SUMMARY
The host specificity of Ralstonia solanacearum, the causal organism of bacterial wilt on many solanaceous crops, is poorly understood. To identify a gene conferring host specificity of the bacterium, SL341 (virulent to hot pepper but avirulent to potato) and SL2029 (virulent to potato but avirulent to hot pepper) were chosen as representative strains. We identified a gene, rsa1, from SL2029 that confers avirulence to SL341 in hot pepper. The rsa1 gene encoding an 11.8‐kDa protein possessed the perfect consensus hrpII box motif upstream of the gene. Although the expression of rsa1 was activated by HrpB, a transcriptional activator for hrp gene expression, Rsa1 protein was secreted in an Hrp type III secretion‐independent manner. Rsa1 exhibited weak homology with an aspartic protease, cathepsin D, and possessed protease activity. Two specific aspartic protease inhibitors, pepstatin A and diazoacetyl‐d,l‐norleucine methyl ester, inhibited the protease activity of Rsa1. Substitution of two aspartic acid residues with alanine at positions 54 and 59 abolished protease activity. The SL2029 rsa1 mutant was much less virulent than the wild‐type strain, but did not induce disease symptoms in hot pepper. These data indicate that Rsa1 is an extracellular aspartic protease and plays an important role for the virulence of SL2029 in potato.
INTRODUCTION
When plants encounter pathogens in nature, the pathogens have a specific range of susceptible hosts that they are able to colonize for successful infection. To differentiate the specificity of host–pathogen interactions, host cultivars possessing various genotypes and different pathogen isolates are used. The host specificity results in races of pathogens, and is often determined by direct or indirect interactions between a resistance (R) protein from the host plant and an avirulence (Avr) protein from the pathogen (Chisholm et al., 2006). Most bacterial Avr proteins are effectors delivered into plant cells via hypersensitive response (HR) and pathogenicity (Hrp) type III secretion systems (T3SSs; Cornelis and Van Gijsegem, 2000; Mudgett, 2005; Staskawicz et al., 2001). As most known effectors possess enzymatic activities to promote bacterial virulence, T3SSs are essential for the successful infection of plant pathogenic bacteria (Mudgett, 2005; Staskawicz et al., 2001). In hosts resistant to certain bacterial pathogens, R proteins recognize bacterial Avr effectors, thereby conferring resistance (Chisholm et al., 2006).
We were interested in the host specificity of Ralstonia solanacearum, which causes bacterial wilt worldwide on economically important hosts, such as tomato, potato, tobacco, peanut and banana, by invading their xylem vessels (Hayward, 1991). Unlike other plant pathogenic bacteria, this bacterium uses different kinds of plants, rather than different cultivars within a given plant species, to differentiate races (Hayward, 1991). Ralstonia solanacearum has broad physiological and genetic diversities, which result in seven biovars and five races, depending on the host range (Hayward, 1991). Race 1 strains are the most prevalent and infect many solanaceous plants, including tomato, tobacco and hot pepper, whereas race 3 strains are highly virulent on potato, but are avirulent on hot pepper (Boucher et al., 1985).
To understand the molecular mechanisms of pathogenicity of R. solanacearum, hrp genes mainly encoding proteins for T3SS have been studied intensively (2004a, 2004b). On the basis of genome information of R. solanacearum race 1 strains GMI1000 and RS1000, inventories of T3SS‐dependent effectors have been reported (Mukaihara et al., 2010; Poueymiro and Genin, 2009). However, it has been difficult to demonstrate gene‐for‐gene interactions, primarily as a result of resistance sources against five races of R. solanacearum. An avrA gene from R. solanacearum strain AW1, responsible for inducing HR in tobacco, was cloned by complementation in tobacco pathogenic strain NC252 (Carney and Denny, 1990). The avrA mutant of strain AW1 abolished the ability to induce HR in tobacco, but failed to be pathogenic to tobacco (Carney and Denny, 1990). This was a result of the presence of an additional avirulence determinant on tobacco (popP1) in the strain. The gene avrA then behaves as a ‘classical’avr gene when this second avr gene is absent (Poueymiro and Genin, 2009).
Two avr genes, popP1 and popP2, of R. solanacearum were identified from the race 1 strain GMI1000 (Deslandes et al., 2003; Lavie et al., 2002). The expression of popP1 and popP2 is dependent on the key transcriptional regulator, HrpB, controlling most hrp gene expression (Deslandes et al., 2003; Lavie et al., 2002; Occhialini et al., 2005). The popP1 gene behaves as a typical avr gene by conferring avirulence to Petunia (Lavie et al., 2002). PopP1 and PopP2 belong to a YopJ/AvrRxv family and have structural similarities to ubiquitin‐like protease 1 and are T3SS effector proteins (Deslandes et al., 2003; Lavie et al., 2002). PopP2 is recognized by RRS1‐R of Arabidopsis thaliana ecotype Nd‐1, and the PopP2 and RRS1 proteins colocalize in the nucleus (Deslandes et al., 2003). However, it is still rare to demonstrate gene‐for‐gene resistance in natural hosts of R. solanacearum compared with other foliar bacterial pathogens.
In this study, we report a gene, rsa1, involved in the host specificity and virulence of R. solanacearum SL2029. We also show that the expression of rsa1 is dependent on HrpB, but that the secretion of Rsa1 is independent of T3SS. Rsa1 possesses aspartic protease activity, and most probably functions in the intercellular spaces of plant cells. This study provides an opportunity to understand how T3SS‐independent secreted proteins trigger host specificity.
