SUMMARY
To survive, plants possess elaborate defence mechanisms to protect themselves against virus or pathogen invasion. Recent studies have suggested that plant mitochondria may play an important role in host defence responses to biotic stresses. In contrast with animal mitochondria, plant mitochondria possess a unique respiratory pathway, the cyanide‐insensitive alternative pathway, which is catalysed by the alternative oxidase (AOX). Much work has revealed that the genes encoding AOX, AOX protein and the alternative respiratory pathway are frequently induced during plant–pathogen (or virus) interaction. This raises the possibility that AOX is involved in host defence responses to biotic stresses. Thus, a key to the understanding of the role of mitochondrial respiration under biotic stresses is to learn the function and regulation of AOX. In this article, we focus on the theoretical and experimental progress made in the current understanding of the function and regulation of AOX under biotic stresses. We also address some speculative aspects to aid further research in this area.
INTRODUCTION
In the natural environment, plants encounter a wide range of microbial pathogens or virus invasions during their lifetime. To survive, plants are equipped with highly elaborate and flexible defence mechanisms to protect themselves against these biotic stresses (Koornneef and Pieterse, 2008; Pieterse and Dicke, 2007). Mitochondrial respiration, as one of the most important metabolic processes of plant cells, should evolve to meet the metabolic demands of the host defence strategy.
In higher plants, electrons produced by the respiratory oxidation of the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) can flow through the usual cytochrome respiratory pathway or the alternative respiratory pathway. It is well known that the alternative respiratory pathway is catalysed by the alternative oxidase (AOX), which is located in the mitochondrial inner membrane and acts as a terminal oxidase in the mitochondrial electron transport chain (mtETC). AOX branches from the main respiratory chain at the level of the ubiquinone pool and catalyses the four‐electron reduction of oxygen to water. Thus, the operation of the alternative pathway bypasses two of three sites of energy conservation supporting oxidative phosphorylation (complexes III and IV) and leads to the release of energy as heat (Millenaar and Lambers, 2003). It is believed that AOX is a unique component of plant mtETC, and its presence allows the flexibility of plant respiratory metabolism, especially under environmental stresses (Mackenzie and McIntosh, 1999; Vanlerberghe and McIntosh, 1997).
Based on studies of the molecular distinction among AOXs from different plant species, it was found that AOX is encoded by a small family of nuclear genes in a wide variety of monocotyledonous and eudicotyledonous plants (Considine et al., 2002). From the data presently known, there exist two discrete AOX gene subfamilies: AOX1‐ and AOX2‐type genes. Generally, AOX1 is most widely known for its induction by stress stimuli in many tissues, and is present in both monocot and eudicot plant species, but AOX2 is usually constitutive or developmentally expressed in eudicot species and is absent from the genomes of all monocot species (Considine et al., 2002). It is supposed that different isoforms of AOX proteins encoded by separate genes could have different catalytic properties and thereby play different physiological roles (Grant et al., 2009).
Most, if not all, stressful conditions, including drought (Bartoli et al., 2005), high salt (Costa et al., 2007), chilling (Mizuno et al., 2008) and aluminium stress (Panda et al., 2008), increase the AOX transcript abundance, the contents of AOX protein or the level of the alternative respiratory pathway. Currently, AOX is receiving considerable attention from plant scientists, and is believed to play an important role in abiotic stress alleviation. In addition, many studies have revealed that the genes encoding AOX, AOX protein and the alternative respiratory pathway are frequently induced during plant–pathogen (or virus) interactions (see below). Thus, it is expected that a key to the understanding of the role of mitochondrial respiration under biotic stresses is to learn the function and regulation of AOX.
THE EFFECTS OF BIOTIC STRESSES ON THE ALTERNATIVE PATHWAY AND AOX EXPRESSION
An increase in the respiratory rate of a host is a widespread phenomenon during plant–pathogen (or virus) interaction (Dwurazna and Weintraub, 1969; Farrar, 1992; Simons et al., 1999). Simons et al. (1999) found that infection of Arabidopsis leaves with avirulent or virulent Pseudomonas syringae pv. tomato strain resulted in an increase in both total respiration and cyanide‐resistant O2 uptake (i.e. the ‘capacity’ of AOX) levels. However, because the electrons flowing through the cytochrome respiratory pathway can be redirected to the alternative pathway on addition of cyanide (Vanlerberghe and McIntosh, 1992), the ‘capacity’ of the AOX pathway measured in the presence of cyanide does not necessarily represent the actual activity of the AOX pathway. The only method to date to accurately determine AOX activity is to use oxygen isotope discrimination (Robinson et al., 1995). With the use of this technique, it was reported that the bacterial elicitor harpin NEa induced a twofold respiratory burst in tobacco leaves, which was essentially a result of the fivefold induction of the activity of the alternative pathway. By contrast, the activity of the cytochrome pathway increased by merely 50% (Vidal et al., 2007). These results indicate an actual and positive contribution of the alternative pathway to the enhancement of total respiration during pathogen–plant interactions.
Many studies have also shown that infection with bacterial pathogens and their elicitors can strongly enhance the content of AOX protein or the level of AOX transcript in either leaves or cell suspensions (Kiba et al., 2007; Lacomme and Roby, 1999; Simons et al., 1999; Vidal et al., 2007). In Arabidopsis cell suspensions, the expression of the AOX gene was induced by infection with the avirulent strain of bacterium Xanthomonas campestris pv. campestris, but no increase in AOX transcript was found in the cells infected by the virulent X. campestris pv. campestris strain (Lacomme and Roby, 1999). On the basis of this observation, it seems that the induction of AOX only occurs during incompatible interaction. However, observations using Arabidopsis leaf tissue appear to show differences from those obtained from Arabidopsis cell suspensions. Simons et al. (1999) found that, compared with infection with the avirulent P. syringae pv. tomato strain, infection with the virulent P. syringae pv. tomato strain led to a similar, but delayed, increase in AOX protein and transcript in Arabidopsis leaves.
Virus infection can also lead to an increase in AOX transcript or AOX protein amounts. Chivasa and Carr (1998) reported that infection with Tobacco mosaic virus (TMV) induced a high level of AOX transcript in the resistant tobacco (Nicotiana tabacum L.) cultivar, whereas no increase in AOX transcript was detected in the infected susceptible tobacco cultivar. Interestingly, Lennon et al. (1997) reported that there were no changes in the activity of the alternative pathway and the partitioning of electrons to the alternative pathway in the leaves of the susceptible tobacco cultivar infected by TMV, despite an increase in the amount of AOX protein. However, Lennon et al. (1997) also speculated that the small percentage of tissue near the infected site could show increased AOX activity and partitioning of electrons during TMV infection.
To date, the responses of the alternative respiratory pathway and AOX genes or proteins to biotic stresses are not well understood. Moreover, as a result of the large differences in experimental materials and approaches used in these different studies, it may be difficult to achieve a comparative understanding of the responses of the AOX pathway or AOX proteins and genes to biotic stresses. In addition, although much work has reported that AOX transcripts are induced in response to pathogen or virus infection, this work did not use specific probes for separate member(s) of the AOX gene family (Table 1), meaning that the expression patterns of the AOX gene family under biotic stresses are still unknown. However, typically, although not exclusively, it can be concluded from most available studies that an increase in the AOX pathway or the expression of AOX is a common result of pathogen or virus infection. Table 1 presents the main studies on the changes in the alternative respiratory pathway and the expression of the AOX gene (or protein) during different types of plant–pathogen (or virus) interaction.
Table 1.
