SUMMARY
Flagellin is a component of bacterial flagella and acts as a proteinaceous elicitor of defence responses in organisms. Flagellin from a phytopathogenic bacterium, Acidovorax avenae strain N1141, induces immune responses in suspension‐cultured rice cells. To analyse the function of flagellin in rice, we fused the N1141 flagellin gene to the cauliflower mosaic virus 35S promoter and introduced it into rice. Many of the resulting transgenic rice plants accumulated flagellin at various levels. The transgenic rice developed pale spots in the leaves. The expression of a defence‐related gene for phenylalanine ammonia‐lyase was induced in the transgenic plants, and H2O2 production and cell death were observed in some plants with high levels of gene expression, suggesting that the flagellin triggers immune responses in the transgenic rice. Transgenic plants inoculated with Magnaporthe grisea, the causal agent of rice blast, showed enhanced resistance to blast, suggesting that the flagellin production confers disease resistance in the transgenic rice.
INTRODUCTION
Flagellin, a component of bacterial flagella, has been identified as a pathogen‐associated molecular pattern that is recognized by the innate immune system in plants, insects and mammals (Felix et al., 1999; Hayashi et al., 2001; Samakovlis et al., 1992). In plants, a 32‐kDa flagellin has been purified from Pseudomonas syringae pv. tabaci as an elicitor responsible for triggering defence responses in tomato cells (Felix et al., 1999). Furthermore, a 22‐amino‐acid peptide (flg22) that corresponds to the most conserved N‐terminal domain of eubacterial flagellin induces some defence responses in various dicotyledonous plants, and thus it appears to behave as a general elicitor (Felix et al., 1999).
Acidovorax avenae is a Gram‐negative bacterium that causes a seedling disease (Kadota et al., 1991). A rice‐incompatible strain of A. avenae (N1141, isolated from finger millet) caused rapid cell death in rice (Che et al., 1999). Che et al. (2000) reported that a 50‐kDa flagellin from strain N1141 caused hypersensitive cell death in rice, whereas flagellin from a compatible strain did not. The N1141 flagellin induces H2O2 generation and the expression of some defence‐related genes prior to cell death in cultured rice cells (Tanaka et al., 2003). By contrast, neither flg22 nor the N‐terminal 22‐amino‐acid peptide of N1141 flagellin that corresponds to flg22 induces any defence response in rice (Felix et al., 1999; Takai et al., 2007), suggesting that the recognition mechanism for flagellin in rice is not the same as that observed in dicotyledonous plants.
Recent studies have elucidated the importance of flagellin perception and the subsequent signal‐transduction mechanisms in plant disease resistance (Asai et al., 2002; Zipfel et al., 2004). However, to date, the development of transgenic plants that produce flagellin has not been reported. In the present study, we developed transgenic rice plants that produce flagellin and examined whether immune responses and disease resistance are induced.
RESULTS
The flagellin gene N1141‐flaA from A. avenae strain N1141, which is incompatible with rice (Che et al., 2000), was fused to the cauliflower mosaic virus 35S promoter, a promoter for constitutive expression in plants. The construct was then introduced into ‘Koshihikari’ rice by means of Agrobacterium‐mediated transformation. We obtained 50 independent primary transgenic plants (the T0 generation) and analysed them to test for the production of the flagellin protein. The level of flagellin production varied among the plants (Fig. 1A). The flagellin was detected in soluble fractions, but not in intercellular fluid fractions (Fig. 1B). Many transgenic plants that produced the flagellin showed small, chlorotic spots on their leaves (Fig. 1C).
Figure 1.
