SUMMARY
The cultivated grapevine, Vitis vinifera, is a member of the Vitaceae family, which comprises over 700 species in 14 genera. Vitis vinifera is highly susceptible to the powdery mildew pathogen Erysiphe necator. However, other species within the Vitaceae family have been reported to show resistance to this fungal pathogen, but little is known about the mechanistic basis of this resistance. Therefore, the frequency of successful E. necator penetration events, in addition to programmed cell death (PCD) responses, were investigated in a representative genotype from a range of different species within the Vitaceae family. The results revealed that penetration resistance and PCD‐associated responses, or combinations of both, are employed by the different Vitaceae genera to limit E. necator infection. In order to further characterize the cellular processes involved in the observed penetration resistance, specific inhibitors of the actin cytoskeleton and secretory/endocytic vesicle trafficking function were employed. These inhibitors were demonstrated to successfully break the penetration resistance in V. vinifera against the nonadapted powdery mildew E. cichoracearum. However, the use of these inhibitors with the adapted powdery mildew E. necator unexpectedly revealed that, although secretory and endocytic vesicle trafficking pathways play a crucial role in nonhost penetration resistance, the adapted powdery mildew species may actually require these pathways to successfully penetrate the plant host.
INTRODUCTION
Grapevine powdery mildew, Erysiphe necator, is the most economically important disease of viticulture worldwide because of the high susceptibility of the cultivated grapevine species, Vitis vinifera, to this pathogen. Erysiphe necator is of North American origin, and has subsequently spread through Europe by the introduction of American grapevines (Gadoury and Pearson, 1991). Several members of the Vitaceae family have been reported as being susceptible to E. necator, including members of the genera Ampelopsis, Cissus, Parthenocissus and Vitis (Boubals, 1961). Conversely, there are several reports of E. necator resistance in different Vitaceae members. For example, North American Vitis species, such as V. riparia, V. aestivalis and V. rupestris, which have co‐evolved with the pathogen, are thought to be less susceptible than V. vinifera to E. necator (Boubals, 1961; Cadle‐Davidson et al., 2010; Fung et al., 2008).
Plants have evolved several layers of defence to prevent pathogen penetration and colonization. Preformed constitutive physical barriers, such as leaf surface wax or preformed antimicrobial secondary metabolites, prevent the entry of the majority of plant pathogens (Thordal‐Christensen, 2003). Powdery mildew is an obligate biotrophic pathogen which needs access to plant host nutrients for growth and reproduction. Therefore, the powdery mildew spore (conidium) forms an infection structure (appressorium) to generate sufficient pressure to rupture the plant cell wall. As the fungus penetrates the plant cell, it forms a feeding structure (haustorium), which is surrounded by the extrahaustorial membrane, a continuum of the plant plasma membrane but with a unique composition (Koh et al., 2005). The plant can respond to this invasion with inducible defences. The endocytic and secretory membrane trafficking pathways are now emerging as important components of penetration and basal resistance. For example, the recognition of pathogen ingress is mediated by plasma membrane‐localized receptors, which become internalized via endocytosis following the binding of pathogen‐associated molecular patterns (PAMPs) (Robatzek et al., 2006). Chitin and ergosterol can be considered as fungal PAMPs (Granado et al., 1995; Miya et al., 2007). The endocytosis of these PAMP receptors activates defence responses, including pathogenesis‐related (PR) gene expression and callose deposition at the site of pathogen contact (Clay et al., 2009; Gomez‐Gomez et al., 1999). Papillae are formed to reinforce the cell wall under the site of pathogen contact through the polarized secretion of materials, such as callose, phenolics and hydrogen peroxide (McLusky et al., 1999; Mellersh et al., 2002; Thordal‐Christensen et al., 1997). In addition to secretory vesicles, many other cellular components also become polarized at the site of pathogen interaction, including the actin cytoskeleton and organelles such as peroxisomes, the nucleus, Golgi and endoplasmic reticulum (Freytag et al., 1994; Lipka et al., 2005; Takemoto et al., 2003). It has been proposed that the actin cytoskeleton may facilitate the delivery of secretory vesicles. The depolymerization of the actin cytoskeleton through the use of fungal‐derived inhibitors (cytochalasins) or, genetically, by the overexpression of actin depolymerization factors (ADF) have both been found to break penetration resistance to powdery mildew (Kobayashi et al., 1997; Miklis et al., 2007; Yun et al., 2003). Furthermore, Kobayashi and Hakuno (2003) have demonstrated that callose deposition at pathogen entry sites is dependent on the actin cytoskeleton, which is also consistent with the model that actin is required for secretory vesicle delivery.
The SNARE (soluble N‐ethylmaleimide‐sensitive factor attachment protein receptor) family includes proteins which mediate vesicle secretion by the facilitation of membrane fusion events (Pratelli et al., 2004). A specific plasma membrane‐localized member of the syntaxin subgroup of SNARE proteins in Arabidopsis (PEN1; SYP121) and in barley (ROR2) has been shown to play an important role in penetration resistance against powdery mildew (Collins et al., 2003). PEN3, another protein required for penetration resistance against powdery mildew in Arabidopsis (Stein et al., 2006), is also implicated in polarized secretion to the papillae. This ATP‐binding cassette (ABC) transporter protein is required for callose deposition and functions in a pathway distinct from PEN1 (Bednarek et al., 2009; Clay et al., 2009; Consonni et al., 2006; Lipka et al., 2005; Stein et al., 2006). Both of these proteins have also been shown to accumulate in papillae at the site of pathogen interaction (Meyer et al., 2009).
In the overwhelming majority of cases, plants successfully block the invasion by pathogens through the effective operation of these preformed and inducible penetration resistance pathways. However, some pathogens have evolved mechanisms to suppress PAMP receptor‐mediated penetration resistance through the secretion of effector (previously virulence factor) proteins (Bent and Mackey, 2007; Caplan et al., 2008; Stergiopoulos and de Wit, 2009). In response to this, some plants have evolved a second layer of defence involving programmed cell death (PCD). PCD is typically triggered when a specific recognition event occurs between a plant resistance (R) gene product and a pathogen‐secreted effector protein (previously avirulence protein) (Flor, 1971). This rapid, localized cell death effectively restricts the growth of biotrophic pathogens, such as powdery mildew (Peterhänsel et al., 1997). The majority of characterized plant R genes encode proteins which contain leucine‐rich repeat (LRR) domains, a central nucleotide‐binding site (NBS) and a variable N‐terminus comprising either a Toll/ interleukin‐1 receptor (TIR) domain or coiled‐coil (CC) domain (Jones and Takemoto, 2004).
Muscadinia rotundifolia, a grapevine species native to south‐eastern USA, is a source of strong resistance to E. necator infection. This resistance cosegregates with the Run1 locus, which has been found to contain a family of TIR‐NBS‐LRR resistance gene candidates (Dry et al., 2009). Similarly, the REN1 locus from the V. vinifera cultivar ‘Kishmish vatkana’, which is native to central Asia, also displays PCD‐associated resistance to E. necator (Hoffmann et al., 2008). However, these are the few examples in which resistance mechanisms against E. necator have been characterized in any detail. We have therefore undertaken a detailed investigation of the resistance responses in a representative genotype from a range of different species within the Vitaceae family to E. necator infection. Our results reveal a range of different responses to limit powdery mildew infection. These include penetration resistance and PCD‐associated resistance, or combinations of both. Furthermore, using inhibitors of endocytic and secretory vesicle trafficking pathways, we demonstrate the different roles played by these pathways in the penetration resistance of grapevine species to both nonadapted and adapted powdery mildew species.
RESULTS
Characterization of resistance mechanisms to E. necator infection within the Vitaceae
In order to investigate possible sources of resistance to E. necator within the Vitaceae, representatives of the genera Vitis, Muscadinia, Ampelopsis, Cissus and Parthenocissus were inoculated with the adapted powdery mildew species E. necator. Forty‐eight hours after inoculation, the infection stage of germinated conidia (appressorium, haustorium), together with the presence or absence of PCD, was scored using trypan blue which stains both fungal structures and dead host cells. Two types of E. necator resistance were observed: penetration resistance and PCD induction. Different frequencies of these resistance mechanisms in the species investigated resulted in four outcomes to E. necator inoculation (Fig. 1): susceptibility, partial resistance, PCD‐associated resistance and penetration resistance.
