SUMMARY
Vascular wilts caused by Verticillium spp. are very difficult to control and, as a result, are the cause of severe yield losses in a wide range of economically important crops. The responses of Arabidopsis thaliana mutant plants impaired in known pathogen response pathways were used to explore the components in defence against Verticillium dahliae. Analysis of the mutant responses revealed enhanced resistance in etr1‐1[ethylene (ET) receptor mutant] plants, but not in salicylic acid‐, jasmonic acid‐ or other ET‐deficient mutants, indicating a crucial role of ETR1 in defence against this pathogen. Quantitative polymerase chain reaction analysis revealed that the decrease in symptom severity shown in etr1‐1 plants was associated with significant reductions in the growth of the pathogen in the vascular tissues of the plants, suggesting that impaired perception of ET via ETR1 results in increased disease resistance. Furthermore, the activation and increased accumulation of the PR‐1, PR‐2, PR‐5, GSTF12, GSTU16, CHI‐1, CHI‐2 and Myb75 genes, observed in etr1‐1 plants after V. dahliae inoculation, indicate that the outcome of the induced defence response of etr1‐1 plants seems to be dependent on a set of defence genes activated on pathogen attack.
INTRODUCTION
Verticillium dahliae Kleb. is a soil‐borne fungal pathogen with a worldwide distribution, causing vascular disease in a wide range of plants, including vegetables (artichoke, aubergine, pepper, potato and tomato), fruits (grapevine, olive and strawberry), flowers (chrysanthemum), oilseed crops (sunflower), fibre crops (cotton, flax) and woody perennials (Pegg and Brady, 2002; Schnathorst, 1981). The control of V. dahliae is especially difficult because the fungus survives in the soil as resting structures, microsclerotia, for several years (Schnathorst, 1981), and there are no effective chemical treatments to combat the disease. Furthermore, as it lacks a high degree of host specificity, it can multiply and survive by colonizing several different plant species (Klimes et al., 2006; Krikun and Bernier, 1987; Subbarao et al., 1995).
On pathogen recognition by plants, several signal transduction pathways are activated. The role of the signalling pathways mediated by salicylic acid (SA), jasmonic acid (JA) and ethylene (ET) in the Arabidopsis innate immune response is well established (Glazebrook, 2005). However, not much is known about induced plant defence responses to soil‐borne pathogens. In some recent studies (Johansson et al., 2006; Tjamos et al., 2005; Veronese et al., 2003), efforts have been made to explore the signal transduction network controlling Arabidopsis resistance to Verticillium spp. by analysing the pathogen susceptibility, at different developmental stages, of mutant plants defective in ET, JA and SA pathways.
In some case studies, enhanced resistance or tolerance to V. dahliae infection was not observed in Arabidopsis genotypes affected in different steps of SA signalling, when compared with wild‐type (WT) plants (Veronese et al., 2003), whereas, in other studies involving V. longisporum, the SA mutants sid2‐1 and pad4‐1 were found to be less affected than WT plants (Johansson et al., 2006). The results obtained on the activity of the biocontrol agent Paenibacillus alvei K165 strain show, however, that the biocontrol‐induced response against V. dahliae in Arabidopsis is dependent on SA via NPR‐1 (Tjamos et al., 2005), which further suggests that SA has a positive influence on resistance. Moreover, JA insensitivity does not appear to influence Verticillium symptom development in Arabidopsis plants (Veronese et al., 2003). In addition, none of the jar1‐1, coi1‐16 or eds8‐1 A. thaliana mutants impaired in JA signalling show any enhancement of susceptibility when compared with WT in response to V. longisporum, which suggests an independence of JA signalling (Johansson et al., 2006). Of the A. thaliana ET‐insensitive mutants tested, some showed enhanced susceptibility, others had similar responses to WT plants and only etr1‐1 was more tolerant than WT on Verticillium inoculation (Johansson et al., 2006; Tjamos et al., 2005; Veronese et al., 2003). However, these studies were either performed in vitro (Johansson et al., 2006; Veronese et al., 2003), or the direct aim of the study was not the elucidation of the ETR1—Verticillium interaction (Tjamos et al., 2005). In addition, a correlation between Verticillium spp. endophytic levels and disease severity in tolerant and susceptible Verticillium spp.–host interactions has never been reported, as it was found that Verticillium spp. colonization reached the same extent in both types of interaction (Brandt et al., 1984; Corsini et al., 1988; Gold et al., 1996; Heinz et al., 1998; Lynch et al., 1997; Schnathorst, 1981; Veronese et al., 2003).
The ability of plants to induce the expression of defence genes in response to V. dahliae via the SA or ET/JA response pathway remains unclear. In a study of the Arabidopsis–V. dahliae interaction, no transcripts of PR‐1 or PDF1.2, two common marker genes for SA, JA and ET signalling pathways, were observed (Veronese et al., 2003); however, in more recent studies, overexpression of the SA, JA and ET marker genes, PR‐1, PR‐2, PR‐4, PR‐5 and PDF1.2 (Johansson et al., 2006; Tjamos et al., 2005), was observed on Verticillium spp. infection.
The published data on the A. thaliana—V. dahliae interaction indicate that the molecular mechanisms that control resistance to this pathogen remain poorly understood. Therefore, there is much interest in determining the molecular basis of plant innate immunity against this major pathogen for a broad spectrum of important crops.
In view of the above, the objectives of this study were as follows: (i) to clarify in planta the role of SA, JA and ET signalling mutants in defence against V. dahliae; (ii) to determine the correlation between disease severity and degree of fungal proliferation in the vascular tissues by real‐time quantitative polymerase chain reaction (qPCR); and (iii) to monitor the expression levels of several defence genes involved in the SA, ET/JA and abscisic acid (ABA) response pathways in tolerant and susceptible V. dahliae—A. thaliana mutant interactions.
RESULTS
Impaired perception of ET via ETR1 reduces V. dahliae wilt symptoms
The role of SA, JA and ET signalling in defence against V. dahliae was assessed in A. thaliana mutant plants impaired in these pathways. The plant responses to this pathogen were evaluated by the recording of wilt symptoms.
The first V. dahliae symptoms appeared in the form of wilting, especially on older leaves at 5 days post‐inoculation (dpi), and were recorded until 30 dpi. Disease severity progressed rapidly in all genotypes, except for etr1‐1, which showed less prominent symptoms and slower disease development (1, 2). At 30 dpi, the disease incidence (percentage of plants with wilt symptoms) in etr1‐1 plants was 93% (data not shown) and the disease severity was 22%, whereas, in the other genotypes, the disease incidence ranged from 82% to 100% (data not shown) and the disease severity from 50% to 95% (Fig. 1A). The relative area under the disease progression curve (AUDPC) in etr1‐1 plants was 5.5%, whereas, in the other genotypes, it ranged from 21% to 43.5% (Fig. 1B).
Figure 1.
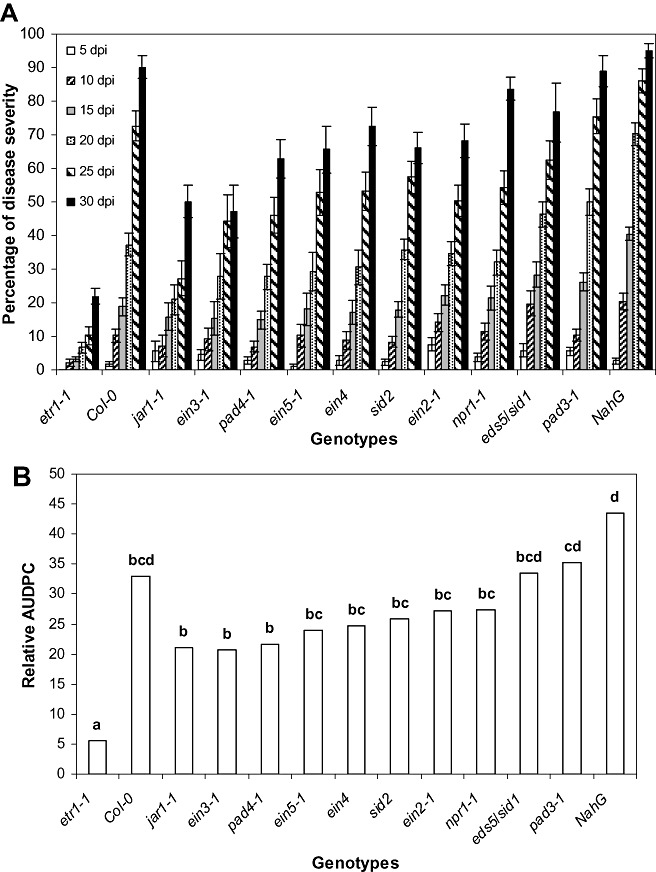
(A) Percentage disease severity of Arabidopsis thaliana plants after inoculation by Verticillium dahliae. Disease severity at each observation was calculated from the number of leaves that showed wilting as a percentage of the total number of leaves of each plant. Each genotype consisted of 15 plants and the experiment was repeated three times with similar results. The columns represent the means of 15 plants and the vertical bars indicate the standard errors. (B) Disease ratings were plotted over time to generate disease progress curves; subsequently, the area under the disease progress curve (AUDPC) was calculated by the trapezoidal integration method (Campbell and Madden, 1990), and disease was expressed as a percentage of the maximum possible area for the whole period of the experiment, which is referred to as the relative AUDPC (Korolev et al., 2001). Columns with different letters are statistically significantly different according to Tukey's multiple range test at P≤ 0.05.
