the translocation of DspA/E into plant cells. Mol. Plant Pathol. 9, 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford, M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Buttner, D. and Bonas, U. (2002) Getting across—bacterial type III effector proteins on their way to the plant cell. EMBO J. 21, 5313–5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charkowski, A.O. , Alfano, J.R. , Preston, G. , Yuan, J. , He, S.Y. and Collmer, A. (1998) The Pseudomonas syringae pv. tomato HrpW protein has domains similar to harpins and pectate lyases and can elicit the plant hypersensitive response and bind to pectate. J. Bacteriol. 180, 5211–5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough, S.J. , Schell, M.A. and Denny, T.P. (1994) Evidence for involvement of a volatile extracellular factor in Pseudomonas solanacearum virulence gene expression. Mol. Plant–Microbe Interact. 7, 621–630. [Google Scholar]
- Cunnac, S. , Occhialini, A. , Barberis, P. , Boucher, C. and Genin, S. (2004) Inventory and functional analysis of the large Hrp regulon in Ralstonia solanacearum: identification of novel effector proteins translocated to plant host cells through the type III secretion system. Mol. Microbiol. 53, 115–128. [DOI] [PubMed] [Google Scholar]
- Denny, T.P. (2000) Ralstonia solanacearum—plant pathogen in touch with its host. Trends Microbiol. 8, 486–489. [DOI] [PubMed] [Google Scholar]
- Deslandes, L. , Olivier, J. , Peeters, N. , Feng, D.X. , Khounlotham, M. , Boucher, C. , Somssich, I. , Genin, S. and Marco, Y. (2003) Physical interaction between RRS1‐R a protein conferring resistance to bacterial wilt and PopP2 a type III effector targeted to the plant nucleus. Proc. Natl Acad. Sci. USA, 100, 8024–8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouts, D.E. , Abramovitch, R.B. , Alfano, J.R. , Baldo, A.M. , Buell, C.R. , Cartinhour, S. , Chatterjee, A.K. , D'Ascenzo, M. , Gwinn, M.L. , Lazarowitz, S.G. , Lin, N.C. , Martin, G.B. , Rehm, A.H. , Schneider, D.J. , Van Dijk, K. , Tang, X.Y. and Collmer, A. (2002) Genome wide identification of Pseudomonas syringae pv. tomato DC3000 promoters controlled by the HrpL alternative sigma factor. Proc. Natl Acad. Sci. USA, 99, 2275–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudriault, S. , Malandrin, L. , Paulin, J.P. and Barny, M.A. (1997) DspA, an essential pathogenicity factor of Erwinia amylovora showing homology with AvrE of Pseudomonas syringae, is secreted via Hrp secretion pathway in a DspB‐dependent way. Mol. Microbiol. 26, 1057–1069. [DOI] [PubMed] [Google Scholar]
- Genin, S. and Boucher, C.A. (2004) Lessons learned from the genome analysis of Ralstonia solanacearum . Annu. Rev. Phytopathol. 42, 107–134. [DOI] [PubMed] [Google Scholar]
- González, E.T. , Brown, D.G. , Swanson, J.K. and Allen, C. (2007) Using the Ralstonia solanacearum Tat secretome to identify bacterial wilt virulence factors. Appl. Environ. Microbiol. 73, 3779–3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guéneron, M. , Timmers, A.C. , Boucher, C. and Arlat, M. (2000) Two novel proteins, PopB, which has functional nuclear localization signals, and PopC, which has a large leucine‐rich repeat domain, are secreted through the hrp‐secretion apparatus of Ralstonia solanacearum . Mol. Microbiol. 36, 261–277. [DOI] [PubMed] [Google Scholar]
- Guidot, A. , Prior, P. , Schoenfeld, J. , Carrere, S. , Genin, S. and Boucher, C. (2007) Genomic structure and phylogeny of the plant pathogen Ralstonia solanacearum inferred from gene distribution analysis. J. Bacteriol. 189, 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward, A.C. (1991) Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum . Annu. Rev. Phytopathol. 29, 65–87. [DOI] [PubMed] [Google Scholar]
- He, S.Y. , Huang, H.C. and Collmer, A. (1993) Pseudomonas syringae pv. syringae harpinpss: a protein that is secreted via the hrp pathway and elicits the hypersensitive response in plants. Cell, 73, 1255–1266. [DOI] [PubMed] [Google Scholar]
