SUMMARY
Soybean mosaic virus (SMV), a member of the genus Potyvirus, is transmitted by aphids in a non‐persistent manner. It has been well documented that the helper component‐proteinase (HC‐Pro) plays a role as a ‘bridge’ between virion particles and aphid stylets in the aphid transmission of potyviruses. Several motifs, including the KITC and PTK motifs on HC‐Pro and the DAG motif on the coat protein (CP), have been found to be involved in aphid transmission. Previously, we have shown strong interaction between SMV CP and HC‐Pro in a yeast two‐hybrid system (YTHS). In this report, we further analysed this CP–HC‐Pro interaction based on YTHS and an in vivo binding assay to identify crucial amino acid residues for this interaction. Through this genetic approach, we identified two additional amino acid residues (H256 on CP and R455 on HC‐Pro), as well as G12 on the DAG motif, crucial for the CP–HC‐Pro interaction. We introduced mutations into the identified residues using an SMV infectious clone and showed that these mutations affected the efficiency of aphid transmission of SMV. We also investigated the involvement of the PTK and DAG motifs in the CP–HC‐Pro interaction and aphid transmission of SMV. Our results support the concept that physical interaction between CP and HC‐Pro is important for potyviral aphid transmission. Based on the combination of our current results with previous findings, the possibility that aphid transmission may be regulated by more complex molecular interactions than the simple involvement of HC‐Pro as a bridge is discussed.
INTRODUCTION
Soybean mosaic virus (SMV), one of the most common viral pathogens of soybean, is a member of the genus Potyvirus (Mayo and Pringle, 1998). SMV causes severe symptoms, including mosaic, mottling and necrosis, in many soybean cultivars, and is easily transmitted by aphids of at least 32 species in a non‐persistent manner (Hill, 1999), thus resulting in significant reductions in soybean yield and quality. Like all potyviruses, the SMV genome encodes one large polyprotein, which is cleaved to yield at least 10 mature proteins, including P1, helper component‐proteinase (HC‐Pro), P3, 6K1, cylindrical inclusion (CI), 6K2, viral protein genome‐linked (VPg), nuclear inclusion a proteinase (NIa‐Pro), nuclear inclusion b (NIb) and coat protein (CP) (Jayaram et al., 1992). Recently, however, an additional small protein, named PIPO, was identified, which is embedded within the P3 cistron of the polyprotein translated in the +2 reading frame (Chung et al., 2008). Several studies have demonstrated that some of these viral proteins act as pathogenic determinants in SMV disease reactions (Eggenberger et al., 2008; 2005, 2006; Seo et al., 2009d).
Accumulating evidence has suggested that HC‐Pro mediates aphid transmission, acting as a ‘bridge’ between virion particles and aphid stylets in the aphid transmission of potyviruses, including Potato virus Y (PVY), Tobacco etch virus (TEV), Tobacco vein mottling virus (TVMV) and Zucchini yellow mosaic virus (ZYMV) (Atreya and Pirone, 1993; 1997, 1998; Govier and Kassanis, 1974a; Peng et al., 1998). These studies also suggest that there are conserved motifs for aphid transmission in HC‐Pro and CP. Two conserved motifs, known as KITC and PTK, were located in HC‐Pro (Atreya et al., 1990; Peng et al., 1998). The KITC motif of TEV seems to be involved in the binding to aphid stylets, whereas the PTK motif of ZYMV has been shown to be implicated in binding to CP (Blanc et al., 1998; Peng et al., 1998). One other conserved motif, DAG, which is found at the N‐terminus of TVMV CP, seems to be essential for binding to HC‐Pro (Blanc et al., 1997). Several biological analyses have demonstrated that these motifs are engaged in direct protein–protein interactions (Blanc et al., 1997; Peng et al., 1998). Concomitantly, several studies have suggested that not only these three conserved motifs but also other amino acid residues may play a critical role in the aphid transmission of potyviruses (Dombrovsky et al., 2005; Flasinski and Cassidy, 1998; Llave et al., 2002).
We have analysed previously the protein–protein interactions between viral proteins of the SMV strain G7H using the yeast two‐hybrid system (YTHS) (Kang et al., 2004). In that study, we found a strong interaction between SMV CP and HC‐Pro. SMV strain G7H, which is thought to be transmissible as this strain is prevalent in soybean fields in Korea (Kim et al., 2003), contains the conserved motifs for aphid transmission, including the KLSC (corresponding to the KITC motif of many other potyviruses) and PTK motifs in HC‐Pro and the DAG motif in CP (GenBank Accession no. FJ807700). In this study, we further analysed the specific interaction between SMV CP and HC‐Pro using YTHS and an in vivo binding assay to identify responsible key amino acid residues or motifs. Based on our approach, we found two additional amino acid residues (histidine‐256 on CP and arginine‐455 on HC‐Pro) important for the direct CP–HC‐Pro interaction. We constructed amino acid substitution mutants by changing the identified residues to alanine within the infectious clone of SMV strain G7H (pSMV‐G7H; Seo et al., 2009c) to test whether these amino acid residues are responsible for the aphid transmission of SMV. We also tested the involvement of the PTK and DAG motifs of SMV in the CP–HC‐Pro interaction and aphid transmission.
RESULTS
Characterization of the interaction between SMV CP and HC‐Pro in yeast
YTHS has been used widely to investigate interactions between proteins from many different organisms, including viruses (Evangelista et al., 1996). This system provides a genetic approach to characterize physical protein–protein interactions and to identify specific domains of proteins responsible for interactions (Fields and Sternglanz, 1994). For potyviruses, several studies with different viruses have been performed to construct interaction maps for all of the major viral proteins (Guo et al., 2001; Kang et al., 2004; Lin et al., 2009).