A summary of the main studies on the changes in the alternative respiratory pathway and the expression of the alternative oxidase (AOX) gene or protein during different types of plant–pathogen (or virus) interaction.
| Experimental system | Events | Reference |
|---|---|---|
| Arabidopsis thaliana; cell suspensions infected with Xanthomonas campestris pv. campestris | AOX transcript was transiently induced by avirulent strain. No detectable AOX expression during virulent infection | Lacomme and Roby, 1999 |
| Arabidopsis thaliana; leaves infected with Pseudomonas syringae pv. tomato | Infiltration with avirulent strain resulted in a rapid increase in cyanide‐resistant O2 uptake, AOX mRNA and AOX protein content, whereas the increase was delayed in the compatible combination | Simons et al., 1999 |
| Nicotiana tabacum L. (susceptible cultivar); leaves infected with Ttobacco mosaic virus (TMV) | An enhancement in AOX protein content was observed in infected leaves, but there were no changes in the partitioning of electrons to the alternative pathway and the activity of the alternative pathway | Lennon et al., 1997 |
| N. tabacum L.; leaves infected with TMV | High levels of AOX transcript in resistant cultivar; no increase in AOX transcript in susceptible cultivar | Chivasa and Carr, 1998 |
| Nicotiana sylvestris; leaves infected with harpin from Erwinia amylovora | Transcript of AOX was transiently induced at 1 h after harpin treatment | Garmier et al., 2002 |
| Lactuca sativa cv. Success; leaves infected with Pseudomonas cichorii (Pc) or P. syringae pv. syringae (Pss) | Continuous increase in AOX transcription during the development of Pc‐induced soft rotting disease. Two phases of AOX gene expression were observed during the Pss‐induced hypersensitive response | Kiba et al., 2007 |
| Nicotiana sylvestris; leaves infected with bacterial elicitor harpin NEa | The activity of the alternative pathway increased within the first 8 h after harpin treatment. AOX transcripts increased transiently at 1 h after harpin treatment | Vidal et al., 2007 |
| Nicotiana tabacum cv. Samsun; leaves infected with tobacco necrosis virus (TNV) or TMV | The levels of AOX transcript displayed a transient suppression within the first 6 h, but increased again at 20 and 24 h. | Künstler et al., 2007 |
| Arabidopsis thaliana; suspension cells infected with harpin from Pseudomonas syringae | Strong induction of AOX transcript within the first 4 h after harpin treatment | Krause and Durner, 2004 |
| Solanum lycopersicum (susceptible cultivar); leaves infected with TMV | Induction of AOX transcript as early as 12 h post‐inoculation | Fu et al., 2010 |
AOX COULD PLAY A ROLE IN MEDIATING THE METABOLIC LINK BETWEEN RESPIRATION AND THE DEFENCE RESPONSE
From a physiological perspective, respiratory metabolism and the defence response to biotic stress could be linked at the biochemical level. Generally, pathogen infection leads to the enhanced biosynthesis of many aromatic secondary metabolites, such as salicylic acid (SA), phytoalexin and lignin. These aromatic compounds play important roles in the host defence response (Bennett and Wallsgrove, 1994). The shikimate pathway is of pivotal importance for the production of these aromatic compounds. The precursors of the shikimate pathway are erythrose‐4‐phosphate and phosphoenolpyruvate, both of which are produced by the respiratory oxidation of glucose (Arcuri et al., 2004). Thus, intermediate products of respiratory carbon metabolism provide substrates for the biosynthesis of these aromatic compounds related to the defence response (Fig. 1).
Figure 1.
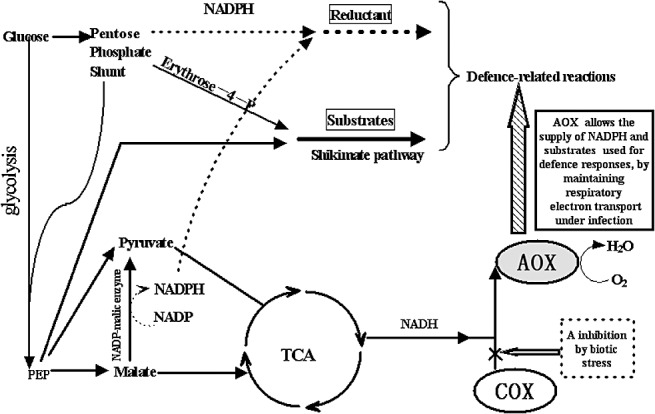
The link between respiratory metabolism and the defence response to infection could be modulated by alternative oxidase (AOX). The reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) and substrates produced by respiratory metabolic pathways could be used for the defence response. AOX may favour the operation of these respiratory metabolic pathways on infection. COX, cyclo‐oxygenase; PEP, phosphoenolpyruvate; TCA, tricarboxylic acid.
Under the condition of infection, the activities of the pentose phosphate shunt and NADP‐malic enzyme are largely enhanced, leading to an increase in the quantity of NADPH (Schaaf et al., 1995; Shaw and Samborski, 1957; Simons et al., 1999; Stryer, 1981). It is conceivable that the biosynthesis of some pathogen defence‐related compounds, such as lignin and flavonoids, requires NADPH as an important reducing energy equivalent, and the provision of NADPH could be attributed to the pentose phosphate shunt and NADP‐malic enzyme (Casati et al., 1999; Pryke and Rees, 1977). Moreover, NADPH provided by NADP‐malic enzyme can be used for the synthesis of reactive oxygen species (ROS) that are produced in order to kill or damage pathogens (Casati et al., 1999). Thus, respiratory carbon metabolism also provides a key source of the cellular reductant NADPH for the biotic stress response.
The production of these intermediates and NADPH from respiratory carbon metabolism can lead to the rapid accumulation of pyruvate as the major end‐product. The resulting pyruvate is subsequently oxidized by the tricarboxylic acid cycle, and thus results in an increase in NADH in the mitochondrial matrix. However, a number of events commonly associated with biotic stress inhibit directly mitochondrial electron transport from NADH to H2O (Amirsadeghi et al., 2007). For example, infection with pathogens and virus can result in the specific accumulation of nitric oxide (NO) (Delledonne, 2005; Delledonne et al., 1998), which, similar to KCN, can cause the strong inhibition of cytochrome oxidase (Brown and Borutaite, 2001; Vieira and Kroemer, 2003). Because upstream carbon metabolism and downstream electron transport are coupled processes, the inhibition of electron transport will cause an imbalance between carbon metabolism and electron transport. As a result, the production of intermediates and reductant from respiratory metabolism could become slow (Vanlerberghe and Ordog, 2002). Thus, under such conditions, there must be mechanisms to correct for such imbalances. AOX has the ability to accept elections from the cytochrome respiratory pathway, especially when the cytochrome pathway is saturated or limited (Vanlerberghe and Ordog, 2002). Accordingly, the AOX pathway has been suggested to play a role in allowing the supply of NADPH and substrates that are used for defence responses by integrating the coupled processes of carbon metabolism and electron transport under the condition of infection (Lennon et al., 1997; Mackenzie and McIntosh, 1999; Simons et al., 1999).
Although there is no direct evidence to support this hypothesis, it fits well with the characteristics of respiratory metabolism in plant defence responses. Moreover, on the basis of molecular evidence, AOX functions in modulating the carbon use efficiency or supporting respiratory carbon metabolism, especially when the cytochrome pathway is inhibited (Sieger et al., 2005; Vanlerberghe et al., 1997).
AOX COULD PLAY A ROLE IN LIMITING THE PRODUCTION OF ROS UNDER BIOTIC STRESS
Ample evidence has proven that mitochondria are a main source of ROS generation (Dat et al., 2000; and references cited therein). Over the years, the mechanism of ROS generation in mitochondria has been studied extensively. An important generalization is that the overall reduction level of the mitochondrial ubiquinone pool will be the primary determinate of mitochondrial ROS (mtROS) output (Sweetlove and Foyer, 2004). Some inhibitors of the components of mtETC, such as antimycin A, can strongly stimulate the production of mtROS. These inhibitors presumably promote the overreduction of the ubiquinone pool by blocking electron transport downstream of the ubiquinone pool (Moller, 2001).
Biotic stress, as other environmental stresses, can lead to a marked increase in the production of ROS in plant cells (Dat et al., 2000; Rhoads et al., 2006; and references cited therein). Some studies have found that some elicitors and toxins of plant pathogens can increase the production of mtROS, suggesting that mitochondria are the likely source of ROS under biotic stress (Krause and Durner, 2004; Rhoads et al., 2006). SA, methyl jasmonate (JAME) and NO, which are generally accumulated in plants infected with pathogens or virus (Koornneef and Pieterse, 2008), are all inhibitors of mtETC and their application can cause an increase in mtROS (Brown and Borutaite, 2001; Norman et al., 2004; Zhang and Xing, 2008). Although it remains unknown whether the physiological compound concentrations generated during plant–pathogen (or virus) interactions are sufficient to inhibit mtETC, such events imply a mechanism of accumulation of mtROS under biotic stress.
It has been confirmed that, by accepting electrons from the ubiquinone pool, AOX can prevent the overreduction of the ubiquinone pool, and therefore has a function in limiting mtROS formation (Maxwell et al., 1999; Wagner and Krab, 1995). It should be noted that mitochondria contribute 20%–30% of the cytosolic steady‐state concentration of hydrogen peroxide (H2O2) (Boveris and Cadenas, 1997). This means that AOX might also have a potential function in forestalling the ROS production of cellular cytosol. This is supported by the observations that the inhibition of catalase activity can enhance AOX mRNA expression, and a lack of AOX is accompanied by an increase in some cytosolic anti‐oxidant defences (Amirsadeghi et al., 2006; Mizuno et al., 2005).