Figure 2.

Typical symptoms caused by Verticillium dahliae on Arabidopsis salicylic acid (SA), jasmonic acid (JA) and ethylene (ET) mutants. The mutants etr1‐1, ein2‐1, ein3‐1, ein4, ein5‐1, jar1‐1, pad3‐1, pad4‐1, sid2, npr1‐1, NahG, eds5/sid1 and the corresponding wild‐type Col‐0 were inoculated with V. dahliae or mock inoculated. V. dahliae‐inoculated etr1‐1 mutants show reduced symptom development, including less severe stunting, wilting and tissue necrosis, when compared with Col‐0 and the other mutant plants. Photographs were taken at 20 days post‐inoculation.
qPCR fungal quantification in ET‐insensitive mutants
The data obtained from the pathogenicity tests revealed that only the etr1‐1 mutant plants were less affected by the pathogen of all the SA, JA and ET mutant plants tested and the WT plants. On the basis of these results, we focused our efforts on the investigation of the interaction of V. dahliae—ET‐insensitive mutants. To determine whether the reduced wilt symptoms of etr1‐1 mutants and the impaired perception of ET via ETR1 have an impact on fungal growth and colonization in vascular tissues, Col‐0 WT plants and the ET‐insensitive mutants etr1‐1, ein2‐1, ein3‐1, ein4 and ein5‐1 were inoculated with V. dahliae, and the level of fungal colonization was assessed in each genotype by real‐time qPCR. In these experiments, we included all ET‐insensitive mutants in an effort to obtain information on each component of the ET signalling pathway and to investigate whether the disease symptoms are associated with the amount of the pathogen in the vascular tissues of the plants.
qPCR analysis showed that, by 5 dpi, V. dahliae was present in the vascular tissues of all genotypes (Fig. 3). The presence of V. dahliae decreased at 10 dpi, except for Col‐0 and ein2‐1, and then increased steadily until 25 dpi (Fig. 3). The same colonization pattern was observed in all genotypes; however, in the ET receptor mutant plants etr1‐1, the levels of V. dahliae were significantly lower than in the WT plants and the rest of the other ET mutants at all sampling time points, except 5 dpi (Fig. 3). At 10 dpi, the amount of V. dahliae in etr1‐1 plants was at least 4.9 times less than that in the other genotypes, and at 15, 20 and 25 dpi the difference ranged from 4.4 to 6.6 times. Interestingly, the amount of V. dahliae in ein4 plants, the other ET receptor mutant, was significantly greater than that in etr1‐1 plants at each sampling time point, ranging from 5.2 times at 5 dpi to a maximum of 11.3 times at 20 dpi.
Figure 3.
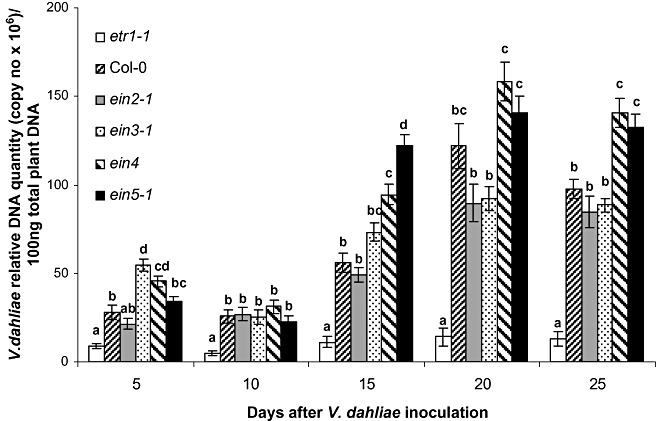
Quantification of the Verticillium dahliae DNA levels in the ethylene (ET) mutants etr1‐1, ein2‐1, ein3‐1, ein4, ein5‐1 and Col‐0 plants. The fungal DNA levels were estimated by quantitative polymerase chain reaction (qPCR) using total plant DNA isolated from the above‐ground parts of 10 plants per genotype and sampling at 5, 10, 15, 20 and 25 days post‐inoculation (dpi). The experiment was repeated three times. The columns represent the means of 30 plants and the vertical bars indicate the standard errors. At each sampling day, columns with different letters differ significantly according to Tukey's multiple range test at P≤ 0.05.
Transcriptional changes in response to V. dahliae infection
Our data from the pathogenicity tests and fungal quantification indicated that etr1‐1 plants showed increased resistance to V. dahliae. We reasoned that the identification of genes up‐regulated in etr1‐1 plants compared with WT plants would elucidate potential components of basal resistance operating in this mutant. We used Affymetrix Arabidopsis ATH1 GeneChips representing approximately 24 000 genes to examine the transcriptional profiles of WT and etr1‐1 plants. By comparing the transcriptional profiles of etr1‐1 with WT plants, we identified 95 genes up‐regulated by more than two‐fold in etr1‐1 plants (Table S1, see Supporting Information). The Affymetrix microarray experiment was not repeated; therefore, we considered these genes as candidates for verification. From the candidate genes, we selected, for qPCR analysis over time, a group of nine genes (Table S1) that were reported in the literature to be involved in the resistance of plants against pathogens. The qPCR expression analyses were performed at different time points after V. dahliae inoculation. In these experiments, we also included 23 marker genes of the SA, ET, JA and ABA defence response pathways (Table 1) in an effort to obtain more information about the defence responses of plants at different sampling days. The experimental set‐up included the ET receptor mutants etr1‐1 and ein4 and Col‐0 WT plants. We included ein4 plants in our analysis in an initial attempt to dissect the role of ET perception between ETR1 and EIN4 in the defence response of plants against V. dahliae, as both ETR1 and EIN4 encode ET receptors. ETR1 is a member of the type I and EIN4 is a member of the type II subfamily of ET receptors (Guo and Ecker, 2004), but, as shown in this study, they exhibited opposite defence responses against V. dahliae. qPCR analysis confirmed constitutively higher expression of the candidate genes GSTF12, GSTU16 (glutathione‐S‐transferases, GSTs), CHI‐1, CHI‐2 (chitinases), PR‐5 (thaumatin‐like), PR‐1, PR‐2 (β‐1,2‐glucanase) and the Myb75 transcription factor in etr1‐1 plants compared with WT and ein4 plants over time (Fig. 4). The mRNA level of this set of genes was induced at 2 dpi in all genotypes (Fig. 4). The expression level of GSTF12 and PR‐5 was greater in etr1‐1 mutants than in ein4 and WT plants, whereas there was no difference between treatments in the expression levels of the other genes at 2 dpi (Fig. 4A,G). Similarly, but to a greater extent, the expression levels of all genes at 6, 10 and 14 dpi were higher in the V. dahliae—etr1‐1 interaction compared with the other treatments. The genes GSTU16 and PR‐2 exhibited the strongest up‐regulation in etr1‐1 plants at 10 dpi, whereas GSTF12 and PR‐1 reached their maximum expression value at 14 dpi (Fig. 4A,B,E,F). CHI‐2 and PR‐5 exhibited similar expression patterns in all treatments, and showed the highest expression values at 10 and 14 dpi in etr1‐1 plants (Fig. 4D,G); the transcript levels of CHI‐1 and Myb75 were strongly expressed from 6 to 14 dpi (Fig. 4C,H). Interestingly, overexpression of the transcript levels of all examined genes was observed in all genotypes in response to V. dahliae, indicating the activation of plant defence mechanisms to fungal attack. It is worth mentioning that the expression of these genes in mock‐inoculated etr1‐1 plants was at least at the same level as that of mock‐inoculated WT and ein4 plants (data not shown).
Table 1.