We have demonstrated previously a strong interaction between SMV CP and HC‐Pro using YTHS (Kang et al., 2004). To further characterize this interaction using YTHS, several truncated mutants were introduced into both CP and HC‐Pro (Fig. 1). Deletions of CP and HC‐Pro were made on the basis of polymerase chain reaction (PCR) amplification and fused downstream of the GAL4 activation domain (GAL4‐AD) and GAL4 DNA‐binding domain (GAL4‐BD), respectively. Interactions between either the CP truncations and intact HC‐Pro (HC‐Pro BD) or intact CP (CP AD) and HC‐Pro truncations were tested using YTHS on synthetic dropout (SD) agar medium lacking leucine, tryptophan, histidine and adenine (–LWHA) (Fig. 1). CP AD consistently interacted with HC‐Pro BD. However, the CP truncations comprising amino acids 1–132 (CP1–132 AD) or amino acids 149–265 (CP149–265 AD) failed to interact with HC‐Pro BD, whereas the CP truncations comprising amino acids 81–265 (CP81–265 AD) or amino acids 127–265 (CP127–265 AD) maintained an interaction with HC‐Pro BD. In addition, the CP truncations comprising amino acids 1–245 (CP1–245 AD) also failed to interact with HC‐Pro BD. Concomitantly, the HC‐Pro truncations comprising amino acids 1–423 (HC‐Pro1–423 BD) failed to interact with CP AD, whereas HC‐Pro truncations comprising amino acids 144–457 (HC‐Pro144–457 BD) or amino acids 309–457 (HC‐Pro309–457 BD) interacted with CP AD. All of these interactions were further confirmed by galactosidase assays on SD/–LW and SD/–LWHA media (Fig. 1). In all, these results suggest that the HC‐Pro region comprising amino acids 424–457 and two regions of CP comprising amino acids 133–148 and 246–265 may be indispensable for the CP–HC‐Pro interaction.
Figure 1.
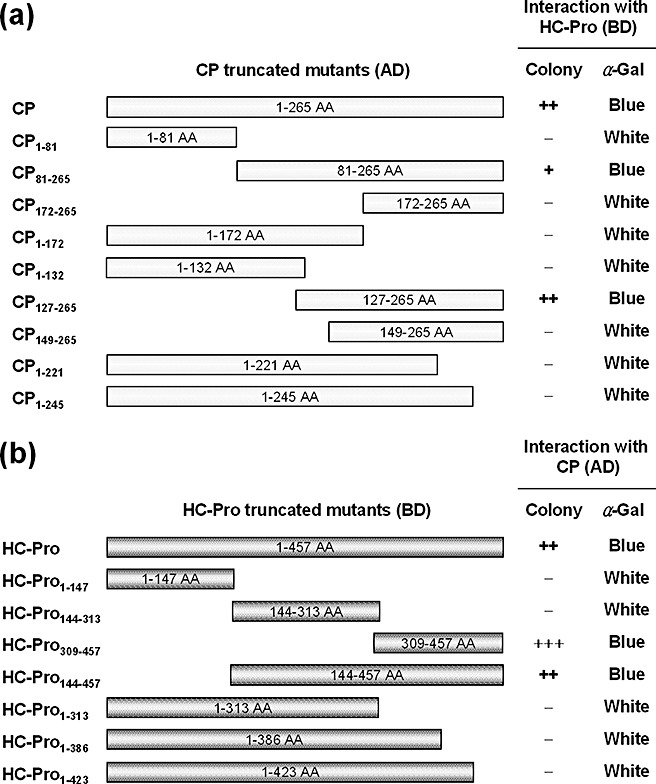
Map of interaction between Soybean mosaic virus (SMV) coat protein (CP) and helper component‐proteinase (HC‐Pro) in a yeast two‐hybrid system (YTHS). Full‐length CP and its truncations were fused downstream of the GAL4 activation domain (GAL4‐AD) of pACT2. Full‐length HC‐Pro and its truncations were fused downstream of the GAL4 DNA‐binding domain (GAL4‐BD) of pAS2‐1. Yeast cells co‐transformed with pACT2 and pAS2‐1 fusion derivatives were selected on synthetic dropout (SD) agar medium lacking leucine, tryptophan, histidine and adenine (–LWHA), and their α‐galactosidase activities were assayed on SD/–LW and SD/–LWHA agar plates containing X‐α‐Gal. The number of ‘+’ symbols indicates the comparative number of colonies formed on SD medium. (a) Interactions between HC‐Pro and CP truncations. (b) Interactions between CP and HC‐Pro truncations.
