SUMMARY
Numerous vegetable crops are susceptible to powdery mildew, but cucurbits are arguably the group most severely affected. Podosphaera fusca (synonym Podosphaera xanthii) is the main causal agent of cucurbit powdery mildew and one of the most important limiting factors for cucurbit production worldwide. Although great efforts have been invested in disease control, by contrast, many basic aspects of the biology of P. fusca remain unknown.
Taxonomy: Podosphaera fusca (Fr.) Braun & Shishkoff. Kingdom Fungi; Phylum Ascomycota; Subdivision Pezizomycotina; Class Leotiomycetes; Order Erysiphales; Family Erysiphaceae; genus Podosphaera; species fusca.
Identification: Superficial persistent mycelium. Conidia in chains, hyaline, ellipsoid to ovoid or doliform, about 24–40 × 15–22 µm, with cylindrical or cone‐shaped fibrosin bodies, which often germinate from a lateral face and produce a broad, clavate germ tube and cylindrical foot‐cells. Unbranched erect conidiophores. Cleistothecia globose, mostly 70–100 µm in diameter, dark brown/black. One ascus per cleistothecium with eight ascospores.
Host range: Angiosperm species that include several families, such as Asteracea, Cucurbitaceae, Lamiaceae, Scrophulariaceae, Solanaceae and Verbenaceae.
Disease symptoms: White colonies develop on leaf surfaces, petioles and stems. Under favourable environmental conditions, the colonies coalesce and the host tissue becomes chlorotic and usually senesces early.
Control: Chemical control and the use of resistant cultivars. Resistance has been documented in populations of P. fusca to some of the chemicals registered for control.
INTRODUCTION
The Cucurbitaceae or cucurbit family is a medium‐sized plant family composed of a diverse group of species grown around the world's warmer regions. It is a major family for economically important species, particularly those with edible fruits, and comprises an important starch resource in many regional diets. The major cultivated types include cucumber, melon, watermelon, squash and pumpkin. Minor cultivated types include chayote, citron, gherkin, gourds, horned cucumber and wild cucumber. They are amongst the earliest cultivated plants in both the Old and New Worlds, and some have medicinal and other uses. Unfortunately, diseases plague the production of cucurbits. There are over 200 known cucurbit diseases of diverse aetiologies (Zitter et al., 1996).
Powdery mildew is probably the most common, conspicuous, widespread and easily recognizable disease of cucurbits. Like other powdery mildew diseases, its symptoms are characterized by the whitish, talcum‐like, powdery fungal growth that develops on both leaf surfaces (Fig. 1A,B), petioles and stems (Sitterly, 1978; Zitter et al., 1996), and rarely on fruits. The disease can be caused by either Golovinomyces cichoracearum or Podosphaera fusca. These obligate biotrophic ectoparasites induce identical symptoms, but can be easily distinguished from each other by light microscopy (Braun et al., 2002). In Spain, as in many other countries around the world, cucurbit powdery mildew is a serious threat, and P. fusca is considered to be the main causal agent of powdery mildew on cucurbits and one of the most important limiting factors for cucurbit production (Fernández‐Ortuño et al., 2006; del Pino et al., 2002).
Figure 1.
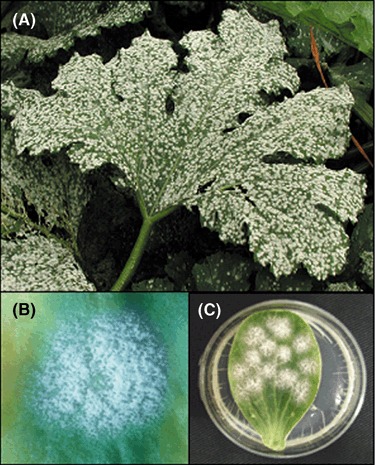
Cucurbit powdery mildew symptoms caused by Podosphaera fusca. (A) Symptoms on a zucchini leaf. (B) Detail of a powdery mildew colony. (C) Zucchini cotyledon maintained in vitro and infected with P. fusca showing typical powdery mildew colonies.
Powdery mildew fungi are biotrophic parasites that usually grow on the plant surface, obtaining nutrients from the host epidermal cells by means of haustoria (Green et al., 2002). Owing to their nature as obligate parasites, they cannot be cultured on nutrient medium, a fact that has significantly hampered research from genetic and molecular points of view. Appropriate disease management programmes require a good understanding of the biology of the responsible pathogen. This review summarizes our current knowledge of the biology of P. fusca, focusing on the molecular aspects of host–pathogen interactions, fungicide resistance and multitrophic interactions between host, P. fusca and biological control agents.
TAXONOMY
The nomenclature of the main causal agent of cucurbit powdery mildew is not yet completely standardized in the literature. The fungus has been designated Sphaerotheca fuliginea, Sphaerotheca fusca, Podosphaera fusca or Podosphaera xanthii. Based on scanning electron microscopy and molecular results, the reduction of the genus Sphaerotheca to synonymy with Podosphaera is now widely accepted (Braun et al., 2002). The separation of P. xanthii from the P. fusca group was proposed according to a morphological species concept based on the teleomorph, by which the organism on cucurbits seems to have large ascomata and an ascus with a large oculus (Braun and Takamatsu, 2000; Braun et al., 2001). In fungi, many morphological characters are plastic, and the natural variation of these characters within a species is difficult to assess. It has therefore become evident for many mycologists that the morphological species concept is largely unsatisfactory (Moncalvo, 2005). This seems to be the case for P. xanthii, because the morphological features proposed to define this species represent doubtful criteria for two main reasons. First, chasmothecia are rarely or never observed in the field (McGrath, 1994), and second, although the production of chasmothecia can be induced in the laboratory (Bardin et al., 1997), these are nonfertile as the ascospores obtained are not able to cause infection on cucurbits (McGrath, 1994). In addition, although some fragmentary molecular data based on internal transcribed spacer (ITS) sequences have become available (Hirata et al., 2000), these data are not sufficient to make a decision because, as convincingly argued by Taylor et al. (2000), a phylogenetic approach for recognizing fungal species should not be based on a single gene phylogeny, but on the concordance of multiple gene genealogies. Thus, this division remains controversial and many authors have continued to consider P. xanthii as synonymous with P. fusca.
