SUMMARY
Nitric oxide (NO) production by Botrytis cinerea and the effect of externally supplied NO were studied during saprophytic growth and plant infection. Fluorescence analysis with 4,5‐diaminofluorescein diacetate and electrochemical studies were conducted in vitro between 4 and 20 h of incubation and in planta between 15 and 75 h post‐inoculation. The production of NO by B. cinerea in vitro was detected inside the germinating spores and mycelium and in the surrounding medium. In planta production of NO showed a large variation that was dependent on the host plant and developmental stage of the infection. The induced production of NO was detected from 16 h of in vitro incubation in response to externally added NO. The production of NO by B. cinerea is probably modulated to promote fungal colonization of the plant tissue. The production of NO which diffuses outside the fungal cells and the induction of NO production by exogenous NO open up the possibility of NO cross‐talk between the fungus and the plant. Finally, the existence of an NO concentration threshold is proposed, which may increase or reduce the plant defence against necrotrophic fungal pathogens.
INTRODUCTION
Nitric oxide (NO) is a potent signal molecule in biological systems. Its production in human cells was first detected at the beginning of the 20th century (Mitchell et al., 1916). However, the biological implications in signalling, as an endogenous regulator of the vascular system, were first studied in the 1980s (Schmidt and Walter, 1994).
In mammals, NO is synthesized predominantly by the enzyme nitric oxide synthase (NOS) (Stuehr, 1999). Several isoforms of the enzyme exist in mammals, all catalysing the same basic reaction: a nicotinamide adenine dinucleotide phosphate (reduced form, NADPH)‐dependent oxidation of l‐arginine to NO and l‐citrulline. In plants, the mechanisms of NO production are not well understood. Evidence from different experimental sources suggests that a NOS‐like enzyme is present. NOS catalytic activity has been characterized in pea (Leshem and Haramaty, 1996), Lupinus (Cueto et al., 1996), soybean (Delledonne et al., 1998), tobacco (Durner et al., 1998) and maize (Ribeiro et al., 1999), and NOS inhibitors, such as Nω‐nitro‐l‐arginine methyl ester (L‐NAME), have been used to suppress NO production in soybean, Arabidopsis and tobacco (Delledonne et al., 1998; Durner et al., 1998). Furthermore, immunological studies have suggested the presence of NOS‐like proteins in plants (Barroso et al., 1999; Corpas et al., 2001); however, the specificity of NOS antibodies has been questioned (Beligni and Lamattina, 2001). Extensive research led finally to the identification of a protein in Arabidopsis, AtNOA1, elimination of which considerably reduced NO production (Zeidler et al., 2004). However, it has recently been demonstrated that AtNOA1 does not participate directly in NO production (Crawford et al., 2006; Moreau et al., 2008). The activity of nitrate reductase is another possible source of NO in plants. Thus, the addition of nitrite under anoxic conditions (Rockel et al., 2002) or without photosynthetic activity (Botrel et al., 1996) increases NO production. Arabidopsis nitrate reductase mutants nia1–nia2 show a decrease in NO production following Pseudomonas syringae infection (Modolo et al., 2005). Furthermore, during the infection of Arabidopsis by Sclerotinia sclerotiorum, both nitrate reductase and NOS pathways constitute the main sources of plant NO synthesis (Perchepied et al., 2010). Finally, nonenzymatic NO production has also been reported in plants (Bethke et al., 2004).
NO is needed to trigger the hypersensitive response (HR) in plants through a tight balance with reactive oxygen species (ROS) (Delledonne et al., 2001), and NO has been shown to play essential signalling roles in processes such as plant growth and development, plant hormone interaction, stomatal closure and seed germination (Cevahir et al., 2007). NO signalling is mediated by protein kinases, Ca2+ channels, Ca2+, cyclic guanosine monophosphate (cGMP) and cyclic ADP ribose (cADPR) (Courtois et al., 2008). NO interacts with other signalling pathways. Indeed, the interaction between salicylic acid, jasmonic acid and NO causes the activation of genes responsible for jasmonic acid synthesis, ethylene synthesis, ethylene signalling and abscisic acid response (Parani et al., 2004; Wendehenne et al., 2004).
Recent studies have indicated that NO may play an important role in signalling in fungi. For instance, the application of external NO to Colletotrichum coccodes delayed spore germination, whereas treatment with NO scavengers stimulated spore germination (Wang and Higgins, 2005). In the biotrophic fungus Blumeria graminis, application of either an NO scavenger or a mammalian NOS inhibitor affected appressorium formation (Prats et al., 2005, 2008).
Fungi are able to produce NO during anaerobic conditions as a result of denitrification processes (Morozkina and Kurakov, 2007; Ye et al., 1994). The activity of NOS in fungi has been detected through biochemical methods (Maier et al., 2001; Ninnemann and Maier, 1996). In the fungus Coniothyrium minitans, mutants that lack the activity of the l‐arginine‐specific carbamoyl‐phosphate synthase enzyme show a reduction in spore germination that is re‐established with the addition of NO, indicating NOS activity (Gong et al., 2007). However, no active mechanism for NO production in fungi has been identified to date.
Botrytis cinerea is a necrotophic pathogen that promotes plant cell necrosis through different enzymes, such as Nep‐1‐like proteins (Schouten et al., 2008) and xylanase Xyn11A (Noda et al., 2010). In addition, the plant HR plays a crucial role in B. cinerea infection (Govrin and Levine, 2000). As mentioned above, NO is essential for the activation of HR in plants and, consequently, the levels of NO in plant cells, fungal mycelium and interaction medium will have important consequences in the success of the fungal infection. Thus, the study of NO production by the fungus is of great interest. van Baarlen et al. (2004) detected, for the first time, the production of NO by germinating conidia and developing mycelium of B. cinerea through the use of fluorescent probes. In parallel, Conrath et al. (2004) detected the production of NO in B. cinerea in vitro by mass spectrometry. Floryszak‐Wieczorek et al. (2007) detected strong NO generation using 4,5‐diaminofluorescein diacetate (DAF‐2DA) during the B. cinerea colonization of pelargonium leaves. Nevertheless, studies on the biosynthetic pathway(s) for NO production by B. cinerea, and its implications for plant infection, have not been conducted. In this work, we present a detailed analysis of NO production during the saprophytic growth of B. cinerea and during the interaction with the host plant to study the role of NO in the establishment of the plant–fungus interaction.
RESULTS
Production of NO in vitro
Botrytis cinerea strain B05.10 was grown in liquid culture and monitored for the production of NO using an NO‐specific fluorescent probe. A fluorescence signal was observed inside spores and in the surrounding culture medium up to 20 h after the start of incubation (Fig. 1). Control experiments conducted with the NO scavenger 2‐(4‐carboxyphenyl)‐4,4,5,5‐tetramethylimidazoline‐1‐oxyl‐3‐oxide (cPTIO) did not show any fluorescence (images not shown). This provided evidence of the specificity of the signal detected in the experiments conducted to investigate the fungal production of NO in vitro.
