SUMMARY
Common scab is a severe disease worldwide affecting tap root crops and potato tubers. It is caused by soil‐borne filamentous bacteria belonging to the genus Streptomyces. Streptomycetes usually are saprophytic microorganisms, but a few species have acquired the ability to infect underground plant tissues. The predominant causal agent of potato scab worldwide is Streptomyces scabies. The production of phytotoxins called thaxtomins is essential for the virulence of common scab‐causing agents. The genes involved in the biosynthetic pathway of thaxtomins and other virulence genes are clustered on a large pathogenicity island. The pathogenicity island can be mobilized and transferred to nonpathogenic relatives, leading to the emergence of new pathogenic streptomycetes. In most pathogenic Streptomyces species, thaxtomin A is the predominant form found. The regulation of thaxtomin A synthesis is complex. Although the plant‐derived compound cellobiose is now recognized as the inducer of thaxtomin A synthesis at a genetic level, other molecules (including aromatic amino acids and some secondary metabolites) show inhibitory effects on the production of the toxin. This paper is an overview of common scab with a focus on S. scabies and its virulence mechanisms.
Taxonomy: Streptomyces scabies (Thaxt.) Lambert and Loria; Kingdom Bacteria; Phylum Actinobacteria; Class Actinomycetes; Order Actinomycetales; Family Streptomycetaceae; genus Streptomyces; species scabies or scabiei.
Host range: Streptomyces scabies (syn. S. scabiei) has a broad host range comprising tuber vegetables and most tap root crops. Streptomyces scabies causes common scab on potato (Solanum tuberosum), beet (Beta vulgaris), carrot (Daucus carota), parsnip (Pastinaca sativa), radish (Raphanus sativus), rutabaga (Brassica napobrassica) and turnip (Brassica rapa).
Disease symptoms: Common scab symptoms appear as randomly distributed shallow, raised or deep‐pitted corky lesions. Their size and colour are quite variable, but lesions typically are brown with a diameter of a few millimetres. No above‐ground symptoms disclose the presence of the disease as aerial tissues of scab‐infected plants remain healthy. Streptomyces scabies also inhibits the growth of seedlings in monocot and dicot plants.
Useful websites: http://www.sanger.ac.uk/Projects/S_scabies, http://www.potatodiseases.org/scab.html, http://www.uri.edu/ce/factsheets/sheets/potatoscab.html
INTRODUCTION
Common scab is a frequent disease of bacterial origin that can thrive in fields in which root and tuber vegetables are grown. The predominant causal agent of potato scab is Streptomyces scabies. It belongs to a wide group of filamentous Gram‐positive soil bacteria (Fig. 1A) that are essentially saprophytic microorganisms. Nevertheless, S. scabies is one of the few members of actinomycetes that is a plant pathogen. Common scab is characterized by visible lesions on the surface of various root and tuber vegetables (Fig. 1B). Symptoms can emerge as shallow, raised or deep‐pitted corky lesions, depending on various environmental conditions (Goyer et al., 1996). The occurrence of common scab in the field does not usually alter crop yields and the consumption of scab‐infected food may not threaten human health. However, as the quality of crops, such as potato, depends on their general appearance, the degree of infection by common scab pathogens directly affects their market value. For instance, the incidence of common scab on potato tubers has been found to generate economic losses of around 15% in Quebec, Canada (Hill and Lazarovits, 2005).
Figure 1.
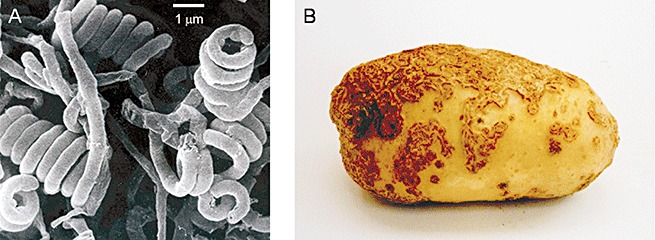
(A) Filamentous mycelium of Streptomyces scabies observed by electron microscopy. Life cycle eventually evolves to the formation of spores borne in spiral chains. (B) Common scab lesions on a potato tuber.
The virulence of pathogenic S. scabies strains is essentially dependent on their capacity to produce a family of phytotoxins called thaxtomins (King et al., 1989). These secondary metabolites are 4‐nitroindol‐3‐yl‐containing 2,5‐dioxopiperazines, consisting of cyclized nitro‐tryptophan and phenylalanine (the biosynthetic pathway of thaxtomins has been reviewed by Loria et al., 2006). The dominant form of thaxtomin produced by S. scabies is thaxtomin A (King et al., 1992; Fig. 2), although phytotoxicity has also been reported for other thaxtomins (Hiltunen et al., 2006; King and Lawrence, 2001). Recently, it has been shown that thaxtomin A biosynthesis is induced by the presence of the plant polysaccharides cellobiose and cellotriose (Johnson et al., 2007; Wach et al., 2007). Cellobiose binds a regulatory protein that upregulates the genes of the biosynthetic pathway of the toxin (Joshi et al., 2007b). However, the presence of cellobiose only weakly induces thaxtomin production when the bacterium is grown in minimal medium. Previous work has also reported weak thaxtomin A production in the presence of suberin, a plant polymer covering the surface of potato tubers (Beauséjour et al., 1999). A combination of suberin and the thaxtomin inducer cellobiose generates the production of large amounts of the phytotoxin by S. scabies grown in minimal medium (S. Lerat et al., unpublished data). The role of suberin in thaxtomin biosynthesis needs to be elucidated, but it is hypothesized that suberin stimulates secondary metabolism and thus might favour thaxtomin production (Lauzier et al., 2008).