RESULTS
Identification of rsa1
To identify a gene involved in the determination of the host specificity of R. solanacearum race 3, we chose strains SL341 belonging to phylotype I and SL2029 belonging to phylotype IV. Strain SL341 is virulent on tomato and hot pepper, but avirulent on potato and tobacco, and strain SL2029 is virulent on potato, but only weakly virulent on tomato and avirulent on hot pepper and tobacco (Table S1, see Supporting Information). We then mobilized the SL2029 genomic library into SL341 and isolated a cosmid clone pRS1 that changed the host specificity of SL341 (Fig. 1A). Strain SL341 carrying pRS1 was avirulent to hot pepper, whereas the wild‐type SL341 caused bacterial wilt on hot pepper (1, 2). Restriction enzyme digestion analysis showed that pRS1 had an insert of approximately 25 kb (Fig. 1A). When pRS13 carrying the 1.0‐kb HindIII/PstI fragment of pRS1 was mobilized into SL341, no bacterial wilt symptoms were observed on hot pepper (Fig. 2A). Complete DNA sequence analysis from the 1.0‐kb HindIII/PstI fragment of pRS13 identified one possible open reading frame (ORF), named rsa1 (Fig. 1B; GenBank accession number EU707808). The rsa1 gene consists of 330 bp and is predicted to encode an approximately 11.8‐kDa protein exhibiting 49% identity and 61% similarity to a putative transmembrane protein (gene RSp1467) from R. solanacearum GMI1000 (race 1), 48% identity and 63% similarity to a hypothetical protein (gene RSMK03424) from R. solanacearum MolK2 (race 2), and 40% identity and 54% similarity to a hypothetical protein (gene RRSL03065) from R. solanacearum UW551 (race 3). The amino acid sequence of Rsa1 had some degree of homology (26%–28% identity from the stretch of amino acids 6–69) with an aspartic protease cathepsin D in the MEROPS peptidase database (Rawlings et al., 2008). Furthermore, two aspartic acid residues characteristic of the aspartic acid protease active site were found at positions 54 and 59 (Fig. 1B). A perfect consensus hrpII box motif consisting of two direct repeat sequences (TTCG) separated by 16 bp was located 280 bp upstream of the putative translation start site of rsa1, and was similar to that found in R. solanacearum GMI1000 (Fig. 1B).
Figure 1.
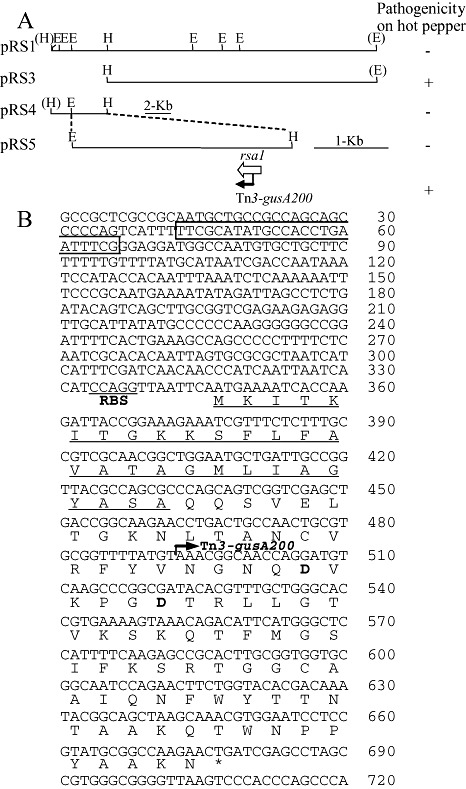
Identification of rsa1 from SL2029. (A) Restriction maps of plasmids carrying rsa1 and pathogenicity of SL341 carrying each plasmid on hot pepper (−, avirulent; +, virulent). (B) Nucleotide and amino acid sequences of rsa1. Vertical bars with arrows indicate the position and direction of the Tn3‐gusA insertion. Nucleotide sequence in the rectangular box indicates the perfect consensus hrpII box motif. Signal peptide sequences are underlined. Restriction endonuclease sites: H, HindIII; E, EcoRI. RBS denotes a putative ribosome binding site.
Figure 2.

Pathogenicity of Ralstonia solanacearum SL341 and SL2029 derivatives in hot pepper (A) and potato (B) plants: 1, water control; 2, SL341; 3, SL2029; 4, SL341(pRS13); 5, SL2029 rsa1::Tn3‐gusA200; 6, SL2029 rsa1::Tn3‐gusA200(pRS13). Photographs were taken 21 and 15 days after inoculation for hot pepper and potato plants, respectively.
Rsa1 is important for the virulence of SL2029
As rsa1 conferred avirulence to SL341 in hot pepper, we mutagenized pRS1 with Tn3‐gusA to generate an rsa1 mutant of SL2029. After screening and mapping of correct Tn3‐gusA insertions in pRS1, we found one insertion, rsa1::Tn3‐gusA200, inserted in the middle of the coding region of rsa1 (Fig. 1B). The plasmid pRS1::Tn3‐gusA200 was mobilized into SL2029, followed by marker exchange into the chromosome. When the resulting SL2029 rsa1::Tn3‐gusA200 mutant was inoculated into hot pepper, no wilting was observed 15 days after inoculation (Fig. 2A). When pRS13 carrying rsa1 was introduced into SL2029 rsa1::Tn3‐gusA200, no phenotypic changes were observed in infected hot pepper plants (i.e. the plants were still resistant to the pathogen) (Fig. 2A). This indicated that there were other avr genes in SL2029 that were able to elicit resistance in hot pepper.
To determine whether rsa1 plays any role in the virulence of SL2029 in potato, we inoculated the SL2029 rsa1::Tn3‐gusA200 mutant, together with the wild‐type strain and SL341. The SL2029 rsa1::Tn3‐gusA200 mutant was much less virulent than the wild‐type strain (Fig. 2B). When pRS13 carrying rsa1 was mobilized into the SL2029 rsa1::Tn3‐gusA200 mutant, virulence was fully recovered in potato (Fig. 2B). This indicates that Rsa1 is important for the virulence of SL2029 in potato.