To identify key amino acid residues responsible for the CP–HC‐Pro interaction, alanine substitution mutations were introduced into the central and C‐terminal regions of CP and the C‐terminal region of HC‐Pro. Both positively and negatively charged amino acids in these regions were substituted with alanine (Fig. 2a, b). In addition, we introduced mutations into the DAG and PTK motifs, i.e. glycine at amino acid position 12 of CP and threonine at amino acid position 310 of HC‐Pro were substituted with glutamic acid and alanine, respectively (CP G12E and HC‐Pro T310A; Fig. 2a, b). Each of these mutations was identified to abolish the in vitro interaction between CP and HC‐Pro (Blanc et al., 1997; Peng et al., 1998). Alanine‐substituted mutants (ASMs) of CP and HC‐Pro were fused downstream of GAL4‐AD and GAL4‐BD, respectively. Interactions between either CP ASMs and HC‐Pro BD or CP AD and HC‐Pro ASMs were tested using YTHS and galactosidase assays. As summarized in 1, 2, only mutants CP H256A and HC‐Pro R455A failed to maintain the CP–HC‐Pro interaction. These results indicate that histidine at position 256 of CP and arginine at position 455 of HC‐Pro are involved in the CP–HC‐Pro interaction. However, all tested ASMs for the central region of CP maintained interaction with HC‐Pro BD (Table 1). Thus, to confirm these interactions using the truncated mutant able to interact with HC‐Pro BD, same single‐alanine substitution mutations were introduced into CP127–265 AD, and the interactions between the resulting CP127–265 ASMs and HC‐Pro BD were tested. The results showed that only the mutant CP127–265 K143A could not interact with HC‐Pro BD (Fig. S1; see Supporting Information). However, because the mutation CP K143A in the full‐length CP maintained interaction with HC‐Pro BD, we concluded that the central region of CP was not directly involved in the interaction between intact CP and HC‐Pro. In addition, we tested whether the mutants CP G12E and HC‐Pro T310A lost the ability to interact with HC‐Pro and CP, respectively, in YTHS. Interestingly, the CP mutation G12E disrupted the CP–HC‐Pro interaction (Table 1 and Fig. S2; see Supporting Information). However, inconsistent with the previous in vitro result (Peng et al., 1998), the HC‐Pro mutation T310A still maintained the CP–HC‐Pro interaction (Table 2 and Fig. S3; see Supporting Information).
Figure 2.
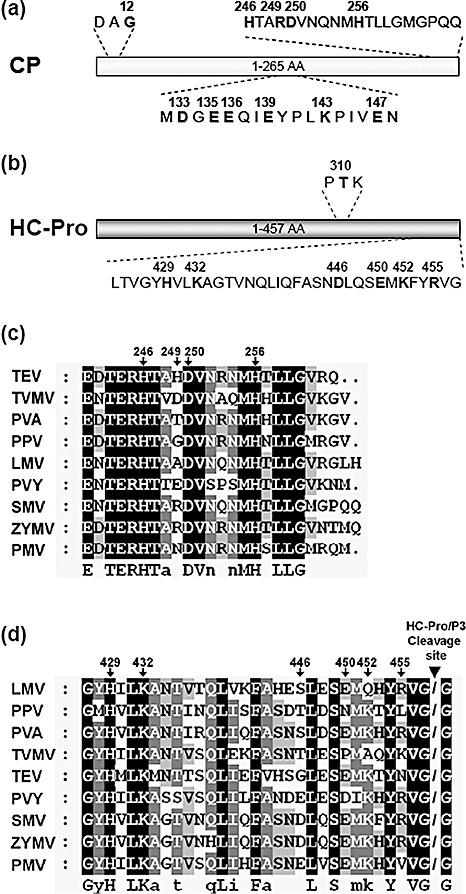
Identification of amino acid residues crucial for the coat protein–helper component‐proteinase (CP–HC‐Pro) interaction in a yeast two‐hybrid system (YTHS). Schematic representations of Soybean mosaic virus (SMV) CP (a) and HC‐Pro (b), showing the amino acid positions of the site‐directed mutations tested in this study. Alignment of the C‐terminal amino acid sequences of potyviral CPs (c) and HC‐Pros (d). Sequences were aligned by ClustalX2. Arrows indicate the amino acid positions on SMV CP and HC‐Pro. Arrowhead indicates the location of the HC‐Pro self‐cleavage site. Potyviral sequence data were obtained from the National Center for Biotechnology Information (NCBI) database: TEV, Tobacco etch virus (NC001555); TVMV, Tobacco vein mottling virus (NC001768); PVA, Potato virus A (NC004039); PPV, Plum pox virus (NC001445); LMV, Lettuce mosaic virus (NC003605); PVY, Potato virus Y (NC001616); SMV, Soybean mosaic virus (FJ807700); ZYMV, Zucchini yellow mosaic virus (NC003224); PMV, Peanut mottle virus (NC002600).
Table 1.
Interactions between coat protein (CP) mutants and wild‐type (wt) helper component‐proteinase (HC‐Pro).
| ASM (AD)* | Interaction with HC‐Pro (BD)† | |
|---|---|---|
| Colony | α‐Gal | |
| CP wt | ++ | Blue |
| CP G12E | − | White |
| CP D133A | ++ | Blue |
| CP E135A | ++ | Blue |
| CP E136A | ++ | Blue |
| CP E139A | ++ | Blue |
| CP K143A | ++ | Blue |
| CP E147A | ++ | Blue |
| CP D133A+E135A | ++ | Blue |
| CP D133A+E136A | ++ | Blue |
| CP E135A+E136A | ++ | Blue |
| CP H246A | ++ | Blue |
| CP R249A | ++ | Blue |
| CP D250A | ++ | Blue |
| CP H256A | − | White |
AD, activation domain; ASM, alanine‐substituted mutant; BD, binding domain.
The amino acid substitution mutants of CP were fused downstream of the GAL4 activation domain (GAL4‐AD) of pACT2.
Yeast cells co‐transformed with pACT2 and pAS2‐1 fusion derivatives were selected on synthetic dropout (SD) agar medium lacking leucine, tryptophan, histidine and adenine (–LWHA), and their α‐galactosidase activities were assayed on SD/–LW and SD/–LWHA agar plates containing X‐α‐Gal. The number of ‘+’ symbols indicates the comparative number of colonies formed on SD medium.