Figure 2.
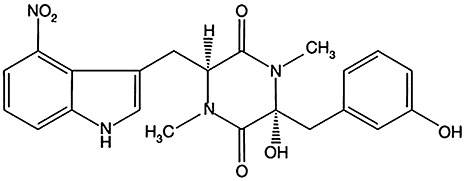
Schematic representation of thaxtomin A, the major phytotoxin produced by Streptomyces scabies.
The effect of thaxtomins on plant physiology has been investigated in several plant species. However, the mechanisms of action of the toxins have been only partially identified to date. The pathogens are thought to derive carbohydrates from expanding underground plant tissues through the inhibition of cellulose synthesis (Fry and Loria, 2002; Scheible et al., 2003). Nonetheless, the presence of thaxtomin has also been shown to trigger plant defence mechanisms. An early plant response to thaxtomin A in Arabidopsis thaliana is Ca2+ influx (Duval et al., 2005; Errakhi et al., 2008; Tegg et al., 2005). Calcium is a key messenger in the reaction of plants challenged by pathogens. In A. thaliana culture cells, the calcium‐mediated plant response to thaxtomin A ultimately leads to programmed cell death (Duval et al., 2005; Errakhi et al., 2008). Moreover, experiments conducted on tobacco and A. thaliana have shown that both S. scabies and thaxtomin A elicit the production of scopoletin, a plant defence phytoalexin (S. Lerat et al., unpublished data). Interestingly, when the pathogenic agent was grown in a thaxtomin‐inducing growth medium, the presence of scopoletin caused a drastic decrease in toxin synthesis (S. Lerat et al., unpublished data).
Pathogenic Streptomyces species do not show a high level of host specificity under controlled conditions. Leiner et al. (1996) reported the effect of inoculation with virulent S. scabies strains on the seedlings of 14 crop plants, including monocot and dicot species. The presence of the pathogenic bacterium negatively altered shoot growth in 11 of the species tested. This is not surprising as the target of thaxtomins is a universal component of plant cell walls (i.e. cellulose). Nevertheless, the range of hosts having a propensity to common scab infection under field conditions appears to be restricted to a limited number of agricultural crops (Goyer and Beaulieu, 1997). Moreover, a few potato cultivars are recognized as resistant or moderately sensitive to the disease (Hiltunen et al., 2005). Therefore, the host infection process that leads to common scab under natural conditions appears to be governed by complex plant–microbe interactions.
COMMON SCAB: A DISEASE ON THE SPREAD
The causal agent of potato common scab was first identified in North America in the late 19th century (Thaxter, 1892). A review of the recent scientific literature reveals that this disease is now present worldwide, wherever potatoes are cultivated. In addition to the predominant and well‐studied species S. scabies, other Streptomyces species are recognized as common scab‐causing agents (Beaulieu et al., 2008). The isolation of actinomycetes from infected potato tubers has led to the discovery of new or unidentified pathogenic agents (Bouchek‐Mechiche et al., 2000; Miyajima et al., 1998; Park et al., 2003; Wanner, 2007, 2006). The exhaustive list of common scab‐causing agents contains various, not closely related Streptomyces species. Worthy of mention are S. turgidiscabies and S. acidiscabies, which appear to be the most widely distributed scab pathogens after S. scabies. They can be distinguished from each other by differences in morphology (Lambert and Loria, 1989a, 1989b; Miyajima et al., 1998), carbon source utilization (Miyajima et al., 1998) and 16S rRNA sequence (Bouchek‐Mechiche et al., 2000; Lehtonen et al., 2004). It is assumed that, in most species, pathogenicity was acquired after the mobilization and lateral transfer of genetic material from common scab‐causing strains to saprophytic recipient strains (Kers et al., 2005).
