SUMMARY
Fungal histidine kinases (HKs) have been implicated in different processes, such as the osmostress response, hyphal development, sensitivity to fungicides and virulence. Members of HK class III are known to signal through the HOG mitogen‐activated protein kinase (MAPK), but possible interactions with other MAPKs have not been explored. In this study, we have characterized fhk1, encoding a putative class III HK from the soil‐borne vascular wilt pathogen Fusarium oxysporum. Inactivation of fhk1 resulted in resistance to phenylpyrrole and dicarboximide fungicides, as well as increased sensitivity to hyperosmotic stress and menadione‐induced oxidative stress. The osmosensitivity of Δfhk1 mutants was associated with a striking and previously unreported change in colony morphology. The Δfhk1 strains showed a significant decrease in virulence on tomato plants. Epistatic analysis between Fhk1 and the Fmk1 MAPK cascade indicated that Fhk1 does not function upstream of Fmk1, but that the two pathways may interact to control the response to menadione‐induced oxidative stress.
INTRODUCTION
Two‐component histidine kinase (HK) phosphorelay protein complexes are important components of the signal‐sensing machinery of bacteria, plants and fungi, allowing them to sense and adapt to their environment (Catlett et al., 2003; West and Stock, 2001). In prokaryotes, this signalling complex is generally composed of two distinct proteins, HK and a response regulator (RR). In response to environmental stimuli, HK autophosphorylates a conserved histidine residue and transfers the phosphoryl group to an aspartic acid residue in the RR protein, leading to changes in gene expression and cell response (West and Stock, 2001). Most eukaryotic two‐component systems are composed of hybrid HKs in which the HK and RR domains are present in a single protein. In this case, the phosphate group on the aspartate residue of the HK RR domain is transferred to a histidine phosphotransfer (HPT) protein which, in turn, phosphorylates an aspartate residue of the receiver domain on a RR protein (West and Stock, 2001). Although bacterial HK signalling systems control the cell response through direct activation of gene transcription by the RR protein, eukaryotic HKs are generally found at the head of intracellular signalling pathways that recruit more conventional downstream signalling modules, such as mitogen‐activated protein kinase (MAPK) cascades (West and Stock, 2001).
Two‐component systems have been well studied in the yeast Saccharomyces cerevisiae. The S. cerevisiae genome contains one HK, Sln1, one HPT protein, Ypd1, and two RRs, Ssk1 and Skn7. Sln1 acts as a sensor HK, transmitting the high‐osmolarity response signal via the Ypd1–Ssk1 phosphorelay to the Ssk2 (Ssk22)/Pbs2/Hog1 MAPK module. Under hyperosmotic conditions, Sln1 is in an unphosphorylated state that is conducive to the activation of the downstream Hog1 MAPK (Hohmann, 2002; Saito and Tatebayashi, 2004). Sln1 also mediates the phosphorylation of Skn7, which acts as a transcription factor during hyperosmotic and oxidative stress and interacts with the cell wall integrity (CWI) MAPK and the calcium/calcineurin pathways (Levin, 2005; Li et al., 1998; Morgan et al., 1997; Williams and Cyert, 2001). In addition to Sln1, the Hog1 MAPK cascade can be activated by an alternative osmosensing branch via Sho1, Ste11 and Pbs2 (Posas and Saito, 1997).
In filamentous fungi, orthologues of the elements of the yeast Hog1 pathways have been identified by functional and in silico analysis (Dixon et al., 1999; Fujimura et al., 2003; Han and Prade, 2002; Izumitsu et al., 2007; Rispail et al., 2009; Segmüller et al., 2007; Yamashita et al., 2008; Zhang et al., 2002). For example, Neurospora crassa Os‐2 is orthologous to yeast Hog1 (Zhang et al., 2002) and is activated via the MAPKKK Os‐4 and the MAPKK Os‐5 (Fujimura et al., 2003). Orthologues of Sln1, Sho1, Ypd1, Ssk1, Skn7 and Ste11 are also present in N. crassa (Jones et al., 2007; Rispail et al., 2009). However, the N. crassa HK responsible for the high‐osmolarity response, Nik‐1/Os‐1, is not the orthologue of yeast Sln1 (Catlett et al., 2003).
Filamentous fungi contain multiple HKs that have been classified into 11 groups on the basis of the protein sequence (Catlett et al., 2003). In filamentous ascomycetes, so far, only members of HK class III have been associated with the high‐osmolarity response, including Nik‐1/Os‐1 in N. crassa (Ochiai et al., 2001), NikA in Aspergillus nidulans (Hagiwara et al., 2007), Daf1/Bos1 in Botrytis cinerea (Cui et al., 2002; Viaud et al., 2006), Nik1 in Cochliobolus heterostrophus and Alternaria brassicicola (Avenot et al., 2005; Yoshimi et al., 2004) and Hik1 in Magnaporthe oryzae (Motoyama et al., 2005a). In addition to osmosensitivity, mutations in class III HKs also confer resistance to certain classes of fungicides, and often result in morphological defects (Hagiwara et al., 2007; Motoyama et al., 2005a; Ochiai et al., 2001; Viaud et al., 2006; Yoshimi et al., 2004).
Class III HKs have been implicated in the dimorphism and virulence of several human pathogens, such as Blastomyces dermatiditis (Nemecek et al., 2006), Candida albicans (Yamada‐Okabe et al., 1999), Histoplasma capsulatum (Nemecek et al., 2006), Aspergillus fumigatus (Clemons et al., 2002) and Cryptococcus neoformans (Bahn et al., 2006). The role of class III HKs in the virulence of plant pathogenic fungi is less clear, and depends not only on the fungal species but also on the type of mutation. Thus, the deletion of class III HKs strongly reduced the virulence in B. cinerea and A. brassicicola (Cho et al., 2009; Liu et al., 2008; Viaud et al., 2006), but had no effect in M. oryzae (Motoyama et al., 2005a). Similarly, point mutations in the class III HK Mf‐os1 decreased the virulence of Monilinia fructicola (Ma et al., 2006), but had no such effect in naturally occurring fungicide‐resistant field isolates of B. cinerea or A. brassicicola (Avenot et al., 2005; Cui et al., 2002).
Fusarium oxysporum, a ubiquitous soil‐borne ascomycete, causes vascular wilt disease on more than 100 plant species, provoking severe losses in important crops, such as banana, cotton, melon and tomato (Gordon and Martyn, 1997). This fungus is also being recognized as an emerging human pathogen which poses a lethal threat to immunocompromised individuals (Nucci and Anaissie, 2007). Its remarkably broad host range and the array of molecular tools available make F. oxysporum an attractive model for studying the different aspects of fungal infection (Di Pietro et al., 2003; Michielse and Rep, 2009). It has been shown previously that Fmk1, an orthologue of the yeast mating and filamentation MAPKs Fus3/Kss1, and one of its downstream targets, the transcription factor Ste12, are crucial for the virulence of F. oxysporum on tomato plants (Di Pietro et al., 2001; Rispail and Di Pietro, 2009). Mutants lacking the fmk1 gene are impaired in multiple virulence‐related functions, such as root adhesion, host penetration, invasive growth on living plant tissue and secretion of cell wall‐degrading enzymes, as well as in vegetative hyphal fusion, a ubiquitous process in filamentous fungi (Delgado‐Jarana et al., 2005; Di Pietro et al., 2001; Prados Rosales and Di Pietro, 2008). In contrast with the pleiotropic role of Fmk1, the function of F. oxysporum Ste12 is restricted to host penetration, invasive growth and virulence (Rispail and Di Pietro, 2009). In addition to Fmk1, F. oxysporum has two additional MAPKs orthologous to yeast Mpk1 and Hog1 (Rispail et al., 2009), respectively, as well as at least 21 HKs (N. Rispail and A. Di Pietro, unpublished work). The potential role of these additional signalling modules in the virulence of F. oxysporum is currently unknown.
In this study, we investigated the role of fhk1, encoding a class III HK of F. oxysporum. Our study addressed three major questions: (i) is Fhk1 required for correct cellular adaptation to stresses?; (ii) is Fhk1 required for root infection and virulence?; and (iii) does Fhk1 interact with the Fmk1/Ste12 signalling pathway? We found that deletion mutants lacking the fhk1 gene were resistant to phenylpyrrole and dicarboximide fungicides, showed increased sensitivity to hyperosmotic and oxidative stresses, and displayed reduced virulence on tomato plants. Interestingly, the increased sensitivity of Δfhk1 strains to oxidative stress was restored to the wild‐type level in a Δfmk1Δfhk1 double mutant, providing new genetic evidence for an interaction between the Fmk1 and Fhk1 signalling pathways.