DISEASE SYMPTOMS AND LIFE CYCLE
The disease caused by P. fusca (similar to other powdery mildews) is easily recognized by the presence of a visual white powdery mass, mainly composed of mycelia and conidia, on leaf surfaces (Fig. 1A,B), petioles and young stems (Zitter et al., 1996). Under favourable environmental conditions, fungal colonies may coalesce, covering the entire top surface of the leaves. The fungus feeds the plant nutrients, reduces photosynthesis and causes yellowing, and sometimes the death of leaves. A severe infection may kill the plant. Crop yields can be reduced because of reduced size or number of fruits. Fruit from affected plants can have low quality (Zitter et al., 1996). Although cucurbit fruits are not directly or rarely attacked by powdery mildew fungi, they may be malformed, sunburned and ripened prematurely or incompletely because of a loss of foliage cover caused by premature senescence of infected leaves (Sitterly, 1978).
The asexual life cycle of P. fusca is very similar to that of other powdery mildew fungi (Fig. 2). Typically, after landing on a susceptible host, conidia produce a short germ tube, ending in a primary differentiated appressorium, from which a primary haustorium forms in an epidermal cell. From the primary appressorium, or from another pole of the conidium, a first hypha (primary hypha) arises that forms secondary appressoria from which secondary haustoria are formed. Later, the primary hypha branches to form secondary hyphae. Conidiophores emerge vertically from some of the secondary hyphae as morphologically distinct structures. At the tip of each conidiophore, 5–10 ovoid‐shaped conidia are produced in chains. The mat of secondary hyphae and conidia forms the white mycelium on the surface of the plant, the typical visible symptom of powdery mildews (Pérez‐García et al., 2001).
Figure 2.
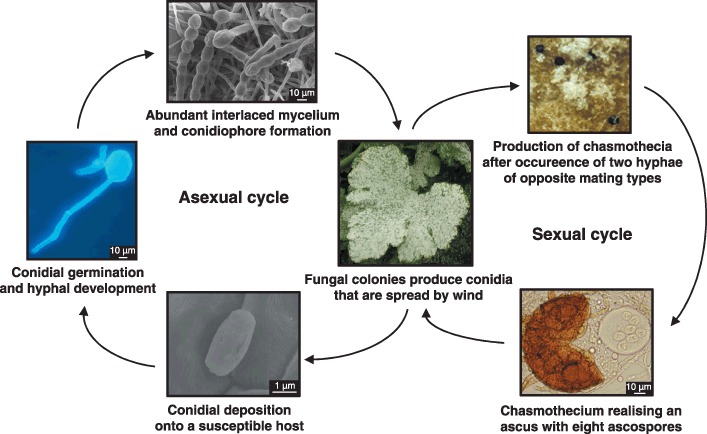
Diagram depicting the life cycle of Podosphaera fusca.
Podosphaera fusca is a heterothallic fungus, and only after the encounter of two hyphae of opposite mating types does sexual reproduction occur (Fig. 2). As a consequence, a type of fruiting body, termed a chasmothecium, is formed which, in the case of P. fusca, contains only one ascus bearing eight ascospores or sexual spores (McGrath, 1994). Chasmothecia are, in general, considered to be overwintering and oversummering sources of inoculum. Although poorly investigated, the outbreak of the disease caused by ascospores is thought to resemble that of asexual conidia (Butt, 1978; Jarvis et al., 2002). In the case of cucurbit powdery mildew, chasmothecia have rarely or never been observed in several of the world's most important cucurbit growing areas (McGrath, 1994). For this reason, the question of the prevalence and epidemiological relevance of the sexual stage of the pathogen remains largely unanswered.
RESEARCH TOOLS
Powdery mildews are comparatively difficult fungi to work with. Their obligate, biotrophic, parasitic nature and consequent inability to grow on artificial culture medium significantly hamper research. Podosphaera fusca has been traditionally cultured and conserved by periodical transfers of conidia to fresh plant material. In the laboratory, P. fusca is usually grown on detached cotyledons of several cucurbit species, such as Lagenaria, melon or zucchini, which are maintained in vitro on agarized medium (Fig. 1C) (Álvarez and Torés, 1997; Bardin et al., 2007). As a conservation system, this method is not very practical for maintaining large numbers of isolates and does not prevent genetic or physiological changes during long‐term and frequent subculturing (Nicot et al., 2002). Cryopreservation in liquid nitrogen is considered to be the best and most widely applicable preservation technique available for filamentous fungi, and has also been reported for the long‐term storage of P. fusca (Bardin et al., 2007; O’Brien and Weinert, 1994). Briefly, conidia are dried in air or using a desiccating agent such as CaCl2, and stored at −196 °C in liquid nitrogen. However, freezing in ultralow freezers is the most commonly used method to preserve microbial culture collections, and the method that adjusts better to the requirements of any standard laboratory (Pérez‐García et al., 2006). Recently, a storage technique for P. fusca has been developed, which has made possible the long‐term preservation of the pathogen in ultralow freezers. Basically, conidia are desiccated in the presence of anhydrous silica gel for 8 h in the dark at 22 °C and then stored at −80 °C (Pérez‐García et al., 2006). The method has been tried for up to 8 years (F. López‐Ruiz, unpublished data). Currently, more than 1500 isolates of P. fusca have been stored at −80 °C using this method in our laboratory. This preservation method also seems to work for other powdery mildew species, such us Blumeria graminis f. sp. tritici (J. Brown, John Innes Centre, Norwich, UK; personal communication).