- Henrissat, B. , Heffron, S.E. , Yoder, M.D. , Lietzke, S. and Jurnak, F. (1995) Functional implications of structure‐based sequence alignment of proteins in the extracellular pectate lyase superfamily. Plant Physiol. 107, 963–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer, H. , Yin, Y.N. , Xue, Q.Y. , Smalla, K. and Guo, J.H. (2007) Repeat domain diversity of avrBs3‐like genes in Ralstonia solanacearum strains and association with host preferences in the field. Appl. Environ. Microbiol. 73, 4379–4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekema, A. , Hirsch, P.R. , Hooykaas, P.J.J. and Schilperoort, R.A. (1983) A binary plant vector strategy based on separation of vir‐ and T‐region of the Agrobacterium tumefaciens Ti‐plasmid. Nature, 303, 179–180. [Google Scholar]
- Hoyos, M.E. , Stanley, C.M. , He, S.Y. , Pike, S. , Pu, X.A. and Novacky, A. (1996) The interaction of HarpinPss, with plant cell walls. Mol. Plant–Microbe Interact. 9, 608–616. [Google Scholar]
- Jones, D.T. (1999) Protein secondary structure prediction based on position‐specific scoring matrices. J. Mol. Biol. 292, 195–202. [DOI] [PubMed] [Google Scholar]
- Kim, J.F. and Beer, S.V. (1998) HrpW of Erwinia amylovora, a new harpin that contains a domain homologous to pectate lyases of a distinct class. J. Bacteriol. 180, 5203–5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.F. , Wei, Z.M. and Beer, S.V. (1997) The hrpA and hrpC operons of Erwinia amylovora encode components of a type III pathway that secretes harpin. J. Bacteriol. 179, 1690–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.G. , Jeon, E. , Oh, J. , Moon, J.S. and Hwang, I. (2004) Mutational analysis of Xanthomonas harpin HpaG identifies a key functional region that elicits the hypersensitive response in nonhost plants. J. Bacteriol. 186, 6239–6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitten, T. and Willis, D.K. (1996) Suppression of a sensor kinase‐dependent phenotype in Pseudomonas syringae by ribosomal proteins L35 and L20. J. Bacteriol. 178, 1548–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. , Klüsener, B. , Tsiamis, G. , Stevens, C. , Neyt, C. , Tampakaki, A.P. , Panopoulos, N.J. , NÖller, J. , Weiler, E.W. , Cornelis, G.R. , Mansfield, J.W. and Nürnberger, T. (2001) HrpZPsph from the plant pathogen Pseudomonas syringae pv. phaseolicola binds to lipid bilayers and forms an ion‐conducting pore in vitro . Proc. Natl Acad. Sci. USA, 98, 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J.G. , Liu, H.X. and Guo, J.H. (2008) PopW of Ralstonia solanacearum, a harpin that can induce tobacco resistance to tobacco mosaic virus. Phytopathology, 98, 89. [Google Scholar]
- Liu, H. , Zhang, S. , Schell, M.A. and Denny, T.P. (2005) Pyramiding unmarked deletions in Ralstonia solanacearum shows that secreted proteins in addition to plant cell‐wall‐degrading enzymes contribute to virulence. Mol. Plant–Microbe Interact. 18, 1296–1305. [DOI] [PubMed] [Google Scholar]
- Marchler‐Bauer, A. , Anderson, J.B. , Derbyshire, M.K. , DeWeese‐Scott, C. , Gonzales, N.R. , Gwadz, M. , Hao, L. , He, S. , Hurwitz, D.I. , Jackson, J.D. , Ke, Z. , Krylov, D. , Lanczycki, C.J. , Liebert, C.A. , Liu, C. , Lu, F. , Lu, S. , Marchler, G.H. , Mullokandov, M. , Song, J.S. , Thanki, N. , Yamashita, R.A. , Yin, J.J. , Zhang, D. and Bryant, S.H. (2007) CDD: a conserved domain database for interactive domain family analysis. Nucleic Acids Res. 35, 237–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuffin, L.J. , Bryson, K. and Jones, D.T. (2000) The PSIPRED protein structure prediction. Bioinformatics, 16, 404–405. [DOI] [PubMed] [Google Scholar]
- Miller, J.H. (1972) Experiments in Molecular Genetics. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Murashige, T. and Skoog, F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473–497. [Google Scholar]
- Niu, X.G. , Chen, Q.J. , Liu, Q. and Wang, X.C. (2005) Cloning, subcellular localization and expression analysis of an inositol 1,3,4‐trisphosphate 5/6‐kinase‐like gene in Oryza sativa. J. Agric. Biotechnol. 13, 815–816. [Google Scholar]
- Occhialini, A. , Cunnac, S. , Reymond, N. , Genin, S. and Boucher, C. (2005) Genome wide analysis of gene expression in Ralstonia solanacearum reveals that the hrpB gene acts as a regulatory switch controlling multiple virulence pathways. Mol. Plant–Microbe Interact. 18, 938–949. [DOI] [PubMed] [Google Scholar]
