SUMMARY
The root‐knot nematode Meloidogyne javanica induces giant cells and feeds from them during its development and reproduction. To study the cellular processes underlying the formation of giant cells, laser microdissection was used to isolate the contents of early‐stage giant cells 4 and 7 days post‐infection (dpi) from tomato, and cDNA libraries from both stages were generated with 87 [250 expressed sequence tag (EST) clones] and 54 (309 EST clones) individual transcripts identified, respectively. These transcripts have roles in metabolism, stress response, protein synthesis, cell division and morphogenesis, transport, signal transduction, protein modification and fate, and regulation of cellular processes. The expression of 25 selected transcripts was studied further by real‐time quantitative reverse transcriptase‐polymerase chain reaction. Among them, 13 showed continuous up‐regulation in giant cells from 4 to 7 dpi. The expression of two transcripts was higher than in controls at 4 dpi and remained at the same level at 7 dpi; a further five transcripts were highly expressed only at 7 dpi. The Phi‐1 protein gene, a cell cycle‐related homologue in tobacco, was expressed 8.5 times more strongly in giant cells than in control cells at 4 dpi, but was reduced to 6.7 times at 7 dpi. Using in situ hybridization, the expression of the Phi‐1 gene was preferentially localized in the cytoplasm of giant cells at 4 dpi, together with a pectinesterase U1 precursor gene. The identification of highly expressed transcripts in developing giant cells adds to the knowledge of the plant genes responsive to nematode infection, and may provide candidate genes for nematode control strategies.
INTRODUCTION
Sedentary endoparasitic nematodes are the most economically important group of plant parasitic nematodes. They are obligate biotrophs and have intimate relationships with their hosts, where they can control the development of host cells after infection (Jones, 1981). Root‐knot nematodes (Meloidogyne spp.) establish feeding sites from provascular cells of the host root, and these act as the sole source of nutrition for the development and reproduction of the nematode. Several distinct processes occur during the establishment of feeding sites. J2 juveniles (J2s) invade roots behind the root tip, and then migrate between cells to reach the developing vascular cylinder. The J2s introduce secretions from their oesophageal gland cells into a group of selected provascular cells which re‐enter the cell cycle (Davis et al., 2000; Jones and Payne, 1978). These cells undergo repeated nuclear divisions without cytokinesis to form larger multinucleate cells, known as giant cells (Jones, 1981). Cortical cells around the developing giant cells also divide rapidly and expand to form a gall.
The expansion of giant cells and cytoskeleton rearrangements end at 10–14 days post‐infection (dpi) (de Almeida Engler et al., 2004; Jones, 1981). They become filled with dense cytoplasm and contain many organelles (Jones, 1981). Giant cells also have a high osmotic potential. From about 3 dpi, transfer cell‐like wall ingrowths form next to vascular tissues; these ingrowths become very extensive and increase the surface area of the cell membrane by up to 10‐fold to facilitate the uptake of nutrients to the developing nematode (Jones and Dropkin, 1976). These physiological and biochemical changes are accompanied by alterations in gene expression, not only in the giant cells, but also in neighbouring cells.
In studies on the responses of host plants to nematode infection, a number of differentially expressed genes have been identified in infected root tissues relative to control roots (Bird and Wilson, 1994; Fuller et al., 2007; Jammes et al., 2005; Wang et al., 2003). Other approaches have involved promoter trapping and transcriptional studies with β‐glucuronidase (GUS) (Favery et al., 1998, 2004; Goddijn et al., 1993), and the localization of expression of nematode responsive genes by in situ hybridization (Escobar et al., 2003; Lohar et al., 2004). However, as giant cells make up only a small fraction of the root cells, the analysis of gene expression at the single‐cell level is required to study these highly specialized nematode feeding cells (Ramsay et al., 2004). Until recently, the isolation of cytoplasmic contents from nematode feeding cells was limited to manual dissection and microaspiration (Bird and Wilson, 1994; Jammes et al., 2005; Juergensen et al., 2003; Wang et al., 2003). The feeding cell‐enriched tissues or giant cell contents obtained from such techniques have been subjected to molecular analysis, including the use of microarrays to study the genome‐wide expression profiles of Arabidopsis infected with root‐knot nematodes (Jammes et al., 2005; Wang et al., 2003). However, there are limitations to these methods, in that they cannot be applied effectively to study the early stages of development of giant cells.
Laser microdissection (LM) provides a solution to the isolation of the cytoplasmic contents from specific plant cells, including early‐stage nematode feeding cells (Day et al., 2005; Ramsay et al., 2006). Klink et al. (2005) constructed a syncytium‐specific cDNA library from mRNA obtained by LM of soybean roots infected with the soybean cyst nematode Heterodera glycines at 8 dpi. LM has also been coupled with the microarray technique to provide a comprehensive gene expression profile of developing syncytia in soybean roots induced by H. glycines (Ithal et al., 2007a). It is well established that there are fundamental differences between the development of syncytia and giant cells (Gheysen and Fenoll, 2002; Jones, 1981). The identification of changes in gene expression in giant cells using LM will advance the current knowledge of the processes of their induction, development and maintenance, and add to our understanding of the differences in development between giant cells and syncytia.
In this study, we have constructed two cDNA libraries using LM to isolate total RNA from early‐stage giant cells, 4 and 7 days after nematode infection. The transcripts known to be involved in a range of key cellular and biological processes, including the genes involved in cell division, metabolism (carbohydrate, lipid, energy, and nucleotide and amino acid) and cellular transport, were identified. The temporal and spatial analysis of gene expression of some of the transcripts identified was also performed. The differences in the cellular processes involved in the formation and development of giant cells and syncytia are discussed.
RESULTS
RNA from developing giant cell contents obtained by LM
A PALM Robot‐Combi LM system was used to isolate the cytoplasmic contents of early‐stage giant cells induced by Meloidogyne javanica from the roots of in vitro‐cultured tomato. Root galls were fixed, embedded in paraffin wax, sectioned and de‐waxed. Giant cell initials were clearly visible in treated sections and the morphology was adequately preserved. This allowed the identification and isolation of the contents of the developing giant cells (Fig. 1). As a control for the quantification of gene expression, cells adjacent to the vascular cylinder were similarly isolated from in vitro‐cultured tomato roots at the same age as the infected roots, but without nematode infection. For cDNA library construction, 100 ng of total RNA obtained from laser microdissected cells was subjected to two rounds of linear amplification using a T7 RNA‐based polymerase system to give up to 100 µg of amplified RNA (aRNA), which was also used for real‐time quantitative reverse transcriptase‐polymerase chain reaction (RT‐PCR) analysis.
Figure 1.
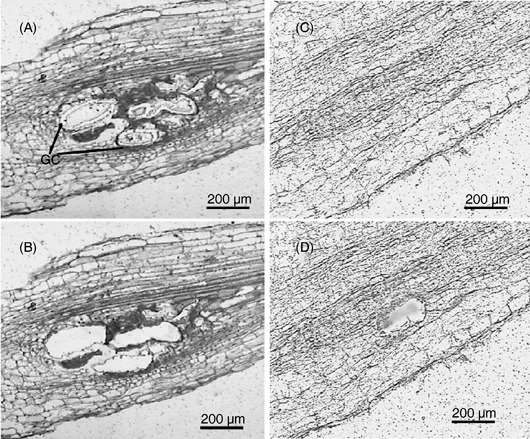
Laser microdissection (LM) of giant cells induced by the root‐knot nematode Meloidogyne javanica on roots of tomato. (A, B) Longitudinal sections through the root showing early‐stage giant cells before dissection (A) and with cell contents removed after LM (B). (C, D) Longitudinal sections through the root of a control plant (C) showing region of cells close to the vasculature (D) captured as control cells to calibrate gene expression of giant cell transcripts. GC, giant cells.
Transcript profile of giant cells at 4 dpi
The 4‐dpi giant cell‐specific cDNA library was constructed from cDNA synthesized from aRNA primed with Oligo‐dT and random hexamers. The expressed sequence tag (EST) clones were initially screened by PCR and restriction enzyme digestion analysis, and those selected for sequencing had an insert of at least 70 bp. The analysis of 250 EST clones generated 87 individual sequences (Table 1). The size of the cDNA inserts ranged from 76 to 648 bp. All the sequences have been submitted to the National Center for Biotechnology Information (NCBI) GenBank with accession numbers ES790116–ES790201. Most of the transcripts were identified stringently as tomato sequences when compared with the tomato gene index database from The Gene Index Project using the ‘blast’ search tool, most of which are known to express in tissues other than the root system. Some other transcripts were identified as homologues of genes sequenced from other plant species from the NCBI database, including Solanum tuberosum, Arabidopsis thaliana, Nicotiana tabacum and Nicotiana benthamiana, and also monocots, such as Oryza spp., Triticum aestivum and Zea mays. Eight of the transcripts did not match any known gene or protein sequence. Instead, they were identified as cDNA sequences from tomato (ES790139, ES790144, ES790156 and ES790178), S. tuberosum (ES790121, ES790125, ES790142 and ES790167) or Nicotiana spp. (ES790125). The transcript ES790177 had no similar match on any database.