RESULTS
Cloning and targeted knockout of the F. oxysporum fhk1 gene
A blastp search for orthologues of class III HK in the annotated genome of F. oxysporum f. sp. lycopersici strain 4287, available at the Broad Institute (http://www.broadinstitute.org/annotation/genome/fusarium_group/MultiHome.html), identified a single gene, FOXG_01684 (subsequently termed fhk1 for Fusarium histidine kinase 1). Comparison with the orthologous gene of the related species F. verticillioides (FVEG_08048; AY456038) suggested that the predicted open reading frame of FOXG_01684 lacked the N‐terminal part of the protein as a result of sequencing errors. A 6.8‐kb genomic region encompassing the complete fhk1 gene, including the promoter, coding region and terminator, was sequenced manually, revealing an open reading frame of 3882 bp interrupted by five putative introns, according to the Fgenesh 2.6 prediction server (http://linux1.softberry.com/berry.phtml?topic=fgenesh&group=programs&subgroup=gfind). The fhk1 gene encodes a putative 1293‐amino‐acid polypeptide with a predicted molecular mass of 141.83 kDa and a pI value of 5.37. The sequence of the F. oxysporum fhk1 gene has been deposited in GenBank under accession number GQ871928. Analysis of the Fhk1 sequence with the InterProScan prediction server (http://www.ebi.ac.uk/Tools/InterProScan/) detected all the characteristic domains of class III HKs, including five HAMP repeats (IPR003660) (Aravind and Ponting, 1999), the HK A signal transducer domain (HK; IPR003661), the HK‐like ATPase domain (HATPase; IPR003594) and the RR domain (REC; IPR001789), as well as the highly conserved H‐, N‐, G1‐, G2‐ and D‐boxes of the HK and REC catalytic site (West and Stock, 2001) (Fig. 1A). Alignment of F. oxysporum Fhk1 with sequences from the databases revealed significant identity with class III HKs from other fungi, including F. verticillioides GmNik1 (98.9% identity), N. crassa Os‐1 (80.0%), M. oryzae Hik1 (77.6%), C. heterostrophus Nik1 (61.4%) and Candida albicans Nik1 (47.3%) (Fig. 1B,C). On the basis of these data, we conclude that F. oxysporum fhk1 is the orthologue of N. crassa os‐1.
Figure 1.
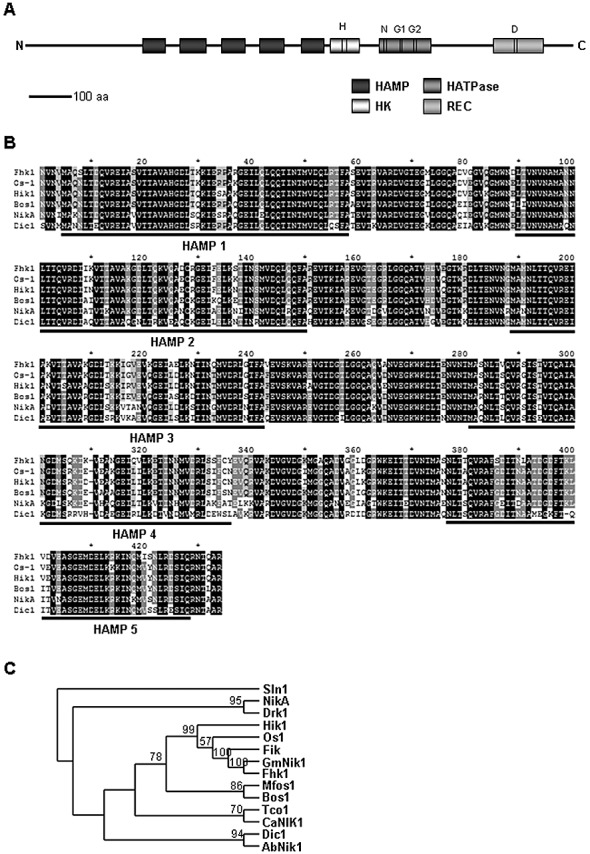
Fhk1 protein structure. (A) Scaled diagram of the domain structure of Fusarium oxysporum Fhk1. The positions of the conserved H‐, N‐, G1‐, G2‐ and D‐boxes are shown. (B) Amino acid alignment of the HAMP repeat region of Fhk1 with Neurospora crassa Os1 (AAB01979), Magnaporthe grisea Hik1 (BAB40947), Botrytis cinerea Bos1 (AAL30826), Aspergillus nidulans NikA (AN4479.3) and Cochliobolus heterostrophus Dic1 (BAC78679). HAMP domain repeat regions are indicated by black lines. Absolutely conserved residues are shaded in black, residues conserved in at least 80% are shaded in dark grey and residues conserved in at least 60% are shaded in light grey. (C) Cladogram generated by the maximum likelihood method after alignment of F. oxysporum Fhk1 with F. verticillioides GmNik1 (AAR30126), F. solani f. sp. pisi Fik (AAD09491), Neurospora crassa Os1 (AAB01979), Magnaporthe grisea Hik1 (BAB40947), Botrytis cinerea Bos1 (AAL30826), Aspergillus nidulans NikA (AN4479.3), Cochliobolus heterostrophus Dic1 (BAC78679), Alternaria brassicicola AbNik1 (AAU10313), Monilinia fructicola Mfos1 (ABF60145), Candida albicans CaNik1 (BAA24952), Ajellomyces dermatiditis Drk1 (ABF13477) and Cryptococcus neoformans Tco1 (ABD49452). Saccharomyces cerevisiae Sln1 (NP_012119) was included as outgroup.
To explore the biological role of Fhk1 in F. oxysporum, a Δfhk1 null allele was generated by replacing most of the fhk1 open reading frame with the hygromycin resistance cassette, using a polymerase chain reaction (PCR) fusion method (Fig. S1A, see Supporting Information; see Experimental procedures for details). The knockout construct was introduced into both the wild‐type strain and the Δfmk1 mutant to study possible epistatic relationships between the two genes. Hygromycin‐resistant transformants were analysed by PCR with different combinations of gene‐specific primers, identifying six and one transformants, respectively, which produced amplification products indicative of homologous integration‐mediated gene replacement (data not shown). Southern blot analysis of these transformants confirmed the replacement of a 6‐kb EcoRI fragment, corresponding to the wild‐type fhk1 allele, by a fragment of 2.8 kb (Fig. S1B, see Supporting Information), demonstrating that these transformants, named Δfhk1 and Δfmk1Δfhk1, respectively, lacked a functional fhk1 gene. By contrast, transformant efhk1‐1 still showed the wild‐type fragment, together with another hybridizing fragment, suggesting that it contained an ectopic insertion of the knockout construct (Fig. S1B, see Supporting Information).
To confirm that the phenotype of the Δfhk1 mutants was indeed caused by a loss of fhk1 function, a 9‐kb DNA fragment encompassing the complete F. oxysporum fhk1 gene was introduced into the Δfhk1‐2 strain by cotransformation with the phleomycin resistance marker. PCR with gene‐specific primers Fhk1‐1 and Fhk1‐2 produced a 6‐kb amplification product, identical to that obtained from the wild‐type strain, in two phleomycin‐resistant transformants, but not in the Δfhk1‐2 mutant (Fig. S1C, see Supporting Information). We conclude that these cotransformants, denominated Δfhk1+fhk1, had integrated an intact copy of the fhk1 gene into the genome.
Fhk1 is not required for vegetative hyphal growth
To test whether fhk1 was required for vegetative hyphal growth, the colony growth rate was determined on synthetic medium (SM) adjusted to pH 4.5 or pH 6.5. As reported previously (Prados Rosales and Di Pietro, 2008), the Δfmk1 strain showed a significant reduction in growth rate compared with the wild‐type at both pH values (Fig. S2, see Supporting Information). By contrast, no significant difference was observed between the wild‐type and Δfhk1 mutants, or between the Δfmk1 single mutant and the Δfmk1Δfhk1 double mutant (Fig. S2, see Supporting Information). This suggests that fhk1 is not required for normal vegetative growth on artificial medium as reported for other class III HKs (Cho et al., 2009; Motoyama et al., 2005a).