Figure 1.
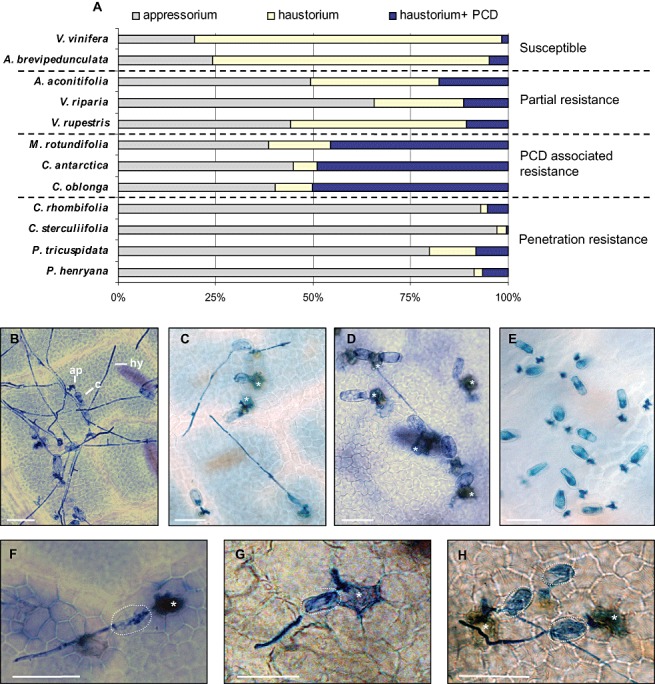
Susceptibility of different Vitaceae species to Erysiphe necator infection. The outcome of E. necator infection on leaves 48 h post‐inoculation. (A) Frequency of E. necator penetration attempts on different Vitaceae members, which concluded in appressorium formation but no penetration, successful penetration and haustorium formation. or a haustorium followed by programmed cell death (PCD) of the penetrated epidermal cell. The frequency of these outcomes in each Vitaceae species leads to susceptibility, partial resistance, PCD‐associated resistance or penetration resistance. Each data point is based on three biological replicates (leaves) on which a minimum of 100 germinated conidia were scored. The data shown are representative of the results obtained in at least two independent experiments. (B–H) Trypan blue staining following E. necator inoculation. (B) Susceptible Vitis vinifera. (C) Partially resistant V. riparia. (D) PCD‐mediated resistance in Muscadinia rotundifolia. (E) Penetration resistance in Parthenocissus tricuspidata. (F) PCD response in M. rotundifolia. (G) PCD response in Cissus antarctica. (H) PCD response in C. oblonga. Asterisks indicate cells which have undergone PCD as stained by trypan blue. Broken white circles indicate the position of a conidium. ap, appressorium; c, conidium; hy, hypha. Scale bars, 50 µm.
Both V. vinifera and A. brevipedunculata were found to be susceptible to E. necator, with the majority (71%–79%) of infection attempts leading to the development of a haustorium within penetrated epidermal cells and subsequent formation of secondary hyphae (Fig. 1A,B). The remaining 19%–24% of germinated conidia formed appressoria, but were unable to penetrate epidermal cells. PCD was only observed in 1%–5% of cells containing haustoria. This high level of successful penetration, combined with a low incidence of PCD in penetrated cells, enables the pathogen to complete its asexual life cycle on these hosts with the development of significant amounts of sporulating hyphae (data not shown).
Ampelopsis aconitifolia, V. riparia and V. rupestris all showed increased resistance to E. necator relative to V. vinifera. Penetration resistance in these vines was greater than that found in V. vinifera, with between 44% and 65% of germinated appressoria failing to penetrate epidermal cells (Fig. 1A,C). Significantly increased levels of PCD were also observed following penetration (10%–17%) compared with those found in the susceptible vines V. vinfera and A. brevipedunculata. The combined action of increased penetration resistance and increased PCD per penetration considerably restricted the development of E. necator on these hosts, in comparison with V. vinifera, such that there was little or no sporulation observed 7 days after inoculation (data not shown)
Muscadinia rotundifolia, C. antarctica and C. oblonga were found to show complete resistance to E. necator and did not support any sporulation. Resistance in these three species was characterized by a rapid induction of PCD responses with 45%–50% of germinated conidia (Fig. 1A,D). The PCD responses of C. antarctica and C. oblonga to E. necator were very similar to that found in M. rotundifolia, where hyphal growth was rapidly arrested following epidermal PCD (Fig. 1F–H). They also exhibited similar levels of penetration resistance (38%–44%) to those found in the partially resistant group, which includes A. aconitifolia, V. riparia and V. rupestris.
The final subgroup of Vitaceae species includes C. rhombifolia, C. sterculiifolia, P. tricuspidata and P. henryana. These species were also completely resistant to E. necator infection. Similar results were also obtained with C. discolor (data not shown). This group displayed high levels of penetration resistance to E. necator, with between 79% and 95% of germinated spores that formed an appressorium failing to successfully penetrate epidermal cells, as shown by the absence of a haustorium (Fig. 1A,E).
Characterization of nonhost penetration resistance in V. vinifera
Figure 1 illustrates that different genotypes within the Vitaceae family show different levels of penetration resistance to E. necator. For example, V. vinifera shows a low (19%) penetration resistance to E. necator, whereas M. rotundifolia and P. tricuspidata show intermediate (38%) and strong (79%) penetration resistances, respectively. Penetration resistance is normally considered to be the major component of nonhost resistance (NHR) to nonadapted powdery mildew pathogens. This penetration resistance requires the actin cytoskeleton, polarized secretion and papilla formation (Kobayashi et al., 1997; Schmelzer, 2002; Staiger, 2000). Therefore, we asked whether the penetration resistance observed in Vitaceae members, to the adapted mildew E. necator, requires the same components as NHR to a nonadapted powdery mildew species.
In order to address this question, we first investigated the components involved in the penetration resistance in V. vinifera against the nonadapted cucurbit powdery mildew species Erysiphe cichoracearum. V. vinifera was found to display strong nonhost penetration resistance to E. cichoracearum, with only 21% ± 5% of penetration attempts successful, compared with 77% ± 10% when infected with the adapted powdery mildew E. necator (Fig. 2). To examine the mechanistic basis of this NHR in V. vinifera, different cell machinery inhibitors were employed to block actin cytoskeleton polymerization and vesicle trafficking pathways. Cytochalasin E (CE) is a fungal‐derived metabolite which blocks the polymerization of the actin cytoskeleton and has been reported previously to break NHR in several plant species (Kobayashi et al., 1997). Brefeldin A (BFA) is a fungal toxin commonly employed to study vesicle‐mediated protein secretion and endocytosis (Robinson et al., 2008), whereas wortmannin (WM) inhibits the endocytic trafficking of plasma membrane proteins and disrupts the vacuolar sorting of these proteins (Kleine‐Vehn et al., 2008; Wang et al., 2009).
Figure 2.
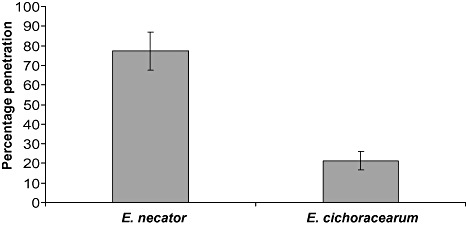
Penetration efficiency of the adapted powdery mildew Erysiphe necator and the nonadapted powdery mildew E. cichoracearum on Vitis vinifera. Successful penetration was scored by the presence/absence of a haustorium. Each data point represents the mean ± standard deviation of three independent experiments within which a minimum of 100 germinated conidia were scored on three leaves.