Table 1.
Transcripts identified in the 4 days post‐infection (dpi) cDNA library generated from laser microdissected giant cells induced in tomato roots by Meloidogyne javanica.
| Clone | GenBank Accession No. | Highest homology search results | Functional category | blast score |
|---|---|---|---|---|
| 4LCMGC001 | ES790116 | Hypothetical protein {Arabidopsis thaliana} | Unclassified protein | 2.10E‐29 |
| 4LCMGC002 | ES790117 | Hydroxymethylglutaryl coenzyme A synthase | Metabolism | 7.70E‐50 |
| 4LCMGC003 | ES790118 | Pyruvate dehydrogenase E1 α subunit | Metabolism | 2.30E‐34 |
| 4LCMGC004 | ES790119 | Fibre protein Fb15 | Unknown function | 1.80E‐116 |
| 4LCMGC005 | ES790120 | 60S ribosomal protein L7A‐like | Cellular component, protein synthesis | 1.60E‐117 |
| 4LCMGC006 | ES790121 | cDNA sequence {Solanum tuberosum} | Unknown function | 5.00E‐94 |
| 4LCMGC007 | ES790122 | Unknown protein {A. thaliana} | Unclassified protein | 7.00E‐61 |
| 4LCMGC008 | ES790123 | Clathrin binding {A. thaliana} | Intercellular transport | 2.60E‐49 |
| 4LCMGC009 | ES790124 | DnaJ‐like protein | DNA processing | 1.90E‐39 |
| 4LCMGC010 | ES790125 | cDNA sequence from Solanum tuberosum, Nicotiana spp. | Unknown function | 8.00E‐11 |
| 4LCMGC011 | ES790126 | Peptidyl‐prolyl cis–trans isomerase (cyclophilin) | Protein modification | 4.00E‐37 |
| 4LCMGC012 | ES790127 | Lipid transfer protein, PVR3‐like protein | Transport | 4.70E‐41 |
| 4LCMGC013 | ES790128 | Expressed protein {A. thaliana} | Unclassified protein | 2.50E‐12 |
| 4LCMGC014 | ES790129 | S‐Adenosylmethionine decarboxylase | Metabolism, amino acid | 1.00E‐15 |
| 4LCMGC015 | ES790256 | Protein translation factor SUI1 homologue (GOS2 protein) | Protein synthesis | 3.00E‐37 |
| 4LCMGC016 | ES790130 | 40S ribosomal protein S8 | Cellular component, protein synthesis | 6.60E‐24 |
| 4LCMGC017 | ES790131 | 28‐kDa small subunit ribosomal protein | Cellular component, protein synthesis | 5.70E‐16 |
| 4LCMGC018 | ES790132 | Transaldolase | Metabolism, carbohydrate | 2.00E‐56 |
| 4LCMGC019 | ES790133 | Unknown protein {A. thaliana} | Unclassified protein | 1.50E‐67 |
| 4LCMGC020 | ES790134 | Calmodulin‐like protein | Regulation of cellular activities | 1.4E‐35 |
| 4LCMGC021 | ES790135 | Cyclopropane fatty acid synthase | Metabolism, lipid | 1.30E‐22 |
| 4LCMGC022 | ES790136 | Histone H1 | DNA processing | 1.20E‐16 |
| 4LCMGC023 | ES790137 | F‐box‐containing protein | Cell cycle regulation, signal transduction | 2.60E‐82 |
| 4LCMGC024 | ES790138 | Hypothetical protein {A. thaliana} | Unclassified protein | 5.30E‐40 |
| 4LCMGC025 | ES790139 | cDNA sequence {Lycopersicon esculentum} | Unknown function | 4.80E‐58 |
| 4LCMGC026 | ES790140 | Unknown protein {A. thaliana} | Unclassified protein | 3.40E‐59 |
| 4LCMGC027 | ES790141 | Early light‐inducible protein | Thylakoid biogenesis and rescue | 2.00E‐108 |
| 4LCMGC028 | ES790142 | cDNA sequence {S. tuberosum} | Unknown function | 4E‐86 |
| 4LCMGC029 | ES790143 | Glyceraldehyde 3‐phosphate dehydrogenase | Metabolism, energy | 5.70E‐44 |
| 4LCMGC030 | ES790144 | cDNA sequence {L. esculentum} | Unknown function | 2.7E‐56 |
| 4LCMGC031 | ES790145 | Cytochrome P450 monooxygenase CYP72B | Electron transfer | 1.50E‐90 |
| 4LCMGC032 | ES790146 | Cytosolic Cu,Zn superoxide dismutase (LeSODCC) | Regulation of cellular processes | 8.90E‐13 |
| 4LCMGC033 | ES790147 | ChaC‐like family protein‐like | Unclassified protein | 2.20E‐34 |
| 4LCMGC034 | ES790148 | 60S ribosomal protein L30 | Cellular component, protein synthesis | 1.50E‐26 |
| 4LCMGC035 | ES790149 | Ethylene response factor 5 | Transcription | 3.50E‐18 |
| 4LCMGC036 | ES790150 | ELI3 protein | Metabolism, carbohydrate | 8.20E‐13 |
| 4LCMGC037 | ES790151 | Chaperonin 21 precursor | Protein modification | 1.80E‐80 |
| 4LCMGC038 | ES790152 | Aspartyl aminopeptidase | Metabolism, amino acid | 6.50E‐20 |
| 4LCMGC039 | ES790153 | LescPth2, serine/threonine protein kinase Fen | Signal transduction | 1.20E‐06 |
| 4LCMGC040 | ES790154 | Unknown protein, heavy‐metal‐associated domain putative | Unknown function | 2.80E‐37 |
| 4LCMGC041 | ES790155 | Proteasome subunit β type 2‐A | Protein modification | 2.20E‐84 |
| 4LCMGC042 | ES790156 | cDNA sequence {L. esculentum} | Unknown function | 3.40E‐27 |
| 4LCMGC043 | ES790157 | 60S ribosomal protein L15 | Cellular component, protein synthesis | 3.60E‐12 |
| 4LCMGC044 | ES790158 | Unknown protein {A. thaliana} | Unclassified protein | 6.80E‐19 |
| 4LCMGC045 | ES790159 | Transcription factor {A. thaliana} | Transcription | 2.50E‐34 |
| 4LCMGC046 | ES790160 | Transaldolase | Metabolism, carbohydrate | 2.00E‐56 |
| 4LCMGC047 | ES790161 | RING‐H2 finger protein RHG1a‐like | Transcription | 9.70E‐20 |
| 4LCMGC048 | ES790162 | NADPH cytochrome P450 reductase CprA {Aspergillus fumigatus Af293} | Electron transfer | 6.40E‐59 |
| 4LCMGC049 | ES790163 | Heat shock protein 70‐3 | Regulation of cellular activities | 1.90E‐29 |
| 4LCMGC050 | ES790164 | Putative CPF 0172 family protein {A. thaliana} | Unclassified protein | 4.00E‐73 |
| 4LCMGC051 | ES790165 | Pectinesterase U1 precursor (pectin methylesterase) | Cell division and cellular morphogenesis | 3.30E‐25 |
| 4LCMGC052 | ES790166 | Calmodulin‐binding protein | Regulation of cellular activities | 1.20E‐49 |
| 4LCMGC053 | ES790167 | cDNA sequence {S. tuberosum} | Unknown function | 2E‐154 |
| 4LCMGC054 | ES790168 | 2‐Oxoglutarate‐dependent dioxygenase | Metabolism, energy | 6.10E‐63 |
| 4LCMGC055 | ES790169 | Cinnamyl alcohol dehydrogenase (CAD) | Metabolism, biosynthesis of secondary metabolites | 8.20E‐20 |
| 4LCMGC056 | ES790170 | Hypothetical protein {A. thaliana} | Unclassified protein | 3.70E‐73 |
| 4LCMGC057 | ES790171 | Ribosomal protein 117 | Cellular component, protein synthesis | 9.8E‐17 |
| 4LCMGC058 | ES790172 | ADP‐ribosylation factor‐like protein | Signal transduction | 2.50E‐24 |
| 4LCMGC059 | ES790173 | Histone H2B | DNA processing | 9.40E‐42 |
| 4LCMGC060 | ES790174 | Phenylalanine ammonia‐lyase | Metabolism, biosynthesis of secondary metabolites | 1.70E‐36 |
| 4LCMGC061 | ES790175 | Zinc finger transcription factor‐like protein | Transcription | 4.90E‐11 |
| 4LCMGC062 | ES790176 | G‐protein‐coupled receptor‐like protein | Signal transduction | 8.60E‐23 |
| 4LCMGC063 | ES790177 | No match to a known sequence | ||
| 4LCMGC064 | ES790178 | cDNA sequence {L. esculentum} | Unknown function | 2.90E‐73 |
| 4LCMGC065 | ES790179 | Clathrin‐binding protein‐like {A. thaliana} | Protein modification | 1.40E‐97 |
| 4LCMGC066 | ES790180 | RNA polymerase II largest subunit (fragment) | Transcription | 2.90E‐09 |
| 4LCMGC067 | ES790181 | Zinc finger protein | Transcription | 2.20E‐32 |
| 4LCMGC068 | ES790182 | Putative receptor protein kinase PERK1 {A. thaliana} | Signal transduction | 7.30E‐79 |
| 4LCMGC069 | ES790183 | RNA‐binding protein putative | Protein synthesis | 3.40E‐19 |
| 4LCMGC070 | ES790184 | Putative uncharacterized protein {A. thaliana} | Unclassified protein | 7.50E‐41 |