The genes conferring pathogenicity are generally clustered within a large pathogenicity island (PAI), which represents a unique report in Gram‐positive bacteria (Kers et al., 2005). Among them are genes involved in thaxtomin biosynthesis, as well as open reading frames showing strong similarities to virulence genes associated with other pathogenic organisms. In an elegant study aimed at the investigation of the genetic variability of common scab‐causing agents, Wanner (2006) tested for the presence of three marker genes of the PAI in the genome of about 100 isolates. The genes under investigation were a gene involved in thaxtomin biosynthesis (txtAB), a necrosis gene (nec1) and a tomatinase (tomA). All isolates that contained the txtAB gene showed pathogenicity on potato or radish. However, some pathogenic isolates lacked the presence of one or both of the nec1 and tomA genes. The absence of the nec1 gene has been reported previously in some pathogenic S. scabies strains isolated in Finland (Kreuze et al., 1999). More recently, Flores‐Gonzáles et al. (2008) have shown that the nec1 gene is missing from one‐half of the pathogenic Streptomyces isolates obtained from infected potatoes. This implies that, unlike the txtAB genes, the nec1 and tomA genes are not essential for pathogenicity. Consequently, polymerase chain reaction (PCR)‐based techniques targeting the txtAB operon (or other genes of the synthetic pathway of thaxtomins) can be legitimately used as the most robust diagnostic tools for the detection of common scab‐inducing pathogens in biological and soil samples (Flores‐Gonzáles et al., 2008; Qu et al., 2008).
ORGANIZATION OF VIRULENCE‐RELATED GENES IN S. SCABIES
Evidence for the existence of a PAI was established in S. turgidiscabies (Kers et al., 2005). At the very least, parts of the PAI were shown to be conserved among pathogenic Streptomyces (Kers et al., 2005; Wanner, 2006). In S. turgidiscabies, the size of the PAI has been estimated to be between 325 and 660 kb. However, the public release of the complete genome of S. scabies (Sanger Institute, http://www.sanger.ac.uk/Projects/S_scabies/) revealed a curious feature regarding the organization of PAI in this species. In S. scabies strain 87.22, typical genes of PAI are found in two remote regions of the bacterial chromosome. For instance, the txtA and nec1 genes are over 4900 kb distant (the whole genome is c. 10 149 kb). Interestingly, this physical separation also mirrors a partition of functions.
Genes associated with toxin production are clustered in the first section of the PAI, which we will call the ‘toxicogenic region’ (http://www.sanger.ac.uk/Projects/S_scabies; estimated coordinates c. 3596–3653 kb; G + C content of 68%). All genes shown to be involved in thaxtomin biosynthesis are found in this region (Table 1). These genes are txtAB (Healy et al., 2000), txtC (Healy et al., 2002), nos (Kers et al., 2004) and txtR (Joshi et al., 2007b). They code for a nonribosomal peptide synthase (cyclization of the dipeptide), a cytochrome P450 monooxygenase (hydroxylation of the cyclic dipeptide), a nitric oxide synthase (nitration of the tryptophan moiety is essential for the toxicity of thaxtomins) and a cellobiose‐binding regulatory protein, respectively. A schematic representation of the organization of these genes in S. scabies has been supplied by Joshi et al. (2007b). An open reading frame coding for a protein sharing 44% similarity with a putative phage integrase in Streptomyces sp. Mg1, and separated from nos by only 2634 bp, is apparently the only complete mobile genetic element associated with this region. The second segment of the PAI, which we will call the ‘colonization region’, contains considerably more genes (http://www.sanger.ac.uk/Projects/S_scabies; estimated coordinates c. 8471–8581 kb; G + C content of 68.5%). This chromosomal region includes genes such as nec1 and tomA which are not essential to pathogenicity, but play a significant role in virulence. Nec1 and TomA may play a role in the infection process through the suppression of plant defences (Joshi et al., 2007a; Seipke and Loria, 2008). Four open reading frames showing similarities with transposases are found in the close vicinity of the nec1 gene. The presence of genes coding for esterase‐like proteins in the same region also deserves to be mentioned, as extracellular esterase activity was measured in S. scabies (Beauséjour et al., 1999; Schottel et al., 1992) and some esterases are thought to play a role in the degradation of suberin, which can act as a physical barrier against microbial invasion (Kolattukudy, 1980). Table 1 presents a list of genes associated with the toxicogenic and colonization regions.
Table 1.
Examples of the main functions* associated with the open reading frames (ORFs) present in Streptomyces scabies pathogenicity island.
