SUMMARY
The type III secretion system (T3SS) is required by plant pathogenic bacteria for the translocation of certain bacterial proteins to the cytoplasm of plant cells or secretion of some proteins to the apoplast. The T3SS of Erwinia amylovora, which causes fire blight of pear, apple and other rosaceous plants, secretes DspA/E, which is an indispensable pathogenicity factor. Several other proteins, including HrpN, a critical virulence factor, are also secreted by the T3SS. Using a CyaA reporter system, we demonstrated that DspA/E is translocated into the cells of Nicotiana tabacum‘Xanthi’. To determine if other T3‐secreted proteins are needed for translocation of DspA/E, we examined its translocation in several mutants of E. amylovora strain Ea321. DspA/E was translocated by both hrpW and hrpK mutants, although with some delay, indicating that these two proteins are dispensable in the translocation of DspA/E. Remarkably, translocation of DspA/E was essentially abolished in both hrpN and hrpJ mutants; however, secretion of DspA/E into medium was not affected in any of the mentioned mutants. In contrast to the more virulent strain Ea273, secretion of HrpN was abolished in a hrpJ mutant of strain Ea321. In addition, HrpN was weakly translocated into plant cytoplasm. These results suggest that HrpN plays a significant role in the translocation of DspA/E, and HrpJ affects the translocation of DspA/E by affecting secretion or stability of HrpN. Taken together, these results explain the critical importance of HrpN and HrpJ to the development of fire blight.
INTRODUCTION
Progress in understanding the genetics of pathogenesis of Erwinia amylovora, which causes the devastating disease known as fire blight in rosaceous plants, has proceeded rapidly in the past two decades (Oh and Beer, 2005). Several pathogenicity or virulence factors of E. amylovora, such as the type III secretion system (T3SS) (Desvaux et al., 2006), proteins secreted by it, extracellular polysaccharides and siderophores, have been described (Bereswill and Geider, 1997; Dellagi et al., 1998; Expert, 1999).
The T3SS provides a dedicated mechanism whereby extracellular bacteria can deliver proteins to the cytosol of host cells or the apoplast (Galán and Wolf‐Watz, 2006; He et al., 2004). The T3SS of plant pathogenic bacteria consists mainly of Hrc proteins, encoded by conserved hrc (hrp‐conserved) genes among plant pathogenic bacteria and Hrp proteins, encoded by hrp (hypersensitive response and pathogenicity) genes (Cornelis and Van Gijsegem, 2000). In E. amylovora, hrc and hrp genes are clustered in a pathogenicity island (PAI) (Oh et al., 2005), which also includes dsp (disease‐specific) genes (Bogdanove et al., 1998a; Steinberger and Beer, 1988). In addition to the T3SS, hrp genes of E. amylovora encode T3‐secreted proteins such as harpins, HrpN (Wei et al., 1992) and HrpW (Kim and Beer, 1998). DspA/E (Bogdanove et al., 1998a; Gaudriault et al., 1997), which is critical to disease development, and Eop1 [or EopB]; (Nissinen et al., 2007; Oh and Beer, 2005), a YopJ homologue, were also shown to be secreted through the T3SS. Recently, 12 proteins, including HrpN, HrpW, HrpK, HrpJ and DspA/E, were determined as T3‐secreted proteins by proteomic analyses (Nissinen et al., 2007).
T3 secretion and translocation is thought to be a one‐step process, whereby certain T3‐secreted proteins, so‐called effector proteins, are translocated directly into host cells through two bacterial membranes and the host plasma membrane. In the case of plant pathogens, effectors must also traverse plant cell walls. This process involves protein secretion machinery similar to the flagellar export system, an extracellular pilus or needle‐like appendage, and particular T3‐secreted proteins called translocators (Mota and Cornelis, 2005). Translocators are required for effector transport across the host membrane, either by forming pores in the membrane (Goure et al., 2005) or by forming a complex with the pore‐formers (Holmstrom et al., 2001). For plant pathogenic bacteria, both HrpF of Xanthomonas campestris pv. vesicatoria (Büttner et al., 2002) and HrpK of Pseudomonas syringae pv. tomato (HrpFxav and HrpKPs) (Petnicki‐Ocwieja et al., 2005) have been characterized as putative pore‐forming translocators.
Thus far, no proteinaceous translocators have been determined in E. amylovora. However, there are several likely candidates based on previous reports and homology to proteins known to be involved in effector translocation. Two harpins, HrpN and HrpW, are candidate translocators in E. amylovora because they are probably targeted to the plant apoplast, where these proteins elicit a hypersensitive response (HR) following infiltration into the intercellular spaces of leaf tissue (Kim and Beer, 1998; Wei et al., 1992). More interestingly, HrpN of E. amylovora, unlike HrpW, is required for full virulence in plants (Barny, 1995; Wei et al., 1992). HrpN of E. amylovora and HrpZPsph from P. syringae pv. phaseolicola have been shown to form ion‐conducting pores in vitro (Lee et al., 2001; J. Lee and T. Nürnberger, personal communication).
HrpW has a pectate‐lyase domain (Kim and Beer, 1998), as does a homologue of the same name of P. syringae (Charkowski et al., 1998) and several similar proteins that have been detected in other plant pathogenic enterobacteria (Bell et al., 2004). The pectate lyase domain has not shown enzymatic activity in either P. syringae or E. amylovora, but HrpW of the former was shown to bind to calcium pectate, a major component of plant cell walls (Charkowski et al., 1998).
Based on in silico analysis, E. amylovora HrpK (HrpKEa) has homology to HrpKPs. The similarity of HrpKEa with the putative translocator HrpKPs (Petnicki‐Ocwieja et al., 2005) suggests the possibility that HrpKEa might function as a translocator in E. amylovora. HrpKEa also has slight homology to HrpF, a T3‐translocator protein from X. campestris pv. vesicatoria (Alfano and Collmer, 2004; Büttner et al., 2002). However, unlike HrpKPs, HrpKEa mutants are not affected in virulence of E. amylovora in either immature pear fruits or in apple shoots (Nissinen et al., 2007; Oh et al., 2005).
E. amylovora HrpJ (HrpJEa) is a member of the HrpJ family identified in several Gram‐negative plant pathogenic bacteria. When compared with other plant pathogen sequences, highest similarity is with homologous sequences in bacteria of the family Enterobacteriaceae. However HrpJEa is also similar to HrpJ of P. syringae pv. tomato (HrpJPst), which has been shown to affect secretion of accessory T3 proteins and translocation of effectors (Fu et al., 2006).