Figure 1.
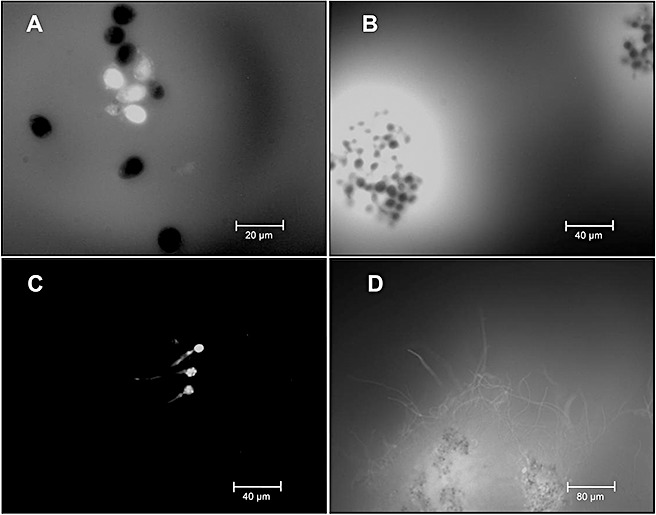
Bio‐imaging of Botrytis cinerea strain B05.10 nitric oxide (NO) production in vitro. NO detection was conducted with 4,5‐diaminofluorescein diacetate (DAF‐2DA) at 4 h (A), 8 h (B), 10 h (C) and 20 h (D) from the beginning of incubation. Spores were grown in B5S/SP medium (Gamborg's B5 salts medium supplemented with 10 mm sucrose and 10 mm KH2PO4; pH 6.0).
After 4 h of incubation, the production of NO was detected inside 40% of the germinating spores (high‐intensity white pixels, Fig. 1A) and in the surrounding medium (low‐intensity white pixels, Fig. 1A). After 8 h of incubation, fluorescence levels increased both inside the spores and in the surrounding culture medium. Interestingly, a substantial proportion of the spores (approximately 50%) displayed no fluorescence signal. After 10 h of incubation, only internal production of NO was detected in germinating spores and germ tubes (Fig. 1C). Following 20 h of incubation, as germ tubes branched and developed into mycelium, the production of NO was observed in the mycelium generated and in its adjacent medium.
The production of NO was analysed in the flavohaemoglobin knockout mutant strain ΔBcfhg1 and compared with the production of NO in the wild‐type strain B05.10. Figure 2 shows the production of NO in vitro by strains B05.10 and ΔBcfhg1 following 12 h of incubation. Both the mutant and wild‐type strain produced NO in germinating spores and mycelium. In previous studies, we observed that ΔBcfhg1 was unable to enzymatically detoxify NO and had a lower rate of NO degradation than wild‐type B05.10 (Turrion‐Gomez et al., 2010). Thus, a larger amount of NO in the mutant was expected when compared with the wild‐type. However, the results shown in Fig. 2 reveal that the mutant ΔBcfhg1strain does not produce more NO than the wild‐type B05.10 strain. Multiple repetitions of the experiments consistently showed a slight reduction in NO production in the mutant ΔBcfhg1 compared with the wild‐type, as observed in Fig. 2. Therefore, the fungus appears to be able to regulate the amount of NO it produces to compensate for the lack of NO degradation.
Figure 2.
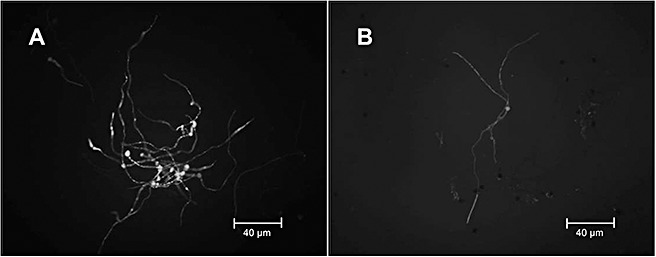
Nitric oxide (NO) production during Botrytis cinerea culture in vitro[in MEB medium (2% weight/volume malt extract broth)]. NO detection was carried out using diaminofluorescein diacetate (DAF‐2DA) fluorescence after 12 h of incubation of the fungal strains B05.10 (A) and ΔBcfhg1 (B).
Production of NO in planta
The production of NO was monitored during the infection of B. cinerea wild‐type strain B05.10 in different hosts in both plant and fungal cells. Control experiments conducted with the NO scavenger cPTIO showed no fluorescence at the same emission wavelength as diaminotriazolfluorescein (DAF‐2T), indicating that the observed green fluorescence was produced by the reaction of NO with DAF‐2DA (images not shown).
Figure 3 shows the production of NO in Solanum lycopersicum (A–D) and Nicotiana tabacum (E–H). This study indicated that the intensity of the green fluorescence signal changed at different incubation times on S. lycopersicum and N. tabacum. In addition, the intensity of the green fluorescence signal was different for different hosts following similar incubation periods.
Figure 3.
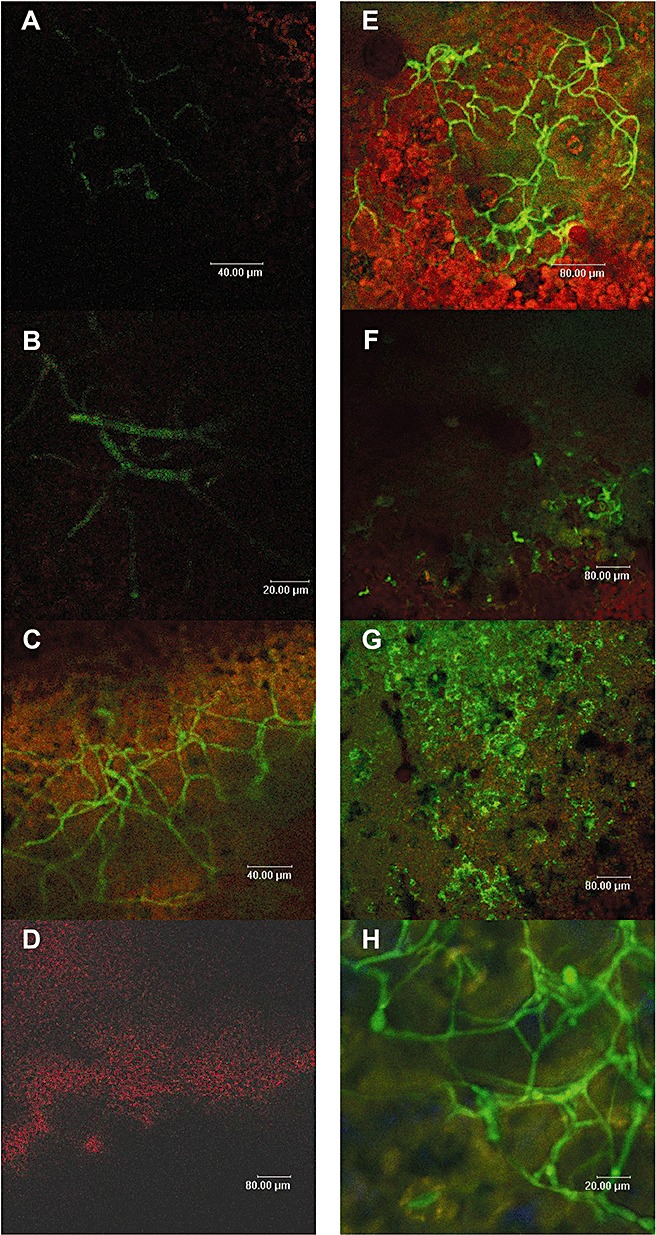
Visualization of nitric oxide (NO) by diaminofluorescein diacetate (DAF‐2DA) during the interaction of Botrytis cinerea with tomato leaves (A–D) and tobacco leaves (E–H). Analyses were conducted at 15 h (A), 24 h (B), 48 h (C), 72 h (D), 20 h (E), 28 h (F), 48 h (G) and 75 h (H) post‐inoculation.