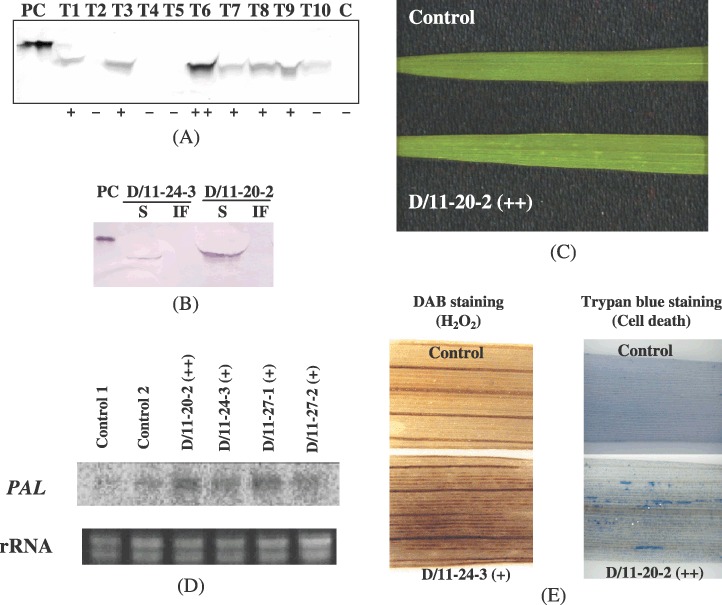
Analysis of flagellin production and the immune response. (A) Detection of N1141 flagellin production in transgenic rice. Thirty micrograms of soluble proteins from the leaves of the primary transgenic plants (T) and ‘Koshihikari’ (C) were subjected to immunoblot analysis . As a reference (positive control, PC), 30 ng of histidine‐tagged N1141 flagellin was used. Results: –, not detected; +, 0.015–0.05% of total soluble leaf proteins; ++, more than 0.05% of total soluble leaf proteins. (B) Localization of flagellin production. S, total soluble leaf protein fractions; IF, intercellular fluid fractions. (C) Chlorotic spots observed on the leaves of a transgenic plant that accumulated flagellin. (D) Northern blot analysis of the expression of the PAL gene. Ten micrograms of total RNA from leaves of primary transgenic plants was used for RNA blotting. (E) Detection of H2O2 (left) and cell death (right) in transgenic rice. H2O2 was detected as brown spots among the main veins of a leaf of the transgenic plant. Cell death was detected as blue spots along the veins of the leaves of transgenic plants. In panels C, D and E, a transgenic plant harbouring pSB24 was used as a control and parentheses indicate the level of flagellin production.
To elucidate whether immune responses were induced in the transgenic plants, we investigated the expression of the rice PAL (phenylalanine ammonia‐lyase) gene, a defence‐related marker gene. Expression of PAL was induced in the transgenic lines in the absence of pathogens, but not in control transgenic plants (Fig. 1D). The level of PAL gene expression in the transgenic plants was three to five times that in the control plants. Further, hydrogen peroxide (H2O2) production (Fig. 1E, left panel) and cell death (Fig. 1E, right panel) were detected in the transgenic plants, but not in the control plants. These results suggest that immune responses were induced in the transgenic rice plants.
To analyse the disease resistance of transgenic rice plants, we performed a disease assay using Magnaporthe grisea, the causal agent of rice blast, on the T1 generation. As controls, we used ‘Koshihikari’ and ‘Ginga’, whose field resistance to the blast fungus were low and high, respectively. Disease symptoms were evaluated according to the disease index (DI) chart in Fig. 2A. The progress of disease symptoms in transgenic plants was clearly retarded compared with that in ‘Koshihikari’ (Fig. 2B). DI frequency distributions in several lines 6 days following infection are shown in Fig. 2C. In D/11‐31‐1, a line that incorporated the flagellin gene but that did not produce flagellin, the frequency distribution of symptoms was similar to that of ‘Koshihikari’. The DI distributions of three of the four flagellin‐producing transgenic lines (D/11‐20‐2, D/11‐20‐3, D/11‐24‐3) and that of ‘Ginga’ were significantly different from that of ‘Koshihikari’ (P < 0.01), indicating that the transgenic lines that produce the flagellin have an enhanced resistance to rice blast. To evaluate the relationship between flagellin production and disease resistance, we analysed the DI distributions of the plants that produce flagellin and those that do not. We performed immunoblotting on 59 transgenic plants used in the disease assay. The median DI of the plants that produced flagellin (n = 40) was 4, the same as that of ‘Ginga’, whereas the median DI of the plants that did not produce flagellin (n = 19) was 6, the same as ‘Koshihikari’ (Fig. 2C). The DI distribution of the plants that produced flagellin was significantly different from that of the plants that did not and from that of ‘Koshihikari’ (P < 0.01). The result shows that the observed resistance in the transgenic rice was caused by the production of flagellin.