| 4LCMGC071 | ES790185 | Pectin methylesterase (PMEU1) | Cell division and cellular morphogenesis | 6.00E‐66 |
| 4LCMGC072 | ES790186 | AP2/ERF‐domain protein | Transcription | 5.80E‐21 |
| 4LCMGC073 | ES790187 | Sugar‐phosphate isomerase‐like protein | Metabolism, carbohydrate | 4.10E‐51 |
| 4LCMGC074 | ES790188 | Ubiquitin‐conjugating enzyme UBC2 | Protein modification | 7.90E‐54 |
| 4LCMGC075 | ES790189 | Phi‐1 protein (Nicotiana tabacum} | Cell division and cellular morphogenesis | 2.20E‐56 |
| 4LCMGC076 | ES790190 | Pectinesterase‐like protein | Cell division and cellular morphogenesis | 4.40E‐21 |
| 4LCMGC077 | ES790191 | Unknown protein {A. thaliana} | Unclassified protein | 1.50E‐29 |
| 4LCMGC078 | ES790192 | Microtubule‐associated protein MAP65‐1a | Unclassified protein | 3.1E‐28 |
| 4LCMGC079 | ES790193 | Methionine synthase | Metabolism, amino acid | 5.50E‐82 |
| 4LCMGC080 | ES790194 | Pathogenesis‐related TSI‐1 protein | Defence related | 4.70E‐20 |
| 4LCMGC081 | ES790195 | Arginine decarboxylase | Metabolism, amino acid | 4.60E‐46 |
| 4LCMGC082 | ES790196 | Resistance gene‐like, S. tuberosum ssp. andigena ry‐1 gene | Defence related | 1E‐25 |
| 4LCMGC083 | ES790197 | Ubiquitin‐protein ligase {A. thaliana} | Protein modification | 2.7E‐82 |
| 4LCMGC084 | ES790198 | Transcription initiation factor IIB (TFIIB) | Transcription | 4.90E‐125 |
| 4LCMGC085 | ES790199 | PIP‐type aquaporin | Cellular transport | 2.70E‐21 |
| 4LCMGC086 | ES790200 | Expressed but uncharacterized protein {A. thaliana} | Unclassified protein | 5.00E‐58 |
| 4LCMGC087 | ES790201 | Probable 60S ribosomal protein L32, mitochondrial precursor | Cellular component, protein synthesis | 3.70E‐22 |
Transcript profile of giant cells at 7 dpi
From aRNA obtained from giant cells at 7 dpi, 54 individual transcripts with sizes ranging from 81 to 610 bp were obtained from the sequencing of 309 cDNA clones (Table 2). These have accession numbers ES790202–ES790255 in the NCBI GenBank. From the blast search results, 42 of the transcripts matched characterized genes of tomato, Medicago truncatula and A. thaliana. The sequences of seven transcripts (ES790212, ES790224, ES790225, ES790232, ES790248, ES790249 and ES790255) did not match any characterized gene in the databases, although mRNA and/or cDNA sequences with significant matches have been identified from other plant species. For instance, clones of identical sequences to ES790212 exist in databases for cDNA from trichomes of N. benthamiana (GenBank Accession No. ES887635.1) and Humulus lupulus (L. cv. Phoenix) (GenBank Accession No. ES658159.1).
Table 2.
Transcripts identified in the 7 days post‐infection (dpi) cDNA library generated from laser microdissected giant cells induced in tomato roots by Meloidogyne javanica.
| Clone | GenBank Accession No. | Highest homology search results | Functional category | blast score |
|---|---|---|---|---|
| 7LCMGC001 | ES790202 | Ribosomal protein L27 | Cellular component, protein synthesis | 7.70E‐17 |
| 7LCMGC002 | ES790203 | Putative protein {Arabidopsis thaliana} | Unclassified proteins | 1.50E‐24 |
| 7LCMGC003 | ES790204 | Putative membrane‐associated protein {A. thaliana} | Unknown function | 3.10E‐14 |
| 7LCMGC004 | ES790205 | Putative protein {A. thaliana} | Unclassified proteins | 4.20E‐23 |
| 7LCMGC006 | ES790207 | Histone H4 {Capsicum annuum} | DNA processing | 4.70E‐47 |
| 7LCMGC007 | ES790208 | 26S proteasome regulatory particle triple‐A ATPase subunit 2b | Protein modification | 2.50E‐26 |
| 7LCMGC008 | ES790209 | Metallothionein‐like protein type 2 | Detoxification (regulation) of heavy metals in cells c | 9.50E‐24 |
| 7LCMGC009 | ES790210 | Peptidyl‐prolyl cis–trans isomerase (cyclophilin) | Protein modification | 5.20E‐14 |
| 7LCMGC010 | ES790211 | 40S ribosomal protein S15‐like | Cellular component, protein synthesis | 2.20E‐42 |
| 7LCMGC011 | ES790212 | cDNA sequence cloned from several plant species | Unknown function | 4.00E‐47 |
| 7LCMGC012 | ES790213 | Unknown protein {A. thaliana} | Unclassified proteins | 2.00E‐49 |
| 7LCMGC013 | ES790214 | Chloroplast rRNA‐operon | Protein synthesis | 3.60E‐64 |
| 7LCMGC014 | ES790215 | Phenylalanine ammonia‐lyase (PAL) | Metabolism, biosynthesis of secondary metabolites | 1.80E‐43 |
| 7LCMGC015 | ES790216 | Lethal leaf spot 1‐like protein | Regulation of cellular processes | 2.40E‐56 |
| 7LCMGC016 | ES790217 | ATPP2‐A15 {A. thaliana} | Unknown function | 8.40E‐38 |
| 7LCMGC017 | ES790218 | Scarecrow‐like 8 transcription factor | Transcription | 7.60E‐08 |
| 7LCMGC018 | ES790219 | Cytochrome oxidase subunit 3 | Metabolism, electron transport | 6.20E‐19 |
| 7LCMGC019 | ES790220 | Probable nucleolar GTP‐binding protein 1 | Signal transduction | 2.10E‐19 |
| 7LCMGC020 | ES790221 | Heat shock cognate 70‐kDa protein | Regulation of cellular processes | 7.70E‐53 |
| 7LCMGC021 | ES790222 | Annexin p34 | Membrane traffic and signal transduction among others | 6.50E‐44 |
| 7LCMGC022 | ES790223 | Expressed protein {A. thaliana} | Unclassified proteins | 1.20E‐09 |
| 7LCMGC023 | ES790224 | cDNA sequence cloned from several plant species | Unknown function | 1.00E‐79 |
| 7LCMGC024 | ES790225 | cDNA sequence {Lycopersicon esculentum} | Unknown function | 3.00E‐92 |
| 7LCMGC025 | ES790226 | 60S ribosomal protein L7A‐like | Cellular component, protein synthesis | 4.00E‐43 |
| 7LCMGC026 | ES790227 | CRT3 (Calreticulin 3) {A. thaliana} | Protein modification | 3.50E‐37 |
| 7LCMGC027 | ES790228 | Manganese superoxide dismutase | Regulation of cellular activities | 1.10E‐15 |
| 7LCMGC028 | ES790229 | RNA‐binding protein RZ‐1 | Protein synthesis | 2.90E‐25 |
| 7LCMGC029 | ES790230 | Glucose‐6‐phosphate 1‐dehydrogenase, cytoplasmic isoform | Carbohydrate metabolism | 3.10E‐64 |
| 7LCMGC030 | ES790231 | 40S ribosomal protein S8 | Cellular component, protein synthesis | 7.0E‐49 |
| 7LCMGC031 | ES790232 | cDNA sequence {L. esculentum} | Unknown function | 3.80E‐08 |
| 7LCMGC032 | ES790233 | 40S ribosomal protein S8 | Cellular component, protein synthesis | 1.2E‐22 |
| 7LCMGC033 | ES790234 | Zinc finger A20 and AN1 domains‐containing protein {A. thaliana} | Transcription | 8.3E‐96 |
| 7LCMGC034 | ES790235 | DNA‐binding protein | Transcription | 1.5E‐37 |
| 7LCMGC035 | ES790236 | DNA‐binding protein, MYB‐CC type transfactor {A. thaliana} | Transcription | 1.40E‐44 |
| 7LCMGC036 | ES790237 | MTD1 {Medicago truncatula} | Unknown function | 4.40E‐38 |
| 7LCMGC037 | ES790238 | Glyceraldehyde‐3‐phosphate dehydrogenase, A subunit (GAPDH) | Metabolism, energy | 4.30E‐28 |
| 7LCMGC038 | ES790239 | Ribosomal protein L27 | Cellular component, protein synthesis | 2.10E‐27 |
| 7LCMGC039 | ES790240 | U2AF (U2 snRNP auxiliary factor) small subunit | Transcription | 6.00E‐45 |
| 7LCMGC040 | ES790241 | Unknown protein {A. thaliana} | Unclassified proteins | 5.90E‐42 |
| 7LCMGC041 | ES790242 | Putative serine/arginine (SR) protein kinase protein | Signal transduction | 4.6E‐43 |
