SUMMARY
Pantoea ananatis causes disease symptoms in a wide range of economically important agricultural crops and forest tree species worldwide. It is regarded as an emerging pathogen based on the increasing number of reports of diseases occurring on previously unrecorded hosts in different parts of the world. Its unconventional nature lies in the fact that, unlike the majority of plant pathogenic microbes, P. ananatis is capable of infecting humans and occurs in diverse ecological niches, such as part of a bacterial community contaminating aviation jet fuel tanks and contributing to growth promotion in potato and pepper.
Taxonomy: Bacteria; Gammaproteobacteria; family Enterobacteriaceae; genus Pantoea.
Microbiological properties: Gram‐negative; facultatively anaerobic; most strains are motile and produce a yellow pigment in culture; indole positive.
Biology: Pantoea ananatis is a common epiphyte; it also occurs endophytically in hosts where it has been reported to cause disease symptoms and in hosts where no such symptoms have been described. Some strains are ice‐nucleating, a feature which has been used as a biological control mechanism against some insect pests of agricultural crops and by the food industry.
Disease symptoms: Pantoea ananatis infects both monocotyledonous and dicotyledonous plants. The symptoms are diverse depending on the host infected, and include leaf blotches and spots, die‐back, and stalk, fruit and bulb rot.
Biological control agent: Pantoea ananatis has both antifungal and antibacterial properties. These characteristics have the potential of being exploited by biological control specialists.
INTRODUCTION
The appearance of new and re‐emerging plant diseases is having a significant constraint on agricultural productivity worldwide (Bandyopadhyay and Frederiksen, 1999; Bright, 1998; Palm, 1999). The extent of this threat has increased dramatically over the past two decades as a result of increased movement of agricultural products between countries. The threat of these emerging diseases is, however, not restricted to cultivated plants, as native and wild plants are also at risk (Anderson et al., 2004). This is of considerable importance in terms of biodiversity conservation.
An emerging plant pathogen is considered to be one which has shown an increase in incidence, host and geographical range (Anderson et al., 2004). Since its initial discovery on pineapple, causing fruitlet rot in the Philippines in 1928 (Serrano, 1928), Pantoea ananatis has been found to cause a wide range of symptoms on both monocotyledonous and dicotyledonous plants. Its occurrence in these hosts leads to sporadic disease outbreaks, resulting in severe economic losses. From 1983 to date, the host range of P. ananatis has increased to eight, and the pathogen has now been reported to cause plant disease symptoms in at least 11 countries. The increase in geographical range is best illustrated by the distribution of palea browning of rice. This disease was initially reported from Japan (Tabei et al., 1988), but now also occurs in Korea (Kim et al., 1989), the Philippines (Xie, 1996 cited by Xie, 2001), China (Xie, 2001) and, more recently, Italy (Cortesi and Pizzatti, 2007).
Pantoea ananatis is a ubiquitous bacterium which, in itself, is not unique as a number of other bacterial plant pathogens share this characteristic. However, P. ananatis, when it is not associated with plants as an epiphyte, endophyte, pathogen or symbiont, also occupies diverse and unusual ecological niches. Here it may function as a saprophyte. In the case of humans, P. ananatis has also been reported to cause bacteraemia (De Baere et al., 2004). With the exception of perhaps only Pantoea agglomerans, no other plant pathogen behaves in such an unconventional manner. An explanation of how P. ananatis has become so broadly adapted to these different habitats will probably only be resolved once species‐specific genes have been identified.
Pantoea ananatis is not only important in the above‐mentioned roles. Its ice nucleation activity has been exploited by both the food industry and biological control specialists of insects. Extracellular ice nucleators from P. ananatis have been tested and applied in the freezing of foods in order to obtain the desired texture (Zasypkin and Lee, 1999), and in the freeze‐drying of foods (Watanabe and Arai, 1994). Ice‐nucleating strains of P. ananatis also markedly reduce the cold hardiness of mulberry pyralid larvae (Watanabe and Sato, 1999), and thus these strains have the potential to act as biological control agents of insect pests. In addition, the accomplishment of researchers in Switzerland to genetically modify rice to produce ‘yellow rice’ owes its success to P. ananatis. In this situation, the phytoene desaturase from P. ananatis (a strain identified as Erwinia uredovora) was used to introduce the β‐carotene biosynthesis pathway into rice (Beyer et al., 2002).
In this review, we focus on the taxonomy, detection and identification of P. ananatis. We also discuss its role in different ecological niches with a focus on its association with plants as an epiphyte, endophyte and plant pathogen. The molecular basis for its role in these different niches is, however, not well understood.
TAXONOMY
Until recently all phytopathogenic Enterobacteriaceae belonged to a single genus Erwinia. The genus was proposed by Winslow et al. (1920) for all plant‐associated, Gram‐negative, non‐spore‐forming, peritrichous, fermentative, rod‐shaped bacteria. Dye (1968, 1969a, 1969b,c) divided the genus into four ‘natural’ clusters, namely the amylovora, carotovora, herbicola and ‘atypical’Erwinia groups. The ‘herbicola’ group, of interest in this review, consisted of Erwinia strains that usually produced a yellow pigment in culture and related non‐pigmented clinical isolates often named Enterobacter agglomerans. This group is referred to in the literature as the Erwinia herbicola–Enterobacter agglomerans complex. Many researchers have subsequently shown that this complex is heterogeneous and contains bacterial strains with different phenotypes and genotypes (Brenner et al., 1984; Mergaert et al., 1983, 1984; Verdonck et al., 1987).
In 1989, Gavini et al. proposed the genus Pantoea (Gavini et al., 1989). The type strains of E. herbicola, Ent. agglomerans and E. milletiae were found to belong to the same DNA hybridization group (Beji et al., 1988), and thus the combined species, P. agglomerans, was proposed for these bacteria (Gavini et al., 1989). Other species included in this genus are P. dispera (Gavini et al., 1989), P. punctata, P. citrea, P. terrea (Kageyama et al., 1992), P. stewartii, P. stewartii ssp. indologenes, P. stewartii ssp. stewartii and P. ananatis (Mergaert et al., 1993). Pantoea ananatis was first described by Serrano (1928) as Erwinia ananas. Mergaert et al. (1993) proposed the name P. ananas, which was corrected to ‘ananatis’ by Trüper and De’Clari (1997).
Pantoea ananatis and P. uredovora are listed in Bergey's Manual of Determinative Bacteriology (Holt, 1977) as differing in their ability to reduce nitrate to nitrite. Both species also differ substantially from each other in host range. Pantoea ananatis was initially described as a pathogen that caused fruitlet rot of pineapple (Serrano, 1928), whereas P. uredovora was described as a pathogen of the basidiomycete rust fungus, Puccinia graminis (Pon et al., 1954). On the basis of a high level of genotypic relatedness, Mergaert et al. (1993) synonymized these two species. They found that seven strains received as either one of these two species, and including their type strains, exhibited between 76% and 100% DNA binding and constituted a single DNA hybridization group. It has previously been shown that the phenotypic differentiation of these strains is extremely difficult. However, this synonymy was supported by numerical analysis (Dye, 1981; Mergaert et al., 1984; Verdonck et al., 1987). Furthermore, Waleron et al. (2002) have recently shown that the two species are in the same recA polymerase chain reaction‐restriction fragment length polymorphism (PCR‐RFLP) group, and Brady et al. (2007) have shown that these strains also form part of the same fluorescent amplified fragment length polymorphism (F‐AFLP) cluster. Despite this research, the synonymy of these two species is not widely accepted, and the name E. uredovora is still commonly used in the literature (Beyer et al., 2002).
ISOLATION, DETECTION AND IDENTIFICATION
Most general media, such as blood agar, nutrient agar, tryptic soy agar or media specifically designed for the isolation of the Enterobacteriaceae, for example MacConkey and Hektoen agar, can be used for the isolation of P. ananatis (Grimont and Grimont, 2005). From diseased plants, the bacterium is usually isolated using nutrient agar (Bruton et al., 1991; Coutinho et al., 2002; Gitaitis and Gay, 1997; Schaad, 2001), but other media, such as yeast extract–dextrose–calcium carbonate agar (Azad et al., 2000) and King's Medium B (Cother et al., 2004), have also been employed.