The genetics of powdery mildew fungi represents a serious challenge to researchers. The range of characters that can be studied is restricted to those that are most important for the adaptation of the fungus to its host, namely host range and race‐specific avirulence, and fungicide resistance (Brown, 2002). In contrast with other powdery mildew fungi, such as B. graminis, the genetics of P. fusca remains virtually unexplored because we lack a system to perform P. fusca meiotic crosses. Although the production of chasmothecia can be induced in the laboratory on detached cotyledons when two strains of opposite mating types are co‐inoculated (McGrath, 1994), the special conditions for chasmothecial maturation and the appropriate development of ascospores have yet to be determined. Thus, this is without doubt the main bottleneck to the performance of genetic analysis in P. fusca.
Molecular studies of powdery mildew fungi are plagued by the intrinsic difficulty of extracting DNA or RNA of suitable quality and sufficient quantity to perform large‐scale molecular analyses. However, the technologies generally termed whole genome amplification (WGA) and whole transcriptome amplification (WTA) have the potential to overcome these limitations. Recently, the WGA method called multiple displacement amplification (MDA) has been used to amplify the whole genome of P. fusca (Fernández‐Ortuño et al., 2007, 2008a). MDA is a technique capable of generating microgram quantities of high‐molecular‐weight DNA from a few nanograms of input DNA, the MDA‐synthesized DNA being highly accurate and representative of the amplified genome and suitable for a diverse set of downstream applications. The MDA method is especially useful in population biology studies, when a large number of samples or isolates need to be analysed, in fields such as molecular epidemiology (e.g. detection of fungicide resistance alleles) or population genetics (e.g. determination of the genetic structure of pathogen populations using polymerase chain reaction‐based approaches, such as multilocus sequence typing) (Fernández‐Ortuño et al., 2007, 2008a). MDA technology can also be very useful in systematics, contributing to overcome the most limiting factor in the advancement of fungal molecular phylogenetics, i.e. the relatively small number of genes that are readily accessible for systematics, especially from fungi recalcitrant to study, such as powdery mildews (Fernández‐Ortuño et al., 2007). Similar to WGA, WTA methods amplify the whole transcriptome of a given organism from a few nanograms of starting RNA (Tomlins et al., 2006). Although WTA methods have yet to be applied to P. fusca, it seems probable that both WGA and WTA technologies will facilitate molecular studies in this and other obligate fungal pathogens.
HOST–PATHOGEN INTERACTIONS
Genetic resistance to powdery mildew in cucurbits is a very important attribute, in that it contributes to higher yields of better quality crops and low fungicide applications, hence increasing environmental and health benefits. Breeding for resistance to powdery mildew in the Cucurbitaceae family has a long and successful history (Jahn et al., 2002), as the use of resistant cultivars represents one of the main means of disease control, although with variable success (Zitter et al., 1996). Many commercial varieties and breeding lines of cucumber, melon and squash have been released with resistance to P. fusca. Several resistance genes (Pm) have been reported, especially in melon (Pitrat, 2002). Resistance is usually conferred by single dominant genes in most cases, although recessive genes have also been described (Jahn et al., 2002). The fourth major crop, watermelon, has been traditionally considered to be resistant to powdery mildew. Unfortunately, in recent years, outbreaks of powdery mildew in watermelon have been reported that appear to be elicited by highly aggressive isolates of P. fusca (Cohen et al., 2000; Jahn et al., 2002; del Pino et al., 2002). Although, as previously mentioned, many commercial varieties have been released with resistance to P. fusca, the development of new races of the pathogen hinders disease management through resistance breeding. This problem is especially serious in melon, where there have been more than 30 reported sources of resistance to the 28 putative races of P. fusca described so far (McCreight, 2006).
Various resistance mechanisms to powdery mildews have been reported, and can be roughly classified as pre‐ and post‐haustorial resistance (Huang et al., 1998). With pre‐haustorial resistance, the formation of haustoria is prevented or reduced by papillae and is not associated with plant cell necrosis (hypersensitive reaction). This mechanism has been reported in quantitative non‐race‐specific types of resistance and also in non‐host resistance to powdery mildew species. To our knowledge, this resistance mechanism has not been reported in cucurbits against cucurbit powdery mildew. Post‐haustorial resistance is usually associated with a hypersensitive reaction. This is the typical mechanism of the major genic race‐specific resistance to powdery mildews. Resistance to races 1 and 2 of P. fusca has been reported as post‐haustorial in several melon cultivars, such as PMR‐6 (Cohen and Eyal, 1988; Cohen et al., 1990; Pérez‐García et al., 2001), and in some resistant cucumber and melon lines, but switched by temperature variations (Munger, 1979; Pérez‐García et al., 2001). Although the interaction between melon and P. fusca is assumed to comply with the gene‐for‐gene concept, DNA sequences encoding Pm genes have not been isolated, nor have avirulence genes been identified.
In post‐haustorial resistance, the cascade of mechanisms identified in melon plants resistant to P. fusca proceeds as follows. Podosphaera fusca conidia are hypothesized to release cell wall monomers, as described for B. graminis. After recognition, the resistant plant triggers a sequence of physiological changes initiated by the rapid accumulation of reactive oxygen species, such as hydrogen peroxide and superoxide anions. These changes take place a few hours after pathogen inoculation and before the formation of the first haustorium (Romero et al., 2008). This oxidative burst is followed by a strong reinforcement of plant cell walls of P. fusca‐invaded and surrounding cells. The reinforcement is caused by the deposition of lignin and callose polymers, which may slow down pathogen ingress into the cell and disrupt nutrient flow (Cohen et al., 1990; Romero et al., 2008). The coordinated spatial–temporal accumulation pattern of cell wall deposits and the production of hydrogen peroxide, combined with the increase in peroxidase activity found in diverse cucurbit species in response to P. fusca, also suggest the involvement of this reactive oxygen species in cell wall strengthening (Lebeda et al., 1999; Reuveni and Bothma, 1985; Romero et al., 2008). Interestingly, the transcriptional levels of phenylalanine ammonia‐lyase, an important enzyme for phenylpropanoid metabolism, do not seem to change in response to P. fusca inoculation (Romero et al., 2008). The additional accurate deployment of pathogenesis‐related proteins and phytoalexins in the zone of penetration has been suggested to arrest P. fusca development directly. A differential expression of β‐glucanase has been reported to occur in susceptible and resistant cultivars in response to infection by P. fusca (Rivera et al., 2002); however, its precise role in P. fusca resistance has not been conclusively established, as immunocytolocalization studies have yet to be undertaken. In any case, the manifestation of the response mechanism, including plant cell necrosis, is typically post‐haustorial (Cohen et al., 1990; Rivera et al., 2002). Recent studies have demonstrated, however, that, in response to inoculation with the same P. fusca race, some resistant lines afford a post‐haustorial resistance pattern slightly modified from that mentioned previously. In this case, the accumulation of callose appears to be the most relevant physiological modification, whereas the typical hypersensitive reaction seems to play a complementary role (Kuzuya et al., 2006).
