SUMMARY
Elsinoë fawcettii and E. australis are important pathogens of citrus. Both species are known to produce red or orange pigments, called elsinochrome. Elsinochrome is a nonhost‐selective phytotoxin and is required for full fungal virulence and lesion formation. This article discusses the taxonomy, epidemiology, genetics and pathology of the pathogens. It also provides a perspective on the cellular toxicity, biosynthetic regulation and pathological role of elsinochrome phytotoxin.
Taxonomy: Elsinoë fawcettii (anamorph: Sphaceloma fawcettii) and E. australis (anamorph: S. australis) are classified in the Phylum Ascomycota, Class Dothideomycetes, Order Myriangiales and Family Elsinoaceae.
Host range: Elsinoë fawcettii causes citrus scab (formerly sour orange scab and common scab) on various species and hybrids in the Rutaceae family worldwide, whereas E. australis causes sweet orange scab, primarily on sweet orange and some mandarins, and has a limited geographical distribution.
Disease symptoms: Citrus tissues infested with Elsinoë often display erumpent scab pustules with a warty appearance.
Toxin production: Elsinochrome and many perylenequinone‐containing phytotoxins of fungal origin are grouped as photosensitizing compounds that are able to absorb light energy, react with oxygen molecules and produce reactive oxygen species, such as superoxide and singlet oxygen. Elsinochrome has been documented to cause peroxidation of cell membranes and to induce rapid electrolyte leakage from citrus tissues. Elsinochrome biosynthesis and conidiation are coordinately regulated in E. fawcettii, and the environmental and physiological inducers commonly involved in both processes have begun to be elucidated.
INTRODUCTION
Citrus scab (formerly sour orange scab or common scab) is caused by Elsinoë fawcettii Bitancourt and Jenkins, and is one of the most important foliage fungal pathogens in many citrus‐producing areas worldwide. Citrus scab affects the fruit, leaves and twigs of many susceptible cultivars of citrus, including lemons, grapefruit and many tangerines and their hybrids, causing external blemishes and reducing the acceptability of the fruit for the fresh market. Citrus scab seldom reduces yield, but may reduce the value of the fruit by as much as 50% if uncontrolled. Sweet orange scab, caused by Elsinoë australis Bitancourt and Jenkins, affects the fruit but not the leaves of most sweet orange [C. sinensis (L.) Osbeck] and some mandarin (C. reticulate Blanco) cultivars. Elsinoë australis is common in humid citrus‐growing areas of Argentina, Bolivia, Brazil, Ecuador, Paraguay and Uruguay, but its presence has not been confirmed elsewhere. The introduction and establishment of E. australis in a new area could have quarantine implications for the marketing of fresh fruit.
Isolates of both E. fawcettii and E. australis grow slowly in culture, often producing red or brown pigments. The pigments have been identified as a nonhost‐selective, perylenequinone‐containing phytotoxin, called elsinochrome. The production of elsinochrome is a prerequisite for full fungal virulence and lesion formation. The cellular toxicity is highly dependent on the production of reactive oxygen species (ROS), such as superoxide and singlet oxygen, under aerobic and illuminated conditions. This article summarizes information on the taxonomy, host ranges and life cycles of E. fawcettii and E. australis. It also presents the current state of our understanding of the mode of action, biosynthesis and regulation of elsinochrome, as well as its role in fungal pathogenesis from molecular and genetic perspectives.
TAXONOMY AND CHARACTERISTICS
Both Elsinoë fawcettii Bitancourt and Jenkins (anamorph: Sphaceloma fawcettii Jenkins), causing citrus scab, and E. australis Bitancourt and Jenkins (anamorph: S. australis Bitancourt and Jenkins), causing sweet orange scab, belong to Kingdom Fungi, Phylum Ascomycota, Class Dothideomycetes, Subclass Dothideomycetidae, Order Myriangiales and Family Elsinoaceae. The causal agent of ‘Tryon's scab’ was once described as Sphaceloma fawcettii var. scabiosa and has been regrouped with S. fawcettii (Timmer et al., 1996). The teleomorph (sexual stage) of both Sphaceloma species was found only in Brazil (Bitancourt and Jenkins, 1936a, 1936b) and its role in the onset of disease remains largely unknown. Elsinoë fawcettii (5–6 µm × 10–12 µm) produces smaller ascospores (sexual spores) compared with those produced by E. australis (12–20 µm × 15–30 µm). Both species produce single‐celled, hyaline, elliptical conidia (asexual spores) with sizes of around 3–4 µm × 4–8 µm. Conidia can be propagated through budding. Elsinoë fawcettii, but not E. australis, also produces dark‐pigmented and spindle‐shaped conidia (Fig. 1) on scab lesions, which may germinate to form hyaline conidia (Timmer et al., 1996; Whiteside, 1975). Elsinoë fawcettii has been documented to form hyphae within hyphae, called intrahyphal hyphae or endohyphae (Kim and Hyun, 2007). In axenic culture, both E. fawcettii and E. australis grow slowly, lack extensive aerial hyphae and form colonies often with red or brown pigments. Fungal colonies and pigments vary substantially among isolates, providing no reliable traits for distinguishing E. fawcettii from E. australis. The two species can be separated by host range and by molecular analysis of random amplified polymerase DNA (RAPD) polymorphism and of the amplified internal transcribed spacer (ITS) of ribosomal DNA digested with various restriction endonucleases (2001, 2007; Tan et al., 1996; Timmer et al., 1996).
Figure 1.
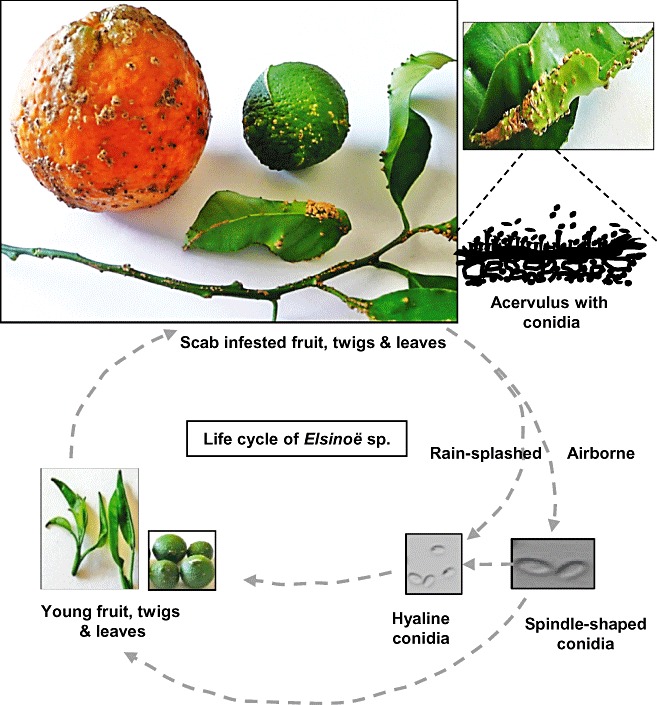
Disease cycle of citrus scab caused by Elsinoë fawcettii on fruit, twigs and leaves, showing corky lesions with erumpent scab pustules.
HOST RANGE AND PATHOGENETIC DIVERSITY
Elsinoë fawcettii has a number of pathogenic variants that are able to attack various citrus species and hybrids in the Rutaceae family. Citrus cultivars, such as ‘Temple’ and ‘Murcott’ tangors, ‘Minneola’ tangelos [Citrus reticulata Blanco ×C. sinensis (L.) Osbeck], lemons [C. limon (L.) Burm. F.] and grapefruit (C. paradisi Macf.), are most susceptible to E. fawcettii. Rootstocks, such as rough lemon (C. jambhiri Lush), sour orange (C. aurantium Lush) and ‘Rangpur’ lime (C. limonia Osbeck), are also susceptible to E. fawcettii. Elsinoë fawcettii has also been reported to affect ornamental citrus and rootstocks such as calamondins (C. madurensis Lour.) and Poncirus trifoliate (L.) Raf. Elsinoë fawcettii rarely attacks Persian lime (C. latifolia Tanaka), kumquats [Fortunella margarita (L.) Swingle], pummelos (C. maxima Burm. F. Merr.), citrons (C. medica L.) or limes (C. aurantifolia Christm. Swingle).
Early studies based on field observations and pathogenicity assays led to the recognition of two different biotypes of E. fawcettii (Whiteside, 1978). One type infected the leaves and fruit of citrus cultivars, including lemon, rough lemon, grapefruit, sour orange, mandarins and some cultivars of tangelos. This biotype also affected the fruit, but not the leaves, of sweet orange, and thus was designated as the ‘Florida Broad Host Range (FBHR)’ pathotype (Timmer et al., 1996). The second biotype was reported to affect only lemon, rough lemon, Cleopatra mandarin and grapefruit, and was designated as the ‘Florida Narrow Host Range (FNHR)’ pathotype. In addition, Tyron's pathotype (formerly S. fawcettii var. scabiosa), reported only in Australia, primarily affects lemon and rough lemon rootstock seedlings, as well as Cleopatra mandarin, but not sour orange. A new ‘Lemon’ pathotype affecting only rough lemon was also found among isolates collected from Australia (Timmer et al., 1996). Pathogenicity assays with the field isolates collected from Florida or other countries revealed that some isolates could not be grouped with any of the described pathotypes of E. fawcettii (Hyun et al., 2009; Wang et al., 2009a), implicating the presence of novel pathogenic variants or cryptic subspecies. Isolates of E. fawcettii have very complex diversities of host range.
