SUMMARY
Tomato yellow leaf curl disease (TYLCD) is one of the most devastating viral diseases affecting tomato crops in tropical, subtropical and temperate regions of the world. Here, we focus on the interactions through recombination between the different begomovirus species causing TYLCD, provide an overview of the interactions with the cellular genes involved in viral replication, and highlight recent progress on the relationships between these viruses and their vector, the whitefly Bemisia tabaci.
Taxonomy: The tomato yellow leaf curl virus‐like viruses (TYLCVs) are a complex of begomoviruses (family Geminiviridae, genus Begomovirus) including 10 accepted species: Tomato yellow leaf curl Axarquia virus (TYLCAxV), Tomato yellow leaf curl China virus (TYLCCNV), Tomato yellow leaf curl Guangdong virus (TYLCGuV), Tomato yellow leaf curl Indonesia virus (TYLCIDV), Tomato yellow leaf curl Kanchanaburi virus (TYLVKaV), Tomato yellow leaf curl Malaga virus (TYLCMalV), Tomato yellow leaf curl Mali virus (TYLCMLV), Tomato yellow leaf curl Sardinia virus (TYLCSV), Tomato yellow leaf curl Thailand virus (TYLCTHV), Tomato yellow leaf curl Vietnam virus (TYLCVNV) and Tomato yellow leaf curl virus(TYLCV). We follow the species demarcation criteria of the International Committee on Taxonomy of Viruses (ICTV), the most important of which is an 89% nucleotide identity threshold between full‐length DNA‐A component nucleotide sequences for begomovirus species. Strains of a species are defined by a 93% nucleotide identity threshold.
Host range: The primary host of TYLCVs is tomato (Solanum lycopersicum), but they can also naturally infect other crops [common bean (Phaseolus vulgaris), sweet pepper (Capsicum annuum), chilli pepper (C. chinense) and tobacco (Nicotiana tabacum)], a number of ornamentals [petunia (Petunia×hybrida) and lisianthus (Eustoma grandiflora)], as well as common weeds (Solanum nigrum and Datura stramonium). TYLCVs also infect the experimental host Nicotiana benthamiana.
Disease symptoms: Infected tomato plants are stunted or dwarfed, with leaflets rolled upwards and inwards; young leaves are slightly chlorotic; in recently infected plants, fruits might not be produced or, if produced, are small and unmarketable. In common bean, some TYLCVs produce the bean leaf crumple disease, with thickening, epinasty, crumpling, blade reduction and upward curling of leaves, as well as abnormal shoot proliferation and internode reduction; the very small leaves result in a bushy appearance.
INTRODUCTION
Tomato yellow leaf curl disease (TYLCD) is one of the most devastating viral diseases affecting tomato (Solanum lycopersicum L.) crops in tropical, subtropical and temperate regions of the world (Moriones and Navas‐Castillo, 2000) (Fig. 1A,B). Epidemics of TYLCD can cause extensive crop losses (up to 100%). Severe outbreaks of the disease were reported in the late 1920s in the Jordan Valley (now Israel) (Cohen and Antignus, 1994) and, since then, TYLCD has become one of the most economically important tomato diseases in many regions of Africa, the Middle East and Southeast Asia, Europe (Czosnek and Laterrot, 1997; Moriones and Navas‐Castillo, 2000) and, more recently, North and South America (Polston et al., 1999; Zambrano et al., 2007). A complex of more than ten virus species and their strains [according to the demarcation criteria of the International Committee on Taxonomy of Viruses (ICTV) (Fauquet et al., 2008)], including the species first described in Israel, TYLCV, have been associated with TYLCD and are hereafter referred to as tomato yellow leaf curl virus‐like viruses (TYLCVs). These viruses belong to the genus Begomovirus (family Geminiviridae) and are transmitted in a circulative, persistent manner by the whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) (Moriones and Navas‐Castillo, 2000) (Fig. 1C). Unlike most whitefly‐transmitted geminiviruses whose genomes are bipartite, the genomes of TYLCVs (with the exception of Tomato yellow leaf curl Thailand virus and Tomato yellow leaf curl Kanchanaburi virus, which possess bipartite genomes) are monopartite, i.e. they contain only one genomic circular single‐stranded DNA (ssDNA) molecule of about 2.8 kb. This genomic DNA encodes six partially overlapping open reading frames (ORFs) that are bidirectionally organized into two transcriptional units separated by an intergenic region of about 300 nucleotides (Kheyr‐Pour et al., 1991; Navot et al., 1991). Of the six ORFs, two are on the virus sense strand [encoding the coat protein (CP) and a movement‐like protein (V2)], and four are on the complementary sense strand [encoding a replication‐associated protein (Rep), a transcription activator protein (TrAP), a replication enhancer protein (REn) and a small C4 protein embedded within the Rep] (Jupin et al., 1994; Laufs et al., 1995; Wartig et al., 1997) (Fig. 1D). In addition to encapsidating the genome and transporting it in and out of the nucleus, the CP of monopartite begomoviruses is required for systemic plant infection and vector transmission, and determines insect vector specificity. Rep and REn are required for efficient viral DNA replication, although only Rep is essential. Recently, the V2 protein of TYLCV has been shown to function as a viral suppressor of RNA silencing (Zrachya et al., 2007). In addition to their role as transcription activators of late viral genes, the TrAP proteins of several begomoviruses, including Tomato yellow leaf curl China virus (TYLCCNV), have also been shown to act as suppressors of RNA silencing (Bisaro, 2006). C4 proteins have been implicated in symptom expression and virus movement (Jupin et al., 1994; Raja et al., 2008; Rojas et al., 2001). The noncoding intergenic region contains key elements for the replication and transcription of the viral genome, including the origin of replication, within a stem‐loop structure conserved in the geminiviruses.
Figure 1.
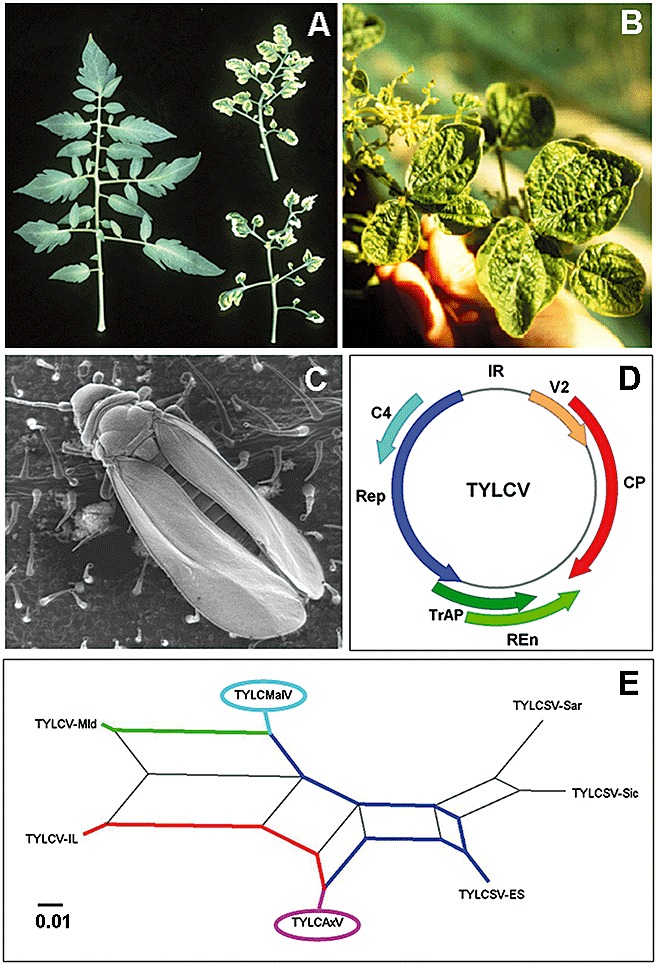
Leaf symptoms caused by Tomato yellow leaf curl virus (TYLCV) on tomato (left, healthy control) (A) and on common bean (B). (C) Adult of Bemisia tabaci on a tomato leaf. (D) Map of the TYLCV genome showing the open reading frames (ORFs) coded by the virus sense [movement‐like protein (V2), coat protein (CP)] and complementary sense [replication‐associated protein (Rep), C4, transcription activator protein (TrAP), replication enhancer protein (REn)] strands. (E) Phylogenetic network of the complete genome sequences of the TYLCVs found in the Mediterranean basin generated using the SplitsTree4 program (Huson and Bryant, 2006). The formation of a reticular network rather than a single bifurcating tree is suggestive of recombination. The recombinant viruses, Tomato yellow leaf curl Malaga virus (TYLCMalV) (TYLCSV‐ES ® TYLCV‐Mld) and Tomato yellow leaf curl Axarquia virus (TYLCAxV) (TYLCSV‐ES ® TYLCV‐IL), are highlighted. Coloured lines link recombinant viruses to their putative parents.