Fhk1 mediates the sensitivity to phenylpyrrole and dicarboximide fungicides
Fungal class III HKs have been shown to confer sensitivity to different classes of fungicide (Motoyama et al., 2005a; Ochiai et al., 2001; Viaud et al., 2006). We thus tested whether the deletion of fhk1 in F. oxysporum affected the sensitivity to phenylpyrrole and dicarboximide fungicides. Both the wild‐type and Δfmk1 strains showed a dramatic decrease in growth rate on SM supplemented with 10 µg/mL of fludioxonil or iprodione, compared with SM without fungicide (Fig. 2). By contrast, growth rates of Δfhk1 and Δfmk1Δfhk1 mutants were not affected by the presence of either type of fungicide. Complementation of Δfhk1 with the native fhk1 gene restored fungicide sensitivity to the wild‐type level (Fig. 2). We conclude that Fhk1 mediates the sensitivity of F. oxysporum to phenylpyrrole and dicarboximide fungicides.
Figure 2.
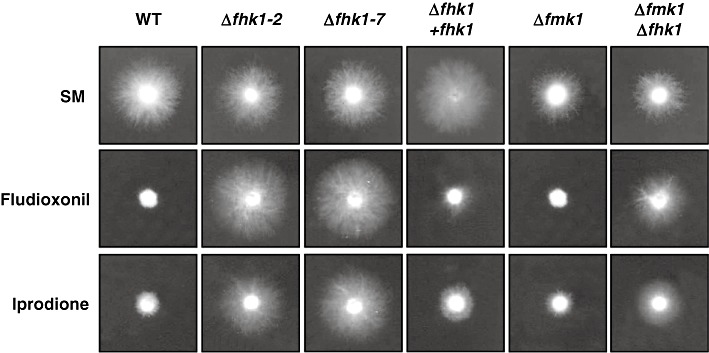
Fhk1 mediates sensitivity to phenylpyrrole and dicarboximide fungicides. Conidia of the indicated strains were spotted onto plates containing synthetic medium (SM), or SM with 10 µg/mL fludioxonil or 10 µg/mL iprodione, and grown at 28 °C for 3 days.
Fhk1 is required for cellular adaptation to hyperosmotic stress
In addition to fungicide sensitivity, fungal class III HKs have been associated with the response to osmotic stress by acting at the head of the Hog1 MAPK pathway (Liu et al., 2008; Yoshimi et al., 2005). To determine whether Fhk1 plays a role in osmoregulation, we determined colony growth on SM containing 0.8 m sodium chloride, 0.8 m potassium chloride or 1.2 m glycerol. All three types of osmolyte produced moderate growth inhibition (30%) in the wild‐type and the complemented Δfhk1+fhk1 strains, but only slight growth inhibition in the Δfmk1 mutant (15%) (Fig. 3A).
Figure 3.
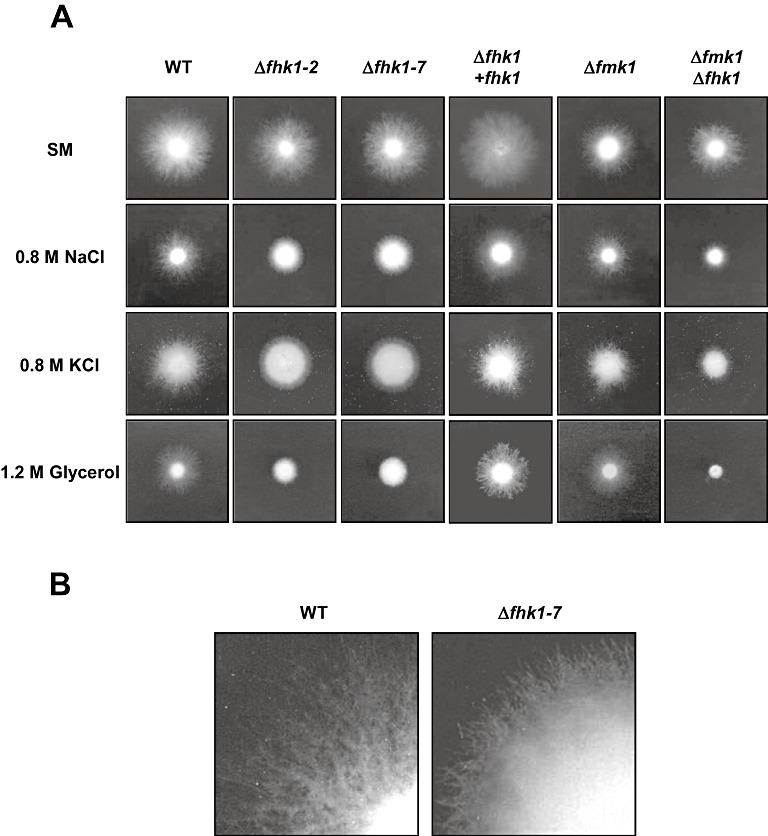
Fhk1 is required for adaptation to osmotic stress. (A) Conidia of the indicated strains were spotted onto plates containing synthetic medium (SM), or SM with 0.8 m NaCl, 0.8 m KCl or 1.2 m glycerol, and grown at 28 °C for 3 days. (B) Detailed view of the colony edge after salt treatment (0.8 m NaCl) for wild‐type and Δfhk1 mutant strain.
Under salt stress (NaCl and KCl), the Δfhk1 mutants showed a similar reduction in radial growth to the wild‐type strain, whereas polyol‐induced osmotic stress led to a more pronounced growth inhibition, indicating that Δfhk1 mutants are more sensitive than the wild‐type to high concentrations of glycerol (Fig. 3A). Under all three conditions of hyperosmotic stress, the colony morphology of the Δfhk1 mutants differed drastically from that of the wild‐type. In contrast with the wild‐type or Δfmk1 strain, whose colony morphology was normal, albeit with a slower growth rate, colonies of the Δfhk1 mutants were very compact with extremely short extending hypha (Fig. 3A,B). The Δfmk1Δfhk1 double mutant showed the same morphological defects as Δfhk1, and a more pronounced decrease in growth rate compared with the Δfmk1 strain. Collectively, these results indicate that: (i) Fhk1 is required for correct adaptation to hyperosmotic stress; (ii) Fmk1 acts as a negative regulator of osmotic stress adaptation; and (iii) Fmk1 and Fhk1 act in independent and additive pathways, as the double mutation leads to a more drastic phenotype.
Fhk1 contributes to the oxidative stress response
The oxidative stress response in fungi has been shown to depend on both the Hog1 MAPK and CWI pathways, depending on the nature of the stress‐inducing compound (Levin, 2005). As class III HKs have been suggested to act at the top of the Hog1 pathway in filamentous fungi (Liu et al., 2008; Yoshimi et al., 2005), we tested the effect of compounds inducing either oxidative (menadione, hydrogen peroxide) or nitrosative (sodium nitrite) stress on F. oxysporum wild‐type and mutant strains. The addition of 10 µg/mL menadione induced only moderate growth inhibition in the wild‐type and Δfmk1 strains, but had a more drastic effect in Δfhk1 mutants (Fig. 4). Interestingly, the double Δfmk1Δfhk1 mutant only had a mild phenotype, similar to the wild‐type and the Δfmk1 strains. No significant differences in growth inhibition between the different strains were observed in response to hydrogen peroxide (Fig. 4). Similarly, no differences were observed between the wild‐type and Δfhk1 strains in response to 1 mm sodium nitrite (Fig. 4). However, nitrosative stress led to a significant decrease in growth rate in the Δfmk1 and Δfmk1fhk1 mutants (Fig. 4). We conclude that: (i) Fhk1 contributes to the tolerance to menadione‐induced oxidative stress; (ii) Fmk1 negatively regulates the response to menadione‐induced oxidative stress, acting either downstream or competing with Fhk1; and (iii) Fmk1 but not Fhk1 is required for nitrosative stress tolerance.
Figure 4.
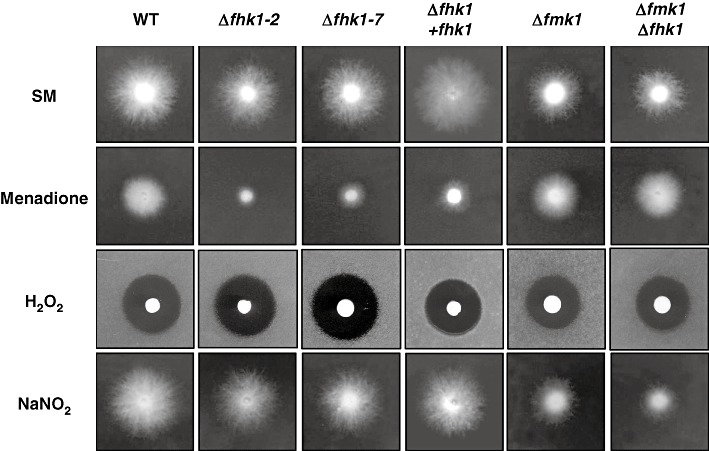
Fhk1 and Fmk1 play opposing roles in the menadione‐induced oxidative stress response. Conidia of the indicated strains were spotted onto plates containing synthetic medium (SM), or SM with 10 µg/mL menadione or 1 mm NaNO2, and grown at 28 °C for 3 days. For H2O2 sensitivity assays, conidia were spread onto SM plates and grown for 1 day at 28 °C before placing a filter disc saturated with 3% H2O2 at the centre of the plates, followed by growth for two additional days at 28 °C. Growth inhibition was assessed by measuring the clear halo surrounding the filter disc.