| Toxicogenic region | Colonization region | ||||
|---|---|---|---|---|---|
| Putative function of the protein‐coding gene | Name | Position† (ORF length, bp) | Putative function of the protein‐coding gene | Name | Position† (ORF length, bp) |
| Gene regulation | Gene regulation | ||||
| AraC family regulatory protein | txtR | 3 610 920 (834) | LacI family regulatory protein | 8 539 752 (912) | |
| Mobile genetic elements | Mobile genetic elements | ||||
| Phage integrase family protein | 3 617 834 (1224) | Transposase | 8 515 735 (1191) | ||
| Phytotoxic compound biosynthesis | Virulence | ||||
| Cytochrome P450 | txtC | 3 598 108 (1188) | Necrosis protein | nec1 | 8 514 341 (555) |
| MbtH‐like protein‡ | 3 600 056 (198) | Tomatinase | tomA | 8 542 763 (1602) | |
| Thaxtomin synthetase B | txtB | 3 600 343 (4467) | Hydrolytic enzymes | ||
| Thaxtomin synthetase A | txtA | 3 604 857 (4377) | Glycosyl hydrolase | 8 528 829 (1260) | |
| Nitric oxide synthase | nos | 3 615 200 (1173) | Glycosyl hydrolase | 8 530 643 (2418) | |
| Bacteriocin biosynthesis | Glycosyl hydrolase | 8 541 164 (1434) | |||
| Lantibiotic precursor§ | 3 630 960 (171) | Esterase/lipase | 8 509 360 (906) | ||
| Lantibiotic dehydratase‐like§ | 3 631 341 (3006) | Epoxide hydrolase | 8 575 654 (714) | ||
| Lanthionine synthetase C‐like§ | 3 634 343 (1233) | Detoxification/stress resistance | |||
| Aerial mycelium development | β‐Lactamase domain‐containing protein | 8 490 519 (1365) | |||
| Sensor histidine kinase¶ | 3 638 794 (1305) | Rhodanese domain‐containing protein | 8 493 161 (5760) | ||
| Roadblock/LC7 domain‐containing protein¶ | 3 640 095 (417) | Bacitracin resistance protein | 8 581 305 (876) | ||
| Transport | |||||
| RarC‐like protein¶ | 3 640 538 (333) | Cellobiose ABC transporter permease | 8 535 066 (822) | ||
| RarD‐like protein¶ | 3 640 896 (573) | Cellobiose ABC transporter permease | 8 537 128 (1230) | ||
| Cytochrome P450¶ | 3 641 465 (1323) | Cellobiose ABC transporter permease | 8 535 977 (987) | ||
| Sugar ABC transporter permease | 8 551 858 (840) | ||||
| ABC‐type antimicrobial peptide transport system | 8 502 763 (780) | ||||
The protein‐coding gene sequence was predicted using the GeneMark.hmm for Prokaryotes program (Version 2.4; Lukashin and Borodovsky, 1998) and protein function was determined using the BlastP program (National Center for Biotechnology Information).
Position in the S. scabies genome sequence release by the Sanger Institute (http://www.sanger.ac.uk/Projects/S_scabies/).
MbtH‐like proteins are a family of proteins encoded by genes often found in clusters responsible for the biosynthesis of peptide antibiotics. There is no experimental evidence that this protein is necessary for thaxtomin synthesis.
Cluster containing genes encoding proteins involved in the biosynthesis of lantibiotic. There is no experimental evidence that this cluster allows bacteriocin synthesis in S. scabies.
Gene cluster similar to rarA‐E cluster of Streptomyces griseus (Komatsu et al., 2003).
EFFECT OF AROMATIC AMINO ACIDS ON S. SCABIES VIRULENCE
Streptomyces scabies possesses the capacity to produce melanoid pigments, as do several bacteria, fungi, plants and animals (Sánchez‐Ferrer et al., 1995). Melanin is a pigment synthesized from the amino acid tyrosine by a multifunctional tyrosinase enzyme (Bell and Wheeler, 1986). In S. scabies, the mechanisms leading to melanin production and thaxtomin synthesis are somewhat correlated. Beauséjour and Beaulieu (2004) generated S. scabies mutants deficient in melanin biosynthesis. Interestingly, thaxtomin A production was negatively affected in most of these mutants, suggesting that some regulatory or biosynthetic pathways leading to toxin synthesis and melanogenesis could be connected. Nevertheless, as thaxtomin synthesis in the melanin‐deficient mutants was not totally abolished, pathogenicity tests performed on potato tubers showed that bacterial virulence was not reduced in all melanin‐deficient mutants (Beauséjour and Beaulieu, 2004). Furthermore, all mutant strains retained the capacity to sporulate under standard conditions, but their growth and sporulation capacities showed higher sensitivity to various stresses than did the wild strain, suggesting that the synthesis of melanin and thaxtomins may be linked to the stress response in S. scabies.
Streptomyces scabies also has the ability to synthesize the plant growth phytohormone indole‐3‐acetic acid (IAA) using tryptophan as a precursor (Manulis et al., 1994). This trait is common amongst soil‐inhabiting bacteria. The stimulating effect on plant growth of some rhizobacteria relies on their capacity to synthesize auxins (Patten and Glick, 1996). In S. scabies, IAA synthesis seems to be regulated by tryptophan availability, as extracellular IAA production increases with tryptophan concentration (G. Legault et al., unpublished data, Université de Sherbrooke). When expressed as a function of tryptophan concentration, bacterial thaxtomin A and IAA display opposite profiles of production (Fig. 3). The role of bacterial biosynthesis of auxins in the interaction between S. scabies and potato needs to be investigated further, but auxins show remarkable effects on common scab. Evidence of reduced common scab incidence on potatoes was observed following foliar applications of auxin analogues in glasshouse and field experiments (1981, 1982). However, auxin treatments were associated with low yields of marketable tubers. Recently, in an attempt to elucidate the mechanisms whereby foliar sprays of auxin analogues reduce common scab severity, Tegg et al. (2008) reported the accumulation of the synthetic auxin 2,4‐dichlorophenoxyacetic acid in tubers of treated plants. This accumulation translated into an enhanced tolerance to thaxtomin A. Auxins had no toxic effect on S. scabies, but 2,4‐dichlorophenoxyacetic acid (at a concentration of 1 mm) showed inhibitory effects on thaxtomin production (Tegg et al., 2008).