HrpJEa is also somewhat similar to YopN of the animal pathogen Yersinia spp. (Bogdanove et al., 1996), which functions indirectly in effector translocation and prevents premature secretion of T3‐secreted proteins (Ferracci et al., 2005). Recently, HrpJEa was shown to be required for pathogenicity and elicitation of the HR, a rapid localized cell death response. In addition, HrpJEa controls secretion of HrpN because its secretion by an hrpJ mutant was reduced significantly relative to secretion of HrpN by the virulent strain Ea273 (Nissinen et al., 2007). However, whether HrpJEa is involved in translocation of effector proteins remains to be determined.
DspA/E is a well‐known protein of 198 kDa encoded by a gene of the E. amylovora PAI (Bogdanove et al., 1998b; Gaudriault et al., 1997), and it belongs to the AvrE family of effector proteins. DspA/E, as well as AvrE, has been shown to suppress basal defence by suppressing callose deposition (DebRoy et al., 2004). In addition, members of our group have shown that DspA/E interacts with the intracellular portion of receptor kinases from apple (Meng et al., 2006) and with preferredoxin in the apple cytoplasm (Bonasera et al., 2006), indicating that DspA/E may function inside host plant cells. Moreover, transient expression of DspA/E in apple and tobacco cells induces an HR (Boureau et al., 2006, Oh et al., 2007). Although DspA/E is secreted via a T3SS (Bogdanove et al., 1998a) and is thought to be delivered into plant cells, no definitive evidence that DspA/E is translocated into plant cells has been presented.
In the present study, we first show that DspA/E of E. amylovora is translocated into tobacco cells in a T3SS‐dependent manner using the CyaA reporter system (Sory and Cornelis, 1994). Similarly, we demonstrate, using several mutant strains of E. amylovora, that HrpN and HrpJ are necessary for translocation of DspA/E. In addition, we show that HrpN is weakly translocated into tobacco plant cells.
RESULTS
DspA/E1‐733‐CyaA is translocated into plant cells in a T3‐dependent manner
To determine whether DspA/E is translocated into plant cells, we constructed plasmid pCPP1553, which expresses the catalytic domain of CyaA, by fusing a 733‐amino‐acid fragment of the N‐terminus of DspA/E, resulting in DspA/E1‐733‐CyaA (Table 1). All wild‐type and mutant strains of Ea321 harbouring pCPP1553 produced cAMP only in the presence of calmodulin in vitro (data not shown). To find a suitable plant for assay of translocation of DspA/E in vivo, the strains harbouring pCPP1553 were inoculated into leaves of apple (Malus x domestica‘Gala’), Nicotiana tabacum‘Xanthi’ and N. benthamiana. Unfortunately, leaves of apple, a natural host of E. amylovora, were consistently difficult to infiltrate due to their hardness and extensive wax/cutin layer (data not shown). Thus, we tested Nicotiana sp. plants, which frequently are used for translocation assays.
Table 1.
Bacterial strains and plasmids used in this study.
| Strain | Characteristics | Reference |
|---|---|---|
| Ea321 | Wild‐type E. amylovora, moderately virulent on apple and pear | ATCC49947 |
| Ea321‐hrcN | Secretion mutant. Ea 321::Tn10 mini‐Km in hrcN (Ea321K178) | Wei and Beer (1993) |
| Ea321‐hrpK | Tn5 inserted in hrpK gene | Oh et al. (2005) |
| Ea321‐hrpW | hrpW::TnphoA (Ea321‐P110) | Kim and Beer (1998) |
| Ea321ΔhrpJ | 777‐bp deletion in frame of hrpJ gene | A. Bogdanove, unpublished data |
| Ea321‐hrpN | Tn5Tac insert in 1.3‐kb hrpN gene (Ea321‐Tn5tac1) | Wei et al. (1992) |
| Ea321hrpNΔhrpJ | Double mutant of hrpN and hrpJ | Present study |
| Ea321ΔhrpN‐W | Deletion of hrpN, orfa, eop1, orfC and hrpW. Region replaced with nptII | J.‐F. Kim, unpublished data |
| Ea273 | Wild‐type E. amylovora, highly virulent on apple and pear | ATCC 49946 |
| Ea273‐hrcN | Secretion mutant of Ea273 equivalent to Ea321‐hrcN | Present study |
| E. coli DH5α |
Escherichia coli, F−φ80dlacZ M15 (lacZYA‐argF) U169 recA1 endA1 hsdR17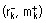 phoA supE44 λ−
thi‐1 gyrA96 relA1
phoA supE44 λ−
thi‐1 gyrA96 relA1 |
Invitrogen (Carlsbad, CA) |
| Plasmid | ||
| pCPP1553 | Codons 2–406 of Cya from pMJH20 cloned in HindIII site of pCPP1249 to yield fusion protein DspA/E1‐733‐CyaA | Present study |
| pMJH20 | pWSK29 containing codons 2–406 of CyaA in SmaI/EcoRI site | Miao et al. (1999) |
| pCPP1249 | 2.2‐kb of N‐terminal fragment of dspE cloned in a pML122 vector (Labes et al., 1990) with a hrpL promoter | A. Bogdanove, unpublished data |
| pCPP1084 | hrpN gene under the control of T7 in M13KS+, expresses HrpN at low levels | Wei et al. (1992) |
| pCPP1729 | Codons 1–323 of hrpN from pCPP1084 cloned in BamHI–SmaI sites of pMJH20 to yield fusion protein HrpN1‐323‐CyaA | Present study |
| pET‐24a(+) | Expression vector. It carries an N‐terminal T7•Tag® plus an optional C‐terminal His•Tag®. Kmr | EMD Chemicals Inc. (San Diego, CA) |
| pCPP1031 | Complete hrpJ gene in pMB1 replicon | A. Bogdanove, unpublished data |
| pCPP1763 | Complete hrpJ gene in pET‐24a(+) | Present study |
E. amylovora induces HR in both N. tabacum and N. benthamiana (Oh and Beer, 2005). The HR reaction in N. benthamiana developed between 5 and 7 h after infiltration of moderate concentrations (5 × 107 cfu/mL) of E. amylovora. In N. tabacum, visible collapse of the infiltrated areas occurred approximately 8 h after infiltration with bacterial suspensions of 4 × 108 cfu/mL (data not shown). As collapse and necrosis of the tissue due to HR would affect cytosolic proteins and cAMP, and thus affect the translocation assays (Casper‐Lindley et al., 2002), we sampled the tissues before visible HR appeared. Thus, we used N. tabacum‘Xanthi’ for translocation assays, because we considered the time for sample collection to be less restrictive with it. In addition, we were able to infiltrate higher concentrations of bacteria. Samples for determination of cAMP were collected 7 h after infiltration, just before visible collapse occurred.