Figure 2.
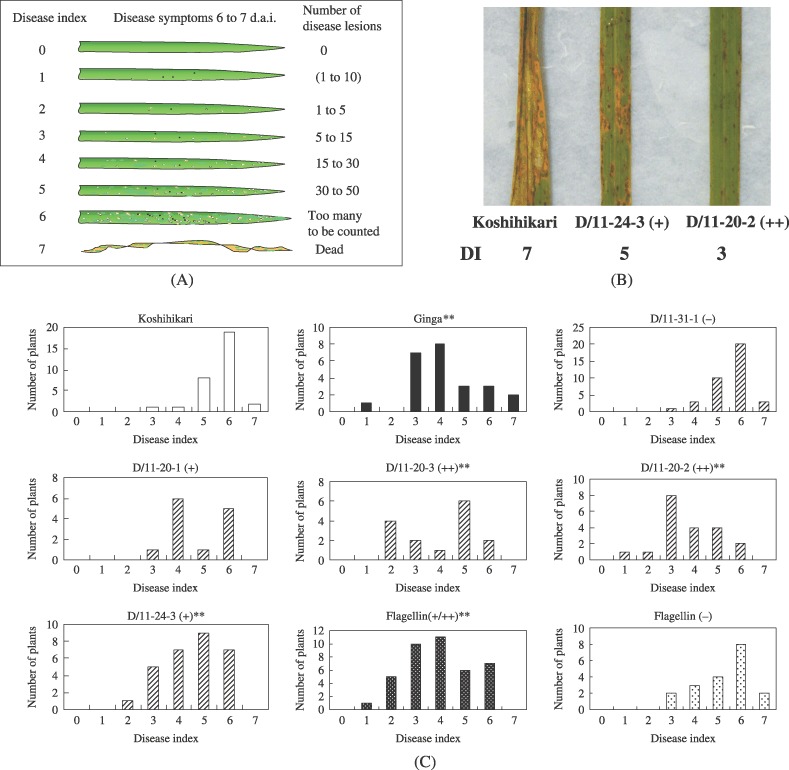
Analysis of resistance to M. grisea in transgenic rice. (A) An illustration of the disease indices used in this experiment. Disease indices were judged based on the number of disease lesions on the leaves. Disease index (1 to 10) in parentheses indicates small dark brownish lesions that are difficult to be judged as representing disease lesions. (B) Photographs of typical disease symptoms on the leaves of the transgenic rice 7 days following infection. DI, disease index. (C) The results of the inoculation test with M. grisea. The frequency distributions of the resulting DI values in various lines are shown. Asterisks (**) represent distributions of DI that differ significantly from that of ‘Koshihikari’ (Steel–Dwass test, P < 0.01). The distributions of DI values for plants that produced flagellin [Flagellin (+/++)] and those that did not [Flagellin (–)] are also shown. In B and C, parentheses indicate the level of flagellin production.
DISCUSSION
Here we have produced the first transgenic plants that express a flagellin gene. Furthermore, we demonstrated that the transgenic rice showed immune responses and enhanced disease resistance. The expression of a defence‐related gene (PAL), hydrogen peroxide production and cell death were detected in the transgenic rice plants that produced flagellin in the absence of pathogens. These responses have also been observed in suspension‐cultured rice cells treated with the purified flagellin from A. avenae N1141 (Che et al., 2000; Tanaka et al., 2003). Hydrogen peroxide is known to be a transducer involved in plant defence reactions (Yang et al., 1997). In addition, PAL catalyses the first step in the phenylpropanoid pathway, which is involved in the biosynthesis of lignin monomers and some types of small antimicrobial molecules known as phytoalexins (Dixon and Paiva, 1995).
