SUMMARY
Fusarium species infect cereal crops worldwide and cause the important diseases Fusarium head blight and crown rot in wheat. Fusarium pathogens reduce yield and some species also produce trichothecene mycotoxins, such as deoxynivalenol (DON), during infection. These toxins play roles in pathogenesis on wheat and have serious health effects if present in grain consumed by humans or animals. In the present study, the response of wheat tissue to DON has been investigated. Infusion of wheat leaves with DON induced hydrogen peroxide production within 6 h followed by cell death within 24 h that was accompanied by DNA laddering, a hallmark of programmed cell death. In addition, real‐time PCR analysis revealed that DON treatment rapidly induced transcription of a number of defence genes in a concentration‐dependent manner. Co‐treatment with DON and the antioxidant ascorbic acid reduced these responses, suggesting their induction may be at least partially mediated by reactive oxygen species (ROS), commonly known to be signalling molecules in plants. Wheat defence genes were more highly expressed in wheat stems inoculated with a DON‐producing fungal strain than those inoculated with a DON‐non‐producing mutant, but only at a late stage of infection. Taken together, the results are consistent with a model in which DON production during infection of wheat induces ROS, which on the one hand may stimulate programmed host cell death assisting necrotrophic fungal growth, whereas, on the other hand, the ROS may contribute to the induction of antimicrobial host defences.
INTRODUCTION
Many plant pathogenic fungi produce secondary metabolites, including mycotoxins, during the infection process. Mycotoxins, such as the trichothecene deoxynivalenol (DON) produced by some Fusarium species, are not always essential for initiating disease but are often linked with increased aggressiveness (Desjardins et al., 1995). The effect of DON on cell function has been most extensively studied in animal cells in attempts to understand its toxicity (Pestka et al., 2004; Rocha et al., 2005). These studies have resulted in a model where trichothecenes inhibit protein synthesis by binding to the 60S ribosomal subunit, activating a cell signalling programme that results in apoptosis. In conjunction with this, there is an induction of cytokine‐regulated gene expression resulting in an inflammatory response. In macrophages, as well as T and B cells of the mammalian immune system, these complex effects mean that trichothecenes such as DON can be either immunostimulatory or immunosuppressive depending on dosage, duration and frequency of exposure (Pestka et al., 2004).
Fusarium head blight (FHB) and crown rot (CR) are both diseases of wheat caused by several Fusarium species, including F. graminearum (Fg) and F. pseudograminearum (Fp) (Akinsanmi et al., 2004). The major difference between these diseases is the site and timing of infection, with FHB infecting wheat heads around the time of anthesis, and CR infecting stem base and crown tissue at all growth stages. DON is produced during both FHB and CR diseases (Blaney and Dodman, 2002; Mudge et al., 2006) and this is a major concern due to its toxicity to humans and animals following consumption of contaminated grain (Larsen et al., 2004).
The role of DON and other trichothecene mycotoxins during pathogenesis has been analysed using mutant fungal strains that do not produce toxin (Hohn and Desjardins, 1992). Studies on a strain of Fg that has a mutation in the Tri5 gene encoding a DON biosynthetic enzyme have shown that Fg strains unable to produce DON are less aggressive during FHB in both wheat and barley (Boddu et al., 2007; Langevin et al., 2004). More specifically in wheat, the mycotoxin appears to be necessary for fungal passage from infected florets into the rachis from where it can further colonize the head (Jansen et al., 2005). These DON‐non‐producing Fg strains are unable to prevent thickening of host cell walls after penetration and so their movement from the point of infection is hindered (Bai et al., 2002; Jansen et al., 2005; Maier et al., 2006). There is also evidence that FHB‐resistant wheat genotypes accumulate far less DON than susceptible ones (Goswami and Kistler, 2005; Wilde and Miedaner, 2006). For FHB, it therefore appears that occurrence of disease may not be dependent on the toxin, but DON production does affect disease levels, and so improving resistance to DON may improve resistance to the disease. The role of DON during CR is less well defined than for FHB, but it appears to be necessary for fungal colonization of upper stem nodes following infection at the crown and stem base (Mudge et al., 2006).
Several studies have shown that fungal derived toxins can elicit responses in plants that have aspects in common with well‐known pathogen‐induced responses. It has recently been reported that another trichothecene produced by some Fusaria, the T‐2 toxin, induces hydrogen peroxide (H2O2) production, inhibits protein synthesis and stimulates cell death in the non‐host plant Arabidopsis thaliana at concentrations as low as 0.4 mg/L (Nishiuchi et al., 2006). This study also showed that DON can induce defences in A. thaliana plants but only at the higher concentration of 30 mg/L. In barley, a comparison of gene expression induced by wild‐type and a DON‐non‐producing strain of F. graminearum revealed that DON is responsible for the induction of genes encoding proteins involved in ubiquitination and programmed cell death (PCD) among others (Boddu et al., 2007). In wheat, differential display analysis has been used to demonstrate that DON affects transcript levels of a few specific host genes in roots, including peroxidase genes (Ansari et al., 2007). However, the physiological and molecular responses of wheat cells to DON exposure have not been described.
We aimed to assess the effects of DON on wheat defence responses and observed the production of H2O2, a well‐known signalling molecule, followed by cell death, a phenomenon frequently observed in mammalian cell lines exposed to DON (Pestka et al., 2004). In addition, DON induced a range of well‐known defence genes and interestingly a DON‐producing fungal strain induced higher levels of defence transcripts than a DON‐non‐producing mutant during disease development. Both cell death and defence gene induction was reduced by co‐treatment of DON and an antioxidant that would scavenge free radicals such as H2O2. These results suggest that DON produced during Fusarium‐related diseases may play a role in the activation of wheat defence responses and cell death, at least partially via the signalling molecule H2O2.
RESULTS
DON elicits ROS production, genomic DNA laddering and cell death in wheat
Wheat stem tissue was infiltrated with a solution of 100 or 200 mg/L DON, resulting in the production of localized H2O2 microbursts (Levine et al., 1994) within 6 h after treatment (Fig. 1A–C; Table 1; supplementary Fig. S1). Co‐infiltration of DON with 40 mm ascorbic acid, an antioxidant capable of scavenging reactive oxygen species (ROS), or 4 mg/L cycloheximide, a eukaryotic protein synthesis inhibitor, reduced the amount of H2O2 produced (Table 1; supplementary Fig. S1). Trypan blue staining to reveal cell death was negative at this 6‐h time point (data not shown). By 24 h post‐infiltration, H2O2 staining was no longer visible in DON‐infiltrated tissue (data not shown), but the tissue stained positively for cell death, primarily in stomatal guard cells and also in mesophyll cells of samples treated with 200 mg/L DON (Fig. 1D–F; Table 1; supplementary Fig. S1). Again, co‐infiltration with ascorbic acid reduced cell death and co‐infiltration with cycloheximide prevented cell death (Table 1; supplementary Fig. S1).