To rule out the possibility that expression of DspA/E1‐733‐CyaA interferes with secretion or translocation of other proteins, we compared the wild‐type strain, with and without pCPP1553, for pathogenesis in apple and induction of HR in tobacco. We detected no difference in either the severity or the timing of development of the HR or disease symptoms based on the presence of pCPP1553 (data not shown). These observations confirmed that DspA/E1‐733‐CyaA did not affect the relevant phenotypes of the strains studied.
To measure the translocation of DspA/E1‐733‐CyaA into plant cells, we followed accumulation of cAMP in tobacco leaves inoculated with Ea321 and an Ea321‐hrcN mutant harbouring pCPP1553 as a negative control. The concentrations of cAMP from wild‐type Ea321, expressing DspA/E1‐733‐CyaA, were significantly (30–500 times) higher than the values from the buffer control and Ea321‐hrcN. These results indicate that DspA/E1‐733‐CyaA is translocated into tobacco cells, and the translocation of DspA/E1‐733‐CyaA is dependent on a functional T3SS (Fig. 1).
Figure 1.
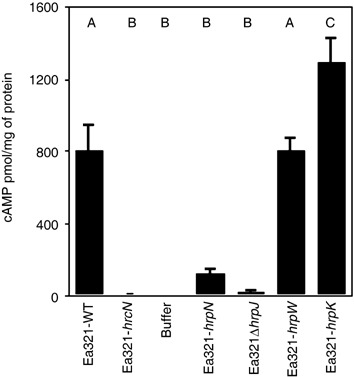
Translocation of DspA/E1‐733‐CyaA into plant cells by Ea321 and several mutants. Plants were infiltrated with suspensions of strains of Ea321 harbouring pCPP1553 at OD600 = 0.4. Leaf samples were taken 7 h after infiltration and the amount of cAMP was determined. cAMP is expressed as pmol/mg of total protein. The values for each strain are the means of 12 samples from one experiment. The bars represent standard errors. Values denoted by the same capital letters do not differ significantly at the P = 0.05 level. WT, wild‐type.
HrpN and HrpJ are required for translocation of DspA/E1‐733‐CyaA into plant cells
E. amylovora secretes several proteins, including HrpK, HrpJ and the two harpins, HrpN and HrpW, which may function in translocation. To determine if any of these proteins is involved in the translocation of DspA/E, we assayed for translocation of DspA/E in strains that had been mutated in genes encoding these proteins. The concentrations of cAMP detected from both Ea321ΔhrpJ and Ea321‐hrpN mutants 7 h after infiltration were significantly reduced and statistically similar to those obtained from the secretion mutant and the buffer treatment (Fig. 1). In contrast, no significant change in cAMP concentration was detected for the hrpW mutant of Ea321, as compared with the Ea321 wild‐type (Fig. 1). Levels of cAMP for the hrpK mutant of Ea321 were higher in half of our experiments (Fig. 1), and not affected in the two other experiments (Fig. 2). Levels of cAMP accumulation from the double mutant, lacking both hrpN and hrpJ, were statistically similar to those of the single mutants in hrpN or hrpJ (data not shown). These results indicate that both HrpJ and HrpN are required for efficient translocation of the DspA/E into plant cells. Similarly, the levels of accumulation from a mutant with a deletion that includes the two genes encoding the harpins HrpW and HrpN, as well as the genes encoding the putative effector Eop1 and its chaperone, were similar to those of the single mutant in hrpN (data not shown). This result rules out any contribution that HrpW might make to the translocation of DspA/E1‐733‐CyaA.
Figure 2.
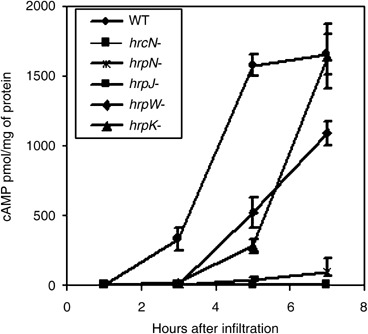
Time‐course translocation of DspA/E1‐733‐CyaA. Plants were infiltrated with suspensions of strains of Ea321 harbouring pCPP1553 at OD600 = 0.4. Leaf samples were taken 1, 3, 5 and 7 h after infiltration and the amount of cAMP was determined. cAMP is expressed as pmol/mg of total protein. The values for each strain are the means of four samples from one experiment. The bars represent standard errors. WT, wild‐type.
To determine translocation of DspA/E1‐733‐CyaA from the wild‐type Ea321 and mutant strains earlier than 7 h after infiltration, levels of cAMP accumulation were monitored at 1, 3, 5 and 7 h after infiltration (Fig. 2). cAMP was first detected 3 h after infiltration and reached a peak in wild‐type Ea321 5 h after infiltration. Very little accumulation of cAMP was detected consistently from the hrpN and hrpJ mutants at all assay times (Fig. 2). Interestingly, similar amounts of cAMP were detected for the wild‐type strain at 3 or 5 h after infiltration as were detected in both the hrpK and the hrpW mutants at 5 or 7 h after infiltration, respectively. These results suggest that translocation of DspA/E might be delayed in the absence of either HrpK or HrpW, but unlike HrpN and HrpJ, the former two proteins are not critical for translocation of DspA/E.
HrpN and HrpJ are not needed for T3‐dependent secretion in vitro of DspA/E1‐733‐CyaA
To determine whether mutation in hrpN or hrpJ affects secretion of DspA/E1‐733‐CyaA from bacterial cells, expression and secretion of the fusion protein under hrp‐inducing conditions was examined by immunoblotting with a CyaA antibody. DspA/E1‐733‐CyaA was expressed by all the tested strains, and it was secreted by all the strains except for the T3 secretion mutant Ea321‐hrcN (Fig. 3). These results indicate that neither HrpN nor HrpJ are needed for expression and secretion of DspA/E1‐733‐CyaA, and that HrpJ may affect translocation of DspA/E1‐733‐CyaA by facilitating HrpN secretion.
Figure 3.
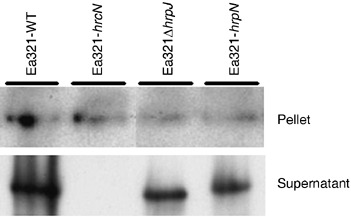
Expression and secretion of DspA/E1‐733‐CyaA by several strains of E. amylovora under hrp‐inducing conditions. Strains of Ea321 (pCPP1553) indicated in the figure were grown in hrp gene‐inducing medium at 18 °C for 36 h. Total proteins from cell pellet and supernatant were collected separately and loaded onto 8% SDS‐PAGE gels. The DspA/E1‐733‐CyaA fusion protein was detected with CyaA antibody. WT, wild‐type.