Recent studies have focused on the role of AOX in controlling ROS production of chloroplasts by means of energy‐dissipating systems (Borecky et al., 2006; Pastore et al., 2007). In photosynthetic organisms, chloroplasts transform light into reducing power. However, because CO2 fixation in most plants only uses about 50% of the light energy absorbed, it is inevitable that excess reducing power is produced during photosynthesis. Excess reducing power, if it is not dissipated, can increase the leakage of electrons from the photosynthetic electron transport chain of the thylakoid membrane. These electrons then reduce molecular oxygen to ROS and consequently cause oxidative damage to the photosynthetic apparatus (Dat et al., 2000; and references cited therein). Environmental stress will further decrease the CO2 fixation of photosynthesis, therefore leading to more ROS accumulation in chloroplasts (Baker, 1991; Leprince et al., 1994). It is therefore essential to dissipate excess redox equivalents to avoid the overreduction of electron transport components and oxidative damage to thylakoid membranes.
It has been proposed that the excess reducing power generated through photosynthesis can be transported as malate to the mitochondria via the malate–oxaloacetate shuttle and be dissipated by AOX (Padmasree et al. 2002; Yoshida et al., 2007) (Fig. 2). Apart from the export of malate, the dissipation of excess reducing power from chloroplasts is also ensured by the export of glycolate, which is formed from the Calvin cycle in chloroplasts and is then metabolized to glycine in peroxisomes (Raghavendra and Padmasree, 2003). Glycine then leaves the peroxisome and is imported into the mitochondria, in which glycine is oxidized to serine (this process is called photorespiration). 1997, 2001) found that the oxidation of glycine in mitochondria is coupled to increased electron flow through the AOX pathway, suggesting that AOX activity is necessary for the operation of photorespiration. On the basis of this evidence, it has been suggested that the AOX pathway, possibly working in combination with photorespiration, may have important benefits for the dissipation of excess reduced equivalents for chloroplasts, and thus act as an anti‐oxidant defence system when green tissues are exposed to environmental stress (Bartoli et al., 2005; van Lis and Atteia, 2004; Padmasree et al., 2002; Raghavendra and Padmasree, 2003; Svensson and Rasmusson, 2001).
Figure 2.
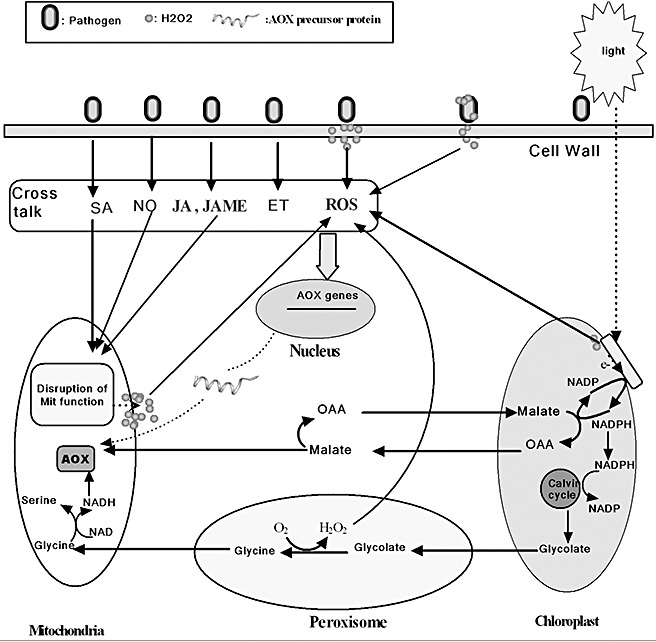
The interaction between mitochondrial alternative oxidase (AOX) and chloroplasts, and the potential signalling pathways for AOX induction under biotic stress. Infection with pathogens could activate the expression of AOX by salicylic acid (SA), nitric oxide (NO), methyl jasmonate (JAME) (or jasmonic acid, JA) or ethylene (ET) signalling pathways. H2O2 produced from cell wall, chloroplasts, mitochondria, peroxisome, and even from the pathogens themselves, has a potential function in inducing the expression of AOX during pathogen–plant interaction. There could exist a complex cross‐talk between these signalling pathways for the induction of AOX. OAA, oxaloacetic acid.
As mentioned above, the expression of AOX in infected Arabidopsis leaf tissue appears to be different from expression in infected Arabidopsis cell suspensions. The discrepancy between these results may originate from the difference in photosynthetic metabolism between cultured suspension cells and green tissues under the condition of infection. Recent work has shown that chloroplasts could be a source of ROS during viral disease development (Díaz‐Vivancos et al., 2008). Thus, compared with suspension cells cultured in the dark, infection‐induced AOX expression in green tissues is expected to play an additional role in reducing the risk of ROS production in chloroplasts, possibly by means of energy dissipation. This should be considered to be an important and interesting issue that needs to be explored in the future.
AOX MAY PLAY A ROLE IN CELL DEATH INDUCED BY BIOTIC STRESSES
During susceptible plant–pathogen interaction (compatible interaction), virulent infection leads to necrosis‐like cell death, which is widespread following disease development in infected tissue (Kiba et al., 2006). During the resistant response to pathogen infection (incompatible interaction), specific recognition by the host triggers the formation of the hypersensitive response (HR), which, as localized programmed cell death (PCD), can restrict pathogen growth and disease development (Alvarez, 2000; Beers and McDowell, 2001; Dickman et al., 2001).
Mitochondria are the first cellular compartments to show PCD responses, which include decreased mitochondrial membrane potential, increased mtROS production and the release of cytochrome c (Lam et al., 2001; Rhoads et al., 2006). It has been reported that, when mtROS production is reduced by ROS scavengers, subsequent PCD‐related events are delayed, suggesting that mtROS is an important factor in PCD signalling (Yao et al., 2002). The treatment of plants with many PCD‐inducing substances, such as O3 and H2O2, can strongly induce the expression of AOX (Amor et al., 2000; Ederli et al., 2006). Recent work has shown that the induction of AOX protein during PCD induced by β‐glucan elicitor can decrease mtROS production (Mizuno et al., 2005). Thus, it is expected that the induction of AOX during the PCD response would delay entry into the PCD pathway. The evidence for this role of AOX comes from the observation that tobacco suspension‐cultured cells with a lack of AOX are much more susceptible to PCD‐inducing substances (Robson and Vanlerberghe, 2002). Moreover, higher levels of AOX protein after anoxic treatment of soybean cells are correlated with an increased resistance to H2O2‐induced PCD (Amor et al., 2000).
Many studies have also reported that the induction of AOX occurs during HR‐like PCD induced by incompatible infection (Chivasa and Carr, 1998; Lacomme and Roby, 1999). Ordog et al. (2002) observed that the overexpression of AOX in tobacco mutants resulted in smaller HR lesions against TMV infection than in wild‐type plants, suggesting that AOX expression can reduce the progression of HR‐like PCD. However, AOX was found to be induced not only during HR‐based resistance, but also during susceptible plant–pathogen interactions (Kiba et al., 2007; Simons et al., 1999). In addition, Kiba et al. (2007) observed that cell death induced by Pseudomonas cichorii (bacterial rot‐causing compatible bacterium) is enhanced in the presence of AOX inhibition in lettuce leaves, suggesting that AOX may also play a role in preventing cell death during compatible plant–pathogen interactions.
Indeed, on the basis of most original articles and opinion papers on AOX research, a common generalization is that AOX may represent a ‘reporter gene’ to evaluate whether mitochondrial oxidative stress occurs during abiotic and biotic stress, and its induction can avoid the disruptions of mitochondrial function via a decrease in mtROS production or maintenance of mitochondrial downstream electron transport (van Aken et al., 2009; Amirsadeghi et al., 2007; Arnholdt‐Schmitt et al., 2006; Vanlerberghe and Ordog, 2002; and references cited therein). More importantly, it has been found that there is much overlap in physiological events between necrosis‐like cell death and HR‐like PCD in plants (van Breusegem and Dat, 2006; Love et al., 2008). For example, although increased mtROS and decreased mitochondrial membrane potential are necessary for subsequent HR‐like PCD, the treatment of plant cells with a number of compatible pathogens or elicitors that do not cause HR‐like cell death also induces mtROS accumulation and a decrease in the mitochondrial membrane potential (Hano et al., 2008; Ren et al., 2002). In particular, it has been reported that cell death during compatible interaction is triggered via mitochondrial oxidative damage (Hano et al., 2008). Thus, the induction of AOX during passive cell death or PCD could be a common response to mitochondrial oxidative stress.
