SUMMARY
Successful host colonization by necrotrophic plant pathogens requires the induction of plant cell death to provide the nutrients needed for infection establishment and progression. We have cloned two genes encoding necrosis and ethylene‐inducing peptides from Sclerotinia sclerotiorum, which we named SsNep1 and SsNep2. The peptides encoded by these genes induce necrosis when expressed transiently in tobacco leaves. SsNep1 is expressed at a very low level relative to SsNep2 during infection. The expression of SsNep2 was induced by contact with solid surfaces and occurred in both the necrotic zone and at the leading margin of the infection. SsNep2 expression was dependent on calcium and cyclic adenosine monophosphate signalling, as compounds affecting these pathways reduced or abolished SsNep2 expression coincident with a partial or total loss of virulence.
INTRODUCTION
Necrotrophic plant pathogens derive their nutrients from dead or dying host tissues. Host programmed cell death (PCD), which is associated with the hypersensitive response, is effective in restricting the growth of biotrophic pathogens, but is counteractive in the defence against necrotrophs (Dickman et al., 2001). Sclerotinia sclerotiorum is a necrotrophic fungus that infects many important crops, such as soybean, lentils, sunflower and oilseed rape (Boland and Hall, 1994). Its main pathogenicity determinants are considered to be oxalic acid and numerous polygalacturonases (PGs). Oxalic acid is particulary important as it lowers ambient pH, sequesters calcium in the middle lamellae, leading to a loss of cell wall integrity (Godoy et al., 1990), and suppresses the oxidative burst (Cessna et al., 2000). Indeed, oxalic acid‐deficient mutants are non‐pathogenic (Godoy et al., 1990). PGs are enzymes that degrade pectin in the plant cell wall and are highly active under acidic conditions. Sclerotinia sclerotiorum produces four different types of PG that are involved in infection establishment, lesion expansion and tissue maceration (2004a, 2004b) In response, plants produce polygalacturonase inhibitor proteins (PGIPs) to inactivate these enzymes (Hegedus et al., 2008). Oxalic acid and lytic enzymes produced by necrotrophic pathogens have additional roles in pathogenesis, including the induction of PCD (Kim et al., 2008; Van Kan, 2006). Botrytis cinerea produces several PGs, some of which possess necrotizing activity (Kars et al., 2005), and a S. sclerotiorum PG has been shown to elevate cellular Ca+2 levels and induce PCD (Zuppini et al., 2005).
Another group of PCD elicitors found in many plant pathogens is the necrosis and ethylene‐inducing peptides (NEPs) (Pemberton and Salmond, 2004). NEP1 was first isolated from Fusarium oxysporum culture filtrates as a 24‐kDa protein that caused necrosis and induced ethylene production in the coca plant, Erythroxylum coca (Bae et al., 2006; Bailey, 1995). In the past few years, NEP1‐like proteins (NLPs) have been identified in taxonomically diverse organisms, including bacteria, fungi and oomycetes. They have also been found in non‐pathogenic organisms, such as Vibrio pommerensis (Jores et al., 2003), and are expressed in the hemibiotrophic fungus Moniliophthora perniciosa during the biotrophic stage (Garcia et al., 2007). Transformation of Colletotrichum coccodes with F. oxysporum Nep1 increased virulence and the spectrum of hosts that could be infected (Amsellem et al., 2002). NLPs elevate internal Ca2+ levels, activate mitogen‐activated protein (MAP) kinases and promote the formation of reactive oxygen species, ethylene and pathogenesis‐related proteins (Fellbrich et al., 2002; Jennings et al., 2001; Keates et al., 2003; Qutob et al., 2006; Veit et al., 2001). In Arabidopsis thaliana, global gene expression patterns in response to the Phytophthora parasitica NLPs were similar to those observed in response to the bacterial flagellin (Qutob et al., 2006). The mechanism by which NLPs bring about these phenomenon is unknown, but they must associate with the outer surface of the plasma membrane to trigger PCD (Qutob et al., 2006; Schouten et al., 2008). NLPs have also been reported to erode the host cuticle (Keates et al., 2003).
Common to all NLP proteins is a conserved GHRHDWE domain and an amino‐terminal register of conserved cysteine residues. Based on the number of cysteine residues, NLPs can be classified as Type I or Type II, which possess either two or four cysteine residues, respectively. Both types of NLP are produced by fungi and bacteria; however, only Type I NLPs have been reported in oomycetes (Gijzen and Nürnberger, 2006). The conserved GHRHDWE domain is not solely responsible for activity (Schouten et al., 2008). Mutation of the conserved cysteine residues or other alterations that affect the tertiary structure abolish the ability to induce PCD in protoplasts or to generate necrotic lesions (Fellbrich et al., 2002; Qutob et al., 2006).
The majority of studies conducted on NLPs have focused on their interaction with their host. Little is known about factors governing the regulation of NLP gene expression. In Erwinia carotovora, Nep was expressed only when the bacteria were grown on solid medium (Mattinen et al., 2004). In this article, we report the characterization of two NLP genes from S. sclerotiorum, referred to as SsNep1 and SsNep2. The peptides encoded by these genes cause necrosis, and SsNep2 is expressed soon after contact of the mycelia with the leaf or other solid surfaces. We also investigated the role of cyclic adenosine monophosphate (cAMP) and Ca2+ in the regulation of SsNep expression. This study further illustrates the significance of having multiple effectors to initiate host PCD and tissue necrosis for successful infection by necrotic pathogens.
RESULTS
Identification of SsNep1 and SsNep2
To identify S. sclerotiorum Nep (SsNep) genes, we conducted a blastx search of the S. sclerotiorum genome sequence (http://www.broad.mit.edu/annotation/genome/sclerotiniasclerotiorum/Home.html) with F. oxysporum Nep1 (AF036580). Two Nep1 homologues were identified with expectation values of 0 (SS1G_03080.1) and 1.06 e‐31 (SS1G_11912.1), which were named SsNep1 and SsNep2, respectively. The SsNep1 gene contains two introns of 53 and 58 bp with the resultant 738‐bp open reading frame encoding a 246‐amino‐acid protein with a predicted molecular weight of 26.7 kDa. The SsNep2 gene contains two introns of 61 and 58 bp with a 735‐bp open reading frame that encodes a 245‐amino‐acid protein predicted to have a molecular weight of 26.0 kDa.