In a previous ‘Pathogen profile’, Gafni (2003) reviewed the intracellular dynamics of TYLCV. Here, we focus on the interactions between different species of TYLCVs through recombination, the major driving force in begomovirus evolution, and provide an overview of the interactions with the cellular proteins involved in viral replication. Finally, we highlight recent progress on the relationships between these viruses and their vector, the whitefly Bemisia tabaci.
THE ROLE OF RECOMBINATION IN TYLCV COMPLEX EVOLUTION
The three major sources of genetic variation exploited by plant viruses are mutation, reassortment and recombination (García‐Arenal et al., 2001; Worobey and Holmes, 1999). Because mixed viral infections are common in nature, genetic exchange through recombination or reassortment (for fragmented genomes) offers viruses the opportunity to rapidly evolve to explore new genome combinations, some of which increase virus pathogenicity or improve environmental adaptation (Fernández‐Cuartero et al., 1994; Froissart et al., 2005; Martin et al., 2005). Recombination seems to contribute greatly to the genetic diversification of begomoviruses, increasing their evolutionary potential and local adaptation (Berrie et al., 2001; Chatchawankanphanich and Maxwell, 2002; Harrison and Robinson, 1999; Moffat, 1999; Monci et al., 2002; Padidam et al., 1999; Pita et al., 2001; 1999, 2000; Umaharan et al., 1998; Zhou et al., 1997). The existence in this group of viruses of a recombination‐dependent replication (RDR) (Jeske et al., 2001) in addition to a rolling‐circle replication (RCR) (Saunders et al., 1991; Stenger et al., 1991), and the evidence of the co‐infection of single cells (Morilla et al., 2004), might explain the frequency of recombination. For recombinants to thrive in nature, the portions of the genomes inherited from different parents must work well together. In this sense, analysis of recombination breakpoint distributions within begomovirus genomes indicates a nonrandom distribution probably associated with structural and functional constraints (Fauquet et al., 2005; García‐Andrés et al., 2007b; Lefeuvre et al., 2009; Stanley, 1995; Stenger et al., 1991). In geminiviruses, recombination has been reported at the level of strain (Hou and Gilbertson, 1996; Kirthi et al., 2002), species (Fondong et al., 2000; García‐Andrés et al., 2006; Martin et al., 2001; Monci et al., 2002; Navas‐Castillo et al., 2000; Sanz et al., 2000; Saunders et al., 2002; Zhou et al., 1997), genus (Briddon et al., 1996; Klute et al., 1996) and even interfamily (Saunders and Stanley, 1999).
Mixed infections of TYLCVs seem to be frequent in epidemics worldwide (Davino et al., 2009; Delatte et al., 2005; García‐Andrés et al., 2006; Moriones et al., 2007; Sánchez‐Campos et al., 1999; Ueda et al., 2004). This is especially relevant because the emergence of recombinant variants derived from interactions between these viruses has been demonstrated to be frequent (García‐Andrés et al., 2007b). Consequently, the emergence of recombinant variants during epidemics might occur and change their development (e.g. Monci et al., 2002). The epidemics of begomoviruses of the TYLCV complex in the western Mediterranean basin are an excellent example of this situation (Davino et al., 2009; García‐Andrés et al., 2007a; Moriones and Navas‐Castillo, 2008) (Fig. 1E). In Spain, the first reports of infections by TYLCV‐related viruses were in the early 1990s and were associated with the presence of the ES strain of Tomato yellow leaf curl Sardinia virus (TYLCSV) (Noris et al., 1994). This initial colonization resulted in a relatively stable population with a low level of genetic diversity (Sánchez‐Campos et al., 2002). However, subsequent introductions of isolates of the Israeli severe (IL) and mild (Mld) strains of TYLCV (Morilla et al., 2003; 1997, 1999) resulted in novel sources of variation and provided conditions for recombination. In fact, only 2 years after the detection of TYLCV, a novel recombinant variant named Tomato yellow leaf curl Málaga virus (TYLCMalV) emerged as a result of a genetic exchange between isolates of the ES strain of TYLCSV and of the Mld strain of TYLCV (Monci et al., 2002). This recombinant variant was ecologically well adapted and spread within the population (García‐Andrés et al., 2007a). Soon after this, a novel recombinant between the ES strain of TYLCSV and the IL strain of TYLCV, Tomato yellow leaf curl Axarquía virus (TYLCAxV), was detected in the population (García‐Andrés et al., 2006). These novel natural recombinants exhibited biological properties that suggested a better ecological adaptation than either parental virus (García‐Andrés et al., 2007b; Monci et al., 2002), with unknown epidemiological consequences. Interestingly, studies conducted with natural populations of Solanum nigrum, a weed host widely distributed in the Mediterranean basin, indicated that mixed infections including recombinant variants were frequent (García‐Andrés et al., 2006), suggesting that wild hosts can be reservoirs of genetic diversity and venues for genetic exchanges that give rise to better adapted recombinant begomoviruses.
In Italy, the presence of mixed infections of TYLCV and TYLCSV provided another interesting opportunity to study how the interaction between these viruses leads to the emergence and spread of recombinants. TYLCSV was reported to be endemic in Sicily and Sardinia in 1989 (Credi et al., 1989; Crespi et al., 1995), and TYLCV was first detected in 2002 (Accotto et al., 2003; Davino et al., 2006). Since then, TYLCSV and TYLCV have coexisted in either single or mixed infections (Davino et al., 2006). This has resulted in the emergence of recombinant variants, as occurred in Spain. Thus, 2 years after the first detection of TYLCV, several recombinant variants were detected (Davino et al., 2009).