Fhk1 is required for full virulence on tomato plants
The Fmk1 MAPK controls invasive growth functions in F. oxysporum, such as the ability to penetrate cellophane sheets (Prados Rosales and Di Pietro, 2008) or to invade and colonize living fruit tissue (Di Pietro et al., 2001). We explored whether these invasive growth functions are also mediated by Fhk1. In contrast with Δfmk1 and the Δfmk1Δfhk1 double mutant, Δfhk1 mutants penetrated cellophane sheets and colonized apple slices as efficiently as the wild‐type strain (Fig. S3, see Supporting Information).
Plant infection was performed by inoculating the roots of tomato seedlings with microconidial suspensions of the different strains. Disease symptoms in plants inoculated with the wild‐type strain increased steadily throughout the experiment, and most of the plants were dead 20 days after inoculation (Fig. 5). As reported previously (Di Pietro et al., 2001), plants inoculated with the Δfmk1 mutant had extremely low disease ratings. Two independent Δfhk1 mutants, Δfhk1‐2 and Δfhk1‐4, showed significantly reduced virulence compared with the wild‐type, with a delay of approximately 10 days in symptom development. The Δfmk1Δfhk1 double mutants behaved similarly to the Δfmk1 single mutant. We conclude that Fhk1 contributes to the virulence of F. oxysporum on tomato plants independent of Fmk1, although it does not mediate invasive growth functions.
Figure 5.
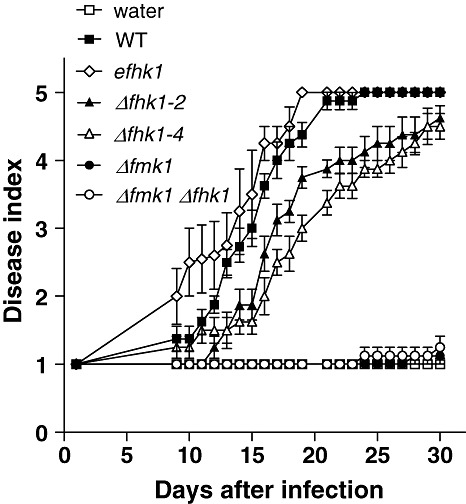
Fhk1 is required for full virulence of Fusarium oxysporum on tomato plants. The graph shows the incidence of Fusarium wilt on tomato plants (cultivar Monica) inoculated with the indicated strains. The severity of disease symptoms was recorded at different times after inoculation, using an index ranging from one (healthy plant) to five (dead plant). Error bars represent standard errors calculated from 10 plants.
DISCUSSION
Fungal HKs are involved in essential cellular processes, such as osmosensing, the oxidative stress response, cell cycle control and virulence (Bahn, 2008). In particular, class III HKs have been shown to mediate the cellular responses to osmotic and oxidative stresses (Motoyama et al., 2005a; Viaud et al., 2006), to control conidiation and asexual morphology (Liu et al., 2008; Viaud et al., 2006), to regulate the mould‐to‐yeast transition of dimorphic fungi (Nemecek et al., 2006) and to mediate pathogenicity (Liu et al., 2008; Nemecek et al., 2006; Viaud et al., 2006; Yamada‐Okabe et al., 1999). However, the role of class III HKs in these different processes differs greatly according to the type of mutation and the fungal species. In this study, we characterized Fhk1, a class III HK from the plant and human pathogen F. oxysporum, and studied its role in the mediation of cellular adaptation to different stresses and virulence. In addition, we explored the potential interaction between this HK and the pathogenicity MAPK Fmk1. As reported in other filamentous fungi, the predicted F. oxysporum Fhk1 protein contains all the characteristic domains of hybrid HKs, as well as the five HAMP repeats specific to class III fungal HKs (Catlett et al., 2003). Although the HK, HATPase and REC domains are strictly required for HK activation and its signal transduction function (Catlett et al., 2003; Motoyama et al., 2005a; West and Stock, 2001), the role of the HAMP domains remains unknown, although mutations in the HAMP domains of os‐1, dic1, abnik1 and bos1 are responsible for the increased osmosensitivity and fungicide resistance of N. crassa, C. heterostrophus, A. brassicicola and B. cinerea, respectively (Avenot et al., 2005; Cui et al., 2002; Ochiai et al., 2001; Yoshimi et al., 2004). These phenotypes, together with the high sequence conservation of the HAMP region in filamentous fungi (Avenot et al., 2005) (Fig. 1B), strongly suggest that the HAMP region plays a pivotal role in Fhk1 function. The elucidation of this role will be an important challenge for future studies.
Function of Fhk1 in the stress response
In this study, we have demonstrated that Fhk1 is required for normal sensitivity of F. oxysporum to phenylpyrrole and dicarboximide fungicides (Fig. 2). This result confirms the involvement of class III HKs in the sensitivity to phenylpyrrole, dicarboximide and aromatic hydrocarbon fungicides reported for N. crassa, M. grisea, C. heterostrophus, B. cinerea, among others (Motoyama et al., 2005a; Ochiai et al., 2001; Viaud et al., 2006; Yoshimi et al., 2004). Although the exact mode of action of these fungicides is still unclear, the finding that the heterologous expression of M. oryzae hik1 in the yeast S. cerevisiae confers susceptibility to this otherwise resistant organism suggests that class III HKs are direct targets of these classes of fungicide (Motoyama et al., 2005b). Several lines of evidence indicate that fungicide‐activated class III HKs recruit the conserved stress‐activated Hog1 MAPK pathway, leading to the aberrant accumulation of glycerol and other stress‐related responses (Hagiwara et al., 2009; Kojima et al., 2004; Motoyama et al., 2005b; Yamashita et al., 2008; Yoshimi et al., 2005). For instance, the application of fungicide or osmotic stress has been shown to induce the hyperphosphorylation of Hog1, and mutants in Hog1 orthologues are resistant to fungicides (Kojima et al., 2004; Noguchi et al., 2007; Yoshimi et al., 2005). In addition, a recent microarray analysis has shown that the transcriptional response to the phenylpyrrole fungicide fludioxonil in Aspergillus nidulans is dependent on Hog1 orthologues and partly overlaps with the transcriptional response to hyperosmotic stress (Hagiwara et al., 2009). The fact that fhk1‐deficient mutants of F. oxysporum show a higher sensitivity to hyperosmotic and menadione‐induced oxidative stresses suggests that recruitment of the Hog1 pathway on activation of Fhk1 also occurs in F. oxysporum.
Higher sensitivity to osmotic stress, including salt and polyols, and to menadione has been reported for most mutants lacking class III HKs, although the exact effect of a given HK mutation on osmotic stress adaptation depends on the fungal species studied (Alex et al., 1996; Hagiwara et al., 2007; Motoyama et al., 2005a; Ochiai et al., 2001; Viaud et al., 2006; Yoshimi et al., 2004). Thus, mutants of N. crassa, C. heterostrophus and B. cinerea show a higher sensitivity to salt (NaCl or KCl) than to polyols (glycerol, sorbitol) (Alex et al., 1996; Viaud et al., 2006; Yoshimi et al., 2004), whereas M. oryzae Δhik1 mutants show a strong growth reduction on sorbitol but not on salt (Motoyama et al., 2005a). In F. oxysporum, we detected a similar trend, as Δfhk1 mutants were more sensitive to glycerol than to salt stress. Strikingly, the growth of Δfhk1 mutants under both types of hyperosmotic stress was associated with a clear morphological phenotype: fungal colonies showed a dense and compact colony morphology with very short hyphae and more aerial mycelium than the wild‐type strain (see Fig. 3B). To our knowledge, such a morphological switch in response to hyperosmotic stress has not been reported previously in filamentous fungi. Our finding indicates that the Hog1‐dependent stress response may be more complex than previously thought, or that Fhk1 may recruit other signalling pathways in addition to Hog1. The fact that knockout mutants in sak1, the B. cinerea Hog1 orthologue, are still sensitive to fungicides supports this second hypothesis and suggests that other signalling pathways could also be recruited by these HKs (Liu et al., 2008).