During the infection of B. cinerea on S. lycopersicum, the production of NO in B. cinerea mycelium was detected from 15 h post‐inoculation (hpi) (Fig. 3A) and transiently increased, reaching a maximum following 48 hpi (Fig. 3C). At 72 hpi (Fig. 3D), NO was not detected. Repetitions of the experiment showed similar results.
The pattern of NO production by B. cinerea during infection of N. tabacum was different from that observed on S. lycopersicum. At 20 hpi, the production of NO by the mycelium was significantly higher than that observed at the other time points (Fig. 3E). At 28 and 48 hpi, the production of NO by the mycelium decreased considerably, whereas the production of NO by the plant was more pronounced (Fig. 3F,G). Following 75 hpi, the production of NO by the mycelium was again observed (Fig. 3H). This transient pattern was consistently observed in three replicates.
During the infection of B. cinerea on Arabidopsis thaliana, a large production of NO by both the plant and the fungus was observed (data not shown). In addition, the intensity of production of NO on A. thaliana was higher than that observed following similar periods of time (late stages of infection, 72–75 hpi) on S. lycopersicum and N. tabacum.
A correlation between the detection of the maximum concentration of NO (Fig. 3) and the spread of B. cinerea infection was observed. Thus, in S. lycopersicum, the fungal infection spread slowly and higher NO concentrations were observed after long incubation times (48 hpi). On tobacco leaves, the fungal lesions started to spread at an earlier time point and spread at a faster rate and, in this host, maximal fungal NO production was detected during earlier incubation times.
Induction of NO production in B. cinerea
The effect of exogenous NO on the production of NO in B. cinerea was investigated in vitro in the mutant strain ΔBcfhg1 and in the wild‐type strain B05.10. Figure 4 presents the concentration of NO in the medium over time during 18 min following the injection of exogenous NO in samples containing no mycelium (negative control) and in those containing mycelium from the wild‐type strain B05.10 precultured for 8 h (positive control) or from strain ΔBcfhg1 precultured for 8 or 16 h.
Figure 4.
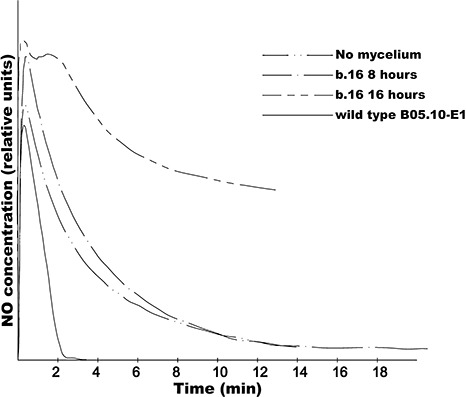
Detection of nitric oxide (NO) with an NO electrode in the Botrytis cinerea mutant ΔBcfhg1 (b.16). Samples were incubated in vitro for 8 and 16 h. NO was applied at time 0 as a gas dissolved in water to obtain a final concentration of 2 µm. The changes in NO concentration were monitored for at least 14 min. The B. cinerea strain B05.10 grown for 8 h (wild‐type B05.10‐E1) was used as a positive control. The sterile culture medium B5S/SP (Gamborg's B5 salts medium supplemented with 10 mm sucrose and 10 mm KH2PO4; pH 6.0) (no mycelium) was used as a negative control.
Following 8 h of incubation, the NO concentration profile over time in the medium with the B05.10 strain spiked with exogenous NO showed a lower concentration of NO than that measured in the sample with no mycelium spiked with exogenous NO. This indicated that NO was removed from the medium. This observation corroborates our previous results, where we demonstrated that B05.10 enzymatically degrades NO (Turrion‐Gomez et al., 2010). Following 8 h of incubation, the temporal profile of the concentration of NO measured in the medium with mycelium from strain ΔBcfhg1 spiked with exogenous NO was similar to that in the absence of fungi (no mycelium), in agreement with previous observations (Turrion‐Gomez et al., 2010). However, following 16 h of incubation, the concentration of NO was considerably higher in the medium with strain ΔBcfhg1 mycelium spiked with exogenous NO than in the medium in the absence of fungal biomass spiked with exogenous NO. The difference in NO concentration between the negative control sample (no mycelium) and the fungal mutant sample shows that the mycelium from the mutant strain ΔBcfhg1 precultured for 16 h produces NO which diffuses into the medium.
To further investigate the behaviour of the wild‐type strain B05.10, in vitro experiments similar to those carried out with strain ΔBcfhg1 were conducted with the wild‐type strain following culture times up to 20 h (Fig. 5). It was observed that, following 12 and 14 h of incubation, strain B05.10 NO concentrations were similar to those observed following 8 h of incubation. Nevertheless, when the incubation time was increased to 16 h, the temporal profile of the concentration of NO changed, and the slope of the curve was smaller than that of the curve registered in the medium with no mycelium, indicating that NO is produced in these conditions. Following longer incubation times (17 and 20 h), even higher concentrations of NO were recorded. Consequently, it can be concluded that the production of NO by the wild‐type strain B05.10 occurred (or not) as a function of the incubation time and therefore of the fungal developmental stage. Following c. 22 min of the experiment conducted with mycelium incubated for 20 h, 2 µL of 50 mm cPTIO was added to the fungal culture (indicated by an arrow in Fig. 5). The application of the NO scavenger resulted in a sudden decrease in NO concentration levels, as expected, confirming that the signal detected with the NO electrode was NO dependent.
Figure 5.
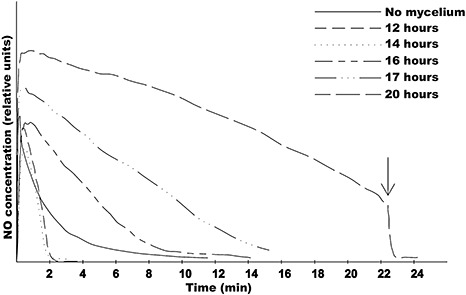
In vitro electrochemical detection of nitric oxide (NO) in Botrytis cinerea strain B05.10. Samples were incubated for 12, 14, 16, 17 and 20 h. The arrow indicates the addition of 1 mm 2‐(4‐carboxyphenyl)‐4,4,5,5‐tetramethylimidazoline‐1‐oxyl‐3‐oxide (cPTIO) to the sample incubated for 20 h. The sterile culture medium B5S/SP (Gamborg's B5 salts medium supplemented with 10 mm sucrose and 10 mm KH2PO4; pH 6.0) (no mycelium) was used as a negative control.