The precise mechanisms controlling recombination in begomoviruses are unknown (Padidam et al., 1999; Seal et al., 2006), and the search for specific sequence features near recombination sites has been unsuccessful (Sanz et al., 2000). It is known that cross‐over sites are not evenly distributed throughout the genome and that recombination hot and cold spots can be located (Fauquet et al., 2005; García‐Andrés et al., 2007b; Lefeuvre et al., 2009; Stanley, 1995; Stenger et al., 1991). Thus, inspection of recombinant viruses reveals that one of the cross‐over sites frequently occurs in the intergenic region, within the stem‐loop structure conserved among geminiviruses, where replication initiates (Gutiérrez, 1999; Hanley‐Bowdoin et al., 1999). Moreover, Lefeuvre et al. (2007), by analysing the available sequences of begomoviruses, found convincing statistical evidence of hot and cold spots for recombination, indicating that either DNA breakage and repair do not occur randomly or that certain recombinants are selected. Furthermore, analysis of recombination breakpoint distributions within the genomes of diverse ssDNA virus families again suggested nonrandom breakpoint distributions, a finding that is only partially attributable to the mechanistic aspects of the recombination process (Lefeuvre et al., 2009). The significant tendency of recombination breakpoints to fall either outside or on the peripheries of genes, such as structural protein genes, suggests that natural selection acting against viruses expressing recombinant proteins is a major determinant of the nonrandom distribution.
In summary, recombination during the interaction of TYLCV‐related viruses contributes significantly to the generation of genetic diversity and novel virus variants better adapted to local ecological conditions. In addition to interacting via recombination, TYLCV‐related viruses interact competitively; because the viruses differ in ecological adaptations, some are selected for and some are selected against depending on the conditions, leading to changes in the virus population structure during epidemics. Thus, displacement of TYLCSV by TYLCV has been associated with better transmission of TYLCV by the vector and more efficient maintenance of TYLCV between epidemics in Spain (Sánchez‐Campos et al., 1999). In addition, the general deployment of tomatoes carrying resistance to TYLCD resulted in displacement of TYLCSV by TYLCV because of the better performance of TYLCV on the resistant genotypes (García‐Andrés et al., 2009).
PLANT HOST GENES AND REPLICATION OF TYLCVs
TYLCVs have small ssDNA genomes that encode only a few proteins. After entering the cell, the ssDNA is converted into a double‐stranded (ds) replicative form that serves as a template for the transcription of the viral replication proteins Rep and REn (Hanley‐Bowdoin et al., 1999). The Rep protein recognizes and binds to the virus replication origin (Behjatnia et al., 1998; Fontes et al., 1994) and catalyses the cleavage and religation reaction in a conserved hairpin loop to initiate the RCR (Laufs et al., 1995). Geminiviruses do not encode their own DNA polymerases and rely on the nuclear DNA replication machinery. They replicate in the nuclei of mature cells, which are inactive in DNA replication. Accumulating evidence strongly supports the notion that geminivirus proteins have a significant impact on a variety of host cell pathways, including cell differentiation, cell cycle control, DNA replication, plasmodesmata function and RNA silencing (Gutiérrez et al., 2004; Hanley‐Bowdoin et al., 2004). Several studies have shown that the begomovirus Rep and REn proteins bind to viral and host proteins. Rep interacts with the plant retinoblastoma homologue pRBR (Ach et al., 1997; Arguello‐Astorga et al., 2004; Kong et al., 2000) to induce the transcription of genes encoding host replicative enzymes required for viral DNA replication (Gutiérrez, 2000; Hanley‐Bowdoin et al., 1999). One of these induced factors is the proliferating cell nuclear antigen (PCNA), an essential component of the eukaryotic replication machinery, that acts as a ‘sliding clamp’ preventing DNA polymerase from dissociating from the template DNA strand. Indeed, Rep from TYLCSV interacts with PCNA, possibly to recruit it to the viral origin and the replisome (whose major components have helicase, gyrase, primase, DNA polymerase and ligase activities) (Castillo et al., 2003). Rep also interacts with a serine/threonine kinase, a kinesin and histone H3 (Kong and Hanley‐Bowdoin, 2002), as well as with the SUMO conjugating enzyme NbSCE1/Ubc9, a component of the sumoylation pathway (Castillo et al., 2004). REn from TYLCV and Tomato golden mosaic virus interacts with Rep, PCNA and pRBR, in addition to itself (Castillo et al., 2003; 2001, 2005). Hence, a complex network of interactions that involves Rep, REn and several plant host factors seems to be important to ensure efficient replication of geminivirus DNA. Several lines of evidence indicate that REn acts primarily through interaction with plant proteins. First, the REn protein sequence shows no homology to any known enzymatic motifs, suggesting that structural changes produced by the Ren–Rep interaction, rather than a putative catalytic activity of REn, enhance viral replication. Second, although REn replication enhancement activity is highly tolerant to mutation, mutated versions of REn protein impaired for replication enhancement activity are also impaired for interaction with REn, Rep and/or PCNA (Settlage et al., 2005). To complicate the network of interacting proteins, REn of Tomato leaf curl virus, a begomovirus related to TYLCVs, was found to interact with a NAC domain protein of tomato (SINAC1). This interaction mediates the enhancement of viral DNA accumulation (Selth et al., 2005). However, how this interaction works is unknown, given that it is unlikely that Ren–SINAC1 interactions are sufficient to explain replication enhancement by REn.
In summary, it is likely that REn protein enhances replication through multiple mechanisms. REn could increase the affinity of Rep for the origin of replication or, by interacting with PCNA and Rep, REn could assist Rep to recruit the replication machinery necessary for viral DNA replication.
INTERACTIONS OF TYLCVs WITH BEMISIA TABACI
Bemisia tabaci is a genetically diverse group that includes more than 40 biotypes (De Barro et al., 2005). Damage by this pest occurs through phloem feeding, excretion of honeydew, induction of phytotoxic disorders and the transmission of plant viruses. The begomoviruses are transmitted exclusively by B. tabaci in a persistent, circulative manner.
Research suggests that TYLCV particles, once ingested by B. tabaci from infected tissues during feeding, enter the gut from where they are transported to the haemolymph and further to the salivary gland, from where they are inoculated back into the plants during subsequent feeding (Czosnek and Ghanim, 2002; Ghanim and Medina, 2007). In contrast, the gut–haemolymph barrier blocks TYLCV in the nonvector glasshouse whitefly Trialeurodes vaporariorum (Ohnishi et al., 2009), suggesting that transcytosis across the gut membranes is the main mechanism governing TYLCV movement in whiteflies. However, Caciagli et al. (2009) found that TYLCSV mutants nontransmissible by B. tabaci move in the haemolymph and cross the haemolymph–salivary gland wall as virions, indicating that the crossing of these barriers does not guarantee transmission. This suggests that interaction with molecular factors in the salivary glands may be fundamental to ensure infectivity. In addition, TYLCV transmission by B. tabaci depends on chaperonin GroEL homologues produced by their endosymbiotic bacteria. The GroEL homologue directly binds TYLCV particles and protects them from degradation in the haemolymph (1999, 2000). Interestingly, transgenic plants overexpressing the GroEL gene were tolerant to TYLCV infection, presumably because they sequestered the virions and thereby interfered with pathogenesis (Akad et al., 2007). This finding will certainly be relevant for the design of strategies to control TYLCD via transmission interference (Edelbaum et al., 2009). In addition, this mechanism could offer broad‐range tolerance against plant viruses that interact with GroEL homologues, a feature shared by several groups of circulative viruses of plants (Hogenhout et al., 2008).