Figure 1.
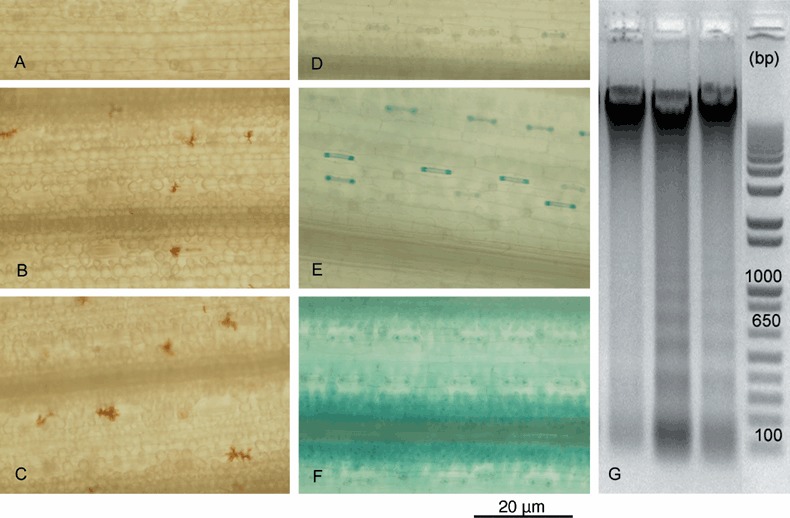
Infiltration of wheat tissue with DON resulted in H2O2 production, cell death and DNA laddering. DAB‐stained wheat leaf tissue from 2‐week‐old seedlings showing H2O2 production at 6 h after (A) mock infiltration, (B) 100 mg/L DON infiltration and (C) 200 mg/L DON infiltration. Trypan blue‐stained wheat leaf tissue 24 h after (D) mock infiltration, (E) 100 mg/L DON infiltration and (F) 200 mg/L DON infiltration. (G) Genomic DNA laddering in wheat 24 h after infiltration with mock solution shown in the first lane, 100 mg/L DON shown in the second lane, 100 mg/L DON combined with 7 g/L ascorbic acid shown in the third lane and the 1‐kb + DNA size ladder (Invitrogen) in the fourth lane. Further images of tissue from all infiltration treatments are shown in supplementary Fig. S1.
Table 1.
Summary of H2O2 and cell death observed in infiltrated wheat leaf tissue stained with DAB 6 h after treatment and stained with trypan blue 24 h after treatment.
| H2O2—6 h | Cell death—24 h | |
|---|---|---|
| Mock | – | + |
| 100 mg/L DON | +++ | ++ |
| 200 mg/L DON | +++ | ++++ |
| 100 mg/L DON + 7 g/L AA | ++ | + |
| 100 mg/L DON + 4 mg/L CHX | ++ | – |
DON, deoxynivalenol; AA, ascorbic acid; CHX, cycloheximide. Scale: +, some stomata affected or sporadic H2O2 microbursts in some tissue; ++, most stomata affected or many H2O2 microbursts in some tissue; +++, all stomata affected or many microbursts in all tissue; ++++, widespread tissue affected including stomata and mesophyll cells. Further images of the tissue described in this table are shown in supplementary Fig. S1.
To investigate the mechanism behind DON‐induced cell death further, genomic DNA collected from wheat stems 24 h after infiltration was analysed for cleavage products by gel electrophoresis. A shown in Fig. 1G, a distinct DNA laddering pattern was observed in DON‐treated leaves when compared with mock‐treated controls. Similar to cell death visualized using trypan blue staining, DNA laddering appeared to be reduced when tissue was co‐infiltrated with ascorbic acid. This effect of ascorbic acid on DNA laddering was observed in two independent experiments. Visually quantifying differences between these DNA laddering profiles was subjective, and therefore to quantify the results, the total number of pixels in each lane was assessed using ImageJ software. This was then compared with the number of pixels in the region of the image showing only degraded DNA (< 5000 bp). This method of quantification showed that DNA from mock‐treated samples was only 5% degraded, while that from DON‐treated samples was 66% degraded; combining ascorbic acid with the DON treatment reduced degradation to 29%. DON‐induced DNA laddering as observed here indicates induction of a host‐mediated PCD process (Ryerson and Heath, 1996).
Fp and Fg are closely related species that are both able to cause CR and FHB in wheat, and both pathogen species produce DON (Mudge et al., 2006). Tissue inoculated with Fp spores was studied in detail and revealed H2O2 production and cell death, indicating defences induced by purified DON are consistent with those induced during a natural CR infection. DON has previously been shown to be produced during CR at levels > 100 mg/L when Fp is the causal agent (Mudge et al., 2006). Within 7 days after inoculation of wheat, using a detached leaf assay, intense H2O2 production was observed in some stomatal guard cells that were in close proximity to Fp spores (Fig. 2A). DAB staining also revealed the presence of H2O2 in some fungal spores (Fig. 2A inset), a phenomenon previously associated with pathogenicity in other host–pathogen interactions (Egan et al., 2007). Host cell death of Fp‐inoculated wheat tissue was observed at multiple infection points and spread in a way that seemed to follow vascular tissue (Fig. 2B; supplementary Fig. S2). Stomatal guard cells showed the most intense and widespread staining for cell death among those cells surrounding infection points. Preliminary examination of Fg infection sites indicated similar host responses to those observed with Fp (data not shown).
Figure 2.
