SUMMARY
Rice yellow mottle virus (RYMV) reaches a high virus content in rice, is genetically highly variable and evolves rapidly. Nevertheless, only a small proportion of isolates overcome rymv1‐2 rice resistance by mutations in the VPg (viral protein genome‐linked). The accumulation rates of wild‐type (WT) and resistance‐breaking (RB) genotypes of the E‐ and T‐pathotypes of RYMV, with average and low virulence, respectively, were assessed. By quantitative reverse transcriptase‐polymerase chain reaction, it was shown that: (i) in resistant plants, both WT genotypes reached a level of 105–107 viral copies per milligram of fresh leaf; (ii) the accumulation of RB genotypes was variable, but was always much higher than the WT, with an RB/WT accumulation ratio of up to 106; (iii) in susceptible plants, the RB genotypes were counter‐selected to a similar level. In competition experiments, there was a straightforward exclusion of WT by RB genotypes in resistant hosts. The mutation rate in VPg was more than 1 × 10−3 mutations per site per year. Overall, a steady supply of highly adaptive RB genotypes was expected in resistant plants. However, the use of the few possible mutational pathways to virulence is tightly regulated by pathotype‐specific genetic constraints: codon usage, mutational bias and sign epistasis. In addition, genetic drift may restrict the fixation of RB mutants. Altogether, both genetic and demographic constraints contribute to the low ability of RYMV to break rymv1‐2 resistance.
INTRODUCTION
A knowledge about host plant resistance to plant viruses, especially that caused by recessive genes, has accumulated steadily in recent years (Maule et al., 2002; Robaglia and Caranta, 2006). In parallel, the complexity of the mechanisms involved in resistance breaking has been unravelled progressively (Ayme et al., 2006; Pinel‐Galzi et al., 2007; Yeam et al., 2007). However, a paradox first raised by Harrison (1981) and substantiated later (Garcia‐Arenal and McDonald, 2003) is still unresolved: despite high mutation rates, short replication cycles and high accumulation levels, plant viruses are often inefficient at breaking host plant resistances. This is exemplified by Rice yellow mottle virus (RYMV) of the genus Sobemovirus. RYMV reaches a high virus content in plants (Ioannidou et al., 2000), is genetically highly variable among plants (Fargette et al., 2004), evolves rapidly (Fargette et al., 2008) and infects rice irrespective of the cultivars or the agro‐ecosystem encountered (Traoréet al., 2005). Nevertheless, only a small proportion of isolates overcome the high resistance caused by rymv1‐2 (Allarangaye, 2008; Pinel‐Galzi et al., 2007; Traoréet al., 2006).
The Rymv1 gene maps to chromosome 4 and encodes a translation initiation factor eIF(iso)4G (Albar et al., 2006). The rymv1‐2 recessive resistance allele is characterized by a substitution of a lysine (K) for a glutamic acid (E) in susceptible cultivars at position 309 of eIF(iso)4G. The breakdown of rymv1‐2 resistance is caused by the emergence of resistance‐breaking (RB) viral genotypes that are derived from wild‐type (WT) genotypes by mutations in VPg (viral protein genome‐linked) (Hébrard et al., 2006; Pinel‐Galzi et al., 2007). The proportion of RYMV isolates able to break rymv1‐2 resistance has been assessed in three large‐scale surveys. The percentages of RB isolates were 9% (Pinel‐Galzi et al., 2007), 15% (Traoréet al., 2006) and 20% (Allarangaye, 2008). The differences were attributed to the proportions of the two main pathotypes with contrasted virulence towards rymv1‐2 and different geographical distribution (Pinel‐Galzi et al., 2007). Virulence is defined here as the genetic ability of a pathogen to overcome a genetically determined resistance leading to disease (Shaner et al., 1992). Up to 20% of the isolates with a glutamic acid (E) at codon 49 of VPg—referred to as the E‐pathotype—can break rymv1‐2 resistance after inoculation of WT isolates (Allarangaye, 2008; Pinel‐Galzi et al., 2007; Traoréet al., 2006). Interestingly, codon 49 of VPg is under diversifying selection (Pinel‐Galzi et al., 2007). There are few possible mutational pathways to virulence and most of them are localized at the adjacent codon 48. The most frequent (named mutational pathway I) consists of two successive transitions at codon 48 of VPg, which first substitutes an arginine (R; codon AGA) by a glycine (G; GGA), and then a glycine by a glutamic acid (E; GAA). An occasional pathway (named mutational pathway II) is composed of a transversion that changes an arginine into an isoleucine (I; ATA) at the same position. Additional RB mutations at other positions of VPg are sometimes observed, but are transitory and displaced after the fixation of 48E (Pinel‐Galzi et al., 2007). By contrast, the 48I RB mutant is never replaced by 48E, even in long‐term experiments.
Isolates with a threonine (T) at codon 49 of VPg—referred to as the T‐pathotype—are hardly able to break rymv1‐2 resistance. Only three RB isolates have been obtained of the 100 tested: one from Mali (Pinel‐Galzi et al., 2007) and two from Niger (O. Traoré and A. Pinel‐Galzi, unpublished results). Altogether, the RB abilities of the E‐ and T‐pathotypes differ by an order of magnitude. This has been attributed to the failure of the T‐pathotype to follow mutational pathway I (Pinel‐Galzi et al., 2007). The virulence of the T‐pathotype is caused by a transversion that changes the arginine at position 48 of VPg (R; codon AGG) into a tryptophan (TGG) (mutational pathway III). Differences in codon usage at codon 48 (AGA vs. AGG) account for some, but not all, differences in the mutational pathways followed by the E‐ and T‐pathotypes. Mutational pathway II cannot be followed by the T‐pathotype by a one‐step substitution, as isoleucine is not coded from the arginine AGG. Conversely, mutational pathway III cannot be used by the E‐pathotype, as tryptophan is not coded from the arginine AGA by a single mutation. However, differences in codon usage do not explain why the T‐pathotype fails to follow mutational pathway I, as glycine and glutamic acid can be coded either from AGA (GGA; GAA) or AGG (GGG; GAG).