Currently, there are only a few semi‐selective media for the isolation of P. ananatis. Hasegawa et al. (2003) developed NSCV‐In medium for the selective isolation of pathogenic P. ananatis from diseased rice plants. PA 20 was developed for the isolation of P. ananatis from onion seed (Goszczynska et al., 2006a,b), and was reported to inhibit the growth of most of the common saprophytes associated with the seed. Similar to the use of general isolation media to obtain P. ananatis, further tests to confirm the identity and pathogenicity of isolates are still required when using selective media.
The tentative identification of isolates as belonging to the genus Pantoea is usually performed using commercial identification systems (Azad et al., 2000; Cortesi and Pizzatti, 2007) or sequencing of the 16S rRNA gene (Coutinho et al., 2002) . For this group of bacteria, 16S rRNA is, however, too highly conserved to differentiate reliably between closely related species (Stackebrand and Goebels, 1994). API 20E or Biolog systems also have problems in identifying specific Pantoea species accurately as they share many phenotypic characteristics. The production of indole by P. ananatis is often used to distinguish between this species and P. agglomerans strains, but this characteristic is shared by strains of P. stewartii ssp. indologenes (Grimont and Grimont, 2005). The identity of P. ananatis strains is therefore often confirmed by means of other techniques, such as whole‐cell fatty acid methyl ester profiles (Azad et al., 2000; Cother et al., 2004; Gitaitis and Gay, 1997; Schwartz and Otto, 2000), PCR‐RFLP analysis of the recA gene (Waleron et al., 2002), DNA–DNA hybridization (Coutinho et al., 2002) or F‐AFLP (Brady et al., 2007).
At present, there is no recommended method for the direct detection of P. ananatis in plant material. Walcott et al. (2002) designed species‐specific primers for the 16S–23S rDNA internal transcribed spacer region, but the best primer pair detected both P. ananatis and P. stewartii ssp. stewartii. These researchers also tried to overcome the interference of saprophytes on onion seed by enriching samples for P. ananatis by means of polyclonal immunomagnetic bead capturing before proceeding with PCR. Cortesi and Pizzatti (2007) used another primer set targeting the same region, but no data on the assay's specificity are available. An oligonucleotide probe for fluorescent in situ hybridization was also not species specific and detected both P. ananatis and P. agglomerans (Nakanishi et al., 2006).
Currently, the most promising approach for species assignment is the use of multilocus sequence analysis (MLSA) (Gevers et al., 2005). Using sequence data from four housekeeping genes, Brady et al. (2008) showed that MLSA could clearly differentiate between all the current Pantoea spp. A high level of congruence was observed between the gyrB sequence data and DNA–DNA hybridization values, and it was recommended that accurate identification of P. ananatis strains could be achieved with comparison of either the gyrB (Fig. 1) or rpoB gene sequences (Brady et al., 2008).
Figure 1.
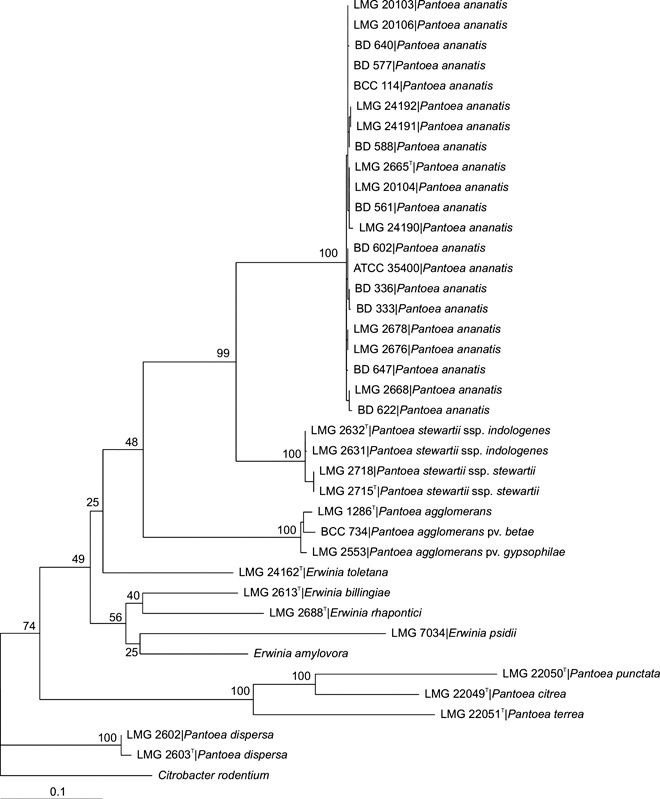
Maximum likelihood tree based on the gyrB sequences of Pantoea species and their closest phylogenetic neighbours. Bootstrap values after 1000 replicates are expressed as percentages. Citrobacter rodentium was included as an outgroup. BD 333 and 336 were isolated from onion seed in South Africa, ATCC 35400 from honeydew melons in the USA, LMG 2676 and 2678 from Puccinia graminis in the USA and Zimbabwe, respectively, LMG 2668 and 2665 from pineapple in Hawaii and Brazil, respectively, LMG 20103, 20104, 20106 and BD 114 from Eucalyptus in South Africa, LMG 24190 from onion in the USA, and BD 561, 577, 588, 602, 622, 640, 647 and LMG 24191 and 24192 from maize in South Africa.
PANTOEA ANANATIS AS AN EPIPHYTE
Pantoea ananatis is a common epiphyte on host and non‐host plants. Gitaitis et al. (2002) detected and cultured this bacterium as an epiphyte from 25 asymptomatic weed species, including crabgrass, sicklepod and yellow nutsedge, and from crop plants, such as Bermuda grass, cowpea and soybean. It has also been reported as an epiphyte on numerous economically important plant species, including rice (Watanabe et al., 1996), maize (Paccola‐Meirelles et al., 2001), barley, buckwheat, uredospores of Ustilago smut of maize (Coplin and Kado, 2001), cotton lint (Chun and Perkins, 1997), mulberry (Takahashi et al., 1995), poplar trees (Zeng et al., 1999) and wheat heads (Legard et al., 1994). In many of these cases, the occurrence of P. ananatis on the plant surface has not been linked to a specific disease on the host from whence it was isolated as an epiphyte. However, these asymptomatic non‐hosts could be providing a source of inoculum, causing disease outbreaks of susceptible hosts grown in their vicinity.
The occurrence of P. ananatis on plant surfaces may be beneficial to these plants. The bacterium has both antibacterial and antifungal activity in vitro and in vivo, thus protecting host plants against infection by other pathogenic fungi and bacteria. A strain, previously described as P. uredovora, was found to have in vitro antibacterial activity against Xanthomonas, Agrobacterium and all species belonging to the ‘amylovora’ group, including Erwinia amylovora (Vantomme et al., 1989). Isolates of P. ananatis from buckweed seed have been shown to have strong antifungal activity against Rhizopus spp. in vitro (Iimura and Hosono, 1998). In a study by Pajk (2004), it was reported that ‘P. uredovora’ reduced the infection of pome fruit trees by E. amylovora. A strain, CPA‐3, of P. ananatis was discovered to rapidly colonize wounds of harvested pome fruit, protecting them against Penicillium expansum (Torres et al., 2005). Similarly, strains of P. ananatis have been found to protect tomato fruit against the grey mould fungus, Botrytis cinerea, by producing antifungal compounds (Enya et al., 2007). Rice blast caused by Pyricularia oryzae has also been controlled by a strain of P. ananatis transformed with a chitinolytic enzyme gene (ChiA) from Serratia marcescens (Simeya et al., 2003).