SUPPRESSED EXPRESSION OF AOX DURING INCOMPATIBLE INTERACTION: A PROGRAMMED EVENT FOR THE FORMATION OF HR?
Interestingly, in some cases, the induction of AOX by incompatible infection is transient and is subsequently suppressed during the early stages of HR (Garmier et al., 2002; Krause and Durner, 2004; Künstler et al., 2007; Lacomme and Roby, 1999; Vidal et al., 2007). What is unclear from the present study is whether this suppression of AOX expression acts to attenuate or promote HR‐like PCD. However, similar to this change in AOX expression, some genes encoding anti‐oxidant enzymes also present suppressed expression during the relatively early stages of HR (Dorey et al., 1998; Fodor et al., 1997; Künstler et al., 2007; Mittler et al., 1998; 1999, 2003). Moreover, it has been proposed that the suppressed expression of these anti‐oxidant enzymes should be a programmed event for the formation of HR (Künstler et al., 2007; Takahashi et al., 1997).
During incompatible plant–pathogen interactions, early weak and transient ROS production is a result of a biologically nonspecific reaction, which is found in plant cells attacked by virulent or avirulent pathogens. However, after some hours, a second, massive and prolonged ROS production event, called the oxidative burst, precedes the onset of HR cell death and only occurs in cells attacked by avirulent pathogens (Chandra et al., 1996; Goodman and Novacky, 1995; Lamb and Dixon, 1997). It is conceivable that the downregulation of ROS scavenging systems during the relatively early stages of HR is required to further promote ROS accumulation and thus contribute to the formation of the oxidative burst (Künstler et al., 2007; Mittler et al., 1998; Shetty et al., 2008; Takahashi et al., 1997).
In particular, earlier studies have shown that the pathogen‐induced oxidative burst can originate from mitochondria (Allan and Fluhr, 1997; Bolwell and Wojtaszek, 1997; Naton et al., 1996). Although AOX is not an anti‐oxidant enzyme, its suppression also leads to dramatic amounts of ROS in mitochondria (Maxwell et al., 1999). Therefore, one possible explanation is that AOX suppression during HR‐like PCD, similar to the genes encoding anti‐oxidant enzymes, would ensure the specific accumulation of ROS at the mitochondrion and thereby contribute to the formation of the oxidative burst. This conjecture seems to be related to a recent observation by Krause and Durner (2004), who described a clear picture of the kinetic changes of AOX expression and ROS production in Arabidopsis suspension cells treated with harpin (a bacterial protein elicitor of HR). In this study, the induction of AOX was associated with an early accumulation of ROS, whereas subsequent suppression of AOX expression was observed during the oxidative burst phase (Krause and Durner, 2004).
To date, however, whether the dynamic change in AOX expression during incompatible interactions can be associated with the progress of HR‐like PCD has been studied only rarely. More detailed research on the biological significance of AOX in PCD induced by biotic stress is still required.
SIGNAL TRANSDUCTION FOR AOX GENE EXPRESSION UNDER BIOTIC STRESS
Infection with pathogens and virus can result in the specific accumulation of H2O2, NO, ethylene (ET), SA and jasmonic acid (JA), which are used as signal molecules for plant defence responses (Koornneef and Pieterse, 2008; and references cited therein). All of these signal molecules have been reported to induce AOX gene expression or increase AOX protein amounts (see below).
Wagner (1995) found that the addition of H2O2 to Petunia hybrida cells resulted in increased cyanide‐resistant respiration and AOX protein amounts. The addition of anti‐oxidants lowered the intracellular ROS level and inhibited AOX1 gene expression (Maxwell et al., 2002). Recent work has also shown that the promoter of AOX1a is responsive to H2O2 (Ho et al., 2008). These observations suggest that ROS could initiate the signal for AOX gene expression (Fig. 2).
In previous studies, an effective means of inducing the expression of the AOX gene was with an artificial chemical inhibitor of mitochondrial electron transport, such as antimycin A (1994, 2002). Treatments with this type of inhibitor can simultaneously increase the production of mtROS (Maxwell et al., 2002; Vanlerberghe et al., 2002). Thus, it is thought that ROS can arise from stressed mitochondria and function as a second messenger to induce the expression of AOX. In addition, many studies have found that an increase in mtROS is a common consequence of biotic stresses (Amirsadeghi et al., 2007; and references cited therein). Thus, it is expected that the expression of AOX under biotic stress could be induced by ROS produced from mitochondria. It should be noted that there are wide sources of ROS during pathogen–plant interactions. In addition to mitochondria, chloroplasts could be an important source of ROS during disease development (Díaz‐Vivancos et al., 2008). Initial pathogen invasion can also lead to strong production of H2O2 at the cell wall of the host (Bolwell et al., 1998). However, whether ROS production from these different sites has the same function in inducing the expression of AOX during pathogen–plant interaction remains to be determined.
In particular, in addition to an increase in ROS in the host cell under biotic stress, our recent work has shown that the phytopathogen itself can also produce substantial amounts of H2O2 from its cell walls (2007, 2008). Although there is still no evidence to show whether H2O2 production from bacterial pathogens has a potential function in inducing the expression of AOX or other defence genes of host plants during interaction, a theoretical possibility still exists, based on the observations that exogenous H2O2 application can initiate the signal for the expression of many genes, including AOX. Future work is expected to reveal the roles of pathogen‐produced H2O2 during plant–pathogen interaction.
In infected plants, SA is accumulated at a high level and acts as an important signal for the induction of the plant resistance response (Delaney et al., 1994; Murphy et al., 1999). On the basis of the observation that exogenous SA can induce a dramatic increase in the level of the AOX transcript and protein (Raskin et al., 1987; Rhoads and McIntosh, 1992, 1993), it is hypothesized that AOX induction during infection is dependent on SA accumulation. However, using transgenic NahG plants, which cannot accumulate SA, it was confirmed that SA is not required for AOX induction in both compatible and incompatible plant–pathogen combinations (Simons et al., 1999). Moreover, recent work has also shown that the promoter of AOX1a is unresponsive to SA (Ho et al., 2008). Indeed, as an uncoupler or a strong inhibitor of electron transfer, SA may cause the disruption of mitochondrial function, consequently increasing the production of mtROS (Norman et al., 2004). Therefore, AOX expression with exogenous SA treatment could be a result of mitochondrial dysfunction and be induced indirectly by mtROS (Finnegan et al., 2004). However, it should also be noted that SA can stimulate ROS production via other mechanisms that do not involve mitochondria (Chen et al., 1993; Durner and Klessig, 1995; Norman et al., 2004; Slaymaker et al., 2002). Moreover, although SA is clearly not essential for AOX expression, the fast induction of AOX during the avirulent plant–pathogen combination is abolished in NahG plants, indicating that SA‐dependent processes still play a role in AOX expression (Simons et al., 1999). Clifton et al. (2005) have suggested that SA could provide a mechanism to amplify the intensity of some signals that lead to the expression of AOX.
NO has been identified as a second messenger during HR induced by incompatible pathogens (Delledonne et al., 1998; Klessig et al., 2000). The Arabidopsis AOX1a gene has been found to be upregulated by exogenous NO (Huang et al., 2002; Parani et al., 2004). Similar to SA, NO can also cause a strong inhibition of cytochrome oxidase and an increase in mtROS (Brown and Borutaite, 2001). Thus, ROS could act downstream of NO signalling to induce AOX expression. However, from other reports, there is a possibility that NO can activate AOX1a expression through cADP Rib[1‐(5‐phospho‐beta‐d‐ribosyl)adenosine 5′‐phosphate cyclic anhydride], which stimulates Ca2+ release into the cytoplasm (Klessig et al., 2000). To date, whether physiological NO generation during plant–pathogen interaction affects AOX expression remains unknown.
Ederli et al. (2006) found that ET boosts AOX mRNA content in tobacco leaf discs. Using the etr‐1 mutant, which shows defective ET perception, Simons et al. (1999) revealed that the expression of AOX in infected leaves could be ET dependent. However, it was found that, although ET is required for the O3‐induced upregulation of AOX1a, there exists an ET‐independent pathway for O3‐induced AOX expression (Ederli et al., 2006), suggesting that AOX induction can occur via other mechanisms that do not involve ET.