We also monitored the growth of SL341 and SL2029, SL341 carrying pRS13, the SL2029 rsa1::Tn3‐gusA200 mutant and the SL2029 rsa1::Tn3‐gusA200 mutant carrying pRS13 in stems of potato plants for a period of 20 days after inoculation. Populations of SL341 slowly decreased after inoculation into potato stems (Fig. 3). The wild‐type SL2029 multiplied well in potato, reaching approximately 1 × 1010 colony‐forming units (cfu)/g stem tissue, 14 days after inoculation; however, the population of the SL2029 rsa1::Tn3‐gusA200 mutant increased slightly up to 20 days (Fig. 3). When pRS13 was transferred into the SL2029 rsa1::Tn3‐gusA200 mutant and SL341, bacterial cell populations of the SL2029 rsa1::Tn3‐gusA200 mutant carrying pRS13 increased, but did not reach the wild‐type level (Fig. 3).
Figure 3.
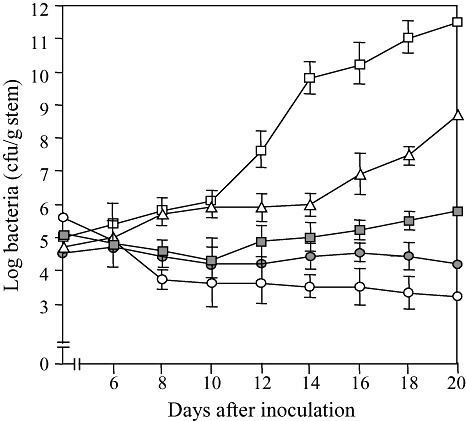
Growth patterns of Ralstonia solanacearum isolates SL341 ( ), SL2029 (
), SL2029 ( ), SL2029 rsa1::Tn3‐gusA200 (
), SL2029 rsa1::Tn3‐gusA200 ( ), SL341(pRS13) (
), SL341(pRS13) ( ) and SL2029 rsa1::Tn3‐gusA200(pRS13) (
) and SL2029 rsa1::Tn3‐gusA200(pRS13) ( ) in potato plants. Bacterial numbers were determined every 2 days after inoculation. The data are shown as the means of five replicates. The vertical bars indicate the error ranges.
) in potato plants. Bacterial numbers were determined every 2 days after inoculation. The data are shown as the means of five replicates. The vertical bars indicate the error ranges.
Regulation of rsa1 by HrpB
As a consensus hrpII box motif was found in the promoter region of the rsa1 gene, we measured β‐glucuronidase activity from the strains of SL2029 rsa1::Tn3‐gusA200, SL2029 hrpB::Ωrsa1::Tn3‐gusA200 and SL2029 hrpB::Ωrsa1::Tn3‐gusA200 carrying hrpB in pRS20 to determine whether the expression of rsa1 is regulated by HrpB. The rsa1 gene was expressed 40‐fold more strongly in the wild‐type background than in the hrpB::Ω background when the cells were grown in minimal medium supplemented with glutamate (MMG) (Fig. 4). The expression level of rsa1 in the hrpB::Ω background was increased by introducing pRS20 carrying the wild‐type hrpB gene into the SL2029 hrpB::Ωrsa1::Tn3‐gusA200 mutant (Fig. 4). These results clearly indicate that the expression of rsa1 depends on HrpB, and thus rsa1 belongs to the HrpB regulon.
Figure 4.
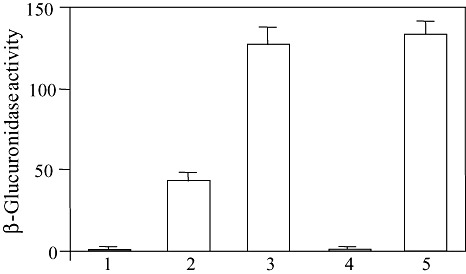
Expression levels of rsa1 in Ralstonia solanacearum SL2029 after growth for 24 h at 28 °C in MMG medium. β‐Glucuronidase activities were measured from strains SL2029 (1), SL2029 rsa1::Tn3‐gusA200 (2), SL2029 rsa1::Tn3‐gusA200(pRS20) (3), SL2029 rsa1::Tn3‐gusA200, hrpB::Ω (4) and SL2029 rsa1::Tn3‐gusA200, hrpB::Ω(pRS13) (5) as described in Experimental procedures and are averages of three experiments. One unit of β‐glucuronidase was defined as 1 nmol of 4‐methylumbelliferone released per bacterium per minute. Vertical bars indicate standard deviations.
Secretion of Rsa1 is independent of Hrp T3SS
To determine whether the secretion of Rsa1 is dependent on Hrp T3SS, Rsa1 secretion in the wild‐type and the hrcN (encoding the inner membrane‐associated ATPase in T3SS; Pozidis et al., 2003) mutant strains was analysed. A typical HR was observed when cells of the wild‐type SL2029 were infiltrated into potato leaves, whereas the hrcN mutant of SL2029 caused no HR (Fig. S1, see Supporting Information), which confirms that our hrcN mutant is a bona fide T3SS mutant. Cellular and extracellular proteins from the wild‐type SL2029 and the SL2029 rsa1::Tn3‐gusA200 and SL2029 hrcN::Tn3‐gusA20 mutants carrying either pLAFR3 as a control or pRS13 after growth in MMG medium were detected by immunoblotting using polyclonal antibodies raised against Rsa1 protein. Rsa1 was detected from the culture supernatants and from cellular fractions of the wild‐type SL2029 and the SL2029 hrcN::Tn3‐gusA20 mutant, whereas no Rsa1 was observed in either cellular or extracellular fractions from the SL2029 rsa1::Tn3‐gusA200 mutant (Fig. 5). When pRS13 carrying rsa1 was introduced into the SL2029 rsa1::Tn3‐gusA200 mutant, Rsa1 was detected from both cellular and extracellular fractions (Fig. 5). This indicates that the secretion of Rsa1 is independent of Hrp T3SS.
Figure 5.