| 7LCMGC042 | ES790243 | Fibre protein Fb11 | Unknown function | 4.50E‐54 |
| 7LCMGC043 | ES790244 | DNA‐directed RNA polymerase II polypeptide K | Protein synthesis | 3.10E‐60 |
| 7LCMGC044 | ES790245 | Ribonucleoprotein complex subunit 2‐like protein (Nhp2‐like protein) | Transcription | 1.20E‐35 |
| 7LCMGC045 | ES790246 | Cytochrome c oxidase polypeptide Vc‐2 | Metabolism, electron transport | 6.90E‐52 |
| 7LCMGC046 | ES790247 | RAD23‐like protein | DNA repair and protein modification | 3.10E‐64 |
| 7LCMGC047 | ES790248 | cDNA sequence {L. esculentum} | Unknown function | 5.50E‐49 |
| 7LCMGC048 | ES790249 | cDNA sequence {Solanum tuberosum} | Unknown function | 1.00E‐26 |
| 7LCMGC049 | ES790250 | Cytosolic ascorbate peroxidase | Metabolism, electron transport | 2.90E‐133 |
| 7LCMGC050 | ES790251 | Putative ethylene‐responsive (ER6) protein (A. thaliana} | Regulation of cellular processes | 2.2E‐39 |
| 7LCMGC051 | ES790252 | Glycoprotein‐like protein | Protein synthesis | 1.00E‐51 |
| 7LCMGC052 | ES790253 | Putative thioredoxin reductase {Oryza sativa cv. japonica} | Metabolism, electron transport | 5.9E‐29 |
| 7LCMGC053 | ES790254 | Glyoxalase I {O. sativa cv. japonica} | Carbohydrate metabolism | 2.2E‐15 |
| 7LCMGC054 | ES790255 | cDNA sequence {S. tuberosum} | Unknown function | 2E‐104 |
Functional characterization of giant cell transcripts
To understand the biological processes that govern the establishment of giant cells, the transcripts obtained from the giant cell‐specific libraries constructed at 4 and 7 dpi were classified into functional groups using UNIProt and the Kyoto Encyclopaedia of Genes and Genomes, and relevant descriptions and motifs of homologues in databases. The classification was performed on the basis of the cellular processes that are likely to be involved in the induction and development of giant cells, including several signal transduction pathways, cell division and expansion, transport, DNA replication and protein synthesis associated with high metabolic activities (1, 2).
Several genes that are putative components of signal transduction pathways were identified. These included LescPth2 (a serine/threonine protein kinase), which is a component of the pathway involved in the M phase of the cell cycle, a putative serine/arginine (SR) protein kinase, a G‐protein‐coupled receptor‐like protein and a homologue of the A. thaliana putative receptor protein kinase PERK1. The presence and initial movement of J2s between cells may activate defence and stress transduction pathways in the host root. This is suggested by the identification of the heat shock cognate 70‐kDa protein, cytosolic copper, zinc and manganese superoxide dismutase, ethylene responsive factors, early light‐inducible protein, pathogenesis‐related proteins TSI‐1 and the S. tuberosum ssp. andigena ry‐1 resistance gene, all of which are known to be involved in defence and cellular responses to biotic and abiotic stresses.
Five individual genes involved in cell division and cell wall morphogenesis were identified in the 4‐dpi library. These included a tomato homologue of the tobacco Phi‐1 protein, two pectin methylesterases and a pectinesterase U1 precursor. An F‐box‐containing protein known to regulate diverse cellular processes, including cell cycle transition, transcriptional regulation and signal transduction, was also identified in the 4‐dpi library. The process of cell re‐differentiation to produce giant cells will employ a rapid DNA processing machinery, evidenced in the identification of several DNA‐binding proteins, including transcription factors [e.g. transcription initiation factor IIB (TFIIB), Scarecrow‐like 8 transcription factor, Zinc finger, AP2/ERF] and other structural and functional components involved in DNA synthesis, such as histones (e.g. Histone 1, 2B‐like and 4), ribonucleoprotein complexes (e.g. ribonucleoprotein complex subunit 2‐like protein) and RNA polymerases.
Many genes directly involved in protein synthesis, modification and degradation were identified from both libraries. These included 15 ribosomal proteins, two RNA‐binding proteins (e.g. RNA‐binding protein RZ‐1) and a DNA‐directed RNA polymerase II polypeptide K. The ubiquitin–proteasome pathway, which plays a key role in many cellular processes, including cell cycle control, hormonal signal transduction, flower development and circadian rhythm, was well represented in the libraries. Enzymes representing the three main enzymatic reactions governing the ubiquitination of target proteins were present in the libraries: 26S proteasome regulatory particle, ubiquitin‐conjugating enzyme UBC2 and ubiquitin‐protein ligase, also known to be involved in the maintenance of cell viability during root development in rice. Other genes identified in the libraries, which are involved in protein folding and fate, included cyclophilins, a chaperone precursor and a homologue of the A. thaliana Calreticulin 3. Consistent with alterations in metabolic activities in nematode feeding sites, many genes encoding proteins involved in different pathways in energy, carbohydrate and amino acid metabolism were identified from both giant cell‐specific libraries. Only one gene, for a cyclopropane fatty acid synthase, was cloned that was directly involved in lipid metabolism.
Genes involved in cellular transport and intercellular communication were also identified, including a PIP‐type aquaporin and a non‐specific lipid transfer protein, similar to the PVR3 protein and known to be a root‐specific protein in beans and pineapple. Clathrin and clathrin‐binding proteins, which are part of an oligomeric complex of coat proteins regulating vesicular traffic through the Golgi complex and from the Golgi to the endoplasmic reticulum, were represented in the library. Calcium‐binding proteins regulate changes in the concentration of cytosolic calcium ions, a process necessary for the control of several biological processes. A gene for each of the two types of calcium‐binding protein was identified in the libraries: a calmodulin and a calmodulin‐binding protein were identified from the 4‐dpi library, whereas an annexin p34 was obtained from the 7‐dpi library.
Temporal and spatial expression of giant cell transcripts
Real‐time quantitative RT‐PCR was used to study the expression levels of selected genes in giant cells. Twenty‐five genes representing various metabolic pathways and cellular components/processes were chosen, including seven genes (ZW0103001–ZW0903001) previously known to be expressed in mature giant cells. Gene expression was quantified at two time points (4 and 7 dpi) using the comparative CT method (ΔΔCT) and the cytosolic glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) gene as an endogenous control. CT values were the means of three replicates. The relative gene expression at each time point was calculated with reference to expression in control cells obtained by LM, and a fold difference of 1.5 was considered as significantly up‐ or down‐regulated (Table 3).
Table 3.