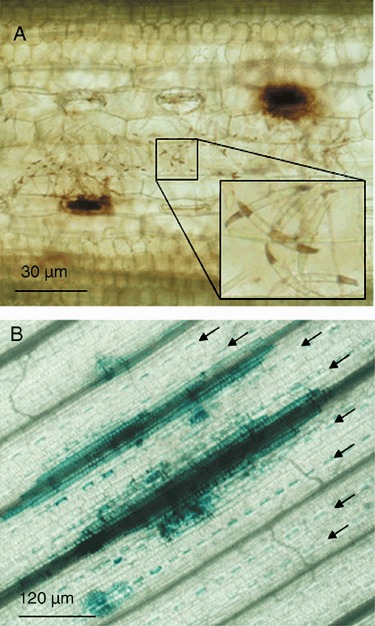
H2O2 production and cell death was observed in wheat leaves after inoculation with F. pseudograminearum spores. (A) Wheat leaf tissue was inoculated using a detached leaf assay stained using DAB 7 days later. H2O2 was visible in stomata that were in close proximity to spores and also in the spore tips (inset). (B) Trypan blue staining of inoculated leaf tissue showed cell death seemed to follow vascular tissue and was most widespread in stomatal guard cells. Rows of affected stomatal guard cells that surround the primary infection site are indicated by arrows. Further images of cell death in inoculated tissue are shown in supplementary Fig. S2.
Induction of wheat defence gene transcripts and proteins by DON
We have previously shown that drop inoculation of wheat with Fp spores leads to the induction of a suite of defence gene transcripts including PR1.1, PR2 (β‐1–3 glucanase), PR3 (chitinase), PR4 (wheatwin), PR5 (thaumatin‐like protein), PR10, peroxidase and germin‐like (Desmond et al., 2005). Herein, a similar drop treatment of wheat seedlings with a solution of DON also induced expression of these well‐known defence genes. Figure 3A shows transcript induction by DON, generally in a concentration‐dependent manner, within the range of 1–100 mg/L. Treatment with 0.1 mg/L DON did not induce defence gene expression (data not shown). Interestingly, the most highly induced genes were germin‐like and peroxidase, both genes implicated in reactive oxygen metabolism.
Figure 3.
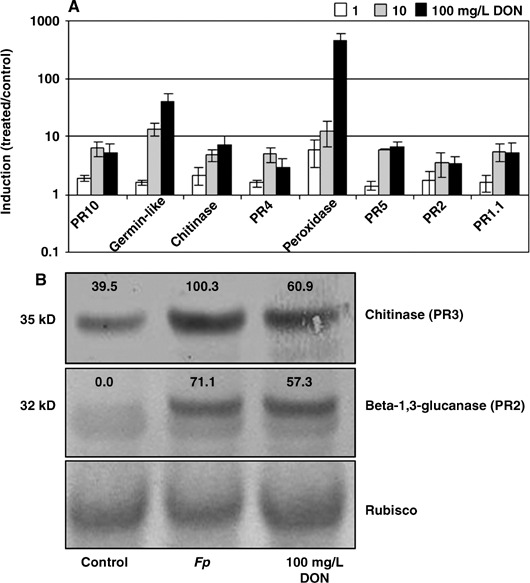
(A) Induction of defence gene expression in 2‐week‐old seedlings at 1 day after treatment with 1, 10 and 100 mg/L DON. Columns represent average induction ratios (± SE; n = 3) of gene transcripts in treated compared with mock‐treated and are plotted on a logarithmic scale. (B) Western blot analysis of total protein extracted from wheat tissue using β‐1,3‐glucanase (PR2) and chitinase (PR3) antibodies 2 days after mock treatment, F. pseudograminearum inoculation, or 100 mg/L DON treatment. Lower panel shows Rubisco stained with Ponceau red to show protein loading. The numbers above bands for chitinase and β‐1,3‐glucanase indicate protein levels as a percentage of Rubisco. Total protein (20 µg) was separated on a 4–12% polyacrylamide gradient gel. Protein molecular masses are indicated on the left.
DON is known to be a potent inhibitor of eukaryotic protein synthesis at concentrations well below 100 mg/L (Nishiuchi et al., 2006). Therefore, a Western blot to assess protein levels was performed on 100 mg/L DON‐treated wheat samples using PR2‐ (β‐1,3‐glucanase) and PR3‐ (chitinase) specific antibodies. Again, ImageJ was used to quantify pixels for each band, and protein abundance as a percentage of Rubisco is shown for each band in Fig. 3B. PR2 and PR3 are clearly more abundant both visually and quantitatively in both Fp‐inoculated and DON‐treated samples compared with controls.
The influence of ROS on DON‐induced defence gene induction was investigated by comparing samples treated with DON alone or DON combined with ascorbic acid. Figure 4 shows that the presence of ascorbic acid reduced the average induction of defence genes at both 6 and 24 h after treatment. Statistical analysis showed this reduction was significant (P < 0.05) for at least PR3, peroxidase and PR2.
Figure 4.
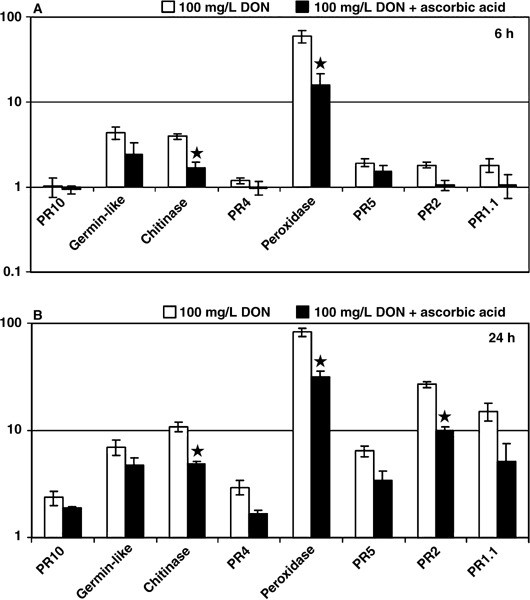
Induction of defence gene expression in 2‐week‐old wheat seedlings (A) 6 h and (B) 24 h after treatment with 100 mg/L DON and a combination of 100 mg/L DON and 7 g/L ascorbic acid. Columns represent average induction ratios (± SE; n = 3) of gene transcripts in treated compared with mock‐treated plants and are plotted on a logarithmic scale. Statistically significant differences in gene induction (Student's t‐test, P < 0.05) resulting from treatments including ascorbic acid are indicated by a star.
Variation in the level of induction following 100 mg/L DON treatment was observed in gene expression analysis presented in 3, 4. The quantitative gene expression data obtained from RT‐qPCR often shows variation in experiments set‐up independently of each other. Slight variations in light conditions, temperature, inoculum quality and developmental stage of treated plants could not be completely prevented, and can affect gene induction during stress responses. Therefore, the question of whether gene induction was consistently observed would be more relevant than the actual fold‐induction observed in each experiment. In the present case, induction was observed in all three biological replicates tested on both occasions that have been reported.