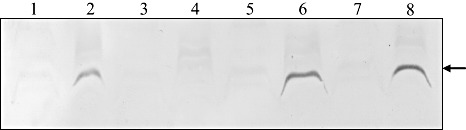
Secretion assay of Rsa1 by Western blot analysis using anti‐Rsa1: 1, cellular proteins of SL2029(pRS13); 2, culture supernatants from SL2029(pRS13); 3, cellular proteins of SL2029 rsa1::Tn3‐gusA200(pLAFR3); 4, culture supernatants from SL2029 rsa1::Tn3‐gusA200(pLAFR3); 5, cellular proteins of SL2029 rsa1::Tn3‐gusA200(pRS13); 6, culture supernatants from SL2029 rsa1::Tn3‐gusA200(pRS13); 7, cellular proteins of SL2029 hrcN::Tn3‐gusA20(pRS13); 8, culture supernatants from SL2029 hrcN::Tn3‐gusA20(pRS13). The arrow indicates Rsa1.
As Rsa1 is secreted from the wild‐type SL2029 and the SL2029 hrcN::Tn3‐gusA20 mutant carrying pRS13, we determined the N‐terminal amino acid sequences from purified Rsa1‐His to determine whether the protein has signal peptide sequences. The N‐terminal amino acid sequences of Rsa1‐His purified from Escherichia coli supernatants started with QQSVEL (Fig. 1B), which indicates that the first 29 amino acid residues were cleaved for the secretion in E. coli. This was consistent with the predicted signal peptide generated by the SignalP 3.0 Server.
Rsa1 is an extracellular aspartic protease
As Rsa1 shares homology with cathepsin D, we tested whether Rsa1 possesses aspartic protease activity in vitro using the casein‐BODIPY proteinase assay kit. Recombinant wild‐type Rsa1‐His exhibited high protease activity, but less than that of cathepsin D or renin (Fig. 6). The optimum pH and temperature for the protease activity of Rsa1‐His were pH 6.5 and 25 °C in 50 mm sodium phosphate buffer (Fig. 7). As cathepsin D belongs to the aspartic peptidase family A1 (Rawlings and Barrett, 1995), we substituted the two conserved aspartic acid residues at 54 and 59 with alanine to generate Rsa1D54A and Rsa1D59A mutant proteins, respectively. Both substitutions abolished Rsa1‐His protease activity (Fig. 6). These data indicate that Rsa1‐His is a protease, and that aspartic acid residues at positions 54 and 59 are important for protease activity.
Figure 6.
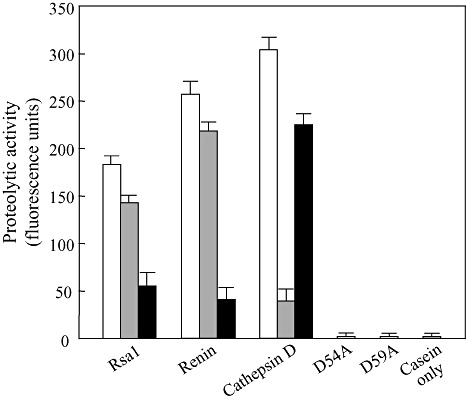
In vitro proteolytic activity assays of purified Rsa1, renin, cathepsin D and Rsa1 mutant proteins. Each enzyme was treated with no inhibitor (□), 2 mm pepstatin A ( ) in 50 mm sodium phosphate buffer (pH 6.5) at 25 °C for 20 min, or 2 mm diazoacetyl‐d,l‐norleucine methyl ester (DAN) (
) in 50 mm sodium phosphate buffer (pH 6.5) at 25 °C for 20 min, or 2 mm diazoacetyl‐d,l‐norleucine methyl ester (DAN) ( ) in 50 mm sodium phosphate buffer (pH 6.5) containing 0.13 mm CuSO4 at 25 °C for 1 h. Values represent the means of triplicate samples. Vertical bars indicate standard deviations.
) in 50 mm sodium phosphate buffer (pH 6.5) containing 0.13 mm CuSO4 at 25 °C for 1 h. Values represent the means of triplicate samples. Vertical bars indicate standard deviations.
Figure 7.
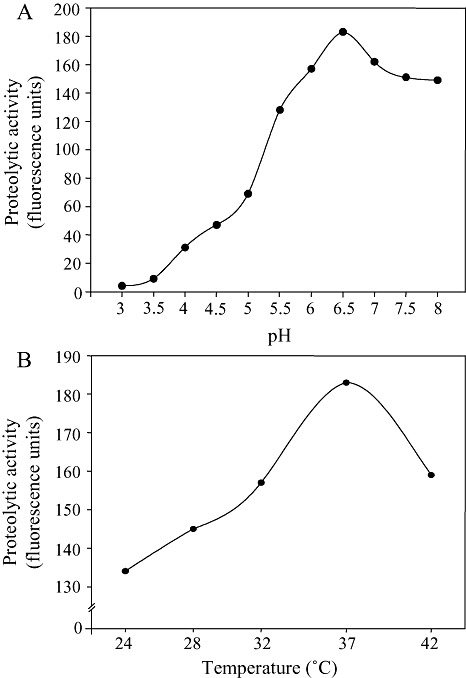
Protease activity of Rsa1 at different pH values (A) and different temperatures (B). Protease activity was measured with 500 nm Rsa1‐His in 50 mm sodium phosphate buffer (pH 6.5) at 25 °C for 1 h using a casein‐BODIPY proteinase assay kit.
As two neighbouring aspartic acid residues were critical for the activity of Rsa1‐His, we tested whether Rsa1‐His belongs to the aspartic proteases. To determine whether Rsa1 activity was inhibited by known aspartic acid protease inhibitors, pepstatin A and diazoacetyl‐d,l‐norleucine methyl ester (DAN), Rsa1‐His, renin and cathepsin D were treated with the two inhibitors. Pepstatin A inhibited 20%, 13% and 87%, and DAN inhibited 70%, 84% and 26%, of Rsa1‐His, renin and cathepsin D activities, respectively, under the given assay conditions (Fig. 6). Taken together with the mutational analysis of rsa1, these results clearly indicate that Rsa1‐His is an aspartic protease.