Changes in expression of 25 selected genes between laser microdissected control cells and giant cells 4 and 7 days post‐infection (dpi) revealed by real‐time quantitative reverse transcriptase‐polymerase chain reaction (RT‐PCR).
| Clone | Gene | Fold increase over uninfected tomato roots | Relative gene* expression pattern from before infection to 7 dpi | |
|---|---|---|---|---|
| 4 dpi | 7 dpi | |||
| ZW0103001 | Cysteine synthase | 34.53 ± 0.31 | 68.59 ± 0.23 | C  4 4  7 7 |
| ZW30050020 | 60S ribosomal protein L37A | 28.84 ± 0.03 | 233.94 ± 0.08 | C  4 4  7 7 |
| 4LCMGC0080 | Pathogenesis‐related TSI‐1 protein | 28.05 ± 1.53 | 107.62 ± 0.59 | C  4 4  7 7 |
| ZW0103003 | SAMDC | 24.25 ± 0.33 | 55.71 ± 0.26 | C  4 4  7 7 |
| 4LCMGC0032 | Cytosolic Cu,Zn superoxide dismutase | 17.87 ± 0.64 | 41.64 ± 0.41 | C  4 4  7 7 |
| 4LCMGC036 | ELI3 protein | 12.29 ± 0.14 | 25.99 ± 0.57 | C  4 4  7 7 |
| ZW3107005 | Cytochrome c reductase subunit | 6.32 ± 0.34 | 19.97 ± 0.46 | C  4 4  7 7 |
| 4LCMGC039 | LescPth2, serine/threonine protein kinase | 4.47 ± 0.18 | 7.41 ± 0.61 | C  4 4  7 7 |
| 4LCMGC061 | Zinc finger transcription factor‐like protein | 4.17 ± 0.27 | 9.71 ± 0.56 | C  4 4  7 7 |
| 4LCMGC002 | Hydroxymethylglutaryl coenzyme A synthase | 3.78 ± 0.31 | 5.24 ± 0.84 | C  4 4  7 7 |
| ZW3107003 | Cytochrome c oxidase subunit VIIa | 1.83 ± 0.31 | 3.50 ± 0.43 | C  4 4  7 7 |
| 4LCMGC052 | Calmodulin‐binding protein | 1.55 ± 0.09 | 2.80 ± 0.53 | C  4 4  7 7 |
| 4LCMGC003 | Pyruvate dehydrogenase E1 α subunit | 1.54 ± 0.32 | 3.20 ± 0.28 | C  4 4  7 7 |
| 4LCMGC0051 | Pectinesterase U1 precursor | 8.00 ± 0.36 | 7.31 ± 0.79 | C  4 ≈ 7 4 ≈ 7 |
| ZW0903001 | 60S ribosomal protein L31 | 1.85 ± 0.36 | 1.85 ± 0.51 | C  4 ≈ 7 4 ≈ 7 |
| 4LCMGC075 | Phi‐1 protein | 8.51 ± 0.26 | 6.73 ± 0.68 | C  4 4  7 7 |
| ZW3107009 | Unknown protein | 1.04 ± 0.37 | 8.57 ± 0.21 | C ≈ 4  7 7 |
| 4LCMGC008 | Clathrin‐binding protein | 0.67 ± 0.33 | 2.99 ± 0.27 | C ≈ 4  7 7 |
| 7LCMGC0036 | MTD1 | 1.29 ± 0.31 | 2.03 ± 0.87 | C ≈ 4  7 7 |
| 4LCMGC001 | Hypothetical protein | 0.30 ± 0.47 | 1.82 ± 0.27 | C ≈ 4  7 7 |
| 4LCMGC0018 | Transaldolase | 0.78 ± 0.26 | 1.64 ± 0.29 | C ≈ 4  7 7 |
| 4LCMGC0012 | Lipid transfer protein | 0.64 ± 0.29 | 0.17 ± 0.30 | C ≈ 4 ≈ 7 |
| 4LCMGC060 | Phenylalanine ammonia‐lyase | 0.30 ± 0.08 | 0.15 ± 0.54 | C ≈ 4 ≈ 7 |
| 4LCMGC0078 | Microtubule‐associated protein MAP65‐1a | 0.07 ± 0.46 | 0.16 ± 0.37 | C ≈ 4 ≈ 7 |
| 4LCMGC0085 | PIP‐type aquaporin | 0.01 ± 0.28 | 0.04 ± 0.04 | C ≈ 4 ≈ 7 |
Relative gene expression pattern was established using the fold change at each time point starting from expression levels in control cells to 4 and 7 dpi. The symbols  ,
,  and ≈ represent lower, higher or similar expression between time points.
and ≈ represent lower, higher or similar expression between time points.
The expression patterns of these selected genes formed five different groups. Thirteen genes in group 1 showed continuously up‐regulated expression from 4 to 7 dpi. This group also includes the highest expressed genes: a cysteine synthase with a 34.5‐fold up‐regulation at 4 dpi, and a 60S ribosomal protein L37A with a 234‐fold increase at 7 dpi. Two genes in group 2, pectinesterase U1 precursor and 60S ribosomal protein L31, were up‐regulated at 4 dpi and the expression remained the same at 7 dpi. Expression of the Phi‐1 gene in group 3 was highly up‐regulated in giant cells at 4 dpi, and then decreased at 7 dpi. Five genes in group 4 showed no significant changes in expression at 4 dpi compared with control cells, but were up‐regulated by 7 dpi. The expression of four genes in group 5 was not affected during the induction and development of giant cells, and the levels were the same as those in control cells at 4 and 7 dpi.
The localization of expression of two genes, Phi‐1 and the pectinesterase U1 precursor, was studied further by in situ hybridization in gall tissues at 4 dpi. Individual EST clones of these genes identified in the libraries were used to synthesize sense and antisense digoxigenin‐labelled probes employing T7 and SP6 RNA polymerase. Using the polymer polyvinyl alcohol, known to enhance formazan formation in alkaline phosphatase reactions, a strong hybridization signal was observed in tissues hybridized with antisense probes, with little background on tissues hybridized with sense probes (Fig. 2). Although most cells exhibited hybridization with the antisense probes, the signal was much stronger in developing giant cells than in the surrounding cells, indicating that these transcripts were preferentially expressed in the cytoplasm of giant cells.
Figure 2.
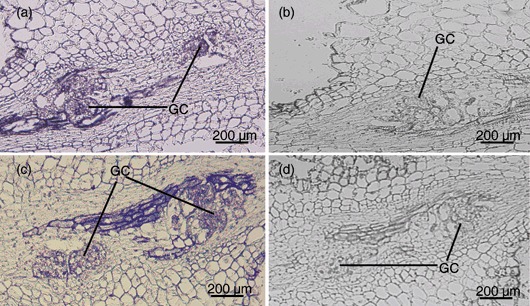
In situ hybridization of Meloidogyne javanica‐infected tomato root tissues at 4 days post‐infection (dpi) with probes generated from a Phi‐1 protein gene (A, B) and a pectinesterase‐like protein gene (C, D). Strong signals are present in giant cells when hybridized with antisense probes (A, C) compared with sense probes (B, D). GC, giant cells.
DISCUSSION
The technique of LM has been applied to isolate specific cells and tissues for gene and protein profiling studies. We first demonstrated the isolation by LM of specific cytoplasmic contents of early developing giant cells induced by M. javanica (Ramsay et al., 2004). Using the same technique, giant cell contents at 4 and 7 dpi have been isolated, and mRNA amplified from the captured cells was used to construct giant cell‐specific cDNA libraries. More than 5000 clones were screened from both libraries by PCR and restriction digestion before sequencing. A total of 141 individual transcripts (87 and 54 from 4‐ and 7‐dpi libraries, respectively) have been identified, which can be expected to play a role in the cellular processes important for giant cell formation, i.e. in metabolism, cell response to stress, protein synthesis, cell division and morphogenesis, transport, signal transduction, protein modification and fate, and regulation of cellular processes.
The isolation of giant cell contents by LM from sections of unstained gall tissue younger than 4 dpi is technically very demanding, because it is difficult to differentiate giant cell initials from surrounding cells (Ramsay et al., 2004). Generally, by 6 dpi, giant cells are clearly multinucleate with enlarged nuclei and nucleoli, cell expansion is obvious and there are well‐developed wall ingrowths (Jones, 1981; Jones and Payne, 1978). The induction and expansion phases of giant cells may be completed by 7–10 dpi, but changes in metabolism can occur up to maturity at 25–30 dpi (Ehsanpour and Jones, 1996). By dividing the analysis of the infection into two time points at 4 and 7 dpi, the aim was to study the processes involved in the induction and early‐stage development of giant cells.