Pantoea ananatis is one of only a few species of bacteria shown to contain ice‐nucleating strains (Abe et al., 1989; Obata et al., 1990). An ice nucleation active (INA) gene, inaA, has been sequenced and found to be similar to that of Pseudomonas species (Abe et al., 1989). This implies that frost formation on sensitive plants is induced at temperatures slightly higher than would normally occur when the bacterium is absent. Examples of frost‐sensitive plants are Eucalyptus, poplar, pea, pome and stone fruit trees (Lindow, 1983). Pantoea ananatis has been found to be the dominant INA bacterium on maize leaves in China (Sun et al. 2005), and has also been reported to cause frost injury to mulberry (Takahashi et al., 1995), tea (Goto et al., 1988), strawberry (Obata et al., 1990), apricot (Zhao et al., 2007) and citrus (Wang et al., 2008). The freezing injury caused by P. ananatis in poplar has resulted in an increased incidence of infection by the fungal pathogen Dothiorella gregaria in China (DePeng et al., 1999; Xiang et al., 2001; Zeng et al., 1999). The exact role of frost injury in outbreaks of many of the diseases caused by P. ananatis has not been elucidated clearly.
PANTOEA ANANATIS AS AN ENDOPHYTE
Although the term ‘endophyte’ is more commonly associated with fungi, bacteria are also capable of colonizing the interior of a plant. In this environment, they may be considered beneficial, neutral or existing as latent pathogens (Lodewyckx et al., 2002). In recent years, P. ananatis has been isolated as an endophyte from a number of plants, including, for example, coffee (Nunes and de Melo, 2006) and ginseng (Cho et al., 2007). Its role as an endophyte has mainly been found to be beneficial to the plant. In pepper, P. ananatis promoted significant growth and induced systemic resistance against Xanthomonas axonopodis pv. vesicatoria (Kang et al., 2007). Likewise, when shoot tips of papaya were inoculated with P. ananatis strains originally acquired as endophytic residents from within papaya tissue, they displayed significantly better root and shoot growth (Thomas et al., 2007). Strains found to occur endophytically in maize kernels have in vitro activity against Lecanicillium aphanocladii (Rijavec et al., 2007).
There are few reports of P. ananatis behaving as a latent pathogen within susceptible host tissue. It has been reported as an endophyte in rice plants (Mano and Morisaki, 2008), and we have also isolated P. ananatis as an endophyte from both susceptible and resistant Eucalyptus grandis × Eu. nitens (GN) clones in South Africa (T. A. Coutinho and S. N. Venter, unpublished results). In susceptible clones, this pathogen causes leaf blight and die‐back (Coutinho et al., 2002). Pantoea ananatis also occurs endophytically in rice seed (Mano et al., 2006; Okunishi et al., 2005) and maize kernels (Rijavec et al., 2007). Both are known hosts of this pathogen and its occurrence within seed may be of considerable epidemiological significance.
SAPROPHYTIC NATURE OF P. ANANATIS
Pantoea ananatis has been isolated from a diverse range of environments as a saprophyte. These include rivers (Morohoshi et al., 2007), soil (Lai and Hsu, 1974), aviation fuel tanks (Rauch et al., 2006), sorghum fermentation (Mohammed et al., 1991), from the rhizosphere of soft rush (Juncus effusus) (Halda‐Alija, 2003) and on ticks (Murrell et al., 2003), and is a common inhabitant of the gut microflora of brown plant hoppers (Nilaparvata lugens) (Watanabe et al., 1996), mulberry pyralid (Glyphodes pyloalis) (Takahashi et al., 1995), cotton fleahoppers (Pseudatomoscelis seriatus) (Bell et al., 2007) and tobacco thrips (Frankliniella fusca) (Gitaitis et al., 2003; Wells et al., 2002). Pantoea ananatis has also been isolated from Pinus elliottii roots colonized by ectomycorrhizal fungi in Australia (Izumi et al., 2008). Many of these studies which show that P. ananatis is present as a saprophyte involved the analyses of bacterial communities either occurring in or contaminating a specific location. The identification of the inhabitants of these sites was mostly based on partial sequencing of the 16S rRNA gene and determining the similarity of sequences to those in GenBank. This introduces an element of caution into the interpretation of these results, and one cannot state with absolute confidence that P. ananatis does indeed reside in these habitats.
PANTOEA ANANATIS AS A PATHOGEN
Host range and symptom expression
Pantoea ananatis infects both monocotyledonous and dicotyledonous plants (Table 1). This bacterium has also been deposited in numerous culture collections, where its role, as an epiphyte or pathogen, is not clearly indicated. These hosts include Cattleya sp. (LMG 2807), Musa sp. (LMG 2628), Cassia pectuta (ICMP 12183) and sugarcane (ICMP 10132). The symptoms caused by P. ananatis are diverse depending on the host infected, and include blotches and spots (Fig. 2A), die‐back, fruit, boll and bulb rot (Fig. 2B), and decay (Fig. 2C). In the case of infection of honeydew melons and cantaloupes, the brown spot symptoms occur only after harvesting (Bruton et al., 1991; Wells et al., 1987). The disease apparently originates in the form of field infections that remain quiescent until the fruit ripens. Following the infection of young Eucalyptus trees or seedlings/cuttings with P. ananatis, the shoots wilt and die‐back occurs (Coutinho et al., 2002). The pathogen appears to spread from the petioles into the main leaf veins, and from these parts into adjacent leaf tissue. In cases in which monocotyledonous plants are infected, symptoms are in the form of blotches and streaks, forming parallel to the main leaf vein (Azad et al., 2000; Paccola‐Meirelles et al., 2001). Outbreaks of diseases caused by P. ananatis are usually sporadic, possibly as a result of its opportunistic nature.
Table 1.
Host range of Pantoea ananatis
| Natural host | After artificial inoculation | Symptoms | Reference |
|---|---|---|---|
| Pineapple | Fruitlet rot | Serrano (1928) | |
| Sugarcane | Leaf streaks | Serrano (1928) | |
| Sudangrass | Leaf blotches and streaks | Azad et al. (2000) | |
| Sorghum and oats | Leaf blotches and streaks | Azad et al. (2000) | |
| Cantaloupe fruit | Brown spot | Bruton et al. (1991) | |
| Honeydew melons | Brown spot | Ceponis et al. (1985) | |
| Onions including giant onions | Leaf blight, seed stalk rot, bulb decay | Gitaitis and Gay (1997) | |
| Yumiko et al. (2005) | |||
| Eucalypts | Leaf blight, shoot tip die‐back | Coutinho et al. (2002) | |
| Maize | Necrotic spots and streaks | Paccola‐Meirelles et al. (2001) | |
| Brown stalk rot | Goszczynska et al. (2007) | ||
| Rice | ‘Palea’ browning | Tabei et al. (1988) | |
| Stalk rot | Cother et al. (2004) | ||
| Tomato | ‘Graywall’ | Stall et al. (1969) | |
| Puccinia graminis (causal agent of leaf rust of wheat) | Parasite of the rust | Pon et al. (1954) | |
| King oyster mushroom | Soft rot | Kim et al. (2007) | |
| Cotton | Internal boll rot | Bell et al. (2007) | |
| Watermelon | Walcott et al. (2003) |
Figure 2.

Disease symptoms caused by Pantoea ananatis. (A) Bacterial blight of Eucalyptus. (B) Brown stalk rot of maize (photograph courtesy of Dr Teresa Goszczynska, Agricultural Research Council‐Plant Protection Research Institute, Pretoria, South Africa). (C) Centre rot of onion (photograph courtesy of Professor Ron Gitaitis, University of Georgia, Tifton, GA, USA).
Of particular interest is that symptoms caused by P. ananatis on the same host may differ from country to country. On maize in Brazil, the symptoms described are necrotic or white leaf spots and streaks (Bomfeti et al., 2008; Paccola‐Meirelles et al., 2001), whereas, in South Africa, infection results in stalk rot (Goszczynska et al., 2007). Similarly, on rice in Japan and elsewhere in the world, the pathogen infects the developing seed causing palea browning, whereas, in Australia, the symptom caused by P. ananatis is stem necrosis (Cother et al., 2004).