DISCUSSION
We identified Rsa1 as an Avr‐like protein of R. solanacearum on the basis of two phenotype changes: SL341 carrying rsa1 became avirulent on hot pepper and SL2029 rsa1 mutant became less virulent on potato. However, the SL2029 rsa1 mutant failed to be virulent on hot pepper, which is similar to the phenotype of the avrA mutant of strain AW1 (Carney and Denny, 1990). In this regard, it appears that rsa1 is not a typical avr gene identified from other plant pathogenic bacteria. Alternatively, the fact that the SL2029 rsa1 mutant is not virulent on hot pepper could be a result of other Avr factors, as observed in the avrA mutant (Poueymiro and Genin, 2009).
Bacterial growth of the SL2029 rsa1 mutant in potato was positively correlated with disease development. These phenotype changes are consistent with those observed from mutants of other avr genes (Bai et al., 2000; Lim and Kunkel, 2005; Ong and Innes, 2006; Ritter and Dangl, 1995). Similar phenotype changes were reported from the popP2 mutant of R. solanacearum GMI1000 studied in the resistant Arabidopsis ecotype Nd‐1 and susceptible ecotype Col‐5 (Deslandes et al., 2003). However, it was not clear whether PopP2 played any role in the virulence of the susceptible Arabidopsis ecotype Col‐5, as the bacterial population of the popP2 mutant of R. solanacearum GMI1000 in the Col‐5 ecotype increased as much as that in the wild‐type (Deslandes et al., 2003). This suggests that Rsa1 and PopP2 might have different roles in resistant and susceptible plants.
The biochemical functions of many Avr proteins identified from various plant pathogenic bacteria mainly constitute enzymatic activities (Axtell et al., 2003; Roden et al., 2004; Shao et al., 2003). In this regard, the fact that Rsa1 possesses aspartic protease activity is not an exception. However, Rsa1 does not belong to any known Avr effector protein group based on the following two reasons. First, Rsa1 is the first example of an aspartic protease that probably functions at the plant apoplast. Second, Rsa1 is not a T3SS substrate, unlike other bacterial Avr effector proteins. As Rsa1 has a signal peptide, as confirmed by N‐terminal amino acid sequencing, Rsa1 is possibly secreted by type II secretion systems (T2SSs). This finding was unexpected because most known bacterial Avr proteins are secreted via T3SSs (Kjemtrup et al., 2000; Yucel et al., 1994). However, there is an example of the secretion of a molecule for avirulence activity independent of T3SS: secretion of a molecule for AvrXa21 activity in Xanthomonas oryzae pv. oryzae depends on RaxABC type I secretion systems (Lee et al., 2009). In the biotrophic fungus Cladosporium fulvum, whose growth is confined to the apoplast space, Avr proteins have signal peptide sequences located in the apoplastic space (Joosten et al., 1994; Stergiopoulos and deWit, 2009).
The fact that the expression of rsa1 is dependent on HrpB implies that HrpB‐mediated gene regulation is not limited to genes encoding T3SSs and their substrates (Delaspre et al., 2007; Occhialini et al., 2005). We have reported previously that 16 proteins among extracellular proteomes of Burkholderia glumae are upregulated by HrpB, but secreted via T2SSs (Kang et al., 2008). Similarly, the expression of cysP2 encoding a cysteine protease homologue in X. oryzae pv. oryzae is under the control of a key hrp gene transcriptional regulator, HrpXo, equivalent to HrpB of R. solanacearum, but CysP2 secretion is dependent on T2SS (Furutani et al., 2004). Therefore, it is becoming clear that two well‐known hrp gene regulators, HrpB and HrpX, of Gram‐negative plant pathogenic bacteria are involved in the regulation of genes encoding not only T3SSs and their substrates, but also T2SS substrates.
Among many cloned bacterial avr genes, a relatively small number of Avr proteins have been characterized biochemically. Characterized Avr effectors are often proteases that modify plant target proteins to promote pathogenicity and suppress pathogen‐associated molecular pattern (PAMP)‐triggered immunity for successful infection in their susceptible hosts (Chisholm et al., 2006). Rsa1 appears to belong to the aspartic protease group A1, because the active site of the group has two neighbouring aspartic acid residues (Rawlings et al., 2008). However, unlike other members of the aspartic protease A1 family that are most active in acidic conditions, Rsa1‐His is most active at pH 6.5, as it was observed that renin is active at neutral pH (Rawlings et al., 2008). Considering the plant apoplastic conditions, the optimum pH for Rsa1 activity is reasonable.
Comparative genomic analysis between the race 1 strain GMI1000 and the race 3 strain UW551 (phylotype II) identified ORFs unique to the race 3 strain UW551 (Gabriel et al., 2006). Several putative avr gene candidates that could limit the host range of race 3 on tobacco were found in UW551; however, no rsa1 homologue is present in the list of putative UW551 T3SS effectors (Gabriel et al., 2006). This is not unexpected because putative avr candidates from UW551 are all T3SS‐dependent effectors. Nonetheless, it is intriguing that Rsa1 homologues are found in race 1, 2 and 3 in the database search. It would be interesting to determine whether the functions of these homologues are similar to those of Rsa1.
If avirulence mediated by Rsa1 of R. solanacearum SL2029 is one of the general gene‐for‐gene interaction models, such as RRS1‐R and PopP2, a counterpart of Rsa1 is likely to be identified from hot pepper and potato in the future. Substrates of Rsa1 in hot pepper and potato could be proteins secreted from plant cells or surface proteins present in the plant cell wall. Once the substrates of Rsa1 are identified, it is very likely that we will unveil a new type of Avr‐like protein function from plant pathogenic bacteria caused by previously unidentified protease functions of Rsa1.
EXPERIMENTAL PROCEDURES
Bacterial strains and culture conditions
The bacterial strains and plasmids used in this study are listed in Table S1 (see Supporting Information). Representative strains, SL341 and SL2029, of R. solanacearum were chosen on the basis of previous studies (Jeong et al., 2007). Escherichia coli strains were grown at 37 °C in Luria–Bertani broth (Sambrook et al., 1989), and R. solanacearum strains were grown at 28 °C in triphenyl tetrazolium chloride (TZC) medium (Kelman, 1954). MMG medium was used as the hrp‐inducing medium (Boucher et al., 1985). Antibiotics were used at the following concentrations: tetracycline, 10 µg/mL; rifampicin, 50 µg/mL; kanamycin, 50 µg/mL; ampicillin, 50 µg/mL; spectinomycin, 25 µg/mL.