Comparison of NLPs from several fungal pathogens revealed a diverse phylogenetic arrangement (Fig. 1B). SsNEP1 and SsNEP2 were most closely related to Botrytis elliptica Nep1 (ABB43266; 82% identity) and Nep2 (CAJ31369; 79% identity), respectively. SsNEP1 was also related to an Aspergillus oryzae NLP (XP_001824353), whereas SsNEP2 was related to an Aspergillus fumigatus NLP (EAL86241). The oomycete NLPs from Phytophthora and Pythium species formed a clade that was distinct from those including SsNEP1 and SsNEP2, although the former was more similar to the oomycete NLP sequences. SsNEP1 and SsNEP2 are highly divergent and exhibit only 35% identity at the amino acid level. The conserved GHRHDWE sequence is located between positions 103 and 110 in SsNEP1 and 109 and 116 in SsNEP2. Both appear to be secreted as they were predicted to possess signal peptides. Conserved cysteine residues define the type or class of NLP. Both SsNEPs are Type I with two conserved cysteine residues at positions 47 and 73 in the mature protein (Fig. 1A). SsNEP1 contains a third cysteine residue, and SsNEP2 contains three additional cysteines. The third cysteine in SsNEP1 and two of the three additional cysteines in SsNEP2 are conserved among the orthologues in Botrytis species, but not so in other NLPs.
Figure 1.
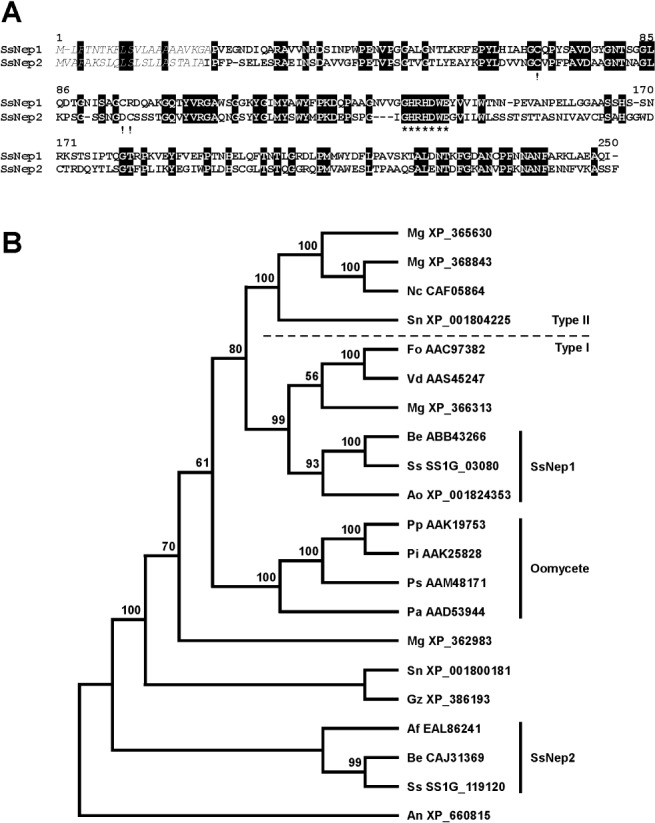
Sclerotinia sclerotiorum necrosis and ethylene‐inducing peptides (SsNEPs). (A) Alignment of SsNEP1 and SsNEP2 showing identical residues (white on black background), signal peptides (italics), conserved cysteine residues (!) indicative of Type I NEP1‐like proteins (NLPs) and the GHRHDWE motif (*). (B) Phylogenetic relationship between NLPs from various fungi and oomycetes. The proteins used in the analysis are coded with the first two letters representing the organism followed by the GenBank accession number. Af, Aspergillus fumigatus; As, Aspergillus nidulans; Ao, Aspergillus oryzae; Be, Botrytis elliptica; Fo, Fusarium oxysporum; Mg, Magnaporthe grisea; Pa, Pythium aphanidermatum NEP1; Pi, Phytophthora infestans; Pp, Phytophthora parasitica; Ps, Phytophthora sojae; Sn, Stagonospora nodorum; Ss, Sclerotinia sclerotiorum; Vd, Verticillium dahliae. Type I and Type II NLPs were classified according to Gijzen and Nürnberger (2006). Confidence values for each node are based on 100 bootstrap analyses.
In planta expression of SsNEPs
Recombinant Potato virus X (PVX) vectors allowing for the expression of SsNep1 and SsNep2 under the direction of the cauliflower mosaic virus (CaMV) promoter were introduced into Nicotiana benthamiana leaves. In each case, endogenous SsNEP signal peptides were included in the heterologous expression vectors, and therefore the proteins were expected to be secreted into the apoplast. Constructs expressing either SsNep1 or SsNep2 developed lesions within 10–12 days post‐inoculation (dpi). The lesion started near the margin of the inoculation site and then proceeded to expand to the surrounding tissue by 14–16 dpi (Fig. 2). We did not observe any differences in the rate of lesion development or the severity of symptoms between the SsNep1 and SsNep2 constructs.
Figure 2.
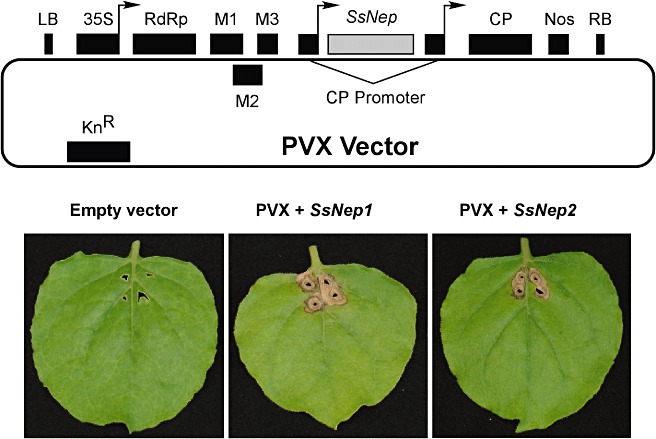
Map of the Potato virus X‐based binary vector pgR107 used for the in planta expression of Sclerotinia sclerotiorum necrosis and ethylene‐inducing peptide genes (SsNep). Elements shown are: LB, left border; 35S, cauliflower mosaic virus 35S promoter; RdRp, viral RNA‐dependent RNA polymerase; M1–M3, viral movement proteins; CP, coat protein, Nos, Agrobacterium tumefaciens nopaline synthetase transcriptional terminator; RB, right border. Necrotic symptoms observed on Nicotiana benthamiana leaves 2 weeks after inoculation with PVX virus, PVX +SsNep1 or PVX +SsNep2.
Expression of SsNep1 and SsNep2 during infection and in response to different surfaces
We examined the expression of SsNep1 and SsNep2 during the course of infection. Necrotic symptoms were visible within 6 h post‐inoculation (hpi) of Brassica napus leaves with S. sclerotiorum. By 24 hpi, most of the area beneath the inocula had become necrotic and, by 48 hpi, the leaf tissue was completely macerated. SsNep1 transcripts were detected at very low levels from 6 to 24 hpi (Fig. 3), but were undetectable at 48 hpi. SsNep2 transcripts were also detected within 6 hpi and peaked at 24 hpi. The expression of SsNep1 was always much less than that of SsNep2, with the former often being barely detectable using either Northern blotting or reverse transcriptase‐polymerse chain reaction (RT‐PCR).