Epidemiology
The epidemiology of plant diseases caused by P. ananatis on different hosts is relatively unknown. What has been established is that the pathogen enters its host through flowers (Hasegawa et al., 2003; Serrano, 1928) and/or wounds created by feeding insects (Gitaitis et al., 2003; Watanabe et al., 1996; Wells et al., 2002), mechanical injury (Serrano, 1928) and plant to plant contact during high winds (Azad et al., 2000; Cother et al., 2004). The development of brown hopper burn symptoms on rice was found to be accelerated when P. ananatis was present on the leaf surfaces. Gitaitis et al. (2003) were also able to show that tobacco thrips vector P. ananatis in onion fields. Although P. ananatis was isolated from Miridae feeding on infected plant tissue in a field outbreak of blight and die‐back of eucalypts in South Africa, its exact role in disease outbreaks is currently unknown (J. Roux and T. A. Coutinho, unpublished results).
Pantoea ananatis is both seed‐borne and seed‐transmitted in onions (Goszczynska et al., 2006a; Walcott et al., 2002), sudangrass (Azad et al., 2000) and rice (Azegami et al., 1983; Tabei et al., 1988). Together with a number of other bacterial species, this bacterium was also found in buckwheat seed (Iimura and Hosono, 1996) and in maize kernels (Rijavec et al., 2007). The recent appearances of bacterial blight and die‐back on Eucalyptus in countries that have purchased seed from South Africa suggest seed transmission (Dr. G. Nakabonge, FABI, University of Pretoria, Pretoria, South Africa, unpublished data). Similarly, outbreaks of centre rot of onions may be a result of the introduction of infested seed into new environments/countries (Gitaitis et al., 2004; Goszczynska et al., 2006a).
Environmental factors influence the severity of the diseases caused by P. ananatis on its different hosts. In the case of maize and Eucalyptus, high humidity and moderate temperature conditions (between 20 and 25 °C) increase the incidence and severity of the disease (Coutinho et al., 2002; Paccola‐Meirelles et al., 2001). In contrast, on sudangrass, infection was found to be worst at a temperature of 32 °C and a high relative humidity (Azad et al., 2000). This is similar to the situation with onions, where P. ananatis was found to be active at bulb formation when moisture was high and temperatures ranged from 28 to 35 °C (Schwartz et al., 2003).
Economic importance
As a plant pathogen, P. ananatis can lead to serious economic losses. In the case of sudangrass, blighted foliage can reach 50% or more of the total leaf area (Azad et al., 2000). Losses of up to 100% have been recorded in the case of onions (Gitaitis and Gay, 1997). Severe infections of maize resulted in leaf senescence and a sharp decrease in grain size and weight (Pinto, 1995 cited by Paccola‐Meirelles et al., 2001). Where an outbreak of P. ananatis occurs in a Eucalyptus plantation, trees either fail to survive or are multistemmed (Coutinho et al., 2002). What is of particular concern is the fact that this pathogen is able to infect a wide range of Eucalyptus species, hybrids and clones. If left unchecked, the disease on its numerous hosts has the potential to reach epidemic proportions under favourable environmental conditions. In addition to being a primary plant pathogen in the field, P. ananatis is also responsible for post‐harvest losses of cantaloupe fruit (Bruton et al., 1991), honeydew melons (Wells et al., 1987) and onions (Gitaitis et al., 2003).
Pathogenesis
Pantoea ananatis has been reported to produce indole‐3‐acetic acid (Enya et al., 2007; Halda‐Alija, 2003; Mano and Morisaki, 2008; Sessitsch et al., 2004), which could play a role in pathogenesis. This plant growth promoter affects plants at very low concentrations and promotes cell wall loosening during cell elongation (Brandl and Lindow, 1998). The major virulence factors of P. ananatis are, however, currently unknown. The genome of a virulent strain of P. ananatis from eucalypts, as well as the type strain from pineapple, have recently been sequenced using 454 pyrosequencing (De Maayer et al., 2008) and solexa technology, respectively, and detailed information will soon become available. Initial comparative genomics revealed the absence of the Type II, Type III and Type IV secretion systems in P. ananatis. These secretion systems are located on pathogenicity islands in a broad range of animal‐ and other plant‐associated bacteria. The genome of P. ananatis does, however, contain a cluster of genes with high homology to members of the novel Type VI secretion system. This system plays a role in diseases caused by several human and animal‐as well as plant‐pathogenic Gram‐negative bacteria, but whose function has yet to be elucidated fully (Mattinen et al., 2008; Pukatzki et al., 2006).
Pantoea ananatis produces quorum sensing‐related signal molecules. Yoshida et al. (2006) revealed that, when this bacterium inhabited wheat heads, it produced at least two N‐acyl‐l‐homoserine lactones (AHLs): N‐hexanoyl‐l‐homoserine lactone (C6‐HSL) and N‐(3‐oxohexanoyl)‐l‐homoserine lactone (3‐oxo‐C6‐HSL). Pomini et al. (2006) reported that P. ananatis produced three AHLs with the major substance identified being C6‐HSL. Morohoshi et al. (2007) identified the LuxRI homologue, EanRI, and C6‐HSL and 3‐oxo‐C6‐HSL in a strain isolated from the Shirakwa river in Japan. They were able to show the involvement of the quorum‐sensing system in the regulation of exopolysaccharide biosynthesis, biofilm formation and the infection of onion leaves.
Control
The control of diseases caused by P. ananatis is usually achieved through the deployment of resistant/tolerant cultivars/clones. Blight and die‐back of eucalypts in South Africa are currently controlled by the use of resistant clones (T. A. Coutinho, unpublished data). They are selected in a cutting production nursery where the incidence of disease is high. Similarly, in a study by Paccola‐Meirelles et al. (2002), it was discovered that, by artificially inoculating maize lines, it was possible to select genotypes resistant to P. ananatis. The control of white spot disease of maize in Brazil has also been achieved by applying the fungicide Mancozeb in the initial phases of disease development (Bomfeti et al., 2007). The use of mulch and irrigation systems has been investigated for the control of centre rot of onion (Gitaitis et al., 2004). Irrigation type had no effect on the incidence and severity of disease. However, the use of straw mulch or bare ground was found to delay symptom development by 7–14 days compared with the use of black plastic. Avoidance and eradication of the initial inoculum are probably the most appropriate management strategies that can be recommended against diseases caused by P. ananatis and most other phytopathogenic bacteria.
CONCLUSIONS
-
•
The conclusive identification of strains of P. ananatis has been difficult in the past. However, the use of gene sequences, such as gyrB or rpoB, now provides plant pathologists with a tool to rapidly and reliably identify this species. In addition, this will allow bacteriologists to clarify the ecological role of this bacterium in the natural environment.
-
•
The association of P. ananatis with plants as a pathogen has been known since 1928. In the past 20 years, new reports of P. ananatis on previously unreported hosts have highlighted the re‐emergence of this bacterium as a potentially economically important plant pathogen.
-
•
Little is known of how P. ananatis induces diseases in its hosts. However, now that the genomes of virulent strains from pineapple and eucalypts have been sequenced, information pertaining to pathogenicity and host specificity will become available. This approach will facilitate the development of novel approaches to pathogen control in the future.
ACKNOWLEDGEMENTS
We wish to thank the National Research Foundation (NRF) and the THRIP initiative of the Department of Trade and Industry for funding. We would also like to thank Jolanda Roux for an early review of the manuscript and Carrie Brady for providing Fig. 1.