Recombinant DNA techniques
All DNA manipulations were performed as described by Sambrook et al. (1989). To construct a genomic library of SL2029, total genomic DNA was partially digested with Sau3A1, and 20–30‐kb fragments were ligated into the BamHI site of pLAFR3, followed by transfection into E. coli HB101 as described previously (Kim et al., 2003). All plasmid clones used for mobilization into R. solanacearum were pLAFR3 derivatives.
Mutagenesis by marker exchange
A cosmid pRS20 carrying hrpB and other hrp genes was isolated from the genomic library of SL2029 by colony hybridization as described previously (Kim et al., 2003). The 1130‐bp SalI fragment containing the hrcQRS region of B. glumae was used as probe DNA in colony hybridization (Kang et al., 2008). The plasmids pRS1 and pRS20, carrying rsa1 and hrp genes, respectively, were mutagenized with Tn3‐gusA, and the exact insertion site and orientation of Tn3‐gusA in each mutant were determined as described previously (Kim et al., 2003). To construct an hrpB:: Ω mutant, the 2.0‐kb EcoRI Ω fragment from pHP45Ω was inserted into the EcoRI site of pRS43 (the 5.0‐kb HindIII fragment of pRS20 in pLAFR3ΔE) to produce pRS46. All marker exchange mutagenesis in SL2029 was performed as described previously (Kim et al., 2003). The marker‐exchanged mutation was confirmed by Southern hybridization (Kim et al., 2003).
β‐Glucuronidase assay
β‐Glucuronidase assays were performed as described previously, except that cells of R. solanacearum were grown in MMG medium for 24 h at 28 °C (Kim et al., 2003).
Plant assays
All plants used in this study were grown in a glasshouse in plastic pots containing commercial soil. For pathogenicity tests on tomato (Lycopersicon esculentum Mill. cv. Kwangsoo), potato (Solanum tuberosum L. cv. Daeji) and pepper (Capsicum annuum L. cv. Nokkwang), the roots of plants at the five‐ or six‐leaf stage were dipped into a bacterial suspension at approximately 1 × 108 cfu/mL for 2 h. To measure bacterial cell growth in potato, a bacterial suspension containing approximately 1 × 106 cfu/mL was injected into the stems of plants at the five‐ or six‐leaf stage. The inoculated plants were grown at 25–35 °C in a glasshouse and were sampled daily. One gram of stem tissue from around the injection point was macerated in 1 mL of sterile water, and the number of bacteria was determined by the plating method.
Secretion assay for Rsa1
The preparation of cellular and extracellular proteins from SL2029 derivatives was followed as described previously (Kim et al., 2003). A synthetic peptide from Rsa1 was used to raise anti‐mouse Rsa1 antibodies, and immunoblot analysis was performed using 1:5000 diluted Rsa1 antibodies as described previously (Kim et al., 2003).
Signal peptide prediction and N‐terminal sequencing of Rsa1
The presence and location of the signal peptide cleavage sites of Rsa1 were predicted using the SignalP 3.0 Server (Bendtsen et al., 2004). To purify Rsa1, the coding region of rsa1 was cloned into the NdeI/BamHI site of pET‐21a to generate pRS69 in which Rsa1 is fused to a His‐tag at the C‐terminus (Table S1, see Supporting Information). The Rsa1‐His tagged protein was purified from the culture supernatants using Ni‐NTA Superflow Columns (Qiagen, Valencia, CA, USA), according to the manufacturer's instructions. Rsa1‐His was separated by 15% sodium dodecylsulphate‐polyacrylamide gel electrophoresis, transferred to a polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA) and stained with Coomassie blue R‐250. The Rsa1‐His band was cut out of the membrane for N‐terminal amino acid sequencing using an Applied Biosystems 477A automatic sequencer (Applied Biosystems, Carlsbad, CA, USA).
Site‐directed mutagenesis of rsa1
Site‐directed mutagenesis of rsa1 was performed to change aspartic acid residues at positions 54 and 59 to alanine as described previously, resulting in Rsa1D54A and Rsa1D59A, respectively (Li and Wilkinson, 1997). Two individual complementary primers that contained the desired mutations were used for polymerase chain reaction (PCR) with pRS13 as template DNA (Table S2, see Supporting Information). After mutagenesis, the mutations were confirmed by DNA sequencing.
Protease activity assay of Rsa1
Protease activity was measured with 500 nm Rsa1‐His in 50 mm sodium phosphate buffer (pH 6.5) at 25 °C for 1 h using a casein‐BODIPY proteinase assay kit as described by the manufacturer (Invitrogen, Carlsbad, CA, USA). The specific inhibition of aspartic protease activity of Rsa1 was determined using pepstatin A (Sigma‐Aldrich Corp., St Louis, MO, USA) or DAN (Sigma). Cathepsin D (Sigma) and renin (Sigma) (Rawlings et al., 2008) were used at a concentration of 500 nm as positive controls. Each inhibitor at 2 mm was mixed with 500 nm Rsa1, 500 nm cathepsin D or 500 nm renin in 50 mm sodium phosphate buffer (pH 6.5) at 25 °C for 1 h, and fluorescence was measured in a fluorescence spectrophotometer F‐4500 (excitation, 505 nm; emission, 480 nm; Hitachi, Tokyo, Japan). Each assay was performed in triplicate.
Supporting information
Fig. S1 Hypersensitive response of potato injected with cells [1 × 108 colony‐forming units (cfu)/mL] of SL2029 and the hrcN mutant of SL2029. Plant responses were observed 24 h after injection.
Table S1 Bacterial strains and plasmids used in this study.
Table S2 Oligonucleotides used for site‐directed mutagenesis.