Figure 3.

Effect of tryptophan on the relative production of thaxtomin A and indole‐3‐acetic acid (IAA) in Streptomyces scabies (strain EF‐35) grown in minimal medium. Increasing tryptophan concentrations totally inhibited thaxtomin A biosynthesis, but stimulated IAA production.
Although tryptophan and phenylalanine are biosynthetic precursors of thaxtomins, the exogenous supply of aromatic amino acids in the culture medium induces the inhibition of thaxtomin biosynthesis. In a study on the regulation of thaxtomin synthesis, Lauzier et al. (2002) investigated the effect of amino acids on toxin production. Aliphatic amino acids had no significant effect on thaxtomin A production at a concentration of 2.5 mm or less. However, the presence in the growth medium of S. scabies of the three aromatic amino acids, tryptophan, phenylalanine and tyrosine, at a concentration of 2.5 mm totally inhibited or greatly reduced the production of thaxtomin A (Lauzier et al., 2002). These results were unexpected as examples of the inhibition of a biosynthetic molecule by its own precursors are rare. No clear explanation has yet been offered to elucidate such a regulatory mechanism. Tryptophan and other aromatic amino acids therefore appear to be key molecules in the metabolism and virulence process of S. scabies.
PERSPECTIVES
This article is an attempt to describe concisely the most recent knowledge on the pathogenicity of S. scabies, the major pathogenic agent responsible for common scab. The mechanisms of virulence may at first appear to be straightforward, as the pathogenic agent that detects the presence of a host plant excretes a phytotoxin that eventually targets a plant function. However, the events leading to toxicity are complex and represent an exciting challenge for researchers. Moreover, in spite of the knowledge accumulated over the past 30 years, no efficient method is yet available to control the disease in the field. In addition, the damage caused to crops by common scab is greater than ever. The fact that the genes responsible for pathogenicity can disseminate by lateral transfer implies that common scab may theoretically develop in any streptomycetes‐bearing soil, propagating the disease to new niches. However, several avenues can be followed to find solutions for the biocontrol of common scab (for example, Beauséjour et al., 2003; Hiltunen et al., 2009; Liu et al., 1995).
Most of the research which is presently being conducted is essentially focused on the bacterial pathogenicity mechanisms. This approach minimizes the importance of the plant in this host–microbe interaction, and more attention should be given to the plant partner. Common scab‐resistant cultivars are of specific relevance as they may help to identify the physiological events restraining the development of the plant pathogens.
The close link between tryptophan, auxins and thaxtomins, possibly caused by chemical similarities, is also of particular interest. Tegg et al. (2005) suggested the possible competition between auxin and thaxtomin A for auxin receptors. Foliar sprays of auxin analogues are accompanied by tuber growth issues, but have been proven to be efficient in limiting the impact of the pathogenic agents. Research into the hormonal aspects may improve the control of the disease in the field.
A comprehensive understanding of the virulence mechanisms of common scab‐causing agents still remains a promising research avenue. The complete sequencing and recent annotation (Loria et al., 2008) of the S. scabies genome opens up a new area. Today's molecular tools, such as DNA microarray techniques, are powerful and should eventually help to unravel the regulation mechanisms triggering pathogenicity.
ACKNOWLEDGEMENTS
The authors thank G. Legault for permission to report unpublished data. Our research work on common scab was supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Fonds Québécois de la Recherche sur la Nature et les Technologies (FQRNT).