Attempts were made to detect and monitor the NO concentration continuously during the first 20 h of growth of the wild‐type strain B05.10 in liquid culture using the NO electrode without spiking exogenous NO. In these conditions, no signal could be detected (not shown), indicating that NO does not accumulate in the medium at levels detectable with the electrode within the time interval considered in these experiments; this observation was confirmed with the addition of 10 µL of 50 mm cPTIO. Therefore, the production of NO by the B. cinerea mycelium appears to be induced by the addition of exogenous NO, and this induction occurs very quickly, within less than 1 min, suggesting that the signal involved in this induction is simple, effective and strong.
NO production systems
NOSs are broadly distributed in the animal kingdom, presenting different copies with an ancient origin and different functions [neural (nNOS), endothelial (eNOS) and inducible (iNOS) in mammals]. Proteins similar to mammalian NOS have been identified in Prokaryotes such as Deinococcus radiodurans (Adak et al., 2002), Bacillus subtilis (Pant et al., 2002) and Staphylococcus aureus (Hong et al., 2003). The NOS‐like proteins from Prokaryotes lack redox domains and have diverse functions in toxin biosynthesis, protection against oxidative damage and signalling to regulate growth responses (Crane et al., 2010). Fungi and plants do not contain NOS‐like sequences in their genomes, except for some fungal species from the genus Aspergillus (A. flavus, A. oryzae and A. niger) and Glomerella graminicola. An extensive genome analysis failed to reveal any NOS‐like sequence in B. cinerea. However, low homology was detected between NOS and the carboxyl‐terminal reductase domain of cytochrome P450 reductase (data not shown).
A pharmacological approach was undertaken. The NOS inhibitor L‐NAME was applied to B. cinerea strain B05.10 mycelium cultured for 16 h in B5S/SP liquid medium [Gamborg's B5 salts medium (AppliChem, Darmstadt, Germany) supplemented with 10 mm sucrose and 10 mm KH2PO4; pH 6.0], 15 min before exogenous NO was spiked. Analysis of the NO curves obtained with the NO electrode demonstrated that the addition of the NOS inhibitor did not prevent the induced production of NO (data not shown). In addition, in planta experiments carried out in the presence of 5 mm L‐NAME and DAF‐2DA showed that the NOS inhibitor did not reduce NO production during B. cinerea infection of N. tabacum leaves following 48 hpi (data not shown). To study the possible production of NO through nitrate reductase, B. cinerea wild‐type B05.10 mycelium was incubated for 16 h in B5S/SP liquid medium and spiked with 50 mm of NO3 –. The NO electrode did not show an increase in NO concentration after the addition of NO3 –, suggesting that nitrate reductase is not the main system responsible for the production of NO in B. cinerea. All of these data suggest that another physiological and genetic system different from NOS and nitrate reductase should be responsible for NO production in B. cinerea.
DISCUSSION
Botrytis cinerea produces NO that diffuses into the medium
Botrytis cinerea produces NO both during saprophytic growth and in planta. In our experimental approach, we were able to detect the production of NO in all fungal developmental stages considered, from spores to mature mycelium, although at varying levels in the different stages. This study showed, for the first time, that B. cinerea generates two different NO signals. One signal is localized within the germinating spores and mycelium, and is only detected in a fraction (percentage) (c. 40%) of the spores and mycelium when the fungus is cultured in artificial liquid synthetic medium. The internal production of NO by B. cinerea has been observed previously in other studies (van Baarlen et al., 2004, 2007; Conrath et al., 2004; Floryszak‐Wieczorek et al., 2007), although the authors did not provide quantitative or qualitative studies of fungal NO production. Fluorescence was also observed in culture medium surrounding the germinating spores and mycelium. This observation is indicative of the diffusion of NO produced inside the fungal tissue into the medium. This NO diffusing from fungal biological units could have important physiological consequences in the establishment and progress of the plant–fungus interaction, as discussed later.
Production of NO in B. cinerea is regulated
Several observations obtained during the course of this work indicate that the production of NO in B. cinerea is a regulated process. First, the internal production of NO is detected in only a percentage of spores, germ tubes and hyphae during saprophytic growth. Interestingly, the internal production of NO in a percentage of spores has been observed previously in the fungus C. coccodes, a system in which NO appears to play a role in the germination of spores (Wang and Higgins, 2005). Although the experimental conditions determine good synchronization of germination and all the spores look the same (Fig. 1A,B), the observation that not all of the spores show NO production indicates the occurrence of a different physiological status between individual spores, suggesting that the production of NO in spores is regulated. Whether the production of NO conditions specific functions of individual spores remains to be determined.
Previous studies have reported the production of NO several days post‐inoculation. For example, van Baarlen et al. (2004) observed that B. cinerea infection on Arabidopsis leaves resulted in the production of NO by the fungus 5 days after inoculation. In this work, an early production of NO (at 15 hpi) was detected in the fungus during in planta infection, corroborating the in vitro results. Interestingly, temporal changes in the production of NO by B. cinerea (and also by the plant) were observed, which resulted in different NO production profiles over time when different host plant species were considered. The specific stimuli determining changes over time on a given host and differences between profiles in different hosts can be various. Our in vitro analysis demonstrates that the developmental status of the fungus and its exposure to NO both influence NO production by the fungus, as the addition of NO stimulated NO production and this (enhanced) production of NO could only be observed after the incubation of fungal spores for 16 h. Remarkably, this production is a response that occurs very rapidly, almost immediately. Therefore, the system responsible for this response must be simple and involve very few elements. One possible system is the deactivation of proteins implicated in NO metabolism through S‐nitrosylation, as occurs in A. thaliana (Mannick and Schonhoff, 2002; Romero‐Puertas et al., 2008), although such a system has not been investigated in fungi. Further research is needed to identify the enzymatic system responsible for the production of NO in fungi and to fully understand the role of NO during plant infection by B. cinerea.
Previous research conducted in our laboratory has provided evidence of the degradation of NO by B. cinerea by means of a flavohaemoglobin enzyme (Turrion‐Gomez et al., 2010). Therefore, the generation of NO in B. cinerea is a dynamic process resulting from opposing mechanisms of production and degradation of NO, which operate simultaneously in this fungus. This situation widens the repertoire of regulatory elements by means of which NO levels could be modulated in B. cinerea. It is remarkable that the expression of the flavohaemoglobin coding gene is developmentally regulated and induced by exposure to exogenous NO (Turrion‐Gomez et al., 2010), as is the production of NO by the fungus.