Supporting info item
ACKNOWLEDGEMENTS
This study was supported by the Technology Development Program for Agriculture and Forestry of the Ministry for Food, Agriculture, Forestry, and Fisheries, and the Crop Functional Genomics Center of the 21st Century Frontier R&D Program.
REFERENCES
- Axtell, M.J. , Chisholm, S.T. , Dahlbeck, D. and Staskawicz, B.J. (2003) Genetic and molecular evidence that the Pseudomonas syringae type III effector protein AvrRpt2 is a cysteine protease. Mol. Microbiol. 49, 1537–1546. [DOI] [PubMed] [Google Scholar]
- Bai, J. , Choi, S.‐H. , Ponciano, G. , Leung, H. and Leach, J. (2000) Xanthomonas oryzae pv. oryzae avirulence genes contribute differently and specifically to pathogen aggressiveness. Mol. Plant–Microbe Interact. 13, 1322–1329. [DOI] [PubMed] [Google Scholar]
- Bendtsen, J.D. , Nielsen, H. , Von Heijne, G. and Brunak, S. (2004) Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340, 783–795. [DOI] [PubMed] [Google Scholar]
- Boucher, C.A. , Barberis, P. , Trigalet, A.P. and Démery, D.A. (1985) Transposon mutagenesis of Pseudomonas solanacearum: isolation of Tn5‐induced avirulence mutants. J. Gen. Microbiol. 131, 2449–2457. [Google Scholar]
- Carney, B.F. and Denny, T.P. (1990) A cloned avirulence gene from Pseudomonas solanacearum determines incompatibility on Nicotiana tabacum at the host species level. J. Bacteriol. 172, 4836–4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm, S.T. , Coaker, G. , Day, B. and Staskawicz, B.J. (2006) Host–microbe interactions: shaping the evolution of the plant immune response. Cell, 124, 803–814. [DOI] [PubMed] [Google Scholar]
- Cornelis, G.R. and Van Gijsegem, F. (2000) Assembly and function of type III secretory systems. Annu. Rev. Microbiol. 54, 735–774. [DOI] [PubMed] [Google Scholar]
- Cunnac, S. , Boucher, C. and Genin, S. (2004a) Characterization of the cis‐acting regulatory element controlling HrpB‐mediated activation of the type III secretion system and effector genes in Ralstonia solanacearum . J. Bacteriol. 186, 2309–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnac, S. , Occhialini, A. , Barberis, P. , Boucher, C. and Genin, S. (2004b) Inventory and functional analysis of the large Hrp regulon in Ralstonia solanacearum: identification of novel effector proteins translocated to plant host cells through the type III secretion system. Mol. Microbiol. 53, 115–128. [DOI] [PubMed] [Google Scholar]
- Delaspre, F. , Nieto Peñalver, C.G. , Saurel, O. , Kiefer, P. , Gras, E. , Milon, A. , Boucher, C. , Genin, S. and Vorholt, J.A. (2007) The Ralstonia solanacearum pathogenicity regulator HrpB induces 3‐hydroxyl‐oxindole synthesis. Proc. Natl. Acad. Sci. USA, 104, 15 870–15 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslandes, L. , Oliver, J. , Peeters, N. , Feng, D.X. , Khounlotham, M. , Boucher, C. , Somssich, I. , Genin, S. and Marco, Y. (2003) Physical interaction between RRS1‐R, a protein conferring resistance to bacterial wilt, and PopP2, a type III effector targeted to the plant nucleus. Proc. Natl. Acad. Sci. USA, 100, 8024–8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furutani, A. , Tsuge, S. , Ohnishi, K. , Hikichi, Y. , Oku, T. , Tsuno, K. , Inoue, Y. , Ochiai, H. , Kaku, H. and Kubo, Y. (2004) Evidence for HrpXo‐dependent expression of type II secretory proteins in Xanthomonas oryzae pv. oryzae . J. Bacteriol. 186, 1374–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel, D.W. , Allen, C. , Schell, M. , Denny, T.P. , Greenberg, J.T. , Duan, Y.P. , Flores‐Cruz, Z. , Huang, Q. , Clifford, J.M. , Presting, G. , González, E.T. , Reddy, J. , Elphinstone, J. , Swanson, J. , Yao, J. , Mulholland, V. , Liu, L. , Farmerie, W. , Patnaikuni, M. , Balogh, B. , Norman, D. , Alvarez, A. , Castillo, J.A. , Jones, J. , Saddler, G. , Walunas, T. , Zhukov, A. and Mikhailova, N. (2006) Identification of open reading frames unique to a select agent: Ralstonia solanacearum race 3 biovar 2. Mol. Plant–Microbe Interact. 19, 69–79. [DOI] [PubMed] [Google Scholar]
- Hayward, A.C. (1991) Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum . Annu. Rev. Phytopathol. 29, 65–87. [DOI] [PubMed] [Google Scholar]
- Jeong, Y. , Kim, J. , Kang, Y. , Lee, S. and Hwang, I. (2007) Genetic diversity and distribution of Korean isolates of Ralstonia solanacearum . Plant Dis. 91, 1277–1287. [DOI] [PubMed] [Google Scholar]
- Joosten, M.H.A.J. , Cozijnsen, T.J. and De Wit, P.J.G.M. (1994) Host resistance to a fungal tomato pathogen lost by a single base‐pair change in an avirulence gene. Nature, 367, 384–386. [DOI] [PubMed] [Google Scholar]
- Kang, Y. , Kim, J. , Kim, S. , Kim, H. , Lim, J.Y. , Kim, M. , Kwak, J. , Moon, J.S. and Hwang, I. (2008) Proteome analysis of total proteins regulated by HrpB from the plant pathogenic bacterium Burkholderia glumae . Proteomics, 8, 106–121. [DOI] [PubMed] [Google Scholar]
- Kelman, A. (1954) The relationship of pathogenicity in Pseudomonas solanacearum to colony appearance on a tetrazolium medium. Phytopathology, 44, 693–695. [Google Scholar]