Figure 3.
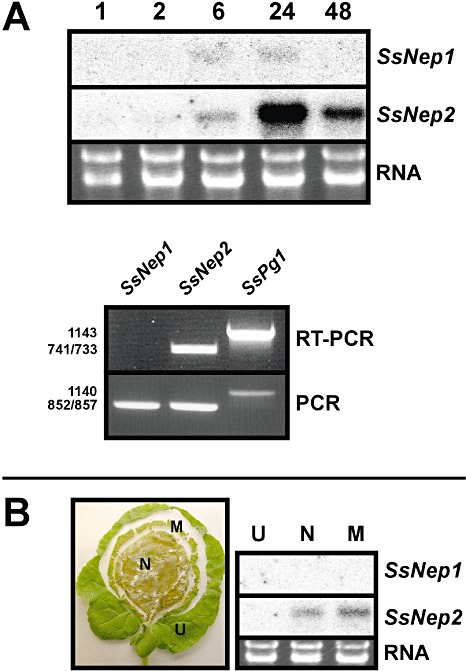
Expression of Sclerotinia sclerotiorum necrosis and ethylene‐inducing peptide genes (SsNep) during infection of Brassica napus leaves. (A) Northern blot analysis showing expression in mycelia at various times (hours) after inoculation. The total RNA loaded in each lane is also shown. Lower panel shows SsNep1, SsNep2 and SsPg1 reverse transcriptase‐polymerase chain reaction (RT‐PCR) products amplified from mycelia 24 h after inoculation. Amplification of the corresponding loci using genomic DNA was conducted as a positive PCR control. (B) Division of the lesion into uninfected (U), margin (M) and necrotic (N) zones and Northern blot analysis showing expression in each zone.
We also examined the expression of SsNep genes in various infection zones, namely necrotic tissue, the lesion margin and uninfected or asymptomatic tissue (Fig. 3). SsNep1 transcripts were not detected using Northern blotting in any of the zones tested, whereas SsNep2 was expressed in the necrotic region and the expanding margin of the lesion.
Expression of SsNep2 on different surfaces
A previous study found that the E. carotovora Nep gene was expressed only when the bacterium was grown on solid medium (Mattinen et al., 2004). Therefore, we examined the effect of different contact surfaces on SsNep2 expression, the more highly expressed of the two S. sclerotiorum Nep genes. SsNep2 transcripts were not detected in liquid medium, including minimal salts (MS) (starvation), MS–glucose or MS supplemented with finely granulated leaf wax (Fig. 4). Conversely, when mycelia were placed on solid or semi‐solid surfaces (intact leaves, leaves stripped of wax, leaf wax on glass or glass), SsNep2 expression was sharply induced within 24 h. Interestingly, SsNep2 was not expressed in response to contact with the hydrophobic material Parafilm™ in either the presence or absence of glucose.
Figure 4.
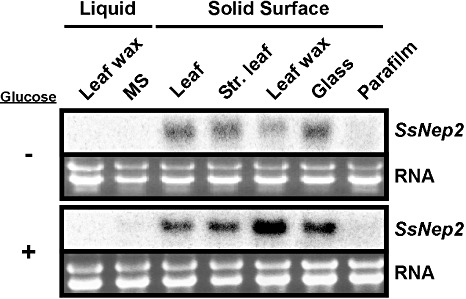
Northern blot analysis showing the expression of Sclerotinia sclerotiorum necrosis and ethylene‐inducing peptide 2 gene (SsNep2) in mycelia in various liquid media or after transfer to solid surfaces in the presence or absence of glucose. The bottom panel shows the total RNA loaded in each lane.
Role of calcium signalling and cAMP in SsNep2 regulation
To study the role of calcium in SsNep2 expression, we tested the effect of several compounds that affect calcium signalling. Caffeine increases cytosolic calcium levels by activating ryanodine receptors on the endoplasmic reticulum, leading to the release of internal calcium stores (Berridge, 1997). The treatment of mycelia with 20 mm caffeine resulted in a near‐complete inability to penetrate the B. napus leaf cuticle, whereas 2.5 mm caffeine did not affect virulence (Fig. 5). Both concentrations of caffeine decreased SsNep2 expression after 24 h on the leaf surface (Fig. 6). Lanthanum chloride, a plasma membrane calcium channel blocker, greatly reduced S. sclerotiorum virulence and expression of SsNep2. Ethylene Glycol Tetraacetic Acid (EGTA), which chelates extracellular calcium ions, had no effect, suggesting that mobilization of internal calcium stores is required for infection and SsNep2 expression. Compounds 48/80 and U73122 inhibit phospholipase C activity, but were found to promote the release of calcium from intracellular stores (Mogami et al., 1997). Treatment with 48/80 slightly reduced virulence; however, both reduced SsNep2 expression. None of the compounds tested above were found to inhibit mycelial growth at the concentrations used (Fig. 5). Calcineurin, a calcium‐ and calmodulin‐dependent Ser/Thr protein phosphatase, has been shown to play a central role in fungal pathogenesis (Fox and Heitman, 2002). The calcineurin inhibitor cyclosporin A reduced virulence and strongly reduced the expression of SsNep2, although mycelial growth was also reduced. Northern blot analysis was conducted with similar amounts of total RNA from each sample; however, to determine the extent to which the treatments affected housekeeping gene expression, the same blots were probed with the SsActin gene. The intensity of the hybridization signal in the control lane for both SsNep2 and SsActin was similar. In all cases in which SsNep2 expression was reduced, the level of actin expression was similar to the control or was affected to a far lesser degree than SsNep2, indicating that the compounds had a greater effect on SsNep2 than SsActin expression.
Figure 5.