REFERENCES
- Abe, K. , Watabe, S. , Emori, Y. , Watanabe, M. and Arai, S. (1989) An ice nucleation active gene of Erwinia ananas. Sequence similarity to those of Pseudomonas species and regions required for ice nucleation activity. FEBS Lett. 258, 297–300. [DOI] [PubMed] [Google Scholar]
- Anderson, P.K. , Cunningham, A.A. , Patel, N.G. , Morales, F.J. , Epstein, P.R. and Daszak, P. (2004) Emerging infectious diseases of plants: pathogen pollution, climate change and agrotechnology drivers. Trends Ecol. Evol. 19, 535–544. [DOI] [PubMed] [Google Scholar]
- Azad, H.R. , Holmes, G.J. and Cooksey, D.A. (2000) A new leaf blotch disease of sudangrass caused by Pantoea ananas and Pantoea stewartii . Plant Dis. 84, 973–979. [DOI] [PubMed] [Google Scholar]
- Azegami, K. , Ozaki, K. and Matsuda, A. (1983) Bacterial palea browning, a new disease of rice caused by Erwinia herbicola . Bull. Nat. Inst. Agric. Sci. Ser. C 39, 1–12. [Google Scholar]
- Bandyopadhyay, R. and Frederiksen, R.A. (1999) Contemporary global movement of emerging pathogens. Ann. NY Acad. Sci. 894, 28–36. [DOI] [PubMed] [Google Scholar]
- Beji, A. , Mergaert, J. , Gavini, F. , Izard, D. , Kersters, K. , Leclec, H. and De Ley, J. (1988) Subjective synonymy of Erwinia herbicola, Erwinia milletiae, and Enterobacter agglomerans and redefinition of the taxon by genotypic and phenotypic data. Int. J. Syst. Bacteriol. 38, 77–88. [Google Scholar]
- Bell, A.A. , Medrano, E.G. , Lopez, J.D. and Luff, R.K. (2007) Transmission and importance of Pantoea ananatis during feeding on cotton buds (Gossypium hirsutum L.) by cotton fleahoppers (Pseudatomoscelis seriatus Reuter). World Cotton Research Conference‐4, Lubbock, TX, USA, 10–14 September 2007 . ( Abstract) http://wcrc.conFex.com/wcrc/2007/techprogram/P1835.HTM
- Beyer, P. , Al‐Babili, S. , Ye, X‐D. , Lucca, P. , Schaub, P. , Welsch, R. and Potrykus, I. (2002) Golden rice: introducing the beta‐carotene biosynthesis pathway into rice endosperm by genetic engineering to defeat Vitamin A deficiency. J. Nutr. 132, 506S–510S. [DOI] [PubMed] [Google Scholar]
- Bomfeti, C.A. , Meirelles, W.F. , Souza‐Paccola, E.A. , Casela, C.R. , Ferreira, A.S. , Marriel, I.E. and Paccola‐Meirelles, L.D. (2007) Evaluation of commercial chemical products in vitro and in vivo in the control of foliar disease, maize white spot, caused by Pantoea ananatis . Summa Phytopathol. 33, 63–67. [Google Scholar]
- Bomfeti, C.A. , Souza‐Paccola, A. , Massola Júnior, N.S. , Marriel, I.E. , Meirelles, W.F. , Casela, C.R. and Paccola‐Meirelles, L.D. (2008) Localization of Pantoea ananatis inside lesions of maize white spot disease using transmission electron microscopy and molecular techniques. Trop. Plant Pathol. 33, 1–6. [Google Scholar]
- Brady, C. , Venter, S.N. , Cleenwerck, I. , Vancanneyt, M. , Swings, J. and Coutinho, T.A. (2007) An FAFLP‐system for the improved identification of plant‐pathogenic and ‐associated species of the genus Pantoea. Syst. Appl. Microbiol. 30, 413–417. [DOI] [PubMed] [Google Scholar]
- Brady, C. , Cleenwerck, I. , Venter, S.N. , Vancanneyt, M. , Swings, J. and Coutinho, T.A. (2008) Phylogeny and identification of Pantoea species associated with plants, humans and the natural environment based on multilocus sequence analysis (MLSA). Syst. Appl. Microbiol. 31, 447–460. [DOI] [PubMed] [Google Scholar]
- Brandl, M.T. and Lindow, S.E. (1998) Contribution of indole‐3‐acetic acid production to the epiphytic fitness of Erwinia herbicola . Appl. Environ. Microbiol. 64, 3256–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner, D.J. , Fanning, G.R. , Leete Knutson, J.K. , Steigerwalt, A.G. and Krichevsky, M.L. (1984) Attempts to classify herbicola group‐Enterobacter agglomerans strains by deoxyribonucleic acid hybridization and phenotypic tests. Int. J. Syst. Bacteriol. 34, 45–55. [Google Scholar]
- Bright, C. (1998) Life Out of Bounds. Bioinvasion in a Borderless World. New York: W.W. Norton. [Google Scholar]
- Bruton, B.D. , Wells, J.M. , Lester, G.E. and Patterson, C.L. (1991) Pathogenicity and characterization of Erwinia ananas causing a postharvest disease of cantaloupe fruit. Plant Dis. 75, 180–183. [Google Scholar]
- Ceponis, M.J. , Wells, J.M. and Cappellini, R.A. (1985) Bacterial brown spot of honeydew melons. HortSci. 20, 302–303. [Google Scholar]
- Cho, K.M. , Hong, S.Y. , Lee, S.M. , Kim, Y.H. , Kahng, G.G. , Lim, Y.P. , Kim, H. and Yun, H.D. (2007) Endophytic bacterial communities in ginseng and their antifungal activity against pathogens. Microb. Ecol. 54, 341–351. [DOI] [PubMed] [Google Scholar]
- Chun, D.T.W. and Perkins, H.H. (1997) Profile of bacterial genera associated with cotton from low endotoxin and high endotoxin growing regions. Ann. Agric. Environ. Med. 4, 233–242. [Google Scholar]
- Coplin, D.L. and Kado, C.I. (2001) Pantoea In: Laboratory Guide for Identification of Plant Pathogenic Bacteria, 3rd edn. (Schaad N.W., Jones J.B. and Chun W., eds), pp. 73–83. St. Paul, MN: APS Press. [Google Scholar]
- Cortesi, P. and Pizzatti, C. (2007) Palea browning, a new disease of rice in Italy caused by Pantoea ananatis . J. Plant Pathol. 89, S76. [Google Scholar]
- Cother, E.J. , Reinke, R. , McKenzie, C. , Lanoiselet, V.M. and Noble, D.H. (2004) An unusual stem necrosis of rice caused by Pantoea ananas and the first record of this pathogen on rice in Australia. Australas. Plant Pathol. 33, 495–503. [Google Scholar]
- Coutinho, T.A. , Preisig, O. , Mergaert, J. , Cnockaert, M.C. , Riedel, K‐H. , Swings, J. and Wingfield, M.J. (2002) Bacterial blight and die‐back of Eucalyptus species, hybrids and clones in South Africa. Plant Dis. 86, 20–25. [DOI] [PubMed] [Google Scholar]
- De Baere, T. , Verhelst, R. , Labit, C. , Verschraegen, G. , Wauters, G. , Claeys, G. and Vaneechoutte, M. (2004) Bacteremic infection with Panteoa ananatis . J. Clin. Microbiol. 42, 4393–4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maayer, P. , Venter, S.N. and Coutinho, T.A. (2008) The genome sequence of Pantoea ananatis—a comparative approach. Proceedings of the 20th Congress of the South African Genetics Society, Pretoria, South Africa, 27–29 March 2008 , p. 59 Department of Genetics, University of Pretoria, Pretoria, South Africa( Abstract .)