In contrast with E. fawcettii, E. australis affects the fruit, but not the leaves, of most sweet orange and mandarin cultivars. Sweet orange scab is primarily restricted to South America, including Argentina, Bolivia, Brazil and Paraguay (Timmer et al., 1996). A novel ‘Natsudaidai (Citrus natsudaidai Hayata)’ pathotype of E. australis that is nonpathogenic to sweet orange has been identified recently (Hyun et al., 2001).
SYMPTOMS
Elsinoë spp. grow inter‐ and intracellularly within host cells. Citrus scab symptoms vary among cultivars and are highly dependent on the age of the tissue when infected. Scab lesions can be seen on leaves as early as 4 days after infection, and on fruit around 7 days after infection. The lesions emerge as tiny spots, often with an irregular and rough appearance. As the host tissues expand, the affected areas become elevated and form erumpent scab pustules comprising fungal and host tissues (Fig. 1). Electron microscopy reveals the formation of dense cork layers beneath the lesions (Kim et al., 2004). The raised warts, probably induced by hormone imbalance, often show light yellow or brown pigments in young tissues, and these darken as the tissues age or are colonized by other microorganisms. The affected leaves may develop lesions with warty or protuberant pustules emerging from one side of the leaf and a correspondingly depressed area on the opposite side. Severely affected leaves often become deformed and scabby. Similar scabby lumps are also formed on twigs and fruit. The affected grapefruit or sweet oranges tend to have a flatter corky appearance.
LIFE CYCLE AND EPIDEMIOLOGY
Elsinoë fawcettii overwinters in scab pustules that are formed in leaves, fruit and twigs (Fig. 1), whereas E. australis probably survives solely in fruit. Both species propagate primarily via conidia, which are produced in acervuli on the edge of scab pustules. The pathological role of ascospores is less certain. Elsinoë spp. are mainly dispersed by rain or water splash of conidia, although dispersal via wind is also possible. The severity of citrus scab is principally dependent on the frequency and duration of humidity, as well as the inoculum source (Agostini et al., 2003). In general, scab incidence is more severe in low‐lying areas with frequent wetting, compared with elevated areas. At optimum temperature (24–28°C), a short wetting period (2–3 h) is sufficient to induce conidia formation, germination and infection (Agostini et al., 2003; Whiteside, 1975). Citrus leaves are more susceptible to Elsinoë spp. soon after they develop from the bud, and become tolerant to infection 7–10 days after emergence. Fruit are vulnerable to infection 2 months after petal fall. Once infected, the pathogen reproduces rapidly inside the lesions and initiates new infection if environmental conditions are conducive and if susceptible hosts with young tissues are available. Infection by Elsinoë spp. occurs mainly during the spring flush and sporadically during the summer flush. Although scab occurring during summer flushes seldom causes severe damage, fungal inocula can build up for the following year and may result in an endemic problem.
CHEMICAL CONTROL
Citrus scab causes severe external blemishes on fruit and decreases their economic value. The disease must be controlled if the fruit are destined for the fresh market. Citrus scab is manageable with appropriate fungicide spray programmes that achieve sustained profitability. In Florida, two or three fungicide applications are required for scab control. The first spray is often applied when new flushes emerge in the early spring, and this is followed by a second application after petal fall and a third application approximately 3 weeks thereafter. If inoculum is low in a given area, the first spray can be disregarded. Copper fungicides, benomyl, ferbam, thiram, captafol and the sterol biosynthesis‐inhibiting fungicides—although some are not registered for use in citrus, and others have been discontinued—are effective for scab control. However, frequent applications of a fungicide may facilitate the occurrence of the fungicide‐resistant strains. Benomyl‐resistant strains of E. fawcettii are widespread in Florida (Whiteside, 1980) and probably in many of the citrus‐growing areas worldwide (Leki, 1994; Tyson and Fullerton, 2001). Apart from fungicides, the avoidance of overhead sprinkler irrigation and the planting of susceptible cultivars on higher ground or in well‐circulated areas may substantially reduce scab incidence.
ELSINOCHROME PIGMENTS PRODUCED BY ELSINOË SPECIES
Many Elsinoë species have long been known to produce red or yellow pigments in culture (Fig. 2). The water‐insoluble pigment, named ‘elsinochrome’, was first isolated from cultures of Elsinoë randii Jenkins and Bitanc. (anamorph: Sphaceloma randii) and characterized by Weiss et al. (1957). The chemical structure of elsinochrome was independently elucidated to be a derivative of 1,2‐dihydrobenzo‐perylenequinone (Fig. 2). The chemical and physical properties of elsinochrome have been studied in detail by several research groups (1969, 1970a; Mebius et al., 1990; Meille et al., 1989; Shirasugi and Misaki, 1992; Weiss et al., 1965). Elsinochrome consists of various derivatives (A, B, C and D) that have common phenolic quinones and vary only in their side‐chains (Weiss et al., 1987). Each elsinochrome contains interchangeable tautomers (Fig. 2). Elsinochromes A, B and C, containing a two‐fold rotation axis with highly symmetrical side‐groups, are red pigments. Elsinochrome D, probably derived from ‘C’ by the formation of a methylenedioxy ring, was identified and characterized from the pigment mixture of Elsinoë annonae Bitanc. and Jenkins (Kurobane et al., 1981; Lousberg et al., 1970b; Shirasugi and Misaki, 1992).
Figure 2.
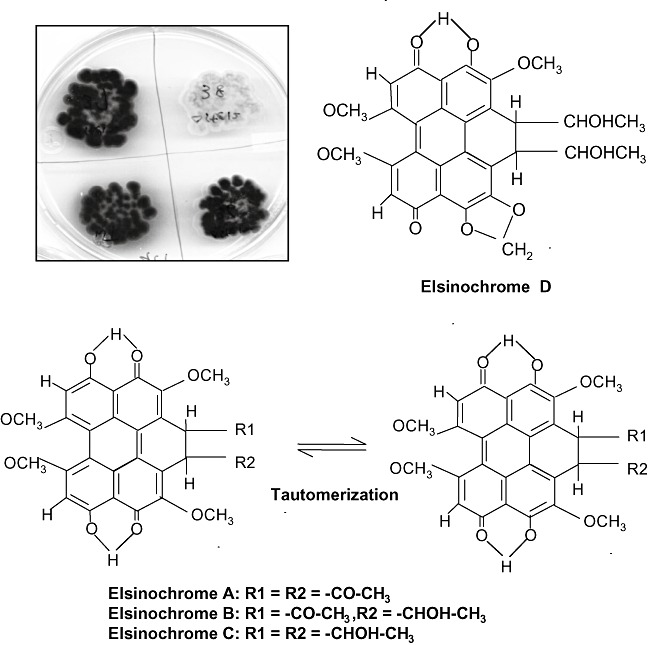
Accumulation of red‐ or yellow‐pigmented elsinochrome phytotoxin by the isolates of Elsinoë fawcettii. The fungal cultures were grown on potato dextrose agar in the light for 4 weeks. The chemical structures and tautomer of elsinochrome (1,2‐dihydrobenzo‐perylenequinone) A–D are also shown.
Some E. fawcettii isolates produce the main A, B or C derivatives, accompanied by small amounts of other pigments, whereas other isolates accumulate large amounts of ‘B’ or ‘C’ with little or no ‘A’ produced. Elsinochrome can be extracted from fungal mycelia with acetone or ethyl acetate. The separation of ‘A’, ‘B’ and ‘C’ derivatives can be achieved by thin‐layer (Fig. 3a) or high‐performance liquid chromatography using various adsorbents (Weiss et al., 1965). In addition to Elsinoë spp., elsinochrome has been reported to be produced by some isolates of the bamboo pathogen Hypocrella bambusae (Berk. and Broome) Sacc. and the bindweed biocontrol fungus Stagonospora convolvuli Dearness and House LA39 strain (Ahonsi et al., 2006; Ma et al., 2003). The latter is genetically distinct from Septoria sp. (Pfirter et al., 1999). A previously described ‘phycaron’ pigment produced by a fungus, which was mistakenly identified as Phyllosticta caryae Peck., was later recognized to be elsinochrome. The producing fungus is actually an asexual stage of Elsinoë randii Bitancourt and Jenkins (Jenkins and Bitancourt, 1938). Moreover, Pyrenochaeta terrestris (H.N. Hansen), a fungal pathogen causing pink root disease of onions, was once reported to synthesize elsinochrome (Kurobane et al., 1981). However, the identity of the producing fungus remains questionable because only one field‐collected isolate was able to produce the red pigments.
Figure 3.