The probability that a mutation is fixed depends on: (i) the mutation rate at that particular site; (ii) its selective advantage; and (iii) the effect of genetic drift (Hartl and Clark, 2007). Low RB ability can be caused by a combination of a slow mutation rate, a low selective advantage of RB mutants or a strong effect of genetic drift (Ayme et al., 2006). The respective importance of these three factors in overcoming rymv1‐2 resistance was assessed. The WT and a few RB genotypes of isolate CI4 (E‐pathotype) and CIa (T‐pathotype) were tested. The virus content reached by WT and RB genotypes in resistant and susceptible plants was quantified by quantitative (or real‐time) reverse transcriptase‐polymerase chain reaction (qRT‐PCR). Competition experiments between WT and RB genotypes in resistant and susceptible plants were also performed. The mutation rate of plant viruses has been estimated experimentally only twice (Malpica et al., 2002; Sanjuan et al., 2009). The upper limits of the mutation rates of four viruses in different hosts were derived from the mutant spectrum (reviewed in Sanjuan et al., 2009). With RYMV, the lower limit of the mutation rate was estimated by a genetic population approach. Under neutral evolution, the mutation rate is equal to the substitution rate, whereas, under negative selection, the mutation rate is lower than the substitution rate (Gillespie, 2004; Rice, 2004). Therefore, the lower bound of the mutation rate in VPg of RYMV was estimated from the substitution rate under a negative evolutionary constraint. Considering the results obtained, a steady supply of highly adaptive RB genotypes is expected in resistant plants. Therefore, the ability of RYMV to break rymv1‐2 resistance is strikingly low. This is attributed to genetic and demographic constraints, exemplified by the contrasted virulence of the E‐ and T‐pathotypes.
RESULTS
Low replication of WT genotypes in resistant hosts
Isolates CI4 and CIa were selected to represent the E‐ and T‐pathotypes, respectively (see Experimental procedures). The accumulation of the WT genotypes CI4 and CIa was evaluated by qRT‐PCR in the two resistant Oryza sativa indica cultivars Gigante and Bekarosaka. The WT genotype was detected erratically in resistant plants by double‐antibody‐sandwich enzyme‐linked immunosorbent assay (DAS‐ELISA) (data not shown). By contrast, the sensitive qRT‐PCR technique detected a low but consistent viral RNA accumulation in non‐inoculated leaves from each resistant host (18 plants). Because of the low replication of WT genotypes, only one of 18 could be sequenced. Genotyping confirmed that the RNA detected 30 days post‐inoculation (dpi) was WT, because no additional mutation was observed in the VPg coding region. At 30 dpi, RNA accumulation of CI4 and CIa isolates was 105–107 RNA copies per milligram of fresh leaf, with no difference between the two WT genotypes (1, 2). A similar accumulation was reached in both resistant cultivars Bekarosaka and Gigante (data not shown), indicating that the indica genetic background of the resistant hosts did not affect the replication of the WT genotypes. With a low multiplication of the WT genotypes, the plant growth of both resistant cultivars was unimpaired, as the dry weight of the aerial parts of the inoculated plants did not differ from that of virus‐free plants (P > 0.1) (1, 2).
Figure 1.
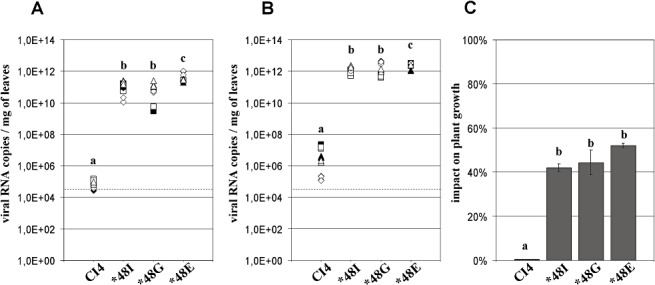
Viral accumulation and impact on plant growth of wild‐type and resistance‐breaking genotypes of isolate CI4 (E‐pathotype) in rymv1‐2 resistant cv. Bekarosaka. Resistance‐breaking genotypes were CI4‐48I, CI4‐48G and CI4‐48E. Numbers of viral RNA copies per milligram of fresh leaf were estimated by quantitative reverse transcriptase‐polymerase chain reaction (qRT‐PCR) at 15 days post‐inoculation (dpi) (A) and 30 dpi (B). Squares, triangles and diamonds, inoculum triplicates; open and filled symbols, reverse transcriptase duplicates; broken lines, detection threshold. (C) Impact of wild‐type and resistance‐breaking genotypes on plant growth at 45 dpi, expressed as a percentage of dry weight loss compared with virus‐free plants. a, b, c, groups significantly different after multiple mean comparison (anova, P= 0.05).
Figure 2.
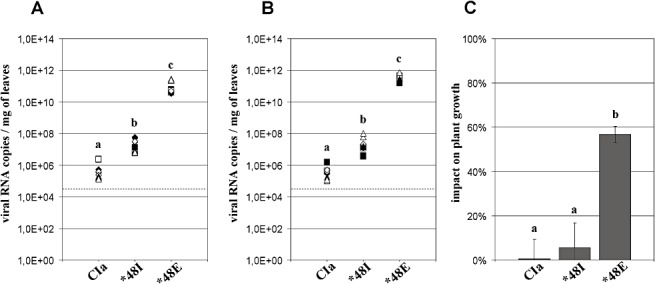
Viral accumulation and impact on plant growth of wild‐type and resistance‐breaking genotypes of isolate CIa (T‐pathotype) in rymv1‐2 resistant cv. Bekarosaka. Resistance‐breaking genotypes were CIa‐48I and CIa‐48E. Numbers of viral RNA copies per milligram of fresh leaf were estimated by quantitative reverse transcriptase‐polymerase chain reaction (qRT‐PCR) at 30 days post‐inoculation (dpi) (A) and 45 dpi (B). Squares, triangles and diamonds, inoculum triplicates; open and filled symbols, reverse transcriptase duplicates; broken lines, detection threshold. (C) Impact of wild‐type and resistance‐breaking genotypes on plant growth at 60 dpi, expressed as a percentage of dry weight loss compared with virus‐free plants. a, b, c, groups significantly different after multiple mean comparison (anova, P= 0.05).