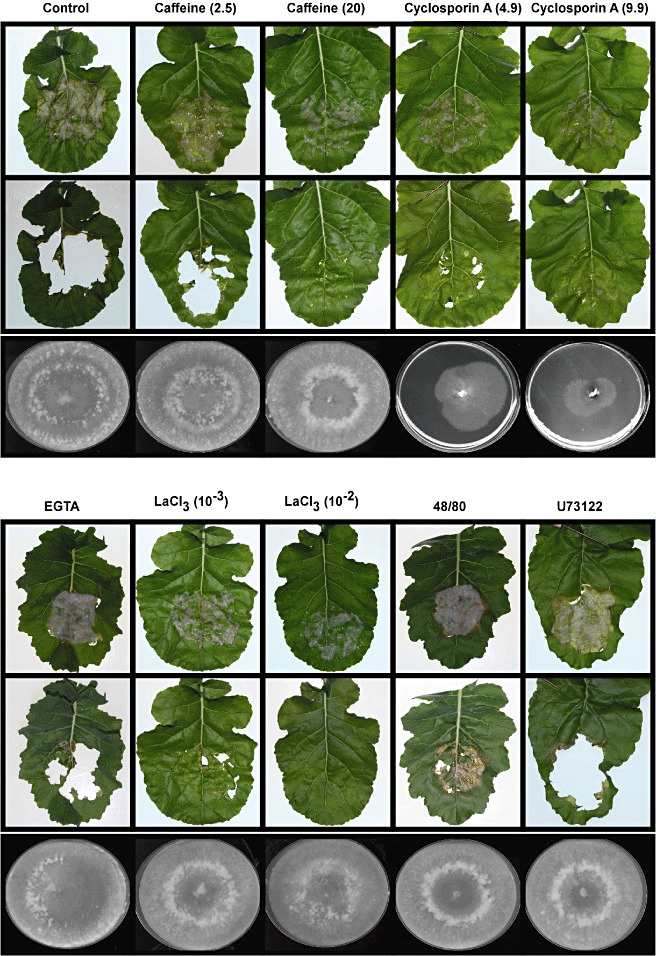
The effect of compounds affecting cyclic adenosine monophosphate (cAMP) levels (caffeine) and calcium signalling on Sclerotinia sclerotiorum infection of Brassica napus leaves and expression of S. sclerotiorum necrosis and ethylene‐inducing peptide 2 gene (SsNep2). The top panels show leaves 16 h after inoculation (upper frame), as well as the lesions beneath (lower frame), for mycelia that were untreated (control) or treated with caffeine (2.5 and 20 mm), cyclosporin A (CspA; 4.9 and 9.9 nm), [ethylenebis(oxonitrilo)]tetraacetic acid (EGTA) (10 mm), lanthanum chloride (LaCl3; 1 and 10 mm), compound 48/80 (5 µm) or compound U73122 (10 mm). The bottom panels show the effect of these compounds on new mycelial growth. A sample of the mycelial preparation used to inoculate the leaves was also placed in the centre of a minimal salts–glucose (MS‐Glu) plate and radial growth was assessed after 5 days.
Figure 6.
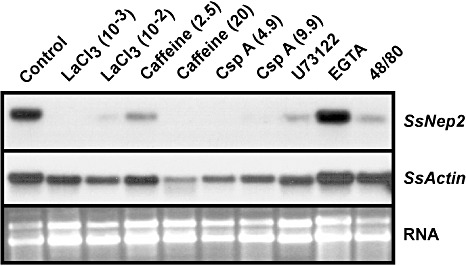
Northern blot analysis showing the expression of Sclerotinia sclerotiorum necrosis and ethylene‐inducing peptide 2 (SsNep2) and SsActin genes in mycelia treated with compounds affecting cyclic adenosine monophosphate (cAMP) levels (caffeine) and calcium signalling and placed on B. napus leaves for 16 h. The bottom panel shows the total RNA loaded in each lane.
Caffeine also elevates internal cAMP levels by inhibiting cAMP‐phosphodiesterase activity, which converts cAMP to a non‐cyclic form (Berridge, 1997). Several studies have reported that cAMP signalling plays a role in many aspects of fungal biology, including virulence and development (D'Souza and Heitman, 2001; Rollins and Dickman, 1998). To test the effect of cAMP on the colonization of B. napus by S. sclerotiorum, we monitored lesion formation and SsNep2 expression after application of exogenous cAMP (Fig. 7). At 0.1 and 1 mm concentrations, cAMP had no effect on disease development or SsNep2 expression; however, at 10 mm, lesion formation was severely reduced. SsNep2 expression also decreased; however, the expression of SsActin also declined with increasing cAMP levels, indicating that the loss of virulence may be a result of factors in addition to reduced SsNep2 expression. The S. sclerotiorum adenylate cyclase deletion mutant, sac1, which is unable to generate cAMP, was also non‐pathogenic (Jurick and Rollins, 2007). This mutant grew very slowly and, although mycelial rRNA levels were similar to those of the wild‐type strain, we were unable to detect transcripts from SsNep2 or SsActin, indicating that the mutation has broad pleiotrophic effects. Furthermore, the application of exogenous cAMP to the sac1 mutant failed to restore pathogenicity or SsNep2 expression, although SsActin expression was not detected in the sac1 mutant.
Figure 7.
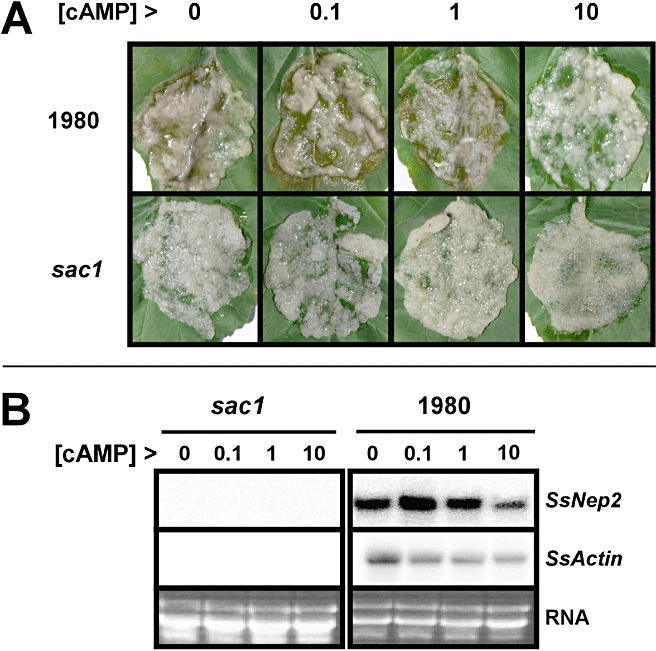
The effect of exogenous cyclic adenosine monophosphate (cAMP) application on Sclerotinia sclerotiorum infectivity and SsNep2 expression. (A) Lesions forming on Brassica napus leaves when mycelia from S. sclerotiorum strain 1980 and the sac1 adenylate cyclase mutant were treated with increasing levels of cAMP (mm) prior to inoculation. (B) Northern blot analysis showing SsNep2 expression 24 h after inoculation of B. napus leaves with mycelia treated with cAMP. The bottom panel shows the total RNA loaded in each lane.
DISCUSSION
The induction of host cell death is crucial for S. sclerotiorum pathogenicity (Dickman et al., 2001) and its genome was found to contain two NLP genes. The Phytophthora sojae and Phytophthora ramorum genomes contain 50–60 NLP genes and pseudogenes, whereas the Magnaporthe grisea and Giberella zeae genomes contain only four (Gijzen and Nürnberger, 2006). The S. sclerotiorum NEP proteins were most similar to those from Botrytis elliptica (Staats et al., 2007), the genus closest to Sclerotinia. Although SsNEPs are highly divergent, each possessed features highly conserved among NLPs, and were effective in causing necrosis when introduced into B. napus leaves using the PVX expression system.