All properties required for vector transmission and specificity, including GroEL homologue interaction, rely on the viral capsid protein. For TYLCSV, a region of the CP critical for transmission by B. tabaci was mapped between amino acids 129 and 134 (Caciagli et al., 2009; Noris et al., 1998). The relevance of this region in transmission was further supported by the characterization of whitefly nontransmissible mutants of two other begomoviruses, Watermelon chlorotic stunt virus (Kheyr‐Pour et al., 2000) and Abutilon mosaic virus (Hohnle et al., 2001). The three‐dimensional structures of the begomovirus African cassava mosaic virus CP and capsomers have been modelled on the basis of cryoelectron microscopy (Bottcher et al., 2004), and the amino acids critical for whitefly transmission are located in an exposed loop (Caciagli et al., 2009).
How TYLCVs are replicated, their genome expressed and transovarially transmitted in their insect hosts remain unclear and controversial. Rubinstein and Czosnek (1997) reported that TYLCV has harmful effects on whiteflies (B biotype), decreasing fecundity and longevity by more than 50%. Jiu et al. (2007) reported opposite results working with TYLCCNV; when B biotype whiteflies feed on TYLCCNV‐infected plants, their fecundity and longevity increase by 18‐ and seven‐fold, respectively. After 56 days, the B biotype whitefly population density was 13 times higher on infected than on healthy plants. In contrast, the indigenous ZHJ1 biotype performed similarly on healthy and infected plants (Jiu et al., 2007). Whether TYLCV can replicate in B. tabaci is controversial. No direct evidence of replication has been obtained so far, but TYLCV transcripts accumulated in B. tabaci after the whitefly fed on tomato infected with TYLCV (Sinisterra et al., 2005). In addition, the quantity of TYLCV DNA increased with time in the insect following a short acquisition period on infected tomato plants (Czosnek et al., 2001), a result recently confirmed by quantitative polymerase chain reaction (A. Mahadav and H. Czosnek, unpublished results). It might be interesting to determine whether TYLCV is able to replicate in whitefly cell cultures (Hunter and Polston, 2001). This approach would increase our understanding of the complex relationship between TYLCVs and their vector.
Ghanim et al. (1998) detected TYLCV in the ovaries of viruliferous B. tabaci females, in the eggs deposited by these females, in the developing instars and in the adults. In contrast, Bosco et al. (2004) found that TYLCSV DNA, but not TYLCV DNA, was transovarially transmitted in B. tabaci. Horizontal transmission of TYLCV and of a number of bipartite begomoviruses during copulation occurs between individuals belonging to the same biotype, whether B or Q, but not between individuals belonging to different biotypes. Transmission probably occurred by contamination of the haemolymph (Ghanim and Czosnek, 2000; Ghanim et al., 2007). Overall, TYLCV has features of a plant as well as an insect pathogen, which is also true of some RNA plant viruses belonging to or related to the families Rhabdoviridae, Reoviridae and Bunyaviridae.
CONCLUSIONS AND PROSPECTS
-
1
Co‐evolution between whiteflies and begomoviruses has probably occurred for more than 100 million years and has produced a very restricted and specific type of interaction involving one insect species, one form of capsid protein, one family of symbiotic chaperonins and, probably, one specialized type of receptor in the insect gut and in the salivary gland that await discovery.
-
2
There are many genetic systems that provide resistance to TYLCVs in tomato (at least five different loci associated with resistance have been mapped), indicating that, in addition to interacting with discovered plant proteins, the virus interacts with other, as yet undiscovered, plant proteins at various stages of its life cycle.
-
3
Recombination is a driving force of diversity in TYLCVs. Recombination probably generates the genetic variability that leads to the selection for increased virulence and the ability to overcome host plant resistance, which otherwise limits virus replication and spread. At present, recombination has been inferred mainly from the fact that the sequence of some viruses is made from sequence fragments of other viruses. It will be interesting to obtain evidence of recombination by showing that two viruses are able to recombine in vitro to generate an infectious new virus. The availability of such an in vitro system will increase our understanding of the molecular mechanism of geminivirus recombination.
-
4
Whether TYLCVs replicate and their genomes are expressed within the whitefly vector remain controversial and unclear. How endosymbionts participate in these processes and how important they are in determining the efficacy of transmission, and even recombination, remain to be established.
ACKNOWLEDGEMENTS
Research in the authors' laboratories was supported by the research grants AGL2007‐66062‐C02‐01 and AGL2007‐66062‐C02‐02 from the Ministerio de Educación y Ciencia (Spain), PO6‐AGR‐01771 and P08‐AGR‐04045 from Junta de Andalucía (Spain) (co‐financed by FEDER), IS‐4062‐07 from the United States–Israel Binational Agricultural Research and Development Fund (BARD) and 884/07 from the Israel Science Foundation. The scanning electron microscopy image of Bemisia tabaci was kindly supplied by A. Fereres, E. Garzo, F. Pinto and M. J. Rodríguez‐López (ICC‐CSIC and IHSM‐UMA‐CSIC, Spain).
REFERENCES
- Accotto, G.P. , Gragaloni, M. , Luison, D. , Davino, S. and Davino, M. (2003) First report of tomato yellow leaf curl virus (TYLCV) in Italy. Plant Pathol. 52, 799. [Google Scholar]
- Ach, R.A. , Durfee, T. , Miller, A.B. , Taranto, P. , Hanley‐Bowdoin, L. , Zambriski, P.C. and Gruissem, W. (1997) RRB1 and RRB2 encode maize retinoblastoma‐related proteins that interact with a plant D‐type cyclin and geminivirus replication protein. Mol. Cell. Biol. 17, 5077–5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akad, F. , Eybishtz, A. , Edelbaum, D. , Gorovits, R. , Dar‐Issa, O. , Iraki, N. and Czosnek, H. (2007) Making a friend from a foe: expressing a GroEL gene from the whitefly Bemisia tabaci in the phloem of tomato plants confers resistance to tomato yellow leaf curl virus. Arch. Virol. 152, 1323–1339. [DOI] [PubMed] [Google Scholar]