FUNGICIDE RESISTANCE
Although great efforts have been invested in plant breeding programmes, growers still have important concerns about disease control, and the application of fungicides continues to be the principal practice for the management of powdery mildew in most cucurbit crops (McGrath, 2001). The impact of chemical control, however, has been very much tempered by the ease with which P. fusca develops resistance, quickly rendering many systemic fungicides ineffective, perhaps because high disease pressures require repeated fungicide treatments (Hollomon and Wheeler, 2002). The phenomenon of fungicide resistance in cucurbit powdery mildew traces back to 1967, when benomyl‐resistant strains were detected in an experimental glasshouse in the USA (Schroeder and Provvidenti, 1969). Since then, the cucurbit powdery mildew fungus has exhibited a high potential for developing resistance in many areas of the world to several fungicide classes, including methyl benzimidazole carbamates, sterol demethylation inhibitors (DMIs), morpholines, organophosphates, hydroxypyrimidines, Qo inhibitors (QoIs) and quinoxalines (McGrath, 2001).
The first molecular data on fungicide resistance in P. fusca were related to resistance to strobilurins (QoI fungicides). The main mechanism conferring resistance to QoIs in phytopathogenic fungi is a target site modification that involves mutations in the cytochrome b gene CYTB, such as the substitution of glycine by alanine at position 143 (G143A) (Fernández‐Ortuño et al., 2008b). The occurrence of the G143A amino acid change in isolates of P. fusca resistant to QoI fungicides was first documented in Spain, where it was reportedly widespread in cucumber (Heaney et al., 2000), and also in Japan (Ishii et al., 2001). However, in a recent study with resistant isolates obtained from melon and other cucurbit crops in south‐central Spain, the mechanism responsible for QoI resistance in P. fusca was not shown to be linked to typical mutations in CYTB (Fernández‐Ortuño et al., 2008a). In that report, neither G143A nor other consistent amino acid substitutions in cytochrome b QoI domains were found to correlate with resistance. In addition, the absence of the G143A substitution could not be explained by an intron following codon 143, as found in several rust fungi. In the same study, the role of alternative respiration in QoI resistance was also ruled out. Considering the pattern of cross‐resistance to different QoIs, the high levels of resistance of the resistant P. fusca isolates and the absence of consistent mutations in CYTB, a structural change in the Rieske‐FeS protein (ISP), the other protein component of the target site of QoI fungicides, may well be responsible for QoI resistance (Fernández‐Ortuño et al., 2008a). Experimental evidence regarding the role of the Rieske protein in resistance to QoI fungicides in P. fusca has yet to be obtained.
Sterol DMI fungicides are one of the most important classes of agricultural fungicides and the current leading class against powdery mildews. Unfortunately, resistance to DMI fungicides in P. fusca has been documented (McGrath, 2001). In contrast with QoI resistance, DMI resistance seems to be quantitative, with resistance resulting from the modification of several interacting genes. Several mechanisms of DMI resistance seem to be operating in plant pathogens, including mutations and overexpression of the target C14α‐demethylase (CYP51) gene (Ma and Michailides, 2005). The molecular basis of resistance to DMI fungicides in P. fusca is being elucidated currently. Recent data on the molecular analysis of the CYP51 gene from azole‐resistant and azole‐sensitive P. fusca isolates showed a correlation between certain amino acid substitutions and the different resistance phenotypes observed (López‐Ruiz et al., 2008), but conclusive data about the precise role of these amino acid changes in DMI resistance have yet to be provided. Furthermore, differences in CYP51 expression do not appear to play a significant role in DMI resistance (F. López‐Ruiz, unpublished data).
MULTITROPHIC INTERACTIONS BETWEEN HOST, PATHOGEN AND BIOLOGICAL CONTROL AGENTS
The increasing problem of fungicide resistance and public concerns about the hazardous effects of chemicals on the environment have led researchers to explore suitable environmentally friendly alternatives or complements to chemicals for the management of cucurbit powdery mildew. Although certain natural products, such as plants and compost extracts, detergents and mineral oils, micronutrient solutions, silicon and even animal‐based products, such as unpasteurized milk, have been proposed in the literature as less harmful alternatives to traditional chemical practices, management approaches based on the use of natural enemies of pathogens (referred to as biological control) are by far the most investigated of such alternatives (Bélanger and Labbé, 2002).