Fungal production of NO could enhance the spread of infection
The capacity of the fungus to produce NO that diffuses outside the fungal cells, and its ability to stimulate its production in mature mycelium when exposed to NO, could have important physiological implications because NO is a potent plant signalling molecule (Delledonne et al., 1998) that is able to cross hydrophobic membranes. As NO is highly reactive (Lancaster, 1994), it might be argued that NO could react before reaching the plant cells. However, in animal systems, NO is able to diffuse from the NO‐producing cells to neighbouring cells at distances longer than 100 µm depending on the presence of haemoglobins (Lancaster, 1994). As indicated in 4, 5, NO remains dissolved in the liquid medium over minutes, enabling enough time to diffuse from the mycelium into the plant cells.
It is well established that plant cells produce NO in response to pathogen attack to activate HR (Hong et al., 2008). In the case of B. cinerea, experimental evidence indicates that HR is needed for the success of plant infection (Govrin and Levine, 2000), and the exploitation of the defence systems by the fungus has been discussed by other authors (van Baarlen et al., 2007; Temme and Tudzynski, 2009). NO produced by the fungus early during germination, as detected in this work, and diffusing into the surrounding environment, could reach the plant cells and contribute to the triggering of the plant HR, in conjunction with other chemicals, such as H2O2 (Rolke et al., 2004), oxalic acid (Kim et al., 2008) or botrydial sesquiterpene (Siewers et al., 2005). The fungus would then start to grow on plant tissues undergoing induced cell death. At later stages of the infection process, the fungus forms spreading lesions on which it grows, actively producing abundant fungal biomass. The dying tissues generate large amounts of H2O2 and NO that contribute to accelerate HR. This NO, when reaching the fungal mycelium, would stimulate the production of NO by B. cinerea, further enhancing plant cell death. Figure 6 presents a scheme that summarizes the equilibrium between NO production and degradation, the NO–ROS interaction, the flow of NO in the plant–fungal interaction and the feedback production of NO.
Figure 6.
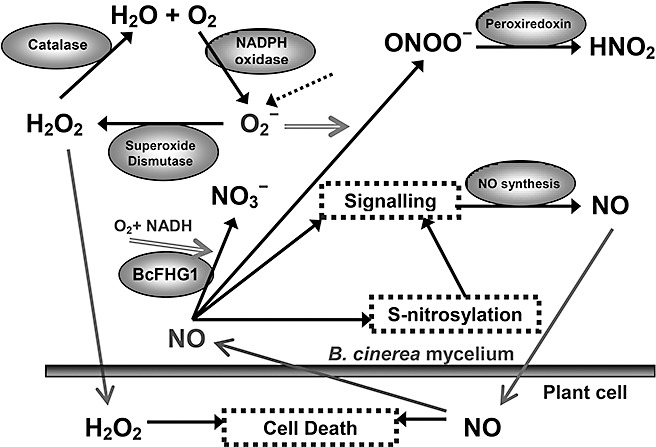
Schematic representation of the role and interaction of nitric oxide (NO) in Botrytis cinerea during plant infection. The fluxes of NO and H2O2 between the plant cells and the mycelium are represented with arrows. Signalling processes are represented in dotted boxes. The dotted arrow represents the O2 ‐ produced during respiration. The enzymes are shown inside ellipses.
Taking into consideration these observations, it is tempting to propose that B. cinerea could enhance the infection of plant cells through its own production of NO. These findings are supported by previous research with B. cinerea reporting biochemical evidence of the increase in fungal infection with the use of NO donors and the reduction of fungal infection with the use of NO scavengers (Malolepsza and Rozalska, 2005). The absence of induced NO production in the early stages of incubation in the mutant ΔBcfhg1, coupled with a similar induction of NO concentration in the wild‐type and the mutant ΔBcfhg1 at later stages, indicates that fungal NO levels are not primarily regulated by the NO degradation enzymatic system. Therefore, the system responsible for the modulation of NO levels in this case depends on NO inputs, but not on NO outputs.
The importance of NO in plant resistance has been reported during B. cinerea infection of pelargonium (Floryszak‐Wieczorek et al., 2007), N. benthamiana (Asai and Yoshioka, 2009) and between Sclerotinia sclerotiorum and Arabidopsis (Perchepied et al., 2010). However, the controlled production of NO by B. cinerea shown in the present study suggests that the NO produced by the fungus could by itself, or in synergy with the NO produced by plant cells, be important for the success of the fungal infection. In Fig. 7, we propose a model to integrate both contradictory hypotheses: (i) NO facilitates fungal infection; and (ii) NO is required for plant resistance against necrotrophic pathogens. We hypothesize that an NO concentration threshold will trigger plant cell death. Below this threshold, NO acts as a signalling molecule to activate diverse plant defence systems against the fungus. Our observations introduce additional considerations into the analysis of the interactions in which B. cinerea participates, and highlight the complexity of the interplay between B. cinerea and the plant during the establishment and progress of the fungal necrotroph–host plant interaction.
Figure 7.
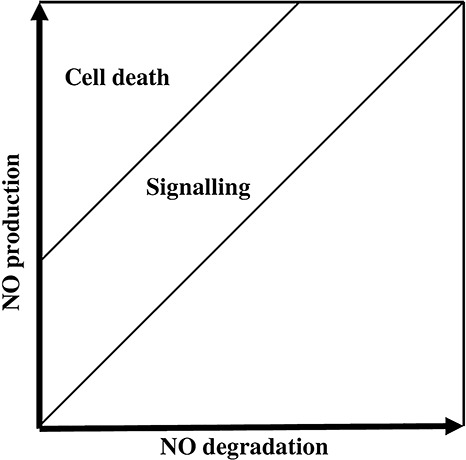
Representation of the nitric oxide (NO) balance produced after cross‐talk between Botrytis cinerea and the host plant. A tight balance between total NO degradation and plant and fungal NO production creates an NO concentration that will lead to signalling or cell death in the plant and/or the fungus.
EXPERIMENTAL PROCEDURES
Biological material and culture
Fungal spores were obtained by cultivating the B. cinerea wild‐type strain B05.10 (Buttner et al., 1994) and the mutant strain ΔBcfhg1 (b.16), which lacks NO degradation activity (Turrion‐Gomez et al., 2010), on potato dextrose agar (PDA; Difco, Franklin Lakes, NJ, USA) plates containing 25% weight/volume of tomato leaf extract. Fresh fungal spores (5 × 105 spores/mL) were then cultivated in B5S/SP medium or MEB medium [2% weight/volume malt extract broth (BD; Difco)]. Incubation was performed in an orbital shaker at 180 rpm and 22 °C.