In the dimorphic human pathogen B. dermatiditis, a class III HK has been shown to control the yeast‐to‐hyphal transition, a process related to pseudohyphal growth in S. cerevisiae which is mediated by the conserved MAPK Kss1 (Nemecek et al., 2006). In plant pathogenic fungi, Kss1 orthologues, such as F. oxysporum Fmk1, play a key role in plant pathogenicity (Di Pietro et al., 2001). As the upstream elements of Fmk1 are still unknown, we reasoned that Fhk1 could function as an upstream element of the Fmk1 pathway. As the Δfmk1 mutant had the same sensitivity to fungicides and to osmotic stress as the wild‐type (see 2, 3), we conclude that Fmk1 is probably not a direct downstream element of Fhk1. However, we found evidence suggesting that the Fmk1 and Fhk1 pathways may interact to control the response to menadione‐induced oxidative stress. Thus, the increased sensitivity to menadione of the Δfhk1 mutant was rescued to wild‐type levels in the Δfmk1Δfhk1 double mutant (see Fig. 4), indicating that Fmk1 may be a negative regulator of the oxidative stress response, and that one function of Fhk1 may be to inhibit Fmk1 during the response to oxidative stress. The role of Fmk1 in stress responses may be even more complex, as we observed that the Δfmk1 mutant is slightly more sensitive to nitrosative stress, which is generally thought to be under the control of the CWI pathway (Brown et al., 2009). Collectively, our results suggest that the pathogenicity MAPK Fmk1 may participate, at least partially, in controlling the responses to different types of stress. In C. heterostrophus, a recent report has shown that the pathogenicity MAPK Chk1, but not Hog1, controls the fungal response to oxidative stress after activation of its upstream element ChSte11 (Izumitsu et al., 2009). However, the phenotype described for the C. heterostrophus chk1‐deficient mutant in response to oxidative stress is somewhat different from that of F. oxysporum, highlighting the complexity of stress signalling in filamentous fungi. The clarification of the connection between HKs, Fmk1, Hog1 and the CWI MAPK will be an important challenge to better understand how filamentous fungi adapt to changes in the environment.
Role of Fhk1 in the virulence of F. oxysporum
In addition to the important role of Fhk1 in fungicide sensitivity and the stress response, we found that fhk1 deletion decreased significantly the virulence of F. oxysporum in tomato plants. HKs have been reported as pathogenicity determinants of human pathogens, such as Candida albicans, B. dermatiditis, Cryptococcus neoformans and Aspergillus fumigatus (see Kruppa and Calderone, 2006 and references therein). In plant pathogens, the involvement of class III HKs in virulence has so far only been studied in air‐borne pathogens, leading to conflicting results, with mutant phenotypes ranging from highly virulent to completely avirulent depending on the type of mutation, genetic background and fungal species studied. HKs are required for full virulence in the necrotrophic fungi B. cinerea and A. brassicicola, but not in the hemibiotroph M. oryzae (Cho et al., 2009; Liu et al., 2008; Motoyama et al., 2005a; Viaud et al., 2006). Thus, there is an important need to address the role of class III HKs in a soil‐borne pathogen, such as F. oxysporum, which exists in a completely different lifestyle and environment. In this study, we have shown that, although Δfhk1 mutants retain their capacity to colonize fruit tissue and penetrate cellophane membranes, they show a clear reduction in disease symptoms in tomato plants. The fact that Δfhk1 mutants are still able to cross cellophane and to colonize living fruit tissue suggests that the role of Fhk1 in virulence is mainly restricted to post‐penetration events. Similarly, Δabnik1 and Δbos1 mutants of A. brassicicola and B. cinerea, respectively, were still able to form appressoria and to penetrate, but were defective in colonizing healthy plant tissue (Cho et al., 2009; Viaud et al., 2006). Two hypotheses could explain the observed attenuated virulence phenotype of Δfhk1 mutants. On the one hand, the higher sensitivity to different types of stress decreases the likelihood of the mutants surviving within the adverse environment of the host. The plant defence response, for example, is well known to involve the generation of different oxidative agents, such as hydrogen peroxide and superoxide (Huckelhoven and Kogel, 2003; Scott and Eaton, 2008). On the other hand, Fhk1 functions upstream of a signalling pathway that partly contributes to virulence. In F. oxysporum and other plant pathogenic fungi, virulence is under the control of the conserved pathogenicity MAPK cascade and its main downstream target, the transcription factor Ste12 (Di Pietro et al., 2001; Rispail and Di Pietro, 2009; Rispail et al., 2009). Our failure to detect any epistatic effects between Fhk1 and Fmk1 [see Fig. 5 and Fig. S3 (Supporting Information)] suggests that the role of Fhk1 in virulence is likely to be independent of the pathogenicity MAPK. In B. cinerea, mutation of the Hog1 orthologue Sak1 led to a similar pathogenicity defect as deletion of the HK Bos1 (Liu et al., 2008; Segmüller et al., 2007). Thus, it is possible that Fhk1 also recruits the Hog1 MAPK in F. oxysporum. The functional link between Fhk1 and the Hog1 pathway, as well as their potential roles in the stress response and virulence of F. oxysporum, will be the subject of future investigations.
EXPERIMENTAL PROCEDURES
Fungal isolates and culture conditions
Fusarium oxysporum f. sp. lycopersici race 2 wild‐type strain 4287 (FGSC 9935) was used in all experiments. The generation and molecular characterization of the F. oxysporumΔfmk1 mutant have been described previously (Di Pietro et al., 2001). All fungal strains were stored as microconidial suspensions at −80 °C with 30% glycerol. For the extraction of genomic DNA and for microconidia production, cultures were grown in potato dextrose broth (PDB; Difco, Detroit, MI, USA) at 28 °C with shaking at 170 rpm (Di Pietro and Roncero, 1998). Cellophane invasion assay was performed as described previously (Prados Rosales and Di Pietro, 2008). For phenotypic analysis of colony growth, aliquots of 2 × 105 microconidia were spotted onto SM containing 1% glucose as unique carbon source and 0.2% NaNO3 as nitrogen source, and buffered at pH 6.5 with 50 mm phosphate buffer (Di Pietro and Roncero, 1998). When needed, SM was supplemented with sodium chloride, potassium chloride, glycerol, menadione or sodium nitrite at the indicated concentrations. For fungicide assay, 1 mg/mL stock solutions of fludioxonil and iprodione, prepared in dimethylsulphoxide (DMSO) or ethanol, respectively, were used to complement SM at the indicated concentration. Fungal growth on fungicide‐supplemented SM was compared with that on SM complemented with 0.01% DMSO or ethanol, respectively. All chemicals were obtained from Sigma‐Aldrich (Sigma‐Aldrich Química SA, Madrid, Spain). To determine growth at pH 4.5 or pH 6.5, media were buffered with 50 mm phosphate buffer. After inoculation, plates were incubated for 3 days at 28 °C. To determine H2O2 sensitivity, 1 × 106 microconidia were evenly spread onto the surface of SM and grown overnight at 28 °C before adding a filter paper disc imbibed with 3% H2O2 (v/v) solution at the centre of the plate. Growth inhibition induced by H2O2 was determined after 3 days of growth at 28 °C by assessing the size of the clear halo surrounding the filter paper disc.
Nucleic acid manipulations
Genomic DNA was extracted from F. oxysporum mycelium following previously reported protocols (Chomczynski and Sacchi, 1987; Raeder and Broda, 1985). Southern analysis and probe labelling were carried out as described previously (Di Pietro and Roncero, 1998) using the nonisotopic digoxigenin labelling kit (Roche Diagnostics SL, Barcelona, Spain). Other routine DNA procedures were performed as described in standard protocols (Sambrook and Russell, 2001).
For sequencing of the fhk1 gene, genomic DNA of F. oxysporum was used for PCR amplification with primers Fhk1‐For/Fhk1‐KO1, Fhk1‐1/Fhk1‐2 and Fhk1‐KO2/Fhk1‐Rev designed from the conserved regions of F. graminearum and F. verticillioides fhk1 and its flanking genes (Table S1, see Supporting Information). The amplified DNA fragments were cloned into the pGEM‐T vector (Promega, Madison, WI, USA). Sequencing was performed at the Servicio de Secuenciación Automática de DNA (SCAI, University of Cordoba, Spain) using the Dyedeoxy Terminator Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) on an ABI Prism 377 Genetic Analyser apparatus (Applied Biosystems) employing the pGEMT vector‐specific primers SP6 and T7 as well as gene‐specific primers (Table S1, see Supporting Information). DNA and protein sequence databases were searched using the blast algorithm (Altschul et al., 1990).