List of examined genes and their primer pairs used in the quantitative polymerase chain reaction (qPCR) experiments.
| Gene | AGI code | Primer pairs |
|---|---|---|
| PR‐1 | AT2G14610 | 5′‐TCACAACCAGGCACGAGGAG‐3′ |
| 5′‐CACCGCTACCCCAGGCTAAG‐3′ | ||
| PR‐2 | AT3G57260 | 5′‐GCTCTCCGTGGCTCTGACATC‐3′ |
| 5′‐TACCGGAATCTGACACCATCTCTG‐3′ | ||
| PR‐3 | AT3G12500 | 5′‐TTATCACCGCTGCAAAGTCCT‐3′ |
| 5′‐TGGCGCTCGGTTCACAGTA‐3′ | ||
| PR‐4 | AT3G04720 | 5′‐ATAATCCGGCGCAGAATAAT‐3′ |
| 5′‐GCGGTCCAGCCATACTTG‐3′ | ||
| PR‐5 | AT1G75040 | 5′‐GACTCCAGGTGCTTCCCGACAG‐3′ |
| 5′‐ACTCCGCCGCCGTTACATCTT‐3′ | ||
| PDF1.2 | AT5G44420 | 5′‐CTGTTACGTCCCATGTTAAATCTACC‐3′ |
| 5′‐CAACGGGAAAATAAACATTAAAACAG‐3′ | ||
| CHI‐1 | AT2G43620 | 5′‐CGGCTGCCCAATCGTTCG‐3′ |
| 5′‐ACCGCGGCCGTAGTAGTCTTTTC‐3′ | ||
| CHI‐2 | AT2G43570 | 5′‐GCTTCGGTGCTTCCATCTCC‐3′ |
| 5′‐GCCATAGTAGCCCTTTCCTTGTG‐3′ | ||
| WRKY18 | AT4G31800 | 5′‐AGCGCAAGTGAGTTACGAG‐3′ |
| 5′‐ATCCGGGTCTTGTTTTCTTTTCT‐3′ | ||
| WRKY22 | AT4G01250 | 5′‐CCCGTAAACAAGTGGAGCGAAATA‐3′ |
| 5′‐CACCGGAGACGATGAATAAGTAGC‐3′ | ||
| WRKY30 | AT2G38470 | 5′‐AGTACGGACAAAAACAGGTGAAA‐3′ |
| 5′‐TCGTTGGACAATTAGGGAAAGT‐3′ | ||
| WRKY40 | AT1G80840 | 5′‐GCTTAAACCGCCACATCTCT‐3′ |
| 5′‐GTAGTCACCGGCACAGTCAAG‐3′ | ||
| WRKY53 | AT4G23810 | 5′‐CAGACGGGGATGCTACGGTTTTC‐3′ |
| 5′‐CGGCGAGGCTAATGGTGGTGT‐3′ | ||
| WRKY60 | AT2G25000 | 5′‐TAATCTTATGGAGGAGTTGC‐3′ |
| 5′‐ACCGTCGCTTTATCGTT‐3′ | ||
| ERF | AT5G50080 | 5′‐GTTTGGCTCGGGACGTTTGATAC‐3′ |
| 5′‐AGAGGAGGGGGAGGAGGAAGAAT‐3′ | ||
| ERF2 | AT3G16770 | 5′‐CGGGGAAACGGAGGAAGAGG‐3′ |
| 5′‐GCGATGACGGCGGAGGAGTAT‐3′ | ||
| Myb32 | AT4G34990 | 5′‐TCATCAAACTACATAGCCTTCTCG‐3′ |
| 5′‐CCCTTTTCTTAATAGCTTCCTCTT‐3′ | ||
| Myb75 | AT1G56650 | 5′‐GATCTTCTTCTTCGCCTTCA‐3′ |
| 5′‐CACGGTTCATGTTTCTTACTCA‐3′ | ||
| VSP1 | AT5G24780 | 5′‐TTTTACGCCAAAGGACTTGC‐3′ |
| 5′‐AATCCCGAGTTCCAAGAGGT‐3′ | ||
| VSP2 | AT5G24770 | 5′‐TCAGTGACCGTTGGAAGTTGTG‐3′ |
| 5′‐GTTCGAACCATTAGGCTTCAATATG‐3′ | ||
| ATGSTF8 | AT2G47730 | 5′‐CTCGAAGGTAAGCTCCAGAAAG‐3′ |
| 5′‐TCACCAGCCAAGAACTCAGATT‐3′ | ||
| ATGSTF12 | AT5G17220 | 5′‐GGTCAAGTTCCAGCCATAGA‐3′ |
| 5′‐TTGCCCAAAAGGTTCGT‐3′ | ||
| ATGSTU16 | AT1G59700 | 5′‐TCGCTCTTCGTCTCAAATCAGTGG‐3′ |
| 5′‐AATCGGTTTGTTGTTGTGGAGGAG‐3′ | ||
| ATGSL05 | AT4G03550 | 5′‐GAATGCATTATGGCCACCCTGAT‐3′ |
| 5′‐TTAAACCCGGCAAAGATGTCCTC‐3′ | ||
| RAB | AT3G02480 | 5′‐AGGATGCTGCTGCTTCA‐3′ |
| 5′‐TTAGTGGCTTTTGTTCAT‐3′ | ||
| RAB18 | AT5G66400 | 5′‐AGTGGTGGTGGCTTGGGAGGAAT‐3′ |
| 5′‐ACCACCGTAGCCACCAGCATCAT‐3′ | ||
| UGT73B1 | AT4G34138 | 5′‐CATATCGGTCCGCTTTCCTTAG‐3′ |
| 5′‐CTTGCCTTTTTGCCTCTTTCTG‐3′ | ||
| SDR1 | AT1G52340 | 5′‐AACTCGCTTTGGCTCATTTG‐3′ |
| 5′‐GTCAGTTCCACCCCTTTTAGATTC‐3′ | ||
| AtMYC2 | AT1G32640 | 5′‐TCATACGACGGTTGCCAGAA‐3′ |
| 5′‐AGCAACGTTTACAAGCTTTGATTG‐3′ | ||
| RD22 | AT5G25610 | 5′‐CTGTTTCCACTGAGGTGGCTAAG‐3′ |
| 5′‐TGGCAGTAGAACACCGCGA‐3′ | ||
| ABI1 | AT4G26080 | 5′‐CGGCAAAACTGCACTTCCAT‐3′ |
| 5′‐CACGAGCTCCATTCCACTGAA‐3′ | ||
| KIN1 | AT5G15960 | 5′‐GCTGGCAAAGCTGAGGAGAA‐3′ |
| 5′‐TTCCCGCCTGTTGTGCTC‐3′ |
AGI, Arabidopsis Genome Initiative.
Figure 4.
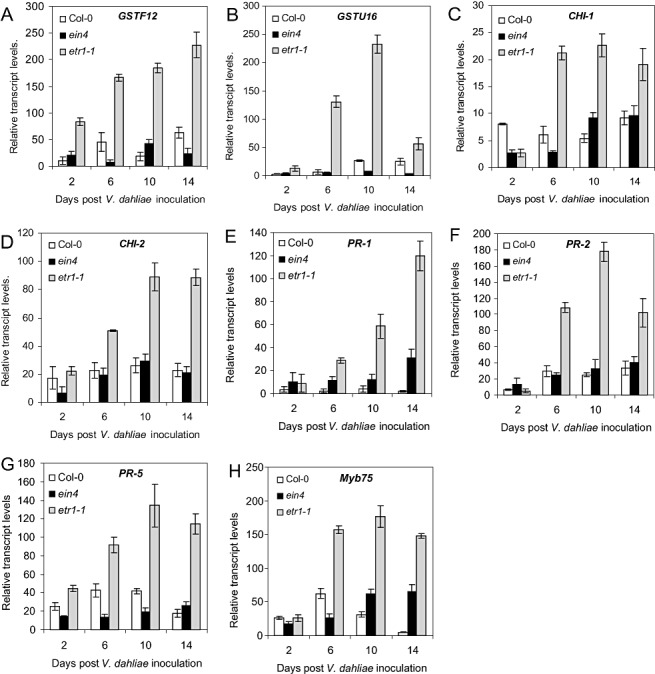
Expression of GSTF12 (A), GSTU16 (B), CHI‐1 (C), CHI‐2 (D), PR‐1 (E), PR‐2 (F), PR‐5 (G) and Myb75 (H) in Arabidopsis thaliana Col‐0, ein4 and etr1‐1 plants in response to infection with Verticillium dahliae. RNA was isolated from the above‐ground parts of 10 plants per genotype with sampling at 2, 6, 10 and 14 days post‐inoculation (dpi). Poly(A)+‐RNA was reverse transcribed to cDNA, and subjected to quantitative polymerase chain reaction (qPCR). Transcript levels in the different samples were normalized to those of the constitutive gene α‐tubulin. The relative mRNA level was calculated with respect to the level of the corresponding transcript in uninfected plants. Each genotype consisted of 80 plants (40 V. dahliae‐ and 40 mock‐inoculated) and the experiment was repeated twice. The columns represent the means of 20 plants and the vertical bars indicate the standard errors.