Differential defence gene induction by a DON‐non‐producing fungal strain at late stages of CR disease development
To test whether DON may play a role during CR infection of wheat, we assessed fungal biomass and the levels of defence gene transcripts in wheat stem tissue inoculated with either the transgenic DON‐non‐producing strain of Fg (Fg ΔTri5) or its wild‐type DON‐producing parental strain. Fg was specifically used for this particular study because its facile transformation system and published genome sequence enabled the construction of the Tri5 deletion strain. Inoculation of detached leaves with Fg ΔTri5 resulted in similar H2O2 production and cell death responses as for tissues inoculated with the wild‐type Fg and Fp strains at early stages of infection (data not shown, Fig. 1; supplementary Fig. S1). Results also indicated that wheat defence gene transcript levels were unchanged between tissues inoculated with these two strains at 1, 2 and 14 days post‐inoculation. However, a significant reduction in defence transcript levels (Fig. 5A) and fungal biomass (Fig. 5B) was observed in wheat stem tissue inoculated with the Fg ΔTri5 strain at 28 days post‐inoculation.
Figure 5.
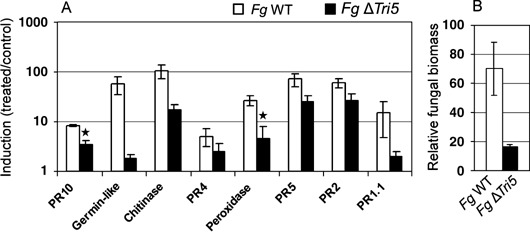
(A) Induction of defence gene expression 28 days after inoculation with the DON‐non‐producing F. graminearum Tri5 deletion line (Fg ΔTri5) and wild‐type. Columns represent average induction ratios (± SE; n = 3) of gene transcripts in treated compared with mock‐treated plants and are plotted on a logarithmic scale. Statistically significant differences in gene induction (Student's t‐test, P < 0.05) resulting from treatments are indicated by a star. (B) Fungal biomass 28 days after inoculation with the DON‐non‐producing F. graminearum Tri5 deletion line and wild‐type. Columns represent average amplification of the Fg 18S rRNA gene relative to wheat actin (± SE; n = 3).
DISCUSSION
Previous work has shown that production of DON plays an important part in fungal colonization of host tissues in both FHB and CR caused by the fungal pathogens Fg and Fp (Jansen et al., 2005; Langevin et al., 2004; Maier et al., 2006; Mudge et al., 2006). Interestingly, the work described here indicates that DON can also induce a range of classical plant defence responses in wheat, including the production of ROS that may be at least partially responsible for the induction of host defence gene transcripts, their protein products and PCD. This suggests that DON may assist necrotrophic growth of the pathogen by promoting host cell death, while also stimulating an antimicrobial defence response in the host. These contrasting effects of DON may influence the rate and extent of disease development during FHB and CR diseases of wheat.
Previous characterization of responses induced in Arabidopsis following exposure to a range of trichothecenes, including T‐2 toxin, HT‐2 toxin, diacetoxyscirpenol and DON, has shown that all of these toxins can cause cell death and the induction of defence gene transcripts (Masuda et al., 2007; Nishiuchi et al., 2006). In addition, it was demonstrated that protein synthesis, assessed by measuring the incorporation of [3H]‐leucine into proteins of an Arabidopsis cell suspension, was 50% inhibited by 1.5 mg/L DON. Although the accumulation of defence proteins was not studied in Arabidopsis, it was suggested that DON may be acting as a protein synthesis inhibitor at concentrations below the threshold required to activate Arabidopsis defence responses (Nishiuchi et al., 2006). Consistent with these studies, our work showed that, in wheat, a concentration‐dependent induction of defence gene transcripts occurred, with larger effects on defence gene induction, including defence protein accumulation, at higher concentrations (100 mg/L), which are known to inhibit protein synthesis in wheat (Miller and Ewen, 1997; Rocha et al., 2005). In our experiments, we applied DON droplets to the surface of wheat tissue and it would have diffused into surrounding tissue. Thus, defence gene transcript and protein induction may have occurred in plant cells prior to DON reaching levels that inhibit protein synthesis. In affected cells, it is also possible that total protein synthesis may be reduced while the expression of defence genes we assessed was preferentially induced. However, this possibility appears to be unlikely in this situation as there was no reduction in Rubisco levels in DON‐treated leaves. It is not clear from current studies if it is specifically DON that is detected by host cells leading to the induction of defence transcripts or if non‐specific cellular stress caused by this toxin leads to the release of endogenous signals that activate defence responses in surrounding cells.
The most highly transcriptionally induced genes following DON treatment were the germin‐like and the peroxidase genes, both of which are related to reactive oxygen metabolism (Liu et al., 2005; Zimmermann et al., 2006). These functional associations are consistent with the production of H2O2 that was also observed in wheat cells following DON infiltration. Application of the antioxidant ascorbate, together with DON, significantly reduced the level of transcriptional activation of the peroxidase gene by DON, as well as that of several other defence genes. The effects of ascorbic acid on the activity of DON are unknown, but it is likely that reactive oxygen production in response to DON is at least partially responsible for the observed induction of host defence genes.
We have demonstrated here that DON causes cell death in wheat and that this was associated with genomic DNA laddering, a hallmark of PCD in plants and other eukaryotes (Ryerson and Heath, 1996; Tada et al., 2001). PCD is known to be an active process that requires de novo protein synthesis (Tada et al., 2001), and treatment with DON combined with cycloheximide, a eukaryotic protein synthesis inhibitor, prevented cell death 24 h after treatment. The mechanisms involved in this protective effect of cycloheximide are unknown, although it is possible that synthesis of a specific protein associated with DON‐inducible cell death is inhibited by cycloheximide. If this is the case, it suggests that the cell death observed here is actively undertaken by host cells and is likely to be PCD rather than necrosis.
H2O2 is known to induce PCD in plant cells, (Houot et al., 2001; Yoda et al., 2006) and as expected, we observed that co‐infiltration of DON and ascorbic acid reduced genomic DNA laddering and cell death, further suggesting that DON elicitation of ROS may be an important signalling event that stimulates PCD. Necrotrophic fungal pathogens often produce toxins to induce PCD during infection (Navarre and Wolpert, 1999; Tada et al., 2001). The toxin fumonisin, produced by pathogens such as F. verticillioides and F. proliferatum (Munkvold et al., 1999), has been demonstrated to induce cell death by depletion of extracellular ATP (Chivasa et al., 2005). Stimulation of cell death by mycotoxins would release nutrients, facilitating necrotrophic fungal growth as well as spread throughout the host. This functional characteristic of DON is consistent with the reduced ability of DON‐non‐producing mutant Fusarium strains to infect spikelets surrounding the point of infection during FHB (Jansen et al., 2005).