Solanum lycopersicum and N. tabacum were cultivated in a vermiculite substrate supplemented with nutrients with a 16‐h photoperiod for 8 weeks at 22 °C. Arabidopsis thaliana plants (ecotype Columbia) were cultivated in a mixture of vermiculite and peat (1:3) substrate with an 8‐h photoperiod at 22 °C for 1 month. Spores of B. cinerea were inoculated on detached S. lycopersicum (tomato, variety Rome) and N. tabacum (tobacco) leaves and nondetached Arabidopsis thaliana leaves. Suspensions (5 µL) of fresh fungal spores (1 × 105 spores/mL) in B5S/SP medium were subsequently placed on plant leaves and incubated at 22 °C with a 16‐h photoperiod in a box closed with transparent film in order to obtain high‐humidity conditions (Benito et al., 1998). The fungal infected material was obtained from S. lycopersicum leaves at 15, 24, 48 and 72 hpi, from N. tabacum leaves at 20, 28, 48 and 75 hpi, and from A. thaliana leaves at 72 hpi. The first incubation time for S. lycopersicum and N. tabacum corresponded to the development of primary lesions when the first visual symptoms were observed; the second incubation time corresponded to the expansion of the lesions; the third and fourth times corresponded to advanced stages of expansion and plant tissue maceration. For A. thaliana, only one incubation time, corresponding to late stages of infection, was included.
Microscopy
The cell‐permeable chemical DAF‐2DA (5 mm dissolved in dimethylsulphoxide; Sigma‐Aldrich, Steinheim, Germany) was used to detect NO in situ and in vivo (Foissner et al., 2000). The reaction of NO with DAF‐2DA produces DAF‐2T, which was monitored with a fluorescence microscope (Leica, St. Gallen, Switzerland) for in vitro experiments and a confocal microscope (Leica) for in planta experiments.
For the detection of NO during saprophytic growth in vitro, 100 mL of fresh fungal cultures of the wild‐type strain B05.10 were incubated in B5S/SP medium. Samples of 1 mL were collected after 4, 8, 10 and 20 h, and centrifuged at 12 000 gfor 5 min. The supernatant was discarded and the pellet was resuspended in 500 µL of 10 mm tris(hydroxymethyl)aminomethane (Tris) (pH 7.5) and 1 µL of 5 mm DAF‐2DA, which was subsequently incubated at room temperature for 10 min. The fluorescent product resulting from the reaction with NO was detected using fluorescence microscopy (excitation/emission wavelengths, 470 nm/525 nm). A similar methodology was applied to fresh fungal cultures of strains B05.10 and ΔBcfhg1 incubated in MEB medium for 12 h. The photographs obtained had similar imaging settings and exposure conditions.
For in planta detection of NO, leaves previously inoculated with fresh fungal spore suspensions were cut in sections (approximately 0.5 cm2), and those that included fungal inoculation sites were used. The leaf cuttings were incubated in 10 µm DAF‐2DA dissolved in 10 mm Tris‐HCl (pH 7.5) at room temperature for 10 min. The resulting fluorescence was monitored by confocal laser scanning microscopy using an excitation wavelength range of 440–470 nm and an emission filter wavelength range of 525–550 nm, which was registered in the green channel. Plant autofluorescence and fluorescence signals not produced by DAF‐2T at wavelengths longer than 600 nm were registered in the red channel as a control. The imaging settings and exposure conditions were the same for all the conditions studied. Control experiments to verify that DAF‐2DA reacts specifically with NO to produce fluorescent DAF‐2T were conducted in parallel for in vitro and in planta experiments, using the same procedure as mentioned above, with the addition of 0.25 mm of the NO scavenger cPTIO (Sigma‐Aldrich) in 10 mm Tris‐HCl (pH 7.5), 15 min prior to the addition of DAF‐2DA.
NO electrode detection
A 2‐mm ISO‐NOP electrode (Word Precision Instruments, Stevenage, Hertfordshire, UK) was used to quantify the NO concentration in B5S/SP medium containing fresh B. cinerea spores incubated for up to 20 h. Samples of 2 mL were collected after 8, 12, 14, 16, 17 and 20 h and spiked with a 2‐µL fresh saturated gas solution of 2 mm NO in distilled water to achieve a final concentration of 2 µm NO in each sample. NO levels in the samples were recorded during the first 15–25 min. Control experiments were conducted to confirm the specificity of the NO measurement by the addition of 1 mm of cPTIO to one sample. This experiment was repeated three times with similar results.
ACKNOWLEDGEMENTS
This work was supported by Grants AGL2009‐08954 (Ministerio de Educación y Ciencia, Spain), SA‐02‐C2‐1 (ITACyL, Junta de Castilla y León, Spain) and GR64 (Junta de Castilla y León, Spain). JLTG was a recipient of a fellowship from the Universidad de Salamanca, Spain (Banco Santander). The authors are grateful to J. P. Bolaños (University of Salamanca, Spain) for the supply of and advice on the NO electrode and to B. Antizar‐Ladislao (University of Edinburgh, UK), P. Swarbrick (University of Nottingham, UK) and J. Cunniff (Rothamsted Research, Harpenden, Hertfordshire, UK) for critical reading of the manuscript.
REFERENCES
- Adak, S. , Bilwes, A.M. , Panda, K. , Hosfield, D. , Aulak, K.S. , McDonald, J.F. , Tainer, J.A. , Getzoff, E.D. , Crane, B.R. and Stuehr, D.J. (2002) Cloning, expression, and characterization of a nitric oxide synthase protein from Deinococcus radiodurans . Proc. Natl. Acad. Sci. USA, 99, 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai, S. and Yoshioka, H. (2009) Nitric oxide as a partner of reactive oxygen species participates in disease. Mol. Plant–Microbe Interact. 22, 619–629. [DOI] [PubMed] [Google Scholar]
- van Baarlen, P. , Staats, M. and van Kan, J.A.L. (2004) Induction of programmed cell death in lily by the fungal pathogen Botrytis elliptica . Mol. Plant Pathol. 5, 559–574. [DOI] [PubMed] [Google Scholar]
- van Baarlen, P. , Ernst, J. , Staats, M. and van Kan, J.A.L. (2007) Histochemical and genetic analysis of host and non‐host interactions of Arabidopsis with three Botrytis species: an important role for cell death control. Mol. Plant Pathol. 8, 41–54. [DOI] [PubMed] [Google Scholar]