An appropriate endogenous control is important for the accuracy of quantitative RT‐PCR analysis. The choice of GAPDH as a reference gene for the comparison of gene expression between nematode feeding cells and control tissues has been well discussed (Fuller et al., 2007). We also confirmed the stability of expression of the GAPDH gene in giant cells. When the same amount of initial laser microdissected RNA was subjected to RT‐PCR, there was no obvious difference in CT values of GAPDH between giant cells and control cells (data not shown). As shown in Table 3, most of the genes studied showed significant changes in expression between the two time points, and from control cells. The highest increase in expression was from a 60S ribosomal protein L37A gene, which was 29‐fold at 4 dpi and 234‐fold at 7 dpi, and a pathogenesis‐related TSI‐1 protein gene (28‐ and 108‐fold, respectively). This 60S ribosomal protein L37A gene was also up‐regulated in mature giant cells (Wang et al., 2003). Another 60S ribosomal protein gene L31 showed increased expression at 4 dpi, and a similar level at 7 dpi. The up‐regulation of other ribosomal protein genes induced in feeding cells has been reported previously (Vaghchhipawala et al., 2001; Wang et al., 2003), and this is consistent with a high level of protein synthesis in nematode feeding cells. Interestingly, there are two 60S ribosomal protein genes, L9 and L11, whose expression was suppressed in syncytia at 3 dpi according to an LM‐associated microarray study (Klink et al., 2007). How the reported suppression of these genes relates to increased ribosomes and protein synthesis in syncytia is not clear. The proliferation of ribosomes in giant cells clearly depends on the increased expression of ribosomal protein genes. However, in syncytia initials, there may be a contribution of ribosomes from neighbouring cells incorporated into syncytia that might account for the increased number of ribosomes.
Except for the Phi‐1 gene, all genes studied by real‐time quantitative RT‐PCR showed up‐regulated or unchanged expression at 7 dpi compared with that at 4 dpi. In contrast, the Phi‐1 gene expression decreased at 7 dpi. Phi‐1 is a cell cycle‐related gene which is also activated in phosphate‐starved tobacco cell cultures when re‐entry of the cell cycle is induced by the addition of phosphate (Farrar et al., 2003; Sano et al., 1999). The transcript level of the Phi‐1 gene in cultured cells then decreases with the start of DNA synthesis (Sano et al., 1999). Cell cycle re‐entry is a typical step of induction of giant cells at the early infection stage, and a number of cycle‐related genes have been found to be up‐regulated in giant cells (de Almeida Engler et al., 1999; Goverse et al., 2000, Ramsay et al., 2004). It is possible that the induced activation of Phi‐1 gene expression occurs before 4 dpi, as giant cell initials re‐enter the cell cycle, and subsequently expression decreases at 7 dpi. In situ hybridization results also indicate that expression of the Phi‐1 gene is located in giant cells at 4 dpi.
Another gene studied by quantitative RT‐PCR and in situ hybridization, the pectinesterase U1 precursor gene, is up‐regulated in giant cells at 4 and 7 dpi compared with that in control cells. Two more pectinesterase genes were identified in the 4‐dpi library, which may be involved in cell wall modification, such as increased extensibility (Gaffe et al., 1994; Wen et al., 1999), during giant cell formation. There are other pectinesterase family genes up‐regulated in the syncytia of soybean at 2 dpi; however, the expression of these genes was depressed in syncytia after 5 dpi (Ithal et al., 2007a). These results are consistent with the different modes of formation of giant cells and syncytia, in that cell wall digestion is a fundamental process in the development of syncytia.
A knowledge of gene expression in nematode feeding cells is very important for an understanding of how feeding cells form and function. Large‐scale gene expression profiling of host cells in response to nematode infection has been carried out by microarray studies on root tissues infected with root‐knot (Jammes et al., 2005) and cyst (Ithal et al., 2007b; Khan et al., 2004; Puthoff et al., 2003, 2007) nematodes, and in more specific studies of individual feeding cells, for example syncytia by LM combined with cDNA library construction (Klink et al., 2005) and microarray analysis (Ithal et al., 2007a; Klink et al., 2007). LM‐associated microarray studies of giant cells have been limited by host plant and the availability of microarray chips covering full genome transcripts, such as for tomato as used in this study. In addition, even when the same Affymetrix soybean GeneChip was used in LM‐associated microarray studies, there were significant differences between independent experiments. For example, Ithal et al. (2007a) identified 1765 genes (1116 up‐regulated and 649 down‐regulated with a 1.5‐fold change cut‐off) from laser microdissected syncytia samples of soybean infected with H. glycines at 2 dpi. Using the same technique, only 351 genes, 79 up‐regulated and 272 down‐regulated with a 1.5‐fold change cut‐off, were identified from laser microdissected soybean syncytia samples also infected with H. glycines at 3 dpi (Klink et al., 2007). Although there were differences in the pathosystems used in these studies, in that different soybean cultivars and different H. glycines lines were used, the variation in the microarray data may result from the different statistical analysis approaches employed. The reliability and reproducibility of microarray results rely on appropriate data analysis practices, standardized if possible (Shi et al., 2008). Nevertheless, microarray studies should be carried out on laser microdissected giant cell samples with the availability of appropriate genome chips and careful interpretation of the data.
In summary, we have successfully produced giant cell‐specific cDNA libraries using LM from early‐stage giant cell contents in tomato, and generated information on a set of genes whose functions and expression suggest possible involvement in the induction and development of giant cells. The temporal and spatial expression of some of the genes contrasts with events in developing syncytia. The genes identified from this work, and that of others, provide an indication of candidate genes for further studies in plant–nematode interactions. In addition, an understanding of the events occurring in nematode feeding sites may be aided by further studies of the uncharacterized transcripts found in the giant cell‐specific libraries. Such work will provide more information for the future development of strategies to confer host resistance to endoparasitic nematodes.
EXPERIMENTAL PROCEDURES
LM of giant cells
In vitro culture of tomato cv. Grosse‐lisse and infection with M. javanica were carried out as described previously (Hutangura et al., 1999). Developing galls from infected roots and uninfected controls were collected at 4 and 7 dpi, and processed through tissue fixation, paraffin embedding and sectioning, as described previously (Ramsay et al., 2004) with modifications. Fixed tissue was dehydrated at 4 °C in a graded ethanol series [(v/v) 75%, 85%, 90%, 100% and 100%] for 15 min each time on a rotator. This was followed by an ethanol : xylene series [(v/v) 3 : 1, 1 : 1, 1 : 3], and then 100% xylene for 1.5 h (3 × 30 min). Tissue sections were then deparaffinized, microdissected (Ramsay et al., 2004) using a PALM Robot‐Combi LM system (PALM, Bernried, Germany) and catapulted into a flat cap of a 0.5‐mL Eppendorf tube containing 40 µL of diethylpyrocarbonate (DEPC)‐treated water with 40 U of RNasin RNase inhibitor (Promega, Sydney, Australia). One hundred cells were collected from galls at 4 and 7 dpi, and also from cells close to the vasculature of uninfected control roots.
RNA extraction and linear amplification
Total RNA from laser microdissected cells was extracted with the PicoPure RNA isolation kit (Arcturus Bioscience, Mountain View, CA, USA) according to the manufacturer's instructions. RNA was eluted with 30 µL of elution buffer and later concentrated in a vacuum centrifuge to approximately 10 µL, and quantified with a NanoDrop ND‐1000 UV–visible spectrophotometer (Biolab Limited, Scoresby, Victoria, Australia). The RNA was then amplified using the SuperScript RNA amplification system (Invitrogen Corporation, Carlsbad, CA, USA) according to the manufacturer's protocol. Typically, this involved first‐strand cDNA synthesis from approximately 100 ng of total RNA with Superscript III reverse transcriptase and T7‐Oligo(dT), followed by second‐strand cDNA synthesis by DNA polymerase I and DNA ligase, purification of cDNA with a spin cartridge and in vitro transcription using T7 polymerase.
cDNA synthesis and cloning
cDNAs for the 4‐ and 7‐dpi giant cell‐specific libraries were synthesized using the SuperScript RNA amplification system with modifications. For each (replicate) cDNA construction, first‐strand cDNA was synthesized from 500 ng of aRNA in separate reactions using random hexamers and Oligo‐dT. Reactions containing random hexamers were incubated at 25 °C to ensure that the primers annealed well before incubation at 46 °C for 2 h. This was immediately followed by double‐stranded cDNA construction at 16 °C for 4 h. The dscDNA was first extracted with tris(hydroxymethyl)aminomethane–ethylenediaminetetraacetic acid (Tris‐EDTA)‐buffered phenol–chloroform–isoamyl alcohol (25 : 24 : 1) and precipitated with 20 µg of Glycogen (Sigma, Castle Hill, New South Wales, Australia), 7.5 m ammonium acetate and 2.5 vol of ethanol. cDNA was then separately A‐tailed with 100 µm of dATP and 5 U of Taq DNA polymerase (Invitrogen Corporation) at 70 °C for 2 h, and 15 U of terminal deoxynucleotidyl transferase (Invitrogen Corporation) at 37 °C for 2 h. A total of 500–550 ng of A‐tailed dscDNA was ligated to pGEM‐T Easy. The ligated DNA was used to transform JM109 competent cells (Promega), and the colonies were selected with isopropyl‐β‐d‐thiogalactopyranoside (IPTG) and 5‐bromo‐4‐chloro‐3‐indolyl‐β‐d‐galactopyranoside (X‐Gal).