Host cell death and H2O2 production all appeared to require the application of relatively high concentrations of DON. Measurements of DON in inoculated crown and head tissues of wheat following inoculation with F. graminearum have shown that DON can accumulate to physiological levels on a fresh weight basis in excess of 100 µg/g (Mudge et al., 2006). However, these high concentrations of DON in infected tissues occur at relatively late stages after stem inoculation (14–28 days) and it is therefore likely that host responses to DON such as PCD are associated with the later stages of CR disease when visible lesions develop. We were able to investigate directly whether DON has a role in inducing wheat defence responses during CR disease development using a Tri5 deletion mutant of Fg. Defence gene induction was not significantly different between the two strains within the first 14 days post‐inoculation and it was only at 28 days post‐inoculation that induction of several defence genes was lower in tissue inoculated with the Fg ΔTri5 compared with wild type. This is consistent with previous observations where infection on wheat stems by the wild‐type Fg resulted in DON levels of approximately 300 µg/g fresh weight at 28 days post‐inoculation (Mudge et al., 2006). According to the results presented herein, these levels should be capable of inducing plant defence genes. However, it was also at this time point that significantly less fungal biomass was observed for the Fg ΔTri5 strain compared with the wild‐type, and so it is unclear if reduced fungus or a lack of DON is influencing defence gene expression at this later stage of infection. Recently, Ansari et al. (2007) also reported that a small suite of genes in wheat heads were inducible by DON, but there was no difference in the expression of these genes in wheat heads during the early stages post‐inoculation with a DON‐non‐producing mutant of Fg and a wild‐type strain (Ansari et al., 2007). So even though we saw several parallels between wheat responses induced by DON and those induced following inoculation with DON‐producing Fp and Fg, it is likely that these fungal pathogens may produce many other elicitors of host responses, and that DON is not likely to be a primary determinant of the host defence responses during early stages of fungal infection.
During later stages of disease, DON production may elicit defences such as the accumulation of the chitinase and glucanase proteins studied here. It may be that the effectiveness of these defence proteins is one of the factors determining fungal colonization rates and therefore quantitative resistance responses. A recent study has indeed suggested that transgenic wheat plants expressing PR2 (β‐1–3 glucanase) showed reduced FHB development (Mackintosh et al., 2007). Our experiments suggest that the level of production of DON affects wheat cellular responses in a way that could either promote necrotrophic fungal growth by initiating PCD or reduce fungal growth by triggering defence gene expression and accumulation of antimicrobial proteins.
EXPERIMENTAL PROCEDURES
F. pseudograminearum isolate, plant material and inoculation procedure
Spore suspensions of the aggressive Fp isolate CS3069 from the CSIRO collection were cultured as previously described (Desmond et al., 2005). The commercial variety of hexaploid wheat (Triticum aestivum) cv. Kennedy was grown in glasshouse conditions as previously described (Desmond et al., 2005). Inoculations were performed using a detached leaf assay where leaves of 2‐week‐old plants were cut into segments ~4 cm long and placed on moist tissue in a Petri dish and then 10‐µL drops of spore solution were evenly spaced along the leaves ~0.5 cm apart. Samples were collected ~7 days later for DAB and trypan blue staining.
Infiltration of wheat tissue for DAB and trypan blue staining
Wheat leaf segments from 2‐week‐old plants were infiltrated with the following solutions: (1) 100 mg/L DON, (2) 200 mg/L DON, (3) 100 mg/L DON and 7 g/L ascorbic acid, (4) 100 mg/L DON and 4 mg/L cycloheximide, and (5) distilled water. Infiltration was performed by covering tissue with treatment solutions and applying a vacuum for 15–20 min. Stem segments were then left in solution for ~10 min before being removed and placed on moist filter paper in a Petri dish. After infiltration, samples were collected 6 and 24 h later and stained using DAB to reveal H2O2 production and trypan blue to reveal cell death. This experiment was performed twice, with similar results produced on both occasions. Images presented are representative of the results.
DAB and trypan blue staining of inoculated and infiltrated tissue
DAB staining to reveal H2O2 was carried out using the DAB Liquid Substrate System (Sigma, St Louis, MO) according to the manufacturer's directions. Tissue was de‐stained by boiling tissue in 90% EtOH for 1 min and viewed using a light microscope. Trypan blue staining was performed to reveal dead plant cells using the procedure described by Belenghi et al. (2003) with a further dilution of the stock solution described with two volumes of 96% ethanol. Tissue was immersed and boiled in the trypan blue solution for 1 min and left to stain overnight before de‐staining with chloral hydrate and viewing using a Leica MZ16FA stereo light microscope.
Infiltration of wheat tissue for DNA cleavage analysis
Wheat stem segments ~2 cm long, collected from 2‐week‐old Kennedy plants, were infiltrated using the same procedure described above with treatments of 100 mg/L DON and a combination of 100 mg/L DON and 7 mg/L ascorbic acid. DNA degradation was assessed by extracting genomic DNA 24 h after infiltration as previously described (Kazan et al., 1993), followed by electrophoresis through a 1% agarose gel made with TAE buffer and stained with ethidium bromide. Digital analysis of the gel images was performed using ImageJ software (http://rsb.info.nih.gov/ij/). First, the image was converted to 8‐bit binary and the threshold was set from 0 to 160. Total pixels was estimated for each lane using the Analyse Particles function with default settings and this was compared with the number of pixels in each lane that were below the 5000‐bp band from the 1‐kb + DNA ladder (Invitrogen, Carlsbad, CA, USA). This method of treatment was found to provide the most consistent results, most likely because infiltration allows even treatment of all cells used for analysis. DNA laddering was observed in samples prepared on three separate occasions, and the images presented are representative of the results observed.