Exogenous JA and JAME can also strongly increase the steady‐state AOX transcript levels (Ederli et al., 2006; Fung et al., 2004). Recently, it has been reported that JAME can induce ROS production from mitochondria and cause the loss of the mitochondrial transmembrane (Zhang and Xing, 2008). Thus, it is possible that JAME could affect AOX expression by stimulating the production of mtROS. However, whether and how endogenous JAME or JA accumulation during infection could affect AOX expression has not been explored.
Although these studies have suggested that AOX expression under the condition of infection may be activated by different signalling molecules, none of the known signalling molecules appears to play a crucial role in determining AOX induction or the level of AOX expression under biotic stress. To date, the signals regulating AOX expression under biotic stress are still unclear. Moreover, much information has shown that the signal molecules reported to induce AOX expression can interplay with each other under conditions of infection. This interaction between signal molecules, either mutually antagonistic or synergistic, can modulate and optimize the adaptive response of plants against pathogen attack (Koornneef and Pieterse, 2008; Pieterse and Dicke, 2007; and references cited therein). Thus, when considering the interactions among these signal molecules, the signals regulating AOX gene expression during plant–pathogen interactions could be more complex than expected (Fig. 2). However, if AOX is an important component of the host adaptive response to biotic stress, it is expected that AOX should process a flexible signalling network under biotic stress (Fig. 2). Future studies are needed to provide a more comprehensive rationalization of the signal transduction of AOX genes.
POTENTIAL ROLE OF AOX IN PATHOGENS
AOX is not only found in host plants. Many animal and plant pathogenic fungi and parasites, such as Trypanosoma brucei, Plasmodium falciparum, Septoria tritici and Cryptococcus neoformans, also contain the genetic capacity to express this protein (Akhter et al., 2003; Huang et al., 2002; and references cited therein).
It is believed that AOX of pathogens plays an important role in the viability of pathogens, especially under the stress of the host environment. For example, it is clear from a variety of studies that both host oxygen‐ and nitrogen‐reactive species are deleterious to pathogenic fungi and parasites (Barja, 1999; Borghouts et al., 2001). On the basis of the observation that NO can inhibit reversibly the cytochrome pathway, but has little effect on AOX activity, Millar and Day (1996) proposed that the presence of AOX in parasites or fungi may be important for the ability of the pathogens to avoid the host's NO toxicity. With the use of the AOX1 mutant strain of Cryptococcus neoformans, it was demonstrated that AOX of C. neoformans plays a role in the yeast's defence against exogenous oxidative stress, and contributes significantly to cellular metabolism, survival within phagocytic cells (can kill invading pathogens by the generation of ROS) and the virulence composite of this yeast (Akhter et al., 2003). Thus, AOX in pathogens seems to have a similar function in counteracting oxidative stress as that in the host plant. From a plant pathology perspective, AOX is equally important for both pathogens and host plants during their interaction.
CONCLUDING REMARKS AND PERSPECTIVES
In recent years, significant progress has been made in the breeding for resistance of many crops to important diseases. However, the resistance available has been quickly exhausted as a result of the ever‐evolving pathogens in natural environments. However, plants must cope with simultaneous interactions with multiple aggressors and different attackers. As an effective alternative, the flexibility of metabolism allows the flexibility of defence responses to cope with changes in pathogens. AOX, as a crucial feature of plant metabolic flexibility, appears to provide a tool to meet the demands of this defence strategy and to attain optimal resistance. Ultimately, a knowledge of the biological significance of AOX in nature should ideally be obtained from a study combining molecular, biological and agricultural perspectives.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (NO. 30900105) and nwnu‐kjcxgc‐03‐77 and 49.
REFERENCES
- Van Aken, O. , Giraud, E. , Clifton, R. and Whelan, J. (2009) Alternative oxidase: a target and regulator of stress responses. Physiol. Plant, 137, 354–361. [DOI] [PubMed] [Google Scholar]
- Akhter, S. , McDade, H.C. , Gorlach, J.M. , Heinrich, G. , Cox, G.M. and Perfect, J.R. (2003) Role of the alternative oxidase gene in pathogenesis of Crytococcus neoformans . Infect. Immun. 71, 5794–5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan, A.C. and Fluhr, R. (1997) Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell, 9, 1559–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez, M.E. (2000) Salicylic acid in the machinery of hypersensitive cell death and disease resistance. Plant Mol. Biol. 44, 429–442. [DOI] [PubMed] [Google Scholar]
- Amirsadeghi, S. , Robson, C.A. , McDonald, A.E. and Vanlerberghe, G.C. (2006) Changes in plant mitochondrial electron transport alter cellular levels of reactive oxygen species and susceptibility to cell death signaling molecules. Plant Cell Physiol. 47, 1509–1519. [DOI] [PubMed] [Google Scholar]
- Amirsadeghi, S. , Robson, C.A. and Vanlerberghe, G.C. (2007) The role of the mitochondrion in plant responses to biotic stress. Physiol. Plant. 129, 253–266. [Google Scholar]
- Amor, Y. , Chevion, M. and Levine, A. (2000) Anoxia pretreatment protects soybean cells against H2O2‐induced cell death: possible involvement of peroxidases and of alternative oxidase. FEBS Lett. 477, 175–180. [DOI] [PubMed] [Google Scholar]
- Arcuri, H.A. , Canduri, F. , Pereira, J.H. , Da Silveira, N.J. , Camera Júnior, J.C. , De Oliveira, J.S. , Basso, L.A. , Palma, M.S. , Santos, D.S. and De Azevedo Júnior, W.F. (2004) Molecular models for shikimate pathway enzymes of Xylella fastidiosa . Biochem. Biophys. Res. Commun. 30, 979–991. [DOI] [PubMed] [Google Scholar]
- Arnholdt‐Schmitt, B. , Costa, J.H. and De Melo, D.F. (2006) AOX—a functional marker for efficient cell reprogramming under stress? Trends Plant Sci. 11, 281–287. [DOI] [PubMed] [Google Scholar]
- Baker, N.R. (1991) A possible role for photosystem I in environmental pertubations of photosynthesis. Physiol. Plant. 81, 563–570. [Google Scholar]
- Barja, G. (1999) Mitochondrial oxygen radical generation and leak: sites of production in states 4 and 3, organ specificity, and relation to aging and longevity. J. Bioenerg. Biomembr. 31, 347–366. [DOI] [PubMed] [Google Scholar]
- Bartoli, C.G. , Gómez, F. , Gergoff, G. , Guiamét, J.J. and Puntarulo, S. (2005) Up‐regulation of the mitochondrial alternative oxidase pathway enhances photosynthetic electron transport under drought conditions. J. Exp. Bot. 56, 1269–1276. [DOI] [PubMed] [Google Scholar]
- Beers, E.P. and McDowell, J.M. (2001) Regulation and execution of programmed cell death in response to pathogens, stress and developmental cues. Curr. Opin. Plant Biol. 4, 561–567. [DOI] [PubMed] [Google Scholar]
- Bennett, R.N. and Wallsgrove, R.M. (1994) Secondary metabolites in plant defence mechanisms. New Phytol. 127, 617–633. [DOI] [PubMed] [Google Scholar]
- Bolwell, G.P. and Wojtaszek, P. (1997) Mechanisms for the generation of reactive oxygen species in plant defense—a broad perspective. Physiol. Mol. Plant Pathol. 51, 347–366. [Google Scholar]