Table 2.
Interactions between wild‐type coat protein (CP) and helper component‐proteinase (HC‐Pro) mutants.
| ASM (BD)* | Interaction with CP (AD)† | |
|---|---|---|
| Colony | α‐Gal | |
| HC‐Pro wt | ++ | Blue |
| HC‐Pro T310A | ++ | Blue |
| HC‐Pro H429A | ++ | Blue |
| HC‐Pro K432A | ++ | Blue |
| HC‐Pro D446A | ++ | Blue |
| HC‐Pro E450A | ++ | Blue |
| HC‐Pro K452A | ++ | Blue |
| HC‐Pro R455A | − | White |
AD, activation domain; ASM, alanine‐substituted mutant; BD, binding domain.
The amino acid substitution mutants of HC‐Pro were fused downstream of the GAL4 DNA‐binding domain (GAL4‐BD) of pAS2‐1.
Yeast cells co‐transformed with pACT2 and pAS2‐1 fusion derivatives were selected on synthetic dropout (SD) agar medium lacking leucine, tryptophan, histidine and adenine (–LWHA), and their α‐galactosidase activities were assayed on SD/–LW and SD/–LWHA agar plates containing X‐α‐Gal. The number of ‘+’ symbols indicates the comparative number of colonies formed on SD medium.
Interaction between SMV CP and HC‐Pro in planta
To further confirm the interaction between CP and HC‐Pro identified by YTHS, we conducted the bimolecular fluorescence complementation (BiFC) assay to investigate the CP–HC‐Pro interaction based on the Agrobacterium infiltration method. The BiFC assay has been developed to study and visualize protein–protein interactions in vivo (Citovsky et al., 2006; Hu and Kerppola, 2003). This assay is based on the formation of a functional fluorescent complex by two fragments of yellow fluorescent protein (YFP) brought together by the physical interaction between target proteins fused to fragmented YFP (Citovsky et al., 2006). To verify the CP–HC‐Pro interaction using this assay in planta, we first developed an Agrobacterium‐mediated protein expression vector based on the pregnancy zone protein‐green fluorescent protein (PZP‐GFP) construct (Fig. 3a; Powers et al., 2008). Both intact and mutated CPs were fused upstream of the N‐terminal region of YFP, comprising amino acids 1–156 (nYFP), and the mutated HC‐Pros and both intact CP and HC‐Pro were fused upstream of the C‐terminal region of YFP comprising amino acids 157–239 (cYFP) (Fig. 3a). Co‐expression of CP‐nYFP and CP‐cYFP as a positive control induced fluorescence signals emitted by YFP under UV light in agroinfiltrated Nicotiana benthamiana epidermal cells (Fig. 3f), whereas co‐expression of nYFP + cYFP, CP‐nYFP + cYFP, nYFP + HC‐cYFP did not induce fluorescence signals (Fig. 3c–e). Next, when either intact or mutated CPs fused to nYFP and HC‐cYFP were co‐expressed in N. benthamiana leaves, strong fluorescence signals were observed in epidermal cells expressing HC‐cYFP together with CP‐nYFP, CPH246A‐nYFP, CPR249A‐nYFP or CPD250A‐nYFP (Fig. 3g, i–k). In contrast, the co‐expression of CPG12E‐nYFP and HC‐cYFP did not induce fluorescence signals (Fig. 3h), and the co‐expression of CPH256A‐nYFP and HC‐cYFP induced fluorescence signals significantly weaker than those observed from the co‐expression of CP‐nYFP and HC‐cYFP (Fig. 3g, l). Subsequently, when CP‐nYFP and the mutated HC‐Pros fused to cYFP were co‐expressed in N. benthamiana leaves, no fluorescence signal was detected in epidermal cells expressing CP‐nYFP and HCR455A‐cYFP (Fig. 3p), whereas obvious fluorescence signals were observed in epidermal cells expressing CP‐nYFP together with HCT310A‐cYFP, HCE450A‐cYFP or HCK452A‐cYFP (Fig. 3m–o). All BiFC assays were repeated more than three times, and similar results were observed. Collectively, these CP–HC‐Pro interaction results, obtained by in planta BiFC assay, were generally consistent with those obtained from YTHS. In contrast, a weak but detectable interaction between CP H256A and HC‐Pro was observed in the BiFC assay, but not in YTHS.
Figure 3.