High replication of RB genotypes in resistant hosts
A maximum accumulation of c. 1012 RNA copies per milligram of leaf was reached, irrespective of the viral genotype inoculated or the resistant cultivar infected (1, 2). The dilution of total RNA samples at 1 : 100 and 1 : 1000 led to proportional detection levels of the number of copies by qRT‐PCR (data not shown). Thus, the level of 1012 RNA copies per milligram of leaf was not an experimental threshold caused by the saturation of either the RT or PCR steps of qRT‐PCR.
The CI4 mutated genotypes (48I, 48G, 48E) accumulated at c. 106 times greater levels than the WT genotype in the resistant hosts. However, significant differences in RNA accumulation between the RB CI4 genotypes occurred. RNA accumulation of CI4‐48E was 10‐fold higher at 15 dpi and two‐fold higher at 30 dpi than that of either CI4‐48I or CI4‐48G, whereas no difference in RNA accumulation was apparent between CI4‐48I and CI4‐48G genotypes. Neither DAS‐ELISA (data not shown) nor the dry weights of the aerial parts showed differences between the three CI4 mutated genotypes (Fig. 1C). Interestingly, sequencing at 30 dpi revealed ongoing changes in three of the 12 resistant plants inoculated with CI4‐48G. E48 mutation was fixed once at codon 48 of VPg. A G/E mixture was apparent through sequencing electrophoregrams in two other plants.
CIa‐48E reached a maximum accumulation of c. 1012 RNA copies per milligram of leaf in the cultivars Bekarosaka and Gigante at 30 dpi, i.e. an amount similar to that of CI4‐48E but attained 2 weeks later. Differences in RNA accumulation between 48I and 48E RB mutants were more pronounced with the CIa than CI4 genotype. CIa‐48I multiplied at c. 107 copies per milligram of leaf (Fig. 2A,B). This was 105 less than CI4‐48I and only 10 times more than WT. The impact of the CIa‐48E genotype on cv. Bekarosaka plant growth was significantly greater than that of CIa‐48I or CIa (P < 0.01, Fig. 2C).
Low replication of RB genotypes in susceptible hosts
In susceptible plants, the highest accumulation of c. 1012 RNA copies per milligram of leaf was attained by the two WT genotypes (CI4 and CIa). This level is similar to the maximum RNA accumulation reached by the RB genotypes in resistant plants. RB accumulation in susceptible plants was lower than that of the WT genotypes by a factor of 10–106, depending on the mutated genotype (3, 4). The mutants CI4‐48I and CIa‐48I were the least impaired. Their accumulation rates were 10 and 103 times lower than those of the WT CI4 and CIa genotypes, respectively. The mutants CI4‐48E and CIa‐48E were most affected, and their RNA accumulation rates were c. 105 times lower than those of the WT genotypes. Interestingly, changes in the RB populations were observed during the course of infection in the susceptible hosts. Reversions from 48E to 48G arose at 30 dpi in three of the six susceptible plants that were inoculated with CI4‐48E at 1012 RNA copies per plant. No other mutations appeared in the VPg coding region.
Figure 3.
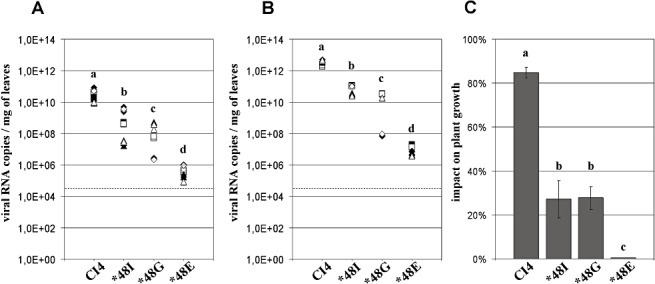
Viral accumulation and impact on plant growth of wild‐type and resistance‐breaking genotypes of isolate CI4 (E‐pathotype) in susceptible cv. IR64. Resistance‐breaking genotypes were CI4‐48I, CI4‐48G and CI4‐48E. Numbers of viral RNA copies per milligram of fresh leaf were estimated by quantitative reverse transcriptase‐polymerase chain reaction (qRT‐PCR) at 15 days post‐inoculation (dpi) (A) and 30 dpi (B). Squares, triangles and diamonds, inoculum triplicates; open and filled symbols, reverse transcriptase duplicates; broken lines, detection threshold. (C) Impact of wild‐type and resistance‐breaking genotypes on plant growth at 45 dpi, expressed as a percentage of dry weight loss compared with virus‐free plants. a, b, c, d, groups significantly different after multiple mean comparison (anova, P= 0.05).
Figure 4.
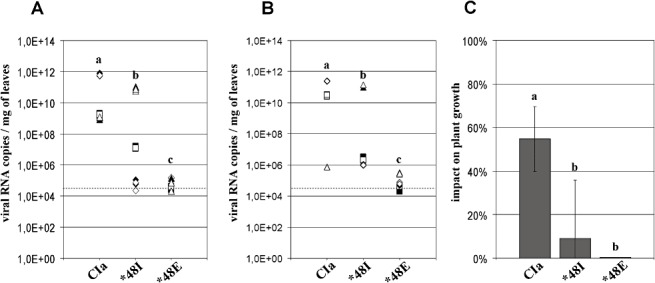
Viral accumulation and impact on plant growth of wild‐type and resistance‐breaking genotypes of isolate CIa (T‐pathotype) in susceptible cv. IR64. Resistance‐breaking genotypes were CIa‐48I and CIa‐48E. Numbers of viral RNA copies per milligram of fresh leaf were estimated by quantitative reverse transcriptase‐polymerase chain reaction (qRT‐PCR) at 30 days post‐inoculation (dpi) (A) and 45 dpi (B). Squares, triangles and diamonds, inoculum triplicates; open and filled symbols, reverse transcriptase duplicates; broken lines, detection threshold. (C) Impact of wild‐type and resistance‐breaking genotypes on plant growth at 60 dpi, expressed as a percentage of dry weight loss compared with virus‐free plants. a, b, c, groups significantly different after multiple mean comparison (anova, P= 0.05).