Several filamentous fungi, yeasts and bacteria have been proposed as biocontrol agents of cucurbit powdery mildew, but only a few have shown significant effectiveness. Much of the work has been performed with the mycoparasites Acremonium alternatum and Ampelomyces quisqualis, and the entomopathogenic fungus Lecanicillium lecanii (Romero et al., 2003). Two main effects on P. fusca development have been attributed to mycoparasites: (i) reduction in nutrient uptake as a result of the limitation of the number of haustoria, leading to a reduction in the growth and size of the colony; (ii) limitation of the number of conidiophores and conidia, essential structures for pathogen multiplication, resulting in a reduction in disease spread (Romero et al., 2003, 2007c). Two different interaction strategies between these mycoparasites and P. fusca have been recognized. The endoparasitic behaviour of A. quisqualis has been well documented by fluorescence microscopy (Romero et al., 2003; Wilson and Backman, 1999). Scanning electron microscopy (SEM) analysis revealed its penetration into P. fusca (Romero et al., 2003; Sztejnberg et al., 1989), which seems to be favoured by the secretion of β‐1,3‐glucanases (Rotem et al., 1999). Acremonium alternatum and L. lecanii, unlike A. quisqualis, behave as ectoparasites (Askary et al., 1997; Malathrakis, 1985). SEM analysis has also revealed the penetration of L. lecanii into P. fusca which, in this case, seems to be favoured by different enzymatic activities, such as proteases and lipases (Askary et al., 1997).
Another successful biocontrol strategy has been the use of antibiotic‐producing microorganisms, such as some bacteria and yeasts. To date, the yeast Pseudozima flocculosa is probably the most promising anti‐powdery mildew biological agent currently available (Avis and Bélanger, 2001). This biocontrol agent produces antifungal compounds closely related to fatty acids, which induce plasmolysis of the target cells in different growth stages (Hajlaoui et al., 1992). With regard to bacterial agents, species belonging to the genus Bacillus deserve special attention. Recent work has demonstrated that strains of Bacillus subtilis, producers of lipopeptide antibiotics, may be suitably applied in glasshouse cropping conditions against P. fusca (Romero et al., 2004, 2007c). Among these compounds, iturin and fengycin lipopeptides have been shown to be key factors in the antagonism of B. subtilis towards P. fusca (Romero et al, 2007b). These lipopeptides, similar to the fatty acids of P. flocculosa, target biological membranes and induce ultrastructural and morphological damage, leading to the failure of germination in P. fusca conidia (Romero et al., 2007a).
Mycoparasitism and antibiosis imply direct interactions between P. fusca and biocontrol agents, and generally require a high relative humidity for optimal disease‐suppressive activity (Romero et al., 2007c). Therefore, their effectiveness may be easier to control in the protected microclimatic environments of glasshouse‐grown crops than in the field. An interesting approach to overcoming this environmental restriction relies on the use of microorganisms that are able to colonize the most buffered plant rhizosphere and promote the induction of natural plant defences, named induced systemic resistance (van Loon et al., 1998). Strains belonging to the genera Pseudomonas and Bacillus, which are widely distributed in nature, have attracted most attention, and have been tested in biocontrol trials against diverse aerial diseases (Ji et al., 2006; Kloepper et al., 2004). The small number of reports dealing with the use of microorganisms as inducers of systemic resistance against P. fusca in cucurbits (García‐Gutiérrez, 2007; Reuveni and Reuveni, 2000) and the gaps in our knowledge with regard to the complex signalling network underlying these beneficial interactions should stimulate more intensive research.
CONCLUSIONS AND FUTURE PROSPECTS
Powdery mildew caused by P. fusca represents one of the most serious threats to cucurbit production worldwide. Despite significant breeding efforts and the development of new fungicides, consistently effective disease control remains elusive to growers. The deployment of resistant varieties, combined with fungicide rotation and the progressive introduction of safer alternatives to chemicals, including inorganic, organic and biological control products, will be used in disease control for several years to come. Although the phase of chasmothecial maturation to the production of viable sexual progeny for genetic analysis is still irresolute, the emerging molecular approaches developed for the pathogen and the forthcoming genome sequences of B. graminis f. sp. hordei and other powdery mildew fungi will be very useful for further research. In particular, these developments will facilitate the analysis of the physiological and molecular processes involved in P. fusca pathogenicity and biology, and an understanding of the intimate molecular dialogue between host and pathogen. This will provide the basis for the design of novel chemicals effective against this powdery mildew fungus, enabling us to face the disease and the design of disease control programmes in a more rational manner.
ACKNOWLEDGEMENTS
We gratefully acknowledge past and ongoing support to the cucurbit powdery mildew project from Plan Nacional de Recursos y Tecnologías Agroalimentarias of the Ministerio de Ciencia e Innovación, Spain (AGF95‐0962; AGF98‐0931; AGL2001‐1837; AGL2004‐06056; AGL2007‐65340). We also acknowledge the past members of our group whose work has contributed to the development of this project.