- DePeng, Z. , LongJun, C. , FuZai, S. and TingChang, X. (1999) The ice nucleation active bacteria on poplar trees and their effects on the causes of freezing injury and induction of fungal canker. Sci. Sil. Sin. 35, 53–57. [Google Scholar]
- Dye, D.W. (1968) A taxonomic study of the genus Erwinia. I. The ‘Amylovora’ Group. N. Z. J. Sci. 11, 590–607. [Google Scholar]
- Dye, D.W. (1969a) A taxonomic study of the genus Erwinia. II. The ‘Carotovora’ Group. N. Z. J. Sci. 12, 81–97. [Google Scholar]
- Dye, D.W. (1969b) A taxonomic study of the genus Erwinia. III. The ‘Herbicola’ group. N. Z. J. Sci. 12, 223–236. [Google Scholar]
- Dye, D.W. (1969c) A taxonomic study of the genus Erwinia. IV. ‘Atypical’ Erwinias Group. N. Z. J. Sci. 12, 833–839. [Google Scholar]
- Dye, D.W. (1981) A numerical taxonomic study of the genus Erwinia . N. Z. J. Agric. Res. 24, 223–229. [Google Scholar]
- Enya, J. , Shinohara, H. , Yoshida, S. , Tsukiboshi, T. , Negishi, H. , Suyama, K. and Tsushima, S. (2007) Culturable leaf‐associated bacteria on tomato plants and their potential as biological control agents. Microb. Ecol. 53, 524–436. [DOI] [PubMed] [Google Scholar]
- Gavini, F. , Mergaert, J. , Beji, A. , Mielcarek, C. , Izard, D. , Kersters, K. and De Ley, J. (1989) Transfer of Enterobacter agglomerans (Beijerinck 1888) Ewing and Fife 1972 to Pantoea gen. nov. as Pantoea agglomerans comb. nov. and description of Pantoea dispersa sp. nov. Int. J. Syst. Bacteriol. 39, 337–345. [Google Scholar]
- Gevers, D. , Cohan, F.M. , Lawrence, J.G. , Spratt, B.G. , Coenye, T. , Feil, E.J. , Stakebrandt, E. , Van De Peer, Y. , Vandamme, P. , Thompson, L. and Swings, J. (2005) Re‐evaluating prokaryotic species. Nat. Rev. Microbiol. 3, 733–739. [DOI] [PubMed] [Google Scholar]
- Gitaitis, R.D. and Gay, J.D. (1997) First report of leaf blight, seed stalk rot, and bulb decay of onion by Pantoea ananas in Georgia. Plant Dis. 81, 1096. [DOI] [PubMed] [Google Scholar]
- Gitaitis, R.D. , Walcott, R. , Culpepper, S. , Sanders, H. , Zolobowska, L. and Langston, D. (2002) Recovery of Pantoea ananatis, causal agent of center rot of onion, from weeds and crops in Georgia, USA. Crop Prot. 21, 983–989. [Google Scholar]
- Gitaitis, R.D. , Walcott, R.R. , Wells, M.L. , Diaz Perez, J.C. and Sanders, F.H. (2003) Transmission of Pantoea ananatis, the causal agent of center rot of onion, by tobacco thrips, Frankliniella fusca . Plant Dis. 87, 675–678. [DOI] [PubMed] [Google Scholar]
- Gitaitis, R.D. , Walcott, R.R. , Sanders, H.F. , Zolobowska, L. and Diaz‐Perez, J.C. (2004) Effects of mulch and irrigation system on sweet onion: II. The epidemiology of center rot. J. Am. Soc. Hortic. Sci. 129, 225–230. [Google Scholar]
- Goszczynska, T. , Moloto, V.M. , Venter, S.N. and Coutinho, T.A. (2006a) Isolation and identification of Pantoea ananatis from onion seed in South Africa. Seed Sci. Technol. 34, 655–668. [Google Scholar]
- Goszczynska, T. , Venter, S.N. and Coutinho, T.A. (2006b) PA 20, a semi‐selective medium for isolation and enumeration of Pantoea ananatis . J. Microbiol. Methods, 64, 225–231. [DOI] [PubMed] [Google Scholar]
- Goszczynska, T. , Venter, S.N. and Coutinho, T.A. (2007) Isolation and identification of the causal agent of brown stalk rot, a new disease of corn in South Africa. Plant Dis. 91, 711–718. [DOI] [PubMed] [Google Scholar]
- Goto, M. , Huang, B.L. , Makino, T. , Goto, T. and Inaba, T. (1988) A taxonomic study on ice nucleation‐active bacteria isolated from gemmisphere of tea (Thea sinensis L.), phylloplane of vegetables and flowers of Magnolia denudata Desr. Ann. Phytopathol. Soc. Jpn. 54, 189–197 (Abstract). [Google Scholar]
- Grimont, P.A.D. and Grimont, F. (2005) Genus: Pantoea In: Bergey's Manual of Systematic Bacteriology, Vol. 2 (Brenner D.J., Krieg N.R. and Staley J.T., eds), pp. 713–720. The Proteobacteria, Part B, The Gammaproteobacteria, 2nd edn. New York: Springer. [Google Scholar]
- Halda‐Alija, L. (2003) Identification of indole‐3‐acetic acid producing freshwater wetland rhizopshere bacteria associated with Juncus effusus L. Can. J Microbiol. 49, 781–787. [DOI] [PubMed] [Google Scholar]
- Hasegawa, M. , Azegami, K. , Yoshida, H. and Otani, H. (2003) Behaviour of Erwinia ananas transformed with bioluminescence genes on rice plants. J. Gen. Plant Pathol. 69, 267–270. [Google Scholar]
- Holt, J.G. (1977) The Shorter Bergey's Manual of Determinative Bacteriology, 8th edn. Baltimore, MD: Williams & Wilkins Company. [Google Scholar]
- Iimura, K. and Hosono, A. (1996) Biochemical characteristics of Enterobacteri agglomerans and related strains found in buckwheat seeds. Int. J. Food Microbiol. 30, 243–253. [DOI] [PubMed] [Google Scholar]
- Iimura, K. and Hosono, A. (1998) Antifungal activities of bacteria endemic to buckwheat seeds. Fagopyrum, 15, 42–54 (Abstract). [Google Scholar]
- Izumi, H. , Cairney, J.W.G. , Killham, K. , Moore, E. , Alexander, I.J. and Anderson, I.C. (2008) Bacteria associated with ectomycorrhizas of slash pine (Pinus elliottii) in south‐eastern Queensland, Australia. Fems Microbiol. Lett. 282, 196–204. [DOI] [PubMed] [Google Scholar]
- Kageyama, B. , Nakae, M. , Yagi, S. and Sonoyama, T. (1992) Pantoea punctata sp. nov., Pantoea citrea sp. nov., and Pantoea terrea sp. nov. isolated from fruit and soil samples. Int. J. Syst. Bacteriol. 42, 203–210. [DOI] [PubMed] [Google Scholar]
- Kang, S.H. , Cho, H.S. , Cheong, H. , Ryu, C.M. , Kim, J.F. and Park, S.H. (2007) Two bacterial endophytes eliciting both growth promotion and plant defense on pepper (Capsicum annum L.). J. Microbiol. Biotechnol. 17, 96–103. [PubMed] [Google Scholar]
- Kim, M.K. , Ryu, J.S. , Lee, Y.H. and Yun, H.D. (2007) First report of Pantoea sp. induced soft rot disease of Pleurotus ergyngii in Korea. Plant Dis. 91, 109. [DOI] [PubMed] [Google Scholar]
- Kim, Y.C. , Kim, K.C. and Choi, B.H. (1989) Palea browning disease of rice caused by Erwinia herbicola and ice nucleation activity of the pathogenic bacterium. Korean J. Plant Pathol. 5, 72–79. [Google Scholar]
- Lai, S‐C. and Hsu, S‐T. (1974) Survival of Erwinia ananas in soil. Plant Protect. Bull., Taiwan, 16, 12–19 (Abstract). [Google Scholar]
- Legard, D.E. , McQuilken, M.P. , Whipps, J.M. , Penlon, J.S. , Fermor, T.R. , Thompson, I.P. , Bailey, M.J. and Lynch, J.M. (1994) Studies on the seasonal changes in the microbial populations on the phyllosphere of spring wheat as a prelude to the release of genetically modified microorganisms. Agric. Ecosys. Environ. 50, 87–101. [Google Scholar]
- Lindow, S.E. (1983) The role of bacterial ice nucleation in frost injury to plants. Annu. Rev. Phytopathol. 21, 363–384. [Google Scholar]