The impact of both WT genotypes on susceptible host fitness was significantly greater than that of their respective RB genotypes (P < 0.001, 3, 4). The low multiplication of the genotypes with the mutation 48E did not affect the growth of susceptible hosts, as the dry weights of the aerial parts did not differ from those of virus‐free plants. The impact of the CI4‐48I and CI4‐48G genotypes on the fitness of the susceptible hosts was significantly greater than that of CI4‐48E (P < 0.0001). Altogether, the RNA content paralleled the dry weight loss in both susceptible and resistant cultivars. The coefficients of correlation between virus titre and weight loss for the CI4 genotypes were 0.90 and 0.97 for cultivars IR64 and Bekarosaka, respectively. With the CIa genotypes, the coefficients of correlation were 0.73 and 0.88 for cultivars IR64 and Bekarosaka, respectively.
Competitive exclusion of WT and RB genotypes in resistant and susceptible hosts
Competitive exclusion of the WT CI4 genotype by the RB genotypes CI4‐48I and CI4‐48G occurred within a few weeks after infection of rymv1‐2 resistant plants (Pinel‐Galzi et al., 2007). After mixed inoculations of resistant plants with CI4 and CI4‐I, only CI4‐I was detected in specific RT‐PCR, indicating exclusion of WT by the RB genotype (Hébrard et al., 2006). Competition between the WT genotype CIa and its RB genotypes (CIa‐48I and CIa‐48E) was tested in the susceptible cv. IR64 and in the resistant cv. Gigante. In susceptible hosts, only WT sequences of the CIa genotype (with 48R in VPg) were detected after co‐infection of either the CIa/CIa‐48I or CIa/CIa‐48E genotypes. Conversely, RB genotypes CIa‐48I and CIa‐48E consistently displaced the WT genotype in resistant hosts. Moreover, no WT/RB mixture was observed in sequencing electrophoregrams. These results were obtained at 30 dpi, when the first test was performed, and the results were similar at 45 and 75 dpi. This indicates a rapid and steady elimination of the counter‐selected genotypes, or at the least their maintenance at a very low level. Altogether, both the high RB/WT accumulation ratio and the rapid exclusion of WT by RB genotypes in competition experiments show the extent of the selective advantage conferred by the RB mutations in rymv1‐2 resistant plants.
Lower limit of the mutation rate in VPg
The rate of substitution of the coat protein (CP) gene was estimated as 1.1 × 10−3 substitutions per site per year from dated sequences of isolates collected from nature (Fargette et al., 2008). The nucleotide diversity of the CP gene and VPg, calculated from sequences of 22 isolates representing the geographical distribution and genetic diversity of RYMV, were 0.09 and 0.08, respectively (Pinel‐Galzi et al., 2007). Assuming proportionality between the substitution rate and nucleotide diversity gives an estimate of 1 × 10−3 substitutions per site per year in VPg. As VPg evolves under a strong purifying selection, even stronger than the CP gene (dN/dS= 0.07 and 0.18, respectively) (Pinel‐Galzi et al., 2007), this value is a lower limit of the mutation rate. Therefore, the mutation rate in VPg is more than 1 × 10−3 mutations per site per year, i.e. more than 3 × 10−6 mutations per site per day. This is quite a conservative value, because of the strong negative selection pressure considered.
DISCUSSION
Trade‐offs and reversion in the rymv1‐2 RB process
Different trade‐offs occurred among the rymv1‐2 RB mutants. The mutant 48E reached the highest accumulation in resistant plants, but the lowest in susceptible cultivars. By contrast, the 48I and 48G mutants conferred less increase in virus accumulation in resistant plants, but were less impaired in susceptible hosts. Consistently, the impact on plant growth of the mutant 48E was higher in resistant cultivars, but lower in susceptible ones, than mutants 48G or 48I. Furthermore, the 48I mutant was maintained in susceptible cultivars during serial passages (Sorho et al., 2005; Hébrard et al., 2006), whereas the 48E mutant was not (A. Pinel‐Galzi and D. Fargette, unpublished results). Accordingly, genotypes 48R (WT) and 48E (RB) show a specialist type of trade‐off for susceptible and resistant plants, respectively, whereas 48I and 48G (RB) behave as generalists (Elena and Lenski, 2003). The contrasted trade‐offs probably reflect differential affinities of pairs of amino acids under a direct site‐to‐site interaction between codon 48 of VPg and codon 309 of eIF(iso)4G (Hébrard et al., 2008). In particular, the E48–K309 interaction between the virulent isolate and the resistant rice restored more efficiently than any other variant the charge complementarity of the R48–E309 interaction between the avirulent isolate and susceptible rice.
The E/K substitution at position 309 of eIF(iso)4G in resistant rice decreased the RNA accumulation of WT isolates by a factor of 106. The accumulation of RB mutants was higher than WT by several orders of magnitude, and the R/E mutation at position 48 of VPg fully restored virus multiplication in resistant plants. This shows the extent of the selection pressure imposed on WT populations in resistant plants, and the selective advantage of the RB mutants. Conversely, the accumulation of RB isolates in susceptible cultivars was reduced to a similar level, although CI4‐48G was less counter‐selected than CI4‐48E. In several instances, CI4‐48E was even displaced by the CI4‐48G mutant in susceptible plants. This is the first evidence of reversion for RYMV. However, the same reversion has been observed recently with another RB isolate of a distantly related strain from East Africa (A. Pinel‐Galzi and D. Fargette, unpublished results). Therefore, the ability to revert may be a general feature in RYMV evolution. No compensatory mutations arose in VPg. Altogether, RYMV restored full plant–virus compatibility in resistant and susceptible rice cultivars along the same reversible pathways.
The trade‐off pattern and reversibility of the evolutionary trajectories are both consistent with the hypothesis of a direct site‐to‐site interaction between codon 48 of VPg and codon 309 of eIF(iso)4G (Hébrard et al., 2008). Site‐to‐site interactions also account for the extent of parallel evolution in the rymv1‐2 RB process and for the limited number of pathways to virulence (Pinel‐Galzi et al., 2007). The identification of the factors that limit the use of the few possible evolutionary trajectories should help us to understand the low ability of RYMV to overcome rymv1‐2 resistance.
Steady production of highly adaptive RB genotypes
The WT populations of both CI4 and CIa isolates accumulated at c. 105–107 viral RNA copies per milligram of fresh leaf in resistant plants. Consequently, rymv1‐2 resistance did not confer a strict immunity against RYMV, but allowed limited replication and systemic movement of the WT genotypes. This multiplication may be caused by either weak binding at other positions in the same interaction domain on chromosome 4 (Hébrard et al., 2008), or an interaction with the other eIF(iso)4G copy located on chromosome 2 (Boisnard et al., 2007).