Vitis vinifera leaves were treated with increasing concentrations of CE, BFA and WM prior to E. cichoracearum inoculation, and the penetration rate of germinated spores was scored. In each case, inhibitor pretreatment was found to partially compromise nonhost penetration resistance to E. cichoracearum in V. vinifera leaves, with the most statistically significant increase in fungal penetration, relative to the control treatment [dimethylsulphoxide (DMSO) only], observed at 10 µm CE, 25 µm BFA and 40 µm WM (Fig. 3). The mean increases in penetration using these optimum concentrations were found to be 26% ± 6%, 17% ± 2% and 19% ± 1% following CE, BFA and WM pretreatment, respectively, over three independent experiments (data not shown). Higher concentrations of CE, BFA and WM decreased the penetration frequency of germinated spores, suggesting that these concentrations were toxic to powdery mildew growth (Fig. 3).
Figure 3.
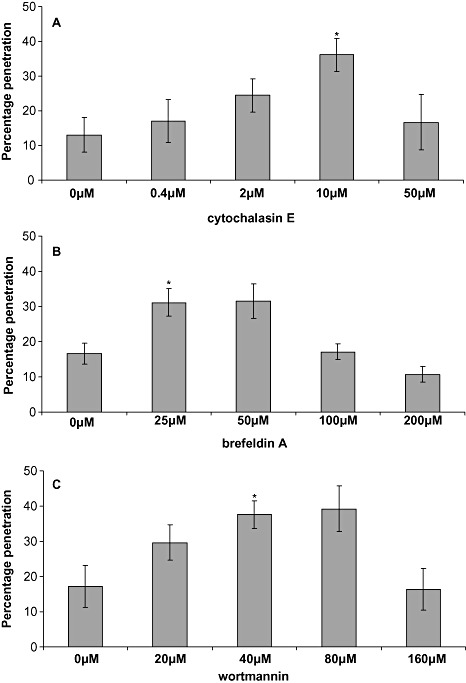
Penetration frequency of the nonadapted powdery mildew Erysiphe cichoracearum on Vitis vinifera following treatment with increasing concentrations of cytochalasin E (A), brefeldin A (B) and wortmannin (C). Successful penetration was scored by the presence/absence of a haustorium. Each data point ± standard deviation is based on three biological replicates (leaves) on which a minimum of 100 germinated conidia were scored. Asterisks indicate a significant difference from the negative dimethylsulphoxide (DMSO) control (P < 0.01; Student's t‐test).
These results confirm that actin cytoskeleton polymerization and vesicle trafficking are important processes for the establishment of nonhost penetration resistance in V. vinifera.
Characterization of host penetration resistance in different Vitaceae species
To investigate whether there was mechanistic overlap between the penetration resistance displayed by different Vitaceae family members against the adapted powdery mildew species E. necator (Fig. 1A) and the nonhost penetration resistance displayed by V. vinifera against the nonadapted species E. cichoracearum (2, 3), the effect of the inhibitors CE, BFA and WM on the penetration resistance displayed by V. vinifera, M. rotundifolia and C. rhombifolia was examined.
The pretreatment of V. vinifera leaves with 10 µm CE increased significantly the penetration efficiency of E. necator from 67% ± 6% in the control treatment (0.2% DMSO) to 80% ± 4% (Fig. 4A). This suggests that a component of the resistance displayed by V. vinifera to E. necator penetration involves the same cellular machinery as that employed against the nonadapted powdery mildew species E. cichoracearum (Fig. 3A), as both require the actin cytoskeleton. However, CE was not found to break the penetration resistance to E. necator in M. rotundifolia or C. rhombifolia (Fig. 4A), which suggests that the penetration resistance displayed in these two species against E. necator is not dependent on actin cytoskeleton function.
Figure 4.
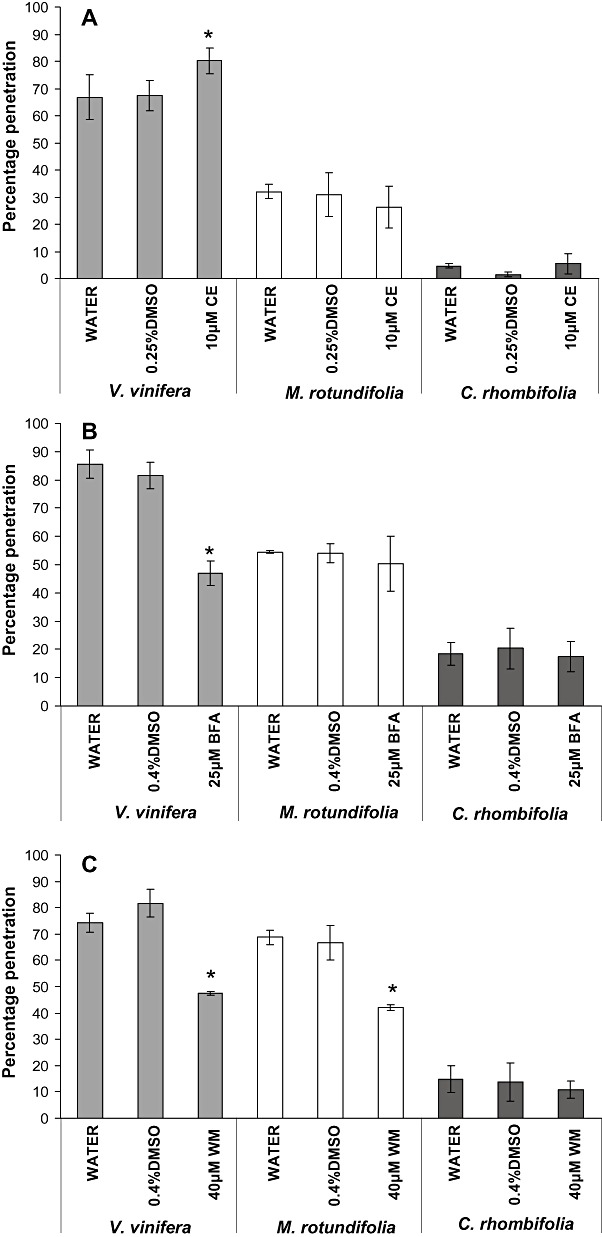
Penetration frequency of the adapted powdery mildew Erysiphe necator on the Vitaceae species Vitis vinifera, Muscadinia rotundifolia and Cissus rhombifolia following treatment with the following inhibitors: (A) cytochalasin E (CE), 0.25% dimethylsulphoxide (DMSO) or water; (B) brefeldin A (BFA), 0.4% DMSO or water; (C) wortmannin (WM), 0.4% DMSO or water. Successful penetration was scored by the presence/absence of a haustorium. Each data point ± standard deviation is based on three biological replicates (leaves) on which a minimum of 100 germinated conidia were scored. The data shown are representative of the results obtained with at least two independent experiments. Asterisks indicate a significant difference from DMSO controls (P < 0.05; Student's t‐test).
In contrast with the results obtained with the nonadapted powdery mildew E. cichoracearum (Fig. 3A), pretreatment with BFA was found to decrease the penetration efficiency of E. necator on V. vinifera from 81% ± 5% in the control treatment (0.4% DMSO) to 47% ± 4% (Fig. 4B). However, as with CE treatment (Fig. 4A), BFA was found to have no effect on the penetration efficiency of E. necator on M. rotundifolia or C. rhombifolia (Fig. 4C). As with BFA, WM treatment was also found to decrease the penetration efficiency of E. necator on V. vinifera from 82% ± 5% in the control treatment (0.4% DMSO) to 47% ± 1% (Fig. 4C). Again this is contrary to the observed effect of WM on the nonhost penetration resistance of V. vinifera against E. cichoracearum (Fig. 3C). Interestingly, WM was also effective at reducing the level of E. necator penetration on M. rotundifolia from 67% ± 6% in the control treatment (0.4% DMSO) to 42% ± 1% (Fig. 4C). However, as with CE and BFA, WM had no significant effect on the penetration efficiency of E. necator on C. rhombifolia (Fig. 4C).
It was surprising that pretreatment with 25 µm BFA and 40 µm WM led to a decrease in penetration efficiency in the compatible interaction between E. necator and V. vinifera (Fig. 4B,C), as the same concentration of inhibitors led to an increase in penetration by the nonadapted powdery mildew species E. cichoracearum (Fig. 3B,C). To rule out the possibility of any direct inhibitory effects of BFA and WM pretreatment on E. necator, at these concentrations, a dose–response curve was constructed using the nonhost species Arabidopsis thaliana treated with increasing concentrations of BFA and WM prior to E. necator inoculation (Fig. S1, see Supporting Information). It was necessary to carry out the dose–response curve on a nonhost plant species for E. necator as, if BFA and WM do decrease the penetration efficiency in compatible interactions, any toxic or inhibitory effects on penetration would be indistinguishable. The penetration efficiency of E. necator on the nonhost Arabidopsis increased significantly following pretreatment with 25 µm BFA and 40 µm WM, demonstrating that these concentrations are not toxic to E. necator (Fig. S1).