Construction of the Δfhk1 null allele and fungal transformation
Knockout of the F. oxysporum fhk1 gene was performed by fusion PCR (Yang et al., 2004). PCRs were routinely performed with the High Fidelity Template PCR system (Roche Diagnostics SL) using a Perkin‐Elmer GeneAmp System 2400. First, 1.1‐ and 1.6‐kb DNA segments flanking the fhk1 coding region were amplified from the genomic DNA of F. oxysporum strain 4287 with primer pairs Fhk1‐1/Fhk1‐KO1 and Fhk1‐KO2/Fhk1‐2, respectively (Table S1, see Supporting Information; Fig. 2). The 5′ regions of Fhk1‐KO1 and Fhk1‐KO2 contained the complementary sequence of the M13 reverse and M13 forward primers, respectively, which were used to amplify the cassette containing the hygromycin B resistance gene under the control of the Aspergillus nidulans gpdA promoter (Punt et al., 1987). In the second reaction, the two fhk1 flanking regions were mixed with the hygromycin B resistance cassette in a molar proportion of 1:1:3 and fused using the external Fhk1‐1 and Fhk1‐2 primers (Table S1, see Supporting Information; Fig. 2). The construct generated was used to transform protoplasts of the F. oxysporum wild‐type strain or the Δfmk1 mutant to hygromycin resistance, and transformants were purified by monoconidial isolation as described previously (Di Pietro and Roncero, 1998). Transformants showing the homologous insertion of the construct were detected by PCR amplification of genomic DNA with primers Fhk1‐1 and Fhk1‐2, and confirmed by Southern analysis of genomic DNA digested with PstI and hybridized with a labelled probe obtained by PCR amplification with primers Fhk1‐KO2 and Fhk1‐2 (Fig. 2). For complementation experiments, a 9‐kb DNA fragment encompassing the entire fhk1 gene was amplified by PCR from F. oxysporum genomic DNA using primers Fhk1‐for and Fhk1‐2 (Table S1, see Supporting Information), and introduced into protoplasts of the Δfhk1 mutant by cotransformation with the phleomycin resistance cassette amplified from plasmid pAN8‐1 (Mattern et al., 1988). Phleomycin‐resistant transformants were selected as described previously (Di Pietro et al., 2001), and the presence of the wild‐type fhk1 allele in the complemented transformants was detected by PCR of genomic DNA with primers Fhk1‐1 and Fhk1‐2.
Plant infection assays
Tomato plant inoculation assays were performed in a growth chamber as described previously (Di Pietro and Roncero, 1998). At different times after inoculation, the severity of disease symptoms was recorded using an index ranging from one (healthy plant) to five (dead plant). Ten plants were used for each treatment, and experiments were performed in triplicate. Invasive growth assays on tomato fruits or apple slices (cultivar Golden Delicious) were carried out as described previously (Di Pietro et al., 2001; Lopez‐Berges et al., 2009) using three replicates.
Phylogenetic analysis
The deduced amino acid sequences of F. oxysporum Fhk1 and fungal orthologues were aligned using the ClustalW algorithm (Thompson et al., 1994) and cleaned by GBlocks v0.91b (Castresana, 2000). The phyml 3.0 program (Guindon and Gascuel, 2003) was used to perform a 1000 nonparametric bootstrap phylogenetic analysis of the resulting alignment of 911 amino acid characters with the maximum likelihood method after optimization of the settings by the ModelGenerator program, version 0.85 (Keane et al., 2006). The analysis was performed using the LG substitution model (Le and Gascuel, 2008), with a gamma distribution parameter alpha of 1.18. The phylogenetic relationship between Fhk1 and other class III HK sequences was depicted in a phylogenetic tree constructed using the TreeView 1.6.6 program (Page, 1996) (Fig. 1C).
Supporting information
Fig. S1 Targeted disruption of the Fusarium oxysporum fhk1 gene. (A) Physical maps of the fhk1 locus and the gene replacement construct obtained by polymerase chain reaction (PCR) fusion (Δfhk1 allele). (B) Southern hybridization analysis of wild‐type strain 4287 (1) and transformants efhk1‐1 (2), Δfhk1‐2 (3), Δfhk1‐4 (4), Δfhk1‐7 (5), Δfmk1 (6) and Δfmk1Δfhk1 (7). Genomic DNA treated with PstI was hybridized with the probe indicated in (A). (C) PCR amplification of genomic DNA of the wild‐type strain 4287 (1), knockout mutant Δfhk1‐2 (2) and complemented strains Δfhk1+fhk1 1 (3) and Δfhk1+fhk1 2 (4), using primers Fhk1‐1 and Fhk1‐2 (fhk1) or M13 Forward and PHL (Phleo).
Fig. S2 Fhk1 is not required for vegetative hyphal growth. Conidia of the indicated strains were spotted onto plates with synthetic medium (SM) buffered at the indicated pH values and grown at 28 °C for 3 days.
Fig. S3 Fhk1 is not required for invasive growth. Microconidial suspensions of the indicated strains were applied for the different invasive growth assays. (A) Invasive growth on apple slices inoculated with microconidia and incubated at 28 °C for 4 days. (B) Penetration of cellophane sheets. Colonies were grown for 4 days on a plate with minimal medium covered by a cellophane sheet (Before); the cellophane sheet with the colony was removed and the plates were incubated for an additional day (After).
Table S1 Oligonucleotides used in this study.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
ACKNOWLEDGEMENTS
This research was supported by the SIGNALPATH Marie Curie research training network (MRTN‐CT‐2005‐019277) and by grant BIO2007‐62661 from the Ministerio de Educación y Ciencia.
REFERENCES
- Alex, L.A. , Borkovich, K.A. and Simon, M.I. (1996) Hyphal development in Neurospora crassa: involvement of a two‐component histidine kinase. Proc. Natl Acad. Sci. USA, 93, 3416–3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul, S.F. , Gish, W. , Miller, W. , Myers, C.W. and Lipman, D.L. (1990) Basic local alignment search tool. J. Mol. Biol. 215, 403–410. [DOI] [PubMed] [Google Scholar]
- Aravind, L. and Ponting, C.P. (1999) The cytoplasmic helical linker domain of receptor histidine kinase and methyl‐accepting proteins is common to many prokaryotic signalling proteins. FEMS Microbiol. Lett. 176, 111–116. [DOI] [PubMed] [Google Scholar]
- Avenot, H. , Simoneau, P. , Iacomi‐Vasilescu, B. and Bataille‐Simoneau, N. (2005) Characterization of mutations in the two‐component histidine kinase gene AbNIK1 from Alternaria brassicicola that confer high dicarboximide and phenylpyrrole resistance. Curr. Genet. 47, 234–243. [DOI] [PubMed] [Google Scholar]
- Bahn, Y.S. (2008) Master and commander in fungal pathogens: the two‐component system and the Hog signaling pathway. Eukaryot. Cell, 7, 2017–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn, Y.S. , Kojima, K. , Cox, G.M. and Heitman, J. (2006) A unique fungal two‐component system regulates stress responses, drug sensitivity, sexual development, and virulence of Cryptococcus neoformans . Mol. Biol. Cell, 17, 3122–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, A.J.P. , Haynes, K. and Quinn, J. (2009) Nitrosative and oxidative stress responses in fungal pathogenicity. Curr. Opin. Microbiol. 12, 384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castresana, J. (2000) Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 17, 540–552. [DOI] [PubMed] [Google Scholar]
- Catlett, N.L. , Yoder, O.C. and Turgeon, B.G. (2003) Whole‐genome analysis of two‐component signal transduction genes in fungal pathogens. Eukaryot. Cell, 2, 1151–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, Y. , Kim, K.H. , La Rota, M. , Scott, D. , Santopietro, G. , Callihan, M. , Mitchell, T.K. and Lawrence, C.B. (2009) Identification of novel virulence factors associated with signal transduction pathways in Alternaria brassicicola . Mol. Microbiol. 72, 1316–1333. [DOI] [PubMed] [Google Scholar]