Drop treatment with DON and ascorbic acid for gene expression analysis
Deoxynivalenol (Sigma) was initially dissolved to 10 g/L in 100% methanol and then further diluted with water to final concentrations of 100, 10 and 1 mg/L. For treatments of DON combined with an antioxidant, a solution of 100 mg/L DON plus 7 g/L ascorbic acid (pH ~7) was used. All treatment solutions also contained 0.05% Tween 20. Wheat plants were treated by placing a 10‐µL drop onto the stem base using the same method previously described for Fp inoculation (Desmond et al., 2005).
Gene expression analysis by reverse transcriptase quantitative PCR
Tissue samples were collected for three independent biological replicates 1 day after treatment with DON or DON combined with ascorbic acid, followed by RNA isolation and cDNA synthesis as previously described (Desmond et al., 2005). Real‐time quantitative PCR conditions, including primer details and analysis of results, have been previously described (Desmond et al., 2005).
Defence protein expression analysis by protein extraction and Western blot
Total protein was extracted from ~200 mg of frozen wheat stem base tissue from 2‐week‐old seedlings as described (Jayaraj et al., 2004), with the following alterations: one tablet of complete protease inhibitor (Roche, Mannheim, Germany) was added freshly to 50 mL of extraction buffer instead of PMSF. After tissue homogenization in the extraction buffer, samples were left on ice for 15 min. Protein concentration was determined using the bicinchoninic acid microtitre plate assay kit (Pierce, Rockford, IL) according to the manufacturer's instructions. Proteins (75 µg) were separated using a Nu‐Page 4–12% bis‐tris pre‐cast gel (Invitrogen) electrophoresed in a Novex Mini‐Cell (Invitrogen) and transferred to a nitrocellulose membrane using an XCell II Blot module (Invitrogen) followed by protein detection, all performed according to the manufacturer's instructions. For size determination, the SeeBlue Plus2 (Invitrogen) pre‐stained standard was included in all gels. Primary antibodies were kindly donated by S. Muthukrishnan, Kansas State University. Secondary antibodies were detected by incubating blots in one‐step NBT/BCIP (Pierce) for 5–10 min before stopping the reaction with water. To quantify protein levels, ImageJ was again used to count pixels for each band and levels relative to Rubisco were determined. ImageJ settings included a threshold of 10–116 and the Analyse Particle function was run with default settings.
Generation of Fg Tri5 deletion lines
Fusarium graminearum isolate CS3005 (Akinsanmi et al., 2006) was used as a parent to construct the Fg ΔTri5 strain. The 2.8 kb of the Tri5 genomic locus was amplified (AATCTATCAGTGCTTAAATGCAGTTCC and TACGTAGGCCGCGCAGAGGTCAGTA), cloned into pCR8/GW‐TOPO (Invitrogen) and the HindIII–NcoI fragment of the Tri5 coding sequence replaced with the hygromycin phosphotransferase cassette from pUChph, leaving two flanks of 1.2 kb. The construct was introduced into CS3005 protoplasts generated from approximately 1 × 107 conidia germinated overnight in 200 mL of TB3 media (0.3% yeast extract, 0.3% Casamino acids, 20% sucrose) at 20 °C with shaking at 150 r.p.m. Germlings were collected on miracloth and washed with 1 m sorbitol before being resuspended in 20 mL of 1 m sorbitol containing 5 U chitinase (Sigma‐Aldrich, St Louis, MO), 500 mg Driselase (Sigma‐Aldrich) and 200 mg lysing enzymes (Sigma‐Aldrich), and incubated for 1–2 h at 28 °C to release protoplasts. Protoplasts were collected by centrifugation at 2600g, 4 °C, washed three times with ice‐cold STC (20% sucrose, 50 mm Tris/HCl, pH 8, 50 mm CaCl2). To a 200‐µL aliquot of STC containing 2 × 107 protoplasts, 10 µg circular plasmid DNA was added, incubated for 20 min at room temperature, then 1 mL of 40% PEG4000, 10 mm Tris HCl, pH 8, and 50 mm CaCl2 was added, incubated for 20 min at room temperature and then 5 mL of TB3 media was added and the tubes incubated overnight at room temperature with gentle agitation. Three millilitres of the transformation mixture was embedded in 30 mL regeneration media (0.2% yeast extract, 0.2% Casamino acids, 0.55% low‐melt agarose, 27% sucrose) and set in 14‐cm Petri dishes, incubated overnight and then overlaid with 30 mL of regeneration media containing hygromycin (Roche, Penzberg, Germany) at 400 mg/L. After 5–7 days of growth at room temperature in the dark, transformants were transferred to individual plates containing hygromycin (200 mg/L), allowed to grow, single spored then checked for insertion of the construct by PCR (data not shown).
Inoculation of wheat with Fg
Wheat (cv. Kennedy) was grown as described above. Seedlings were inoculated with the wild‐type F. graminearum isolate CS3005 and the Fg ΔTri5 strain in parallel at the stem base as described (Mitter et al., 2006).
Estimation of fungal biomass in wheat stems inoculated with Fg ΔTri5 and parent Fg
Samples were collected 1, 2, 14 and 28 days post‐inoculation by collecting stem from the crown to the first leaf. Each time point consisted of three biological replicates taken in parallel and each replicate was a pool of eight stem bases. DNA was extracted from the samples using a QIAGEN Dneasy® Plant Mini Kit according to the manufacturer's instructions. Real‐time quantitative PCR was performed as described above using the Fg 18S rRNA gene and wheat actin primers (Mudge et al., 2006). Internal PCR amplification efficiencies were calculated by using the program LinRegPCR 7.5 (Ramakers et al., 2003) and absolute DNA amplification was calculated by the average efficiencies raised to the negative crossing threshold. Fg biomass was estimated as the absolute amplification of fungal DNA relative to the absolute amplification of wheat DNA.
Analysis of gene expression in Fg inoculated tissue
RNA was extracted from the same samples used for estimating fungal biomass followed by cDNA synthesis as described above. RT‐qPCR was performed to assess defence gene expression using the primers, procedures and analysis described above.
Supporting information
Fig. S1 H2O2 production, detected using DAB stain, in wheat leaf tissue 6 h after infiltration with (A) mock, (B) 100 mg/L DON, (C) 100 mg/L DON and 7 mg/L ascorbic acid, and (D) 100 mg/L DON and 4 mg/L cycloheximide. Cell death, detected using trypan blue stain, in wheat leaf tissue 24 h after infiltration with (E) mock, (F) 100 mg/L DON, (G) 100 mg/L DON and 7 mg/L ascorbic acid, and (H) 100 mg/L DON and 4 mg/L cycloheximide.