HrpJ protein is secreted by an hrpN mutant
Our laboratory has shown previously that mutation in hrpJ significantly reduces secretion of HrpN in E. amylovora strain Ea273 (Nissinen et al., 2007). First, we determined secretion of HrpN in an Ea321ΔhrpJ mutant. In our experiment, no secreted HrpN protein was detected in this mutant strain, in contrast to the reduced secretion shown previously for an Ea273 hrpJ mutant (data not shown). Next, to examine if mutation of the hrpN gene affects HrpJ secretion, HrpJ proteins were assayed in culture supernatant, using a polyclonal HrpJ antibody raised in this study. Interestingly, HrpJ proteins were secreted by the hrpN mutant, just as with the wild‐type strain (Fig. 4). These results suggest that HrpJ could function as a chaperone‐like protein for secretion of HrpN.
Figure 4.
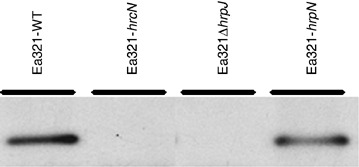
Secretion of HrpJ by several strains of E. amylovora under hrp gene‐inducing conditions. Strains of Ea321 (pCPP1553) indicated in the figure were grown in hrp‐inducing medium at 18 °C for 36 h. Proteins from supernatant were collected and loaded in 8% SDS‐PAGE gels; detection by Western blotting was carried out with HrpJ polyclonal antibody. WT, wild‐type.
HrpN1‐323‐CyaA is weakly translocated into plant cells in a T3‐dependent manner
Because the putative translocator HrpK of P. syringae had been shown to be translocated into plant cells (Petnicki‐Ocwieja et al., 2005), we tested whether HrpN is also translocated into plant cells. A portion of the hrpN gene that encodes the first 323 amino acids (80%) from the N‐terminus of the protein was fused with the catalytic domain of CyaA in a low‐copy‐number vector with a lacZ promoter (pCPP1729; Table 1). cAMP accumulation in tobacco cells was measured following infiltration of the highly virulent strain Ea273, as described previously (Schecter et al., 2004). cAMP levels from wild‐type Ea273, expressing HrpN1‐323‐CyaA, was significantly higher (32‐fold) than those from the T3‐secretion mutant (Fig. 5). However, the absolute levels of cAMP accumulation were lower (59 pmol/mg) than those for DspA/E1‐733‐CyaA (318 pmol/mg) (Fig. 5). These results indicate that HrpN1‐323‐CyaA is translocated into tobacco cells.
Figure 5.
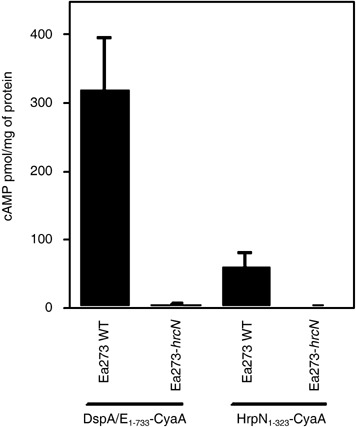
Translocation of HrpN1‐323‐CyaA into tobacco cells. Plants were infiltrated with bacterial suspensions of the wild‐type Ea273 and T3SS‐deficient mutant, Ea273‐hrcN, harbouring pCPP1553 or pCPP1729 at OD600 = 0.4. Leaf samples were taken 7 h after infiltration and the amount of cAMP was determined. cAMP is expressed as pmol/mg of total protein. The values for each strain are the means of six samples from one experiment. The bars represent standard errors. WT, wild‐type.
DISCUSSION
Based on indirect evidence (Boureau et al., 2006; Meng et al., 2006; Oh et al., 2007), the major effector protein of E. amylovora, DspA/E, has been considered to function inside plant cells. In the present study, we provide direct biochemical evidence that DspA/E of E. amylovora is translocated into plant cells by the T3SS. Furthermore, we show that the first 733 N‐terminal amino acids of the 1837‐amino‐acid protein are sufficient for both secretion and translocation. Translocation is detectable as soon as 3 h after inoculation (Fig. 2), indicating that the translocation process starts in the first few hours after bacteria invade plant tissue.
The translocation of T3 effectors from plant pathogenic bacteria through plant plasma membranes is poorly understood. For translocation of effectors into mammalian cells, Mota et al. (2005) proposed that a dedicated set of proteins, termed translocators, is needed to mediate the delivery of effector proteins through host plasma membranes. A subset of translocators is thought to insert themselves into the plasma membranes of host cells and form pores through which the effectors can traverse the host plasma membrane to the cytosol (Büttner et al., 2002; Marenne et al., 2003; Meyer et al., 2006; Petnicki‐Ocwieja et al., 2005). Translocators are needed for translocation, but they are not needed for secretion of the effector proteins (Mota et al., 2005); the translocators themselves are secreted via the T3SS (Hakansson et al., 1996; Sory and Cornelis, 1994), and they also can be translocated into host cells (Francis and Wolf‐Watz, 1998; Petnicki‐Ocwieja et al., 2005).
The critical role of HrpN in the virulence of the fire blight pathogen has been a mystery since its characterization (Wei et al., 1992) and further study (Barny, 1995). Here, we have shown that HrpN is required for translocation of DspA/E from the bacterial cytoplasm to the plant cytoplasm. Thus, the function of HrpN in the translocation of DspA/E, an essential pathogenicity effector of E. amylovora, is fully consistent with its importance for disease development. Although several other harpins, including HrpW, have been characterized for plant pathogenic bacteria, their precise role in virulence remains unclear. Our observation that HrpN1‐323‐CyaA induced significantly more cAMP accumulation than the negative control (Fig. 5) suggests that HrpN itself is translocated into host plant cells. However, cAMP accumulation by HrpN1‐323‐CyaA was much lower than for the DspA/E1‐733‐CyaA fusion protein, suggesting that HrpN proteins are perhaps embedded in plasma membranes rather than fully translocated into the plant cytoplasm as is the DspA/E1‐733‐CyaA fusion protein. This assumption would be consistent with the presence of two putative transmembrane helices in the N‐terminal portion of HrpN, predicted by the ‘TMpred’ tool, available at the ExPASy proteomics server of the Swiss Institute of Bioinformatics. Interestingly, HrpN has also been shown to function as a pore‐former (J. Lee and T Nürnberger, personal communication), as does HrpZ1, a harpin of P. syringae pv. phaseolicola (Lee et al., 2001).
Although HrpN of E. amylovora is required for translocation of normal levels of DspA/E, other proteins could be involved in the translocation process. Accumulation of cAMP by the DspA/E1‐733‐CyaA in the hrpN mutant of Ea321 is greater than in the hrcN mutant or the buffer control (1, 2). This suggests that other proteins might contribute to the translocation of DspA/E. This is consistent with the results of virulence tests in apple shoots, where hrpN mutants show severely reduced virulence, but are still pathogenic (this study, results not shown; and Barny, 1995).