- Barroso, J.B. , Corpas, F.J. , Carreras, A. , Sandalio, L.M. , Valderrama, R. , Palma, J.M. , Lupianez, J.A. and Rio, L.A. (1999) Localization of nitric‐oxide synthase in plant peroxisomes. J. Biol. Chem. 274, 36 729–36 733. [DOI] [PubMed] [Google Scholar]
- Beligni, M.V. and Lamattina, L. (2001) Nitric oxide in plants: the history is just beginning. Plant Cell Environ. 24, 267–278. [Google Scholar]
- Benito, E.P. , Have, A. , Klooster, J.W. and van Kan, J.A.L. (1998) Fungal and plant gene expression during synchronized infection of tomato leaves by Botrytis cinerea . Eur. J. Plant Pathol. 104, 207–220. [Google Scholar]
- Bethke, P.C. , Gubler, F. , Jacobsen, J.V. and Jones, R.L. (2004) Dormancy of Arabidopsis seeds and barley grains can be broken by nitric oxide. Planta, 219, 847–855. [DOI] [PubMed] [Google Scholar]
- Botrel, A. , Magne, C. and Kaiser, W.M. (1996) Nitrate reduction, nitrite reduction and ammonium assimilation in barley roots in response to anoxia. Plant Physiol. Biochem. 34, 645–652. [Google Scholar]
- Buttner, P. , Koch, F. , Voigt, K. , Quidde, T. , Risch, S. , Blaich, R. , Bruckner, B. and Tudzynski, P. (1994) Variations in ploidy among isolates of Botrytis cinerea: implications for genetic and molecular analyses. Curr. Genet. 25, 445–450. [DOI] [PubMed] [Google Scholar]
- Cevahir, G. , Aytamka, E. and Erol, C. (2007) The role of nitric oxide in plants. Biotechnol. Biotechnol. Equip. 21, 13–17. [Google Scholar]
- Conrath, U. , Amoroso, G. , Kohle, H. and Sultemeyer, D.F. (2004) Non‐invasive online detection of nitric oxide from plants and some other organisms by mass spectrometry. Plant J. 38, 1015–1022. [DOI] [PubMed] [Google Scholar]
- Corpas, F.J. , Barroso, J.B. and del Rio, L.A. (2001) Peroxisomes as a source of reactive oxygen species and nitric oxide signal molecules in plant cells. Trends Plant Sci. 6, 145–150. [DOI] [PubMed] [Google Scholar]
- Courtois, C. , Besson, A. , Dahan, J. , Bourque, S. , Dobrowolska, G. , Pugin, A. and Wendehenne, D. (2008) Nitric oxide signalling in plants: interplays with Ca2+ and protein kinases. J. Exp. Bot. 59, 155–163. [DOI] [PubMed] [Google Scholar]
- Crane, B.R. , Sudhamsu, J. and Patel, B.A. (2010) Bacterial nitric oxide synthases. Annu. Rev. Biochem. 79, 445–470. [DOI] [PubMed] [Google Scholar]
- Crawford, N.M. , Galli, M. , Tischner, R. , Heimer, Y.M. , Okamoto, M. and Mack, A. (2006) Plant nitric oxide synthase: back to square one—response. Trends Plant Sci. 11, 526–527. [Google Scholar]
- Cueto, M. , HernandezPerera, O. , Martin, R. , Bentura, M.L. , Rodrigo, J. , Lamas, S. and Golvano, M.P. (1996) Presence of nitric oxide synthase activity in roots and nodules of Lupinus albus . FEBS Lett. 398, 159–164. [DOI] [PubMed] [Google Scholar]
- Delledonne, M. , Xia, Y. , Dixon, R.A. and Lamb, C. (1998) Nitric oxide functions as a signal in plant disease resistance. Nature, 394, 585–588. [DOI] [PubMed] [Google Scholar]
- Delledonne, M. , Zeier, J. , Marocco, A. and Lamb, C. (2001) Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc. Natl. Acad. Sci. USA, 98, 13 454–13 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durner, J. , Wendehenne, D. and Klessig, D.F. (1998) Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP‐ribose. Proc. Natl. Acad. Sci. USA, 95, 10 328–10 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floryszak‐Wieczorek, J. , Arasimowicz, M. , Milczarek, G. , Jelen, H. and Jackowiak, H. (2007) Only an early nitric oxide burst and the following wave of secondary nitric oxide generation enhanced effective defence responses of pelargonium to a necrotrophic pathogen. New Phytol. 175, 718–730. [DOI] [PubMed] [Google Scholar]
- Foissner, I. , Wendehenne, D. , Langebartels, C. and Durner, J. (2000) In vivo imaging of an elicitor‐induced nitric oxide burst in tobacco. Plant J. 23, 817–824. [DOI] [PubMed] [Google Scholar]
- Gong, X.Y. , Fu, Y.P. , Jiang, D.H. , Li, G.Q. , Yi, X.H. and Peng, Y.L. (2007) L‐arginine is essential for conidiation in the filamentous fungus Coniothyrium minitans . Fungal Genet. Biol. 44, 1368–1379. [DOI] [PubMed] [Google Scholar]
- Govrin, E.M. and Levine, A. (2000) The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea . Curr. Biol. 10, 751–757. [DOI] [PubMed] [Google Scholar]
- Hong, I.S. , Kim, Y.K. , Choi, W.S. , Seo, D.W. , Yoon, J.W. , Han, J.W. , Lee, H.Y. and Lee, H.W. (2003) Purification and characterization of nitric oxide synthase from Staphylococcus aureus . FEMS Microbiol. Lett. 222, 177–182. [DOI] [PubMed] [Google Scholar]
- Hong, J.K. , Yun, B.W. , Kang, J.G. , Raja, M.U. , Kwon, E. , Sorhagen, K. , Chu, C. , Wang, Y. and Loake, G. (2008) Nitric oxide function and signalling in plant disease resistance. J. Exp. Bot. 59, 147–154. [DOI] [PubMed] [Google Scholar]
- Kim, K.S. , Min, J.Y. and Dickman, M.B. (2008) Oxalic acid is an elicitor of plant programmed cell death during Sclerotinia sclerotiorum disease development. Mol. Plant–Microbe Interact. 21, 605–612. [DOI] [PubMed] [Google Scholar]
- Lancaster, J.R. (1994) Simulation of the diffusion and reaction of the endogenously produced nitric oxide. Proc. Natl. Acad. Sci. USA, 91, 8137–8141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshem, Y.Y. and Haramaty, E. (1996) The characterization and contrasting effects of the nitric oxide free radical in vegetative stress and senescence of Pisum sativum Linn foliage. J. Plant Physiol. 148, 258–263. [Google Scholar]
- Maier, J. , Hecker, R. , Rockel, P. and Ninnemann, H. (2001) Role of nitric oxide synthase in the light‐induced development of sporangiophores in Phycomyces blakesleeanus . Plant Physiol. 126, 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malolepsza, U. and Rozalska, S. (2005) Nitric oxide and hydrogen peroxide in tomato resistance—nitric oxide modulates hydrogen peroxide level in o‐hydroxyethylorutin‐induced resistance to Botrytis cinerea in tomato. Plant Physiol. Biochem. 43, 623–635. [DOI] [PubMed] [Google Scholar]
- Mannick, J.B. and Schonhoff, C.M. (2002) Nitrosylation: the next phosphorylation? Arch. Biochem. Biophys. 408, 1–6. [DOI] [PubMed] [Google Scholar]