These results suggest that the vesicle trafficking pathways are not required for host penetration resistance to the adapted powdery mildew, E. necator, in the Vitaceae species examined. On the contrary, BFA‐ and WM‐inhibited pathways appear to be partially required for successful E. necator penetration on V. vinifera, whereas only the WM‐inhibited endocytic pathway is required for successful E. necator penetration on M. rotundifolia.
Penetration resistance in C. rhombifolia is not dependent on papillae formation
The results in Fig. 4 show that the penetration resistance of C. rhombifolia to E. necator was not influenced significantly by treatment with the inhibitors CE, BFA or WM, despite the fact that at least one of these cell machinery inhibitors was found to affect the penetration resistance in V. vinifera and M. rotundifolia. This suggests that the strong penetration resistance observed in C. rhombifolia to E. necator may not be caused by an active cellular response, but may be a result of a preformed physical or chemical barrier to penetration.
In order to investigate this further, we looked for the presence of callose‐containing papillae in C. rhombifolia epidermal cells in response to E. necator inoculation. Cissus rhombifolia leaves were inoculated with E. necator and analysed for callose deposition by aniline blue staining. Over three inoculations, callose‐containing papillae (Fig. 5A) were detected beneath 65% ± 7% E. necator appressoria on C. rhombifolia. As callose deposition, at the site of pathogen entry, has been shown to be dependent on actin (Kobayashi and Hakuno, 2003), we also investigated the effect of CE treatment on papillary callose formation in C. rhombifolia. CE was found to reduce the incidence of callose‐containing papillae from 58% ± 10% to 30% ± 3% (Fig. 5B). Thus, although the inhibition of actin cytoskeleton function by CE does not lead to a reduction in penetration resistance to E. necator in C. rhombifolia (Fig. 4A), it does reduce the incidence of papillary callose deposition (Fig. 5B), suggesting that the resistance of C. rhombifolia to E. necator penetration is not dependent on papillary callose deposits.
Figure 5.
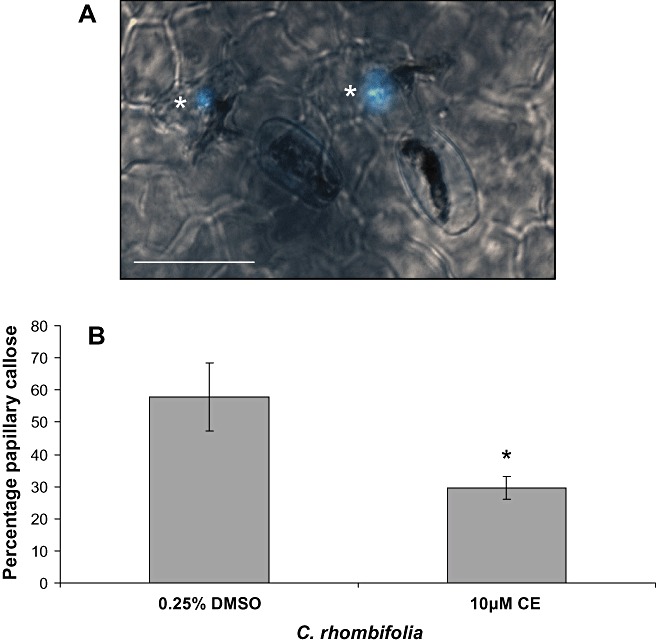
Erysiphe necator‐induced callose deposition in the papillae of Cissus rhombifolia. (A) Merged bright field and fluorescence image of callose under appressoria marked by an asterisk. (B) Frequency of papillary callose following cytochalasin E or 0.25% dimethylsulphoxide (DMSO) treatment, scored by the presence of callose under spores with an appressorium. Leaves were stained with 0.1% aniline blue. Each data point ± standard deviation is based on three biological replicates (leaves) on which a minimum of 100 germinated conidia were scored. The data shown are representative of the results obtained in two independent experiments. Scale bar, 50 µm. Asterisk indicates a significant difference from DMSO control (P < 0.05; Student's t‐test).
DISCUSSION
Previous studies have investigated the susceptibility of Vitis species, including V. vinifera cultivars and wild Vitis species from North America and China, to grapevine powdery mildew, E. necator (Doster and Schnathorst, 1985; Eibach, 1994; Staudt, 1997; Wan et al., 2007). Some information is also available regarding the susceptibility of species from the Vitaceae genera Ampelopsis, Cissus and Parthenocissus (Boubals, 1961). More recently, Cadle‐Davidson et al. (2010) have reported the results of a comprehensive study involving 1025 Vitis accessions analysed under the same conditions, with the same E. necator isolate, to look at the variation in foliar resistance to powdery mildew. However, none of these studies has investigated the biological basis of any observed resistance to E. necator infection.
This study has characterized, for the first time, the relative importance, in different members of the Vitaceae family, of the two major factors regulating susceptibility to E. necator: penetration efficiency and PCD induction in penetrated cells. However, it is important to note that the quantification of these two resistance mechanisms in the different Vitaceae species examined only relates to the specific E. necator isolate used in this study. The relative levels of penetration and PCD‐associated resistance observed may vary significantly when challenged with different isolates which have undergone pathogenic specialization (Gadoury and Pearson, 1991). Furthermore, intraspecific variation in powdery mildew susceptibility has been observed (Cadle Davidson et al., 2010).
Different members of the Vitaceae family display a diverse range of responses to attempted invasion by E. necator (Fig. 1A). The cultivated European species V. vinifera and the ornamental Asian species A. brevipedunculata are highly susceptible to E. necator, because of the low levels of penetration resistance and low levels of PCD induction, following penetration, which are insufficient to prevent E. necator from completing its life cycle. Conversely, members of the Cissus and Parthenocissus genera were found to be completely resistant to E. necator infection because of the very high levels of penetration resistance.
The remaining Vitaceae species examined appear to utilize a combination of both penetration resistance and PCD‐associated resistance to restrict E. necator growth, with varying levels of success. All show significantly higher levels of resistance than V. vinifera to E. necator penetration. However, complete resistance (i.e. absence of any sporulation) was only observed in those species in which this reduced penetration was combined with a highly efficient PCD response in penetrated epidermal cells, i.e. M. rotundifolia, C. antarctica and C. oblonga (Fig. 1A,F–H). Thus, plants within the Vitaceae family appear to have evolved a number of different biological responses in an attempt to restrict E. necator invasion.
Characterization of penetration resistance to powdery mildew in the Vitaceae
Penetration resistance is normally considered to be the major component of NHR to nonadapted powdery mildews, and has been shown to involve the actin cytoskeleton and polarized secretion (Underwood and Somerville, 2008). However, little attention has been paid to the mechanistic basis of penetration resistance to an adapted powdery mildew species, and whether this is a distinct resistance pathway or involves similar components to NHR. In order to investigate this, specific cell machinery inhibitors were employed on members of the Vitaceae family showing a wide variation in penetration resistance against E. necator. CE blocks the polymerization and elongation of the actin cytoskeleton (Kobayashi et al., 1997). BFA is a fungal toxin that interferes with guanine‐nucleotide exchange factors (GEFs) that catalyse the activation of the small GTPase ADP ribosylation factor (ARF) (Geldner et al., 2003; Jackson and Casanova, 2000), required for vesicle formation. Wortmaninn inhibits phosphatidylinositol 3‐kinase (PI3PK), an enzyme involved in the production of phosphatidylinositol 3‐phosphate (PI3P), a lipid predominantly of endosomal membranes (Wang et al., 2009). CE has been reported previously to break NHR in several plant species (Kobayashi et al., 1997); however, to our knowledge, the vesicle trafficking inhibitors BFA and WM have not been used previously to study penetration resistance against powdery mildew.