- Bolwell, G.P. , Davies, D.R. , Gerrish, C. , Auh, C.K. and Murphy, T.M. (1998) Comparative biochemistry of the oxidative burst produced by rose and French bean cells reveals two distinct mechanisms. Plant Physiol. 116, 1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borecky, J. , Nogueira, F.T.S. , De Oliveira, K.A.P. , Maia, I.G. , Vercesi, A.E. and Arruda, P. (2006) The plant energy‐dissipating mitochondrial systems: depicting the genomic structure and the expression profiles of the gene families of uncoupling protein and alternative oxidase in monocots and dicots. J. Exp. Bot. 57, 849–864. [DOI] [PubMed] [Google Scholar]
- Borghouts, C. , Werner, A. , Elthon, T. and Osiewacz, H.D. (2001) Copper‐modulated gene expression and senescence in the filamentous fungus Podospora anserina . Mol. Cell Biol. 21, 390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris, A. and Cadenas, E. (1997) Oxygen, Gene Expression, and Cellular Function (Mazzaro D. and Clerch L., eds), pp. 1–25. New York: Marcel Dekker Inc. [Google Scholar]
- Van Breusegem, F. and Dat, J.F. (2006) Reactive oxygen species in plant cell death. Plant Physiol. 141, 384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, G.C. and Borutaite, V. (2001) Nitric oxide, mitochondria, and cell death. IUBMB Life, 52, 189–195. [DOI] [PubMed] [Google Scholar]
- Casati, P. , Drincovich, M.F. , Edwards, G.E. and Andreo, C.S. (1999) Malate metabolism by NADP‐malic enzyme in plant defense. Photosynth. Res. 61, 99–105. [Google Scholar]
- Chandra, S. , Martin, G.B. and Low, P.S. (1996) The Pto kinase mediates a signaling pathway leading to the oxidative burst in tomato. Proc. Natl Acad. Sci. USA, 93, 13393–13397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z. , Silva, H. and Klessig, D.F. (1993) Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science, 262, 1883–1886. [DOI] [PubMed] [Google Scholar]
- Chivasa, S. and Carr, J.P. (1998) Cyanide restores N gene‐mediated resistance to tobacco mosaic virus in transgenic tobacco expressing salicylic acid hydroxylase. Plant Cell, 10, 1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton, R. , Lister, R. , Parker, K.L. , Sappl, P.G. , Elhafez, D. , Millar, A.H. , Day, D.A. and Whelan, J. (2005) Stress‐induced co‐expression of alternative respiratory chain components in Arabidopsis thaliana . Plant Mol. Biol. 58, 193–212. [DOI] [PubMed] [Google Scholar]
- Considine, M.J. , Holtzapffel, R.C. , Day, D.A. , Whelan, J. and Millar, A.H. (2002) Molecular distinction between alternative oxidase from monocots and dicots. Plant Physiol. 129, 949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa, J.H. , Jolivet, Y. , Hasenfratz‐Sauder, M.P. , Orellano, E.G. , Da Guia Silva Lima, M. , Dizengremel, P. and Fernandes de Melo, D. (2007) Alternative oxidase regulation in roots of Vigna unguiculata cultivars differing in drought/salt tolerance. J. Plant Physiol. 164, 718–727. [DOI] [PubMed] [Google Scholar]
- Dat, J. , Vandenabeele, S. , Vranová, E. , Van Montagu, M. , Inzé, D. and Van Breusegem, F. (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol. Life. Sci. 57, 779–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney, T.P. , Uknes, S. , Vernooij, B. , Friedrich, L. , Weymann, K. , Negrotto, D. , Gaffney, T. , Gut‐Rella, M. , Kessmann, H. and Ward, E. (1994) A central role of salicylic acid in plant disease resistance. Science, 266, 1247–1250. [DOI] [PubMed] [Google Scholar]
- Delledonne, M. (2005) NO news is good news for plants. Curr. Opin. Plant Biol. 8, 390–396. [DOI] [PubMed] [Google Scholar]
- Delledonne, M. , Xia, Y. , Dixon, R.A. and Lamb, C. (1998) Nitric oxide functions as a signal in plant disease resistance. Nature, 394, 585–588. [DOI] [PubMed] [Google Scholar]
- Díaz‐Vivancos, P. , Clemente‐Moreno, M.J. , Rubio, M. , Olmos, E. , García, J.A. , Martínez‐Gómez, P. and Hernández, J. (2008) Alteration in the chloroplastic metabolism leads to ROS accumulation in pea plants in response to plum pox virus. J. Exp. Bot. 59, 2147–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman, M.B. , Park, Y.K. , Oltersdorf, T. , Li, W. , Clemente, T. and French, R. (2001) Abrogation of disease development in plants expressing animal antiapoptotic genes. Proc. Natl Acad. Sci. USA, 98, 6957–6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorey, S. , Baillieul, F. , Saindrenan, P. , Fritig, B. and Kaufmann, S. (1998) Tobacco class I and II catalases are differentially expressed during elicitor‐induced hypersensitive cell death and localized acquired resistance. Mol. Plant–Microbe Interact. 11, 1102–1109. [Google Scholar]
- Durner, J. and Klessig, D.F. (1995) Inhibition of ascorbate peroxidase by salicylic acid and 2,6‐dichloroisonicotinic acid, two inducers of plant defense responses. Proc. Natl Acad. Sci. USA, 92, 11 312–11 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwurazna, M.M. and Weintraub, M. (1969) The respiratory pathways of tobacco leaves infected with potato virus X. Can. J. Bot. 47, 731–736. [Google Scholar]
- Ederli, L. , Morettini, R. , Borgogni, A. , Wasternack, C. , Miersch, O. , Reale, L. , Ferranti, F. , Tosti, N. and Pasqualini, S. (2006) Interaction between nitric oxide and ethylene in the induction of alternative oxidase in ozone‐treated tobacco plants. Plant Physiol. 142, 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar, J.F. (1992) Beyond photosynthesis: the translocation and respiration of diseased leaves In: Pests and Pathogens (Ayres P.G., ed.), pp. 107–127. Oxford: BIOS Scientific Publishers. [Google Scholar]
- Finnegan, P.M. , Soole, K.L. and Umbach, A.L. (2004) Alternative mitochondrial electron transport proteins in higher plants In: Plant Mitochondria: From Genome to Function (Day D.A., Millar A.H. and Whelan J., eds), pp. 163–230. Dordrecht: Kluwer Academic Publishers. [Google Scholar]
- Fodor, J. , Gullner, G. , Adám, A.L. , Barna, B. , Komíves, T. and Király, Z. (1997) Local and systemic responses of antioxidants to tobacco mosaic virus infection and to salicylic acid. Plant Physiol. 114, 1443–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, L.J. , Shi, K. , Gu, M. , Zhou, Y.H. , Dong, D.K. , Liang, W.S. , Song, F.M. and Yu, J.Q. (2010) Systemic induction and role of mitochondrial alternative oxidase and nitric oxide in a compatible tomato–tobacco mosaic virus interaction. Mol. Plant–Microbe. Interact. 23, 39–48. [DOI] [PubMed] [Google Scholar]
- Fung, R.W.M. , Wang, C.Y. , Smith, D.L. , Gross, K.C. and Tian, M.S. (2004) MeSA and MeJA increase steady‐state transcript levels of alternative oxidase and resistance against chilling injury in sweet peppers (Capsicum annuum L.). Plant Sci. 166, 711–719. [Google Scholar]
- Garmier, M. , Dutilleul, C. , Mathieu, C. , Chétrit, P. , Boccara, M. and De Paepe, R. (2002) Changes in antioxidant expression and harpin‐induced hypersensitive response in a Nicotiana sylvestris mitochondrial mutant. Plant Physiol. Biochem. 40, 561–566. [Google Scholar]
- Goodman, R.N. and Novacky, A. (1995) The Hypersensitive Defense Reaction in Plants. St. Paul, MN: American Phytopathological Society Press. [Google Scholar]
- Grant, N. , Onda, Y. , Kakizaki, Y. , Ito, K. , Watling, J. and Robinson, S. (2009) Two Cys or not two Cys? That is the question; alternative oxidase in the thermogenic plant sacred lotus. Plant Physiol. 150, 987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hano, C. , Addi, M. , Fliniaux, O. , Bensaddek, L. , Duverger, E. , Mesnard, F. , Lamblin, F. and Lainé, E. (2008) Molecular characterization of cell death induced by a compatible interaction between Fusarium oxysporum f. sp. linii and flax (Linum usitatissimum) cells. Plant Physiol. Biochem. 46, 590–600. [DOI] [PubMed] [Google Scholar]
- Ho, L.H. , Giraud, E. , Uggalla, V. , Lister, R. , Clifton, R. , Glen, A. , Thirkettle‐Watts, D. , Van Aken, O. and Whelan, J. (2008) Identification of regulatory pathways controlling gene expression of stress‐responsive mitochondrial proteins in Arabidopsis . Plant Physiol. 147, 1858–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, X. , Von‐Rad, U. and Durner, J. (2002) Nitric oxide induces transcriptional activation of the nitric oxide‐tolerant alternative oxidase in Arabidopsis suspension cell. Planta, 215, 914–923. [DOI] [PubMed] [Google Scholar]