In addition to HrpN, HrpJ is necessary for translocation of DspA/E, although it is not required for secretion of DspA/E. However, depending on the aggressiveness of the strain, secretion of both harpins, HrpN and HrpW, is reduced or abolished in hrpJ mutants (Nissinen et al., 2007; this study). These data suggest that HrpJ acts indirectly on the translocation of DspA/E by facilitating the secretion of HrpN. Interestingly, we observed interaction of HrpJ with both HrpN and HrpW in yeast (see supplementary Fig. S1). These interactions support the hypothesis that HrpJ might be involved in secretion of HrpN and/or HrpW. We also observed strong interaction of HrpJ with itself (supplementary Fig. S1), which suggests that HrpJ might function in a complex. Finally, we observed that there is no interaction between HrpJ and DspA/E in yeast (results not shown), consistent with our other results suggesting that HrpJ does not function directly on the effector DspA/E but indirectly through interaction with the putative translocator HrpN.
The function of HrpJ seems to be similar to that of InvE of Salmonella enterica sv. typhimurium, which is required for normal accumulation of the extracellular translocator proteins SipB, SipC and SipD (Kubori and Galán, 2002). Similarly, HrpJ of P. syringae pv. tomato recently was shown to be required for secretion of HrpZ1 in vitro and for the translocation of several effector proteins (Fu et al., 2006).
In contrast to HrpN and HrpJ, our tests of HrpW and HrpK indicate that these proteins are not critical to either secretion (Fig. 3) or translocation of DspA/E (Fig. 1) in Ea321. However, our time‐course measurements of cAMP accumulation imply that both proteins have some effect on translocation of DspA/E because accumulation of cAMP was delayed significantly for up to 2 h (Fig. 2). This is an interesting observation, as one would expect a delay in translocation to be reflected in virulence. However, hrpW and hrpK mutants of E. amylovora are as virulent as the wild‐type strain in host plants (Kim and Beer, 1998; Nissinen et al., 2007; Oh et al., 2005). This could be either because a delay of 2 h is insufficient to affect virulence or because our testing methods cannot measure a difference in virulence over such short periods.
HrpW does not contribute to the translocation of DspA/E based on our observations that a deletion mutant spanning the genes encoding both harpins, the putative effector Eop1 and its chaperone, produced a level of cAMP accumulation similar to that of the hrpN single mutant (data not shown).
HrpK is a T3‐secreted protein of E. amylovora (Nissinen et al., 2007), and it is similar to HrpK of Pseudomonas sp. Interestingly, HrpK of P. syringae pv. tomato DC3000 has been shown to contribute to virulence in host plants, translocation of effector proteins and HR elicitation (Petnicki‐Ocwieja et al., 2005). In contrast, hrpK mutants in E. amylovora Ea273, as well as Ea321, are fully virulent and HR‐positive (Nissinen et al., 2007; this study). Moreover, an hrpK mutant of Ea321 translocated DspA/E as well (Fig. 2) or better (Fig. 1) than the wild‐type strain. The only indication that HrpK could be involved in translocation of DspA/E is the delay in onset of translocation (Fig. 2). Despite the similarity of HrpKEa and HrpKPst, their phenotypes are quite different, possibly because they translocate different effectors. While the effectors of P. syringae that HrpKPst putatively translocate are important for development of disease in its hosts (Petnicki‐Ocwieja et al., 2005), the effectors associated with HrpKEa in E. amylovora might have a redundant function. Under this assumption we cannot dismiss the possibility that HrpK or HrpW are possible translocators of other effector(s), although they clearly do not play a major role in translocation of DspA/E. To ascertain the possible roles of HrpW and HrpK, further translocation tests would be needed with other putative effectors, several of which have been identified in E. amylovora recently (Zhao et al., 2006).
The mechanistic role of HrpN of E. amylovora in the fire blight disease has been an intriguing question since the early 1990s when the protein was characterized and hrpN mutants of E. amylovora were found to be drastically reduced in virulence to host plants such as apple and pear (Wei et al., 1992). From the present study, it is clear that HrpN plays a pivotal role in disease development by facilitating translocation of DspA/E into plant cells. In the absence of HrpN, translocation of the critical disease‐specific protein DspA/E into host cells is dramatically reduced, and consequently the hrpN mutant is essentially non‐virulent.
EXPERIMENTAL PROCEDURES
Bacterial strains, plasmids and culture conditions
Bacterial strains and plasmids used were grown in Luria–Bertani (LB) broth or LB agar with appropriate antibiotics for selection (Table 1). The DspA/E1‐733‐CyaA fusion protein was constructed by cloning a fragment encoding the catalytic domain of CyaA from Bordetella pertussis from plasmid pMJH20 (Miao et al., 1999) into pCPP1249 (A. J. Bogdanove, unpublished data), in frame, after codons for the first 733 amino acids of DspA/E. The N‐terminal fragment of DspA/E contains the putative secretion signal necessary for secretion and presumably for translocation. pCPP1249 is a low‐copy vector with the native promoter sequence for dspA/E under control of the T3‐specific sigma factor hrpL (Wei and Beer, 1995); it also includes the nptII promoter. The resulting plasmid, pCPP1553, which expresses the fusion protein, was transformed into several strains of E. amylovora (Table 1). The HrpN1‐323‐CyaA fusion was constructed by cloning the fragment encoding the first 323 amino acids (80% of the protein) from pCPP1084 (Table 1) into pMJH20, in frame with the catalytic domain of CyaA. The fusion protein in pMJH20 is expressed under control of the lacZ promoter, which is inducible with isopropyl β‐D‐1‐thiogalactopyranoside (IPTG).
Production of polyclonal HrpJ antibodies
Polyclonal antisera against HrpJ was raised at the College of Veterinary Medicine, Cornell University, by injecting intramuscularly 100 µg of purified HrpJ into a rabbit three times at 3‐week intervals. Blood was collected 2 weeks after the final injection. The immunoglobulin fraction was cross‐absorbed with heat‐treated lysate of Escherichia coli BL21 (DE3) [pET24a)(+)]. To produce the HrpJ protein for immunization, the hrpJ gene was amplified from pCPP1031 and cloned into pET‐24a(+) tagging it with the HIS‐tag at HrpJ C‐terminus. HrpJ was produced in high quantities by E. coli BL21 (DE3), and purified with TALON® Metal Affinity Resins (CLONTECH, Palo Alto, CA).