REFERENCES
- Álvarez, B. and Torés, J.A. (1997) Cultivo in vitro de Sphaerotheca fuliginea (Schlecht. ex Fr.), efecto de diferentes fuentes de carbono sobre su desarrollo. Bol. San. Veg. Plagas 23, 283–288. [Google Scholar]
- Askary, H. , Benhamou, N. and Brodeur, J. (1997) Ultrastructural and cytochemical investigations of antagonistic effect of Verticillium lecanii on cucumber powdery mildew. Phytopathology, 87, 359–368. [DOI] [PubMed] [Google Scholar]
- Avis, T.J. and Bélanger, R.R. (2001) Specificity and mode of action of the antifungal fatty acid cis‐9‐heptadecenoic acid produced by Pseudozyma flocculosa . Appl. Environ. Microbiol. 67, 956–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin, M. , Nicot, P.C. , Normand, P. and Lemaire, J.M. (1997) Virulence variation and DNA polymorphism in Sphaerotheca fuliginea, causal agent of powdery mildew in cucurbits. Eur. J. Plant Pathol. 103, 545–554. [Google Scholar]
- Bardin, M. , Suliman, M.E. , Sage‐Palloix, A.‐M. , Mohamed, Y.F. and Nicot, P.C. (2007) Inoculum production and long‐term conservation methods for cucurbits and tomato powdery mildews. Mycol. Res. 111, 740–747. [DOI] [PubMed] [Google Scholar]
- Bélanger, R.R. and Labbé, C. (2002) Control of powdery mildews without chemicals: prophylactic and biological alternatives for horticultural crops In: The Powdery Mildews. A Comprehensive Treatise (Bélanger R.R., Bushnell W.R., Dik A.J. and Carver T.L.W., eds), pp. 256–267. Saint Paul, MN: APS Press. [Google Scholar]
- Braun, U. and Takamatsu, S. (2000) Phylogeny of Erysiphe, Microsphaera, Uncinula (Erysipheae) and Cystotheca, Podosphaera, Sphaerotheca (Cystotheceae) inferred from rDNA ITS sequences—some taxonomic consequences. Schlechtendalia, 4, 1–33. [Google Scholar]
- Braun, U. , Shishkoff, N. , and Takamatsu, S. (2001) Phylogeny of Podosphaera sect. Sphaerotheca subsect. Magnicellulatae (Sphaerotheca fuliginea auct. s. lat.) inferred from rDNA ITS sequences—a taxonomic interpretation. Schlechtendalia, 7, 45–52. [Google Scholar]
- Braun, U. , Cook, T.A. , Inman, A.J. and Shin, H.D. (2002) The taxonomy of powdery mildew fungi In: The Powdery Mildews. A Comprehensive Treatise (Bélanger R.R., Bushnell W.R., Dik A.J. and Carver T.L.W., eds), pp. 13–55. Saint Paul, MN: APS Press. [Google Scholar]
- Brown, J.K.M. (2002) Comparative genetics of avirulence and fungicide resistance in the powdery mildew fungi In: The Powdery Mildews. A Comprehensive Treatise (Bélanger R.R., Bushnell W.R., Dik A.J. and Carver T.L.W., eds), pp. 56–65. Saint Paul, MN: APS Press. [Google Scholar]
- Butt, D.J. (1978) Epidemiology of powdery mildews In: The Powdery Mildews (Spencer D.M., ed.), pp. 51–81. London: Academic Press. [Google Scholar]
- Cohen, Y. and Eyal, H. (1988) Epifluorescence microscopy of Sphaerotheca fuliginea race 2 on susceptible and resistant genotypes of Cucumis melo . Phytopathology, 78, 144–148. [Google Scholar]
- Cohen, Y. , Eyal, H. and Hanania, J. (1990) Ultrastructure, autofluorescence, callose deposition and lignification in susceptible and resistant muskmelon leaves infected with the powdery mildew fungus Sphaerotheca fuliginea . Physiol. Mol. Plant Pathol. 36, 191–204. [Google Scholar]
- Cohen, Y. , Baider, A. , Petrov, L. , Sheck, L. and Voloisky, V. (2000) Cross‐infectivity of Sphaerotheca fuliginea to watermelon, melon and cucumber. Acta Hort. 510, 85–88. [Google Scholar]
- Fernández‐Ortuño, D. , Pérez‐García, A. , López‐Ruiz, F. , Romero, D. , De Vicente, A. and Torés, J.A. (2006) Occurrence and distribution of resistance to QoI fungicides in populations of Podosphaera fusca in south central Spain. Eur. J. Plant Pathol. 115, 215–222. [Google Scholar]
- Fernández‐Ortuño, D. , Torés, J.A. , De Vicente, A. and Pérez‐García, A. (2007) Multiple displacement amplification, a powerful tool for molecular genetic analysis of powdery mildew fungi. Curr. Genet. 51, 209–219. [DOI] [PubMed] [Google Scholar]
- Fernández‐Ortuño, D. , Torés, J.A. , De Vicente, A. and Pérez‐García, A. (2008a) Field resistance to QoI fungicides in Podosphaera fusca is not supported by typical mutations in the mitochondrial cytochrome b gene. Pest Manag. Sci. 64, 694–702. [DOI] [PubMed] [Google Scholar]
- Fernández‐Ortuño, D. , Torés, J.A. , De Vicente, A. and Pérez‐García, A. (2008b) Mechanisms of resistance to QoI fungicides in phytopathogenic fungi. Int. Microbiol. 11, 1–9. [PubMed] [Google Scholar]
- García‐Gutiérrez, L. (2007) Control biológico de oídio de cucurbitáceas mediante rizobacterias inductoras de resistencia sistémica. Master Thesis, Málaga: Universidad de Málaga. [Google Scholar]
- Green, J.R. , Carver, T.L.W. and Gurr, S.J. (2002) The formation and function of infection feeding structures In: The Powdery Mildews. A Comprehensive Treatise (Bélanger R.R., Bushnell W.R., Dik A.J. and Carver T.L.W., eds), pp. 66–82. Saint Paul, MN: APS Press. [Google Scholar]
- Hajlaoui, M.R. , Benhamou, N. and Belanger, R.R. (1992) Cytochemical study of the antagonistic activity of Sporothrix flocculosa on rose powdery mildew, Sphaerotheca pannosa var. rosae . Phytopathology, 82, 583–589. [Google Scholar]