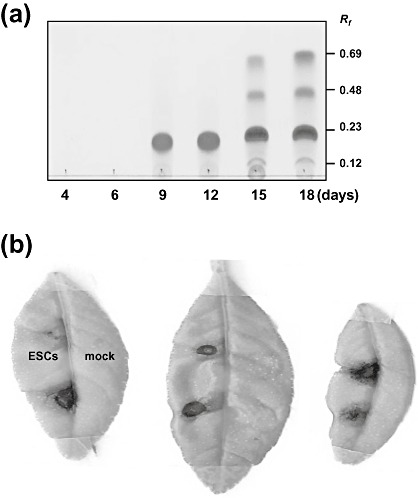
(a) Thin‐layer chromatography (TLC) analysis of elsinochrome produced by Elsinoë fawcettii, causing citrus scab. Elsinochrome was separated on silica gel using a solvent containing chloroform and ethyl acetate (1 : 1, v/v). (b) Induction of necrotic lesions on citrus leaves treated with elsinochrome (ESC). The mock controls were treated with acetone alone.
TOXICITY OF ELSINOCHROME: A NONHOST‐SELECTIVE PHYTOTOXIN
The toxicity of elsinochrome to Gram‐negative bacteria has long been recognized to be dependent on both light and oxygen (Weiss et al., 1957). Elsinochrome structurally resembles the perylenequinone phytotoxins produced by a number of fungal species. These toxins include: aspergillin produced by Aspergillus niger (Lund et al., 1953); alteichin, altertoxin, alterlosin and stemphyltoxin produced by Alternaria spp. (Davis and Stack, 1991; Stack et al., 1986; Stierle and Cardellina, 1989) and Stemphylium botryosum (Davis and Stack, 1991); cercosporin produced by many Cercospora spp. (Daub et al., 2005) and Scolicotrichum graminis (Tabuchi et al., 1994); hypomycin A produced by Hypomyces spp. (Liu et al., 2001); hypocrellin A/B produced by Hypocrella bambusae; shiraiachromes produced by another bamboo pathogen, Shiraia bambusicola (Wu et al., 1989); and phleichrome, calphostin C, cladochrome and ent‐isophleichrome produced by Cladosporium spp. (Arnone et al., 1988; Weiss et al., 1987; Yoshihara et al., 1975). All of these compounds contain a core 3,10‐dihydro‐oxy‐4,9‐perylenequinone chromophore. They are grouped as photosensitizing reagents because of their ability to sensitize cells to visible light and to produce ROS, such as superoxides (O2 ·−), hydrogen peroxide (H2O2), hydroxyl radical (OH·) and/or singlet oxygen (1O2) (Daub, 1982; Daub and Chung, 2009; Daub et al., 2005; Yamazaki et al., 1975). Oxygen and light are pivotal determinants for the photodynamic function and toxicity of these compounds. On illumination, photosensitizing compounds are converted to a stable electronically excited state, called the triplet state. The activated photosensitizers are able to react with oxygen, primarily via two different mechanisms, and generate toxic ROS (Daub and Chung, 2009; Dobrowolski and Foote, 1983; Girotti, 1990; Spikes, 1989). The triplet photosensitizers react with oxygen molecules by transferring a hydrogen atom or electron from reducing substrates, and produce reduced oxygen species including O2 ·− (Type I reaction). The activated photosensitizers are also able to transfer energy directly to oxygen molecules to produce the electronically reactive 1O2 (Type II reaction). Both 1O2 and O2 ·− are capable of inducing the oxidation of fatty acids, membranes, proteins/enzymes, sugars and nucleic acids, and hence are very toxic to cells (Daub et al., 2005). These photodynamic reactions often result in cell death.
The phytotoxic effect of elsinochrome towards host and nonhost plant cells in vitro and in vivo has been documented recently to be light dependent (Liao and Chung, 2008a). Elsinochrome purified from an isolate of E. fawcettii is highly toxic to citrus (host) and tobacco (nonhost) cells in the light. The toxicity of elsinochrome has been demonstrated to be mediated through the production of ROS, which could damage cell membranes and cause electrolyte leakage from the cells. The incubation of citrus leaf discs with elsinochrome in the light induced a steady increment of electrolyte leakage (Liao and Chung, 2008a). The toxicity was greatly alleviated by adding 1O2 quenchers, such as carotenoid carboxylic acid (bixin), 1,4‐diazabicyco‐octane (dabco), reduced glutathione or ascorbate (vitamin C). Application of elsinochrome alone to citrus leaves produced necrotic lesions resembling those caused by the fungal pathogen (Fig. 3b). Moreover, lesion development induced by elsinochrome could be inhibited by the addition of bixin, dabco or ascorbate, but not by α‐tocopherol (Liao and Chung, 2008a). Elsinochrome is a highly effective 1O2 producer with a quantum yield (0.83–0.98 per quantum of light absorbed) comparable with that of two photosensitizing compounds, haematoporphyrin and cercosporin, that are well known 1O2 generators (Aminian‐Saghafi et al., 1992; Ma et al., 2003; Zhang et al., 2009). The addition of β‐carotene, a potent quencher, prevents 1O2 production considerably. Elsinochrome has also been documented experimentally to generate O2 ·− in vitro, and this accumulation was suppressed by superoxide dismutase (SOD), a scavenger of O2 ·−, but was not suppressed by the 1O2 quencher, dabco (Liao and Chung, 2008a). In addition, elsinochrome is a substantial hydroperoxide producer after irradiation with visible light (Arnone et al., 1988). These studies demonstrate that elsinochrome functions as a photosensitizing compound that produces 1O2 and O2 ·− and exerts toxic effects on plant cells. Similar to many photosensitizing compounds, elsinochrome has anti‐tumour activities. Elsinochrome and its derivatives have been shown to generate significant amounts of 1O2 and to induce apoptosis of human colorectal carcinoma cells, as well as primate embryonic stem cells, in a light‐dependent manner (Ma et al., 2003; Zhang et al., 2009).
BIOSYNTHESIS AND REGULATION OF ELSINOCHROME
The production of elsinochrome under laboratory conditions is growth phase dependent and is highly regulated by a variety of environmental and physiological factors (Wang et al., 2009b). Light has been demonstrated to be the most essential factor, not only for toxicity, but also for elsinochrome production. Elsinochrome is primarily produced when the fungus is incubated in light, and its production is markedly suppressed when the fungus is grown in continuous darkness or in a light–dark cycle. In addition to this light response, E. fawcettii also produces large quantities of elsinochrome when grown under alkaline conditions. In contrast, pH has little effect on the production of cercosporin toxin by Cercospora nicotianae (You et al., 2008). Elsinoë fawcettii has been shown to accumulate large quantities of elsinochrome in the absence of nitrogen, or when glucose or mannitol is used as the sole carbon source. The accumulation of elsinochrome is suppressed completely when ammonium chloride or ammonium nitrate is used as the sole nitrogen source. The presence of Ca2+, Co2+ or Li+ decreases elsinochrome production by E. fawcettii. In contrast, ions including K+, Na+, Cu2+, Mg2+, Mn2+, Zn2+ and Fe3+ have been shown to stimulate elsinochrome production to various levels, depending on the concentration of the compound tested. Metal ions could affect the production of fungal toxins by controlling the expression of the genes whose products are required for toxin biosynthesis. In contrast, calcium is required for cercosporin production (Chung, 2003; You et al., 2008). The production of elsinochrome is also affected by antioxidants (Wang et al., 2009b). Dabco or bixin increases elsinochrome production, whereas butylated hydroxyanisole at 5 mm inhibits elsinochrome production. Ascorbate, chlorogenic acid, catechin and gallic acid increase substantially elsinochrome production. Pyridoxine (vitamin B6), cysteine, α‐tocopherol (vitamin E), vanillic acid, caffeic acid and reduced glutathione have little or no effect on elsinochrome accumulation. Phenolic antioxidants also inhibit ochratoxin A production by Aspergillus spp. (Palumbo et al., 2007; Passone et al., 2005). Ascorbate enhances aflatoxin biosynthesis by Aspergillus parasiticus, yet caffeine, flavonoids, gallic acid and phenolics repress its biosynthesis (Holmes et al., 2008; Patel et al., 1990). How antioxidants affect elsinochrome production remains unknown at this point. Some antioxidants may alter the environmental and physiological conditions that normally would be conducive for elsinochrome biosynthesis; others may modulate signal transduction networks that lead to elsinochrome biosynthesis. Alternatively, some compounds may have direct effects on gene expression or enzyme activity of dedicated steps in the elsinochrome biosynthetic pathway.
All known hydroxyperylenequinones of fungal origin are polyketide derivatives containing two highly symmetrical structures. Their biosynthesis via phenol coupling of two polyhydroxynaphthalenes has been well studied (Weiss et al., 1987). The synthesis of elsinochrome in Elsinoë spp. also apparently occurs via a fungal polyketide pathway, a process resembling fatty acid biosynthesis (Kennedy et al., 1999). The biosynthesis of elsinochrome was first investigated by the analysis of culture filtrates after feeding Elsinoë spp. with radioisotope‐labelled 1‐14C acetate and examining the subsequent products (Chen et al., 1966). The study revealed that the 4,9‐dihydroxyperylene‐3,10‐quinone chromophore of elsinochrome A is indeed derived from 4,4′,5,5′‐tetrahydroxy‐1,1′‐dinaphthyl by oxidation of phenols. In addition, 14C formate was incorporated only into the methoxy groups of elsinochrome. Chen et al. (1966) postulated that elsinochrome A is synthesized by iterative decarboxylation to condense one acetate and six malonate units, followed by hydroxylation, methylation and dimerization. The biosynthesis of elsinochromes C and D was also investigated by a separate group, using dually labelled 2‐13C and 2‐2H3 acetates (Kurobane et al., 1981). The analysis of the distribution of radioisotope in elsinochrome C and acetate in elsinochrome ‘D’ by 13C and 2H NMR spectrometry also confirmed that the carbon skeleton of elsinochrome is formed from a naphthalenoid heptaketide intermediate, followed by oxidative coupling of phenols. Elsinochrome D (Fig. 2), containing a methylenedioxy ring, is formed from ‘C’ by dehydroxylation between the C‐3 methoxy and C‐4 hydroxy moieties (Kurobane et al., 1981).