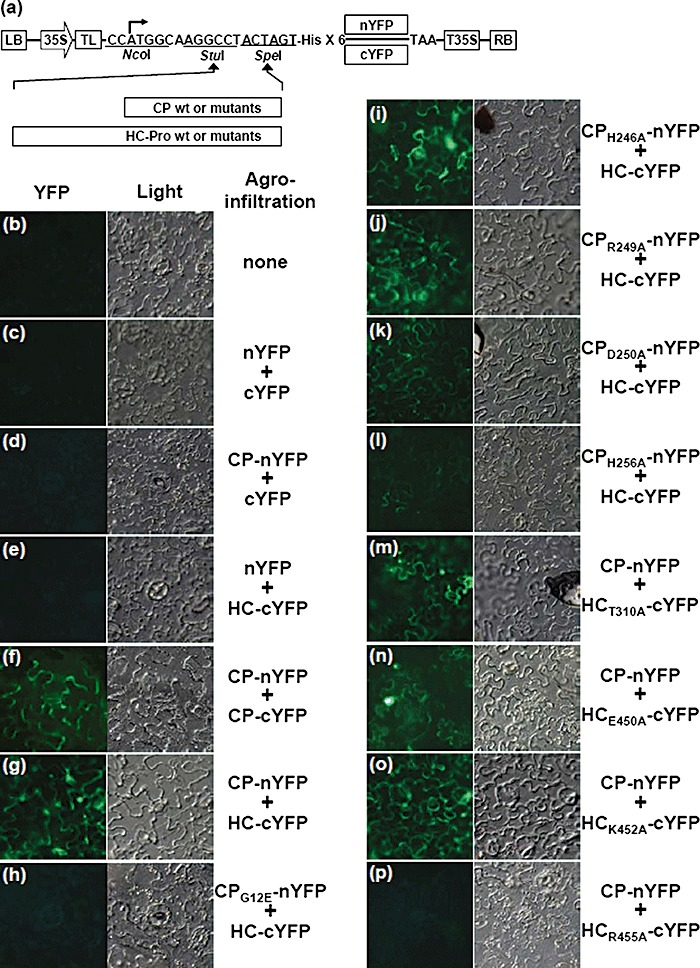
Detection of in planta interactions between coat protein (CP) and helper component‐proteinase (HC‐Pro) by bimolecular fluorescence complementation (BiFC) assay. (a) Schematic representation of the constructs used for BiFC assay. The basal BiFC vector contains, in sequential order, a left border of T‐DNA (LB), a double 35S promoter (35S), cloning sites, six histidine residues, either nYFP or cYFP sequences, a 35S terminator (T35S) and a right border of T‐DNA (RB). CP and HC‐Pro cistrons and their mutants were in‐frame inserted into the basal BiFC vectors utilizing StuI and SpeI sites. The constructed clones were transferred into Nicotiana benthamiana leaves by agroinfiltration in pairwise combinations as follows: none (healthy; b), nYFP + cYFP (c), CP‐nYFP + cYFP (d), nYFP + HC‐cYFP (e), CP‐nYFP + CP‐cYFP (f), CP‐nYFP + HC‐cYFP (g), CPG12E‐nYFP + HC‐cYFP (h), CPH246A‐nYFP + HC‐cYFP (i), CPR249A‐nYFP + HC‐cYFP (j), CPD250A‐nYFP + HC‐cYFP (k), CPH256A‐nYFP + HC‐cYFP (l), CP‐nYFP + HCT310A‐cYFP (m), CP‐nYFP + HCE450A‐cYFP (n), CP‐nYFP + HCK452A‐cYFP (o) and CP‐nYFP + HCR455A‐cYFP (p). The reconstructed yellow fluorescent protein (YFP) signals were observed in the epidermal cells using epifluorescence microscopy at 3 days post‐infiltration (dpi). Co‐expression of (g), (i), (j), (k), (m), (n) and (o) induced fluorescence signals. Co‐expression of (l) induced weak but detectable fluorescence. YFP signals from the co‐expression of (f) were used as a positive control. Images for fluorescence emitted by YFP (left) and for the transmitted light mode (right) are shown.
Effect of CP and HC‐Pro mutations on infectivity and virus accumulation
To test whether the identified mutations abolishing the CP–HC‐Pro interaction are really involved in the aphid transmission of SMV, we introduced these mutations into the pSMV‐G7H clone (Seo et al., 2009c). The resulting constructs containing mutations on CP were named pSMV‐CPG12E, pSMV‐CPH246A, pSMV‐CPR249A, pSMV‐CPD250A and pSMV‐CPH256A. For HC‐Pro mutants, we first introduced the HC‐Pro mutation R455A into pSMV‐G7H (pSMV‐HCR455A) to examine the effect of this mutation on SMV infectivity. Despite many attempts, soybean plants (cv. Somyungkong) were not infected by pSMV‐HCR455A (data not shown). It seems likely that residue R455 of HC‐Pro may be involved in the recognition by HC‐Pro for autoproteolysis, as this residue is located in the conserved cleavage site (Fig. 2d; Adams et al., 2005; Chen et al., 2004). Thus, we used another strategy to introduce the R455A mutation into the infectious SMV construct. To utilize the NIa‐Pro proteolytic activity for the precessing of HC‐Pro from polyprotein, we first constructed an HC‐Pro‐deleted SMV construct (pSMVdHC; Fig. 4b) based on pSMV‐G7H by replacing the HC‐Pro cistron with a cloning site (XbaI) and an additional NIa‐Pro cleavage site (ESVSLQ/S). The intact and mutated HC‐Pros were re‐inserted into pSMVdHC by utilizing the XbaI site to create an in‐frame translational fusion. The amino acid sequences surrounding the HC‐Pro self‐cleavage site are highly conserved (Adams et al., 2005), and it has been shown previously that substitutions of glycine, which is the first residue of P3 protein, eliminate the HC‐Pro self‐cleavage activity (Carrington and Herndon, 1992). Therefore, proteolysis of the viral polyprotein by P1 in cis and NIa‐Pro in trans was predicted to yield the inserted HC‐Pro with two additional amino acids (SR) at its N‐terminus and eight amino acids (SRESVSLQ) at its C‐terminus (Fig. 4c), although this was not verified experimentally. When infectivity of pSMVdHC containing intact HC‐pro (pSMVdHC‐HCWT) was tested on soybean plants, typical SMV G7H‐induced disease symptoms were observed on several soybean cultivars, indicating that this recombinant construct was infectious (data not shown). Thus, the SMV HC‐Pro mutants were constructed utilizing pSMVdHC, and the resultant constructs were named pSMVdHC‐HCT310A, pSMVdHC‐HCE450A, pSMVdHC‐HCK452A and pSMVdHC‐HCR455A.