Exogenously applied N1141 flagellin induces programmed cell death and the expression of some defence‐related genes in suspension‐cultured rice cells (Che et al., 2000; Tanaka et al., 2003). Therefore, flagellin seems to be perceived within the cell wall or the plasma membrane of rice. In Aradopsis thaliana, a receptor of flg22 has been identified as FLS2 (Gómez‐Gómez and Boller, 2000), a membrane protein with an extracellular leucine‐rich reapeat (LRR) domain and an intracellular kinase domain, and flg22 is believed to bind to the LRR domain (Chinchilla et al., 2006; Dunning et al., 2007). In our transgenic rice, flagellin could be extracted from the soluble fraction but was not detected in the intercellular fluids, indicating that it does not accumulate extracellularly. Nevertheless, the immune response was activated in the absence of pathogens, suggesting that the N1141 flagellin was recognized intracellularly in the transgenic rice. An hrp‐deficient mutant of A. avenae strain 1141 did not induce a hypersensitive response, as was the case in flagellin‐deficient mutants, and the expression of most of the genes induced by flagellin perception was found to depend on the hrp‐secretion system according to microarray analysis (F. S. Che, unpublished data). This may suggest that the flagellin is transferred into the rice cells from a bacterium through a type III secretion system. Further analysis will be necessary to elucidate the mechanisms of flagellin perception in rice.
Recently, Zipfel et al. (2004) reported that treatment of plants with flg22 induces the expression of defence‐related genes and triggers resistance to pathogenic bacteria in Aradopsis thaliana. Herein, we have clearly demonstrated that transgenic rice producing flagellin showed immune responses and an enhanced resistance to rice blast. Our results suggest that the flagellin approach might provide one strategy for developing genetically engineered disease‐resistant plants. It will be interesting to investigate whether the transgenic rice is also resistant to other pathogens.
EXPERIMENTAL PROCEDURES
The flagellin expression cassette and rice transformation
A flagellin gene from A. avenae strain N1141 (N1141‐flaA; Che et al., 2000) was inserted into the binary vector pSB21 (Komari et al., 1996) to produce the plasmid containing an expression cassette CaMV 35S promoter::N1141‐flaA::nos terminator. The resultant plasmid was transferred to Agrobacterium tumefaciens strain LBA4404, as described (Komari et al., 1996). Rice (‘Koshihikari’) was transformed as described by Hiei et al. (1994). As a transformation control, transgenic rice harbouring pSB24 (CaMV 35S pro::I‐GUS::nos ter; Komari et al., 1996) was also produced. Rice plants were grown in a greenhouse at 28 °C (day) and 25 °C (night).
Immunoblot and northern blot analyses
Immunoblot analysis was performed according to the method of Takakura et al. (2004). Rabbit anti‐N1141 flagellin serum [Che et al. (2000), 1 : 1000 (v/v) dilution] was used as the primary antibody. The intercellular fluid was collected from leaves by the method of Nishizawa et al. (1999) using 50 mm potassium phosphate buffer (pH 6.0) with 500 mm NaCl. The flagellin produced in transgenic rice was quantified using a densitometer with the histidine‐tagged flagellin produced by Escherichia coli (F. S. Che, unpublished data) used as a calibration standard. Northern blot analysis was performed according to the method of Takakura et al. (2000). The blot was probed using [32P]‐dCTP‐labelled OsPAL (Tanaka et al., 2003) gene.
Detection of hydrogen peroxide and cell death
Hydrogen peroxide (H2O2) was detected using 3,3′‐diaminobenzidine (DAB) tetrahydrochloride (Sigma, St Louis, MO) as described by Fitzgerald et al. (2004). Cell death was detected using trypan blue (Sigma) as described by Ger et al. (2002).