- Lodewyckx, C. , Vangronsveld, J. , Porteous, F. , Moore, E.R.B. , Taghavi, S. , Mezgeay, M. and Van der Lelie, D. (2002) Endophytic bacteria and their potential applications. Crit. Rev. Plant Sci. 21, 583–606. [Google Scholar]
- Mano, J. and Morisaki, H. (2008) Endophytic bacteria in the rice plant. Microbes Environ. 23, 109–117. [DOI] [PubMed] [Google Scholar]
- Mano, J. , Tanaka, F. , Watanabe, A. , Kaga, H. , Okunishi, S. and Morisaki, H. (2006) Culturable surface and endophytic bacterial flora of the maturing seeds of rice plants (Oryza sativa) cultivated in a paddy field. Microbes Environ. 21, 86–100. [Google Scholar]
- Mattinen, L. , Somervuo, P. , Nykyri, J. , Nissenen, R. , Kouvonen, P. , Corthals, G. , Auvinen, P. , Aittamaa, M. , Valkonen, J.P.T. and Pirhonen, M. (2008) Microarray profiling of host‐extract‐induced genes and characterization of the type VI secretion cluster in the potato pathogen Pectobacterium atrosepticum. Microbiology, 154, 2387–2396. [DOI] [PubMed] [Google Scholar]
- Mergaert, J. , Gavini, J. , Kersters, K. , Leclerc, H. and De Ley, J. (1983) Phenotypic and protein electrophoretic similarities between strains of Enterobacter agglomerans, Erwinia herbicola, and Erwinia milletiae from clinical or plant origin. Curr. Microbiol. 8, 327–331. [Google Scholar]
- Mergaert, J. , Verdonck, L. , Kersters, K. , Swings, J. , Boeufgras, J‐M. and De Ley, J. (1984) Numerical taxonomy of Erwinia species using API systems. J. Gen. Microbiol. 130, 1893–1810. [Google Scholar]
- Mergaert, J. , Verdonck, L. and Kersters, K. (1993) Transfer of Erwinia ananas (synonym, Erwinia uredovora) and Erwinia stewartii to the Genus Pantoea emend. as Pantoea ananas (Serrano 1928) comb. nov. and Pantoea stewartii (Smith 1898) comb. nov., respectively, and description of Pantoea stewartii subsp. indologenes subsp. nov. Int. J. Syst. Bacteriol. 43, 162–173. [Google Scholar]
- Mohammed, S.I. , Steenson, L.R. and Kirleis, A.W. (1991) Isolation and characterization of microorganisms associated with traditional sorghum fermentation for production of Sudanese Kisra. Appl. Environ. Microbiol. 57, 2529–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohoshi, T. , Nakamura, Y. , Yamazaki, G. , Ishida, A. , Kato, N. and Ikeda, T. (2007) The plant pathogen Pantoea ananatis produces N‐acylhomoserine lactone and causes center rot disease of onion by quorum sensing. J. Bacteriol. 189, 8333–8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell, A. , Dobson, S.J. , Yang, X. , Lacey, E. and Barker, S.C. (2003) A survey of bacterial diversity in ticks, lice and fleas from Australia. Parasitol. Res. 89, 326–334. [DOI] [PubMed] [Google Scholar]
- Nakanishi, Y. , Adanonon, A. , Okabe, I. , Hoshino, Y.T. and Matsumoto, N. (2006) An oligonucleotide probe for the detection of Erwinia herbicola and Erwinia ananas . J. Gen. Plant Pathol. 72, 328–333. [Google Scholar]
- Nunes, F.V. and De Melo, I.S. (2006) Isolation and characterisation of endophytic bacteria of coffee plants and their potential in caffeine degradation. Environ. Toxicol. 1, 293–297. [Google Scholar]
- Obata, H. , Takinami, K. , Tanishita, J. , Hasegawa, Y. , Kawate, S. , Tokuyama, T. and Ueno, T. (1990) Identification of a new ice‐nucleating bacterium and its ice nucleation properties. Agric. Biol. Chem. 54, 725–730. [Google Scholar]
- Okunishi, S. , Sako, K. , Mano, H. , Imamura, A. and Morisaki, H. (2005) Bacterial flora of endophytes in the maturing seed of cultivated rice (Oryza sativa ). Microbes Environ. 20, 168–177. [Google Scholar]
- Paccola‐Meirelles, L.D. , Ferreira, A.S. , Meirelles, W.F. , Marriel, I.E. and Casela, C.R. (2001) Detection of a bacterium associated with leaf spot disease of maize in Brazil. J. Phytopathol. 149, 275–279. [Google Scholar]
- Paccola‐Meirelles, L.D. , Meirelles, W.F. , Parentoni, S.N. , Marriel, I.E. , Ferreira, A.S. and Casela, C.R. (2002) Reaction of maize inbred lines to the bacterium Pantoea ananas isolated from Phaeosphaeria leaf spot lesions. Crop Breed. Appl. Biotechnol. 2, 587–589. [Google Scholar]
- Pajk, P. (2004) Possibilities of application of abiotic methods for suppression of bacteria Erwinia amylovora (Burr.) Winsl. et al. in Slovenia Zbornik Referatov 1. Slovenskega Sadjarskega Kongresa z Mednarodno Udeležbo, Krško, Slovenia, 24–26 March 2004, Del 2, 2004 (Huaina M., ed.) pp. 449–453. Krško, (Abstract). [Google Scholar]
- Palm, M.E. (1999) Mycology and world trade: a view from the front line. Mycologia 91, 1–12. [Google Scholar]
- Pomini, A.M. , Araǔjo, W.L. and Marsaioli, A.J. (2006) Structural elucidation and biological activity of acyl‐homoserine lactones from the phytopathogen Panteoa ananatis Serrano 1928. J. Chem. Ecol. 32, 1769–1778. [DOI] [PubMed] [Google Scholar]
- Pon, D.S. , Townsend, C.E. , Wessman, G.E. , Schmitt, C.G. and Kingsolver, C.H. (1954) A Xanthomonas parasitic on uredia of cereal rust. Phytopathology, 44, 707–710. [Google Scholar]
- Pukatzki, S. , Ma, M.T. , Sturtevant, D. , Krastins, B. , Sarracino, D. , Nelson, W.C. , Heidelberg, J.F. and Mekalanos, J.J. (2006) Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc. Natl. Acad. Sci. USA, 103, 1528–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch, M.E. , Graef, H.W. , Rozenzhak, S.M. , Jones, S.E. , Bleckmann, C.A. , Kruger, R.L. , Naik, R.R. and Stone, M.O. (2006) Characterization of microbial contamination in United States Air Force aviation fuel tanks. J. Ind. Microbiol. Biotech. 33, 29–36. [DOI] [PubMed] [Google Scholar]
- Rijavec, T. , Lapanje, A. , Dermastia, M. and Rupnik, M. (2007) Isolation of bacterial endophytes from germinated maize kernels. Can. J. Microbiol. 53, 802–808. [DOI] [PubMed] [Google Scholar]
- Schaad, N.W. (2001) Initial identification of common genera In: Laboratory Guide for Identification of Plant Pathogenic Bacteria, 3rd edn. (Schaad N.W., Jones J.B. and Chun W., eds), pp. 1–16. St. Paul, MN: APS Press. [Google Scholar]
- Schwartz, H.F. and Otto, K. (2000) First report of a leaf blight and bulb decay of onion by Pantoea ananatis in Colorado. Plant Dis. 84, 808. [DOI] [PubMed] [Google Scholar]
- Schwartz, H.F. , Otto, K.L. and Gent, D.H. (2003) Relation of temperature and rainfall to development of Xanthomonas and Pantoea leaf blights of onion in Colorado. Plant Dis. 87, 11–14. [DOI] [PubMed] [Google Scholar]
- Serrano, F.B. (1928) Bacterial fruitlet brown‐rot of pineapple in the Philippines. Philippine J. Sci. 36, 271–324. [Google Scholar]
- Sessitsch, A. , Reiter, B. and Berg, G. (2004) Endophytic bacterial communities of field‐grown potato plants and their plant‐growth‐promoting and antagonistic abilities. Can. J. Microbiol. 50, 239–249. [DOI] [PubMed] [Google Scholar]