Figure 4.
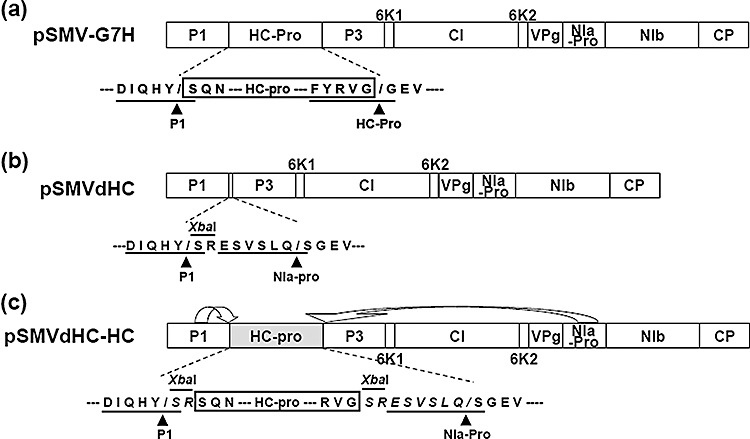
Schematic representation of Soybean mosaic virus (SMV) constructs used for the introduction of helper component‐proteinase (HC‐Pro) mutations. (a) SMV genome organization. HC‐Pro is processed by P1 and by HC‐Pro by itself in an in cis manner. Amino acid sequences of the peptide cleavage sites recognized by either P1 or HC‐Pro are underlined, and arrowheads indicate the location of the cleaved peptide bond. (b) Construction of an available insertion cassette between P1 and P3. A cloning site (XbaI) and the NIa‐Pro cleavage site (ESVSLQ/S) were inserted into the polyprotein open reading frame (ORF) between the P1 and P3 cistrons. The amino acid sequences of the peptide cleavage sites recognized by either P1 or NIa‐Pro are underlined, and arrowheads indicate the location of the cleaved peptide bond. (b) Schematic representation of HC‐Pro processing from the reconstructed SMV genome. HC‐Pro, which is inserted into pSMVdHC utilizing the XbaI site, is processed by P1 in cis and by NIa‐Pro in an in cis manner, and is predicted to have two additional amino acids (SR) at its N‐terminus and eight amino acids (SRESVSLQ) at its C‐terminus.
Prior to examining the aphid transmissibility of the SMV mutant constructs, we investigated their relative infectivity. Plasmid DNAs of all constructed SMV mutants were inoculated onto the primary leaves of soybean seedlings (cv. Somyungkong) by direct rub‐inoculation, as described previously (Seo et al., 2009c). At 9–14 days post‐infiltration (dpi), typical mild mosaic symptoms appeared in most inoculated soybeans, except soybeans inoculated with either pSMV‐CPH246A or pSMV‐CPD250A (data not shown). To verify whether these inoculated soybeans were infected with each SMV mutant, total RNAs extracted from the upper uninoculated leaves were analysed by reverse transcriptase (RT)‐PCR. The RT‐PCR results revealed that soybeans inoculated with pSMV‐CPH246A or pSMV‐CPD250A were not infected, whereas all other SMV mutants were infectious on soybean plants (data not shown). Thus, it seems likely that residues H246 and D250 of CP may be critical for systemic infection or virus assembly of SMV (Kang et al., 2006). Furthermore, to examine the effect of the CP and HC‐Pro mutations on virus accumulation, viral RNA levels were quantified by real‐time RT‐PCR from total RNAs extracted from fully expanded third trifoliolate leaves at 18 dpi, as described previously (Seo et al., 2009c). As summarized in Table 3, the accumulations of viral RNAs in plants infected with pSMV‐CPG12E and pSMVdHC‐HCWT, pSMVdHC‐HCE450A, pSMVdHC‐HCK452A and pSMVdHC‐HCR455A were about 90% of that of pSMV‐G7H, indicating that these mutations and our genetic reconstruction of SMV did not affect virus accumulation significantly. In contrast, the accumulations of viral RNAs in plants infected with pSMV‐CPR249A and pSMV‐CPH256A and pSMVdHC‐HCT310A were about 75% of that of pSMV‐G7H. This implies that residues CP R249, CP H256 and HC‐Pro T310 may have some functions in virus movement or replication. In particular, HC‐Pro has silencing suppression activity. Thus, we cannot exclude the possibility that introductions of mutations into HC‐Pro affect its silencing suppression activity, resulting in decreasing viral accumulation (Kasschau and Carrington, 2001).
Table 3.
Effect of coat protein (CP) and helper component‐proteinase (HC‐Pro) mutations on virus accumulation and aphid transmission of Soybean mosaic virus (SMV).