In S. sclerotiorum strain 1980, SsNep2 was expressed at a much higher level than SsNep1 during the infection of B. napus leaves, which was confirmed by both Northern blot and RT‐PCR analysis. The differential expression of genes encoding pathogenicity factors has been shown elsewhere. For example, in Fusarium solani f. sp. pisi, the pectate lyase gene pelD was expressed only in infected pea tissue, whereas pelA expression was undetectable (Guo et al., 1996; Rogers et al., 2000). We also noted that SsNep2 was expressed soon after mycelial inoculation and in both the necrotic region and at the expanding margin of the lesion. In the hemibiotrophic pathogen P. sojae, the highest level of Nep gene expression coincided with the transition from the biotrophic to the necrotrophic stage (Qutob et al., 2002). There is an accumulating body of evidence that NLPs contribute to virulence. Transgenic C. coccodes expressing Nep1 from F. oxysporum was more virulent on the original host and was able to infect tobacco and tomato lines that were resistance to wild‐type C. coccodes (Amsellem et al., 2002). Similarly, expression of the E. carotovora Nip gene, which encodes an NLP, resulted in larger lesions and more rotting of host tissues (Mattinen et al., 2004).
The expression of SsNep2 was also found to be regulated by the physical properties of the contact surface. SsNep2 transcripts were not detected in MS‐based liquid cultures, but were induced by mycelial contact with a solid or semi‐solid surface. Many studies have examined the effect of physical properties and chemical signals associated with the leaf surface on pathogen development and the differentiation of infectious structures. Appressoria formation by Colletotrichum species was related to leaf topography (Kolattukudy et al., 1995), whereas, in Blumeria graminis f. sp. hordei, these were formed in response to barley leaf wax (Tsuba et al., 2002). The induction of SsNep2 expression in response to contact with a solid surface is in accordance with the observation that it is expressed at very early stages (detectable within 6 h by Northern blot analysis) of the infection. Interestingly, NLPs may affect the structure of the host cuticle, as application of F. oxysporum NEP1 to leaves of several plant species results in a thinning and/or sloughing of the cuticle (Keates et al., 2003). This may be a result of the direct interaction of NEP1 with lipophilic compounds in the cuticle, or to chloroplast disruption and reprofiling of gene expression that occurs soon after NEP1 application (Keates et al., 2003). Regardless of the underlying mechanism, the rapid induction of NLP gene expression by contact with the leaf surface and the ability to erode the cuticle probably allows NLPs to work in concert with cutinases and plant cell wall‐degrading enzymes to breach this primary plant defence.
In Colletotrichum, several genes are induced in response to contact with solid surfaces, including Colletotrichum Hard Surface Induced Protein 1 (CHIP1), which encodes a ubiquitin‐conjugating enzyme, CHIP2, encoding a transcription factor, and CHIP3, which has been predicted to encode a membrane‐localized protein. The expression of these genes under these circumstances is mediated by calcium and calmodulin signalling pathways (1998, 2000; Liu and Kolattukudy, 1998). We showed that compounds affecting intracellular calcium levels (caffeine, compounds 48/80 and U73122) also affect SsNep2 expression, although the last two compounds had little if any effect on virulence at the concentrations tested. Conversely, the downregulation of SsNep2 expression after treatment with the general calcium channel blocker lanthanum chloride coincided with a reduction in virulence. Such compounds are known to have pleiotrophic effects and the expression of the housekeeping gene SsActin was also affected, although not to the same degree as SsNep2. The importance of calcium‐dependent signalling on SsNep2 expression was also indicated by the inhibition of calcineurin, a calcium‐ and calmodulin‐dependent Ser/Thr protein phosphatase, by cyclosporin A, which resulted in a reduction in virulence and the expression of SsNep2. Harel et al. (2006) showed that inhibition of calcineurin impairs sclerotial development without affecting the secretion of oxalic acid, which is another important virulence factor.
cAMP was also found to be involved in the regulation of SsNep2 expression and S. sclerotiorum pathogenicity; however, this may be related to the fine control of cAMP levels. The S. sclerotiorum sac1 mutant, in which the adenylate cyclase gene was deleted, is cAMP deficient and exhibits altered mycelial development and is non‐pathogenic (Jurick and Rollins, 2007). We observed that the elevation of cAMP levels by the application of exogenous cAMP also reduced pathogenicity. Some of the developmental abnormalities associated with the adenylate cyclase mutant were corrected by the application of exogenous cAMP (Jurick and Rollins, 2007), however, this was not the case for pathogenicity in our study. Interestingly, the expression of SsNep2 or SsActin was not observed in the sac1 mutant under any conditions, suggesting that the mutation may have far‐reaching pleiotrophic effects.
In summary, we have shown that S. sclerotiorum NEPs are able to cause necrosis when expressed in planta, but are differentially expressed during infection. The initial expression of, at least, SsNep2 is dependent on the interaction with solid surfaces, and calcium and cAMP signalling are probably involved. Although the lack of SsNep2 expression often coincided with reduced virulence or a loss of pathogenicity, some pharmacological treatments reduced SsNep2 expression without any apparent affect on virulence. Interestingly, expressed sequence tags generated from cDNA libraries made from infected B. napus (Li et al., 2004a) or infection cushions (Sexton et al., 2006) failed to identify genes encoding either SsNEP. As such, further studies, such as the testing of SsNep mutants, are required to determine their overall contribution to pathogenesis.
EXPERIMENTAL PROCEDURES
Cultivation of fungi
Sclerotinia sclerotiorum strain 1980 (ATCC18683) was propagated on minimal salts–glucose (1% w/v) (MS‐Glu) agar according to Li et al. (2004a). The adenylate cyclase deletion mutant, sac1, was obtained from J. Rollins (Jurick and Rollins, 2007). The inoculum for all experiments was prepared by grinding 2 g of sclerotia in 200 mL of MS‐Glu in a Waring blender for 4 min. The volume was increased to 500 mL in a 1 L baffled flask, and the culture was incubated at 20 °C with shaking (60 r.p.m.) for 3 days.
Isolation and analysis of SsNep1 and SsNep2
RNA was extracted from B. napus leaves infected with S. sclerotiorum as described below. cDNA was synthesized using an oligo dt20 primer and Superscript 2 reverse transcriptase (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Using the cDNA as template, the SsNep1 and SsNep2 open reading frames were amplified using SsNep1‐F (5′‐ATCAGGATCCATGCTTTTCACCAACACA‐3′) and SsNep1‐R (5′‐ATAAGAATGCGGCCGCTTAGATTTGTGCCTCTGCGAG‐3′) or SsNep2‐F (5′‐TACTGAATTCGTCGACGGATCCATGGTTGCCTTTGCCAAATCTC‐3′) and SsNep2‐R (5′‐ACAACTCGAGGCGGCCGCCTAGAAACTACTAGCCTTCAC‐3′), respectively. As a result of the low levels of SsNep1 transcripts, the product from the first PCR (50 cycles) was purified and re‐amplified to generate sufficient material for cloning. SsPg1 was amplified using SsPg1‐F (5′‐CTCGAGAAGAGAGCTCCAGCACCAGCACCA‐3′) and SsPg1‐R (5′‐GGAGGAAGAGACGGTACCGTGAACG‐3′).