- Igamberdiev, A.U. , Bykova, N.V. and Gardeström, P. (1997) Involvement of cyanide‐resistant and rotenone‐insensitive pathways of mitochondrial electron transport during oxidation of glycine in higher plants. FEBS Lett. 412, 265–269. [DOI] [PubMed] [Google Scholar]
- Igamberdiev, A.U. , Bykova, N.V. , Lea, P.J. and Gardeström, P. (2001) The role of photorespiration in redox and energy balance of photosynthetic plant cells: a study with a barley mutant deficient in glycine decarboxylase. Plant Physiol. 111, 427–438. [DOI] [PubMed] [Google Scholar]
- Kiba, A. , Takata, O. , Ohnishi, K. and Hikichi, Y. (2006) Comparative analysis of induction pattern of programmed cell death and defense‐related responses during hypersensitive cell death and development of bacterial necrotic leaf spots in eggplant. Planta, 224, 981–994. [DOI] [PubMed] [Google Scholar]
- Kiba, A. , Lee, K.Y. , Ohnishi, K. and Hikichi, Y. (2007) Comparative expression analysis of genes induced during development of bacterial rot and induction of hypersensitive cell death in lettuce. J. Plant Physiol. 165, 1757–1773. [DOI] [PubMed] [Google Scholar]
- Klessig, D.F. , Durner, J. , Noad, R. , Navarre, D.A. , Wendehenne, D. , Kumar, D. , Zhou, J.M. , Shah, J. , Zhang, S. , Kachroo, P. , Trifa, Y. , Pontier, D. , Lam, E. and Silva, H. (2000) Nitric oxide and salicylic acid signaling in plant defense. Proc. Natl Acad. Sci. USA, 97, 8849–8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef, A. and Pieterse, C.M.J. (2008) Cross talk in defense signaling. Plant Physiol. 146, 839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause, M. and Durner, J. (2004) Harpin inactivates mitochondria in Arabidopsis suspension cells. Mol. Plant–Microbe Interact. 17, 131–139. [DOI] [PubMed] [Google Scholar]
- Künstler, A. , Hafez, Y.M. and Király, L. (2007) Transient suppression of a catalase and an alternative oxidase gene during virus‐induced local lesion formation (hypersensitive response) is independent of the extent of leaf necrotization. Acta Phytopathol. Entomol. Hung. 2, 185–196. [Google Scholar]
- Lacomme, C. and Roby, D. (1999) Identification of new early markers of the hypersensitive response in Arabidopsis thaliana . FEBS Lett. 459, 149–153. [DOI] [PubMed] [Google Scholar]
- Lam, E. , Kato, N. and Lawton, M. (2001) Programmed cell death, mitochondria and the plant hypersensitive response. Nature, 411, 848–853. [DOI] [PubMed] [Google Scholar]
- Lamb, C. and Dixon, R.A. (1997) The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. 48, 251–275. [DOI] [PubMed] [Google Scholar]
- Lennon, A.M. , Neuenschwander, U.H. , Ribas‐Carbo, M. , Giles, L. , Ryals, J.A. and Siedow, J.N. (1997) The effect of salicylic acid and tobacco mosaic virus infection on the alternative oxidase of tobacco. Plant Physiol. 115, 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leprince, O. , Hendry, G.A.F. and Atherton, N.M. (1994) Free radical processes induced by desiccation in germinating maize: the relationship with respiration and loss of desiccation tolerance. Proc. R. Soc. Edinb. B, 102, 211–218. [Google Scholar]
- Li, X. , Li, H. , Pang, X. , Feng, H. , Zhi, D. , Wen, J. and Wang, J. (2007) Localization changes of endogenous hydrogen peroxide during cell division cycle of Xanthomonas . Mol. Cell Biochem. 300, 207–221. [DOI] [PubMed] [Google Scholar]
- Li, X. , Feng, H.Q. , Pang, X.Y. and Li, H.Y. (2008) Mesosome formation is accompanied by hydrogen peroxide accumulation in bacteria during the rifampicin effect. Mol. Cell Biochem. 311, 241–247. [DOI] [PubMed] [Google Scholar]
- Van Lis, R. and Atteia, A. (2004) Control of mitochondrial function via photosynthetic redox signals. Photosynth. Res. 79, 133–148. [DOI] [PubMed] [Google Scholar]
- Love, A.J. , Milner, J.J. and Sadanandom, A. (2008) Timing is everything: regulatory overlap in plant cell death. Trends Plant Sci. 13, 589–595. [DOI] [PubMed] [Google Scholar]
- Mackenzie, S. and McIntosh, L. (1999) Higher plant mitochondria. Plant Cell, 11, 571–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell, D.P. , Wang, Y. and McIntosh, L. (1999) The alternative oxidase lowers mitochondrial reactive oxygen production in plant cell. Proc. Natl Acad. Sci. USA, 96, 8271–8276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell, D.P. , Nickels, R. and McIntosh, L. (2002) Evidence of mitochondrial involvement in the transduction of signals required for the induction of genes associated with pathogen attack and senescence. Plant J. 29, 269–279. [DOI] [PubMed] [Google Scholar]
- Millar, A.H. and Day, D.A. (1996) Nitric oxide inhibits the cytochrome oxidase but not the alternative oxidase of plant mitochondria. FEBS Lett. 398, 155–158. [DOI] [PubMed] [Google Scholar]
- Millenaar, F.F. and Lambers, H. (2003) The alternative oxidase: in vivo regulation and function. Plant Biol. 5, 2–15. [Google Scholar]
- Mittler, R. , Feng, X. and Cohen, M. (1998) Post‐transcriptional suppression of cytosolic ascorbate peroxidase expression during pathogen‐induced programmed cell death in tobacco. Plant Cell, 10, 461–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno, M. , Tada, Y. , Uchii, K. , Kawakami, S. and Mayama, S. (2005) Catalase and alternative oxidase cooperatively regulate programmed cell death induced by β‐glucan elicitor in potato suspension cultures. Planta, 220, 849–853. [DOI] [PubMed] [Google Scholar]
- Mizuno, N. , Sugie, A. , Kobayashi, F. and Takumi, S. (2008) Mitochondrial alternative pathway is associated with development of freezing tolerance in common wheat. J. Plant Physiol. 165, 462–467. [DOI] [PubMed] [Google Scholar]
- Moller, I.M. (2001) Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 561–591. [DOI] [PubMed] [Google Scholar]
- Murphy, A.M. , Chivasa, S. , Singh, D.P. and Carr, J.P. (1999) Salicylic acid‐induced resistance to viruses and other pathogens: a parting of the ways? Trends Plant Sci. 4, 155–160. [DOI] [PubMed] [Google Scholar]
- Naton, B. , Hahlbrock, K. and Schmelzer, E. (1996) Correlation of rapid cell death with metabolic changes in fungus infected, cultured parsley cells. Plant Physiol. 112, 433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman, C. , Howell, K.A. , Millar, A.H. , Whelan, J.M. and Day, D.A. (2004) Salicylic acid is an uncoupler and inhibitor of mitochondrial electron transport. Plant Physiol. 134, 492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordog, S.H. , Higgins, V.J. and Vanlerberghe, G.C. (2002) Mitochondrial alternative oxidase is not a critical component of plant viral resistance but may play a role in the hypersensitive response. Plant Physiol. 129, 1858–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmasree, K. , Padmavathi, L. and Raghavendra, A.S. (2002) Essentiality of mitochondrial oxidative metabolism for photosynthesis: optimization of carbon assimilation and protection against photoinhibition. Crit. Rev. Biochem. Mol. Biol. 37, 71–119. [DOI] [PubMed] [Google Scholar]
- Panda, S.K. , Yamamoto, Y. , Kondo, H. and Matsumoto, H. (2008) Mitochondrial alterations related to programmed cell death in tobacco cells under aluminium stress. C. R. Biol. 331, 597–610. [DOI] [PubMed] [Google Scholar]
- Parani, M. , Rudrabhatla, S. , Myers, R. , Weirich, H. , Smith, B. , Leaman, D.W. and Goldman, S.L. (2004) Microarray analysis of nitric oxide responsive transcript in Arabidopsis . Plant Biotechnol. J. 2, 359–366. [DOI] [PubMed] [Google Scholar]
- Pastore, D. , Trono, D. , Laus, M.N. , Di Fonzo, N. and Flagella, Z. (2007) Possible plant mitochondria involvement in cell adaptation to drought stress. A case study: durum wheat mitochondria. J. Exp. Bot. 58, 195–210. [DOI] [PubMed] [Google Scholar]
- Pieterse, C.M.J. and Dicke, M. (2007) Plant interactions with microbes and insects: from molecular mechanisms to ecology. Trends Plant Sci. 12, 564–569. [DOI] [PubMed] [Google Scholar]