DISCUSSION
Verticillium dahliae is a soil‐borne plant pathogen with a worldwide distribution which causes vascular diseases resulting in severe yield losses in a wide range of economically important crops. Vascular wilts caused by V. dahliae constitute serious threats for their hosts, as there are no effective chemical control treatments. Although numerous studies have examined the interaction of Verticillium spp. with many hosts, the genetic basis and molecular mechanisms that control resistance to this pathogen remain poorly understood.
SA, JA and ET are plant hormones that act as signalling molecules in basal defence responses of plants, as well as in gene‐for‐gene‐mediated defence (Thomma et al., 2001). The role of these signalling pathways in the reaction of plants to Verticillium spp. has been documented in a number of recent studies (Johansson et al., 2006; Tjamos et al., 2005; Veronese et al., 2003), but their influence remains unclear.
Veronese et al. (2003) demonstrated in vitro that V. dahliae infection in A. thaliana SA mutants did not result in enhanced resistance or tolerance to the pathogen; however, in a more recent in vitro study implicating V. longisporum, the SA mutants sid2‐1 and pad4‐1 were less affected compared with WT plants, suggesting a role of SA in resistance to V. longisporum (Johansson et al., 2006). In addition, the role of SA has been proposed in the protection of cotton callus cells from V. dahliae toxins (Zhen and Li, 2004). In the present study, it was shown in planta that all the SA screened mutants had similar responses to Col‐0 after V. dahliae inoculation (1, 2), suggesting that SA is not involved in the resistance of plants against this vascular wilt pathogen. Our results are in agreement with the in vitro studies of Veronese et al. (2003), in which a V. dahliae isolate was used.
Mutants impaired in JA signalling (jar1‐1, coi1‐16 and eds8‐1) did not appear to influence disease severity caused by V. dahliae or V. longisporum in in vitro experiments (Johansson et al., 2006; Veronese et al., 2003). In our study, jar1‐1 plants showed similar levels of disease severity to WT plants after V. dahliae inoculation (1, 2), indicating that JA does not influence the disease outcome incited by this pathogen.
Furthermore, the role of ET is even more complicated in disease resistance mechanisms and it appears to be involved in only particular classes of pathogens (Hoffman et al., 1999; Knoester et al., 1998; Thomma et al., 1999). In recent in vitro studies (Johansson et al., 2006; Veronese et al., 2003), it was shown that, on Verticillium spp. inoculation, the etr1‐1 mutant was more tolerant than WT plants, whereas the ein3‐1 and eto1‐1 mutants showed similar responses, and the ein2‐1, ein4‐1 and ein6‐1 mutants exhibited enhanced susceptibility, compared with WT plants (Johansson et al., 2006). Of the ET‐insensitive mutants tested in planta in the present study, we observed that the etr1‐1 plants were the only resistant mutants compared with WT against V. dahliae (1, 2). ETR1 is a member of the type I and EIN4 is a member of the type II subfamily of ET receptors (Guo and Ecker, 2004), and this might explain the different responses of etr1‐1 and ein4 mutant plants to V. dahliae. The observed difference in disease severity between etr1‐1 and ein2‐1, ein3‐1 and ein5‐1 may originate from the fact that EIN2, EIN3 and EIN5 genes are positive regulators of ET responses, acting downstream of ETR1, which acts as a negative regulator of ET (Guo and Ecker, 2004).
A lack of a positive correlation between pathogen growth within plant tissues and symptom severity has been noted in several fungal (Brandt et al., 1984; Corsini et al., 1988; Gold et al., 1996; Heinz et al., 1998; Lynch et al., 1997; Schnathorst, 1981; Veronese et al., 2003), bacterial (Bent et al., 1992; Lund et al., 1998; O'Donnell et al., 2001) and viral (Cecchini et al., 2002) plant–pathogen interactions. A possible explanation of this interesting decoupling could be that symptoms can result from pathogen‐induced signalling events that cause changes in normal plant growth and development, such as accelerated flowering, senescence and programmed cell death (Cecchini et al., 2002; Dietrich et al., 1994; Lund et al., 1998; O'Donnell et al., 2001; Piloff et al., 2002). However, in this study, the extent of V. dahliae growth within the vascular tissues of plants was determined to be positively correlated (r 2= 0.744; d.f. = 28; P < 0.01) with disease severity. qPCR analysis revealed that the level of V. dahliae DNA in the ET mutant etr1‐1, which exhibited the most resistant phenotype against V. dahliae, was significantly lower than that in WT plants and the other ET mutant plants at each sampling day, except for 5 dpi (Fig. 3).
In the V. dahliae—Arabidopsis bioassays, it was observed that the pathogen DNA level reached a maximum at 20 dpi and then declined (Fig. 3), whereas the maximum disease severity was observed at 30 dpi (Fig. 1A). This failure to increase after 20 dpi probably reflects reduced sporulation in the stem and coincides with the cyclical periods of fungal elimination that characterize the lifestyle of V. dahliae in the vascular system of plants, such as tomato and oilseed rape (Chen et al., 2004; Eynck et al., 2007; Heinz et al., 1998). Overall, qPCR analysis revealed that the pathogen levels in the vascular tissues of resistant etr1‐1 plants remained substantially lower than in WT and the other ET mutant plants at all sampling days. It is evident that the etr1‐1 resistance response is based on the activation of defence mechanisms. These mechanisms have been partially investigated in plant—V. dahliae interactions, but never in etr1‐1 mutant plants.
To effectively intercept the attack by pathogens, plants have evolved complex defence strategies and responses (Dicke and Hilker, 2003; Jones and Dangl, 2006; Pieterse and Van Loon,, 2004) regulated by SA, JA and ET signal transduction pathways (Glazebrook, 2001; Pieterse and Van Loon, 1999; Reymond and Farmer, 1998; Thomma et al., 2001). SA, JA and ET accumulate in response to pathogen infection, leading to the activation of distinct sets of defence‐related genes (Glazebrook et al., 2003; Schenk et al., 2000). Earlier studies on the induction of SA, JA and ET marker genes in the Arabidopsis–Verticillium interaction exhibited conflicting results. In one case, no transcripts of PR‐1 and PDF1.2 were detected (Veronese et al., 2003), whereas, in other studies, the expression levels of PR‐1, PR‐2, PR‐4, PR‐5 and PDF1.2 genes were induced after Verticillium inoculation (Johansson et al., 2006; Tjamos et al., 2005). However, no data are available on the expression of marker genes of the different signalling pathways in SA‐, JA‐ and ET‐deficient mutant plants showing either a susceptible or tolerant response after V. dahliae inoculation.
Preliminary results of microarray genome analysis (DNA Vision S.A., Charleroi, Belgium) on RNA isolated from WT and etr1‐1 plants indicated an overexpression of a set of genes in etr1‐1 plants 6 days after V. dahliae inoculation (Table S1). The expression level of a group of 32 defence‐related genes (Table 1) was determined by qPCR analysis at different time points after inoculation with V. dahliae. The results revealed that eight genes (GSTF12, GSTU16, CHI‐1, CHI‐2, PR‐1, PR‐2, PR‐5, Myb75) were overexpressed in etr1‐1 plants compared with WT and ein4 plants (Fig. 4), suggesting that this set of genes might be possible determinants for the resistant phenotype against the pathogen. In a previous study based on microarrays, transcription profiles were determined for stem tissue of Craigella tomato plants infected with two different V. dahliae isolates, Vd1 and E6, resulting in a compatible and a tolerant interaction, respectively (Robb et al., 2007). In this study, as with our findings, up‐regulation of PR1 (P4, P6), PR2 (β‐1,3‐glucanase), endo‐β‐1‐3‐glucanase (SGN‐U144863), acidic 25‐kDa endochitinase (SGN‐U144297) and class IV chitinase (SGN‐U145299) was observed in both types of interaction, whereas only endochitinase 3 and a putative GST (SGN‐U143283) were elevated in the tolerant interaction (Robb et al., 2007). In a more recent study, tomato transcriptional responses to V. dahliae race1 were monitored after inoculation on MoneyMaker (susceptible genotype) and Motelle (resistant genotype against race 1 Verticillium strains) tomato plants using microarrays (van Esse et al., 2009). van Esse et al. (2009) noted the induction of PR5 and chitinase genes in both the roots and foliage of the incompatible interaction, which is in agreement with our results.