Library screening and sequencing
Positively identified transformed colonies were further analysed by PCR using T7 and SP6 primers. The PCRs were made up to 20 µL with water containing 0.2 mm deoxynucleoside triphosphate (dNTP), 2 mm MgCl2, 5 pmol of primers, 0.25 U of recombinant Taq DNA polymerase and 1 × Taq buffer (Invitrogen Corporation), and 5 µL of individual colonies were resuspended in 20 µL of water as template. Selected cDNA clones were sequenced with the universal T7 primer; ambiguous bases were clarified by a further sequencing of the opposite strand with SP6 primer using Big Dye Terminator v3.1 chemistry, and electrophoresed with an AB3730 capillary sequencer (Perkin Elmer Life Sciences, Melbourne, Australia).
Sequence analysis and transcripts in the library
Raw sequences of the transcripts were edited using FinchTV Version 1.4.0 (http://www.geospiza.com/finchtv). For each library, multiple alignment using SeqEd V1.0.3 (Applied Biosystems, Foster City, CA, USA) and clustering of all sequences using ClustalW2 (http://www.ebi.ac.uk/Tools/clustalw2/index.html) were carried out to detect and remove duplicate sequences. ClustalW2 was also used to compare transcripts from both libraries to identify common transcripts. Edited sequences were employed to search for homologous sequences using blast (Altschul et al., 1990) made available by The Gene Index (TGI) Project (http://compbio.dfci.harvard.edu/tgi), with the tomato gene index database from the TGI Project as a primary database to identify tomato genes. The nucleotide and EST collection maintained by the NCBI was also used to search for transcripts with no homologues on the primary database. Sequences with significant matches had a cut‐off expected value of 1E‐5 for which the most homologous was presented. The functions or functional categories of the transcripts were inferred from descriptions or homologues deposited in the TGI project and NCBI, and were confirmed using UNIProt (http://beta.uniprot.org) and the Kyoto Encyclopaedia of Genes and Genomes (http://www.genome.jp/kegg).
Real‐time quantitative RT‐PCR
Real‐time quantitative RT‐PCR was carried out using a Corbett Rotor Gene RG‐3000 (Corbett Research, Brisbane, Queensland, Australia). Specific primers for the target genes were designed with Primer 3 (v.0.4.0) (http://frodo.wi.mit.edu). aRNA used as template for quantitative PCR was reverse transcribed using the High‐Capacity cDNA Reverse‐Transcription Kit, according to the manufacturer's protocol (Applied Biosystems, CA, USA). Quantitative PCRs were performed in triplicate with Power SYBR Green PCR Master Mix, and contained 1 µL of template cDNA and 500 nm of each primer made up to a volume of 20 µL with RNase‐free sterile water. The PCR conditions used were as follows: a hold at 95 °C for 10 min, 45 cycles of denaturation at 95 °C for 10 s, and annealing and acquisition at 55 °C for 1 min, except for three genes where the annealing and acquisition of the data were performed at 50 °C for 1 min. Gene expression was quantified using the comparative CT method (ΔΔCT), as described by the Real Time PCR Handbook, with a tomato GAPDH gene as an endogenous control (http://www.uic.edu/depts/rrc/cgf/realtime/deltact.html).
In situ hybridization
Gall tissues were trimmed from infected tomato roots at 4 dpi and fixed immediately in formalin–acetic acid (50% ethanol; 10% formalin containing 37% formaldehyde; 5% acetic acid) at room temperature for 4 h, with vacuum infiltration at 400 mmHg once every hour, followed immediately by a change of the fixative. Fixed tissues were dehydrated in an ethanol series: 50% for 3 × 30 min, 75% for 1.5 h (both at room temperature) and 85% at 4 °C overnight. The tissues were transferred to a Leica TP 1020 (Leica Microsystems, North Ryde, New South Wales, Australia) automated tissue processing system for further dehydration in a series of 90% and 100% ethanol (1.5 h each time) and then chloroform, before embedding in molten wax. Paraffin‐embedded tissues were sectioned into ribbons, 10 µm thick, floated in DEPC‐treated water at 42 °C and mounted onto silane‐coated slides. PCR products with T7 and SP6 promoter sequences appended to the termini were used to produce labelled RNA probes which were synthesized using the DIG RNA Labelling Kit (Roche Applied Science, Castle Hill, New South Wales, Australia) according to the manufacturer's instructions. In situ hybridizations were performed essentially as described by DeBlock and Debrouwer (2002) using polyvinyl alcohol (40–88 kDa) for enhanced visualization.
ACKNOWLEDGEMENTS
We thank Dr Kathryn Heel‐Miller (Lotteries Laser Microdissection Facility, University of Western Australia, Perth, Australia) for assistance with LM. We also thank Mr Gordon Thomson (Murdoch University, Perth, Australia) for advice in tissue processing. This research was supported an Australian Research Council Discovery Grant (A00105534).
REFERENCES
- Altschul, S.F. , Gish, W. , Miller, W. , Myers, E.W. and Lipman, D.J. (1990) Basic local alignment search tool. J. Mol. Biol. 215, 403–410. [DOI] [PubMed] [Google Scholar]
- Bird, D.M. and Wilson, M.A. (1994) DNA‐sequence and expression analysis of root‐knot nematode‐elicited giant‐cell transcripts. Mol. Plant–Microbe Interact. 7, 419–424. [DOI] [PubMed] [Google Scholar]
- Davis, E.L. , Hussey, R.S. , Baum, T.J. , Bakker, J. , Schots, A. , Rosso, M. and Abad, P. (2000) Nematode parasitism genes. Annu. Rev. Phytopathol. 38, 365–396. [DOI] [PubMed] [Google Scholar]
- Day, R.C. , Grossniklaus, U. and Macknight, R.C. (2005) Be more specific! Laser‐assisted microdissection of plant cells. Trends Plant Sci. 10, 397–406. [DOI] [PubMed] [Google Scholar]
- De Almeida Engler, J. , De Vleesschauwer, V. , Burssens, S. , Celenza, J.L. , Inzè, D. , Von Montagu, M. , Engler, G. and Gheysen, G. (1999) Molecular markers and cell cycle inhibitors show the importance of cell cycle progression in nematode‐induced galls and syncytia. Plant Cell, 11, 793–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Almeida Engler, J. , Van Poucke, K. , Karimi, M. , De Groodt, R. , Gheysen, G. , Engler, G. and Gheysen, G. (2004) Dynamic cytoskeleton rearrangements in giant cells and syncytia of nematode‐infected roots. Plant J. 38, 12–26. [DOI] [PubMed] [Google Scholar]
- DeBlock, M. and Debrouwer, D. (2002) RNA–RNA in situ hybridization using DIG‐labeled probes: the effect of high molecular weight polyvinyl alcohol on the alkaline phosphatase indoxyl‐nitroblue tetrazolium reaction In: Procedures for In Situ Hybridization to Chromosomes, Cells, and Tissue Sections (Doris Eisel, Stefanie Grünewald‐Janho, Bettina Kruchen, eds). Penzberg, Germany: Roche Applied Science, pp. 172–176. [Google Scholar]
- Ehsanpour, A.A. and Jones, M.G.K. (1996) Glucuronidase expression in transgenic tobacco roots with a Parasponia promoter on infection with Meloidogyne javanica . J. Nematol. 28, 407–413. [PMC free article] [PubMed] [Google Scholar]
- Escobar, C. , Barcala, M. , Portillo, M. , Almoguera, C. , Jordano, J. and Fenoll, C. (2003) Induction of the Hahsp17.7G4 promoter by root‐knot nematodes: involvement of heat‐shock elements in promoter activity in giant cells. Mol. Plant–Microbe Interact. 16, 1062–1068. [DOI] [PubMed] [Google Scholar]