MOLECULAR ANALYSES OF THE ELSINOCHROME BIOSYNTHETIC GENES
Further support for the notion that elsinochrome is synthesized via a polyketide pathway comes from evidence of a gene that encodes a type‐I fungal polyketide synthase (PKS), and was cloned and characterized from an isolate of E. fawcettii (Liao and Chung, 2008b). The cloned gene, designated EfPKS1, encodes a polypeptide containing all of the functional domains characteristic of fungal nonreducing PKSs (Yang et al., 1996). These include a β‐keto‐synthase domain, an acyltransferase domain, two acyl carrier protein domains and a thioesterase/Claisen cyclase domain. Thus, EfPKS1 seemingly functions to condense acetate and malonate molecules by iterative decarboxylation, chain elongation of the polyketide and formation of aromatic rings from polyketomethylenes (Watanabe and Ebizuka, 2004). Northern blotting revealed that the expression of EfPKS1 is upregulated on exposure to light, in the presence of large amounts of glucose, during nitrogen starvation or at alkaline pH—all conditions highly conducive to elsinochrome accumulation. The insertion of a bacterial hygromycin phosphotransferase gene (HYG) cassette under the control of the Aspergillus nidulans trpC gene promoter within the EfPKS1 gene, via double crossover recombination, resulted in fungal mutants that completely failed to accumulate any detectable elsinochrome (Liao and Chung, 2008b). The study concluded that the translational product of EfPKS1 is required for elsinochrome biosynthesis. The introduction and expression of a functional copy of EfPKS1 in a null mutant fully restored its capacity for elsinochrome production, thus conclusively demonstrating that fungal PKS is essential for the biosynthesis of elsinochrome.
The genes required for the biosynthesis of secondary metabolites in filamentous fungi often reside in a cluster (Keller and Hohn, 1997; Keller et al., 2005). A DNA sequence analysis upstream and downstream of the EfPKS1 locus was conducted to determine whether any genes adjacent to EfPKS1 might also be involved in elsinochrome production in E. fawcettii (Chung and Liao, 2008). The combination of sequencing and chromosomal walking strategies further identified nine open reading frames (ORFs), designated EfHP1, EfHP2, EfHP3, PRF1, ECT1, TSF1, RDT1, OXR1 and EfHP4, in addition to EfPKS1 (Fig. 4). Some of these may have roles in elsinochrome biosynthesis. A PRF1 gene, located immediately downstream from EfPKS1, encodes a polypeptide showing similarity to prefoldin protein subunit 3. An ECT1 gene, located further downstream of PRF1, possesses a translational product highly similar to fungal nitrate transporters. A TSF1 gene, which is located upstream and transcribed divergently from EfPKS1, encodes a putative protein having strong similarity to fungal transcriptional factors that contain a Cys2His2‐type zinc finger and a GAL4‐like Zn2Cys6 binuclear cluster. A RDT1 gene, located further upstream from TSF1, encodes a polypeptide similar to a number of fungal reductases, such as 1,3,8‐trihydroxynaphthalene (T3HN) reductase that is involved in melanin biosynthesis (Kimura and Tsuge, 1993; Tsuji et al., 2003). A small ORF, designated OXR1, was found between EfPKS1 and TSF1. The predicted OXR1 protein, rich in proline, displays low similarity (39%) to bacterial malate:quinone oxidoreductases. The EfHP1 product shows low similarity (46%) to cation/multidrug efflux pumps in Marinomonas spp. The EfHP1, EfHP3 and EfHP4 genes encode hypothetical proteins with unknown functions.
Figure 4.
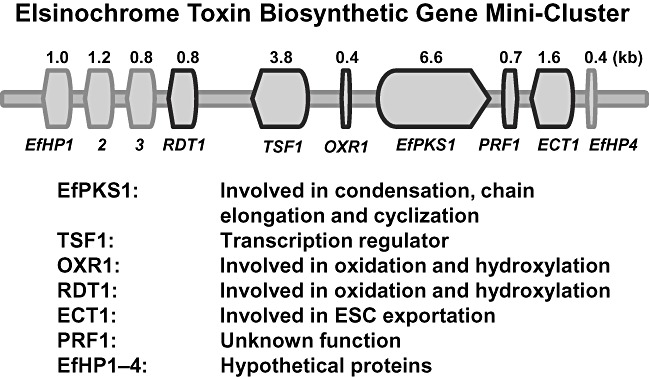
Schematic diagram showing the elsinochrome (ESC) biosynthetic gene cluster with predicted functions identified in Elsinoë fawcettii. This figure shows data reprinted from fig. 1 of Chung and Liao (2008) [reprinted with permission from the Society for General Microbiology (UK)].
Northern blot analysis revealed that the accumulation of transcripts of the identified genes does not completely correspond to the conditions conducive for elsinochrome production (Chung and Liao, 2008). All putative ORFs, except EfHP3, were upregulated under nitrogen starvation and in response to light. TSF1, EfPKS1, PRF1, ECT1 and EfHP2 were preferentially expressed under alkaline conditions, whereas pH had no effect on the expression of EfHP3, EfHP4 or OXR1. Glucose promoted the accumulation of EfPKS1, PRF1, ECT1 and OXR1 gene transcripts and suppressed the expression of TSF1. Expression of the RDT1, EfHP1, EfHP2 and EfHP4 genes was apparently not regulated by glucose.
To determine whether the genes clustered with EfPKS1 are also required for the biosynthesis and regulation of elsinochrome, the TSF1 gene was inactivated by targeted gene disruption and the mutants were characterized in detail. Similar to the EfPKS1 null mutant, the TSF1 null mutant failed to accumulate any detectable elsinochrome in axenic cultures (Chung and Liao, 2008). Although TSF1 is situated next to the EfPKS1 gene in the genome, and is absolutely required for elsinochrome production, its expression profiles did not fully correlate with those of EfPKS1 under certain conditions. However, TSF1 was observed to regulate the expression of the RDT1, EfPKS1, PRF, ECT1 and EfHP1 genes. Interestingly, impairment of EfPKS1 also led to a marked reduction in transcripts of the RDT1, TSF1, PRF1, ECT1 and EfHP1 genes, most probably as a result of a feedback inhibition effect. TSF1 or EfPKS1 had no effect on the expression of the OXR1, EfHP2 or EfHP3 genes. Nevertheless, TSF1 acts as a core transcriptional activator and plays a profound role in elsinochrome biosynthesis. Although we could not demarcate precisely the elsinochrome biosynthetic gene cluster, the determination of gene expression patterns in the EfPKS1‐ or TSF1‐disrupted mutants suggested that some newly identified genes are needed for elsinochrome production. However, the function of the elsinochrome genes, other than EfPKS1 and TSF1, in the biochemical pathway leading to elsinochrome production cannot be conclusively determined until each of the genes is functionally inactivated.
A recent study has further revealed that the E. fawcettii EfSTE12 gene, encoding a polypeptide analogue to the yeast STE12 transcription factor, plays a regulatory role in the biosynthesis of elsinochrome (Yang and Chung, 2010). EfSTE12 is not clustered with the elsinochrome biosynthetic genes. In Saccharomyces cerevisiae, transcriptional activation of STE12 is induced by a pheromone response mitogen‐activated protein kinase (MAPK) pathway, and is required for the regulation of the genes involved in developmental and physiological functions (Madhani et al., 1999; Schwartz and Madhani, 2004). Disruption of the EfSTE12 gene led to a reduced expression of the EfPKS1 gene, but not the TSF1 gene. It appears that EfSTE12 bypasses the pathway‐specific TSF1 regulator and activates the biosynthetic genes directly.
A PROPOSED ROUTE LEADING TO THE PRODUCTION OF ELSINOCHROME
Elsinochrome is synthesized via a pathway resembling the biosynthesis of fatty acids and fungal polyketides. Thus, the assembly of the polyketide backbone of elsinochrome is probably accomplished by the function of EfPKS1 (Fig. 5). One proposal suggests that EfPKS1 iteratively catalyses decarboxylation between acetyl‐CoA and malonyl‐CoA subunits for polyketide chain elongation. In each condensation cycle, the malonyl‐CoA subunit is first attached to the acyl carrier protein domains of EfPKS1 to form a phosphopantheine (PPT) complex, which accepts the acetate unit from acetyl‐CoA via the function of the acyltransferase domain. The β‐keto‐synthase domain of EfPKS1 catalyses the condensation to produce a linear polyketide chain. In each cycle, the keto group of malonyl‐CoA is reduced and results in the integration of two carbons into the polyketide chain. Once the chain is completed, the polyketide is unattached from the complex, probably via the function of the thioesterase/Claisen cyclase domain. The released polyketide undergoes cyclization to form an aromatic ring, and proceeds via serial modification steps to produce the heptaketide backbone of elsinochrome. As elsinochrome has a symmetrical structure, two identical heptaketides are fused to form a core 1,2‐dihydrobenzo‐perylene ring structure, which can then be successively modified to produce the various derivatives of elsinochrome. Some of these reactions may be cooperatively carried out, at least in part, by the products of RDT1, OXR1 and EfPKS1.