In the present study, the SA marker genes PR‐1, PR‐2 and PR‐5 were clearly up‐regulated in the etr1‐1–V. dahliae resistance response, even though SA mutant plants did not show a tolerant or a more susceptible phenotype than WT plants (Fig. 1), indicating the occurrence of cross‐talk between the different signalling pathways (De Vos et al., 2005; Koornneef and Pieterse, 2008; Koornneef et al., 2008). Previous studies have shown that PR‐1, PR‐2 and PR‐5 proteins have a role in resistance to fungal infection (Abad et al., 1996; Alexander et al., 1993; Li et al., 2003; Menu‐Bouaouiche et al., 2003; Niderman et al., 1995; Pressey, 1997; Wessels and Sietsma, 1981). PR‐1 represents a dominant group of PRs induced by pathogens or SA, and is commonly used as a marker for systemic acquired resistance. There is increasing evidence that PR‐1 proteins may play a role in the resistance to fungal infection, but their mode of action and relationship to other proteins are unknown (van Loon and van Strien, 1999). The PR‐2 family consists of β‐1,3‐glucanases that hydrolyse β‐1,3‐glucans, which are major fungal cell wall components (Wessels and Sietsma, 1981), therefore weakening the fungal cell wall and preventing hyphal colonization. However, reaction products of their enzymatic activity may act as elicitors of host defence responses (Menu‐Bouaouiche et al., 2003). Recent work has demonstrated the different timing and level of β‐1,3‐glucanase activity between a V. dahliae‐susceptible and V. dahliae‐tolerant cotton cultivar in response to treatment with a V. dahliae toxin, with the activity of β‐1,3‐glucanase increasing to a higher level at an earlier time point in the resistant cultivar (Li et al., 2003). The PR‐5 family includes proteins that are homologous to the protein thaumatin with in vitro‐demonstrated antifungal activity against V. dahliae (Abad et al., 1996; Pressey, 1997).
Another class of proteins that has been studied extensively in plant defence is the family of chitinases, because of their potential in defence reactions against various pathogens. Chitinases of all families were found to have antifungal activity in vitro (Melchers et al., 1994; Ponstein et al., 1994) against fungi that contain chitin in their cell walls. However, only a few fungi are sensitive to chitinases alone. Most of the fungi are sensitive to the synergistic action of chitinases and β‐1,3‐glucanases (Mauch et al., 1988; Sela‐Buurlage et al., 1993). In vivo, the constitutive expression of a chitinase in transgenic wheat resulted in enhanced resistance against Fusarium graminearum (Shin et al., 2008). In the present study, two putative chitinases have been found to be highly expressed in etr1‐1 plants after V. dahliae inoculation. qPCR analysis revealed an early transcript accumulation of the two putative chitinases at 2 dpi in all genotypes. The high expression levels of PR‐2, CHI‐1 and CHI‐2 in etr1‐1 plants may indicate a synergistic action of the enzymatic products of these genes against V. dahliae, without excluding the participation of PR‐1 and PR‐5 (Mauch et al., 1988; Sela‐Buurlage et al., 1993).
In addition to the aforementioned pathogenesis‐related proteins, several studies have indicated that plants respond to infection by pathogens with an increased expression of signalling or transcription factors, such as GST and Myb (Hahn and Strittmatter, 1994; Mauch and Dudler, 1993; Wagner et al., 2002). In a recent work, GST gene expression was induced more rapidly and more strongly in a V. dahliae‐resistant cotton cultivar than in a susceptible cultivar (Jia et al., 2007). In the present study, two GST genes, GSTF12 and GSTU16, were up‐regulated in all genotypes after V. dahliae inoculation. GSTF12 was found to be expressed more strongly in etr1‐1 plants compared with WT and ein4 plants at each sampling day. Interestingly, the expression level of the GSTU16 gene was not substantially up‐regulated in V. dahliae‐inoculated ein4 plants compared with mock‐inoculated ein4 plants. It is evident that, in addition to the observed differences in pathogenesis‐related genes (PR1, PR2, PR5 and chitinases), differences in the signalling cascade may also be responsible for the observed V. dahliae tolerance of etr1‐1 plants, as revealed by the overexpression of GSTF12 and GSTU16 genes in etr1‐1 plants.
Recent genetic analyses have shown that several genes encoding Myb transcription factors have important roles in plant immune responses, with some Myb factors binding to promoters of defence‐associated genes (Rushton and Somssich, 1998). A T‐DNA insertion in a Myb‐encoding gene of Arabidopsis resulted in enhanced disease symptoms after infection with several biotrophic and necrotrophic pathogens (Mengiste et al., 2003). In the present study, the transcription factor‐encoding gene Myb75 was induced at 2 dpi after V. dahliae inoculation in all genotypes. It was highly expressed in etr1‐1 plants at 6, 10 and 14 dpi.
A molecular basis for the control of increased resistance to V. dahliae in A. thaliana plants has been shown in the present study, indicating for the first time in the literature that impaired ET perception via ETR1 induces altered expression of a subset of defence‐related genes. These transcriptional changes led to reduced fungal growth in vascular tissues and symptom development in etr1‐1 plants. It is an open question whether V. dahliae employs ET perception via ETR1 to escape the activation of the plant defence mechanisms, as it is known that V. dahliae produces ET in vitro (Tzeng and DeVay, 1984). Verticillium dahliae constitutes a serious threat for a number of crops, as there is no chemical treatment; in the present study, we have attempted to elucidate and show the key role of the ETR1 gene in a plant defence mechanism against this vascular wilt pathogen as a step towards the understanding and uncoupling of a plant–pathogen interaction that threats and reduces the agricultural capita in a world that demands additional safer plant products.
EXPERIMENTAL PROCEDURES
Origin of seeds
Seeds of A. thaliana ecotype Columbia (Col‐0) and the transgenic line NahG (Delaney et al., 1994) were provided by Syngenta (Basle, Switzerland); Col‐0 accession mutants etr1‐1 (Bleecker et al., 1988), ein2‐1, ein3‐1, ein4, ein5‐1 (Roman et al., 1995), jar1‐1 (Staswick et al., 1992), npr1‐1 (Cao et al., 1997), pad3‐1 (Glazebrook and Ausubel, 1994) and pad4‐1 (Glazebrook et al., 1996) were obtained from the Nottingham Arabidopsis Stock Centre (Nottingham, UK), and eds5/sid1 and sid2 (Nawrath and Métraux, 1999) were provided by J. P. Métraux (University of Fribourg, Switzerland). All seeds were stored at 4 °C. Seeds were sown directly into 6‐cm‐diameter pots, each containing approximately 200 cm3 of soil (Potground; Klasmann, Deilmann, Germany). The pots were placed at 25 °C with a 14 h photoperiod at 65–70% relative humidity in a controlled‐environment growth chamber. The plants were watered and fed with a nutrient solution (XL 60, Hortifeeds, Lincoln, UK) as needed.
Fungal strains and inoculum preparation
The V. dahliae (Tjamos et al., 2005) isolate with known pathogenicity against A. thaliana plants was used in the experiments. The fungal strain was cryopreserved by freezing a suspension of 4 × 107 conidia/mL in 25% aqueous glycerol at −80 °C (Maniatis et al., 1982). Before being used, fungus was transferred to potato dextrose agar (Merck, Darmstadt, Germany) at 25 °C for 5 days. For the bioassays, conidia of the fungus were prepared in sucrose sodium nitrate (Sinha and Wood, 1968) in Erlenmeyer flasks of 250 mL capacity containing 100 mL of medium. The V. dahliae isolate was incubated in an orbital incubator at 120 rpm and 22 °C for 5 days. Suspensions were centrifuged at 10 000 g at 12 °C for 10 min, and resuspended by vortexing in sterile distilled water before treatment of the plants.
Verticillium dahliae—Arabidopsis bioassays
Three‐week‐old plants were inoculated with V. dahliae by root drenching 10 mL of 1 × 107 conidia/mL sterile distilled water (Tjamos et al., 2005). Control plants were mock inoculated with 10 mL of sterile distilled water. Disease severity at each observation was calculated from the number of leaves that showed wilting as a percentage of the total number of leaves of each plant, and was periodically recorded for 30 days after inoculation. Disease ratings were plotted over time to generate disease progression curves. AUDPC was calculated by the trapezoidal integration method (Campbell and Madden, 1990). Disease was expressed as a percentage of the maximum possible area for the whole period of the experiment, which is referred to as the relative AUDPC. The experiment was repeated three times with 15 replicates per experiment.