The increase in penetration of the nonadapted powdery mildew, E. cichoracearum (Fig. 3A), and adapted powdery mildew species, E. necator (Fig. 4A), on V. vinifera leaves, treated with CE, demonstrates that the actin cytoskeleton is partially required for both nonhost and host penetration resistance. This is in agreement with previous inhibitor and genetic studies, which have demonstrated the importance of the actin cytoskeleton in several plant species for NHR (Kobayashi et al., 1997; Yun et al., 2003) and host penetration resistance in barley (Miklis et al., 2007). The actin cytoskeleton is required for callose deposition at pathogen entry sites (Kobayashi and Hakuno, 2003). The Arabidopsis mutants of pen3 show impaired callose secretion responses (Clay et al., 2009; Stein et al., 2006). Interestingly, the accumulation of PEN3 at attempted barley powdery mildew (Blumeria graminis f.sp. hordei) infection sites is also disrupted by CE (Underwood and Somerville, 2008).
As with CE, the penetration of V. vinifera epidermal cells by the nonadapted powdery mildew species E. cichoracearum was also increased significantly in response to treatment with the vesicle trafficking inhibitors BFA and WM. BFA has been reported to inhibit both endosomal vesicle trafficking and Golgi‐derived vesicle secretion (Driouich et al., 1993; Geldner et al., 2003; Sciaky et al., 1997; Steinmann et al., 1999). WM also inhibits the endocytic trafficking of plasma membrane proteins (Kleine‐Vehn et al., 2008; Wang et al., 2009). For example, WM inhibits the endocytosis of the plasma membrane‐localized PAMP receptor FLAGELLIN SENSITIVE 2 (FLS2) in response to the bacterial PAMP flagellin (Robatzek et al., 2006). Thus, the increase in penetration observed with the nonadapted powdery mildew E. cichoracearum following BFA and WM treatment, suggests that endocytosis and/or vesicle secretion are important for nonhost penetration resistance, possibly through PAMP receptor endocytosis or the exocytosis of cell wall materials at the site of pathogen interaction.
Surprisingly, however, BFA and WM were both found to produce the opposite response against the adapted powdery mildew species E. necator. BFA decreased significantly E. necator penetration on V. vinifera, and WM produced a similar result in both V. vinifera and M. rotundifolia (Fig. 4B,C). These results suggest that endocytic and secretory vesicle trafficking may, in fact, be required for successful infection by an adapted powdery mildew species. One possible explanation for this result may be that the extramembrane material required to form the host extrahaustorial membrane is provided by these vesicle trafficking pathways. The extrahaustorial membrane is of a unique composition and therefore is probably formed de novo by vesicle trafficking (Frei dit Frey and Robatzek, 2009; Koh et al., 2005). An alternative explanation may be that the proteins required for pathogen entry are internalized and trafficked by endocytosis. For example, the plasma membrane‐localized host protein MLO is required for adapted powdery mildew pathogenicity (Büschges et al., 1997; Consonni et al., 2006), and has been shown to accumulate at the site of pathogen infection (Bhat et al., 2005). A family of VvMLOs has been identified recently in V. vinifera (Feechan et al., 2008; Winterhagen et al., 2008). It is conceivable that the inhibition of vesicle trafficking with BFA and WM could interfere with the turnover and accumulation of VvMLO at the site of E. necator infection, leading to reduced penetration efficiency. There is also emerging evidence that fungal pathogen effectors (virulence factors) can enter the plant cell in the absence of the pathogen (Catanzariti et al., 2006; Manning and Ciuffetti, 2005; Rafiqi et al., 2010), suggesting that the plant host cell machinery may be exploited for effector entry inside the plant cell. Therefore, it is possible that WM and BFA could interfere with the delivery of effectors into the plant cell that are required for the suppression of PAMP‐triggered immunity. Support for this theory comes from the recent demonstration that WM inhibits the entry of fungal effectors into plant cells by blocking lipid raft‐mediated endocytosis (Kale et al., 2010). In summary, our data clearly indicate that the role of endocytosis in adapted powdery mildew infection requires further investigation.
Representative members of the four Vitaceae species examined, C. rhombifolia, C. sterculiifolia, P. tricuspidata and P. henryana, were found to be completely resistant to E. necator infection, and this was mediated by very high levels of penetration resistance (Fig. 1). However, attempts to modify penetration resistance in C. rhombifolia (Fig. 3A–C) or P. tricuspidata (data not shown) using CE, BFA or WM were unsuccessful, despite the fact that CE was shown to reduce papillary callose deposition (Fig. 5). These results suggest that the high level of penetration resistance observed in these plants is not dependent on inducible cellular processes involving the actin cytoskeleton and vesicle trafficking. Another explanation may be that the strong penetration resistance found in these plants is the result of a preformed barrier, such as leaf surface wax or preformed antimicrobial secondary metabolites (Thordal‐Christensen, 2003). It has been reported that barley powdery mildew Blumeria graminis secretes a lipase (LIP1) which has lipolytic activity on epicuticular wax components of the host leaf. The released wax derivatives are important for fungal attachment, growth and pathogenicity (Feng et al., 2009). If the composition of the epicuticular wax of a particular plant species is not readily degraded by the powdery mildew‐secreted lipase, this may hinder infection.
Sources of PCD‐associated resistance to powdery mildew in the Vitaceae
R proteins typically trigger PCD following interaction directly or indirectly with specific pathogen effectors (Axtell and Staskawicz, 2003; Dodds et al., 2006; Ellis et al., 2007; 2002, 2003). These effectors (avirulence factors) are deployed by the pathogen to gain access to host nutrients and/or to suppress basal plant immunity (Bent and Mackey, 2007; Caplan et al., 2008). Therefore, effectors and R genes tightly co‐evolve, as the effector is under selection pressure to evade detection, whereas the R gene is under pressure to retain the ability to detect the effector. As E. necator is endemic to the USA, it might be expected that Vitaceae species from this region have evolved R gene‐mediated resistance to E. necator infection pressure.
PCD‐mediated resistance to E. necator has been reported previously in back‐cross progeny derived from a cross between the North American species M. rotundifolia and V. vinifera, and this resistance cosegregates with a cluster of TIR‐NBS‐LRR resistance gene candidates at the Run1 locus (Barker et al., 2005; Dry et al., 2009). Our data show that the powdery mildew R gene(s) in M. rotundifolia mount a very effective PCD response to E. necator infection, leading to PCD in approximately 75% of penetrated cells (Fig. 1A,B,F). Other North American species, V. rupestris and V. riparia, also show increased PCD induction in penetrated cells (approximately 20% and 30%, respectively) compared with V. vinifera, as does the Chinese species A. aconitifolia (36%; Fig. 1A). Further research is required to determine whether the elevated level of E. necator‐induced PCD in these species represents recognition by a weak powdery mildew R gene or an alternative resistance mechanism.
Surprisingly, very high levels of PCD induction were observed in E. necator‐penetrated cells of C. antarctica and C. oblonga, similar to the levels found in M. rotundifolia (Fig. 1F–H). This suggests the existence of R gene products in these two Australian Cissus species, which are capable of recognizing effectors secreted from E. necator. Such a hypothesis would appear to be at odds with the model of plant resistance gene evolution, which proposes that R gene‐mediated resistance is the result of a significant period of co‐evolution between the host and adapted pathogen. To our knowledge, E. necator has only been in Australia since the 1860s (B. Emmett, Dept. of Primary Industries, Victoria, Australia, personal communication). However, this is not the first report of E. necator‐induced PCD in a Vitaceae species originating from outside of North America. Hoffmann et al. (2008) reported the existence of a cultivated grapevine Vitis vinifera‘Kishmish vatkana’ from Central Asia that showed elevated levels of PCD to E. necator. Furthermore, this resistance has been mapped to a single dominant locus (designated REN1) that contains a family of NBS‐LRR genes. Hoffmann et al. (2008) proposed that wild V. vinifera populations in Central Asia, unlike the clonally propagated cultivated grapevines in Europe, are undergoing sexual propagation, and may have evolved resistance since the arrival of E. necator from North America. An alternative explanation may be that closely related powdery mildew species exist that are endemic to Asia and Australia which share effectors with E. necator.