Fig. S2 Pathogen‐induced cell death stained using trypan blue 7 days after inoculation. Infection follows vascular tissue and is most widespread in stomatal guard cells.
Please note: Blackwell Publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
ACKNOWLEDGEMENTS
We would like to thank Dr Bruno Dombrecht and Anca Rusu for assistance with Western blot procedures, Dr S. Muthukrishnan for supplying protein antibodies, Dr Anne Rae for assistance with staining procedures, and the Grains Research and Development Corporation for supplying a postgraduate scholarship to O.J.D. ALM is funded by National Health and Medical Research Council of Australia, Project Grant 252750.
REFERENCES
- Akinsanmi, O.A. , Backhouse, D. , Simpfendorfer, S. and Chakraborty, S. (2006) Genetic diversity of Australian Fusarium graminearum and F. pseudograminearum . Plant Pathol. 55, 494–504. [Google Scholar]
- Akinsanmi, O.A. , Mitter, V. , Simpfendorfer, S. , Backhouse, D. and Chakraborty, S. (2004) Identity and pathogenicity of Fusarium spp. isolated from wheat fields in Queensland and northern New South Wales. Aust. J. Agr. Res. 55, 97–107. [Google Scholar]
- Ansari, K.I. , Walter, S. , Brennan, J.M. , Lemmens, M. , Kessans, S. , McGahern, A. , Egan, D. and Doohan, F.M. (2007) Retrotransposon and gene activation in wheat in response to mycotoxigenic and non‐mycotoxigenic‐associated Fusarium stress. Theor. Appl. Genet. 114, 927–937. [DOI] [PubMed] [Google Scholar]
- Bai, G.H. , Desjardins, A.E. and Plattner, R.D. (2002) Deoxynivalenol‐nonproducing Fusarium graminearum causes initial infection, but does not cause disease spread in wheat spikes. Mycopathologia, 153, 91–98. [DOI] [PubMed] [Google Scholar]
- Belenghi, B. , Acconcia, F. , Trovato, M. , Perazzolli, M. , Bocedi, A. , Polticelli, F. , Ascenzi, P. and Delledonne, M. (2003) AtCYS1, a cystatin from Arabidopsis thaliana, suppresses hypersensitive cell death. Eur. J. Biochem. 270, 2593–2604. [DOI] [PubMed] [Google Scholar]
- Blaney, B.J. and Dodman, R.L. (2002) Production of zearalenone, deoxynivalenol, nivalenol, and acetylated derivatives by Australian isolates of Fusarium graminearum and F‐pseudograminearum in relation to source and culturing conditions. Aust. J. Agr. Res. 53, 1317–1326. [Google Scholar]
- Boddu, J. , Cho, S. and Muehlbauer, G.J. (2007) Transcriptome analysis of trichothecene‐induced gene expression in barley. Mol. Plant–Microbe Interact. 20, 1364–1375. [DOI] [PubMed] [Google Scholar]
- Chivasa, S. , Ndimba, B.K. , Simon, W.J. , Lindsey, K. and Slabas, A.R. (2005) Extracellular ATP functions as an endogenous external metabolite regulating plant cell viability. Plant Cell, 17, 3019–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins, A. , Plattner, R. , Nelsen, T. and Leslie, J. (1995) Genetic analysis of fumonisin production and virulence of Gibberella fujikuroi mating population A (Fusarium moniliforme) on maize (Zea mays) seedlings. Appl. Environ. Microbiol. 61, 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond, O.J. , Edgar, C.I. , Manners, J.M. , Maclean, D.J. , Schenk, P.M. and Kazan, K. (2005) Methyl jasmonate induced gene expression in wheat delays symptom development by the crown rot pathogen Fusarium pseudograminearum . Physiol. Mol. Plant Pathol. 67, 171–179. [Google Scholar]
- Egan, M.J. , Wang, Z.Y. , Jones, M.A. , Smirnoff, N. and Talbot, N.J. (2007) Generation of reactive oxygen species by fungal NADPH oxidases is required for rice blast disease. Proc. Natl Acad. Sci. USA, 104, 11772–11777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami, R.S. and Kistler, H.C. (2005) Pathogenicity and in planta mycotoxin accumulation among members of the Fusarium graminearum species complex on wheat and rice. Phytopathology, 95, 1397–1404. [DOI] [PubMed] [Google Scholar]
- Hohn, T.M. and Desjardins, A.E. (1992) Isolation and gene disruption of the Tox5 gene encoding trichodiene synthase in Gibberella pulicaris . Mol. Plant–Microbe Interact. 5, 249–256. [DOI] [PubMed] [Google Scholar]
- Houot, V. , Etienne, P. , Petitot, A.‐S. , Barbier, S. , Blein, J.‐P. and Suty, L. (2001) Hydrogen peroxide induces programmed cell death features in cultured tobacco BY‐2 cells, in a dose‐dependent manner. J. Exp. Bot. 52, 1721–1730. [PubMed] [Google Scholar]
- Jansen, C. , Von Wettstein, D. , Schafer, W. , Kogel, K.‐H. , Felk, A. and Maier, F.J. (2005) Infection patterns in barley and wheat spikes inoculated with wild‐type and trichodiene synthase gene disrupted Fusarium graminearum . Proc. Natl Acad. Sci. USA, 102, 16892–16897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraj, J. , Muthukrishnan, S. , Liang, G.H. and Velazhahan, R. (2004) Jasmonic acid and salicylic acid induce accumulation of beta‐1,3‐glucanase and thaumatin‐like proteins in wheat and enhance resistance against Stagonospora nodorum . Biol. Plantarum, 48, 425–430. [Google Scholar]
- Kazan, K. , Manners, J.M. and Cameron, D.F. (1993) Genetic‐variation in agronomically important species of Stylosanthes determined using random amplified polymorphic DNA markers. Theor. Appl. Genet. 85, 882–888. [DOI] [PubMed] [Google Scholar]
- Langevin, F. , Eudes, F. and Comeau, A. (2004) Effect of trichothecenes produced by Fusarium graminearum during Fusarium head blight development in six cereal species. Eur. J. Plant Pathol. 110, 735–746. [Google Scholar]
- Larsen, J.C. , Hunt, J. , Perrin, I. and Ruckenbauer, P. (2004) Workshop on trichothecenes with a focus on DON: summary report. Toxicol. Lett. 153, 1–22. [DOI] [PubMed] [Google Scholar]