With 105–107 viral copies per milligram of leaf, and a mutation rate of more than 1 × 10−3 mutations per site per year, a continuous supply of mutants is expected in an RYMV‐resistant rice plant with an average fresh weight of 10 g. Consistently, estimates of the viral content and mutation rate of human immunodeficiency virus‐1 (HIV‐1) have suggested that every single point mutation occurs at least once each day in an infected individual (Parera et al., 2009). With an RB/WT accumulation ratio of c. 106, the RB genotype should outperform WT rapidly, as observed in competition experiments. Consequently, the ability of RYMV to break rymv1‐2 resistance is strikingly low. This is attributed to genetic and demographic constraints, exemplified by the contrasted virulence of the E‐ and T‐pathotypes.
Genetic constraints to resistance breaking
Mutational pathways I and II result from the displacement of a genotype by one with a higher accumulation rate, either WT by an RB genotype, or RB by a fitter RB genotype. However, the use of the few possible mutational pathways is tightly regulated by pathotype‐specific genetic constraints. This was first apparent through differences in codon usage which prevent pathotypes T and E following mutational pathways II and III, respectively (Pinel‐Galzi et al. 2007; see Introduction). This is further exemplified by the following three case studies.
-
1
The 48G and 48I RB mutants of the E‐pathotype have similar accumulation rates in resistant plants, whereas transitions are seven times more frequent than transversions in VPg (Pinel‐Galzi et al., 2007). Therefore, the higher frequency of the 48G (first step of mutational pathway I) over the 48I (mutational pathway II) RB mutant (Pinel‐Galzi et al., 2007) probably reflects the fact that glycine is coded from an arginine by a transition, whereas isoleucine is coded by a transversion. Consistently, mutational pathway III is uncommon, possibly because tryptophan is coded from arginine by a transversion. Similarly, the differences in frequency between RB mutants towards the pvr23 resistance to Potato virus Y in pepper have been attributed to the relative fitness of the mutants and to mutational bias (transition vs. transversion) (Ayme et al., 2006).
-
2
Mutant 48I was never displaced by 48E despite the higher accumulation rate of the latter. By contrast, the RB mutants at other positions were readily outcompeted by 48E (Pinel‐Galzi et al., 2007). Two reasons probably explain why the rymv1‐2 RB process is locked in a suboptimal fitness level. First, glutamic acid (GAA or GAG) cannot be coded from an isoleucine (ATA) by a single mutation. Second, the 48I mutant, once fixed, is unlikely to be outgrown by the 48G genotype emerging from WT, which has a similar accumulation rate.
-
3
Among the plausible causes of the inability of the T‐pathotype to use mutational pathway I, two are now excluded and one is strengthened. It is not caused by a smaller selective advantage of the 48E mutant over WT, as the RB/WT accumulation ratios of the CIa and CI4 genotypes in the resistant plants are comparable. It is not caused either by a slower rate of production of RB mutants, as the multiplication rates of CIa and CI4 WT genotypes in resistant plants are similar. The alternative hypothesis of a sign epistasis—in which a mutation is beneficial in a genetic background and deleterious in another (Weinreich et al., 2005)—is supported. In the E‐pathotype, the 48G mutation is highly beneficial in resistant plants, with an RB/WT accumulation ratio of c. 106. In the T‐pathotype, there is evidence that the 48G mutation is not infectious (Pinel‐Galzi et al., 2007). Consequently, in the E‐pathotype, a steady flow of highly adaptive mutations along mutational pathway I is expected. In the T‐pathotype, the emergence of 48E from 48R through 48G is not observed, and the ability to break rymv1‐2 resistance is restricted to the uncommon mutational pathway III. Not only is the 48G mutation unfit in the T‐context (Pinel‐Galzi et al., 2007), but the accumulation of the 48I genotype is also 105 times lower in resistant plants than in the E‐pathotype. Possibly, the threonine at position 49 of VPg, instead of glutamic acid, alters the ability of both the glycine and isoleucine at the adjacent codon 48 to bind site 309 of the eIF(iso)4G gene (Hébrard et al., 2008; Pinel‐Galzi et al., 2007). The rymv1‐2 RB process exemplifies the role of sign epistasis in limiting the number of mutational trajectories available for selection, because some paths to an optimum include fitness decreases (Weinreich et al., 2005). Altogether, the use of mutational pathways to virulence is tightly regulated by pathotype‐specific genetic constraints: codon usage, mutational bias and sign epistasis.
Demographic constraints to resistance breaking
The ability of the E‐pathotype to follow mutational pathway I is not restricted by genetic constraints. Consequently, factors other than genetics must limit the ability to break rymv1‐2 resistance. Genetic drift may restrict the fixation in RB mutants at the early phase of the RB process. At the early stage of infection, the number of particles in systemically infected leaves can be as low as a few units (French and Stenger, 2003; Li and Roossinck, 2004; Sacristan et al., 2003). The bottlenecks are narrow (Ali and Roossinck, 2008; Li and Roossinck, 2004) and random genetic drift dominates over selection (Sacristan and Garcia‐Arenal, 2008). Accordingly, from a small population of WT genotypes and an initial minimal proportion of RB genotypes, fixation of rymv1‐2 RB mutants in resistant plants is restricted or delayed, despite their selective advantage. Stochasticity is another consequence of genetic drift (Lenormand et al., 2009). This is apparent in the pattern of rymv1‐2 resistance breakdown, as the order of appearance and the fixation time of the RB mutants are quite variable (Pinel‐Galzi et al., 2007). Once an RB mutant is fixed, even a transient one, the virus content increases dramatically. The selection subsequently becomes the main driving force, and the outcome of the RB process is determined. It results nearly always in the fixation of the 48E mutation, which confers the highest accumulation rate in resistant plants.