- Chomczynski, P. and Sacchi, N. (1987) Single‐step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal. Biochem. 162, 156–159. [DOI] [PubMed] [Google Scholar]
- Clemons, K.V. , Miller, T.K. , Selitrennikoff, C.P. and Stevens, D.A. (2002) fos‐1, a putative histidine kinase as a virulence factor for systemic aspergillosis. Med. Mycol. 40, 259–262. [DOI] [PubMed] [Google Scholar]
- Cui, W. , Beever, R.E. , Parkes, S.L. , Weeds, P.L. and Templeton, M.D. (2002) An osmosensing histidine kinase mediates dicarboximide fungicide resistance in Botryotinia fuckeliana (Botrytis cinerea). Fungal Genet. Biol. 36, 187–198. [DOI] [PubMed] [Google Scholar]
- Delgado‐Jarana, J. , Martinez‐Rocha, A.L. , Roldan‐Rodriguez, R. , Roncero, M.I.G. and Di Pietro, A. (2005) Fusarium oxysporum G‐protein beta subunit Fgb1 regulates hyphal growth, development, and virulence through multiple signalling pathways. Fungal Genet. Biol. 42, 61–72. [DOI] [PubMed] [Google Scholar]
- Di Pietro, A. and Roncero, M.I.G. (1998) Cloning, expression, and role in pathogenicity of pg1 encoding the major extracellular endopolygalacturonase of the vascular wilt pathogen Fusarium oxysporum . Mol. Plant–Microbe Interact. 11, 91–98. [DOI] [PubMed] [Google Scholar]
- Di Pietro, A. , Garcia‐Maceira, F.I. , Meglecz, E. and Roncero, M.I.G. (2001) A MAP kinase of the vascular wilt fungus Fusarium oxysporum is essential for root penetration and pathogenesis. Mol. Microbiol. 39, 1140–1152. [PubMed] [Google Scholar]
- Di Pietro, A. , Madrid, M.P. , Caracuel, Z. , Delgado‐Jarana, J. and Roncero, M.I.G. (2003) Fusarium oxysporum: exploring the molecular arsenal of a vascular wilt fungus. Mol. Plant Pathol. 4, 315–326. [DOI] [PubMed] [Google Scholar]
- Dixon, K.P. , Xu, J.R. , Smirnoff, N. and Talbot, N.J. (1999)Independent signaling pathways regulate cellular turgor during hyperosmotic stress and appressorium‐mediated plant infection by Magnaporthe grisea . Plant Cell, 11, 2045–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura, M. , Ochiai, N. , Oshima, M. , Motoyama, T. , Ichiishi, A. , Usami, R. , Horikoshi, K. and Yamaguchi, I. (2003) Putative homologs of SSK22 MAPKK kinase and PBS2 MAPK kinase of Saccharomyces cerevisiae encoded by os‐4 and os‐5 genes for osmotic sensitivity and fungicide resistance in Neurospora crassa . Biosci. Biotechnol. Biochem. 67, 186–191. [DOI] [PubMed] [Google Scholar]
- Gordon, T.R. and Martyn, R.D. (1997) The evolutionary biology of Fusarium oxysporum . Annu. Rev. Phytopathol. 35, 111–128. [DOI] [PubMed] [Google Scholar]
- Guindon, S. and Gascuel, O. (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52, 696–704. [DOI] [PubMed] [Google Scholar]
- Hagiwara, D. , Matsubayashi, Y. , Marui, J. , Furukawa, K. , Yamashino, T. , Kanamaru, K. , Kato, M. , Abe, K. , Kobayashi, T. and Mizuno, T. (2007) Characterization of the NikA histidine kinase implicated in the phosphorelay signal transduction of Aspergillus nidulans, with special reference to fungicide responses. Biosci. Biotechnol. Biochem. 71, 844–847. [DOI] [PubMed] [Google Scholar]
- Hagiwara, D. , Asano, Y. , Marui, J. , Yoshimi, A. , Mizuno, T. and Abe, K. (2009) Transcriptional profiling for Aspergillus nidulans HogA MAPK signaling pathway in response to fludioxonil and osmotic stress. Fungal Genet. Biol. 46, 868–878. [DOI] [PubMed] [Google Scholar]
- Han, K.H. and Prade, R.A. (2002) Osmotic stress‐coupled maintenance of polar growth in Aspergillus nidulans . Mol. Microbiol. 43, 1065–1078. [DOI] [PubMed] [Google Scholar]
- Hohmann, S. (2002) Osmotic stress signaling and osmoadaptation in yeasts. Microbiol. Mol. Biol. Rev. 66, 300–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckelhoven, R. and Kogel, K.H. (2003) Reactive oxygen intermediates in plant–microbe interactions: who is who in powdery mildew resistance? Planta, 216, 891–902. [DOI] [PubMed] [Google Scholar]
- Izumitsu, K. , Yoshimi, A. and Tanaka, C. (2007) Two‐component response regulators Ssk1p and Skn7p additively regulate high‐osmolarity adaptation and fungicide sensitivity in Cochliobolus heterostrophus . Eukaryot. Cell, 6, 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumitsu, K. , Yoshimi, A. , Kubo, D. , Morita, A. , Saitoh, Y. and Tanaka, C. (2009) The MAPKK kinase ChSte11 regulates sexual/asexual development, melanization, pathogenicity, and adaptation to oxidative stress in Cochliobolus heterostrophus . Curr. Genet. 55, 439–448. [DOI] [PubMed] [Google Scholar]
- Jones, C.A. , Greer‐Phillips, S.E. and Borkovich, K.A. (2007) The response regulator RRG‐1 functions upstream of a mitogen‐activated protein kinase pathway impacting asexual development, female fertility, osmotic stress, and fungicide resistance in Neurospora crassa . Mol. Biol. Cell, 18, 2123–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane, T.M. , Creevey, C.J. , Pentony, M.M. , Naughton, T.J. and McInerney, J.O. (2006) Assessment of methods for amino acid matrix selection and their use on empirical data shows that ad hoc assumptions for choice of matrix are not justified. BMC Evol. Biol. 6, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima, K. , Takano, Y. , Yoshimi, A. , Tanaka, C. , Kikuchi, T. and Okuno, T. (2004) Fungicide activity through activation of a fungal signalling pathway. Mol. Microbiol. 53, 1785–1796. [DOI] [PubMed] [Google Scholar]
- Kruppa, M. and Calderone, R. (2006) Two‐component signal transduction in human fungal pathogens. FEMS Yeast Res. 6, 149–159. [DOI] [PubMed] [Google Scholar]
- Le, S.Q. and Gascuel, O. (2008) An improved general amino acid replacement matrix. Mol. Biol. Evol. 25, 1307–1320. [DOI] [PubMed] [Google Scholar]
- Levin, D.E. (2005) Cell wall integrity signaling in Saccharomyces cerevisiae . Microbiol. Mol. Biol. Rev. 69, 262–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S. , Ault, A. , Malone, C.L. , Raitt, D. , Dean, S. , Johnston, L.H. , Deschenes, R.J. and Fassler, J.S. (1998) The yeast histidine protein kinase, Sln1p, mediates phosphotransfer to two response regulators, Ssk1p and Skn7p. EMBO J. 17, 6952–6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, W. , Leroux, P. and Fillinger, S. (2008) The HOG1‐like MAP kinase Sak1 of Botrytis cinerea is negatively regulated by the upstream histidine kinase Bos1 and is not involved in dicarboximide‐ and phenylpyrrole‐resistance. Fungal Genet. Biol. 45, 1062–1074. [DOI] [PubMed] [Google Scholar]
- Lopez‐Berges, M.S. , Di Pietro, A. , Daboussi, M.J. , Wahab, H.A. , Vasnier, C. , Roncero, M.I.G. , Dufresne, M. and Hera, C. (2009) Identification of virulence genes in Fusarium oxysporum f. sp. lycopersici by large‐scale transposon tagging. Mol. Plant Pathol. 10, 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Z. , Luo, Y. and Michailides, T. (2006) Molecular characterization of the two‐component histidine kinase gene from Monilinia fructicola . Pest Manag. Sci. 62, 991–998. [DOI] [PubMed] [Google Scholar]
- Mattern, I.E. , Punt, P.J. and Van Den Hondel, C.A. (1988) A vector of Aspergillus transformation conferring phleomycin resistance. Fungal Genet. Newsl. 35, 25. [Google Scholar]
- Michielse, C.B. and Rep, M. (2009) Pathogen profile update: Fusarium oxysporum . Mol. Plant Pathol. 10, 311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, B.A. , Banks, G.R. , Toone, W.M. , Raitt, D. , Kuge, S. and Johnston, L.H. (1997) The Skn7 response regulator controls gene expression in the oxidative stress response of the budding yeast Saccharomyces cerevisiae . EMBO J. 16, 1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoyama, T. , Kadokura, K. , Ohira, T. , Ichiishi, A. , Fujimura, M. , Yamaguchi, I. and Kudo, T. (2005a) A two‐component histidine kinase of the rice blast fungus is involved in osmotic stress response and fungicide action. Fungal Genet. Biol. 42, 200–212. [DOI] [PubMed] [Google Scholar]