- Kim, J.‐G. , Park, B.K. , Yoo, C.‐H. , Jeon, E. , Oh, J. and Hwang, I. (2003) Characterization of the Xanthomonas axonopodis pv. glycines Hrp pathogenicity island. J. Bacteriol. 185, 3155–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjemtrup, S. , Nimchuk, Z. and Dangl, J.L. (2000) Effector proteins of phytopathogenic bacteria: bifunctional signals in virulence and host recognition. Curr. Opin. Microbiol. 3, 73–78. [DOI] [PubMed] [Google Scholar]
- Lavie, M. , Shillington, E. , Eguiluz, C. , Grimsley, N. and Boucher, C. (2002) PopP1, a new member of the YopJ/AvrRxv family of type III effector proteins, acts as a host‐specificity factor and modulates aggressiveness of Ralstonia solanacearum . Mol. Plant–Microbe Interact. 15, 1058–1068. [DOI] [PubMed] [Google Scholar]
- Lee, S.‐W. , Han, S.‐W. , Sririyanum, M. , Park, C.‐J. , Seo, Y.‐S. and Ronald, P.C. (2009) A type I‐secreted, sulfated peptide triggers Xa21‐mediated innate immunity. Science, 326, 850–853. [DOI] [PubMed] [Google Scholar]
- Li, S. and Wilkinson, M.F. (1997) Site‐directed mutagenesis: a two‐step method using PCR and DpnI. Biotechniques, 23, 588–590. [DOI] [PubMed] [Google Scholar]
- Lim, M.T. and Kunkel, B.N. (2005) The Pseudomonas syringae avrRpt2 gene contributes to virulence on tomato. Mol. Plant–Microbe Interact. 18, 626–633. [DOI] [PubMed] [Google Scholar]
- Mudgett, M.B. (2005) New insights to the function of phytopathogenic bacterial type III effectors in plants. Annu. Rev. Plant Biol. 56, 509–531. [DOI] [PubMed] [Google Scholar]
- Mukaihara, T. , Tamura, N. and Iwabuchi, M. (2010) Genome‐wide identification of a large repertoire of Ralstonia solanacearum type III effector proteins by a new functional screen. Mol. Plant–Microbe Interact. 23, 251–262. [DOI] [PubMed] [Google Scholar]
- Occhialini, A. , Cunnac, S. , Reymond, N. , Genin, S. and Boucher, C. (2005) Genome‐wide analysis of gene expression in Ralstonia solanacearum reveals that the hrpB gene acts as a regulatory switch controlling multiple virulence pathways. Mol. Plant–Microbe Interact. 18, 938–949. [DOI] [PubMed] [Google Scholar]
- Ong, L.E. and Innes, R.W. (2006) AvrB mutants lose both virulence and avirulence activities on soybeans and Arabidopsis. Mol. Microbiol. 60, 951–962. [DOI] [PubMed] [Google Scholar]
- Poueymiro, M. and Genin, S. (2009) Secreted proteins from Ralstonia solanacearum: a hundred tricks to kill a plant. Curr. Opin. Microbiol. 12, 44–52. [DOI] [PubMed] [Google Scholar]
- Pozidis, C. , Chalkiadaki, A. , Gomez‐Serrano, A. , Stahlberg, H. , Brown, I. , Tampakaki, A.P. , Lustig, A. , Sianidis, G. , Politou, A.S. , Engel, A. , Panopoulos, N.J. , Mansfield, J. , Pugsley, A.P. , Karamanou, S. and Economou, A. (2003) Type III protein translocase: HrcN is a peripheral ATPase that is activated by oligomerization. J. Biol. Chem. 278, 25 816–25 824. [DOI] [PubMed] [Google Scholar]
- Rawlings, N.D. and Barrett, A.J. (1995) Families of aspartic peptidases, and those of unknown mechanism. Methods Enzymol. 248, 105–120. [DOI] [PubMed] [Google Scholar]
- Rawlings, N.D. , Morton, F.R. , Kok, C.Y. , Kong, J. and Barrett, A.J. (2008) MEROPS: the peptidase database. Nucleic Acids Res. 36, 320–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter, C. and Dangl, J.L. (1995) The avrRpm1 gene of Pseudomonas syringae pv. maculicola is required for virulence on Arabidopsis. Mol. Plant–Microbe Interact. 8, 444–453. [DOI] [PubMed] [Google Scholar]
- Roden, J. , Eardley, L. , Hotson, A. , Cao, Y. and Mudgett, M.B. (2004) Characterization of the Xanthomonas AvrXv4 effector, a SUMO protease translocated into plant cells. Mol. Plant–Microbe Interact. 17, 633–643. [DOI] [PubMed] [Google Scholar]
- Sambrook, J. , Fritsch, E.F. and Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Shao, F. , Golstein, C. , Ade, J. , Stoutemyer, M. , Dixon, J.E. and Innes, R.W. (2003) Cleavage of Arabidopsis PBS1 by a bacterial type III effector. Science, 301, 1230–1233. [DOI] [PubMed] [Google Scholar]
- Staskawicz, B.J. , Mudgett, M.B. , Dangle, J.L. and Galan, J.E. (2001) Common and contrasting themes of plant and animal diseases. Science, 292, 2285–2289. [DOI] [PubMed] [Google Scholar]
- Stergiopoulos, L. and deWit, P.J.G.M. (2009) Fungal effector proteins. Annu. Rev. Phytopathol. 47, 233–263. [DOI] [PubMed] [Google Scholar]
- Yucel, I. , Boyd, C. , Debnam, Q. and Keen, N.T. (1994) Two different classes of avrD alleles occur in pathovars of Pseudomonas syringae . Mol. Plant–Microbe Interact. 7, 131–139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Hypersensitive response of potato injected with cells [1 × 108 colony‐forming units (cfu)/mL] of SL2029 and the hrcN mutant of SL2029. Plant responses were observed 24 h after injection.
Table S1 Bacterial strains and plasmids used in this study.
Table S2 Oligonucleotides used for site‐directed mutagenesis.
Supporting info item