In conclusion, several novel sources of resistance to grapevine powdery mildew have been identified within the Vitaceae family which include both PCD‐associated and penetration‐based resistance. The characterization of penetration resistance in V. vinifera indicates commonalities with other plant species in terms of the role of the actin cytoskeleton and vesicle trafficking pathways against a nonadapted powdery mildew species. However, these results also suggest that the adapted powdery mildew species may actually require the plant host endocytic and secretory pathways for pathogenicity. Further studies are now underway to elucidate which proteins are trafficked by the endocytic and vesicle secretion pathways that are required by adapted powdery mildew species for successful infection.
EXPERIMENTAL PROCEDURES
Plant cultivation
Cuttings of A. brevipedunculata, A. aconitifolia, C. antarctica, C. oblonga, C. rhombilfolia, C. sterculiifolia, P. tricuspidata and P. henryana were obtained from Adelaide Botanic Garden (Adelaide, SA, Australia). Vitis riparia, V. rupestris and M. rotundifolia were obtained from the variety collection in the Coombe Vineyard of the University of Adelaide (Urrbrae, SA, Australia). At least two individual vines were maintained for each Vitaceae member, with the exception of P. henryana and C. oblonga. Leaves were sampled from potted vines grown in a temperature‐controlled glasshouse at Waite Campus, University of Adelaide (Adelaide, SA, Australia) maintained between 23 and 25 °C. Arabidopsis thaliana (Col‐0) plants were grown at 24 °C with a 14‐h light/10‐h dark cycle.
Powdery mildew inoculation studies
Erysiphe necator (isolate APC, kindly provided by Eileen Scott, University of Adelaide, SA, Australia) was maintained on detached leaves of V. vinifera cv. Cabernet Sauvignon using an 8–10‐day rotation as described previously (Donald et al., 2002). Erysiphe cichoracearum was maintained on cucumber plants (Cucumis sativus) grown at 22 °C with a 16‐h light/8‐h dark cycle.
Young glossy detached vine leaves of a similar developmental stage (approximately 6 cm in diameter) were inoculated with E. necator conidia or E. cichoracearum by gently tapping conidia from infected leaves (8 days post‐inoculation) above open dishes. The Petri dishes were sealed with Parafilm® and the leaves were incubated at 25 °C under a 16‐h light/8‐h dark cycle for 48 h. Leaves from Arabidopsis thaliana Col‐0 plants were infected with E. cichoracearum, using a fine paintbrush, and plants were incubated at 25 °C for 48 h.
Inhibitor studies
All chemical inhibitors were obtained from Sigma‐Aldrich (Castle Hill, NSW, Australia). Inhibitor stocks were dissolved and maintained in DMSO at the following concentrations: BFA, 2 mg/mL; WM, 4 mg/mL; CE, 2 mg/mL. Inhibitor stocks were dissolved in sterile water to achieve the indicated working concentrations. Leaves from V. vinifera cv. Cabernet Sauvignon, M. rotundifolia and C. rhombifolia were immersed in inhibitors, or an appropriate control DMSO solution, or sterile water for 1 h. Vine leaves were rinsed in sterile water and air dried before powdery mildew inoculation. Inhibitors were infiltrated into the abaxial side of Arabidopsis thaliana Col‐0 leaves using a blunt syringe before powdery mildew inoculation.
Microscopy
Leaf material was harvested 48 h after powdery mildew inoculation and stained in trypan blue with heat for 1 h, according to Koch and Slusarenko (1990). Fungal structures were visualized using a Zeiss (Göttingen, Germany) Axioscop 2 light microscope. Successful penetration was determined by the presence of a fungal haustorium, and PCD by the presence of a blue fungal‐penetrated epidermal cell.
To stain for callose, detached leaf material was incubated in 5% NaHCO3 for 2 min before staining with 0.1% aniline blue solution in 50 mm potassium phosphate buffer (pH 7.5) for 1 h. Leaves were rinsed in 10 mm potassium phosphate buffer. Papillary callose was visualized using a Zeiss Axioscope FS CFP (blue light) filter set.
Supporting information
Fig. S1. Penetration frequency of the nonadapted powdery mildew Erysiphe necator on Arabidopsis thaliana following treatment with increasing concentrations of brefeldin A (A) and wortmannin (B). Successful penetration was scored by the presence/absence of a haustorium. Each data point represents the mean ± standard deviation of two independent experiments within which a minimum of 100 germinated conidia were scored on three leaves. Asterisks indicate a significant difference from the negative dimethylsulphoxide (DMSO) control (P < 0.01; Student's t‐test).
Supporting info item
ACKNOWLEDGEMENTS
This work was funded by the Grape and Wine Research Development Corporation. We thank Nicole Kempster and Angelica Jermakow for excellent technical assistance.
REFERENCES
- Axtell, M.J. and Staskawicz, B.J. (2003) Initiation of RPS2‐specified disease resistance in Arabidopsis is coupled to the AvrRpt2‐directed elimination of RIN4. Cell, 112, 369–377. [DOI] [PubMed] [Google Scholar]
- Barker, C.L. , Donald, T. , Pauquet, J. , Ratnaparkhe, M.B. , Bouquet, A. , Adam‐Blondon, A.F. , Thomas, M.R. and Dry, I. (2005) Genetic and physical mapping of the grapevine powdery mildew resistance gene, Run1, using a bacterial artificial chromosome library. Theor. Appl. Genet. 111, 370–377. [DOI] [PubMed] [Google Scholar]
- Bednarek, P. , Pislewska‐Bednarek, M. , Svatos, A. , Schneider, B. , Doubsky, J. , Mansurova, M. , Humphry, M. , Consonni, C. , Panstruga, R. , Sanchez‐Vallet, A. , Molina, A. and Schulze‐Lefert, P. (2009) A glucosinolate metabolism pathway in living plant cells mediates broad‐spectrum antifungal defense. Science, 323, 101–106. [DOI] [PubMed] [Google Scholar]
- Bent, A.F. and Mackey, D. (2007) Elicitors, effectors, and R genes: the new paradigm and a lifetime supply of questions. Annu. Rev. Phytopathol. 45, 399–436. [DOI] [PubMed] [Google Scholar]
- Bhat, R.A. , Miklis, M. , Schmelzer, E. , Schulze‐Lefert, P. and Panstruga, R. (2005) Recruitment and interaction dynamics of plant penetration resistance components in a plasma membrane microdomain. Proc. Natl. Acad. Sci. USA, 102, 3135–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boubals, D. (1961) Etude des causes de la résistance des Vitacées a l'oidium de la vigne Uncinula necator (Schw. Burr.) et leur moed de transmission hèrèditaire. Ann. L'Amél des Plantes, 11, 401–500. [Google Scholar]
- Büschges, R. , Hollricher, K. , Panstruga, R. , Simons, G. , Wolter, M. , Frijters, A. , van Daelen, R. , van der Lee, T. , Diergaarde, P. , Groenendijk, J. , Topsch, S. , Vos, P. , Salamini, F. and Schulze‐Lefert, P. (1997) The barley Mlo gene: a novel control element of plant pathogen resistance. Cell, 88, 695–705. [DOI] [PubMed] [Google Scholar]
- Cadle‐Davidson, L. , Chicoine, D.R. and Consolie, N.H. (2010) Variation within and between Vitis species for foliar resistance to the powdery mildew pathogen Erysiphe necator . Plant Dis. in press. [DOI] [PubMed] [Google Scholar]
- Caplan, J. , Padmanabhan, M. and Dinesh‐Kumar, S.P. (2008) Plant NB‐LRR immune receptors: from recognition to transcriptional reprogramming. Cell Host Microbe, 3, 126–135. [DOI] [PubMed] [Google Scholar]