Figure 5.
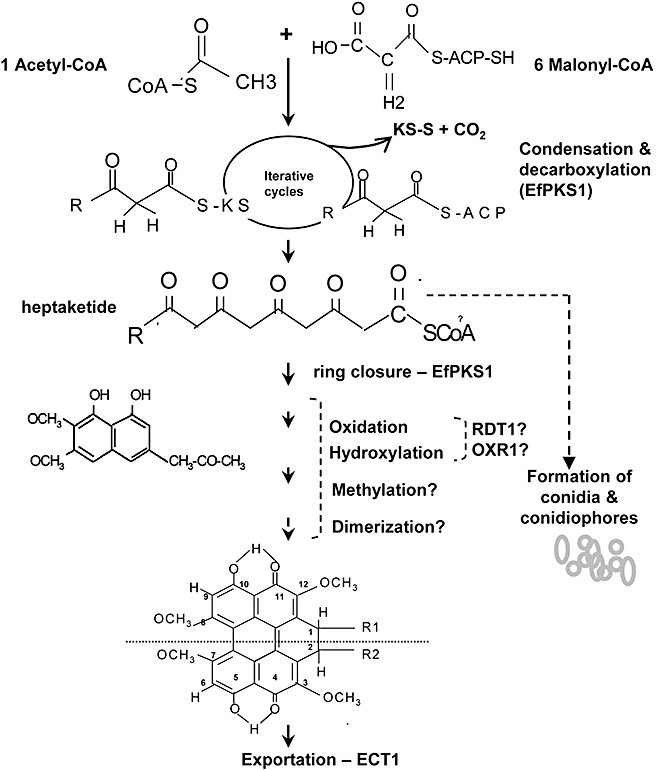
Proposed biosynthetic pathway and involvement of the gene products leading to the formation of elsinochrome and conidia by Elsinoë fawcettii.
The PRF1 gene, embedded within the elsinochrome cluster, produces a protein displaying high similarity to prefoldin protein subunit 3. The prefoldin protein comprises multiple subunits, and forms part of a molecular chaperone system that binds and stabilizes newly synthesized polypeptides to ensure appropriate folding in eukaryotes and archaea (Vainberg et al., 1998). Although the expression of the PRF1 gene coincided with the conditions conducive for elsinochrome production and was markedly repressed in the EfPKS1 or TSF1 null mutants, the actual function of the polypeptide of the PRF1 gene still remains uncertain. If the PRF1 gene product is actually involved in elsinochrome production, it is very possible that the PRF1 protein functions to stabilize some of the biosynthetic enzymes required for elsinochrome production. As prefoldin is a hexamer containing two α and four β subunits; additional prefoldin subunits, whose coding genes may not immediately link to the elsinochrome biosynthetic gene cluster, are required to fulfill the chaperone function. In addition, no methyltransferase‐coding gene exists within the biosynthetic gene cluster, even though elsinochrome has four methyl groups at positions C3, C7, C8 and C12 (Fig. 5). Apparently, the identified gene cluster does not contain the entire entourage of genes responsible for elsinochrome biosynthesis. Once elsinochrome is synthesized, it must be exported outside the fungal cells, which is probably accomplished by the ECT1 transporter, to avoid toxicity.
CONNECTIONS BETWEEN ELSINOCHROME AND CONIDIATION
In filamentous fungi, the production of secondary metabolites is often closely associated with cell development or differentiation (Adams and Yu, 1998; Brodhagen and Keller, 2006; Calvo et al., 2002; Yu and Keller, 2005). Similarly, elsinochrome production and the process of conidiation may have an intimate relationship in Elsinoë spp. In addition to complete obliteration of elsinochrome production, the E. fawcettii mutants disrupted in EfPKS1 or TSF1 also failed to produce conidia (Chung and Liao, 2008; Liao and Chung, 2008b). Conidiation and elsinochrome production can be genetically reverted to wild‐type levels by expressing a functional EfPKS1 gene in a null mutant. Surprisingly, TSF1 restored elsinochrome production, but not conidiation, in a TSF1 null mutant (Chung and Liao, 2008). Although EfSTE12 was required for the regulation of elsinochrome biosynthesis, it was apparently totally dispensable for conidiation (Yang and Chung, 2010).
Environmental and physiological cues that trigger both conidiation and the production of secondary metabolites are very probably perceived and transduced by common signalling pathways in fungi. Environmental factors, such as light, carbon/nitrogen sources and ambient pH, have long been known to regulate both the developmental differentiation and biosynthesis of secondary metabolites in fungi (Calvo et al., 2002). The genetic mechanisms connecting both processes are complex, but are beginning to unfold through molecular studies. An involvement of a G‐protein/cyclic adenosine monophosphate/protein kinase A‐mediated pathway and many cellular regulators has been documented in both asexual sporulation and mycotoxin production in Aspergillus spp. (Calvo et al., 2002; Tsitsigiannis and Keller, 2006). Similarly, the MAPK and cyclin‐dependent kinase pathways have also been documented to regulate both conidiation and the production of cercosporin in Cercospora zeae‐maydis (Shim and Dunkle, 2003) and fumonisins in Fusarium verticillioides (Shim and Woloshuk, 2001), respectively.
The coordinate regulation of conidiation and the production of elsinochrome via similar regulatory pathways, as described in Aspergillus species, remains to be proven in Elsinoë spp. However, the EfPKS1, TSF1 and PRF1 promoters contain one or multiple 5′‐(A/C)(A/G)AGGG(A/G)‐3′ motifs that serve as binding sites for the conidial formation‐related Bristle (BrlA) transcription activator of A. nidulans (Adams et al., 1998). Each of the promoter regions of the RDT1, OXR1, EfPKS1 and ECT1 genes has a consensus sequence, 5′‐CATTC(C/T)‐3′, which acts as a binding site for the AbaA transcription activator involved in conidiophore development in A. nidulans (Andrianopoulos and Timberlake, 1994). In A. nidulans, AbaA is regulated by BrlA and both are required for conidiation. Thus, elsinochrome or intermediates generated during the course of biosynthesis may be involved directly in conidial formation by Elsinoë spp.
GENETIC DETERMINATION OF FUNGAL PATHOGENICITY
Many phytopathogenic fungi are capable of producing nonhost‐selective, light‐activated perylenequinone toxins. For example, cercosporin produced by many Cercospora species has been documented to play a profound role in plant diseases (Callahan et al., 1999; Chen et al., 2007; 2005, 2007; Dekkers et al., 2007; Shim and Dunkle, 2003). However, the role of other perylenequinone toxins required for fungal pathogenesis and for the onset of plant diseases has not yet been investigated widely. Purified elsinochrome has been documented to be toxic to suspension‐cultured citrus and tobacco cells (Liao and Chung, 2008a). Elsinochrome also caused lipid peroxidation and severe electrolyte leakage of host leaves after irradiation, owing to the generation of ROS. Elsinochrome could be obtained from necrotic lesions on Elsinoë‐affected citrus leaves (Wang et al., 2009c), indicating its production during the course of disease development. Furthermore, the application of 1O2 quenchers, such as bixin, ascorbate and reduced glutathione, or of O2 ·− scavengers, such as α‐tocopherol, l‐cysteine and SOD, mitigated the cellular toxicity of elsinochrome (Liao and Chung, 2008a). Most intriguingly, elsinochrome alone induced necrotic lesions on citrus leaves on exposure to visible light, and the development of such lesions could be impeded by the co‐application of 1O2 quenchers. These results imply an important role of elsinochrome in the formation of necrotic lesions.
Elsinochrome is a virulence determinant in Elsinoë spp., although no strong correlation exists between the levels of elsinochrome production in culture and pathogenicity among isolates (Wang et al., 2009c). As stated above, both EfPKS1 and TSF1 null mutants failed to produce any detectable levels of elsinochrome. Fungal pathogenicity assayed on detached citrus leaves revealed that these null mutants defective in EfPKS1 or TSF1 induced fewer necrotic lesions compared with the number induced by wild‐type or genetically reverted strains (Chung and Liao, 2008; Liao and Chung, 2008b). Both conidiation and fungal pathogenicity were fully restored in an EfPKS1 null mutant on acquisition and expression of a functional EfPKS1. Although the TSF1 complementation strain failed to fully restore conidiation, the fungus caused necrotic lesions on citrus leaves at a rate and magnitude that was indistinguishable from that of the wild‐type. However, the EfSTE12 null mutant, which is partially defective in elsinochrome production, is still fully pathogenic to citrus (Yang and Chung, 2010). The EfSTE12 null mutant still produces elsinochrome, which might be at levels sufficient to facilitate fungal invasion.