DNA extraction and qPCR fungal quantification
Ten plants from each treatment were harvested for real‐time qPCR analysis at 5‐day intervals from 5 dpi to 25 dpi. For each sampled plant, the above‐ground parts were cut at soil level, rinsed with sterile distilled water and ground to a fine powder using an autoclaved mortar and pestle in the presence of liquid nitrogen. Roots were sampled as well, but they are not included in the analysis as there was a statistical fluctuation of fungal quantification. The fluctuation probably reflected the impossibility of discriminating fungal biomass within the roots from fungus partly attached to the root surface through inoculation (Eynck et al., 2007). Total DNA was isolated according to Dellaporta et al. (1983), and was quantified by spectrophotometry and agarose gel electrophoresis. qPCR assays for the quantification of V. dahliae were conducted using the primer pair Vd‐F (5′‐CCGCCGGTCCATCAGTCTCTCTGTTTATAC‐3′) and Vd‐R (5′‐CGCCTGCGGGACTCCGATGCGAGCTGTAAC‐3′) designed on the ITS1 and ITS2 regions of the 5.8S ribosomal RNA gene (Z29511) of V. dahliae. qPCR was performed in a Stratagene Mx3005P™ thermocycler and, for the amplification reactions, QuantiFast™ SYBR® Green PCR (Qiagen, Valencia, CA, USA) master mix was used. The results were analysed with MxPro qPCR software. The levels of the Arabidopsisα2‐tubulin gene (M84696), detected using the primer pair TUBa‐F (5′‐TCCGCGAAACGAAAATG‐3′) and TUBa‐R (5′‐TGGCTCAAGATCAACAAAGAC‐3′), were used as internal standards to normalize the differences in DNA template amounts. PCR cycling started with an initial step of denaturation at 95 °C for 5 min, followed by 40 cycles of 95 °C for 10 s and 60 °C for 30 s. The primer specificity and formation of primer dimers were monitored by dissociation curve analysis. All qPCRs were performed in duplicate. To quantify the DNA levels of V. dahliae, a PCR product of the primer pair Vd‐F/Vd‐R was cloned into the commercial vector pGEM (Promega, Madison, WI, USA) and seven 10‐fold dilutions of the plasmids, ranging from 3 × 109 copies to 3 × 103 copies, were used to generate a standard curve (r 2≥ 0.99). The experiments were repeated three times.
RNA isolation and qPCR determination of transcript levels
For Verticillium‐caused wilt diseases, the resistance or susceptibility of the host plant is generally considered to be determined mainly by the cellular interactions of plant against fungus, occurring in the stem (Beckman, 1987; Beckman and Talboys, 1981; Heinz et al., 1998). In the present study, above‐ground parts from the pathogen‐ and mock‐inoculated plants were collected for RNA analysis. Samples were collected from 10 randomly selected plants per treatment per experiment and were immediately frozen in liquid nitrogen and stored at −80 °C. For each sample, total RNA was extracted from 100 mg of tissue ground with liquid nitrogen using TRIzol® Reagent (Invitrogen, Paisley, Renfrewshire, UK), according to the manufacturer's instructions. The RNA samples were treated with DNase I (Invitrogen) to eliminate traces of contaminating genomic DNA. The RNA concentration was measured on a Nanodrop ND‐1000 spectrophotometer (Saveen Werner, Malmö, Sweden). First‐strand cDNA was synthesized using SuperScript II (Invitrogen) following the manufacturer's procedure. Gene‐specific primers for the analysed genes were designed (Table 1). PCR efficiency for each amplicon was calculated by employing the linear regression method on log(fluorescence) per cycle number data, using LinRegPCR software (Remakers et al., 2003). The qPCRs were performed in duplicate, as described previously. The absence of nonspecific products and primer dimers was confirmed by the analysis of melting curves. The A. thalianaα2‐tubulin gene (M84696) was used as an internal standard to normalize small differences in cDNA template amounts. For data analysis, average threshold cycle (C t) values were calculated for each gene of interest (Pfaffl, 2001) on the basis of two independent biological samples.
Microarray sample preparation and data analyses
Total RNA from the same biological samples as used for qPCR at 6 dpi was employed for microarray analysis. Material harvested from mock‐inoculated WT and etr1‐1 plants at 6 dpi was used as the reference sample with which V. dahliae‐inoculated WT and etr1‐1 samples, respectively, were compared. RNA extraction was performed as described previously. Total RNA was sent to DNA Vision S.A. for further processing. RNA was hybridized onto the Affymetrix Arabidopsis ATH1 GeneChip Genome Array (Affymetrix, Inc. US, Santa Clara, CA, USA), which contains more than 22 500 probe sets representing approximately 24 000 gene sequences. Probe preparations, GeneChip hybridizations, washes and chip reading were conducted at DNA Vision S.A. following standard Affymetrix procedures.
To isolate candidate genes from the microarray expression data, the FiRe macro was used (Garcion et al., 2006). The selection of candidate genes was based on their fold‐change ratios after V. dahliae inoculation compared with mock‐inoculated plants. Genes showing at least twofold mRNA up‐regulation in etr1‐1 plants and not in WT plants were selected for further analysis by qPCR.
Statistics
Data on relative AUDPC and V. dahliae DNA quantification were transformed with the 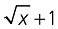 transformation before analysis of variance (anova) was applied. When a significant (P≤ 0.05) F‐test was obtained for treatments, data were subjected to means separation by Tukey's multiple range test.
transformation before analysis of variance (anova) was applied. When a significant (P≤ 0.05) F‐test was obtained for treatments, data were subjected to means separation by Tukey's multiple range test.
Supporting information
Table S1 Genes showing a twofold or greater difference in normalized expression in etr1‐1 plants inoculated with Verticillium dahliae relative to mock‐inoculated etr1‐1 plants from the Affymetrix ATH1 GeneChip experiment.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
ACKNOWLEDGEMENTS
I. S. Pantelides acknowledges IKY (State Scholarships Foundation) for a PhD scholarship.
REFERENCES
- Abad, L.R. , D'Urzo, M.P. , Liu, D. , Narasimhan, M.L. , Reuveni, M. , Zhu, J.K. , Niu, X. , Singh, N.K. , Hasegawa, P.M. and Bressan, R. (1996) Antifungal activity of tobacco osmotin has specificity and involves plasma membrane permeabilization. Plant Sci. 118, 11–23. [Google Scholar]
- Alexander, D. , Goodman, R.M. , Gut‐Rella, M. , Glascock, C. , Weymann, K. , Friedrich, L. , Maddox, D. , Ahl‐Goy, P. , Luntz, T. , Ward, E. and Ryals, J. (1993) Increased tolerance to two oomycete pathogens in transgenic tobacco expressing pathogenesis‐related protein 1a. Proc. Natl. Acad. Sci. USA, 90, 7327–7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman, C.H. (1987) The Nature of Wilt Diseases of Plants. St. Paul, MN: The American Phytopathological Society. [Google Scholar]
- Beckman, C.H. and Talboys, P.W. (1981) Anatomy of resistance In: Fungal Wilt Disease of Plants (Mace M.E., Bell A.A. and Beckman C.H., eds), pp. 431–486. New York: Academic Press. [Google Scholar]
- Bent, A.F. , Innes, R.W. , Ecker, J.R. and Staskawicz, B.J. (1992) Disease development in ethylene‐insensitive Arabidopsis thaliana infected with virulent and avirulent Pseudomonas and Xanthomonas pathogens. Mol. Plant–Microbe Interact. 5, 372–378. [DOI] [PubMed] [Google Scholar]