Altogether, the rymv1‐2 RB process follows a selection drift regime. This describes the transition between stochastic evolution in small populations of viruses, where random drift dominates over selection, and deterministic evolution in large populations when selection prevails (Rouzine et al., 2001). A comparable scenario was observed recently with the breakdown of the dominant Rz1 resistance in sugar beet by Beet necrotic yellow vein virus. Small and highly heterogeneous populations were recovered after the inoculation of resistant plants by the avirulent‐type virus, whereas large and homogeneous populations were isolated after infection with the RB variants (Acosta‐Leal et al., 2008). The selection drift regime also accounts for the failure to detect RB mutants or RB/WT mixtures in susceptible plants. The large populations of WT and their strong selective advantage lead to the rapid elimination of any RB mutants. Altogether, the RB genotypes are more likely to be generated through the limited multiplication of WT in resistant plants than selected from a pool of pre‐existing RB variants in susceptible plants.
EXPERIMENTAL PROCEDURES
Viral isolates and RB genotypes
The virus accumulation of WT and RB genotypes of RYMV was assessed in O. sativa indica cultivars Gigante and Bekarosaka which contain the rymv1‐2 resistance allele (Ndjiondjop et al., 1999; Rakotomalala et al., 2008), and in the susceptible cultivar IR64. The plants were kept in a growth chamber under 12 h of illumination at 120 µE/m2/s at 28 °C and 60% humidity. Two isolates from Ivory Coast were selected. Isolates CI4 and CIa represent the E‐ and T‐pathotypes, with average and low ability to break rymv1‐2 resistance, respectively. The CIa RB genotypes were produced from directed mutagenesis of an infectious clone CIa (Brugidou et al., 1995): CIa‐48I (Hébrard et al., 2006); CIa‐48E (Pinel‐Galzi et al., 2007); CIa4‐48G was not infectious (Pinel‐Galzi et al., 2007). There was no infectious clone of the E‐pathotype available; therefore, the RB genotypes originated from previous passage experiments: CI4‐48I (Fargette et al., 2002), CI4‐48G and CI4‐48E (Pinel‐Galzi et al., 2007). The inoculum was prepared by grinding infected frozen leaves in 0.1 mm phosphate buffer (1 mm KH2PO4 and 1 mm Na2HPO4, pH 7.2) (0.1 g/mL). Extracts were mixed with 600‐mesh carborundum and rubbed on the leaves of 14‐day‐old rice seedlings.
Viral quantification
qRT‐PCR assays were performed on plants that were inoculated with the same concentration of each viral genotype. For the CI4 and CIa experiments, c. 1011 copies of viral RNA were inoculated per plant, except for one additional experiment in which c. 1012 copies of viral RNA of isolate CI4 per plant were used for inoculation. Nine plants were inoculated with each genotype, and three plants of each host genotype. The last expanded leaf of each plant (non‐inoculated leaves) was collected at 15, 30 and 45 dpi. The total RNA from 0.05 g of leaves was purified (RNeasy Plant Mini Kit, Qiagen, Hilden, Germany). Two replicates of a two‐step reverse transcription of a region overlapping the VPg cistron of RYMV were performed for each RNA extract under the following conditions: denaturation of 7.5 µL of total RNA at 65 °C for 5 min with 0.5 mm of dNTPs and 5 mm of reverse primer R0 (nucleotides 1748–1767: 5′‐GGCCGGACTTACGACGTTCC‐3′; conserved region between CI4 and CIa genotypes); addition of 10 mm of dithiothreitol (DTT) (Invitrogen, Carlsbad, CA, USA) and 1 U/µL of ribonuclease inhibitor (Invitrogen) followed by incubation at 37 °C for 2 min; and addition of 10 U/µL of M‐MLV‐Reverse Transcriptase (Invitrogen) with appropriate buffer (final volume, 20 µL) followed by incubation at 37 °C for 50 min and then 70 °C for 15 min.
The qPCRs were run on a Stratagene (Cedar Creek, TX, USA) Mx3005 machine. Measurement of the RNA copy numbers was performed with 5 µL of cDNA (diluted at 1 : 1250) mixed with 12.5 µL of Full Velocity Master Mix (Stratagene) and 300 nm of sense and antisense primers (sense: hybridizing from nucleotides 1360 to 1377, 5′‐GAGGCTCGTCATCTACTG‐3′; antisense: hybridizing from nucleotides 1503 to 1520, 5′‐GTATATCCACGACGCCTA‐3′; conserved regions between CI4 and CIa genotypes) (final volume, 25 µL). DNA amplifications were performed under the following cycling conditions: one cycle at 95 °C for 5 min; 40 cycles at 95 °C for 10 s and 60 °C for 30 s; one cycle at 95 °C for 1 min, 60 °C for 30 s and 95 °C for 30 s. All reactions were performed in duplicate, including the negative control and the standard curve samples. Two standards were used: an infectious clone of CIa (Brugidou et al., 1995) and a cDNA of a purified virus (BF1, strain S1) from infected leaves. The standards were quantified by spectrophotometry (Spectronic Genesys8, Rochester, NY, USA).
Serial dilutions were performed with a range of DNA standards from 5 × 108 to five copies per reaction, aliquoted and stored at −20 °C. The number of copies per reaction was estimated using Stratagene Mx3005 software version 2.02, which was also used to estimate the number of viral RNA copies per milligram of infected leaf. Products below 200–250 copies per reaction were poorly reproducible, and so the detection threshold was set at 500 copies per reaction, which corresponds to 2 × 104 copies per milligram of leaf. RNA replication of genotypes was compared by analysis of variance (anova) (Statistica software version 6.0, Tulsa, OK, USA). To follow the dynamics of mutational events during the experiments, the VPg coding region and its 5′ and 3′ neighbouring regions (nucleotides 1479–2131) were sequenced as described previously (Hébrard et al., 2006) from the total RNA extracts used for qRT‐PCR. The virus content was also assessed by DAS‐ELISA, as performed by N'Guessan et al. (2001).