- Arguello‐Astorga, G. , Lopez‐Ochoa, L. , Kong, L.‐J. , Orozco, B.M. , Settlage, S.B. and Hanley‐Bowdoin, L. (2004) A novel motif in geminivirus replication proteins interacts with the plant retinoblastoma‐related protein. J. Virol. 78, 4817–4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behjatnia, S.A.A. , Dry, I.B. and Rezaian, M.A. (1998) Identification of the replication‐associated protein binding domain within the intergenic region of tomato leaf curl geminivirus. Nucleic Acids Res. 26, 925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrie, L.C. , Rybicki, E.P. and Rey, M.E.C. (2001) Complete nucleotide sequence and host range of South African cassava mosaic virus: further evidence for recombinations amongst begomoviruses. J. Gen. Virol. 82, 53–58. [DOI] [PubMed] [Google Scholar]
- Bisaro, D.M. (2006) Silencing suppression by geminivirus proteins. Virology, 344, 158–168. [DOI] [PubMed] [Google Scholar]
- Bosco, D. , Mason, G. and Accotto, G.P. (2004) TYLCSV DNA, but not infectivity, can be transovarially inherited by the progeny of the whitefly vector Bemisia tabaci (Gennadius). Virology, 323, 276–283. [DOI] [PubMed] [Google Scholar]
- Bottcher, B. , Unseld, S. , Ceulemans, H. , Russell, R.B. and Jeske, H. (2004) Geminate structures of African cassava mosaic virus. J. Virol. 78, 6758–6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briddon, R.W. , Bedord, I.D. , Tsai, J.H. and Markham, P.G. (1996) Analysis of the nucleotide sequence of the treehopper‐transmitted geminivirus, tomato pseudo‐curly top virus, suggests a recombinant origin. Virology, 219, 387–394. [DOI] [PubMed] [Google Scholar]
- Caciagli, P. , Medina, P.V. , Marian, D. , Vecchiati, M. , Masenga, V. , Mason, G. , Falcioni, T. and Noris, E. (2009) Virion stability is important for the circulative transmission of Tomato yellow leaf curl Sardinia virus by Bemisia tabaci, but virion access to salivary glands does not guarantee transmissibility. J. Virol. 83, 5784–5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo, A.G. , Collinet, D. , Deret, S. , Kashoggi, A. and Bejarano, E.R. (2003) Dual interaction of plant PCNA with geminivirus replication accessory protein (Ren) and viral replication protein (Rep). Virology, 312, 381–394. [DOI] [PubMed] [Google Scholar]
- Castillo, A.G. , Kong, L.J. , Hanley‐Bowdoin, L. and Bejarano, E.R. (2004) Interaction between a geminivirus replication protein and the plant sumoylation system. J. Virol. 78, 2758–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatchawankanphanich, O. and Maxwell, D.P. (2002) Tomato leaf curl Karnataka virus from Bangalore, India, appears to be a recombinant begomovirus. Phytopathology, 92, 637–645. [DOI] [PubMed] [Google Scholar]
- Cohen, S. and Antignus, Y. (1994) Tomato yellow leaf curl virus (TYLCV), a whitefly‐borne geminivirus of tomatoes In: Advances in Disease Vector Research, Vol. 10. (Harris K.H., ed.), pp. 259–288. New York: Springer‐Verlag. [Google Scholar]
- Credi, R. , Betti, L. and Canova, A. (1989) Association of a geminivirus with a severe disease of tomato in Sicily. Phytopathol. Mediterr. 28, 223–226. [Google Scholar]
- Crespi, S. , Noris, E. , Vaira, A. and Accotto, G.P. (1995) Molecular characterization of cloned DNA from a tomato yellow leaf curl virus isolate from Sicily. Phytopathol. Mediterr. 34, 93–99. [Google Scholar]
- Czosnek, H. and Ghanim, M. (2002) The circulative pathway of begomoviruses in the whitefly vector Bemisia tabaci—insights from studies with Tomato yellow leaf curl virus . Ann. Appl. Biol. 140, 215–231. [Google Scholar]
- Czosnek, H. and Laterrot, H. (1997) A worldwide survey of tomato yellow leaf curl viruses. Arch. Virol. 142, 1391–1406. [DOI] [PubMed] [Google Scholar]
- Czosnek, H. , Ghanim, M. , Morin, S. , Rubinstein, G. , Fridman, V. and Zeidan, M. (2001) Whiteflies: vectors, and victims (?) of geminiviruses In: Virus–Vector–Plant Interactions (Harris K.H., Smith O.P. and Duffy J.E., eds), pp. 1–27. New York: Academic Press. [DOI] [PubMed] [Google Scholar]
- Davino, S. , Napoli, C. , Davino, M. and Accotto, G.P. (2006) Spread of tomato yellow leaf curl virus in Sicily: partial displacement of another geminivirus originally present. Eur. J. Plant Pathol. 114, 293–299. [Google Scholar]
- Davino, S. , Napoli, C. , Dellacroce, C. , Miozzi, L. , Noris, E. , Davino, M. and Accotto, G.P. (2009) Two new natural begomovirus recombinants associated with the tomato yellow leaf curl disease co‐exist with parental viruses in tomato epidemics in Italy. Virus Res. 143, 15–23. [DOI] [PubMed] [Google Scholar]
- De Barro, P.J. , Trueman, J.W. and Frohlich, D.R. (2005) Bemisia argentifolii is a race of B. tabaci (Hemiptera: Aleyrodidae): the molecular genetic differentiation of B. tabaci populations around the world. Bull. Entomol. Res. 95, 193–203. [DOI] [PubMed] [Google Scholar]
- Delatte, H. , Holota, H. , Naze, F. , Peterschmitt, M. , Reynaud, B. and Lett, J.M. (2005) The presence of both recombinant and nonrecombinant strains of Tomato yellow leaf curl virus on tomato in Réunion island. Plant Pathol. 54, 262. [Google Scholar]
- Edelbaum, D. , Gorovits, R. , Sasaki, S. , Ikegami, M. and Czosnek, H. (2009) Expressing a whitefly GroEL protein in Nicotiana benthamiana plants confers tolerance to tomato yellow leaf curl virus and cucumber mosaic virus, but not to grapevine virus A or tobacco mosaic virus. Arch. Virol. 154, 399–407. [DOI] [PubMed] [Google Scholar]
- Fauquet, C.M. , Sawyer, A. , Idris, A.M. and Brown, J.K. (2005) Sequence analysis and classification of apparent recombinant begomoviruses infecting tomato in the Nile and Mediterranean basins. Phytopathology, 95, 549–555. [DOI] [PubMed] [Google Scholar]
- Fauquet, C.M. , Briddon, R.W. , Brown, J.K. , Moriones, E. , Stanley, J. , Zerbini, M. and Zhou, X. (2008) Geminivirus strain demarcation and nomenclature. Arch. Virol. 153, 783–821. [DOI] [PubMed] [Google Scholar]
- Fernández‐Cuartero, B. , Burgyan, J. , Aranda, M.A. , Salánki, K. , Moriones, E. and García‐Arenal, F. (1994) Increase in the relative fitness of a plant virus RNA associated with its recombinant nature. Virology, 203, 373–377. [DOI] [PubMed] [Google Scholar]
- Fondong, V.N. , Pita, J.S. , Rey, M.E.C. , De Kochko, A. , Beachy, R.N. and Fauquet, C.M. (2000) Evidence of synergism between African cassava mosaic virus and a new double‐recombinant geminivirus infecting cassava in Cameroon. J. Gen. Virol. 81, 287–297. [DOI] [PubMed] [Google Scholar]
- Fontes, E.P.B. , Eagle, P.A. , Sipe, P.S. , Luckow, V.A. and Hanley‐Bowdoin, L. (1994) Interaction between a geminivirus replication protein and origin DNA is essential for viral replication. J. Biol. Chem. 269, 8459–8465. [PubMed] [Google Scholar]