- Bleecker, A.B. , Estelle, M.A. , Somerville, C. and Kende, H. (1988) Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana . Science, 241, 1086–1089. [DOI] [PubMed] [Google Scholar]
- Brandt, W.H. , Lacy, M.L. and Horner, C.E. (1984) Distribution of Verticillium in stems of resistant and susceptible species of mint. Phytopathology, 74, 587–591. [Google Scholar]
- Campbell, C.L. and Madden, L.V. (1990) Introduction to Plant Disease Epidemiology. New York: Wiley. [Google Scholar]
- Cao, H. , Glazebrook, J. , Clarke, J.D. , Volko, S. and Dong, X.N. (1997) The Arabidopsis NPR‐1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell, 88, 57–63. [DOI] [PubMed] [Google Scholar]
- Cecchini, E. , Geri, C. , Love, A.J. , Coupland, G. , Covey, S.N. and Milner, J.J. (2002) Mutations that delay flowering in Arabidopsis de‐couple symptom response from cauliflower mosaic virus accumulation during infection. Mol. Plant Pathol. 3, 81–90. [DOI] [PubMed] [Google Scholar]
- Chen, P. , Lee, B. and Robb, J. (2004) Tolerance to a non‐host isolate of Verticillium dahliae in tomato. Physiol. Mol. Plant Pathol. 64, 283–291. [Google Scholar]
- Corsini, D.L. , Pavek, J.J. and Davis, J.R. (1988) Verticillium wilt resistance in noncultivated tuber‐bearing Solanum species. Plant Dis. 72, 148–151. [Google Scholar]
- De Vos, M. , Van Oosten, V.R. , Van Poecke, R.M.P. , Van Pelt, J.A. , Pozo, M.J. , Mueller, M.J. , Buchala, A.J. , Métraux, J.P. , Van Loon, L.C. , Dicke, M. and Pieterse, C.M.J. (2005) Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol. Plant–Microbe Interact. 18, 923–937. [DOI] [PubMed] [Google Scholar]
- Delaney, T. , Uknes, S. , Vernooij, B. , Friedrich, L. , Weymann, K. , Negrotto, D. , Gaffney, T. , Gut‐Rella, M. , Kessmann, H. , Ward, E. and Ryals, J. (1994) A central role of salicylic acid in plant disease resistance. Science, 266, 1247–1250. [DOI] [PubMed] [Google Scholar]
- Dellaporta, S.L. , Wood, J. and Hicks, J.B. (1983) A plant DNA minipreparation, version 2. Plant Mol. Biol. Rep. 1, 19–21. [Google Scholar]
- Dicke, M. and Hilker, M. (2003) Induced plant defences, from molecular biology to evolutionary ecology. Basic Appl. Ecol. 4, 3–14. [Google Scholar]
- Dietrich, R.A. , Delaney, T.P. , Uknes, S.J. , Ward, E.R. , Ryals, J.A. and Dangl, J.L. (1994) Arabidopsis mutants simulating disease resistance response. Cell, 77, 565–577. [DOI] [PubMed] [Google Scholar]
- Van Esse, H.P. , Fradin, E.F. , De Groot, P.J. , De Wit, P.J.G. and Thomma, B.P.H.J. (2009) Tomato transcriptional responses to a foliar and a vascular fungal pathogen are distinct. Mol. Plant–Microbe Interact. 22, 245–258. [DOI] [PubMed] [Google Scholar]
- Eynck, C. , Koopmann, B. , Grunewaldt‐Stoecker, G. , Karlovsky, P. and Von Tiedemann, A. (2007) Differential interactions of Verticillium longisporum and V. dahliae with Brassica napus detected with molecular and histological techniques. Eur. J. Plant Pathol. 118, 259–274. [Google Scholar]
- Garcion, C. and Applimath, J.P. (2006) FiRe and microarrays: a fast answer to burning questions. Trends Plant Sci. 11, 320–322. [DOI] [PubMed] [Google Scholar]
- Glazebrook, J. (2001) Genes controlling expression of defense responses in Arabidopsis . Curr. Opin. Plant Biol. 4, 301–308. [DOI] [PubMed] [Google Scholar]
- Glazebrook, J. (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 43, 205–227. [DOI] [PubMed] [Google Scholar]
- Glazebrook, J. and Ausubel, F.M. (1994) Isolation of phytoalexin‐deficient mutants of Arabidopsis thaliana and characterization of their interactions with bacterial pathogens. Proc. Natl. Acad. Sci. USA, 91, 8955–8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook, J. , Chen, W.J. , Estes, B. , Chang, H.S. , Nawrath, C. , Métraux, J.P. , Zhu, T. and Katagiri, F. (2003) Topology of the network integrating salicylate and jasmonate signal transduction derived from global expression phenotyping. Plant J. 34, 217–228. [DOI] [PubMed] [Google Scholar]
- Glazebrook, J. , Rogers, E.E. , Ausubel, F.M. (1996) Isolation of Arabidopsis mutants with enhanced disease susceptibility by direct screening. Genetics, 143, 973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold, J. , Lee, B. and Robb, J. (1996) Colonization of tomatoes by Verticillium dahliae, determinative phase II. Can. J. Bot. 74, 1279–1288. [Google Scholar]
- Guo, H. and Ecker, J.R. (2004) The ethylene signalling pathway: new insights. Curr. Opin. Plant Biol. 7, 40–49. [DOI] [PubMed] [Google Scholar]
- Hahn, C. and Strittmatter, G. (1994) Pathogen‐defense gene prp1‐1 from potato encodes an auxin responsive glutathione S‐transferase. Eur. J. Biochem. 226, 619–626. [DOI] [PubMed] [Google Scholar]
- Heinz, R. , Lee, S.W. , Saparno, A. , Nazar, R.N. and Robb, J. (1998) Cyclical systemic colonization in Verticillium‐infected tomato. Physiol. Mol. Plant Pathol. 52, 385–396. [Google Scholar]
- Hoffman, T. , Schmidt, J.S. , Zheng, X.Y. and Bent, A.F. (1999) Isolation of ethylene‐insensitive soybean mutants that are altered in pathogen susceptibility and gene‐for‐gene disease resistance. Plant Physiol. 119, 935–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, Z.Q. , Yuan, H.Y. and Li, Y.Z. (2007) NO and H2O2 induced by Verticillium dahliae toxins and its influence on the expression of GST gene in cotton suspension cells. Chin. Sci. Bull. 52, 1347–1354. [Google Scholar]
- Johansson, A. , Staal, J. and Dixelius, C. (2006) Early responses in the Arabidopsis–Verticillium longisporum pathosystem are dependent on NDR1, JA‐ and ET‐associated signals via cytosolic NPR‐1 and RFO1 . Mol. Plant–Microbe Interact. 19, 958–969. [DOI] [PubMed] [Google Scholar]
- Jones, J.D.G. and Dangl, J.L. (2006) The plant immune system. Nature, 444, 323–329. [DOI] [PubMed] [Google Scholar]
- Klimes, A. , Neumann, M.J. , Grant, S.J. and Dobinson, K.F. (2006) Characterization of the glyoxalase I gene from the vascular wilt fungus Verticillium dahliae . Can. J. Microbiol. 52, 816–822. [DOI] [PubMed] [Google Scholar]
- Knoester, M. , Van Loon, L.C. , Heuvel, J. , Henning, J. , Bol, J.F. and Linthorst, H.J.M. (1998) Ethylene‐insensitive tobacco lacks nonhost resistance against soil‐borne fungi. Proc. Natl. Acad. Sci. USA, 95, 1933–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef, A. and Pieterse, C.M.J. (2008) Cross talk in defense signalling. Plant Physiol. 146, 839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef, A. , Verhage, A. , Leon‐Reyes, A. , Snetselaar, R. , Van Loon, L.C. and Pieterse, C.M.J. (2008) Towards a reporter system to identify regulators of cross‐talk between salicylate and jasmonate signaling pathways in Arabidopsis. Plant Signal. Behav. 3, 543–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
Korolev, N.
,
Pérez‐Artés, E.
,
Bejarano‐Alcázar, J.
,
Rodríguez‐Jurado, D.
,
Katan, J.
,
Katan, T.
and
 , R.M. (2001) Comparative study of genetic diversity and pathogenicity among populations of Verticillium dahliae from cotton in Spain and Israel.
Eur. J. Plant Pathol. 107, 443–456.
[Google Scholar]
, R.M. (2001) Comparative study of genetic diversity and pathogenicity among populations of Verticillium dahliae from cotton in Spain and Israel.
Eur. J. Plant Pathol. 107, 443–456.