CONCLUDING REMARKS
Elsinochrome produced by many Elsinoë spp. is a photosensitizing polyketide. Great strides are being made in the elucidation of its mode of action and in the identification of its biosynthetic and regulatory genes. Elsinochrome clearly is a critical virulence factor for E. fawcettii and, perhaps, for E. australis as well. As with many photoactivated toxins containing perylenequinones (Daub and Chung, 2009), elsinochrome toxin operates by absorbing light energy and generating ROS, which kills host cells. The production of elsinochrome may provide Elsinoë spp. with access to nutrients and allow the pathogen to thrive within host tissues. The analysis of elsinochrome‐deficient mutants has confirmed an important role in normal disease development. Recent studies have also documented that the biosynthesis of elsinochrome is regulated by diverse environmental and physiological cues. Such regulation is mediated by the pathway‐specific TSF1 transcription activator (Fig. 6). The TSF1 gene is located upstream of the EfPKS1 gene, and is clustered with several other genes whose products are implicated in the production of elsinochrome. Environmental signals could be transduced down a number of signalling pathways. For example, elsinochrome biosynthesis, but not conidiation, is regulated, at least in part, by the EfSTE12 transcription factor, whose activity is probably controlled by a MAPK‐mediated signalling pathway. Bypassing the TSF1 regulator, EfSTE12 functions in elsinochrome biosynthesis by direct activation of biosynthetic genes. Elsinochrome is favourably produced by E. fawcettii grown in light, with large amounts of glucose, under nitrogen deprivation or at alkaline pH. Other transcription regulators, such as the nitrogen‐induced AreA (Marzluf, 1997), the light‐regulated WC1/WC2 (Linden and Macino, 1997), the ambient pH‐regulated PacC (Espeso et al., 1997) and the conidial formation‐related BrlA and AbaA (Adams et al., 1998), might also be involved in elsinochrome biosynthesis and conidiation. Elsinochrome biosynthesis and conidial formation are very probably coordinately regulated, at least in part, by common transcriptional regulators in a complex and intertwined network. Alternatively, the proteins or intermediates involved in elsinochrome production might participate directly in conidia formation.
Figure 6.
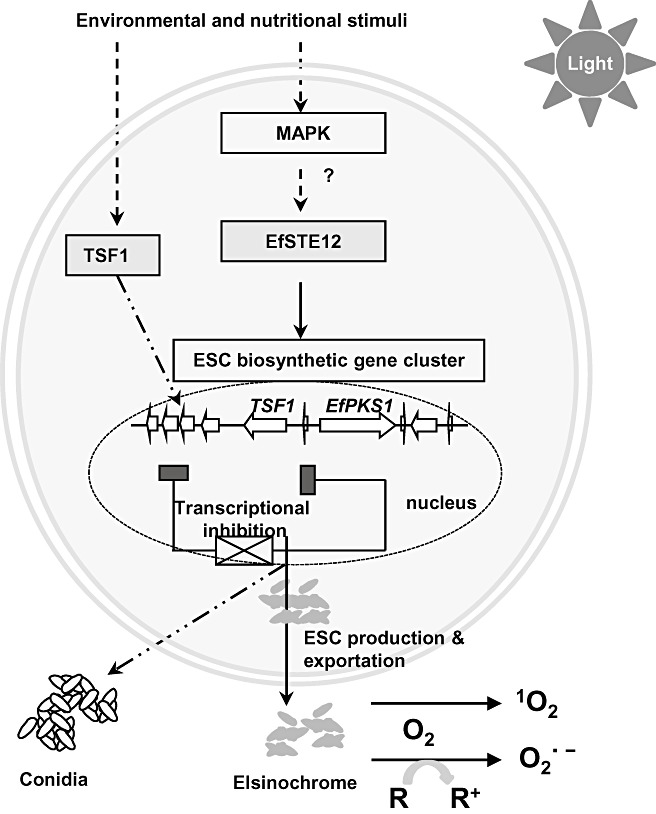
Proposed regulatory pathways involving two transcriptional activators, TSF1 and EfSTE12, and leading to the production of elsinochrome (ESC) and conidia in Elsinoë fawcettii. On exposure to light, ESC assumes an activated triplet state which reacts with oxygen to produce superoxide (O2 ·−) through a reducing substrate (R) or singlet oxygen (1O2) via an energy relay reaction. A cross indicates an inhibitory effect for ESC biosynthesis, presumably by blocking the expression of TSF1 and thus of the elsinochrome‐biosynthetic genes. A filled rectangle indicates the inhibitory activity of expression of the ESC biosynthetic genes. TSF1 is also required for conidiation. The regulation of ESC biosynthesis by EfSTE12 bypasses TSF1 and is probably controlled by a mitogen‐activated protein kinase (MAPK)‐mediated signalling pathway.
ACKNOWLEDGEMENTS
The author thanks S. L. Yang for technical assistance and two anonymous reviewers for their helpful comments. The author's research described in this article was supported by the Florida Agricultural Experiment Station and grants from the Florida Citrus Production Research Advisory Council.
REFERENCES
- Adams, T.H. and Yu, J.H. (1998) Coordinate control of secondary metabolite production and asexual sporulation in Aspergillus nidulans . Curr. Opin. Microbiol. 1, 674–677. [DOI] [PubMed] [Google Scholar]
- Adams, T.H. , Wieser, J.K. and Yu, J.‐K. (1998) Asexual sporulation in Aspergillus nidulans . Microbiol. Mol. Biol. Rev. 62, 35–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostini, J.P. , Bushong, P.M. , Bhatia, A. and Timmer, L.W. (2003) Influence of environmental factors on severity of citrus scab and melanose. Plant Dis. 87, 1102–1106. [DOI] [PubMed] [Google Scholar]
- Ahonsi, M.O. , Boss, D. , Maurhofer, M. and Défago, G. (2006) Potential environmental fate of elsinochrome A, a perylenequinone toxin produced in culture by bindweed biocontrol fungus Stagonospora convolvuli LA39. Environmentalists, 26, 183–193. [Google Scholar]
- Aminian‐Saghafi, T. , Nasini, G. , Caronna, T. , Braun, A.M. and Oliveros, E. (1992) Quantum yields of singlet‐oxygen production by some natural quinoid fungal metabolites and derivatives. Helv. Chem. Acta, 75, 531–538. [Google Scholar]
- Andrianopoulos, A. and Timberlake, W.E. (1994) The Aspergillus nidulans abaA gene encodes a transcriptional activator that acts as a genetic switch to control development. Mol. Cell. Biol. 14, 2503–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone, A. , Assante, G. , Di Modugno, V. , Merlini, L. and Nasini, G. (1988) Perylenequinones from cucumber seedlings infected with Cladosporium cucumerinum . Phytochemistry, 6, 1675–1678. [Google Scholar]
- Bitancourt, A.A. and Jenkins, A.E. (1936a) The perfect stage of the sweet orange scab fungus. Mycologia, 28, 489–492. [Google Scholar]
- Bitancourt, A.A. and Jenkins, A.E. (1936b) Elsinoë fawcettii, the perfect stage of the citrus scab fungus. Phytopathology, 26, 393–396. [Google Scholar]
- Brodhagen, M. and Keller, N.P. (2006) Signalling pathways connecting mycotoxin production and sporulation. Mol. Plant Pathol. 7, 285–301. [DOI] [PubMed] [Google Scholar]
- Callahan, T.M. , Rose, M.S. , Meade, M.J. , Ehrenshaft, M. and Upchurch, R.G. (1999) CFP, the putative cercosporin transporter of Cercospora kikuchii, is required for wild type cercosporin production, resistance, and virulence on soybean. Mol. Plant–Microbe Interact. 12, 901–910. [DOI] [PubMed] [Google Scholar]
- Calvo, A.M. , Wilson, R.A. , Bok, J.W. and Keller, N.P. (2002) Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 66, 447–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C.T. , Nakanishi, K. and Natori, S. (1966) Biosynthesis of elsinochrome A, the perylenequinone from Elsinoë spp. Chem. Pharm. Bull. (Tokyo), 14, 1434–1437. [DOI] [PubMed] [Google Scholar]
- Chen, H.Q. , Lee, M.H. , Daub, M.E. and Chung, K.‐R. (2007) Molecular analysis of the cercosporin biosynthetic gene cluster in Cercospora nicotianae . Mol. Microbiol. 64, 755–770. [DOI] [PubMed] [Google Scholar]
- Choquer, M. , Dekkers, K.A. , Chen, H.Q. , Ueng, P.P. , Daub, M.E. and Chung, K.‐R. (2005) The CTB1 gene encoding a fungal polyketide synthase is required for cercosporin biosynthesis and fungal virulence of Cercospora nicotianae . Mol. Plant–Microbe Interact. 18, 468–476. [DOI] [PubMed] [Google Scholar]
- Choquer, M. , Lee, M.H. , Bau, H.J. and Chung, K.‐R. (2007) Deletion of a MFS transporter‐like gene in Cercospora nicotianae reduces cercosporin toxin accumulation and fungal virulence. FEBS Lett. 581, 489–494. [DOI] [PubMed] [Google Scholar]
- Chung, K.‐R. (2003) Involvement of calcium/calmodulin signaling in cercosporin toxin biosynthesis by Cercospora nicotianae . Appl. Environ. Microbiol. 69, 1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, K.‐R. and Liao, H.L. (2008) Determination of a transcriptional regulator‐like gene involved in biosynthesis of elsinochrome phytotoxin by the citrus scab fungus, Elsinoë fawcettii . Microbiology, 154, 3556–3566. [DOI] [PubMed] [Google Scholar]