- Pryke, J.A. and Rees, T. (1977) The pentose phosphate pathway as a source of NADPH for lignin synthesis. Phytochemistry, 16, 557–560. [Google Scholar]
- Raghavendra, A.S. and Padmasree, K. (2003) Beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Trends Plant Sci. 85, 46–53. [DOI] [PubMed] [Google Scholar]
- Raskin, I. , Ehmann, A. , Melander, W.R. and Meeuse, B.J.D. (1987) Salicylic acid: a natural inducer of heat production in Arum lilies. Science, 237, 1601–1602. [DOI] [PubMed] [Google Scholar]
- Ren, D. , Yang, H. and Zhang, S. (2002) Cell death mediated by MAPK is associated with hydrogen peroxide production in Arabidopsis . J. Biol. Chem. 277, 559–565. [DOI] [PubMed] [Google Scholar]
- Rhoads, D.M. and McIntosh, L. (1992) Salicylic acid regulation of respiration in higher plants: alternative oxidase expression. Plant Cell, 1131–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads, D.M. and McIntosh, L. (1993) Cytochrome and alternative pathway respiration in tobacco. Effects of salicylic acid. Plant Physiol. 103, 877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads, D.M. , Umbach, A.L. , Subbaiah, C.C. and Siedow, J.N. (2006) Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant Physiol. 141, 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, S.A. , Ribas‐Carbo, M. , Yakir, D. , Giles, L. , Reuveni, Y. and Berry, J.A. (1995) Beyond SHAM and cyanide: opportunities for studying the alternative oxidase in plant respiration using oxygen‐isotope discrimination. Aust. J. Plant Physiol. 22, 487–496. [Google Scholar]
- Robson, C.A. and Vanlerberghe, G.C. (2002) Transgenic plant cells lacking mitochondrial alternative oxidase have increased susceptibility to mitochondria‐dependent and ‐independent pathways of programmed cell death. Plant Physiol. 129, 1908–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf, J. , Walter, M.H. and Hess, D. (1995) Primary metabolism in plant defense. Regulation of a bean malic enzyme gene promoter in transgenic tobacco by developmental and environmental cues. Plant Physiol. 108, 949–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, M. and Samborski, D.J. (1957) The physiology of host–parasite relations: the pattern of respiration in rusted and mildewed cereal leaves. Can. J. Bot. 35, 389–407. [Google Scholar]
- Shetty, N.P. , Jørgensen, H.J.L. , Jensen, J.D. , Collinge, D.B. and Shetty, H.S. (2008) Roles of reactive oxygen species in interactions between plants and pathogens. Eur. J. Plant Pathol. 121, 267–280. [Google Scholar]
- Sieger, S.M. , Kristensen, B.K. , Robson, C.A. , Amirsadeghi, S. , Eng, E.W. , Abdel‐Mesih, A. , Moller, I.M. and Vanlerberghe, G.C. (2005) The role of alternative oxidase in modulating carbon use efficiency and growth during macronutrient stress in tobacco cells. J. Exp. Bot. 56, 1499–1515. [DOI] [PubMed] [Google Scholar]
- Simons, B.H. , Millenaar, F.F. , Mulder, L. , Van Loon, L.C. and Lambers, H. (1999) Enhanced expression and activation of the alternative oxidase during infection of Arabidopsis with Pseudomonas syringae pv. tomato . Plant Physiol. 120, 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaymaker, D.H. , Navarre, D.A. , Clark, D. , Pozo, O. , Martin, G.B. and Klessig, D.F. (2002) The tobacco salicylic acid‐binding protein 3 (SABP3) is the chloroplast carbonic anhydrase, which exhibits antioxidant activity and plays a role in the hypersensitive defense response. Proc. Natl Acad. Sci. USA, 99, 11640–11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer, L. (1981) Biochemistry, 2nd edn. San Francisco, CA: W.H. Freeman and Company. [Google Scholar]
- Svensson, A.S. and Rasmusson, A.G. (2001) Light‐dependent gene expression for proteins in the respiratory chain of potato leaves. Plant J. 28, 73–82. [DOI] [PubMed] [Google Scholar]
- Sweetlove, L.J. and Foyer, C.H. (2004) Roles for reactive oxygen species and antioxidants in plant mitochondria In: Plant Mitochondria: From Genome to Function, Vol. 1, Advances in Photosynthesis and Respiration (Day D.A., Millar A.H. and Whelan J., eds), pp. 307–320. Dordrecht: Kluwer Academic Press. [Google Scholar]
- Takahashi, H. , Chen, Z. , Du, H. , Liu, Y. and Klessig, D. (1997) Development of necrosis and activation of disease resistance in transgenic tobacco plants with severely reduced catalase levels. Plant J. 11, 995–1005. [DOI] [PubMed] [Google Scholar]
- Vanlerberghe, G.C. and McIntosh, L. (1992) Coordinate regulation of cytochrome and alternative pathway respiration in tobacco. Plant Physiol. 100, 1846–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe, G.C. and McIntosh, L. (1997) Alternative oxidase: from gene to function. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 703–734. [DOI] [PubMed] [Google Scholar]
- Vanlerberghe, G.C. and Ordog, S.H. (2002) Alternative oxidase: integrating carbon metabolism and electron transport in plant respiration In: Advances in Photosynthesis and Respiration, Vol. 12, Photosynthetic Nitrogen Assimilation and Associated Carbon and Respiratory Metabolism (Foyer C.H. and Noctor G., eds), pp. 173–191. Dordrecht: Kluwer Academic Publishers. [Google Scholar]
- Vanlerberghe, G.C. , Vanlerberghe, A.E. and McIntosh, L. (1994) Molecular genetic alteration of plant respiration: silencing and over‐expression of alternative oxidase in transgenic tobacco. Plant Physiol. 106, 1503–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe, G.C. , Alison, E. and McIntosh, L. (1997) Molecular genetic evidence of the ability of alternative oxidase to support respiratory carbon metabolism. Plant Physiol. 113, 657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe, G.C. , Robson, C.A. and Yip, J.Y.H. (2002) Induction of mitochondrial alternative oxidase in response to a cell signal pathway down‐regulating the cytochrome pathway prevents programmed cell death. Plant Physiol. 129, 1829–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal, G. , Ribas‐Carbo, M. , Garmier, M. , Dubertret, G. , Rasmusson, A.G. , Mathieu, C. , Foyer, C.H. and De Paepe, R. (2007) Lack of respiratory chain complex I impairs alternative oxidase engagement and modulates redox signaling during elicitor‐induced cell death in tobacco. Plant Cell, 19, 640–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira, H. and Kroemer, G. (2003) Mitochondria as targets of apoptosis regulation by nitric oxide. IUBMB Life, 55, 613–616. [DOI] [PubMed] [Google Scholar]
- Wagner, A.M. (1995) A role for active oxygen species as second messengers in the induction of alternative oxidase gene expression in Petunia hybrida cells. FEBS Lett. 368, 339–342. [DOI] [PubMed] [Google Scholar]
- Wagner, A.M. and Krab, K. (1995) The alternative respiration pathway in plants: role and regulation. Physiol. Plant. 95, 318–325. [Google Scholar]
- Yao, N. , Tada, Y. , Sakamoto, M. , Nakayashiki, H. , Park, P. , Tosa, Y. and Mayama, S. (2002) Mitochondrial oxidative burst involved in apoptotic response in oats. Plant J. 30, 567–579. [DOI] [PubMed] [Google Scholar]
- Yi, S.Y. , Yu, S.H. and Choi, D. (1999) Molecular cloning of a catalase cDNA from Nicotiana glutinosa L. and its repression by tobacco mosaic virus infection. Mol. Cells, 9, 320–325. [PubMed] [Google Scholar]
- Yi, S.Y. , Yu, S.H. and Choi, D. (2003) Involvement of hydrogen peroxide in repression of catalase in TMV‐infected resistant tobacco. Mol. Cells, 15, 364–369. [PubMed] [Google Scholar]
- Yoshida, K. , Terashima, I. and Noguchi, K. (2007) Up‐regulation of mitochondrial alternative oxidase concomitant with chloroplast over‐reduction by excess light. Plant Cell Physiol. 48, 606–614. [DOI] [PubMed] [Google Scholar]
- Zhang, L. and Xing, D. (2008) Methyl jasmonate induces production of reactive oxygen species and alterations in mitochondrial dynamics that precede photosynthetic dysfunction and subsequent cell death. Plant Cell Physiol. 49, 1092–1111. [DOI] [PubMed] [Google Scholar]