- Heaney, S.P. , Hall, A.A. , Davies, S.A. and Olaya, G. (2000) Resistance to fungicides in the QoI‐STAR cross‐resistance group: current perspectives In: Proceedings of the British Crop Protection Council Conference—Pests and Diseases, Brighton, UK, pp. 755–762. Alton, Hampshire, UK: British Crop Protection Council. [Google Scholar]
- Hirata, T. , Cunnington, J.H. , Paksiri, U. , Limkaisang, S. , Shishkoff, N. , Grigaliunaite, B. , Sato, Y. and Takamatsu, S. (2000) Evolutionary analysis of subsection Magnicellulatae of Podosphaera section Sphaerotheca (Erysiphales) based on the rDNA internal transcribed spacer sequences with special reference to host plants. Can. J. Bot. 78, 1521–1530. [Google Scholar]
- Hollomon, D.W. and Wheeler, I.E. (2002) Controlling powdery mildews with chemistry In: The Powdery Mildews. A Comprehensive Treatise (Bélanger R.R., Bushnell W.R., Dik A.J. and Carver T.L.W., eds), pp. 249–255. Saint Paul, MN: APS Press. [Google Scholar]
- Huang, C.C. , Groot, T. , Meijer‐Dekens, F. , Niks, R.E. and Lindhout, P. (1998) The resistance to powdery mildew (Oidium lycopersicum) in Lycopersicon species is mainly associated with hypersensitive response. Eur. J. Plant Pathol. 104, 399–407. [Google Scholar]
- Ishii, H. , Fraaije, B.A. , Sugiyama, T. , Noguchi, K. , Nishimura, K. , Takeda, T. , Amano, T. and Hollomon, D.W. (2001) Occurrence and molecular characterization of strobilurin resistance in cucumber powdery mildew and downy mildew. Phytopathology, 91, 1166–1171. [DOI] [PubMed] [Google Scholar]
- Jahn, M. , Munger, H.M. and McCreight, J.D. (2002) Breeding cucurbit crops for powdery mildew resistance In: The Powdery Mildews. A Comprehensive Treatise (Bélanger R.R., Bushnell W.R., Dik A.J. and Carver T.L.W., eds), pp. 239–248. Saint Paul, MN: APS Press. [Google Scholar]
- Jarvis, W.R. , Gubler, W.D. and Grove, G.G. (2002) Epidemiology of powdery mildews in agricultural pathosystems In: The Powdery Mildews. A Comprehensive Treatise (Bélanger R.R., Bushnell W.R., Dik A.J. and Carver T.L.W., eds), pp. 169–199.Saint Paul, MN: APS Press. [Google Scholar]
- Ji, P. , Campbell, H.L. , Kloepper, J.W. , Jones, J.B. , Suslow, T.V. and Wilson, M. (2006) Integrated biological control of bacterial speck and spot of tomato under field conditions using foliar biological control agents and plant growth‐promoting rhizobacteria. Biol. Control, 36, 358–367. [Google Scholar]
- Kloepper, J.W. , Ryu, C.M. and Zhang, S.A. (2004) Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology, 94, 1259–1266. [DOI] [PubMed] [Google Scholar]
- Kuzuya, M. , Yashiro, K. , Tomita, K. and Ezura, H. (2006) Powdery mildew (Podosphaera xanthii) resistance in melon is categorized into two types based on inhibition of the infection processes. J. Exp. Bot. 57, 2093–2100. [DOI] [PubMed] [Google Scholar]
- Lebeda, A. , Kristkova, E. and Dolezal, K. (1999) Peroxidase isozyme polymorphism in Cucurbita pepo cultivars with various morphotypes and different level of field resistance to powdery mildew. Sci. Hortic. 81, 103–112. [Google Scholar]
- Van Loon, L.C. , Bakker, P.A.H.M. and Pieterse, C.M.J. (1998) Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 36, 453–483. [DOI] [PubMed] [Google Scholar]
- López‐Ruiz, F. , Ridout, C.J. , Pérez‐García, A. , De Vicente, A. and Torés, J.A. (2008) Molecular analysis of sterol C14α‐demethylase gene (CYP51) from azole resistant and sensitive Podosphaera fusca isolates, causal agent of powdery mildew of cucurbits In: Meeting Abstracts, 9th European Conference on Fungal Genetics, Edinburgh, UK, p. 108 Nottingham, UK. [Google Scholar]
- Ma, Z. and Michailides, T.J. (2005) Advances in understanding molecular mechanisms of fungicide resistance and molecular detection of resistant genotypes in phytopathogenic fungi. Crop Prot. 24, 853–863. [Google Scholar]
- Malathrakis, N.E. (1985) The fungus Acremonium alternatum Linc: Fr., a hyperparasite of the cucurbits powdery mildew pathogen Sphaerotheca fuliginea . J. Plant Dis. Prot. 92, 509–515. [Google Scholar]
- McCreight, J.D. (2006) Melon–powdery mildew interactions reveal variation in melon cultigens and Podosphaera xanthii races 1 and 2. J. Am. Soc. Hort. Sci. 131, 59–65. [Google Scholar]
- McGrath, M.T. (1994) Heterothallism in Sphaerotheca fuliginea . Mycologia, 86, 517–523. [Google Scholar]
- McGrath, M.T. (2001) Fungicide resistance in cucurbit powdery mildew: experiences and challenges. Plant Dis. 85, 236–245. [DOI] [PubMed] [Google Scholar]
- Moncalvo, J.‐M. (2005) Molecular systematics: major fungal phylogenetic groups and fungal species concepts In: Evolutionary Genetics of Fungi (Xu J., ed.), pp. 1–33. Wymondham, Norfolk: Horizon Bioscience. [Google Scholar]
- Munger, H.M. (1979) The influence of temperature on powdery mildew resistance in cucumber. Cucurbit Genet. Coop. Rep. 2, 9–10. [Google Scholar]
- Nicot, P.C. , Bardin, M. and Dik, A.J. (2002) Basic methods for epidemiological studies of powdery mildews: culture and preservation of isolates, production and delivery of inoculum, and disease assessment In: The Powdery Mildews. A Comprehensive Treatise (Bélanger R.R., Bushnell W.R., Dik A.J. and Carver T.L.W., eds), pp. 83–99. Saint Paul, MN: APS Press. [Google Scholar]