- Catanzariti, A.M. , Dodds, P.N. , Lawrence, G.J. , Ayliffe, M.A. and Ellis, J.G. (2006) Haustorially expressed secreted proteins from flax rust are highly enriched for avirulence elicitors. Plant Cell, 18, 243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay, N.K. , Adio, A.M. , Denoux, C. , Jander, G. and Ausubel, F.M. (2009) Glucosinolate metabolites required for an Arabidopsis innate immune response. Science, 323, 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, N.C. , Thordal‐Christensen, H. , Lipka, V. , Bau, S. , Kombrink, E. , Qiu, J.L. , Huckelhoven, R. , Stein, M. , Freialdenhoven, A. , Somerville, S.C. and Schulze‐Lefert, P. (2003) SNARE‐protein‐mediated disease resistance at the plant cell wall. Nature, 425, 973–977. [DOI] [PubMed] [Google Scholar]
- Consonni, C. , Humphry, M.E. , Hartmann, H.A. , Livaja, M. , Durner, J. , Westphal, L. , Vogel, J. , Lipka, V. , Kemmerling, B. , Schulze‐Lefert, P. , Somerville, S.C. and Panstruga, R. (2006) Conserved requirement for a plant host cell protein in powdery mildew pathogenesis. Nat. Genet. 38, 716–720. [DOI] [PubMed] [Google Scholar]
- Dodds, P.N. , Lawrence, G.J. , Catanzariti, A.M. , Teh, T. , Wang, C.I. , Ayliffe, M.A. , Kobe, B. and Ellis, J.G. (2006) Direct protein interaction underlies gene‐for‐gene specificity and coevolution of the flax resistance genes and flax rust avirulence genes. Proc. Natl. Acad. Sci. USA, 103, 8888–8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald, T.M. , Pellerone, F. , Adam‐Blondon, A.F. , Bouquet, A. , Thomas, M.R. and Dry, I.B. (2002) Identification of resistance gene analogs linked to a powdery mildew resistance locus in grapevine. Theor. Appl. Genet. 104, 610–618. [DOI] [PubMed] [Google Scholar]
- Doster, M.A. and Schnathorst, W.C. (1985) Comparative susceptibility of various grapevine cultivars to the powdery mildew fungus Uncinula‐necator . Am. J. Enol. Vitic. 36, 101–104. [Google Scholar]
- Driouich, A. , Zhang, G.F. and Staehelin, L.A. (1993) Effect of brefeldin A on the structure of the Golgi apparatus and on the synthesis and secretion of proteins and polysaccharides in sycamore maple (Acer pseudoplatanus) suspension‐cultured cells. Plant Physiol. 101, 1363–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dry, I.B. , Feechan, A. , Anderson, C. , Jermakow, A.M. , Bouquet, A. , Adam‐Blondon, A.F. and Thomas, M.R. (2009) Molecular strategies to enhance the genetic resistance of grapevines to powdery mildew. Aust. J. Grape Wine Res. 16, 94–105. [Google Scholar]
- Eibach, R. (1994) Investigations about the genetic‐resources of grapes with regard to resistance characteristics to powdery mildew (Oidium‐tuckeri). Vitis, 33, 143–150. [Google Scholar]
- Ellis, J.G. , Dodds, P.N. and Lawrence, G.J. (2007) Flax rust resistance gene specificity is based on direct resistance–avirulence protein interactions. Annu. Rev. Phytopathol. 45, 289–306. [DOI] [PubMed] [Google Scholar]
- Feechan, A. , Jermakow, A.M. , Torregrosa, L. , Panstruga, R. and Dry, I.B. (2008) Identification of grapevine MLO gene candidates involved in susceptibility to powdery mildew. Funct. Plant Biol. 35, 1255–1266. [DOI] [PubMed] [Google Scholar]
- Feng, J. , Wang, F. , Liu, G. , Greenshields, D. , Shen, W. , Kaminskyj, S. , Hughes, G.R. , Peng, Y. , Selvaraj, G. , Zou, J. and Wei, Y. (2009) Analysis of a Blumeria graminis‐secreted lipase reveals the importance of host epicuticular wax components for fungal adhesion and development. Mol. Plant–Microbe Interact. 22, 1601–1610. [DOI] [PubMed] [Google Scholar]
- Flor, H.H. (1971) Current status of gene‐for‐gene concept. Annu. Rev. Phytopathol. 9, 275. [Google Scholar]
- Frei dit Frey, N. and Robatzek, S. (2009) Trafficking vesicles: pro or contra pathogens? Curr. Opin. Plant Biol. 12, 437–443. [DOI] [PubMed] [Google Scholar]
- Freytag, S. , Arabatzis, N. , Hahlbrock, K. and Schmelzer, E. (1994) Reversible cytoplasmic rearrangements precede wall apposition, hypersensitive cell‐death and defense‐related gene activation in potato–Phytophthora infestans interactions. Planta, 194, 123–135. [Google Scholar]
- Fung, R.W. , Gonzalo, M. , Fekete, C. , Kovacs, L.G. , He, Y. , Marsh, E. , McIntyre, L.M. , Schachtman, D.P. and Qiu, W. (2008) Powdery mildew induces defense‐oriented reprogramming of the transcriptome in a susceptible but not in a resistant grapevine. Plant Physiol. 146, 236–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadoury, D.M. and Pearson, R.C. (1991) Heterothallism and pathogenic specialization in Uncinula‐necator . Phytopathology, 81, 1287–1293. [Google Scholar]
- Geldner, N. , Anders, N. , Wolters, H. , Keicher, J. , Kornberger, W. , Muller, P. , Delbarre, A. , Ueda, T. , Nakano, A. and Jurgens, G. (2003) The Arabidopsis GNOM ARF‐GEF mediates endosomal recycling, auxin transport, and auxin‐dependent plant growth. Cell, 112, 219–230. [DOI] [PubMed] [Google Scholar]
- Gomez‐Gomez, L. , Felix, G. and Boller, T. (1999) A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana . Plant J. 18, 277–284. [DOI] [PubMed] [Google Scholar]
- Granado, J. , Felix, G. and Boller, T. (1995) Perception of fungal sterols in plants—subnanomolar concentrations of ergosterol elicit extracellular alkalinization in tomato cells. Plant Physiol. 107, 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, S. , Di Gaspero, G. , Kovacs, L. , Howard, S. , Kiss, E. , Galbacs, Z. , Testolin, R. and Kozma, P. (2008) Resistance to Erysiphe necator in the grapevine ‘Kishmish vatkana’ is controlled by a single locus through restriction of hyphal growth. Theor. Appl. Genet. 116, 427–438. [DOI] [PubMed] [Google Scholar]
- Jackson, C.L. and Casanova, J.E. (2000) Turning on ARF: the Sec7 family of guanine‐nucleotide‐exchange factors. Trends Cell Biol. 10, 60–67. [DOI] [PubMed] [Google Scholar]
- Jones, D.A. and Takemoto, D. (2004) Plant innate immunity—direct and indirect recognition of general and specific pathogen‐associated molecules. Curr. Opin. Immunol. 16, 48–62. [DOI] [PubMed] [Google Scholar]
- Kale, S.D. , Gu, B. , Capelluto, D.G. , Dou, D. , Feldman, E. , Rumore, A. , Arredondo, F.D. , Hanlon, R. , Fudal, I. , Rouxel, T. , Lawrence, C.B. , Shan, W. and Tyler, B. (2010) External lipid PI3P mediates entry of eukaryotic pathogen effectors into plant and animal host cells. Cell, 142, 284–295. [DOI] [PubMed] [Google Scholar]
- Kleine‐Vehn, J. , Leitner, J. , Zwiewka, M. , Sauer, M. , Abas, L. , Luschnig, C. and Friml, J. (2008) Differential degradation of PIN2 auxin efflux carrier by retromer‐dependent vacuolar targeting. Proc. Natl. Acad. Sci. USA, 105, 17 812–17 817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, I. and Hakuno, H. (2003) Actin‐related defense mechanism to reject penetration attempt by a non‐pathogen is maintained in tobacco BY‐2 cells. Planta, 217, 340–345. [DOI] [PubMed] [Google Scholar]
- Kobayashi, Y. , Yamada, M. , Kobayashi, I. and Kunoh, H. (1997) Actin microfilaments are required for the expression of nonhost resistance in higher plants. Plant Cell Physiol. 38, 725–733. [Google Scholar]
- Koch, E. and Slusarenko, A. (1990) Arabidopsis is susceptible to infection by a downy mildew fungus. Plant Cell, 2, 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh, S. , Andre, A. , Edwards, H. , Ehrhardt, D. and Somerville, S. (2005) Arabidopsis thaliana subcellular responses to compatible Erysiphe cichoracearum infections. Plant J. 44, 516–529. [DOI] [PubMed] [Google Scholar]