| SMV mutant | Virus accumulation* | Aphid transmission† | |||||
|---|---|---|---|---|---|---|---|
| Mean ± SE | P value 1 | P value 2 | Exp. 1 | Exp. 2 | Exp. 3 | P value 3 | |
| Mock | 0 | 0/10 | 0/10 | 0/10 | |||
| pSMV‐G7H | 100.0 ± 0.0 | 10/10 | 9/10 | 10/10 | |||
| pSMV‐CPG12E | 86.3 ± 10.0 | 0.30 | 0/5 | 0/5 | 0/5 | 0.001 | |
| pSMV‐CPH246A | 0 | NA | NA | NA | |||
| pSMV‐CPR249A | 75.6 ± 17.8 | 0.14 | 4/5 | 5/5 | 5/5 | 0.686 | |
| pSMV‐CPD250A | 0 | NA | NA | NA | |||
| pSMV‐CPH256A | 77.7 ± 13.4 | 0.25 | 2/5 | 2/5 | 3/5 | 0.007 | |
| pSMVdHC‐HCWT | 93.0 ± 7.9 | 0.43 | 5/5 | 5/5 | 5/5 | 0.422 | |
| pSMVdHC‐HCT310A | 75.5 ± 14.8 | 0.10 | 0.19 | 0/5 | 0/5 | 0/5 | 0.001 |
| pSMVdHC‐HCE450A | 91.2 ± 4.5 | 0.22 | 0.82 | 5/5 | 4/5 | 5/5 | 0.686 |
| pSMVdHC‐HCK452A | 94.2 ± 4.4 | 0.31 | 0.87 | 4/5 | 5/5 | 5/5 | 0.686 |
| pSMVdHC‐HCR455A | 88.5 ± 11.5 | 0.23 | 0.64 | 0/5 | 0/5 | 0/5 | 0.001 |
*Virus accumulation was quantified by real‐time reverse transcriptase‐polymerase chain reaction (RT‐PCR). Total RNA was extracted from the third trifoliolate leaves of the inoculated soybean plants at 18 days post‐infiltration (dpi). Each value is the mean compared with that of pSMV‐G7H as a percentage of the wild‐type (100%) and the standard error (SE) compiled from at least three independent experiments. P values were estimated by Student's t‐test: 1for virus accumulation of pSMV‐G7H; 2for virus accumulation of pSMVdHC‐HCWT.
†Number of plants infected over number of plants inoculated by aphid transmission. Virus infection was confirmed by RT‐PCR using SMV‐specific primers. NA, not available. P values were estimated by Student's t‐test: 3for aphid transmissibility of pSMV‐G7H.
Effect of CP and HC‐Pro mutations on aphid transmission
To assess the effect of the CP and HC‐Pro mutations on aphid transmission, we conducted a plant‐to‐plant transmission assay using aphids (Aphis glycines). The transmission assays were performed three times independently, comprising a total of 15 plants for each SMV mutant (Table 3). Progeny viruses of pSMV‐G7H and pSMV‐CPR249A and pSMVdHC‐HCWT, pSMVdHC‐HCE450A and pSMVdHC‐HCK452A were successfully transmitted, whereas progeny viruses of pSMV‐CPG12E and pSMVdHC‐HCR455A were not transmitted by aphids (Table 3). However, progenies of pSMV‐CPH256A were transmitted with efficiencies ranging from 40% to 60% (Table 3). It is possible that poor transmission of pSMV‐CPH256A is the result of a poor accumulation of virus in the plant. However, progenies of pSMV‐CPR249A accumulated to similar levels as those of pSMV‐CPH256A but were transmitted successfully by aphids (Table 3). Moreover, aphid transmissions were performed with sufficient numbers of aphids (Domier et al., 2003). In all, these findings lead us to conclude that the poor transmission of pSMV‐CPH256A was not significantly affected by the virus accumulation level in our experiments. In general, our transmission results, together with the yeast and in planta results, suggest that there is a strong correlation between the CP–HC‐Pro interaction and the aphid transmissibility of SMV. In contrast, progeny viruses of pSMVdHC‐HCT310A were not transmitted by aphids, but the HC‐Pro mutation T310A retained physical interaction with CP in our yeast and in planta assays. In fact, this was unexpected, as the corresponding P309A mutation on the PTK motif of ZYMV resulted in a loss of HC‐Pro activity in aphid transmission (Huet et al., 1994; Peng et al., 1998), and the P309A mutation on the PTK motif of ZYMV abolished the in vitro binding between purified virions and HC‐Pros (Peng et al., 1998). It is possible that the HC‐Pros of SMV and ZYMV might have different modes of interaction with their CPs. Other possible reasons for this discrepancy are further considered in the ‘Discussion’ section.
To ensure that progeny viruses of the SMV mutant constructs maintained the introduced mutations during infection and aphid transmission, HC‐Pro and CP cistrons of progeny viruses were recovered by RT‐PCR from infected plants, and the PCR products were directly sequenced. Sequence analyses showed that all introduced mutations were maintained through infection and aphid transmission (data not shown).
DISCUSSION
Since the pioneering studies of 1974a, 1974b) for the aphid transmission of PVY, it has been well documented that HC‐Pro mediates the aphid transmission of potyviruses, and interactions between HC‐Pro and either CP or aphid stylets are important for this mediation (Atreya and Pirone, 1993; 1997, 1998; Huet et al., 1994; Peng et al., 1998). These studies have demonstrated that three conserved motifs, KITC and PTK on HC‐Pro and DAG on CP, are essential for potyviral aphid transmission. These conserved motifs were found on the basis of comparisons of CP and HC‐Pro sequences between naturally occurring aphid transmissible and non‐transmissible potyviruses (Atreya and Pirone, 1993; Atreya et al., 1990; Huet et al., 1994).
As the strong interaction between SMV CP and HC‐Pro was detected in YTHS (Kang et al., 2004), we further analysed this interaction using the truncated and point‐mutated CP and HC‐Pro constructs based on YTHS and in planta binding assay to confirm and further characterize the mode of interaction between CP and HC‐Pro. Through this genetic approach, we identified the additional amino acids crucial for the CP–HC‐Pro interaction and showed that mutation of these amino acid positions affected the efficiency of aphid transmission of SMV.