REFERENCES
- Beaulieu, C. , Goyer, C. and Beaudoin, N. (2008) Interaction between pathogenic streptomycetes and plants: the role of thaxtomins In: Plant–Microbe Interactions (Ait‐Barka A. and Clément C. , eds), pp. 117–133. Trivandrum: Research Signpost. [Google Scholar]
- Beauséjour, J. and Beaulieu, C. (2004) Characterization of Streptomyces scabies mutants deficient in melanin biosynthesis. Can. J. Microbiol. 50, 705–709. [DOI] [PubMed] [Google Scholar]
- Beauséjour, J. , Clermont, N. and Beaulieu, C. (2003) Effect of Streptomyces melanosporofaciens strain EF‐76 and of chitosan on common scab of potato. Plant Soil, 256, 463–468. [Google Scholar]
- Beauséjour, J. , Goyer, C. , Vachon, J. and Beaulieu, C. (1999) Production of thaxtomin by Streptomyces scabies strains in plant extract containing media. Can. J. Microbiol. 45, 764–768. [Google Scholar]
- Bell, A.A. and Wheeler, M.H. (1986) Biosynthesis and functions of fungal melanins. Annu. Rev. Phytopathol. 24, 411–451. [Google Scholar]
- Bouchek‐Mechiche, K. , Gardan, L. , Normand, P. and Jouan, B. (2000) DNA relatedness among strains of Streptomyces pathogenic to potato in France: description of three new species, S. europeiscabiei sp. nov. and S. stelliscabiei sp. nov. associated with common scab, and S. reticuliscabiei sp. nov. associated with netted scab. Int. J. Syst. Evol. Microbiol. 50, 91–99. [DOI] [PubMed] [Google Scholar]
- Duval, I. , Brochu, V. , Simard, M. , Beaulieu, C. and Beaudoin, N. (2005) Thaxtomin A induces programmed cell death in Arabidopsis thaliana suspension‐cultured cells. Planta, 222, 820–831. [DOI] [PubMed] [Google Scholar]
- Errakhi, R. , Dauphin, A. , Meimoun, P. , Lehner, A. , Reboutier, D. , Vatsa, P. , Briand, J. , Madiona, K. , Rona, J.P. , Barakate, M. , Wendehenne, D. , Beaulieu, C. and Bouteau, F. (2008) An early Ca2+ influx is a prerequisite to thaxtomin A‐induced cell death in Arabidopsis thaliana cells. J. Exp. Bot. 59, 4259–4270. [DOI] [PubMed] [Google Scholar]
- Flores‐Gonzáles, R. , Velasco, I. and Montes, F. (2008) Detection and characterization of Streptomyces causing potato common scab in Western Europe. Plant Pathol. 57, 162–169. [Google Scholar]
- Fry, B.A. and Loria, R. (2002) Thaxtomin A: evidence for a plant cell wall target. Physiol. Mol. Plant Pathol. 60, 1–8. [Google Scholar]
- Goyer, C. and Beaulieu, C. (1997) Host range of streptomycetes strains causing common scab. Plant Dis. 81, 901–904. [DOI] [PubMed] [Google Scholar]
- Goyer, C. , Otrysko, B. and Beaulieu, C. (1996) Taxonomic studies on streptomycetes causing potato common scab: a review. Can. J. Plant Pathol. 18, 107–113. [Google Scholar]
- Healy, F.G. , Krasnoff, S.B. , Wach, M. , Gibson, D.M. and Loria, R. (2002) Involvement of a cytochrome P450 monooxygenase in thaxtomin A biosynthesis by Streptomyces acidiscabies . J. Bacteriol. 184, 2019–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy, F.G. , Wach, M. , Krasnoff, S.B. , Gibson, D.M. and Loria, R. (2000) The txtAB genes of the plant pathogen Streptomyces acidiscabies encode a peptide synthetase required for phytotoxin thaxtomin A production and pathogenicity. Mol. Microbiol. 38, 794–804. [DOI] [PubMed] [Google Scholar]
- Hill, J. and Lazarovits, G. (2005) A mail survey of growers to estimate potato common scab prevalence and economic loss in Canada. Can. J. Plant Pathol. 27, 46–52. [Google Scholar]
- Hiltunen, L.H. , Laakso, I. , Chobot, V. , Hakala, K.S. , Weckman, A. and Valkonen, J.P.T. (2006) Influence of thaxtomins in different combinations and concentrations on growth of micropropagated potato shoot cultures. J. Agric. Food Chem. 54, 3372–3379. [DOI] [PubMed] [Google Scholar]
- Hiltunen, L.H. , Ojanperä, T. , Kortemaa, H. , Richter, E. , Lehtonen, M.J. and Valkonen, J.P.T. (2009) Interactions and biocontrol of pathogenic Streptomyces strains co‐occurring in potato scab lesions. J. Appl. Microbiol. 106, 199–212. [DOI] [PubMed] [Google Scholar]
- Hiltunen, L.H. , Weckman, A. , Ylhäinen, A. , Rita, H. , Richter, E. and Valkonen, J.P.T. (2005) Responses of potato cultivars to the common scab pathogens, Streptomyces scabies and S. turgidiscabies . Ann. Appl. Biol. 146, 395–403. [Google Scholar]