Competition experiments
Competition experiments between CIa WT and its RB genotypes (CIa‐48I and CIa‐48E) were conducted in the susceptible O. sativa indica cv. IR64 and in the resistant cv. Gigante. For each competition experiment, c. 108 copies of viral RNA of each genotype, as determined by qRT‐PCR, were mixed and then inoculated to three plants of each host genotype. Two to three infected plants per cultivar were randomly chosen at 30, 45 or 75 dpi, and the last expanded leaf of each plant was collected. The VPg coding region and its 5′ and 3′ neighbouring regions (nucleotides 1479–2131) were sequenced by the analysis of RT‐PCR fragments produced from total RNA purified by the RNeasy Plant Mini Kit (Qiagen), as described previously (Hébrard et al., 2006). A single peak in a sequencing electrophoregram at one position indicated the genotype which gained the competition, by comparison with the original sequence of each genotype. Multiple peaks at the same position reflected a nucleotide polymorphism and indicated a mixture of genotypes (Pinel‐Galzi et al., 2007).
Assessment of viral impact on host fitness
The impact of WT and RB genotypes of CIa and CI4 isolates on the host fitness of susceptible and resistant cultivars was assessed. Aerial parts of infected plants were sampled at 45 and 60 dpi for CI4 and CIa, respectively. The dry weight was measured after 4 days of incubation at 65 °C. The viral impact on host fitness, which is expressed as the percentage decrease in dry weight of aerial parts in comparison with virus‐free plants, was analysed by anova (Statistica software version 6.0).
ACKNOWLEDGEMENTS
We thank J. Aribi for plant care, and we are grateful to C. Chevillon, R. Froissart, B.D. Harrison, T. de Meeûs, and J.M. Thresh for helpful discussions and constructive criticisms of the manuscript. The useful comments of two anonymous referees are also gratefully acknowledged. This work was partly supported by the CNRS program MIE (Maladies Infectieuses Emergentes) 2008. Nils Poulicard was granted a fellowship from the French Ministry of Research.
REFERENCES
- Acosta‐Leal, R. , Fawley, M. and Rush, C. (2008) Changes in the intraisolate genetic structure of beet necrotic yellow vein virus populations associated with plant resistance breakdown. Virology, 376, 60–68. [DOI] [PubMed] [Google Scholar]
- Albar, L. , Bangratz‐Reyser, M. , Hébrard, E. , Ndjiondjop, M.N. , Jones, M. and Ghesquière, A. (2006) Mutations in the eIF(iso)4G translation initiation factor confer high resistance of rice to Rice yellow mottle virus. Plant J. 47, 417–426. [DOI] [PubMed] [Google Scholar]
- Ali, A. and Roossinck, M.J. (2008) Genetic bottlenecks In: Plant Virus Evolution (Roossinck M.J. ed.), pp. 123–131. Berlin: Springer. [Google Scholar]
- Allarangaye, M. (2008) Epidémiologie de la panachure jaune du riz en zone soudano‐sahélienne: interactions entre le virus et les plantes hôtes sauvages. PhD Thesis, University of Ouagadougou, Burkina Faso, 108 pp.
- Ayme, V. , Souche, S. , Caranta, C. , Jacquemond, M. , Chadoeuf, J. , Palloix, A. and Moury, B. (2006) Different mutations in the genome‐linked protein VPg of Potato virus Y confer virulence on the pvr23 resistance in pepper. Mol. Plant–Microbe Interact. 19, 557–563. [DOI] [PubMed] [Google Scholar]
- Boisnard, A. , Albar, L. , Thiéméle, D. , Rondeau, M. and Ghesquière, A. (2007) Evaluation of genes from eIF4E and eIF4G multigenic families as potential candidates for partial resistance QTLs to Rice yellow mottle virus in rice. Theor. Appl. Genet. 116, 53–62. [DOI] [PubMed] [Google Scholar]
- Brugidou, C. , Holt, C. , Yassi, M.N. , Zhang, S. , Beachy, R. and Fauquet, C. (1995) Synthesis of an infectious full‐length cDNA clone of Rice yellow mottle virus and mutagenesis of the coat protein. Virology, 206, 108–115. [DOI] [PubMed] [Google Scholar]
- Elena, S.F. and Lenski, R.E. (2003) Evolution experiments with micro‐organisms: the dynamics and genetic bases of adaptation. Nature Rev. Genet. 4, 457–469. [DOI] [PubMed] [Google Scholar]
- Fargette, D. , Pinel, A. , Traoré, O. , Ghesquière, A. and Konaté, G. (2002) Emergence of resistance‐breaking isolates of Rice yellow mottle virus during serial inoculations. Eur. J. Plant Pathol. 108, 585–591. [Google Scholar]
- Fargette, D. , Pinel, A. , Abubakar, Z. , Traoré, O. , Brugidou, C. , Fatogoma, S. , Hébrard, E. , Choisy, M. , Séré, Y. , Fauquet, C. and Konaté, G. (2004) Inferring the evolutionary history of Rice yellow mottle virus from genomic, phylogenetic, and phylogeographic studies. J. Virol. 78, 3252–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fargette, D. , Pinel, A. , Rakotomalala, M. , Sangu, E. , Traoré, O. , Sérémé, D. , Sorho, F. , Issaka, S. , Hébrard, E. , Séré, Y. , Kanyeka, Z. and Konaté, G. (2008) Rice yellow mottle virus, an RNA plant virus, evolves as rapidly as most RNA animal viruses. J. Virol. 82, 3584–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French, R. and Stenger, D.C. (2003) Evolution of wheat streak mosaic virus: dynamics of population growth within plants may explain limited variation. Annu. Rev. Phytopathol. 41, 199–214. [DOI] [PubMed] [Google Scholar]
- Garcia‐Arenal, F. and McDonald, B.A. (2003) An analysis of the durability of resistance to plant viruses. Phytopathology, 93, 941–952. [DOI] [PubMed] [Google Scholar]
- Gillespie, J.H. (2004) Population Genetics: a Concise Guide, 2nd edn. Baltimore, MD: The Johns Hopkins University Press. [Google Scholar]
- Harrison, B.D. (1981) Plant virus ecology: ingredients, interactions and environmental influences. Ann. Appl. Biol. 99, 195–209. [Google Scholar]