Inoculation test with M. grisea
Seedlings with five leaves were used for the disease assay. Magnaporthe grisea (race 007) conidia adjusted to a concentration of 2 × 105 mL−1 in 0.02% Tween 20 were inoculated by spraying. The inoculated rice plants were incubated in a growth chamber (SLPH‐550‐RDS, NK System, Osaka, Japan) for 24 h at 25 °C and 100% humidity to enhance fungal infection. The rice plants were then transferred to a moist vinyl tunnel in a greenhouse and grown at 25 °C (day) for 16 h and at 22 °C (night) for 8 h. The number of progressive disease lesions on the most‐expanded leaf at the time of inoculation (the fifth leaf) was counted and used as a disease index based on an indicator chart (Fig. 2A). The data were analysed using the Steel–Dwass test based on the median values and range of disease indices for each rice line.
ACKNOWLEDGEMENTS
We are grateful to the Japan Agricultural Research Station of Zeneca K.K. for kindly providing samples of Magnaporthe grisea race 007, and Dr Maarten Stuiver of Syngenta‐MOGEN for helpful discussion. We also thank Ms Eriko Usami, Ms Michiko Ohki and Ms Ayaka Yamashita for technical assistance.
REFERENCES
- Asai, T. , Tena, G. , Plotnikova, J. , Willmann, M.R. , Chiu, W.L. , Gomez‐Gomez, L. , Boller, T. , Ausubel, F.M. and Sheen, J. (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature, 415, 977–983. [DOI] [PubMed] [Google Scholar]
- Che, F.S. , Iwano, M. , Tanaka, N. , Takayama, S. , Minami, E. , Shibuya, N. , Kadota, I. and Isogai, A. (1999) Biochemical and morphological features of rice cell death induced by Pseudomonas avenae . Plant Cell Physiol. 40, 1036–1045. [Google Scholar]
- Che, F.S. , Nakajima, Y. , Tanaka, N. , Iwano, M. , Yoshida, T. , Takayama, S. , Kadota, I. and Isogai, A. (2000) Flagellin from an incompatible strain of Pseudomonas avenae induces a resistance response in cultured rice cells. J. Biol. Chem. 275, 32347–32356. [DOI] [PubMed] [Google Scholar]
- Chinchilla, D. , Bauer, Z. , Regenass, M. , Boller, T. and Felix, G. (2006) The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception. Plant Cell, 18, 465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, R.A. and Paiva, N.L. (1995) Stress‐induced phenylpropanoid metabolism. Plant Cell, 7, 1085–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning, F.M. , Sun, W. , Jansen, K.L. , Helft, L. and Bent, A.F. (2007) Identification and mutational analysis of Arabidopsis FLS2 leucine‐rich repeat domain residues that contribute to flagellin perception. Plant Cell, 19, 3297–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix, G. , Duran, J.D. , Volko, S. and Boller, T. (1999) Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 18, 265–276. [DOI] [PubMed] [Google Scholar]
- Fitzgerald, H.A. , Chern, M.S. , Navarre, R. and Ronald, P.C. (2004) Overexpression of (At)NPR1 in rice leads to a BTH‐and environment‐induced lesion‐mimic/cell death phenotype. Mol. Plant–Microbe Interact. 17, 140–151. [DOI] [PubMed] [Google Scholar]
- Ger M.J., Chen, C.H. , Hwang, S.Y. , Huang, H.E. , Podile, A.R. , Dayakar, B.V. and Feng, T.Y. (2002) Constitutive expression of hrap gene in transgenic tobacco plant enhances resistance against virulent bacterial pathogens by induction of a hypersensitive response. Mol. Plant–Microbe Interact. 15, 764–773. [DOI] [PubMed] [Google Scholar]