Protein secretion assay
Strains of E. amylovora Ea321 harbouring pCPP1553 or mutant strains were grown in Hyunh's minimal medium (Huynh et al., 1989), for 36 h at 18 °C on a shaker at 200 r.p.m. to induce T3‐dependent protein secretion. Suspensions were treated with 0.5 mm phenyl methylsulfonyl fluoride (PMSF) to avoid protein degradation, centrifuged and the supernatants were concentrated 2500 times with Centriplus Centrifugal Filters YM‐10 (Millipore, Bedford, MA). Cell pellets, recovered after centrifugation, were resuspended in one‐tenth of the original volume of SDS loading buffer. Samples were electrophoresed in 8% SDS‐PAGE gels, then proteins were transferred to PVDF membranes. DspA/E1‐733‐CyaA was detected by adenylate cyclase murine monoclonal antibody 3D1 (List Biological Laboratories, Campbell, CA), and HrpN antibody (Wei et al., 1992) and HrpJ antibody (this study) were used to detect HrpN and HrpJ, respectively.
Quantification of cAMP in planta
Strains of E. amylovora were infiltrated at OD600 = 0.4 into leaf panels of N. tabacum‘Xanthi’ as previously described (Wei et al., 1992). Leaf discs (8 mm in diameter) were harvested from the infiltrated areas 7 h after infiltration, placed individually in microfuge tubes, immediately frozen in liquid nitrogen and stored at –80 °C for later processing. The development of HR in infiltrated areas was assessed 24 h after infiltration. Frozen leaf discs were prepared for cAMP quantification as per Schechter et al. (2004), and the cAMP content in samples was determined with the Correlate‐EIA Direct Cyclic AMP Enzyme Immunoassay Kit (Assay Designs, Inc., Ann Arbor, MI), following the manufacturer's instructions. Protein concentration in the samples was determined with a bicinchoninic acid (BCA) test kit (Pierce, Rockford, IL).
Quantification of cAMP in vitro
Strains of E. amylovora were grown overnight at 26 °C with shaking in LB with appropriate antibiotics. One hundred microlitres of overnight culture were transferred into fresh LB with appropriate antibiotics. The bacterial strains were grown to OD600 = 0.4. Bacterial suspensions were centrifuged and the pellet was resuspended in 20 mm Tris containing 10 mm MgCl2 at pH 8. The cells were lysed by sonication for 90 s with a micro‐tip (Model 550 Sonic Dismembrator, Fisher Scientific, Pittsburg, PA). The lysate sample was centrifuged at 4600 g for 10 min and the supernatant was stored in microfuge tubes at –20 °C until further processing. The CyaA assay reaction mixture was prepared as previously described (Schechter et al., 2004; Sory and Cornelis, 1994) with and without calmodulin (0.2 µm). The mixture was incubated at 30 °C for 10 min before determining the content of cAMP, as described for quantification of cAMP in planta (above).
Statistical tests
The program S‐PLUS version 7.0.0 for Windows (Insightful Corp., Seattle, WA.) was used for the statistical analysis. For the anova, a fixed‐effects model and the multiple comparisons (Tukey option) were used to compare the mean values for cAMP accumulated by the different strains in each in planta experiment.
Supporting information
Fig. S1 Direct interaction in yeast between HrpJ and HrpN, HrpW and itself. The hrpJ gene in the bait vector (pGilda) and hrpN, hrpW and hrpJ in the prey vector (pB42AD; EV) were co‐transformed into the yeast strain, EYG48(p8oplacZ), using LiAc/PEG method (Yeast Protocols Handbook; Clontech, Mountain View, CA). Yeast transformants were screened on synthetic Drop‐Out medium (SD) with glucose, but without histidine, tryptophan and uracil (‐HTU). Ten‐microlitre cell suspensions at OD600 = 0.2 and 10‐fold dilutions were plated and incubated for 5 days on SD‐HTU with galactose in the presence or absence of leucine (Leu) to determine protein–protein interaction. Growth on plates regardless of the presence of leucine represents positive interactions. pLexA‐p53 (LexA‐p53) and pB42AD‐T (T‐antigen) were used as positive controls.
Please note: Blackwell Publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
ACKNOWLEDGEMENTS
We thank Lisa Schechter and Alan Collmer for providing protocols and helpful comments on the CyaA reporter system. We thank Kent Loeffler for photography. We thank Jacqueline Nock and Christopher Watkins for storing, in controlled atmospheres, the immature pear fruits used in this study. We thank Sam Cartinhour and James Giovannoni for critically reading the manuscript. This work was supported, in part, by USDA‐CSREES Special Grants for research on fire blight of apple, an USDA‐NSF Microbial Genome Sequencing grant, and royalty funds of Eden Biosciences Corporation to S.V.B.
Present addresses
The second and third authors contributed equally to the work reported.
REFERENCES
- Alfano, J.R. and Collmer, A. (2004) Type III secretion system effector proteins: double agents in bacterial disease and plant defense. Annu. Rev. Phytopathol. 42, 385–414. [DOI] [PubMed] [Google Scholar]
- Barny, M.A. (1995) Erwinia‐amylovora HrpN mutants, blocked in harpin synthesis, express a reduced virulence on host plants and elicit variable hypersensitive reactions on tobacco. Eur. J. Plant Pathol. 101, 333–340. [Google Scholar]
- Bell, K.S. , Sebaihia, M. , Pritchard, L. , Holden, M.T.G. , Hyman, L.J. , Holeva, M.C. , Thomson, N.R. , Bentley, S.D. , Churcher, L.J.C. , Mungall, K. , Atkin, R. , Bason, N. , Brooks, K. , Chillingworth, T. , Clark, K. , Doggett, J. , Fraser, A. , Hance, Z. , Hauser, H. , Jagels, K. , Moule, S. , Norbertczak, H. , Ormond, D. , Price, C. , Quail, M.A. , Sanders, M. , Walker, D. , Whitehead, S. , Salmond, G.P.C. , Birch, P.R.J. , Parkhill, J. and Toth, I.K. (2004) Genome sequence of the enterobacterial phytopathogen Erwinia carotovora subsp. atroseptica and characterization of virulence factors. Proc. Natl Acad. Sci. USA, 101, 11105–11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereswill, S. and Geider, K. (1997) Characterization of the rcsB gene from Erwinia amylovora and its influence on exopolysaccharide synthesis and virulence of the fire blight pathogen. J. Bacteriol. 