- Froissart, R. , Roze, D. , Uzest, M. , Galibert, L. , Blanc, S. and Michalakis, Y. (2005) Recombination every day: abundant recombination in a virus during a single multi‐cellular host infection. PLoS Biol. 3, 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafni, Y. (2003) Tomato yellow leaf curl virus, the intracellular dynamics of a plant DNA virus. Mol. Plant Pathol. 4, 9–15. [DOI] [PubMed] [Google Scholar]
- García‐Andrés, S. , Monci, F. , Navas‐Castillo, J. and Moriones, E. (2006) Begomovirus genetic diversity in the native plant reservoir Solanum nigrum: evidence for the presence of a new virus species of recombinant nature. Virology, 350, 433–442. [DOI] [PubMed] [Google Scholar]
- García‐Andrés, S. , Accotto, G.P. , Navas‐Castillo, J. and Moriones, E. (2007a) Founder effect plant host, and recombination shape the emergent population of begomoviruses that cause the tomato yellow leaf curl disease in the Mediterranean basin. Virology, 359, 302–312. [DOI] [PubMed] [Google Scholar]
- García‐Andrés, S. , Tomás, D.B. , Sánchez‐Campos, S. , Navas‐Castillo, J. and Moriones, E. (2007b) Frequent occurrence of recombinants in mixed infections of tomato yellow leaf curl disease‐associated begomoviruses. Virology, 365, 210–219. [DOI] [PubMed] [Google Scholar]
- García‐Andrés, S. , Tomás, D.M. , Navas‐Castillo, J. and Moriones, E. (2009) Resistance‐driven selection of begomoviruses associated with the tomato yellow leaf curl disease. Virus Res. 146, 66–72. [DOI] [PubMed] [Google Scholar]
- García‐Arenal, F. , Fraile, A. and Malpica, J.M. (2001) Variability and genetic structure of plant virus populations. Annu. Rev. Phytopahol. 39, 157–186. [DOI] [PubMed] [Google Scholar]
- Ghanim, M. and Czosnek, H. (2000) Tomato yellow leaf curl geminivirus (TYLCV‐Is) is transmitted among whiteflies (Bemisia tabaci) in a sex‐related manner. J. Virol. 74, 4738–4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanim, M. and Medina, V. (2007) Localization of Tomato yellow leaf curl virus in its whitefly vector Bemisia tabaci In: Tomato Yellow Leaf Curl Virus Disease: Management, Molecular Biology, Breeding for Resistance (Czosnek H., ed.), pp. 171–183. Dordrecht: Springer. [Google Scholar]
- Ghanim, M. , Morin, S. , Zeidan, M. and Czosnek, H. (1998) Evidence for transovarial transmission of tomato yellow leaf curl virus by its vector, the whitefly Bemisia tabaci . Virology, 240, 295–303. [DOI] [PubMed] [Google Scholar]
- Ghanim, M. , Sobol, I. , Ghanim, M. and Czosnek, H. (2007) Horizontal transmission of begomoviruses between Bemisia tabaci biotypes. Arthropod Plant Interact. 1, 195–204. [Google Scholar]
- Gutiérrez, C. (1999) Geminivirus DNA replication. Cell. Mol. Life Sci. 56, 313–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez, C. (2000) Geminivirus and plant cell cycle. Plant Mol. Biol. 43, 763–772. [DOI] [PubMed] [Google Scholar]
- Gutiérrez, C. , Ramírez‐Parra, E. , Castellano, M.M. , Sanz‐Burgos, A.P. , Luque, A. and Missich, R. (2004) Geminivirus DNA replication and cell cycle interactions. Vet. Microbiol. 98, 111–119. [DOI] [PubMed] [Google Scholar]
- Hanley‐Bowdoin, L. , Settlage, S.B. , Orozco, B.M. , Nagar, S. and Robertson, D. (1999) Geminiviruses: models for plant DNA replication, transcription, and cell cycle regulation. Crit. Rev. Plant Sci. 18, 71–106. [PubMed] [Google Scholar]
- Hanley‐Bowdoin, L. , Settlage, S.B. and Robertson, D. (2004) Reprogramming plant gene expression: a prerequisite to geminivirus DNA replication. Mol. Plant Pathol. 5, 149–156. [DOI] [PubMed] [Google Scholar]
- Harrison, B.D. and Robinson, D.J. (1999) Natural genomic and antigenic variation in whitefly‐transmitted geminiviruses (Begomoviruses). Annu. Rev. Phytopathol. 37, 369–398. [DOI] [PubMed] [Google Scholar]
- Hogenhout, S.A. , Ammar, E.D. , Whitfield, A.E. and Redinbaugh, M.G. (2008) Insect vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 46, 327–359. [DOI] [PubMed] [Google Scholar]
- Hohnle, M.F. , Hofer, P.F. , Bedford, I.D. , Briddon, R.W. , Markham, P.G. and Frischmuth, T. (2001) Exchange of three amino acids in the coat protein results in efficient whitefly transmission of a nontransmissible Abutilon mosaic virus isolate. Virology, 290, 164–171. [DOI] [PubMed] [Google Scholar]
- Hou, Y.M. and Gilbertson, R.L. (1996) Increased pathogenicity in a pseudorecombinant bipartite geminivirus correlates with intermolecular recombination. J. Virol. 70, 5430–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter, W.B. and Polston, J.E. (2001) Development of a continuous whitefly cell line [Homoptera: Aleyrodidae: Bemisia tabaci (Gennadius)] for the study of begomovirus. J. Invert. Pathol. 77, 33–36. [DOI] [PubMed] [Google Scholar]
- Huson, D.H. and Bryant, D. (2006) Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 23, 254–267. [DOI] [PubMed] [Google Scholar]
- Jeske, H. , Lütgemeier, M. and Preiss, W. (2001) DNA forms indicate rolling circle and recombination‐dependent replication of Abutilon mosaic virus. EMBO J. 20, 6158–6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiu, M. , Zhou, X.P. , Tong, L. , Xu, J. , Yang, X. , Wan, F.H. and Liu, S.S. (2007) Vector–virus mutualism accelerates population increase of an invasive whitefly. PLoS ONE 2, e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupin, I. , De Kouchkovsky, F. , Jouanneau, F. and Gronenborn, B. (1994) Movement of tomato yellow leaf curl geminivirus (TYLCV): involvement of the protein encoded by ORF C4. Virology, 204, 82–90. [DOI] [PubMed] [Google Scholar]
- Kheyr‐Pour, A. , Bendahmane, M. , Matzeit, V. , Accotto, G.P. , Crespi, S. and Gronenborn, B. (1991) Tomato yellow leaf curl virus from Sardinia is a whitefly‐transmitted monopartite geminivirus. Nucleic Acids Res. 19, 6763–6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheyr‐Pour, A. , Bananej, K. , Dafalla, G.A. , Caciagli, P. , Noris, E. , Ahoonmanesh, A. , Lecoq, H. and Gronenborn, B. (2000) Watermelon chlorotic stunt virus from the Sudan and Iran: sequence comparisons and identification of a whitefly‐transmission determinant. Phytopathology, 90, 629–635. [DOI] [PubMed] [Google Scholar]
- Kirthi, N. , Maiya, S.P. , Murthy, M.R.N. and Savithr, H.S. (2002) Evidence for recombination among the tomato leaf curl virus strains/species from Bangalore, India. Arch. Virol. 147, 255–272. [DOI] [PubMed] [Google Scholar]
- Klute, K.A. , Nadler, S.A. and Stenger, D.C. (1996) Horseradish curly top virus is a distinct subgroup II geminivirus species with rep and C4 gene derived from a subgroup III ancestor. J. Gen. Virol. 77, 1369–1378. [DOI] [PubMed] [Google Scholar]