- Gómez‐Gómez, L. and Boller, T. (2000) FLS2: an LRR receptor‐like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol. Cell, 5, 1003–1011. [DOI] [PubMed] [Google Scholar]
- Hayashi, F. , Smith, K.D. , Ozinsky, A. , Hawn, T.R. , Yi, E.C. , Goodlett, D.R. , Eng, J.K. , Akira, S. , Underhill, D.M. and Aderem, A. (2001) The innate immune response to bacterial flagellin is mediated by Toll‐like receptor 5. Nature, 410, 1099–1103. [DOI] [PubMed] [Google Scholar]
- Hiei, Y. , Ohta, S. , Komari, T. and Kumashiro, T. (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T‐DNA. Plant J. 6, 271–282. [DOI] [PubMed] [Google Scholar]
- Kadota, I. , Ohuchi, A. and Nishiyama, K. (1991) Serological properties and specificity of Pseudomonas avenae Manns 1909, the causal agent of bacterial brown stripe of rice. Ann. Phytopathol. Soc. Jpn, 57, 268–273. [Google Scholar]
- Komari, T. , Hiei, Y. , Saito, Y. , Murai, N. and Kumashiro, T. (1996) Vectors carrying two separate T‐DNAs for co‐transformation of higher plants mediated by Agrobacterium tumefaciens and segregation of transformants free from selection markers. Plant J. 10, 165–174. [DOI] [PubMed] [Google Scholar]
- Nishizawa, Y. , Nishio, Z. , Nakazono, K. , Soma, M. , Nakajima, E. , Ugaki, M. and Hibi, T. (1999) Enhanced resistance to blast (Magnaporthe grisea) in transgenic Japonica rice by constitutive expression of rice chitinase. Theor. Appl. Genet. 99, 383–390. [DOI] [PubMed] [Google Scholar]
- Samakovlis, C. , Asling, B. , Boman, H.G. , Gateff, E. and Hultmark, D. (1992) In vitro induction of cecropin genes—an immune response in a Drosophila blood cell line. Biochem. Biophys. Res. Commun. 188, 1169–1175. [DOI] [PubMed] [Google Scholar]
- Takai, R. , Kaneda, T. , Isogai, A. , Takayama, S. and Che, F.S. (2007) A new method of defense response analysis using a transient expression system in rice protoplasts. Biosci. Biotechnol. Biochem. 71, 590–593. [DOI] [PubMed] [Google Scholar]
- Takakura, Y. , Ishida, Y. , Inoue, Y. , Tsutsumi, F. and Kuwata, S. (2004) Induction of a hypersensitive response‐like reaction by powdery mildew in transgenic tobacco expressing harpinpss . Physiol. Mol. Plant Pathol. 64, 83–89. [Google Scholar]
- Takakura, Y. , Ito, T. , Saito, H. , Inoue, T. , Komari, T. and Kuwata, S. (2000) Flower‐predominant expression of a gene encoding a novel class I chitinase in rice (Oryza sativa L.). Plant Mol. Biol. 42, 883–897. [DOI] [PubMed] [Google Scholar]
- Tanaka, N. , Che, F.S. , Watanabe, N. , Fujiwara, S. , Takayama, S. and Isogai, A. (2003) Flagellin from an incompatible strain of Acidovorax avenae mediates H2O2 generation accompanying hypersensitive cell death and expression of PAL, Cht‐1, and PBZ1, but not of Lox in rice. Mol. Plant–Microbe Interact. 16, 422–428. [DOI] [PubMed] [Google Scholar]
- Yang, Y. , Shah, J. and Klessig, D.F. (1997) Signal perception and transduction in plant defense responses. Genes Dev. 11, 1621–1639. [DOI] [PubMed] [Google Scholar]
- Zipfel, C. , Robatzek, S. , Navarro, L. , Oakeley, E.J. , Jones, J.D. , Felix, G. and Boller, T. (2004) Bacterial disease resistance in Arabidopsis through flagellin perception. Nature, 428, 764–767. [DOI] [PubMed] [Google Scholar]