- Lipka, V. , Dittgen, J. , Bednarek, P. , Bhat, R. , Wiermer, M. , Stein, M. , Landtag, J. , Brandt, W. , Rosahl, S. , Scheel, D. , Llorente, F. , Molina, A. , Parker, J. , Somerville, S. and Schulze‐Lefert, P. (2005) Pre‐ and postinvasion defenses both contribute to nonhost resistance in Arabidopsis. Science, 310, 1180–1183. [DOI] [PubMed] [Google Scholar]
- Mackey, D. , Holt, B.F., 3rd , Wiig, A. and Dangl, J.L. (2002) RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for RPM1‐mediated resistance in Arabidopsis. Cell, 108, 743–754. [DOI] [PubMed] [Google Scholar]
- Mackey, D. , Belkhadir, Y. , Alonso, J.M. , Ecker, J.R. and Dangl, J.L. (2003) Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2‐mediated resistance. Cell, 112, 379–389. [DOI] [PubMed] [Google Scholar]
- Manning, V.A. and Ciuffetti, L.M. (2005) Localization of Ptr ToxA produced by Pyrenophora tritici‐repentis reveals protein import into wheat mesophyll cells. Plant Cell, 17, 3203–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLusky, S.R. , Bennett, M.H. , Beale, M.H. , Lewis, M.J. , Gaskin, P. and Mansfield, J.W. (1999) Cell wall alterations and localized accumulation of feruloyl‐3′‐methoxytyramine in onion epidermis at sites of attempted penetration by Botrytis allii are associated with actin polarisation, peroxidase activity and suppression of flavonoid biosynthesis. Plant J. 17, 523–534. [Google Scholar]
- Mellersh, D.G. , Foulds, I.V. , Higgins, V.J. and Heath, M.C. (2002) H2O2 plays different roles in determining penetration failure in three diverse plant–fungal interactions. Plant J. 29, 257–268. [DOI] [PubMed] [Google Scholar]
- Meyer, D. , Pajonk, S. , Micali, C. , O'Connell, R. and Schulze‐Lefert, P. (2009) Extracellular transport and integration of plant secretory proteins into pathogen‐induced cell wall compartments. Plant J. 57, 986–999. [DOI] [PubMed] [Google Scholar]
- Miklis, M. , Consonni, C. , Bhat, R.A. , Lipka, V. , Schulze‐Lefert, P. and Panstruga, R. (2007) Barley MLO modulates actin‐dependent and actin‐independent antifungal defense pathways at the cell periphery. Plant Physiol. 144, 1132–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miya, A. , Albert, P. , Shinya, T. , Desaki, Y. , Ichimura, K. , Shirasu, K. , Narusaka, Y. , Kawakami, N. , Kaku, H. and Shibuya, N. (2007) CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA, 104, 19 613–19 618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterhänsel, C. , Freialdenhoven, A. , Kurth, J. , Kolsch, R. and Schulze‐Lefert, P. (1997) Interaction analyses of genes required for resistance responses to powdery mildew in barley reveal distinct pathways leading to leaf cell death. Plant Cell, 9, 1397–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratelli, R. , Sutter, J.U. and Blatt, M.R. (2004) A new catch in the SNARE. Trends Plant Sci. 9, 187–195. [DOI] [PubMed] [Google Scholar]
- Rafiqi, M. , Gan, P.H. , Ravensdale, M. , Lawrence, G.J. , Ellis, J.G. , Jones, D.A. , Hardham, A.R. and Dodds, P.N. (2010) Internalization of flax rust avirulence proteins into flax and tobacco cells can occur in the absence of the pathogen. Plant Cell, 22, 2017–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robatzek, S. , Chinchilla, D. and Boller, T. (2006) Ligand‐induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes Dev. 20, 537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, D.G. , Langhans, M. , Saint‐Jore‐Dupas, C. and Hawes, C. (2008) BFA effects are tissue and not just plant specific. Trends Plant Sci. 13, 405–408. [DOI] [PubMed] [Google Scholar]
- Schmelzer, E. (2002) Cell polarization, a crucial process in fungal defence. Trends Plant Sci. 7, 411–415. [DOI] [PubMed] [Google Scholar]
- Sciaky, N. , Presley, J. , Smith, C. , Zaal, K.J. , Cole, N. , Moreira, J.E. , Terasaki, M. , Siggia, E. and Lippincott‐Schwartz, J. (1997) Golgi tubule traffic and the effects of brefeldin A visualized in living cells. J. Cell Biol. 139, 1137–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger, C.J. (2000) Signaling to the actin cytoskeleton in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51, 257–288. [DOI] [PubMed] [Google Scholar]
- Staudt, G. (1997) Evaluation of resistance to grapevine powdery mildew (Uncinula necator [Schw.] Burr., anamorph Oidium tuckeri Berk.) in accessions of Vitis species. Vitis, 36, 151–154. [Google Scholar]
- Stein, M. , Dittgen, J. , Sanchez‐Rodriguez, C. , Hou, B.H. , Molina, A. , Schulze‐Lefert, P. , Lipka, V. and Somerville, S. (2006) Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to nonhost resistance to inappropriate pathogens that enter by direct penetration. Plant Cell, 18, 731–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann, T. , Geldner, N. , Grebe, M. , Mangold, S. , Jackson, C.L. , Paris, S. , Galweiler, L. , Palme, K. and Jurgens, G. (1999) Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science, 286, 316–318. [DOI] [PubMed] [Google Scholar]
- Stergiopoulos, I. and de Wit, P.J. (2009) Fungal effector proteins. Annu. Rev. Phytopathol. 47, 233–263. [DOI] [PubMed] [Google Scholar]
- Takemoto, D. , Jones, D.A. and Hardham, A.R. (2003) GFP‐tagging of cell components reveals the dynamics of subcellular re‐organization in response to infection of Arabidopsis by oomycete pathogens. Plant J. 33, 775–792. [DOI] [PubMed] [Google Scholar]
- Thordal‐Christensen, H. (2003) Fresh insights into processes of nonhost resistance. Curr. Opin. Plant Biol. 6, 351–357. [DOI] [PubMed] [Google Scholar]
- Thordal‐Christensen, H. , Zhang, Z.G. , Wei, Y.D. and Collinge, D.B. (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley–powdery mildew interaction. Plant J. 11, 1187–1194. [Google Scholar]
- Underwood, W. and Somerville, S.C. (2008) Focal accumulation of defences at sites of fungal pathogen attack. J. Exp. Bot. 59, 3501–3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, Y.Z. , Schwaninger, H. , He, P.C. and Wang, Y.J. (2007) Comparison of resistance to powdery mildew and downy mildew in Chinese wild grapes. Vitis, 46, 132–136. [Google Scholar]
- Wang, J. , Cai, Y. , Miao, Y. , Lam, S.K. and Jiang, L. (2009) Wortmannin induces homotypic fusion of plant prevacuolar compartments. J. Exp. Bot. 60, 3075–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterhagen, P. , Howard, S.F. , Qui, W. and Kovács, L.G. (2008) Transcriptional upregulation of grapevine MLO genes in response to powdery mildew infection. Am. J. Enol. Vitic. 59, 159–168. [Google Scholar]
- Yun, B.W. , Atkinson, H.A. , Gaborit, C. , Greenland, A. , Read, N.D. , Pallas, J.A. and Loake, G.J. (2003) Loss of actin cytoskeletal function and EDS1 activity, in combination, severely compromises non‐host resistance in Arabidopsis against wheat powdery mildew. Plant J. 34, 768–777. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Penetration frequency of the nonadapted powdery mildew Erysiphe necator on Arabidopsis thaliana following treatment with increasing concentrations of brefeldin A (A) and wortmannin (B). Successful penetration was scored by the presence/absence of a haustorium. Each data point represents the mean ± standard deviation of two independent experiments within which a minimum of 100 germinated conidia were scored on three leaves. Asterisks indicate a significant difference from the negative dimethylsulphoxide (DMSO) control (P < 0.01; Student's t‐test).
Supporting info item