Signal peptides were identified using SignalP and N‐linked or O‐linked glycosylation sites employing NetNGlyc 1.0 and NetOGlyc 2.0 (http://www.expasy.org), respectively. Intron–exon boundaries were inferred using NNSPLICE 0.9 (http://www.fruitfly.org/seq_tools/splice.html). Dendograms arising from phylogenetic analysis were constructed according to the neighbour‐joining method, and confidence values for the branches were determined using bootstrap analysis, where 100 trees were generated from randomly resampled data obtained by clustalw.16 as provided in phylip version 3.5C (distributed by J. Felsenstein at http://www.cbr.nrc.ca/cgi‐bin/WebPhylip/index.html). The clustalw alignment was performed using the Blosum scoring matrix with the following gap penalties; opening gap (10), end gap (10), extending gap (0.05) and separation gap (0.05).
In planta production of SsNEPs via the PVX system
The amplified SsNep1 fragment was digested with BamHI and NotI and cloned into the GATEWAY entry vector pTK172 (a modified GATEWAY entry 1A vector with zeocin resistance; a gift from K. Rozwadowski, AAFC, Saskatoon). To convert pGR107 (Chapman et al., 1992) into a GATEWAY destination vector, the GATEWAY cassette A was cloned into the SmaI site of pGR107 as a blunt end fragment. This vector was named pGR107‐GWY. The SsNep1 open reading frame was transferred from pTK172 to pGR107‐GWY by recombination according to the GATEWAY protocol (Invitrogen). The SsNep2 open reading frame was amplified and cloned into pGEM‐T Easy (Promega, Madison, WI, USA). The insert was excised from the pGEM‐T Easy vector by digestion with SalI and XhoI and cloned into the SalI site of the PVX vector pGR107 (Chapman et al., 1992). The PVX constructs were transferred to Agrobacterium tumefaciens Gv3101 and grown on Luria broth agar. Leaves were inoculated by piercing the base of the leaf with a bacteria‐laden toothpick near the main vein of 4–6‐week‐old N. benthamiana plants.
Expression of SsNep genes during infection
To study the expression of SsNep1 and SsNep2 in different infection zones, 1 g of mycelia (wet weight) was spread over a 5‐cm‐diameter circle of a B. napus cv. Westar leaf surface and incubated in a humidified chamber. Newer leaves of approximately 7 cm in diameter from 45‐day‐old plants were used. Tissue was collected from several regions, including unaffected tissues beyond the lesion, a 0.5‐cm zone at the edge of the lesion and necrotic tissue. In cases in which the leaf was degraded, the lesion was collected and mycelia were separated from any remaining leaf tissue in a Petri dish. A time course study was also conducted in which the samples were collected and processed for transcript analysis.
Expression of SsNep2 in response to surface contact
Mycelia were spread over the surface of intact B. napus leaves, leaves stripped of surface wax, Parafilm™ and glass Petri dishes with or without a coating of leaf wax. Leaf wax was isolated by dipping B. napus leaves in a chloroform bath for 30 s and evaporating the chloroform under a stream of liquid nitrogen. Wax was applied by dissolving the crystals in a small volume of chloroform, spreading it to form a thin film on the Petri dish and allowing the chloroform to evaporate. Samples were incubated on the various surfaces in a humidified chamber at 20 °C, after which the mycelia were harvested, frozen in liquid nitrogen and RNA was extracted for Northern blot analysis.
Pharmacological studies
Mycelia were grown in MS‐Glu liquid medium as described above, washed with MS medium and then resuspended in MS‐Glu with compounds known to affect calcium signalling and cAMP levels. The mycelia were incubated with the compounds for 18 h at 20 °C in the dark prior to use. Compound U73122 was dissolved in 95% ethanol and used at a final concentration of 10 mm. EGTA was used at 10 mm, caffeine at 2.5 and 20 mm, compound 48/80 at 5 µm and lanthanum chloride at 1 and 10 mm dissolved in water. Cyclosporin A was dissolved in dimethylsulfoxide (DMSO) and used at final concentrations of 4.9 and 9.9 nm. Mycelia were transferred to B. napus leaves and maintained under humid conditions for 16 h, at which time mycelia were collected and used for Northern blot analysis.
The effect of these compounds on mycelial growth was tested by placing mycelia, treated in the same manner as B. napus leaves, on MS‐Glu agar and incubating at 20 °C.
RNA isolation, Northern blotting and RT‐PCR analysis
To isolate total fungal RNA, 150 mg of mycelia (wet weight) was ground in liquid N2 and dispensed into a 1.5‐mL microcentrifuge tube containing 600 µL of extraction buffer [0.1 m NaCl, 2% sodium dodecylsulfate (SDS), 50 mm Tris–HCl (pH 9.0), 10 mm ethylenediaminetetraacetic acid (EDTA)] and 600 µL phenol–chloroform–isoamyl alcohol (25 : 24 : 1). Samples were mixed for 30 s using a vortex and centrifuged for 10 min at 15 000 g. The aqueous phase was extracted again with an equal volume of chloroform, transferred to a new microcentrifuge tube and the RNA was precipitated using lithium chloride (2 m final concentration) overnight at 4 °C. The pellet was washed once with 2 m lithium chloride and once with 75% ethanol. RNA was air dried for 10 min and dissolved in 35 µL diethylpyrocarbonate (DEPC)‐treated double‐distilled H2O. Northern blotting was performed as described in Li et al. (2004b). SsNep cDNA was amplified using the same primers as mentioned above, labelled with 32P‐CTP and used as a probe. A segment of the SsActin gene (GenBank accession no. XP_001589969) was amplified using SsActinF (5′‐CAGCGTTCTACGTCTCTATC‐3′) and SsActinR (5′‐CGAACATCAACATCACAC‐3′) primers.
For RT‐PCR analysis, mRNA was converted to cDNA using the Superscript First Strand Synthesis System (Invitrogen), followed by PCR, employing the primer pairs described above. The PCR mixture contained 2.5 µL of 10 × buffer, 2 µL of deoxynucleoside triphosphates (dNTPs) (5 mm), 2 µL of forward primer (2.5 mm), 2 µL of reverse primer (2.5 mm), 0.5 µL of rTaq polymerase I, 2 µL of a 1/20th dilution of cDNA and 14 µL of distilled H2O. A 2‐µL aliquot of genomic DNA (40 ng/µL) was used as a control. The PCR conditions used were as follows: initial denaturation at 94 °C for 5 min, followed by 27 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 30 s, and a final extension of 72 °C for 7 min.