- Daub, M.E. (1982) Cercosporin, a photosensitizing toxin from Cercospora species. Photopathology, 72, 370–374. [Google Scholar]
- Daub, M.E. and Chung, K.‐R. (2009) Photoactivated perylenequinone toxins in plant pathogenesis In: Plant Relationships, 2nd edn. The Mycota V (Deising H., ed.), pp. 201–218. Berlin, Heidelberg: Springer‐Verlag. [Google Scholar]
- Daub, M.E. , Herrero, S. and Chung, K.‐R. (2005) Photoactivated perylenequinone toxins in fungal pathogenesis of plants. FEMS Microbiol. Lett. 252, 197–206. [DOI] [PubMed] [Google Scholar]
- Davis, V.M. and Stack, M.E. (1991) Mutagenicity of stemphyltoxin III, a metabolite of Alternaria alternata . Appl. Environ. Microbiol. 57, 180–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekkers, L.A. , You, B.J. , Gowda, V.S. , Liao, H.L. , Lee, M.H. , Bau, H.J. , Ueng, P.P. and Chung, K.‐R. (2007) The Cercospora nicotianae gene encoding dual O‐methyltransferase and FAD‐dependent monooxygenase domains mediates cercosporin toxin biosynthesis. Fungal Genet. Biol. 44, 444–454. [DOI] [PubMed] [Google Scholar]
- Dobrowolski, D.C. and Foote, C.S. (1983) Chemistry of singlet oxygen 46. Quantum yield of cercosporin‐sensitized singlet oxygen formation. Angew. Chem. 95, 729–730. [Google Scholar]
- Espeso, E.A. , Tilburn, J. , SaÂnchez‐Pulido, L. , Brown, C.V. , Valencia, A. , Arst, H.N., Jr and Peñalva, M. (1997) Specific DNA recognition by the Aspergillus nidulans three zinc finger transcription factor PacC. J. Mol. Biol. 274, 466–480. [DOI] [PubMed] [Google Scholar]
- Girotti, A.W. (1990) Photodynamic lipid peroxidation in biological systems. Photochem. Photobiol. 51, 497–509. [DOI] [PubMed] [Google Scholar]
- Holmes, R.A. , Boston, R.S. and Payne, G.A. (2008) Diverse inhibitors of aflatoxin biosynthesis. Appl. Microbiol. Biotechnol. 78, 559–572. [DOI] [PubMed] [Google Scholar]
- Hyun, J.W. , Timmer, L.W. , Lee, S.C. , Yun, S.H. , Ko, S.W. and Kim, K.S. (2001) Pathological characterization and molecular analysis of Elsinoë isolates causing scab diseases of citrus in Jeju island in Korea. Plant Dis. 85, 1013–1017. [DOI] [PubMed] [Google Scholar]
- Hyun, J.W. , Peres, N.A. , Yi, S.Y. , Timmer, L.W. , Kim, K.S. , Kwon, H.M. and Lim, H.C. (2007) Development of PCR assays for identification of species and pathotypes of Elsinoë causing scab on citrus. Plant Dis. 91, 865–870. [DOI] [PubMed] [Google Scholar]
- Hyun, J.W. , Yi, S.Y. , MacKenzie, S.J. , Timmer, L.W. , Kim, K.S. , Kang, S.K. , Kwon, H.M. and Lim, H.C. (2009) Pathotypes and genetic relationship of worldwide collections of Elsinoë spp. causing scab diseases of citrus. Phytopathology, 99, 721–728. [DOI] [PubMed] [Google Scholar]
- Jenkins, A.E. and Bitancourt, A.A. (1938) An Elsinoë causing anthracnose on Hickoria pecan . Phytopathology, 28, 75–80. [Google Scholar]
- Keller, N.P. and Hohn, T.M. (1997) Metabolic pathway gene clusters in filamentous fungi. Fungal Genet. Biol. 21, 17–29. [PubMed] [Google Scholar]
- Keller, N.P. , Turner, G. and Bennett, J.W. (2005) Fungal secondary metabolism—from biochemistry to genomics. Nat. Rev. Microbiol. 3, 937–947. [DOI] [PubMed] [Google Scholar]
- Kennedy, J. , Auclair, K. , Kendrew, S.G. , Park, C. , Vederas, J.C. and Hutchinson, C.R. (1999) Modulation of polyketide synthase activity by accessory proteins during lovastatin biosynthesis. Science, 284, 1368–1372. [DOI] [PubMed] [Google Scholar]
- Kim, K.W. and Hyun, J.W. (2007) Nonhost‐associated proliferation of intrahyphal hyphae of citrus scab fungus Elsinoë fawcettii: refining the perception of cell‐within‐a‐cell organization. Micron, 38, 565–571. [DOI] [PubMed] [Google Scholar]
- Kim, K.W. , Hyun, J.W. and Park, E.W. (2004) Cytology of cork layer formation of citrus and limited growth of Elsinoë fawcettii in scab lesions. Eur. J. Plant Pathol. 110, 129–138. [Google Scholar]
- Kimura, N. and Tsuge, T. (1993) Gene cluster involved in melanin biosynthesis of the filamentous fungus Alternaria alternata . J. Bacteriol. 175, 4427–4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurobane, I. , Vining, L. , McInnes, A.G. , Smith, D.G. and Walter, J.A. (1981) Biosynthesis of elsinochromes C and D. Pattern of acetate incorporation determined by 13C and 2H NMR. Can. J. Chem. 59, 422–430. [Google Scholar]
- Leki, H. (1994) Emergence of benzimidazole‐resistant strains of Elsinoë fawcettii Jenkins in citrus orchards in Japan. Ann. Phytopathol. Soc. Jpn. 60, 501–506. [Google Scholar]
- Liao, H.L. and Chung, K.‐R. (2008a) Cellular toxicity of elsinochrome phytotoxins produced by the pathogenic fungus, Elsinoë fawcettii causing citrus scab. New Phytol. 117, 239–250. [DOI] [PubMed] [Google Scholar]
- Liao, H.L. and Chung, K.‐R. (2008b) Genetic dissection defines the roles of elsinochrome phytotoxin for fungal pathogenesis and conidiation of the citrus pathogen Elsinoë fawcettii . Mol. Plant–Microbe Interact. 21, 469–479. [DOI] [PubMed] [Google Scholar]
- Linden, H. and Macino, G. (1997) White collar‐2, a partner in blue light signal transduction, controlling expression of light‐regulated genes in Neurospora crassa . EMBO J. 16, 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, W.Z. , Shen, Y.X. , Liu, X.F. , Chen, Y.T. and Xie, J.L. (2001) A new perylenequinone from Hypomyces sp. Chin. Chem. Lett. 12, 431–432. [Google Scholar]
- Lousberg, R.J.J. , Salemink, C.A. , Weiss, U. and Batterham, T.J. (1969) Pigments of Elsinoë species II. Structure of elsinochromes A, B and C. J. Chem. Soc. (C), 1219–1227. [Google Scholar]
- Lousberg, R.J.J. , Paolillo, L. , Kon, H. and Weiss, U. (1970a) Pigments of Elsinoë species IV. Confirmatory evidence for the structure of elsinochrome A and its ethers from studies of nuclear magnetic resonance (solvent and Overhauser effects) and electron spin resonance. J. Chem. Soc. (C), 2154–2159. [Google Scholar]
- Lousberg, R.J.J. , Salemink, C.A. and Weiss, U. (1970b) Pigments of Elsinoë species II. Structure of elsinochrome D. J. Chem. Soc. (C), 1259–1262. [Google Scholar]
- Lund, N.A. , Robertson, A. and Whalley, W.B. (1953) The chemistry of Fungi. Part XXI. Asperxanthone and a preliminary examination of aspergillin. J. Chem. Soc. 494, 2324–2439. [Google Scholar]
- Ma, L. , Tai, H. , Li, C. , Zhang, Y. , Wang, Z.H. and Ji, W.Z. (2003) Photodynamic inhibitory effects of three perylenequinones on human colorectral carcinoma cell line and primate embryonic stem cell line. World J. Gastroenterol. 9, 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhani, H.D. , Galitski, T. , Lander, E.S. and Fink, G.R. (1999) Effectors of a developmental mitogen‐activated protein kinase cascade revealed by expression signatures of signaling mutants. Proc. Natl. Acad. Sci. USA, 96, 12 530–12 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzluf, G.A. (1997) Genetic regulation of nitrogen metabolism in the fungi. Microbiol. Mol. Biol. Rev. 61, 17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebius, H.J. , Krabbendam, H. and Duisenberg, A.J.M. (1990) Structure of elsinochrome A. Acta Crystallogr. C46, 267–271. [Google Scholar]
- Meille, S.V. , Malpezzi, L. , Allegra, G. and Nasini, G. (1989) Structure of elsinochrome A: a perylenequinone metabolite. Acta Crystallogr. C45, 628–632. [DOI] [PubMed] [Google Scholar]
- Palumbo, J.D. , O'Keeffe, T.L. and Mahoney, N.E. (2007) Inhibition of ochratoxin A production and growth of Aspergillus species by phenolic antioxidant compounds. Mycopathologia, 164, 241–248. [DOI] [PubMed] [Google Scholar]