- Levine, A. , Tenhaken, R. , Dixon, R. and Lamb, C. (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell, 79, 583–593. [DOI] [PubMed] [Google Scholar]
- Liu, G. , Sheng, X. , Greenshields, D. L. , Ogieglo, A. , Kaminskyj, S. , Selvaraj, G. and Wei, Y. (2005) Profiling of wheat class III peroxidase genes derived from powdery mildew‐attacked epidermis reveals distinct sequence‐associated expression patterns. Mol. Plant–Microbe Interact. 18, 730–741. [DOI] [PubMed] [Google Scholar]
- Mackintosh, C. , Lewis, J. , Radmer, L.E. , Shin, S. , Heinen, S.J. , Smith, L.A. , Wyckoff, M.N. , Dill‐Macky, R. , Evans, C.K. , Kravchenko, S. , Baldridge, G.D. , Zeyen, R.J. and Muehlbauer, G.J. (2007) Overexpression of defense response genes in transgenic wheat enhances resistance to Fusarium head blight. Plant Cell Rep. 26, 479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier, F.J. , Miedaner, T. , Hadeler, B. , Felk, A. , Salomon, S. , Lemmens, M. , Kassner, H. and Schafer, W. (2006) Involvement of trichothecenes in fusarioses of wheat, barley and maize evaluated by gene disruption of the trichodiene synthase (Tri5) gene in three field isolates of different chemotype and virulence. Mol. Plant Pathol. 7, 449–461. [DOI] [PubMed] [Google Scholar]
- Masuda, D. , Ishida, M. , Yamaguchi, K. , Yamaguchi, I. , Kimura, M. and Nishiuchi, T. (2007) Phytotoxic effects of trichothecenes on the growth and morphology of Arabidopsis thaliana . J. Exp. Bot. 58, 1617–1626. [DOI] [PubMed] [Google Scholar]
- Miller, J.D. and Ewen, M.A. (1997) Toxic effects of deoxynivalenol on ribosomes and tissues of the spring wheat cultivars Frontana and Casavant. Nat. Toxins. 5, 234–237. [DOI] [PubMed] [Google Scholar]
- Mitter, V. , Zhang, M.C. , Liu, C.J. , Ghosh, R. and Chakraborty, S. (2006) A high throughput glasshouse bioassay to detect crown rot resistance in wheat germplasm. Plant Pathol. 55, 433–441. [Google Scholar]
- Mudge, A.M. , Dill‐Macky, R. , Dong, Y. , Gardiner, D.M. , White, R.G. and Manners, J.M. (2006) A role for the mycotoxin deoxynivalenol in stem colonisation during crown rot disease of wheat caused by Fusarium graminearum and F. pseudograminearum. Physiol. Mol. Plant Pathol. 69, 73–85. [Google Scholar]
- Munkvold, G.P. , Hellmich, R.L. and Rice, L.G. (1999) Comparison of fumonisin concentrations in kernels of transgenic Bt maize hybrids and nontransgenic hybrids. Plant Dis. 83, 130–138. [DOI] [PubMed] [Google Scholar]
- Navarre, D.A. and Wolpert, T.J. (1999) Victorin induction of an apoptotic/senescence‐like response in oats. Plant Cell, 11, 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiuchi, T. , Masuda, D. , Nakashita, H. , Ichimura, K. , Shinozaki, K. , Yoshida, S. , et al (2006) Fusarium phytotoxin trichothecenes have an elicitor‐like activity in Arabidopsis thaliana, but the activity differed significantly among their molecular species. Mol. Plant–Microbe Interact. 19, 512–520. [DOI] [PubMed] [Google Scholar]
- Pestka, J.J. , Zhou, H.R. , Moon, Y. and Chung, Y.J. (2004) Cellular and molecular mechanisms for immune modulation by deoxynivalenol and other trichothecenes: unravelling a paradox. Toxicol. Lett. 153, 61–73. [DOI] [PubMed] [Google Scholar]
- Ramakers, C. , Ruijter, J.M. , Deprez, R.H.L. and Moorman, A.F.M. (2003) Assumption‐free analysis of quantitative real‐time polymerase chain reaction (PCR) data. Neurosci. Lett. 339, 62–66. [DOI] [PubMed] [Google Scholar]
- Rocha, O. , Ansari, K. and Doohan, F.M. (2005) Effects of trichothecene mycotoxins on eukaryotic cells: a review. Food Addit. Contam. 22, 369–378. [DOI] [PubMed] [Google Scholar]
- Ryerson, D.E. and Heath, M.C. (1996) Cleavage of nuclear DNA into oligonucleosomal fragments during cell death induced by fungal infection or by abiotic treatments. Plant Cell, 8, 393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada, Y. , Hata, S. , Takata, Y. , Nakayashiki, H. , Tosa, Y. and Mayama, S. (2001) Induction and signaling of an apoptotic response typified by DNA laddering in the defence response of oats to infection and elicitors. Mol. Plant–Microbe Interact. 14, 477–486. [DOI] [PubMed] [Google Scholar]
- Wilde, F. and Miedaner, T. (2006) Selection for Fusarium head blight resistance in early generations reduces the deoxynivalenol (DON) content in grain of winter and spring wheat. Plant Breeding, 125, 96–98. [Google Scholar]
- Yoda, H. , Hiroi, Y. and Sano, H. (2006) Polyamine oxidase is one of the key elements for oxidative burst to induce programmed cell death in tobacco cultured cells. Plant Physiol. 142, 193–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann, G. , Baumlein, H. , Mock, H.‐P. , Himmelbach, A. and Schweizer, P. (2006) The multigene family encoding germin‐like proteins of barley. Regulation and function in basal host resistance. Plant Physiol. 142, 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 H2O2 production, detected using DAB stain, in wheat leaf tissue 6 h after infiltration with (A) mock, (B) 100 mg/L DON, (C) 100 mg/L DON and 7 mg/L ascorbic acid, and (D) 100 mg/L DON and 4 mg/L cycloheximide. Cell death, detected using trypan blue stain, in wheat leaf tissue 24 h after infiltration with (E) mock, (F) 100 mg/L DON, (G) 100 mg/L DON and 7 mg/L ascorbic acid, and (H) 100 mg/L DON and 4 mg/L cycloheximide.
Fig. S2 Pathogen‐induced cell death stained using trypan blue 7 days after inoculation. Infection follows vascular tissue and is most widespread in stomatal guard cells.
Please note: Blackwell Publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item