ACKNOWLEDGEMENTS
We thank the Saskatchewan Agriculture Development Fund, the Saskatchewan Canola Development Commission and the Agriculture and Agri‐Food Canada Matching Investments Initiative for providing funding to support this work. We thank Diana Bekkaoui and Elena Beynon for technical assistance.
REFERENCES
- Amsellem, Z. , Cohen, B.A. and Gressel, J. (2002) Engineering hypovirulence in a mycoherbicidal fungus for efficient weed control. Nat. Biotechnol. 20, 1035–1039. [DOI] [PubMed] [Google Scholar]
- Bae, H. , Moon, S.K. , Richard, C.S. , Bae, H.‐J. and Bailey, B. (2006) Necrosis and ethylene inducing peptide from Fusarium oxysporum induces a complex cascade of transcripts associated with signal transduction and cell death in Arabidopsis. Plant Physiol. 141, 1056–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey, B.A. (1995) Purification of a protein from culture filtrate of Fusarium oxysporum that induces ethylene and necrosis in leaves of Erythroxylum coca . Phytopathology, 85, 1250–1255. [Google Scholar]
- Berridge, M.J. (1997) Elementary and global aspects of calcium signaling. J. Physiol. 499, 291–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland, G.J. and Hall, R. (1994) Index of plant hosts of Sclerotinia sclerotiorum . Can. J. Plant Pathol. 16, 93–100. [Google Scholar]
- Cessna, S.G. , Sears, V.E. , Dickman, M.B. and Low, P.S. (2000) Oxalic acid, a pathogenicity factor for Sclerotinia sclerotiorum, suppresses the oxidative burst of the host plant. Plant Cell, 12, 2191–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman, S. , Kavanagh, T.A. and Baulcombe, D.C. (1992) Potato virus X as a vector for gene expression in plants. Plant J. 2, 549–557. [DOI] [PubMed] [Google Scholar]
- Dickman, M.B. , Park, Y.K. , Oltersdorf, T. , Li, W. , Clemente, T. and French, R. (2001) Abrogation of disease development in plants expressing animal antiapoptotic genes. Proc. Natl. Acad. Sci. USA, 98, 6957–6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza, C.A. and Heitman, J. (2001) Conserved cAMP signalling cascades regulate fungal development and virulence. FEMS Microbiol. Rev. 25, 349–364. [DOI] [PubMed] [Google Scholar]
- Fellbrich, G. , Romanski, A. , Varet, A. , Blume, B. , Brunner, F. , Enelhardt, S. , Felix, G. , Kemmerling, B. , Krzymowska, M. and Nurnberger, T. (2002) NPP1, a Phytophthora‐associated trigger of plant defense in parsley and Arabidopsis. Plant J. 32, 375–390. [DOI] [PubMed] [Google Scholar]
- Fox, D.S. and Heitman, J. (2002) Good fungi gone bad: the corruption of calcineurin. BioEssays, 24, 894–903. [DOI] [PubMed] [Google Scholar]
- Garcia, O. , Macedo, J. , Tiburcio, R. , Zaparoli, G. , Rincones, J. , Bittencourt, L. , Ceita, G. , Micheli, F. , Gesteira, A. , Mariano, A. , Schiavinato, M. , Medrano, F. , Meinhardt, L. , Pereira, G. and Cascardo, J. (2007) Characterization of necrosis and ethylene‐inducing proteins (NEP) in the basidiomycete Moniliophthora perniciosa, the causal agent of witches' broom in Theobroma cacao . Mycol. Res. 3, 443–455. [DOI] [PubMed] [Google Scholar]
- Gijzen, M. and Nürnberger, T. (2006) Nep1‐like proteins from plant pathogens: recruitment and diversification of the NPP1 domain across taxa. Phytochemistry, 67, 1800–1807. [DOI] [PubMed] [Google Scholar]
- Godoy, G. , Steadman, J.R. , Dickman, M.B. and Dam, R. (1990) Use of mutants to demonstrate the role of oxalic acid in pathogenicity of Sclerotinia sclerotiorum on Phaseolus vulgaris . Physiol. Mol. Plant Pathol. 37, 179–191. [Google Scholar]
- Guo, W. , Gonzalez‐Candelas, L. and Kolattukudy, P.E. (1996) Identification of a novel pelD gene expressed uniquely in planta by Fusarium solani f. sp. pisi (Nectria haematococca, mating type VI) and characterization of its protein product as an endo‐pectate lyase. Arch. Biochem. Biophys. 332, 305–312. [DOI] [PubMed] [Google Scholar]
- Harel, A. , Bercovic, S. and Yarden, O. (2006) Calcineurin is required for sclerotial development and pathogenicity of Sclerotinia sclerotiorum in an oxalic acid‐independent manner. Mol. Plant–Microbe Interact. 19, 682–693. [DOI] [PubMed] [Google Scholar]
- Hegedus, D. , Li, R. , Buchwaldt, L. , Parkin, I. , Whitwill, S. , Coutu, C. , Bekkaoui, D. and Rimmer, R. (2008) Brassica napus possesses an expanded set of polygalacturonase inhibitor protein genes that are differentially regulated in response to Sclerotinia sclerotiorum infection, wounding and defence hormone treatment. Planta, 228, 241–253. [DOI] [PubMed] [Google Scholar]
- Jennings, J.C. , Apel‐Birkhold, P.C. , Mock, N.M. , Baker, C.J. , Anderson, J.D. and Bailey, B.A. (2001) Induction of defense responses in tobacco by the protein Nep1 from Fusarium oxysporum . Plant Sci. 161, 891–899. [Google Scholar]
- Jores, J. , Appel, B. and Lewin, A. (2003) Cloning and molecular characterization of a unique hemolysin gene of Vibrio pommerensis sp. nov.: development of a DNA probe for the detection of the hemolysin gene and its use in identification of related Vibrio spp. from the Baltic Sea. FEMS Microbiol. Lett. 229, 223–229. [DOI] [PubMed] [Google Scholar]
- Jurick, W.M. and Rollins, J.A. (2007) Deletion of the adenylate cyclase (sac1) gene affects multiple developmental pathways and pathogenicity in Sclerotinia sclerotiorum . Fungal Genet. Biol. 44, 521–530. [DOI] [PubMed] [Google Scholar]
- Kars, I. , Krooshof, G.H. , Wagemakers, L. , Joosten, R. , Benen, J.A.E. and Van Kan, J.A.L. (2005) Necrotizing activity of five Botrytis cinerea endopolygalacturonases produced in Pichia pastoris . Plant J. 43, 213–225. [DOI] [PubMed] [Google Scholar]