- Mitchell, H.H. , Shonle, H.A. and Grindley, H.S. (1916) The origin of the nitrates in the urine. J. Biol. Chem. 24, 461–490. [Google Scholar]
- Modolo, L.V. , Augusto, O. , Almeida, I.M.G. , Magalhaes, J.R. and Salgado, I. (2005) Nitrite as the major source of nitric oxide production by Arabidopsis thaliana in response to Pseudomonas syringae . FEBS Lett. 579, 3814–3820. [DOI] [PubMed] [Google Scholar]
- Moreau, M. , Lee, G.I. , Wang, Y. , Crane, B.R. and Klessig, D.F. (2008) AtNOS/AtNOA1 is a functional Arabidopsis thaliana cGTPase and not a nitric‐oxide synthase. J. Biol. Chem. 283, 32 957–32 967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozkina, E.V. and Kurakov, A.V. (2007) Dissimilatory nitrate reduction in fungi under conditions of hypoxia and anoxia: a review. Appl. Biochem. Microbiol. 43, 544–549. [PubMed] [Google Scholar]
- Ninnemann, H. and Maier, J. (1996) Indications for the occurrence of nitric oxide synthases in fungi and plants and the involvement in photoconidiation of Neurospora crassa . Photochem. Photobiol. 64, 393–398. [DOI] [PubMed] [Google Scholar]
- Noda, J. , Brito, N. and Gonzalez, C. (2010) The Botrytis cinerea xylanase Xyn11A contributes to virulence with its necrotizing activity, not with its catalytic activity. BMC Plant Biol. 10, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant, K. , Bilwes, A.M. , Adak, S. , Stuehr, D.J. and Crane, B.R. (2002) Structure of a nitric oxide synthase heme protein from Bacillus subtilis . Biochemistry, 41, 11 071–11 079. [DOI] [PubMed] [Google Scholar]
- Parani, M. , Rudrabhatla, S. , Myers, R. , Weirich, H. , Smith, B. , Leaman, D.W. and Goldman, S.L. (2004) Microarray analysis of nitric oxide responsive transcripts in Arabidopsis . Plant Biotechnol. J. 2, 359–366. [DOI] [PubMed] [Google Scholar]
- Perchepied, L. , Balague, C. , Riou, C. , Claudel‐Renard, C. , Riviere, N. , Grezes‐Besset, B. and Roby, D. (2010) Nitric oxide participates in the complex interplay of defense‐related signaling pathways controlling disease resistance to Sclerotinia sclerotiorum in Arabidopsis thaliana . Mol. Plant–Microbe Interact. 23, 846–860. [DOI] [PubMed] [Google Scholar]
- Prats, E. , Mur, L.A.J. , Sanderson, R. and Carver, T.L.W. (2005) Nitric oxide contributes both to papilla‐based resistance and the hypersensitive response in barley attacked by Blumeria graminis f. sp. hordei . Mol. Plant Pathol. 6, 65–78. [DOI] [PubMed] [Google Scholar]
- Prats, E. , Carver, T.L.W. and Mur, L.A.J. (2008) Pathogen‐derived nitric oxide influences formation of the appressorium infection structure in the phytopathogenic fungus Blumeria graminis . Res. Microbiol. 159, 476–480. [DOI] [PubMed] [Google Scholar]
- Ribeiro, E.A. , Cunha, F.Q. , Tamashiro, W. and Martins, I.S. (1999) Growth phase‐dependent subcellular localization of nitric oxide synthase in maize cells. FEBS Lett. 445, 283–286. [DOI] [PubMed] [Google Scholar]
- Rockel, P. , Strube, F. , Rockel, A. , Wildt, J. and Kaiser, W.M. (2002) Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro . J. Exp. Bot. 53, 103–110. [PubMed] [Google Scholar]
- Rolke, Y. , Liu, S.J. , Quidde, T. , Williamson, B. , Schouten, A. , Weltring, K.M. , Siewers, V. , Tenberge, K.B. , Tudzynski, B. and Tudzynski, P. (2004) Functional analysis of H2O2‐generating systems in Botrytis cinerea: the major Cu–Zn‐superoxide dismutase (BCSOD1) contributes to virulence on French bean, whereas a glucose oxidase (BCGOD1) is dispensable. Mol. Plant Pathol. 5, 17–27. [DOI] [PubMed] [Google Scholar]
- Romero‐Puertas, M.C. , Campostrini, N. , Matte, A. , Righetti, P.G. , Perazzolli, M. , Zolla, L. , Roepstorff, P. and Delledonne, M. (2008) Proteomic analysis of S‐nitrosylated proteins in Arabidopsis thaliana undergoing hypersensitive response. Proteomics, 8, 1459–1469. [DOI] [PubMed] [Google Scholar]
- Schmidt, H. and Walter, U. (1994) NO at work. Cell, 78, 919–925. [DOI] [PubMed] [Google Scholar]
- Schouten, A. , van Baarlen, P. and van Kan, J.A.L. (2008) Phytotoxic Nep1‐like proteins from the necrotrophic fungus Botrytis cinerea associate with membranes and the nucleus of plant cells. New Phytol. 177, 493–505. [DOI] [PubMed] [Google Scholar]
- Siewers, V. , Viaud, M. , Jimenez‐Teja, D. , Collado, I.G. , Gronover, C.S. , Pradier, J.M. , Tudzynski, B. and Tudzynski, P. (2005) Functional analysis of the cytochrome P450 monooxygenase gene bcbot1 of Botrytis cinerea indicates that botrydial is a strain‐specific virulence factor. Mol. Plant–Microbe Interact. 18, 602–612. [DOI] [PubMed] [Google Scholar]
- Stuehr, D.J. (1999) Mammalian nitric oxide synthases. Biochim. Biophys. Acta Bioenerg. 1411, 217–230. [DOI] [PubMed] [Google Scholar]
- Temme, N. and Tudzynski, P. (2009) Does Botrytis cinerea ignore H2O2‐induced oxidative stress during infection? Characterization of botrytis activator protein 1. Mol. Plant–Microbe Interact. 22, 987–998. [DOI] [PubMed] [Google Scholar]
- Turrion‐Gomez, J.L. , Eslava, A.P. and Benito, E.P. (2010) The flavohemoglobin BCFHG1 is the main NO detoxification system and confers protection against nitrosative conditions but is not a virulence factor in the fungal necrotroph Botrytis cinerea . Fungal Genet. Biol. 47, 484–496. [DOI] [PubMed] [Google Scholar]
- Wang, J. and Higgins, V.J. (2005) Nitric oxide has a regulatory effect in the germination of conidia of Colletotrichum coccodes . Fungal Genet. Biol. 42, 284–292. [DOI] [PubMed] [Google Scholar]
- Wendehenne, D. , Durner, J. and Klessig, D.F. (2004) Nitric oxide: a new player in plant signalling and defence responses. Curr. Opin. Plant Biol. 7, 449–455. [DOI] [PubMed] [Google Scholar]
- Ye, R.W. , Averill, B.A. and Tiedje, J.M. (1994) Denitrification—production and consumption of nitric oxide. Appl. Environ. Microbiol. 60, 1053–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler, D. , Zahringer, U. , Gerber, I. , Dubery, I. , Hartung, T. , Bors, W. , Hutzler, P. and Durner, J. (2004) Innate immunity in Arabidopsis thaliana: lipopolysaccharides activate nitric oxide synthase (NOS) and induce defense genes. Proc. Natl. Acad. Sci. USA, 101, 15 811–15 816. [DOI] [PMC free article] [PubMed] [Google Scholar]