[Google Scholar] - Krikun, J. and Bernier, C.C. (1987) Infection of several crop species by two isolates of Verticillium dahliae . Can. J. Plant Pathol. 9, 241–245. [Google Scholar]
- Li, Y. , Zheng, X. , Tang, H. , Zhu, J. and Yang, J. (2003) Increase of b‐1,3‐glucanase and chitinase activities in cotton callus cells treated by salicylic acid and toxin of Verticillium dahliae Kleb. Acta. Bot. Sin. 45, 802–808. [Google Scholar]
- Van Loon, L.C. and Van Strien, E.A. (1999) The families of pathogenesis‐related proteins, their activities, and comparative analysis of PR‐1 type proteins. Physiol. Mol. Plant Pathol. 55, 85–97. [Google Scholar]
- Lund, S.T. , Stall, R.E. and Klee, H.J. (1998) Ethylene regulates the susceptible response to pathogen infection in tomato. Plant Cell, 10, 371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, D.R. , Kawchuk, M.L. , Hachey, J. , Bains, P.S. and Howard, R.J. (1997) Identification of a gene conferring high levels of resistance to Verticillium wilt in Solanum chacoense . Plant Dis. 81, 1011–1014. [DOI] [PubMed] [Google Scholar]
- Maniatis, T. , Fritsch, E.F. and Sambrook, J. (1982) Molecular Cloning, A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Mauch, F. and Dudler, R. (1993) Differential induction of distinct glutathione S‐transferases of wheat by xenobiotics and by pathogen attack. Plant Physiol. 102, 1193–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch, F. , Mauch‐Mani, B. and Boller, T. (1988) Antifungal hydrolases in pea tissue. 2. Inhibition of fungal growth by combinations of chitinase and β‐1,3‐glucanase. Plant Physiol. 88, 936–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers, L.S. , Apotheker‐de Groot, M. , Van Der Knaap, J. , Ponstein, A.S. , Sela‐Buurlage, M.B. , Bol, J.F. , Cornelissen, B.J.Z. , Elzen, P.J.M. and Linthorst, H.J.M. (1994) A new class of tobacco chitinases homologous to bacterial exo‐chitinases displays antifungal activity. Plant J. 5, 469–480. [DOI] [PubMed] [Google Scholar]
- Mengiste, T. , Chen, X. , Salmeron, J. and Dietrich, R. (2003) The BOTRYTIS SUSCEPTIBLE1 gene encodes an R2R3MYB transcription factor protein that is required for biotic and abiotic stress responses in Arabidopsis . Plant Cell, 15, 2551–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menu‐Bouaouiche, L. , Vriet, C. , Peumans, W.J. , Barre, A. , Van Damme, E.J.M. and Rouge, P. (2003) A molecular basis for the endo‐β1,3‐glucanase activity of the thaumatin‐like proteins from edible fruits. Biochimie, 85, 123–131. [DOI] [PubMed] [Google Scholar]
- Nawrath, C. and Métraux, J.P. (1999) Salicylic acid induction deficient mutants of Arabidopsis express PR‐2 and PR‐5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell, 11, 1393–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niderman, T. , Genetet, I. , Bruyère, T. , Gees, R. , Stinzi, A. , Legrand, M. , Fritig, B. and Mösinger, E. (1995) Pathogenesis‐related PR‐1 proteins are antifungal. Isolation and characterization of three 14 kilodalton proteins of tomato and of a basic PR‐1 of tobacco with inhibitory activity against Phytophthora infestans . Plant Physiol. 108, 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell, P.J. , Jones, J.B. , Antoine, F.R. , Ciardi, J. and Klee, H.J. (2001) Ethylene‐dependent salicylic acid regulates an expanded cell death response to a plant pathogen. Plant J. 25, 315–323. [DOI] [PubMed] [Google Scholar]
- Pegg, G.F. and Brady, B.L. (2002) Verticillium Wilts. Wallingford, Oxfordshire: CABI Publishing. [Google Scholar]
- Pfaffl, M.W. (2001) A new mathematical model for relative quantification in real‐time RT‐PCR. Nucleic Acids Res. 29, 2002–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse, C.M.J. and Van Loon, L.C. (1999) Salicylic acid‐independent plant defence pathways. Trends Plant Sci. 4, 52–58. [DOI] [PubMed] [Google Scholar]
- Pieterse, C.M.J. and Van Loon, L.C. (2004) NPR‐1, the spider in the web of induced resistance signaling pathways. Curr. Opin. Plant Biol. 7, 456–464. [DOI] [PubMed] [Google Scholar]
- Piloff, R.K. , Devadas, S.K. , Enyedi, A. and Raina, R. (2002) The Arabidopsis gain‐of‐function mutant dill spontaneously develops lesions mimicking cell death associated with disease. Plant J. 30, 61–70. [DOI] [PubMed] [Google Scholar]
- Ponstein, A.S. , Bles‐Vloemans, S.A. , Sela‐Buurlage, M.B. , Elzen, P.J.M. , Melchers, L.S. and Cornelissen, B.J.C. (1994) A novel pathogen‐ and wound‐inducible tobacco protein with antifungal activity. Plant Physiol. 104, 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressey, R. (1997) Two isoforms of NP24, a thaumatin‐like protein in tomato fruit. Phytochemistry, 44, 1241–1245. [DOI] [PubMed] [Google Scholar]
- Remakers, C. , Ruijter, J.M. , Deprez, R.H. and Moorman, A.F. (2003) Assumption‐free analysis of quantitative real‐time polymerase chain reaction (PCR) data. Neurosci. Lett. 339, 62–66. [DOI] [PubMed] [Google Scholar]
- Reymond, P. and Farmer, E.E. (1998) Jasmonate and salicylate as global signals for defense gene expression. Curr. Opin. Plant Biol. 1, 404–411. [DOI] [PubMed] [Google Scholar]
- Robb, J. , Lee, B. and Nazar, R.N. (2007) Gene suppression in a tolerant tomato–vascular pathogen interaction. Planta, 226, 299–309. [DOI] [PubMed] [Google Scholar]
- Roman, G. , Lubarsky, B. , Kieber, J.J. , Rothenberg, M. and Ecker, J.R. (1995) Genetic analysis of ethylene signal transduction in Arabidopsis thaliana. Five novel mutant loci integrated into a stress response pathway. Genetics, 139, 1393–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton, P.J. and Somssich, I.E. (1998) Transcriptional control of plant genes responsive to pathogens. Curr. Opin. Plant Biol. 1, 311–315. [DOI] [PubMed] [Google Scholar]
- Schenk, P.M. , Kazan, K. , Wilson, I. , Anderson, J.P. , Richmond, T. , Somerville, S.C. and Manners, J.M. (2000) Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc. Natl. Acad. Sci. USA, 97, 11655–11660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnathorst, W.C. (1981) Life cycle and epidemiology of Verticillium In: Fungal Wilt Diseases of Plants (Mace M.A., Bell A.A. and Beckman C.H., eds), pp. 81–111. New York: Academic Press. [Google Scholar]
- Sela‐Buurlage, M.B. , Ponstein, A.S. , Bres‐Vloemans, S.A. , Melchers, L.S. , Elzen, P.J.M. and Cornelissen, B.J.C. (1993) Only specific tobacco (Nicotiana tabacum) chitinases and β‐1,3‐glucanases exhibit antifungal activity. Plant Physiol. 101, 857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, S. , Mackintosh, C.A. , Lewis, J. , Heinen, S.J. , Radmer, L. , Dill‐Macky, R. , Baldridge, G.D. , Zeyen, R.J. and Muehlbauer, G.J. (2008) Transgenic wheat expressing a barley class II chitinase gene has enhanced resistance against Fusarium graminearum . J. Exp. Bot. 59, 2371–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha, A.K. and Wood, R.K.S. (1968) Studies on the nature of resistance in tomato plants to Verticillium albo‐atrum . Ann. Appl. Biol. 62, 319–327. [Google Scholar]
- Staswick, P.E. , Su, W.P. and Howell, S.H. (1992) Methyl jasmonate inhibition of root‐growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc. Natl. Acad. Sci. USA, 89, 6837–6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao, K.V. , Chassot, A. , Gordon, T.R. , Hubbard, J.C. , Bonello, P. , Mullin, R. , Okamoto, D. , Davis, R.M. and Koike, S.T. (1995) Genetic relationship and cross pathogenicities of Verticillium dahliae isolates from cauliflower and other crops. Phytopathology, 85, 1105–1112. [Google Scholar]
- Thomma, B.P.H. , Eggermont, K. , Tierens, K.F.M.J. and Broekaert, W.F. (1999) Requirement of functional Ethylene‐Insensitive 2 gene for efficient resistance of Arabidopsis to infection by Botrytis cinerea . Plant Physiol. 121, 1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma, B.P.H. , Penninckx, I.A.M.A. , Cammue, B.P.A. and Broekaert, W.F. (2001) The complexity of disease signaling in Arabidopsis. Curr. Opin. Immunol. 13, 63–68. [DOI] [PubMed] [Google Scholar]
- Tjamos, S.E. , Flemetakis, E. , Paplomatas, E.J. and Katinakis, P. (2005) Induction of resistance to Verticillium dahliae in Arabidopsis thaliana by biocontrol agent K‐165 and pathogenesis‐related proteins gene expression. Mol. Plant–Microbe Interact. 18, 555–561. [DOI] [PubMed] [Google Scholar]
- Tzeng, D.D. and DeVay, J.E. (1984) Ethylene production and toxigenicity of methionine and its derivatives with riboflavin in cultures of Verticillium, Fusarium and Colletotrichum species exposed to light. Physiol. Plant. 62, 545–552. [Google Scholar]
- Veronese, P. , Narasimhan, M.L. , Stevenson, R.A. , Zhu, J.K. , Weller, S.C. , Subbarao, K.V. and Bressan, R.A. (2003) Identification of a locus controlling Verticillium disease symptom response in Arabidopsis thaliana . Plant J. 35, 574–587. [DOI] [PubMed] [Google Scholar]
- Wagner, U. , Edwards, R. , Dixon, D.P. and Mauch, F. (2002) Probing the diversity of the Arabidopsis glutathione S‐transferase family. Plant Mol. Biol. 49, 515–532. [DOI] [PubMed] [Google Scholar]
- Wessels, J.G.H. and Sietsma, J.H. (1981) Fungal cell walls, a survey In: Encyclopedia of Plant Physiology. Plant Carbohydrates II, Vol. 13B (Tanner W. and Loewus F.A., eds), p. 352 Berlin: Springer Verlag. [Google Scholar]
- Zhen, X.H. and Li, Y.Z. (2004) Ultrastructural changes and location of beta‐1,3‐glucanase in resistant and susceptible cotton callus cells in response to treatment with toxin of Verticillium dahliae and salicylic acid. J. Plant Physiol. 161, 1367–1377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Genes showing a twofold or greater difference in normalized expression in etr1‐1 plants inoculated with Verticillium dahliae relative to mock‐inoculated etr1‐1 plants from the Affymetrix ATH1 GeneChip experiment.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item