179, 1354–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanove, A.J. , Bauer, D.W. and Beer, S.V. (1998a) Erwinia amylovora secretes DspE, a pathogenicity factor and functional AvrE homolog, through the hrp (type III secretion) pathway. J. Bacteriol. 180, 2244–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanove, A.J. , Kim, J.F. , Wei, Z.M. , Kolchinsky, P. , Charkowski, A.O. , Conlin, A.K. , Collmer, A. and Beer, S.V. (1998b) Homology and functional similarity of an hrp‐linked pathogenicity locus, dspEF, of Erwinia amylovora and the avirulence locus avrE of Pseudomonas syringae pathovar tomato . Proc. Natl Acad. Sci. USA, 95, 1325–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanove, A.J. , Wei, Z.M. , Zhao, L.P. and Beer, S.V. (1996) Erwinia amylovora secretes harpin via a type III pathway and contains a homolog of YopN of Yersinia spp. J. Bacteriol. 178, 1720–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonasera, J.M. , Meng, X.D. , Beer, S.V. , Owens, T. and Kim, W.‐S. (2006) Interaction of DspE/A, a pathogenicity protein of Erwinia amylovora, with pre‐ferredoxin from apple and its relationship to photosynthetic efficiency. Acta Hort. 704, 473–478. [Google Scholar]
- Boureau, T. , ElMaarouf‐Bouteau, H. , Garnier, A. , Brisset, M.N. , Perino, C. , Pucheu, I. and Barny, M.A. (2006) DspA/E, a type III effector essential for Erwinia amylovora pathogenicity and growth in planta, induces cell death in host apple and nonhost tobacco plants. Mol. Plant–Microbe Interact. 19, 16–24. [DOI] [PubMed] [Google Scholar]
- Büttner, D. , Nennstiel, D. , Klüsener, B. and Bonas, U. (2002) Functional analysis of HrpF, a putative type III translocon protein from Xanthomonas campestris pv. vesicatoria . J. Bacteriol. 184, 2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper‐Lindley, C. , Dahlbeck, D. , Clark, E.T. and Staskawicz, B.J. (2002) Direct biochemical evidence for type III secretion‐dependent translocation of the AvrBs2 effector protein into plant cells. Proc. Natl Acad. Sci. USA, 99, 8336–8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charkowski, A.O. , Alfano, J.R. , Preston, G. , Yuan, J. , He, S.Y. and Collmer, A. (1998) The Pseudomonas syringae pv. tomato HrpW protein has domains similar to harpins and pectate lyases and can elicit the plant hypersensitive response and bind to pectate. J. Bacteriol. 180, 5211–5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis, G.R. and Van Gijsegem, F. (2000) Assembly and function of type III secretory systems. Ann. Rev. Microbiol. 54, 735–774. [DOI] [PubMed] [Google Scholar]
- DebRoy, S. , Thilmony, R. , Kwack, Y.B. , Nomura, K. and He, S.Y. (2004) A family of conserved bacterial effectors inhibits salicylic acid‐mediated basal immunity and promotes disease necrosis in plants. Proc. Natl Acad. Sci. USA, 101, 9927–9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellagi, A. , Brisset, M.N. , Paulin, J.P. and Expert, D. (1998) Dual role of desferrioxamine in Erwinia amylovora pathogenicity. Mol. Plant–Microbe Interact. 11, 734–742. [DOI] [PubMed] [Google Scholar]
- Desvaux, M. , Hebraud, M. , Henderson, I.R. and Pallen, M.J. (2006) Type III secretion: what's in a name? Trends Microbiol. 14, 157–160. [DOI] [PubMed] [Google Scholar]
- Expert, D. (1999) Withholding and exchanging iron: interactions between Erwinia spp. and their plant hosts. Ann. Rev. Phytopathol. 37, 307–334. [DOI] [PubMed] [Google Scholar]
- Ferracci, F. , Schubot, F.D. , Waugh, D.S. and Plano, G.V. (2005) Selection and characterization of Yersinia pestis YopN mutants that constitutively block Yop secretion. Mol. Microbiol. 57, 970–987. [DOI] [PubMed] [Google Scholar]
- Francis, M.S. and Wolf‐Watz, H. (1998) YopD of Yersinia pseudotuberculosis is translocated into the cytosol of HeLa epithelial cells: evidence of a structural domain necessary for translocation. Mol. Microbiol. 29, 799–813. [DOI] [PubMed] [Google Scholar]
- Fu, Z.Q. , Guo, M. and Alfano, J.R. (2006) Pseudomonas syringae HrpJ is a type III secreted protein that is required for plant pathogenesis, injection of effectors, and secretion of the HrpZ1 harpin. J. Bacteriol. 188, 6060–6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán, J.E. and Wolf‐Watz, H. (2006) Protein delivery into eukaryotic cells by type III secretion machines. Nature, 444, 567–573. [DOI] [PubMed] [Google Scholar]
- Gaudriault, S. , Malandrin, L. , Paulin, J.P. and Barny, M.A. (1997) DspA, an essential pathogenicity factor of Erwinia amylovora showing homology with AvrE of Pseudomonas syringae, is secreted via the Hrp secretion pathway in a DspB‐dependent way. Mol. Microbiol. 26, 1057–1069. [DOI] [PubMed] [Google Scholar]
- Goure, J. , Broz, P. , Attree, O. , Cornelis, G.R. and Attree, I. (2005) Protective anti‐V antibodies inhibit Pseudomonas and Yersinia translocon assembly within host membranes. J. Infect. Dis. 192, 218–225. [DOI] [PubMed] [Google Scholar]
- Hakansson, S. , Schesser, K. , Persson, C. , Galyov, E.E. , Rosqvist, R. , Homble, F. and Wolf‐Watz, H. (1996) The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact‐dependent membrane disrupting activity. EMBO J. 15, 5812–5823. [PMC free article] [PubMed] [Google Scholar]
- He, S.Y. , Nomura, K. and Whittam, T.S. (2004) Type III protein secretion mechanism in mammalian and plant pathogens. Biochim. Biophys. Acta, 1694, 181–206. [DOI] [PubMed] [Google Scholar]
- Holmstrom, A. , Olsson, J. , Cherepanov, P. , Maier, E. , Nordfelth, R. , Pettersson, J. , Benz, R. , Wolf‐Watz, H. and Forsberg, A. (2001) LcrV is a channel size‐determining component of the Yop effector translocon of Yersinia . Mol. Microbiol. 39, 620–632. [DOI] [PubMed] [Google Scholar]
- Huynh, T.V. , Dahlbeck, D. and Staskawicz, B.J. (1989) Bacterial‐blight of soybean‐regulation of a pathogen gene determining host cultivar specificity. Science, 245, 1374–1377. [DOI] [PubMed] [Google Scholar]