- Kong, L.J. and Hanley‐Bowdoin, L. (2002) A geminivirus replication protein interacts with a protein kinase and a motor protein that display different expression patterns during plant development and infection. Plant Cell, 14, 1817–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, L.J. , Orozco, B.M. , Roe, J.L. , Nagar, S. , Ou, S. , Feiler, H.S. , Durfee, T. , Miller, A.B. , Gruissem, W. , Robertson, D. and Hanley‐Bowdoin, L. (2000) A geminivirus replication protein interacts with the retinoblastoma protein through a novel domain to determine symptoms and tissue specificity of infection in plants. EMBO J. 19, 3485–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs, J. , Traut, W. , Heyraud, F. , Matzeit, V. , Rogers, S.G. , Schell, J. and Gronenborn, B. (1995) In vitro cleavage and joining at the viral origin of replication by the replication initiator protein of tomato yellow leaf curl virus. Proc. Natl Acad. Sci. USA, 92, 3879–3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefeuvre, P. , Martin, D.P. , Hoareau, M. , Naze, F. , Delatte, H. , Thierry, M. , Varsani, A. , Becker, N. , Reynaud, B. and Lett, J.M. (2007) Begomovirus ‘melting pot’ in the south‐west Indian Ocean islands: molecular diversity and evolution through recombination. J. Gen. Virol. 88, 3458–3468. [DOI] [PubMed] [Google Scholar]
- Lefeuvre, P. , Lett, J.M. , Varsani, A. and Martin, D.P. (2009) Widely conserved recombination patterns among single‐stranded DNA viruses. J. Virol. 83, 2697–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, D.P. , Willment, J.A. , Billharz, R. , Velders, R. , Odhiambo, B. , Njuguna, J. , James, D. and Rybicki, E. (2001) Sequence diversity and virulence in Zea mays of Maize streak virus isolates. Virology, 288, 247–255. [DOI] [PubMed] [Google Scholar]
- Martin, D.P. , Van Der Walt, E. , Posada, D. and Rybicki, E.P. (2005) The evolutionary value of recombination is constrained by genome modularity. PLoS Genet. 1, 475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat, A.S. (1999) Plant pathology. Geminiviruses emerge as serious crop threat. Science, 286, 1835. [Google Scholar]
- Monci, F. , Sánchez‐Campos, S. , Navas‐Castillo, J. and Moriones, E. (2002) A natural recombinant between the geminiviruses Tomato yellow leaf curl Sardinia virus and Tomato yellow leaf curl virus exhibits a novel pathogenic phenotype and is becoming prevalent in Spanish populations. Virology, 303, 317–326. [DOI] [PubMed] [Google Scholar]
- Morilla, G. , Antúnez, C. , Bejarano, E.R. , Janssen, D. and Cuadrado, I.M. (2003) A new tomato yellow leaf curl virus strain in southern Spain. Plant Dis. 87, 1004. [DOI] [PubMed] [Google Scholar]
- Morilla, G. , Krenz, V. , Jeske, H. , Bejarano, E.R. and Wege, C. (2004) Tête a tête of Tomato yellow leaf curl virus and Tomato yellow leaf curl Sardinia virus in single nuclei. J. Virol. 78, 10715–10723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin, S. , Ghanim, M. , Zeidan, M. , Czosnek, H. , Verbeek, M. and Heuvel, J.F. (1999) A GroEL homologue from endosymbiotic bacteria of the whitefly Bemisia tabaci is implicated in the circulative transmission of tomato yellow leaf curl virus. Virology, 256, 75–84. [DOI] [PubMed] [Google Scholar]
- Morin, S. , Ghanim, M. , Sobol, I. and Czosnek, H. (2000) The GroEL protein of the whitefly Bemisia tabaci interacts with the coat protein of transmissible and nontransmissible begomoviruses in the yeast two‐hybrid system. Virology, 276, 404–416. [DOI] [PubMed] [Google Scholar]
- Moriones, E. and Navas‐Castillo, J. (2000) Tomato yellow leaf curl virus, an emerging virus complex causing epidemics worldwide. Virus Res. 71, 123–134. [DOI] [PubMed] [Google Scholar]
- Moriones, E. and Navas‐Castillo, J. (2008) Rapid evolution of the population of begomoviruses associated with the tomato yellow leaf curl disease after invasion of a new ecological niche. Span. J. Agric. Res. 6, 147–159. [Google Scholar]
- Moriones, E. , García‐Andrés, S. and Navas‐Castillo, J. (2007) Recombination in the TYLCV complex: a mechanism to increase genetic diversity. Implications for plant resistance development In: Tomato Yellow Leaf Curl Virus Disease: Management, Molecular Biology, Breeding for Resistance (Czosnek H., ed.), pp. 119–138. Dordrecht: Springer. [Google Scholar]
- Navas‐Castillo, J. , Sánchez‐Campos, S. , Díaz, J.A. , Sáez‐Alonso, E. and Moriones, E. (1997) First report of tomato yellow leaf curl‐Is in Spain: coexistence of two different geminiviruses in the same epidemic outbreak. Plant Dis. 81, 1461. [DOI] [PubMed] [Google Scholar]
- Navas‐Castillo, J. , Sánchez‐Campos, S. , Díaz, J.A. , Sáez‐Alonso, E. and Moriones, E. (1999) Tomato yellow leaf curl virus‐Is causes a novel disease of common bean and severe epidemics in tomato in Spain. Plant Dis. 83, 29–32. [DOI] [PubMed] [Google Scholar]
- Navas‐Castillo, J. , Sánchez‐Campos, S. , Noris, E. , Louro, D. , Accotto, G.P. and Moriones, E. (2000) Natural recombination between Tomato yellow leaf curl virus‐Is and Tomato leaf curl virus . J. Gen. Virol. 81, 2797–2801. [DOI] [PubMed] [Google Scholar]
- Navot, N. , Pichersky, E. , Zeidan, M. , Zamir, D. and Czosnek, H. (1991) Tomato yellow leaf curl virus: a whitefly‐transmitted geminivirus with a single genomic component. Virology, 185, 151–161. [DOI] [PubMed] [Google Scholar]
- Noris, E. , Hidalgo, E. , Accotto, G.P. and Moriones, E. (1994) High similarity among the tomato yellow leaf curl virus isolates from the west Mediterranean basin: the nucleotide sequence of an infectious clone from Spain. Arch. Virol. 135, 165–170. [DOI] [PubMed] [Google Scholar]
- Noris, E. , Vaira, A.M. , Caciagli, P. , Masenga, V. , Gronenborn, B. and Accotto, G.P. (1998) Amino acids in the capsid protein of tomato yellow leaf curl virus that are crucial for systemic infection, particle formation, and insect transmission. J. Virol. 72, 10050–10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi, J. , Kitamura, T. , Terami, F. and Honda, K.I. (2009) A selective barrier in the midgut epithelial cell membrane of the nonvector whitefly Trialeurodes vaporariorum to Tomato yellow leaf curl virus uptake. J. Gen. Plant Pathol. 75, 131–139. [Google Scholar]
- Padidam, M. , Sawyer, S. and Fauquet, C.M. (1999) Possible emergence of new geminiviruses by frequent recombination. Virology, 265, 218–225. [DOI] [PubMed] [Google Scholar]
- Pita, J.S. , Fondong, V.N. , Sangare, A. , Otim‐Nape, G.W. , Ogwal, S. and Fauquet, C.M. (2001) Recombination, pseudorecombination and synergism of geminiviruses are determinant keys to the epidemic of severe cassava mosaic disease in Uganda. J. Gen. Virol. 82, 655–665. [DOI] [PubMed] [Google Scholar]