- Farrar, K. , Evans, I.M. , Topping, J.F. , Souter, M.A. , Nielsen, J.E. and Lindsey, K. (2003) EXORDIUM—a gene expressed in proliferating cells and with a role in meristem function, identified by promoter trapping in Arabidopsis. Plant J. 33, 61–73. [DOI] [PubMed] [Google Scholar]
- Favery, B. , Lecomte, P. , Gil, N. , Bechtold, N. , Bouchez, D. , Dalmasso, A. and Abad, P. (1998) RPE, a plant gene involved in early developmental steps of nematode feeding cells. EMBO J. 17, 6799–6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favery, B. , Chelysheva, L.A. , Lebris, M. , Jammes, F. , Marmagne, A. , De Almeida‐Engler, J. , Lecomte, P. , Vaury, C. , Arkowitz, R.A. and Abad, P. (2004) Arabidopsis formin AtFH6 is a plasma membrane associated protein upregulated in giant cells induced by parasitic nematodes. Plant Cell, 16, 2529–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller, V.l. , Lilley, C.J. , Atkinson, H.J. and Urwin, P.E. (2007) Differential gene expression in Arabidopsis following infection by plant‐parasitic nematodes Meloidogyne incognita and Heterodera schachtii . Mol. Plant Pathol. 8, 595–609. [DOI] [PubMed] [Google Scholar]
- Gaffe, J. , Tieman, D.M. and Handa, A.K. (1994) Pectin methylesterase isoforms in tomato (Lycopersicon esculentum) tissues. Plant Physiol. 105, 199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheysen, G. and Fenoll, C. (2002) Gene expression in nematode feeding sites. Annu. Rev. Phytopathol. 40, 191–219. [DOI] [PubMed] [Google Scholar]
- Goddijn, O.J.M. , Lindsey, K. , Van Der Lee, F.M. , Klap, J.C. and Sijmons, P.C. (1993) Differential gene expression in nematode‐induced feeding structures of transgenic plants harbouring promoter‐gusA fusion constructs. Plant J. 4, 863–873. [DOI] [PubMed] [Google Scholar]
- Goverse, A. , De Almeida Engler, J. , Verhees, J. , Van Der Krol, S. , Helder, J. and Gheysen, G. (2000) Cell cycle activation by plant parasitic nematodes. Plant Mol. Biol. 43, 747–761. [DOI] [PubMed] [Google Scholar]
- Hutangura, P. , Mathesius, U. , Jones, M.G.K. and Rolfe, B.G. (1999) Auxin induction is a trigger for root gall formation caused by root‐knot nematodes in white clover and is associated with the activation of the flavonoid pathway. Aust. J. Plant Pathol. 26, 221–231. [Google Scholar]
- Ithal, N. , Recknor, J. , Nettleton, D. , Hearne, L. , Maier, T. , Baum, T.J. and Mitchum, M.G. (2007a) Parallel genome‐wide expression profiling of host and pathogen during soybean cyst nematode infection of soybean. Mol. Plant–Microbe Interact. 3, 293–305. [DOI] [PubMed] [Google Scholar]
- Ithal, N. , Recknor, J. , Nettleton, D. , Maier, T. , Baum, T.J. and Mitchum, M.G. (2007b) Developmental transcript profiling of cyst nematode feeding cells in soybean roots. Mol. Plant–Microbe Interact. 20, 510–525. [DOI] [PubMed] [Google Scholar]
- Jammes, F. , Lecomte, P. , De Almeida Engler, J. , Bitton, F. , Martin‐Magniette, M.L. , Renou, J.P. , Abad, P. and Favery, B. (2005) Genome‐wide expression profiling of the host response to root‐knot nematode infection in Arabidopsis. Plant J. 44, 447–458. [DOI] [PubMed] [Google Scholar]
- Jones, M.G.K. (1981) Host cell responses to endoparasitic nematode attack; structure and function of giant cells and syncytia. Annu. Appl. Biol. 97, 353–372. [Google Scholar]
- Jones, M.G.K. and Dropkin, V.H. (1976) Scanning electron microscopy of nematode‐induced giant transfer cells. Cytobios, 15, 149–161. [PubMed] [Google Scholar]
- Jones, M.G.K. and Payne, H.L. (1978) Early stages of nematode‐induced giant‐cell formation of roots of Impatiens balsamina . J. Nematol. 10, 70–83. [PMC free article] [PubMed] [Google Scholar]
- Juergensen, K. , Scholz‐Starke, J. , Sauer, N. , Hess, P. , Van Bel, A.J.E. and Grundler, F.M.W. (2003) The companion cell‐specific Arabidopsis disaccharide carrier AtSUC2 is expressed in nematode‐induced syncytia. Plant Physiol. 131, 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, R. , Alkharouf, N. , Beard, H. , MacDonald, M. , Chouikha, I. , Meyer, S. , Grefenstette, J. , Knap, H. and Matthews, B. (2004) Microarray analysis of gene expression in soybean roots susceptible to the soybean cyst nematode two days post invasion. J. Nematol. 36, 241–248. [PMC free article] [PubMed] [Google Scholar]
- Klink, V.P. , Alkharouf, N. , MacDonald, M. and Matthews, B. (2005) Laser capture microdissection (LCM) and expression analyses of Glycine max (soybean) syncytium containing root regions formed by the plant pathogen Heterodera glycines (soybean cyst nematode). Plant Mol. Biol. 59, 965–979. [DOI] [PubMed] [Google Scholar]
- Klink, V.P. , Overall, C.C. , Alkharouf, N.W. , MacDonald, M.H. and Matthews, B.F. (2007) Laser capture microdissection (LCM) and comparative microarray expression analysis of syncytia cells isolated from incompatible and compatible soybean (Glycine max) roots infected by the soybean cyst nematode (Heterodera glycines ). Planta, 226, 1389–1409. [DOI] [PubMed] [Google Scholar]
- Lohar, D.P. , Schaff, J.E. , Laskey, J.G. , Kieber, J.J. , Bilyeu, K.D. and Bird, D.M. (2004) Cytokinins play opposite roles in lateral root formation, and nematode and rhizobial symbioses. Plant J. 38, 203–214. [DOI] [PubMed] [Google Scholar]
- Puthoff, D.P. , Nettleton, D. , Rodermel, S.R. and Baum, T.J. (2003) Arabidopsis gene expression changes during cyst nematode parasitism revealed by statistical analyses of microarray expression profiles. Plant J. 33, 911–921. [DOI] [PubMed] [Google Scholar]
- Puthoff, D.P. , Ehrenfried, M.L. , Vinyard, B.T. and Tucker, M.L. (2007) GeneChip profiling of transcriptional responses to soybean cyst nematode, Heterodera glycines, colonization of soybean roots. J. Exp. Bot. 58, 3407–3418. [DOI] [PubMed] [Google Scholar]
- Ramsay, K. , Wang, Z. and Jones, M.G.K. (2004) Using laser capture microdissection to study gene expression in early stages of giant cells induced by root‐knot nematodes. Mol. Plant Pathol. 5, 587–592. [DOI] [PubMed] [Google Scholar]
- Ramsay, K. , Jones, M.G.K. and Wang, Z. (2006) Laser capture microdissection: a novel approach to microanalysis of plant–microbe interactions. Mol. Plant Pathol. 7, 429–435. [DOI] [PubMed] [Google Scholar]
- Sano, T. , Kuraya, Y. , Amino, S. and Nagata, T. (1999) Phosphate as a limiting factor for the cell division of tobacco BY‐2 cells. Plant Cell Physiol. 40, 1–8. [DOI] [PubMed] [Google Scholar]
- Shi, L. , Perkin, R.G. , Fang, H. and Tong, W. (2008) Reproducible and reliable microarray results through quality control: good laboratory proficiency and appropriate data analysis practices are essential. Curr. Opin. Biotechnol. 19, 10–18. [DOI] [PubMed] [Google Scholar]
- Vaghchhipawala, Z. , Bassüner, R. , Clayton, K. , Lewers, K. , Shoemaker, R. and Mackenzie, S. (2001) Modulations in gene expression and mapping of genes associated with cyst nematode infection of soybean. Mol. Plant–Microbe Interact. 14, 42–54. [DOI] [PubMed] [Google Scholar]
- Wang, Z. , Potter, R.H. and Jones, M.G.K. (2003) Differential display analysis of gene expression in the cytoplasm of giant cells induced in tomato roots by Meloidogyne javanica . Mol. Plant Pathol. 4, 361–371. [DOI] [PubMed] [Google Scholar]
- Wen, F. , Zhu, Y. and Hawes, M.C. (1999) Effect of pectin methylesterase gene expression on pea root development. Plant Cell, 11, 1129–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]