- Motoyama, T. , Ohira, T. , Kadokura, K. , Ichiishi, A. , Fujimura, M. , Yamaguchi, I. and Kudo, T. (2005b) An Os‐1 family histidine kinase from a filamentous fungus confers fungicide‐sensitivity to yeast. Curr. Genet. 47, 298–306. [DOI] [PubMed] [Google Scholar]
- Nemecek, J.C. , Wuthrich, M. and Klein, B.S. (2006) Global control of dimorphism and virulence in fungi. Science, 312, 583–588. [DOI] [PubMed] [Google Scholar]
- Noguchi, R. , Banno, S. , Ichikawa, R. , Fukumori, F. , Ichiishi, A. , Kimura, M. , Yamaguchi, I. and Fujimura, M. (2007) Identification of OS‐2 MAP kinase‐dependent genes induced in response to osmotic stress, antifungal agent fludioxonil, and heat shock in Neurospora crassa . Fungal Genet. Biol. 44, 208–218. [DOI] [PubMed] [Google Scholar]
- Nucci, M. and Anaissie, E. (2007) Fusarium infections in immunocompromised patients. Clin. Microbiol. Rev. 20, 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai, N. , Fujimura, M. , Motoyama, T. , Ichiishi, A. , Usami, R. , Horikoshi, K. and Yamaguchi, I. (2001) Characterization of mutations in the two‐component histidine kinase gene that confer fludioxonil resistance and osmotic sensitivity in the os‐1 mutants of Neurospora crassa . Pest Manag. Sci. 57, 437–442. [DOI] [PubMed] [Google Scholar]
- Page, R.D. (1996) TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12, 357–358. [DOI] [PubMed] [Google Scholar]
- Posas, F. and Saito, H. (1997) Osmotic activation of the HOG MAPK pathway via Ste11p MAPKKK: scaffold role of Pbs2p MAPKK. Science, 276, 1702–1705. [DOI] [PubMed] [Google Scholar]
- Prados Rosales, R.C. and Di Pietro, A. (2008) Vegetative hyphal fusion is not essential for plant infection by Fusarium oxysporum . Eukaryot. Cell, 7, 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punt, P.J. , Oliver, R.P. , Dingemanse, M.A. , Pouwels, P.H. and Van Den Hondel, C.A. (1987) Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli . Gene, 56, 117–124. [DOI] [PubMed] [Google Scholar]
- Raeder, U. and Broda, P. (1985) Rapid preparation of DNA from filamentous fungi. Lett. Appl. Microbiol. 1, 17–20. [Google Scholar]
- Rispail, N. and Di Pietro, A. (2009) Fusarium oxysporum Ste12 controls invasive growth and virulence downstream of the Fmk1 MAPK cascade. Mol. Plant–Microbe Interact. 22, 830–839. [DOI] [PubMed] [Google Scholar]
- Rispail, N. , Soanes, D.M. , Ant, C. , Czajkowski, R. , Grünler, A. , Huguet, R. , Perez‐Nadales, E. , Poli, A. , Sartorel, E. , Valiante, V. , Yang, M. , Beffa, R. , Brakhage, A.A. , Gow, N.A.R. , Kahmann, R. , Lebrun, M.H. , Lenasi, H. , Perez‐Martin, J. , Talbot, N.J. , Wendland, J. and Di Pietro, A. (2009) Comparative genomics of MAP kinase and calcium–calcineurin signalling components in plant and human pathogenic fungi. Fungal Genet. Biol. 46, 287–298. [DOI] [PubMed] [Google Scholar]
- Saito, H. and Tatebayashi, K. (2004) Regulation of the osmoregulatory HOG MAPK cascade in yeast. J. Biochem. 136, 267–272. [DOI] [PubMed] [Google Scholar]
- Sambrook, J. and Russell, D.W. (2001) Molecular cloning: a laboratory manual. New York: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Scott, B. and Eaton, C.J. (2008) Role of reactive oxygen species in fungal cellular differentiations. Curr. Opin. Microbiol. 11, 488–493. [DOI] [PubMed] [Google Scholar]
- Segmüller, N. , Ellendorf, U. , Tudzynski, B. and Tudzynski, P. (2007) BcSAK1, a stress‐activated mitogen‐activated protein kinase, is involved in vegetative differentiation and pathogenicity in Botrytis cinerea . Eukaryot. Cell, 6, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, J.D. , Higginsm, D.G. and Gibson, T.J. (1994) Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position‐specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaud, M. , Fillinger, S. , Liu, W. , Polepalli, J.S. , Le Pecheur, P. , Kunduru, A.R. , Leroux, P. and Legendre, L. (2006) A class III histidine kinase acts as a novel virulence factor in Botrytis cinerea . Mol. Plant–Microbe Interact. 19, 1042–1050. [DOI] [PubMed] [Google Scholar]
- West, A.H. and Stock, A.M. (2001) Histidine kinases and response regulator proteins in two‐component signaling systems. Trends Biochem. Sci. 26, 369–376. [DOI] [PubMed] [Google Scholar]
- Williams, K.E. and Cyert, M.S. (2001) The eukaryotic response regulator Skn7p regulates calcineurin signaling through stabilization of Crz1p. EMBO J. 20, 3473–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada‐Okabe, T. , Mio, T. , Ono, N. , Kashima, Y. , Matsui, M. , Arisawa, M. and Yamada‐Okabe, H. (1999) Roles of three histidine kinase genes in hyphal development and virulence of the pathogenic fungus Candida albicans . J. Bacteriol. 181, 7243–7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita, K. , Shiozawa, A. , Watanabe, S. , Fukumori, F. , Kimura, M. and Fujimura, M. (2008) ATF‐1 transcription factor regulates the expression of ccg‐1 and cat‐1 genes in response to fludioxonil under OS‐2 MAP kinase in Neurospora crassa . Fungal Genet. Biol. 45, 1562–1569. [DOI] [PubMed] [Google Scholar]
- Yang, L. , Ukil, L. , Osmani, A. , Nahm, F. , Davies, J. , De Souza, C.P. , Dou, X. , Perez‐Balaguer, A. and Osmani, S.A. (2004) Rapid production of gene replacement constructs and generation of a green fluorescent protein‐tagged centromeric marker in Aspergillus nidulans . Eukaryot. Cell, 3, 1359–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimi, A. , Tsuda, M. and Tanaka, C. (2004) Cloning and characterization of the histidine kinase gene Dic1 from Cochliobolus heterostrophus that confers dicarboximide resistance and osmotic adaptation. Mol. Genet. Genomics, 271, 228–236. [DOI] [PubMed] [Google Scholar]
- Yoshimi, A. , Kojima, K. , Takano, Y. and Tanaka, C. (2005) Group III histidine kinase is a positive regulator of Hog1‐type mitogen‐activated protein kinase in filamentous fungi. Eukaryot. Cell, 4, 1820–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Lamm, R. , Pillonel, C. , Lam, S. and Xu, J.R. (2002) Osmoregulation and fungicide resistance: the Neurospora crassa os‐2 gene encodes a HOG1 mitogen‐activated protein kinase homologue. Appl. Environ. Microbiol. 68, 532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Targeted disruption of the Fusarium oxysporum fhk1 gene. (A) Physical maps of the fhk1 locus and the gene replacement construct obtained by polymerase chain reaction (PCR) fusion (Δfhk1 allele). (B) Southern hybridization analysis of wild‐type strain 4287 (1) and transformants efhk1‐1 (2), Δfhk1‐2 (3), Δfhk1‐4 (4), Δfhk1‐7 (5), Δfmk1 (6) and Δfmk1Δfhk1 (7). Genomic DNA treated with PstI was hybridized with the probe indicated in (A). (C) PCR amplification of genomic DNA of the wild‐type strain 4287 (1), knockout mutant Δfhk1‐2 (2) and complemented strains Δfhk1+fhk1 1 (3) and Δfhk1+fhk1 2 (4), using primers Fhk1‐1 and Fhk1‐2 (fhk1) or M13 Forward and PHL (Phleo).
Fig. S2 Fhk1 is not required for vegetative hyphal growth. Conidia of the indicated strains were spotted onto plates with synthetic medium (SM) buffered at the indicated pH values and grown at 28 °C for 3 days.
Fig. S3 Fhk1 is not required for invasive growth. Microconidial suspensions of the indicated strains were applied for the different invasive growth assays. (A) Invasive growth on apple slices inoculated with microconidia and incubated at 28 °C for 4 days. (B) Penetration of cellophane sheets. Colonies were grown for 4 days on a plate with minimal medium covered by a cellophane sheet (Before); the cellophane sheet with the colony was removed and the plates were incubated for an additional day (After).
Table S1 Oligonucleotides used in this study.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item