- Johnson, E.G. , Joshi, M.V. , Gibson, D.M. and Loria, R. (2007) Cello‐oligosaccharides released from host plants induce pathogenicity in scab‐causing Streptomyces species. Physiol. Mol. Plant Pathol. 71, 18–25. [Google Scholar]
- Joshi, M. , Rong, X. , Moll, S. , Kers, J. , Franco, C. and Loria, R. (2007a) Streptomyces turgidiscabies secretes a novel virulence protein, Nec1, which facilitates infection. Mol. Plant–Microbe Interact. 20, 599–608. [DOI] [PubMed] [Google Scholar]
- Joshi, M.V. , Bignell, D.R.D. , Johnson, E.G. , Sparks, J.P. , Gibson, D.M. and Loria, R. (2007b) The AraC/XylS regulator TxtR modulates thaxtomin biosynthesis and virulence in Streptomyces scabies . Mol. Microbiol. 66, 633–642. [DOI] [PubMed] [Google Scholar]
- Kers, J.A. , Cameron, K.D. , Joshi, M.V. , Bukhalid, R.A. , Morello, J.E. , Wach, M.J. , Gibson, D.M. and Loria, R. (2005) A large, mobile pathogenicity island confers plant pathogenicity on Streptomyces species. Mol. Microbiol. 55, 1025–1033. [DOI] [PubMed] [Google Scholar]
- Kers, J.A. , Wach, M.J. , Krasnoff, S.B. , Widom, J. , Cameron, K.D. , Bukhalid, R.A. , Crane, B.R. and Loria, R. (2004) Nitration of a peptide phytotoxin by bacterial nitric oxide synthase. Nature, 429, 79–82. [DOI] [PubMed] [Google Scholar]
- King, R.R. and Lawrence, C.H. (2001) Herbicidal properties of the thaxtomin group of phytotoxins. J. Agric. Food Chem. 49, 2298–2301. [DOI] [PubMed] [Google Scholar]
- King, R.R. , Lawrence, C.H. and Calhoun, L.A. (1992) Chemistry of phytotoxins associated with Streptomyces scabies, the causal organism of potato scab. J. Agric. Food Chem. 40, 834–837. [Google Scholar]
- King, R.R. , Lawrence, C.H. and Clark, M.C. (1989) Isolation and characterization of phytotoxins associated with Streptomyces scabies . J. Chem. Soc. Chem. Commun. 13, 849–850. [Google Scholar]
- Kolattukudy, P.E. (1980) Biopolyester membranes of plants: cutin and suberin. Science, 208, 990–1000. [DOI] [PubMed] [Google Scholar]
- Komatsu, M. , Kuwahara, Y. , Hiroishi, A. , Hosono, K. , Beppu, T. and Ueda, K. (2003) Cloning of the conserved regulatory operon by its aerial mycelium‐inducing activity in an amfR mutant of Streptomyces griseus . Gene, 306, 79–89. [DOI] [PubMed] [Google Scholar]
- Kreuze, J.F. , Suomalainen, S. , Paulin, L. and Valkonen, J.P.T. (1999) Phylogenetic analysis of 16S rRNA genes and PCR analysis of the nec1 gene from Streptomyces spp. causing common scab, pitted scab, and netted scab in Finland. Phytopathology, 89, 462–469. [DOI] [PubMed] [Google Scholar]
- Lambert, D.H. and Loria, R. (1989a) Streptomyces scabies sp. nov., nom. rev. Int. J. Syst. Bacteriol. 39, 387–392. [Google Scholar]
- Lambert, D.H. and Loria, R. (1989b) Streptomyces acidiscabies sp. nov. Int. J. Syst. Bacteriol. 39, 393–396. [Google Scholar]
- Lauzier, A. , Goyer, C. , Ruest, L. , Brzezinski, R. , Crawford, D.L. and Beaulieu, C. (2002) Effect of amino acids on thaxtomin A biosynthesis by Streptomyces scabies . Can. J. Microbiol. 48, 359–364. [DOI] [PubMed] [Google Scholar]
- Lauzier, A. , Simao‐Beaunoir, A.‐M. , Bourassa, S. , Poirier, G.G. , Talbot, B. and Beaulieu, C. (2008) Effect of potato suberin on Streptomyces scabies proteome. Mol. Plant Pathol. 9, 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen, M.J. , Rantala, H. , Kreuze, J.F. , Bång, H. , Kuisma, L. , Koski, P. , Virtanen, E. , Vihlman, K. and Valkonen, J.P.T. (2004) Occurrence and survival of potato scab pathogens (Streptomyces species) on tuber lesions: quick diagnosis based on a PCR‐based assay. Plant Pathol. 53, 280–287. [Google Scholar]
- Leiner, R.H. , Fry, B.A. , Carling, D.E. and Loria, R. (1996) Probable involvement of thaxtomin A in pathogenicity of Streptomyces scabies on seedlings. Phytopathology, 86, 709–713. [Google Scholar]
- Liu, D. , Anderson, N.A. and Kinkel, L.L. (1995) Biological control of potato scab in the field with antagonistic Streptomyces scabies . Phytopathology, 85, 827–831. [Google Scholar]