- Keates, S.E. , Kostman, T.A. , Anderson, J.D. and Bailey, B.A. (2003) Altered gene expression in three plant species in response to treatment with Nep1, a fungal protein that causes necrosis. Plant Physiol. 132, 1610–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y.K. , Li, D. and Kolattukudy P.E. (1998) Induction of Ca2+‐calmodulin signalling by hard‐surface contact primes Colletotrichum gloeosporioides conidia to germinate and form appressoria. J. Bacteriol. 180, 5144–5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y.K. , Liu, Z.M. , Li, D. and Kolattukudy, P.E. (2000) Two novel genes induced by hard‐surface contact of Colletotrichum gloeosporioides conidia. J. Bacteriol. 182, 4688–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, K.S. , Min, J.‐Y. and Dickman, M.B. (2008) Oxalic acid is an elicitor of plant programmed cell death during Sclerotinia sclerotiorum disease development. Mol. Plant–Microbe Interact. 21, 605–612. [DOI] [PubMed] [Google Scholar]
- Kolattukudy, P.E. , Rogers, L.M. , Li, D. , Hwang, C.S. and Flaishman, M.A. (1995) Surface signaling in pathogenesis. Proc. Natl. Acad. Sci. USA, 92, 4080–4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, R. , Rimmer, R. , Buchwaldt, L. , Sharpe, A. , Séguin‐Swartz, G. , Coutu, C. and Hegedus, D.D. (2004a) Interaction of Sclerotinia sclerotiorum with a resistant Brassica napus cultivar: expressed sequence tag analysis identifies genes associated with fungal pathogenesis. Fungal Genet. Biol. 41, 735–753. [DOI] [PubMed] [Google Scholar]
- Li, R. , Rimmer, R. , Buchwaldt, L. , Sharpe, A. , Seguin‐Swartz, G. and Hegedus, D.D. (2004b) Interaction of Sclerotinia sclerotiorum with Brassica napus: cloning and characterization of endo‐ and exo‐polygalacturonases expressed during saprophytic and parasitic modes. Fungal Genet. Biol. 41, 754–765. [DOI] [PubMed] [Google Scholar]
- Liu, Z.M. and Kolattukudy, P.E. (1998) Identification of a gene product induced by hard‐surface contact of Colletotrichum gloeosporioides conidia as a ubiquitin‐conjugating enzyme by yeast complementation. J. Bacteriol. 180, 3592–3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattinen, L. , Tshuikina, M. , Mae, A. and Pirhonen, M. (2004) Identification and characterization of Nip, Necrosis‐Inducing Virulence Protein of Erwinia carotovora subsp. carotovora . Mol. Plant–Microbe Interact. 17, 1366–1375. [DOI] [PubMed] [Google Scholar]
- Mogami, H. , Lloyd Mills, C. and Gallacher, D.V. (1997) Phospholipase C inhibitor, U73122, releases intracellular Ca2+, potentiates Ins(1,4,5)P3‐mediated Ca2+ release and directly activates ion channels in mouse pancreatic acinar cells. Biochem. J. 324, 645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton, C.L. and Salmond, G.P.C. (2004) The Nep1‐like proteins—a growing family of microbial elicitors of plant necrosis. Mol. Plant Pathol. 5, 353–359. [DOI] [PubMed] [Google Scholar]
- Qutob, D. , Kamoun, S. and Gijzen, M. (2002) Expression of Phytophthora sojae necrosis‐inducing protein occurs during transition from biotrophy to necrotrophy. Plant J. 32, 361–373. [DOI] [PubMed] [Google Scholar]
- Qutob, D. , Kemmerling, B. , Brunner, F. , Kufner, I. , Engelhardt, S. , Gust, A.A. , Luberacki, B. , Seitz, U.H. , Stahl, D. , Rauhut, T. , Glawischnig, E. , Schween, G. , Lacombe, B. , Watanabe, N. , Lam, E. , Schlichting, R. , Scheel, D. , Nau, K. , Dodt, G. , Hubert, D. , Gijzen, M. and Numberger, T. (2006) Phytotoxicity and innate immune responses induced by Nep1 like proteins. Plant Cell, 18, 3721–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, L.M. , Kim, Y.K. , Guo, W. , Gonzalez‐Candelas, L. , Li, D. and Kolattukudy, P.E. (2000) Requirement for either a host‐ or pectin‐induced pectate lyase for infection of Pisum sativum by Nectria haematococca . Proc. Natl. Acad. Sci. USA, 97, 9813–9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins, J.A. and Dickman, M.B. (1998) Increase in endogenous and exogenous cyclic AMP levels inhibits sclerotial development in Sclerotinia sclerotiorum . Appl. Environ. Microbiol. 64, 2539–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten, A. , Van Baarlen, P. and Van Kan, J. (2008) Phytotoxic Nep1 like proteins from the necrotrophic fungus Botrytis cinerea associate with membranes and the nucleus of plant cells. New Phytol. 177, 493–505. [DOI] [PubMed] [Google Scholar]
- Sexton, A.C. , Cozijnsen, A.J. , Keniry, A. , Jewell, E. , Love, C.G. , Batley, J. , Edwards, D. and Howlett, B.J. (2006) Comparison of transcription of multiple genes at three developmental stages of the plant pathogen Sclerotinia sclerotiorum . FEMS Microbiol. Lett. 258, 150–160. [DOI] [PubMed] [Google Scholar]
- Staats, M. , Van Baarlen, P. , Schouten, A. and Van Kan, J. (2007) Functional analysis of NLP genes from Botrytis elliptica . Mol. Plant Pathol. 8, 209–214. [DOI] [PubMed] [Google Scholar]
- Tsuba, M. , Katagiri, C. , Takeuchi, Y. , Takada, Y. and Yamaoka, N. (2002) Chemical factors of the leaf surface involved in the morphogenesis of Blumeria graminis . Physiol. Mol. Plant Pathol. 60, 51–57. [Google Scholar]
- Van Kan, J. (2006) Licensed to kill: the lifestyle of a necrotrophic plant pathogen. Trends Plant Sci. 11, 247–253. [DOI] [PubMed] [Google Scholar]
- Veit, S. , Worle, J.M. , Nurnberger, T. , Koch, W. and Seitz, H.U. (2001) A novel protein elicitor (PaNie) from Pythium aphanidermatum induces multiple defence responses in carrot, Arabidopsis, and tobacco. Plant Physiol. 127, 832–841. [PMC free article] [PubMed] [Google Scholar]
- Zuppini, A. , Navazio, L. , Sella, L. , Castiglioni, C. , Favaron, F. and Mariani, P. (2005) An endopolygalacturonase from Sclerotinia sclerotiorum induces calcium‐mediated signalling and programmed cell death in soybean cells. Mol. Plant–Microbe Interact. 18, 849–855. [DOI] [PubMed] [Google Scholar]