- Pike, S.M. , Adam, A.L. , Pu, X.A. , Hoyos, M.E. , Laby, R. , Beer, S.V. and Novacky, A. (1998) Effects of Erwinia amylovora harpin on tobacco leaf cell membranes are related to leaf necrosis and electrolyte leakage and distinct from perturbations caused by inoculated E. amylovora . Physiol. Mol. Plant Pathol. 53, 39–60. [Google Scholar]
- Popham, P. , Pike, S. and Novacky, A. (1995) The effect of harpin from Erwinia amylovora on the plasmalemma of suspension‐cultured tobacco cells. Physiol. Mol. Plant Pathol. 47, 39–50. [Google Scholar]
- Poussier, S. , Trigalet‐Demery, D. , Vandewalle, P. , Goffinet, B. , Luisetti, J. and Trigalet, A. (2000a) Genetic diversity of Ralstonia solanacearum as assessed by PCR‐RFLP of the hrp gene region, AFLP and 16S rRNA sequence analysis and identification of an African subdivision. Microbiology, 146, 1679–1692. [DOI] [PubMed] [Google Scholar]
- Poussier, S. , Prior, P. , Luisetti, J. , Hayward, C. and Fegan, M. (2000b) Partial sequencing of the hrpB and endoglucanase genes confirms and expands the known diversity within the Ralstonia solanacearum species complex. Syst. Appl. Microbiol. 23, 479–486. [DOI] [PubMed] [Google Scholar]
- Pühler, A. , Arlat, M. , Becker, A. , GÖttfert, M. , Morrissey, J.P. and O'Gara, F. (2004) What can bacterial genome research teach us about bacteria–plant interactions? Curr. Opin. Plant Biol. 7, 137–147. [DOI] [PubMed] [Google Scholar]
- Racapé, J. , Belbahri, L. , Engelhardt, S. , Lacombe, B. , Lee, J. , Lochman, J. , Marais, A. , Nicole, M. , Nurnberger, T. , Parlange, F. , Puverel, S. and Keller, H. (2005) Ca2+‐dependent lipid binding and membrane integration of PopA, a harpin‐like elicitor of the hypersensitive response in tobacco. Mol. Microbiol. 58, 1406–1420. [DOI] [PubMed] [Google Scholar]
- Roberts, D.P. , Denny, T.P. and Scbell, M.A. (1988) Cloning of the egl gene of Pseudomonas solanacearum and analysis of its role in phytopathogenicity. J. Bacteriol. 170, 1445–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanoubat, M. , Genin, S. , Artiguenave, F. , Gouzy, J. , Mangenot, S. , Arlat, M. , Billault, A. , Brottier, P. , Camus, J.C. , Cattolico, L. , Chandler, M. , Choisne, N. , Claudel‐RenardI, C. , Cunnac, S. , Demange, N. , Gaspin, C. , Lavie, M. , Moisan, A. , Robert, C. , Saurin, W. , Schiex, T. , Siguier, P. , Thébault, P. , Whalen, M. , Wincker, P. , Levy, M. , Weissenbach, J. and Boucher, C.A. (2002) Genome sequence of the plant pathogen Ralstonia solanacearum . Nature, 415, 497–502. [DOI] [PubMed] [Google Scholar]
- Sambrook, J. , Fritsch, E.F. and Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual, 2nd edn. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Sinn, J.P. , Oh, C.S. , Jensen, P.J. , Carpenter, S.C. , Beer, S.V. and McNellis, T.W. (2008) The C‐terminal half of the HrpN virulence protein of the fire blight pathogen Erwinia amylovora is essential for its secretion and for its virulence and avirulence activities. Mol. Plant–Microbe Interact. 21, 1387–1397. [DOI] [PubMed] [Google Scholar]
- Valls, M. , Genin, S. and Boucher, C. (2006) Integrated regulation of the Type III secretion system and other virulence determinants in Ralstonia solanacearum . PLoS Pathog. 2, e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gijsegem, F. , Genin, S. and Boucher, C.A. (1993) Conservation of secretion pathways for pathogenicity determinants of plant and animal bacteria. Trends Microbiol. 1, 175–180. [DOI] [PubMed] [Google Scholar]
- Wei, Z.M. , Laby, R.J. , Zumoff, C.H. , Bauer, D.W. , He, S.Y. , Collmer, A. and Beer, S.V. (1992) Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora . Science, 257, 85–88. [DOI] [PubMed] [Google Scholar]
- Willis, D.K. , Rich, J.J. and Hrabak, E.M. (1991) hrp genes of phytopathogenic bacteria. Mol. Plant–Microbe Interact. 4, 132–138. [Google Scholar]
- Yabuuchi, E. , Kosako, Y. , Yano, I. , Hotta, H. and Nishiuchi, Y. (1995) Transfer of two Burkholderia and an Alcaligenes species to Ralstonia general nov: proposal of Ralstonia pickettii (Ralston, Palleroni and Doudoroff 1973) comb. nov, Ralstonia solanacearum (Smith 1896) comb. nov and Ralstonia eutropha (Davis 1969) comb. Microbiol. Immunol. 39, 897–904. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Phylogenetic tree of the popW sequences of 20 Ralstonia solanacearum strains. The numbers at the nodes represent the bootstrap values.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item