- Hartl, D. and Clark, A. (2007) Principles of Population Genetics, 4th edn. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Hébrard, E. , Pinel‐Galzi, A. , Bersoult, A. , Siré, C. and Fargette, D. (2006) Emergence of a resistance‐breaking isolate of Rice yellow mottle virus during serial inoculations is due to a single substitution in the genome‐linked viral protein VPg. J. Gen. Virol. 87, 1369–1373. [DOI] [PubMed] [Google Scholar]
- Hébrard, E. , Pinel‐Galzi, A. and Fargette, D. (2008) Virulence domain of the RYMV genome‐linked viral protein VPg towards rice rymv1‐2‐mediated resistance. Arch Virol. 153, 1161–1164. [DOI] [PubMed] [Google Scholar]
- Ioannidou, D. , Lett, J. , Pinel, A. , Assigbetse, K. , Brugidou, C. , Ghesquière, A. , Nicole, M. and Fargette, D. (2000) Responses of Oryza sativa japonica sub‐species to infection with Rice yellow mottle virus . Phys. Mol. Plant Pathol. 57, 177–188. [Google Scholar]
- Lenormand, T. , Roze, D. and Rousset, F. (2009) Stochasticity in evolution. Trends Ecol. Evol. 24, 157–166. [DOI] [PubMed] [Google Scholar]
- Li, H. and Roossinck, M.J. (2004) Genetic bottlenecks reduce population variation in an experimental RNA virus population. J. Virol. 78, 10 582–10 587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpica, M. , Fraile, A. , Moreno, I. , Obies, C. , Drake, J. and Garcia‐Arenal, F. (2002) The rate and character of spontaneous mutation in an RNA virus. Genetics 162, 1505–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maule, A. , Leh, V. and Lederer, C. (2002) The dialogue between viruses and hosts in compatible interactions. Curr. Opin. Plant Biol. 5, 4279–4284. [DOI] [PubMed] [Google Scholar]
- Ndjiondjop, M.N. , Albar, L. , Fargette, D. , Fauquet, C. and Ghesquière, A. (1999) The genetic basis of high resistance to Rice yellow mottle virus (RYMV) in cultivars of two cultivated rice species. Plant Dis. 83, 931–935. [DOI] [PubMed] [Google Scholar]
- N'Guessan, P. , Pinel, A. , Sy, A.A. , Ghesquière, A. and Fargette, D. (2001) Distribution , pathogenicity, and interactions of two strains of Rice yellow mottle virus in forested and savannah zones of West Africa. Plant Dis. 85, 59–64. [DOI] [PubMed] [Google Scholar]
- Parera, M. , Perez‐Alvarez, N. , Clotet, B. and Martinez, M. (2009) Epistasis among deleterious mutations in the HIV‐1 protease. J. Mol. Evol. 392, 243–250. [DOI] [PubMed] [Google Scholar]
- Pinel‐Galzi, A. , Rakotomala, M. , Sangu, E. , Sorho, F. , Kanyeka, Z. , Traoré, O. , Sérémé, D. , Poulicard, N. , Rabenantoandro, Y. , Séré, Y. , Konaté, G. , Ghesquière, A. , Hébrard, E. and Fargette, D. (2007) Theme and variations in the evolutionary pathways to virulence of an RNA plant virus species. PLoS Pathog. 3, e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakotomalala, M. , Pinel‐Galzi, A. , Albar, L. , Ghesquière, A. , Rabenantoandro, Y. , Ramavovololona, P. and Fargette, D. (2008) Resistance to Rice yellow mottle virus in germplasm in Madagascar. Eur. J. Plant Pathol. 122, 277–286. [Google Scholar]
- Rice, S.H. (2004) Evolutionary Theory: Mathematical and Conceptual Foundations. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Robaglia, C. and Caranta, C. (2006) Translation initiation factors: a weak link in plant RNA virus infection. Trends Plant Sci. 11, 40–45. [DOI] [PubMed] [Google Scholar]
- Rouzine, I. , Rodrigo, A. and Coffin, J. (2001) Transition between stochastic evolution and deterministic evolution in the presence of selection: general theory and application to virology. Microbiol. Mol. Biol. Rev. 65, 151–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacristan, S. and Garcia‐Arenal, F. (2008) The evolution of virulence and pathogenicity in plant pathogen populations. Mol. Plant Pathol. 9, 369–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacristan, S. , Fraile, A. , Malpica, J.M. and Garcia‐Arenal, F. (2003) Estimation of population bottlenecks during systemic movement of Tobacco mosaic virus in tobacco plants. J. Virol. 77, 9906–9911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjuan, R. , Agudelo‐Romero, P. and Santiago‐Elena, F. (2009) Upper‐limit mutation rate estimation for a plant virus. Biol. Lett. 5, 394–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner, G. , Stromberg, E. , Lacy, G. , Barker, K. and Pirone, T. (1992) Nomenclature and concepts of pathogenicity and virulence. Annu. Rev. Phytopathol. 30, 47–66. [DOI] [PubMed] [Google Scholar]
- Sorho, F. , Pinel, A. , Traoré, O. , Bersoult, A. , Ghesquière, A. , Hébrard, E. , Konaté, G. , Séré, Y. and Fargette, D. (2005) Durability of natural and transgenic resistances in rice to Rice yellow mottle virus . Eur. J. Plant Pathol. 112, 349–359. [Google Scholar]
- Traoré, O. , Sorho, F. , Pinel, A. , Abubakar, Z. , Banwo, O. , Maley, J. , Hébrard, E. , Winter, S. , Sere, Y. , Konaté, G. and Fargette, D. (2005) Processes of diversification and dispersion of Rice yellow mottle virus inferred from large‐scale and high‐resolution phylogeographic studies. Mol. Ecol. 14, 2097–2110. [DOI] [PubMed] [Google Scholar]
- Traoré, O. , Pinel, A. , Hébrard, E. , Gumedzoé, M.Y. , Fargette, D. , Traoré, A.S. and Konaté, G. (2006) Occurrence of resistance‐breaking isolates of Rice yellow mottle virus in West and Central Africa. Plant Dis. 90, 259–263. [DOI] [PubMed] [Google Scholar]
- Weinreich, D. , Watson, R. and Chao, L. (2005) Sign epistasis and genetic constraint on evolutionary trajectories. Evolution, 59, 1165–1173. [PubMed] [Google Scholar]
- Yeam, I. , Cavatorta, J.R. , Ripoll, D.R. , Kang, B.C. and Jahn, M.M. (2007) Functional dissection of naturally occurring amino acid substitutions in eIF4E that confers recessive potyvirus resistance in plants. Plant Cell, 19, 2913–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]