- Polston, J.E. , McGovern, R.J. and Brown, L.G. (1999) Introduction of tomato yellow leaf curl virus in Florida and implications for the spread of this and other geminiviruses of tomato. Plant Dis. 83, 984–988. [DOI] [PubMed] [Google Scholar]
- Raja, P. , Sanville, B.C. , Buchmann, R.C. and Bisaro, D.M. (2008) Viral genome methylation as an epigenetic defense against geminiviruses. J. Virol. 82, 8997–9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas, M.R. , Jiang, H. , Salati, R. , Xoconostle‐Cazares, B. , Sudarshana, M.R. , Lucas, W.J. and Gilbertson, R.L. (2001) Functional analysis of proteins involved in movement of the monopartite begomovirus, Tomato yellow leaf curl virus. Virology, 291, 110–125. [DOI] [PubMed] [Google Scholar]
- Rubinstein, G. and Czosnek, H. (1997) Long‐term association of tomato yellow leaf curl virus with its whitefly vector Bemisia tabaci: effect on the insect transmission capacity, longevity and fecundity. J. Gen. Virol. 78, 2683–2689. [DOI] [PubMed] [Google Scholar]
- Sánchez‐Campos, S. , Navas‐Castillo, J. , Camero, R. , Soria, C. , Díaz, J.A. and Moriones, E. (1999) Displacement of tomato yellow leaf curl virus (TYLCV)‐Sr by TYLCV‐Is in tomato epidemics in Spain. Phytopathology, 89, 1038–1043. [DOI] [PubMed] [Google Scholar]
- Sánchez‐Campos, S. , Díaz, J.A. , Monci, F. , Bejarano, E.R. , Reina, J. , Navas‐Castillo, J. , Aranda, M.A. and Moriones, E. (2002) High genetic stability of the begomovirus Tomato yellow leaf curl Sardinia virus in southern Spain over an 8‐year period. Phytopathology, 92, 842–849. [DOI] [PubMed] [Google Scholar]
- Sanz, A.I. , Fraile, A. , Gallego, J.M. , Malpica, J.M. and García‐Arenal, F. (1999) Genetic variability of natural populations of cotton leaf curl geminivirus, a single‐stranded DNA virus. J. Mol. Evol. 49, 672–681. [DOI] [PubMed] [Google Scholar]
- Sanz, A.I. , Fraile, A. , García‐Arenal, F. , Zhou, X.P. , Robinson, D.J. , Khalid, S. , Butt, T. and Harrison, B.D. (2000) Multiple infection, recombination and genome relationships among begomovirus isolates found in cotton and other plants in Pakistan. J. Gen. Virol. 81, 1839–1849. [DOI] [PubMed] [Google Scholar]
- Saunders, K. and Stanley, J. (1999) A nanovirus‐like DNA component associated with yellow vein disease of Ageratum conyzoides: evidence for interfamilial recombination between plant DNA viruses. Virology, 264, 142–152. [DOI] [PubMed] [Google Scholar]
- Saunders, K. , Lucy, A. and Stanley, J. (1991) DNA forms of the geminivirus African cassava mosaic virus consistent with a rolling circle mechanism of replication. Nucleic Acids Res. 19, 2325–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders, K. , Salim, N. , Mali, V.R. , Malathi, V.G. , Briddon, R. , Markham, P.G. and Stanley, J. (2002) Characterisation of Sri Lankan cassava mosaic virus and Indian cassava mosaic virus: evidence for acquisition of a DNA B component by a monopartite begomovirus. Virology, 293, 63–74. [DOI] [PubMed] [Google Scholar]
- Seal, S.E. , Bosch, F. and Jeger, M.J. (2006) Factors influencing begomovirus evolution and their increasing global significance: implications for sustainable control. Crit. Rev. Plant Sci. 35, 23–46. [Google Scholar]
- Selth, L.A. , Dogra, S.C. , Rasheed, M.S. , Healy, H. , Randles, J.W. and Rezaian, M.A. (2005) A NAC domain protein interacts with tomato leaf curl virus replication accessory protein and enhances viral replication. Plant Cell, 17, 311–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settlage, S.B. , Miller, A.B. , Gruissem, W. and Hanley‐Bowdoin, L. (2001) Dual interaction of a geminivirus replication accessory factor with a viral replication protein and a plant cell cycle regulator. Virology, 279, 570–576. [DOI] [PubMed] [Google Scholar]
- Settlage, S.B. , See, R.G. and Hanley‐Bowdoin, L. (2005) Geminivirus C3 protein: replication enhancement and protein interactions. J. Virol. 79, 9885–9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinisterra, X.H. , McKenzie, C.L. , Hunter, W.B. , Powell, C.A. and Shatters, R.G., Jr (2005) Differential transcriptional activity of plant‐pathogenic begomoviruses in their whitefly vector (Bemisia tabaci, Gennadius: Hemiptera Aleyrodidae). J. Gen. Virol. 86, 1525–1532. [DOI] [PubMed] [Google Scholar]
- Stanley, J. (1995) Analysis of African cassava mosaic virus recombinants suggests strand nicking occurs within the conserved nonanucleotide motif during the initiation of rolling circle DNA replication. Virology, 206, 707–712. [DOI] [PubMed] [Google Scholar]
- Stenger, D.C. , Revington, G.N. , Stevenson, M.C. and Bisaro, D.M. (1991) Replicational release of geminivirus genomes from tandemly repeated copies. Evidence for rolling circle replication of a plant viral DNA. Proc. Natl Acad. Sci. USA, 88, 8029–8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda, S. , Kimura, T. , Onuki, M. , Hanada, K. and Iwanami, T. (2004) Three distinct groups of isolates of Tomato yellow leaf curl virus in Japan and construction of an infectious clone. J. Gen. Plant Pathol. 70, 232–238. [Google Scholar]
- Umaharan, P. , Padidam, M. , Phelps, R.H. , Beachy, R.N. and Fauquet, C.M. (1998) Distribution and diversity of geminiviruses in Trinidad and Tobago. Phytopathology, 88, 1262–1268. [DOI] [PubMed] [Google Scholar]
- Wartig, L. , Kheyr‐Pour, A. , Noris, E. , De Kouchkovsky, F. , Jouanneau, F. , Gronenborn, B. and Jupin, I. (1997) Genetic analysis of the monopartite tomato yellow leaf curl geminivirus: roles of V1, V2, and C2 ORFs in viral pathogenesis. Virology, 228, 132–140. [DOI] [PubMed] [Google Scholar]
- Worobey, M. and Holmes, E.C. (1999) Evolutionary aspects of recombination in RNA viruses. J. Gen. Virol. 80, 2535–2543. [DOI] [PubMed] [Google Scholar]
- Zambrano, K. , Carballo, O. , Geraud, F. , Chirinos, D. , Fernández, C. and Marys, E. (2007) First report of Tomato yellow leaf curl virus in Venezuela. Plant Dis. 91, 768. [DOI] [PubMed] [Google Scholar]
- Zhou, X.P. , Liu, Y.L. , Calvert, L. , Munoz, C. , Otim‐Nape, G.W. , Robinson, D.J. and Harrison, B.D. (1997) Evidence that DNA‐A of a geminivirus associated with severe cassava mosaic disease in Uganda has arisen by interspecific recombination. J. Gen. Virol. 78, 2101–2111. [DOI] [PubMed] [Google Scholar]
- Zrachya, A. , Glick, E. , Levy, Y. , Arazi, T. , Citovsky, V. and Gafni, Y. (2007) Suppressor of RNA silencing encoded by Tomato yellow leaf curl virus‐Israel. Virology, 358, 159–165. [DOI] [PubMed] [Google Scholar]