- Kim, J.F. and Beer, S.V. (1998) HrpW of Erwinia amylovora, a new harpin that contains a domain homologous to pectate lyases of a distinct class. J. Bacteriol. 180, 5203–5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubori, T. and Galán, J.E. (2002) Salmonella type III secretion‐associated protein InvE controls translocation of effector proteins into host cells. J. Bacteriol. 184, 4699–4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labes, M. , Puhler, A. and Simon, R. (1990) A new family of Rsf1010‐derived expression and Lac‐fusion broad‐host‐range vectors for Gram‐negative bacteria. Gene, 89, 37–46. [DOI] [PubMed] [Google Scholar]
- Lee, J. , Klusener, B. , Tsiamis, G. , Stevens, C. , Neyt, C. , Tampakaki, A.P. , Panopoulos, N.J. , Noller, J. , Weiler, E.W. , Cornelis, G.R. , Mansfield, J.W. and Nurnberger, T. (2001) HrpZ(Psph) from the plant pathogen Pseudomonas syringae pv. phaseolicola binds to lipid bilayers and forms an ion‐conducting pore in vitro. Proc. Natl Acad. Sci. USA, 98, 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marenne, M.N. , Journet, L. , Mota, L.J. and Cornelis, G.R. (2003) Genetic analysis of the formation of the Ysc‐Yop translocation pore in macrophages by Yersinia enterocolitica: role of LcrV, YscF and YopN. Microb. Pathog. 35, 243–258. [DOI] [PubMed] [Google Scholar]
- Meng, X.D. , Bonasera, J.M. , Kim, J.F. , Nissinen, R.M. and Beer, S.V. (2006) Apple proteins that interact with DspA/E, a pathogenicity effector of Erwinia amylovora, the fire blight pathogen (vol. 18, pg 1, 2006). Mol. Plant–Microbe Interact. 19, 359–359. [DOI] [PubMed] [Google Scholar]
- Meyer, D. , Cunnac, S. , Gueneron, M. , Declercq, C. , Van Gijsegem, F. , Lauber, E. , Boucher, C. and Arlat, M. (2006) PopF1 and PopF2, two proteins secreted by the type III protein secretion system of Ralstonia solanacearum, are translocators belonging to the HrpF/NopX family. J. Bacteriol. 188, 4903–4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao, E.A. , Scherer, C.A. , Tsolis, R.M. , Kingsley, R.A. , Adams, G. , Baumler, A.J. and Miller, S.I. (1999) Salmonella typhimurium leucine‐rich repeat proteins are targeted to the SPI1 and SPI2 type III secretion systems. Mol. Microbiol. 34, 850–864. [DOI] [PubMed] [Google Scholar]
- Mota, L.J. and Cornelis, G.R. (2005) The bacterial injection kit: Type III secretion systems. Annals Med. 37, 234–249. [DOI] [PubMed] [Google Scholar]
- Mota, L.J. , Sorg, I. and Cornelis, G.R. (2005) Type III secretion: the bacteria–eukaryotic cell express. Fems Microbiol. Lett. 252, 1–10. [DOI] [PubMed] [Google Scholar]
- Nissinen, R.M. , Ytterberg, A.J. , Bogdanove, A.J. , Van Wijk, K.J. and Beer, S.V. (2007) Analyses of the secretomes of Erwinia amylovora and selected hrp mutants reveal novel type III secreted proteins and an effect of HrpJ on extracellular harpin levels. Mol. Plant Pathol. 8, 55–67. [DOI] [PubMed] [Google Scholar]
- Oh, C.S. and Beer, S.V. (2005) Molecular genetics of Erwinia amylovora involved in the development of fire blight. FEMS Microbiol. Lett. 253, 185–192. [DOI] [PubMed] [Google Scholar]
- Oh, C.S. , Kim, J.F. and Beer, S.V. (2005) The Hrp pathogenicity island of Erwinia amylovora and identification of three novel genes required for systemic infection. Mol. Plant Pathol. 6, 125–138. [DOI] [PubMed] [Google Scholar]
- Oh, C.S. , Martin, G.B. and Beer, S.V. (2007) DspA/E, a type III effector of Erwinia amylovora, is required for early rapid growth in Nicotiana benthamiana and causes NbSGT1‐dependent cell death. Mol. Plant Pathol. 8, 255–265. [DOI] [PubMed] [Google Scholar]
- Petnicki‐Ocwieja, T. , Van Dijk, K. and Alfano, J.R. (2005) The hrpK operon of Pseudomonas syringae pv. tomato DC3000 encodes two proteins secreted by the type III (Hrp) protein secretion system: HopB1 and HrpK, a putative type III translocator. J. Bacteriol. 187, 649–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter, L.M. , Roberts, K.A. , Jamir, Y. , Alfano, J.R. and Collmer, A. (2004) Pseudomonas sytingae type III secretion system targeting signals and novel effectors studied with a Cya translocation reporter. J. Bacteriol. 186, 543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sory, M.P. and Cornelis, G.R. (1994) Translocation of a hybrid YopE‐adenylate cyclase from Yersinia enterocolitica into Hela‐Cells. Mol. Microbiol. 14, 583–594. [DOI] [PubMed] [Google Scholar]
- Steinberger, E.M. and Beer, S.V. (1988) Creation and complementation of pathogenicity mutants of Erwinia amylovora . Mol. Plant–Microbe Interact. 1, 135–144. [Google Scholar]
- Wei, Z.M. and Beer, S.V. (1993) HrpI of Erwinia amylovora functions in secretion of harpin and is a member of a new protein family. J. Bacteriol. 175, 7958–7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, Z.M. and Beer, S.V. (1995) hrpL activates Erwinia amylovora hrp gene transcription and is a member of the ECF subfamily of sigma factors. J. Bacteriol. 177, 6201–6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, Z.M. , Laby, R.J. , Zumoff, C.H. , Bauer, D.W. , He, S.Y. , Collmer, A. and Beer, S.V. (1992) Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia‐amylovora . Science, 257, 85–88. [DOI] [PubMed] [Google Scholar]
- Zhao, Y.F. , He, S.Y. and Sundin, G.W. (2006) The Erwinia amylovora avrRpt2 (E) gene contributes to virulence on pear and AvrRpt2(E) is recognized by Arabidopsis RPS2 when expressed in Pseudomonas syringae . Mol. Plant–Microbe Interact. 19, 644–654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Direct interaction in yeast between HrpJ and HrpN, HrpW and itself. The hrpJ gene in the bait vector (pGilda) and hrpN, hrpW and hrpJ in the prey vector (pB42AD; EV) were co‐transformed into the yeast strain, EYG48(p8oplacZ), using LiAc/PEG method (Yeast Protocols Handbook; Clontech, Mountain View, CA). Yeast transformants were screened on synthetic Drop‐Out medium (SD) with glucose, but without histidine, tryptophan and uracil (‐HTU). Ten‐microlitre cell suspensions at OD600 = 0.2 and 10‐fold dilutions were plated and incubated for 5 days on SD‐HTU with galactose in the presence or absence of leucine (Leu) to determine protein–protein interaction. Growth on plates regardless of the presence of leucine represents positive interactions. pLexA‐p53 (LexA‐p53) and pB42AD‐T (T‐antigen) were used as positive controls.
Please note: Blackwell Publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item