- O’Brien, R.G. and Weinert, M. (1994) A storage technique for cucurbit powdery mildew (Sphaerotheca fuliginea ). Australas. Plant Pathol. 23, 86–87. [Google Scholar]
- Pérez‐García, A. , Olalla, L. , Rivera, E. , Del Pino, D. , Cánovas, I. , De Vicente, A. and Torés, J.A. (2001) Development of Sphaerotheca fusca on susceptible, resistant, and temperature‐sensitive resistant melon cultivars. Mycol. Res. 105, 1216–1222. [Google Scholar]
- Pérez‐García, A. , Mingorance, E. , Rivera, M.E. , Del Pino, D. , Romero, D. , Torés, J.A. and De Vicente, A. (2006) Long‐term preservation of Podosphaera fusca using silica gel. J. Phytopathol. 154, 190–192. [Google Scholar]
- Del Pino, D. , Olalla, L. , Pérez‐García, A. , Rivera, M.E. , García, S. , Moreno, R. , De Vicente, A. and Torés, J.A. (2002) Occurrence of races and pathotypes of cucurbit powdery mildew in southeastern Spain. Phytoparasitica, 30, 459–466. [Google Scholar]
- Pitrat, M. (2002) 2002 gene list for melon. Cucurbit Genet. Coop. Rep. 25 http://cuke.hort.ncsu.edu/cgc/cgc25/2002toc.html, http://cuke.hort.ncsu.edu/cgc/cgc25/cgc25‐26.pdf [Google Scholar]
- Reuveni, M. and Reuveni, R. (2000) Prior inoculation with non‐pathogenic fungi induces systemic resistance to powdery mildew on cucumber plants. Eur. J. Plant Pathol. 106, 633–638. [Google Scholar]
- Reuveni, R. and Bothma, G.C. (1985) The relationship between peroxidase activity and resistance to Sphaerotheca fuliginea in melons. J. Phytopathol, 114, 260–267. [Google Scholar]
- Rivera, M.E. , Codina, J.C. , Olea, F. , De Vicente, A. and Pérez‐García, A. (2002) Differential expression of β‐1,3‐glucanase in susceptible and resistant melon cultivars in response to infection by Sphaerotheca fusca. Physiol. Mol. Plant Pathol. 61, 257–265. [Google Scholar]
- Romero, D. , Rivera, M.E. , Cazorla, F.M. , De Vicente, A. and Pérez‐García, A. (2003) Effect of mycoparasitic fungi on the development of Sphaerotheca fusca in melon leaves. Mycol. Res. 107, 64–71. [DOI] [PubMed] [Google Scholar]
- Romero, D. , Pérez‐García, A. , Rivera, M.E. , Cazorla, F.M. and De Vicente, A. (2004) Isolation and evaluation of antagonistic bacteria towards the cucurbit powdery mildew fungus Podosphaera fusca . Appl. Microbiol. Biotechnol. 64, 263–269. [DOI] [PubMed] [Google Scholar]
- Romero, D. , De Vicente, A. , Olmos, J.L. , Dávila, J.C. and Pérez‐García, A. (2007a) Effect of lipopeptides of antagonistic strains of Bacillus subtilis on the morphology and ultrastructure of the cucurbit fungal pathogen Podosphaera fusca . J. Appl. Microbiol. 103, 969–976. [DOI] [PubMed] [Google Scholar]
- Romero, D. , De Vicente, A. , Rakotoaly, R.H. , Dufour, S.E. , Veening, J.W. , Arrebola, E. , Cazorla, F.M. , Kuipers, O.P. , Paquot, M. and Pérez‐García, A. (2007b) The iturin and fengycin families of lipopeptides are key factors in antagonism of Bacillus subtilis toward Podosphaera fusca . Mol. Plant–Microbe Interact. 20, 430–440. [DOI] [PubMed] [Google Scholar]
- Romero, D. , De Vicente, A. , Zeriouh, H. , Cazorla, F.M. , Fernández‐Ortuño, D. , Torés, J.A. and Pérez‐García, A. (2007c) Evaluation of biological control agents for managing cucurbit powdery mildew on greenhouse‐grown melon. Plant Pathol. 56, 976–986. [Google Scholar]
- Romero, D. , Rivera, M.E. , Cazorla, F.M. , Codina, J.C. , Fernández‐Ortuño, D. , Torés, J.A. , Pérez‐García, A. and De Vicente, A. (2008) Comparative histochemical analyses of oxidative burst and cell‐wall reinforcement in compatible and incompatible melon–powdery mildew (Podosphaera fusca) interactions. J. Plant Physiol. 165, 1895–1905. [DOI] [PubMed] [Google Scholar]
- Rotem, Y. , Yarden, O. and Sztejnberg, A. (1999) The mycoparasite Ampelomyces quisqualis expresses exgA encoding an exo‐β‐1,3‐glucanase in culture and during mycoparasitism. Phytopathology, 89, 631–638. [DOI] [PubMed] [Google Scholar]
- Schroeder, W.T. and Provvidenti, R. (1969) Resistance to benomyl in powdery mildew of cucurbits. Plant Dis. Rep. 53, 271–275. [Google Scholar]
- Sitterly, W.P. (1978) Powdery mildew of cucurbits In: The Powdery Mildews ( Spencer, D.M. ed.), pp. 359–379. London: Academic Press. [Google Scholar]
- Sztejnberg, A. , Galper, S. , Mazar, S. and Lisker, N. (1989) Ampelomyces quisqualis for biological and integrated control of powdery mildews in Israel. J. Phytopathol. 124, 285–295. [Google Scholar]
- Taylor, J.W. , Jacobson, D.J. , Kroken, S. , Kasuga, T. , Geiser, D.M. , Hibbett, D.S. , and Fisher, M.C. (2000) Phylogenetic species recognition and species concepts in fungi. Fungal Genet. Biol. 31, 21–32. [DOI] [PubMed] [Google Scholar]
- Tomlins, S.A. , Mehra, R. , Rhodes, D.R. , Shah, R.B. , Rubin, M.A. , Bruening, E. , Makarow, V. and Chinnaiyan, A.M. (2006) Whole transcriptome amplification for gene expression profiling and development of molecular archives. Neoplasia, 8, 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, M. and Backman, P.A. (1999) Biological control of plant pathogens In: Handbook of Pest Management (Ruberson J.R., ed.), pp. 309–335. New York: Marcel Dekker. [Google Scholar]
- Zitter, T.A. , Hopkins, D.L. and Thomas, C.E. (1996) Compendium of Cucurbits Diseases. Saint Paul, MN: APS Press. [Google Scholar]