- Passone, M.A. , Resnik, S.L. and Etcheverry, M.G. (2005) In vitro effect of phenolic antioxidants on germination, growth and aflatoxin B1 accumulation by peanut Aspergillus section Flavi. J. Appl. Microbiol. 99, 682–691. [DOI] [PubMed] [Google Scholar]
- Patel, U.D. , Bapat, S.R. and Dave, P.J. (1990) Induction of aflatoxin biosynthesis in Aspergillus parasiticus by ascorbic acid‐mediated lipid peroxidation. Curr. Microbiol. 20, 159–164. [Google Scholar]
- Pfirter, H.A. , Marquis, F. and Défago, G. (1999) Genetic and pathogenic characterization of different Stagonospora sp. isolated from bindweed. Biocontrol Sci. Technol. 9, 555–566. [Google Scholar]
- Schwartz, M.A. and Madhani, H.D. (2004) Principles of MAP kinase signaling specificity in Saccharomyces cerevisiae . Annu. Rev. Genet. 38, 725–748. [DOI] [PubMed] [Google Scholar]
- Shim, W.‐B. and Dunkle, L.D. (2003) CZK3, a MAP kinase kinase kinase homolog in Cercospora zeae‐maydis, regulates cercosporin biosynthesis, fungal development, and pathogenesis. Mol. Plant–Microbe Interact. 16, 760–768. [DOI] [PubMed] [Google Scholar]
- Shim, W.‐B. and Woloshuk, C.P. (2001) Regulation of fumonisin B1 biosynthesis and conidiation in Fusarium verticillioides by a cyclin‐like (C‐type) gene, FCC1 . Appl. Environ. Microbiol. 67, 1607–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasugi, N. and Misaki, A. (1992) Isolation, characterization, and antitumor activities of the cell wall polysaccharides from Elsinoë leucospila . Biosci. Biotechnol. Biochem. 56, 29–33. [DOI] [PubMed] [Google Scholar]
- Spikes, J.D. (1989) Photosensitization In: The Science of Photobiology (Smith K.C., ed.), pp. 79–110. New York: Plenum Press. [Google Scholar]
- Stack, M.E. , Mazzola, E.P. , Page, S.W. , Pohland, A.E. , Highet, R.S. , Tempesta, M.S. and Corely, D.G. (1986) Mutagenic perylenequinone metabolites of Alternaria alternata: altertoxins I, II, and III. J. Nat. Prod. 49, 866–871. [DOI] [PubMed] [Google Scholar]
- Stierle, A.C. and Cardellina, J.H. (1989) Phytotoxins from Alternaria alternata, a pathogen of spotted knapweed. J. Nat. Prod. 52, 42–47. [Google Scholar]
- Tabuchi, H. , Tajimi, A. and Ichihara, A. (1994) Phytotoxic metabolites isolated from Scolecotrichum graminis Fuckel. Biosci. Biotech. Biochem. 58, 1956–1959. [Google Scholar]
- Tan, M.K. , Timmer, L.W. , Broadbent, P. , Priest, M. and Cain, P. (1996) Differentiation by molecular analysis of Elsinoë spp. causing scab diseases of citrus and its epidemiological implications. Phytopathology, 86, 1039–1044. [Google Scholar]
- Timmer, L.W. , Priest, M. , Broadbent, P. and Tan, M.K. (1996) Morphological and pathological characterization of species of Elsinoë causing scab disease of citrus. Phytopathology, 86, 1032–1038. [Google Scholar]
- Tsitsigiannis, D.I. and Keller, N.P. (2006) Oxylipins act as determinants of natural product biosynthesis and seed colonization in Aspergillus nidulans . Mol. Microbiol. 59, 882–892. [DOI] [PubMed] [Google Scholar]
- Tsuji, G. , Sugahara, T. , Fujii, I. , Mori, Y. , Ebizuka, Y. , Shiraishi, T. and Kubo, Y. (2003) Evidence for involvement of two naphthol reductases in the first reduction step of melanin biosynthesis pathway of Colletotrichum lagenarium . Mycol. Res. 107, 854–860. [DOI] [PubMed] [Google Scholar]
- Tyson, J.L. and Fullerton, R.A. (2001) First report of benomyl resistance in Elsinoë fawcettii in New Zealand citrus orchards. Australas. Plant Pathol. 30, 69. [Google Scholar]
- Vainberg, I.E. , Lewis, S.A. , Rommelaere, H. , Ampe, C. , Vandekerckhove, J. , Klein, H.L. and Cowan, N.J. (1998) Prefoldin, a chaperone that delivers unfolded proteins to cytosolic chaperonin. Cell, 93, 863–873. [DOI] [PubMed] [Google Scholar]
- Wang, L.Y. , Liao, H.L. , Bau, H.J. and Chung, K.‐R. (2009a) Characterization of pathogenic variants of Elsinoë fawcettii of citrus implies the presence of new pathotypes and cryptic species in Florida. Can. J. Plant Pathol. 31, 28–37. [Google Scholar]
- Wang, L.Y. , Bau, H.J. , Liao, H.L. and Chung, K.‐R. (2009b) Factors affecting the production of elsinochrome phytotoxin by the citrus scab pathogen, Elsinoë fawcettii . Open Mycol. J. 3, 1–8. [Google Scholar]
- Wang, L.Y. , Bau, H.J. and Chung, K.‐R. (2009c) Accumulation of elsinochrome phytotoxin does not correlate with fungal virulence among Elsinoë fawcettii isolates in Florida. J. Phytopathol. 157, 602–608. [Google Scholar]
- Watanabe, A. and Ebizuka, Y. (2004) Unprecedented metabolism of chain length determination in fungal aromatic polyketide synthases. Chem. Biol. 11, 1101–1106. [DOI] [PubMed] [Google Scholar]
- Weiss, U. , Flon, H. and Burger, W.C. (1957) The photodynamic pigment of some species of Elsinoë and Sphaceloma . Arch. Biochem. Biophys. 69, 311–319. [DOI] [PubMed] [Google Scholar]
- Weiss, U. , Ziffer, H. , Batterham, T.J. , Blumer, M. , Hackeng, W.H.L. , Copier, H. and Salemink, C.A. (1965) Pigments of Elsinoë species I. Pigment production by Elsinoë species: isolation of pure elsinochromes A, B, and C. Can. J. Microbiol. 11, 57–66. [DOI] [PubMed] [Google Scholar]
- Weiss, U. , Merlini, L. and Nasini, G. (1987) Naturally occurring perylenequinones In: Progress in the Chemistry of Organic Natural Products (Herz W., Grisebach H., Kirby G.W. and Tamm C., eds), pp. 2–71. New York: Springer‐Verlag. [DOI] [PubMed] [Google Scholar]
- Whiteside, J.O. (1975) Biological characteristics of Elsinoë fawcettii pertaining to the epidemiology of sour orange scab. Phytopathology, 65, 1170–1177. [Google Scholar]
- Whiteside, J.O. (1978) Pathogenicity of two biotypes of Elsinoë fawcettii to sweet orange and some other cultivars. Phytopathology, 68, 1128–1131. [Google Scholar]
- Whiteside, J.O. (1980) Detection of benomyl‐tolerant strains of Elsinoë fawcettii in Florida citrus groves and nurseries. Plant Dis. 64, 871–872. [Google Scholar]
- Wu, H. , Lao, X.F. , Wang, Q.W. and Lu, R.R. (1989) The shiraiachromes: novel fungal perylenequinone pigments from Shiraia bambusicola . J. Nat. Prod. 5, 948–951. [Google Scholar]
- Yamazaki, S. , Okube, A. , Akiyama, Y. and Fuwa, K. (1975) Cercosporin, a novel photodynamic pigment isolated from Cercospora kikuchii . Agric. Biol. Chem. 39, 287–288. [Google Scholar]
- Yang, G. , Rose, M. , Turgeon, B.G. and Yoder, O.C. (1996) A polyketide synthase is required for fungal virulence and production of the polyketide T‐toxin. Plant Cell, 8, 2139–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, S.L. and Chung, K.‐R. (2010) Transcriptional regulation of elsinochrome phytotoxin biosynthesis by an EfSTE12 activator in the citrus scab pathogen Elsinoë fawcettii . Fungal Biol. 114, 64–73. [DOI] [PubMed] [Google Scholar]
- Yoshihara, T. , Shimanuki, T. , Araki, T. and Sakamura, S. (1975) Phleichrome, a new phytotoxic compound produced by Cladosporium phlei . Agric. Biol. Chem. 39, 1683–1684. [Google Scholar]
- You, B.J. , Lee, M.H. and Chung, K.R. (2008) Production of cercosporin toxin by the phytopathogenic Cercospora fungi is affected by diverse environmental signals. Can. J. Microbiol. 54, 259–269. [DOI] [PubMed] [Google Scholar]
- Yu, J.H. and Keller, N. (2005) Regulation of secondary metabolism in filamentous fungi. Annu. Rev. Phytopathol. 43, 437–458. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Xie, J. , Zhang, L. , Li, C. , Chen, H. , Gu, Y. and Zhao, J. (2009) A novel elsinochrome A derivative: a study of drug delivery and photodynamic activity. Photochem. Photobiol. Sci. 8, 1676–1682. [DOI] [PubMed] [Google Scholar]