- Loria, R. , Bignell, D.R.D. , Moll, S. , Huguet‐Tapia, J.C. , Joshi, M.V. , Johnson, E.G. , Seipke, R.F. and Gibson, D.M. (2008) Thaxtomin biosynthesis: the path to plant pathogenicity in the genus Streptomyces . Antonie van Leeuwenhoek, 94, 3–10. [DOI] [PubMed] [Google Scholar]
- Loria, R. , Kers, J. and Joshi, M. (2006) Evolution of plant pathogenicity in Streptomyces . Annu. Rev. Phytopathol. 44, 469–487. [DOI] [PubMed] [Google Scholar]
- Lukashin, A. and Borodovsky, M. (1998) GeneMark.hmm: new solutions for gene finding. Nucleic Acids Res. 26, 1107–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manulis, S. , Shafrir, H. , Epstein, E. , Lichter, A. and Barash, I. (1994) Biosynthesis of indole‐3‐acetic acid via the indole‐3‐acetamide pathway in Streptomyces spp. Microbiology, 140, 1045–1050. [DOI] [PubMed] [Google Scholar]
- McIntosh, A.H. , Bateman, G.L. , Chamberlain, K. , Dawson, G.W. and Burrell, M.M. (1981) Decreased severity of potato common scab after foliar sprays of 3,5‐dichlorophenoxyacetic acid, a possible antipathogenic agent. Ann. Appl. Biol. 99, 275–281. [Google Scholar]
- McIntosh, A.H. , Burrell, M.M. and Hawkins, J.H. (1982) Field trials of foliar sprays of 3,5‐dichlorophenoxyacetic acid (3,5‐D) against common scab on potatoes. Potato Res. 25, 347–350. [Google Scholar]
- Miyajima, K. , Tanaka, F. , Takeuchi, T. and Kuninaga, S. (1998) Streptomyces turgidiscabies sp. nov. Int. J. Syst. Bacteriol. 48, 495–502. [DOI] [PubMed] [Google Scholar]
- Park, D.H. , Kim, J.S. , Kwon, S.W. , Wilson, C. , Yu, Y.M. , Hur, J.H. and Lim, C.K. (2003) Streptomyces luridiscabiei sp. nov., Streptomyces puniciscabiei sp. nov. and Streptomyces niveiscabiei sp. nov., which cause potato common scab disease in Korea. Int. J. Syst. Evol. Microbiol. 53, 2049–2054. [DOI] [PubMed] [Google Scholar]
- Patten, C.L. and Glick, B.R. (1996) Bacterial biosynthesis on indole‐3‐acetic acid. Can. J. Microbiol. 42, 207–220. [DOI] [PubMed] [Google Scholar]
- Qu, X. , Wanner, L.A. and Christ, B.J. (2008) Using the TxtAB operon to quantify pathogenic Streptomyces in potato tubers and soil. Phytopathology, 98, 405–412. [DOI] [PubMed] [Google Scholar]
- Sánchez‐Ferrer, A. , Rodríguez‐López, J.N. , García‐Cánovas, F. and García‐Carmona, F. (1995) Tyrosinase: a comprehensive review of its mechanism. Biochem. Biophys. Acta, 1247, 1–11. [DOI] [PubMed] [Google Scholar]
- Scheible, W.‐R. , Fry, B. , Kochevenko, A. , Schindelasch, D. , Zimmerli, L. , Somerville, S. , Loria, R. and Somerville, C.R. (2003) An Arabidopsis mutant resistant to thaxtomin A, a cellulose synthesis inhibitor from Streptomyces species. Plant Cell, 15, 1781–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottel, J.L. , Hale, V. and Babcock, M.J. (1992) Regulation and secretion of an extracellular esterase from Streptomyces scabies . Gene, 115, 27–31. [DOI] [PubMed] [Google Scholar]
- Seipke, R.F. and Loria, R. (2008) Streptomyces scabies 87‐22 possesses a functional tomatinase. J. Bacteriol. 190, 7684–7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegg, R.S. , Gill, W.M. , Thompson, H.K. , Davies, N.W. , Ross, J.J. and Wilson, C.R. (2008) Auxin‐induced resistance to common scab disease of potato linked to inhibition of thaxtomin A toxicity. Plant Dis. 92, 638–648. [DOI] [PubMed] [Google Scholar]
- Tegg, R.S. , Melian, L. , Wilson, C.R. and Shabala, S. (2005) Plant cell growth and ion flux responses to the streptomycete phytotoxin thaxtomin A: calcium and hydrogen flux patterns revealed by the non‐invasive MIFE technique. Plant Cell Physiol. 46, 638–648. [DOI] [PubMed] [Google Scholar]
- Thaxter, R. (1892) Potato scab. Conn. Agric. Exp. Stn. 1891, 153–160. [Google Scholar]
- Wach, M.J. , Krasnoff, S.B. , Loria, R. and Gibson, D.M. (2007) Effect of carbohydrates on the production of thaxtomin A by Streptomyces acidiscabies . Arch. Microbiol. 188, 81–88. [DOI] [PubMed] [Google Scholar]
- Wanner, L.A. (2007) A new strain of Streptomyces causing common scab in potato. Plant Dis. 91, 352–359. [DOI] [PubMed] [Google Scholar]
- Wanner, L.A. (2006) A survey of genetic variation in Streptomyces isolates causing potato common scab in the United States. Phytopathology, 96, 1363–1371. [DOI] [PubMed] [Google Scholar]