- Simeya, N. , Numata, S. , Nakjima, M. , Haseba, A. , Hibi, T. and Akutsu, K. (2003) Biological control of rice blast by the epiphytic bacterium Erwinia ananas transformed with a chitinolytic enzyme gene from an antagonistic bacterium, Serratia marcescens . J. Gen. Plant Pathol. 69, 276–282. [Google Scholar]
- Stackebrand, E. and Goebels, B.M. (1994) Taxonomic note: a place for DNA–DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Bacteriol. 44, 846–849. [Google Scholar]
- Stall, R.E. , Alexander, L.J. and Hall, C.B. (1969) Effect of tobacco mosaic virus and bacterial infections on occurrence of graywall of tomato (Erwinia ananas). Fla. State Hortic. Soc. Proc. 81, 157–161. [Google Scholar]
- Sun, F.‐Z. , Zhao, T.‐C. , Wang, J.‐J. , Mu, F.‐S. , An, J.‐Y. and Jin, Q.‐M. (2005) Dynamic changes of ice nucleation active bacterial populations inhabiting corn in northern China. Acta Ecol. Sin. 25, 785–790. [Google Scholar]
- Tabei, H. , Azegami, K. and Fukuda, T. (1988) Infection site of rice grain with Erwinia herbicola, the causal agent of bacterial palea browning of rice. Ann. Phytopathol. Soc. Jpn. 54, 637–639 (Abstract). [Google Scholar]
- Takahashi, K. , Watanabe, K. and Sato, M. (1995) Survival and characteristics of ice nucleation‐active bacteria on mulberry trees (Morus spp.) and in mulberry pyralid (Glyphodes pyloalis ). Ann. Phytopathol. Soc. Jpn. 61, 439–443 (Abstract). [Google Scholar]
- Thomas, P. , Kumari, S. , Swarna, G.K. and Gowda, T.K.S. (2007) Papaya shoot tip associated endophytic bacteria isolated from in vitro cultures and host–endophyte interaction in vitro and in vivo. Can. J. Microbiol. 53, 380–390. [DOI] [PubMed] [Google Scholar]
- Torres, R. , Teixidó, N. , Usali, J. , Abadias, M. and Viñas, I. (2005) Post‐harvest control of Penicillium expansum on pome fruits by the bacterium Pantoea ananatis CPA‐3. J. Hortic. Sci. Biotechnol. 80, 75–81. [Google Scholar]
- Trüper, H.G. and De’Clari, L. (1997) Taxonomic note: necessary correction of specific epithets formed as substantives (nouns) ‘in apposition’. Int. J. Syst. Bacteriol. 47, 908–909. [Google Scholar]
- Vantomme, R. , Mergaert, J. , Verdonck, L. and De Ley, J. (1989) Antagonistic effect in vitro of Erwinia uredovora LMG 2678 against some other bacteria. J. Phytopathol. 124, 372–376. [Google Scholar]
- Verdonck, L. , Mergaert, J. , Rijckaert, C. , Swings, J. , Kersters, K. and De Ley, J. (1987) The genus Erwinia: a numerical analysis of phenotypic features. Int. J. Syst. Bacteriol. 37, 4–18. [Google Scholar]
- Walcott, R.R. , Gitaitis, R.D. , Castro, A.C. , Sanders, F.H. Jr. and Diaz‐Perez, J.C. (2002) Natural infestation of onion seed by Pantoea ananatis, causal agent of center rot. Plant Dis. 86, 106–111. [DOI] [PubMed] [Google Scholar]
- Walcott, R.R. , Gitaitis, R.D. and Castro, A.C. (2003) Role of blossoms in watermelon seed infestation of Acidovorax avenae subsp. citrulli . Phytopathology, 93, 528–534. [DOI] [PubMed] [Google Scholar]
- Waleron, M. , Waleron, K. , Podhajska, A.J. and Lojkowska, E. (2002) Genotyping of bacteria belonging to the former Erwinia genus by PCR‐RFLP analysis of a recA gene fragment. Microbiology, 148, 583–595. [DOI] [PubMed] [Google Scholar]
- Wang, Z.H. , Liao, M. , Yang, W.Y. , Chen, S.B. , Huang, L. and Bian, Q.J. (2008) Isolation and identification of INA bacteria in hybrid ‘Skiranui tangerine’ citrus cultivars and their indoor control. Acta Phytophyl. Sin. 35, 51–57 (Abstract). [Google Scholar]
- Watanabe, K. and Sato, M. (1999) Gut colonization of an ice nucleation active bacterium, Erwinia (Pantoea) ananas, reduces the cold hardiness of mulberry pyralid larvae. Cryobiology, 38, 281–289. [DOI] [PubMed] [Google Scholar]
- Watanabe, K. , Kawakita, H. and Sato, M. (1996) Epiphytic bacterium, Erwinia ananas, commonly isolated from rice plants and brown planthoppers (Nilaparvata lugens) in hopperburn patches. Appl. Entomol. Zool. 31, 459–462. [Google Scholar]
- Watanabe, M. and Arai, S. (1994) Bacterial ice‐nucleation activity and its application to freeze concentration of fresh foods for modification of their properties. J. Food Eng. 22, 453–473. [Google Scholar]
- Wells, J.M. , Sheng, W‐S. , Ceponis, M.J. and Chen, T.A. (1987) Isolation and characterization of strains of Erwinia ananas from honeydew melons. Phytopathology, 77, 511–514. [Google Scholar]
- Wells, M.L. , Gitaitis, R.D. and Sanders, F.H. (2002) Association of tobacco thrips, Frankliniella fusca (Thysanoptera: Thripidae), with two species of bacteria of the genus Pantoea . Ann. Entomol. Soc. Am. 95, 719–723. [Google Scholar]
- Winslow, C.E.A. , Broadhurst, J. , Buchanan, R.E. , Krumwiede, C. Jr. , Rogers, L.A. and Smith, G.H. (1920) The families and genera of the bacteria. Final report of the Committee of the Society of American Bacteriologists on characterization and classification of bacterial types. J. Bacteriol. 5, 191–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang, C.T. , Song, F.Q. , Liu, J.H. , Dong, A.J. , Xue, Y. , Yuan, S.Z. , Zhang, J.H. , Jiang, X.D. , Shi, X.F. , Wang, C.W. , Lin, H.B. and Han, W.X. (2001) The dominant factors caused by INA bacterial canker. J. N.E. For. Uni. 29, 109–113 (Abstract). [Google Scholar]
- Xie, G.L. (2001) First report of palea browning in China and characterization of the causal organism by phenotypic tests and Biolog. Int. Rice Res. Notes 26, 25–26. [Google Scholar]
- Yoshida, S. , Kinkei, L.L. , Shinohara, H. , Numajiri, N. , Hiradate, S. , Koitabashi, M. , Suyama, K. , Negishi, H. and Tsushima, S. (2006) Production of quorum‐sensing‐related signal molecules by epiphytic bacteria inhabiting wheat heads. Can. J. Microbiol. 52, 411–418. [DOI] [PubMed] [Google Scholar]
- Yumiko, T. , Toshiyuki, M. and Yoshiaki, C. (2005) Bacterial rot of Allium giganteum caused by Erwinia ananas (=Pantoea ananatis), and basal rot of Belamcanda chinensis caused by Aphanomyces sp. Bull. Toyama Agric. Res. Cent. 22, 1–6 (Abstract). [Google Scholar]
- Zasypkin, D.V. and Lee, T‐C. (1999) Extracellular ice nucleators from Pantoea ananas: effect of freezing on model foods. J. Food Sci. 64, 473–478. [Google Scholar]
- Zeng, D.P. , Chao, L.J. , Sun, S.Z. and Zhou, T.C. (1999) The ice nucleation active bacteria on poplar trees and their effects on the courses of freezing injury and induction of fungal canker. Sci. Sil. Sin. 35, 53–57 (Abstract). [Google Scholar]
- Zhao, R.Y. , Xu, M. , Fu, Z.F. , Sun, F.Z. , Li, S.T. , Yang, J.M. and Li, S.H. (2007) Ice‐nucleation‐active bacterial species and their dynamics in Beijing apricot trees. Sci. Agric. Sin. 40, 1174–1180 (Abstract). [Google Scholar]