We initially found that the C‐terminal region of HC‐Pro and both the central and C‐terminal regions of CP were crucial for the CP–HC‐Pro interaction in YTHS (Fig. 1). These results were contrary to our expectations because the DAG and PTK motifs are located on the N‐terminal region of CP and central region of HC‐Pro, respectively. However, as the CP–HC‐Pro interaction is still detectable in plants infected with non‐transmissible potyvirus derivatives containing N‐terminal deletions of CP lacking the DAG motif, it has been suggested previously that different modes of CP–HC‐Pro interaction could be related to other biological functions of CP and HC‐Pro (Roudet‐Tavert et al., 2002). In our mutational analysis based on YTHS, we found that residue K143 was involved in the interaction between the N‐terminus‐deleted CP and HC‐Pro (Fig. S1; see Supporting Information). However, when the same mutation was introduced into full‐length CP, the CP–HC‐Pro interaction was not affected (Table 1). These results suggest that there might be conformational differences between the intact CP and N‐terminus‐deleted CP and that a new interaction site might be generated by deletion of the N‐terminus from CP. For this reason, we could not examine the N‐terminal region of CP to identify crucial residues for the CP–HC‐Pro interaction. However, we found that the DAG motif is involved in the CP–HC‐Pro interaction by introducing a mutation in residue G12 (Table 1 and Fig. S2; see Supporting Information). In addition, we identified another amino acid residue (H256) from the C‐terminus of CP involved in the interaction with HC‐Pro. The H256 mutation on CP abolished the CP–HC‐Pro interaction in YTHS, but exhibited weak interaction with HC‐Pro in the BiFC assay (Table 1, Fig. 3l, and Fig. S2; see Supporting Information). This is possible because YTHS generally selects for strong interactions under high stringency conditions (SD/–LWHA medium). In BiFC, the intensity of fluorescence depends on the interaction strength (Magliery et al., 2005). Thus, the CP mutant H256A appears to interact weakly with HC‐Pro. It has been shown previously that the CP–HC‐Pro binding affinity correlates with the frequency of aphid transmission (Blanc et al., 1997). Indeed, progeny viruses of pSMV‐CPH256A were aphid‐transmitted with low efficiencies (40–60%), whereas progenies of pSMV‐G7H were successfully transmitted and progenies of pSMV‐CPG12E were not transmitted (Table 3). In addition, both termini of SMV CP containing the H256 residue as well as the DAG motif are thought to be exposed on the virion surface (Baratova et al., 2001; Kendall et al., 2008; Shukla et al., 1988), and the amino acid residue corresponding to the H256 residue of SMV CP is highly conserved among potyviruses (Fig. 2c; Shukla et al., 1988). This lends support to the conclusion that residue H256 has some function in binding to/with HC‐Pro.
We also identified residue R455 on the C‐terminus of HC‐Pro as being implicated in the interaction with CP, and that this residue is critical for the aphid transmission of SMV (2, 3, Fig. 3p, and Fig. S3; see Supporting Information). As noted above, as residue R455 is located in the cleavage site, we tested the effect of this residue on aphid transmissibility using pSMVdHC‐HCR455A. HC‐Pro proteins produced from pSMVdHC+HC were predicted to carry two and eight additional amino acids at their N‐ and C‐terminus, respectively (Fig. 4c). However, as progenies of pSMVdHC‐HCWT were successfully transmitted by aphids (Table 3), these additional amino acids did not seem to interfere with vector transmission. Furthermore, we were able to confirm that the PTK motif is important for aphid transmission of SMV using pSMVdHC‐HCT310A (Table 3). However, our protein interaction assays revealed that the HC‐Pro mutation T310A did not abolish the physical CP–HC‐Pro interaction. As noted above, this result is contrary to the previous result obtained by Peng et al., (1998). Several factors may explain this contrast. First, the discrepancy could be a result of differences in the experimental approach. The previous study used virions and HC‐Pros purified from ZYMV‐infected plants to examine the effects of HC‐Pro mutations on binding to virions (Peng et al., 1998), whereas we examined the CP–HC‐Pro interactions between single proteins. A recent report has demonstrated that the majority of HC‐Pro is in the oligomeric state, such as dimers, tetramers and hexamers, in purified solution, and this purified oligomeric HC‐Pro is active in transmission assays (Ruiz‐Ferrer et al., 2005). Thus, it is possible that different oligomers may have different functional activities, and that one of these oligomers can mediate aphid transmission of potyviruses. In this regard, the mutations on the PTK motif of HC‐Pro might disrupt some oligomerization states mediating transmission, although this possibility was not examined in either the present or previous studies (Peng et al., 1998). Furthermore, it cannot be ruled out that the PTK motif may be activated to interact with CP by conformational changes to HC‐Pro during oligomerization because the PTK motif is located within the hinge domain of HC‐Pro (Plisson et al., 2003). Our protein interaction assays have a limited ability to detect these kinds of interactions as our experimental set‐ups are designed to detect interactions between single proteins. However, based on our experimental approach, we demonstrated that residue R455 of HC‐Pro was obviously involved in the CP–HC‐Pro interaction and aphid transmission of SMV. In addition, it has been suggested recently that, in particular, some virions of PVY and Potato virus A (PVA) have a protruding tip at the 5′‐end of the viral genome and that this tip contains VPg together with HC‐Pro (Torrance et al., 2006). Furthermore, HC‐Pros were found only at one end of the virions rather than along the entire length of the virions, and detected in approximately 7% of purified virions (Torrance et al., 2006). Therefore, the authors suggested that HC‐Pro may interact with both VPg and CP to form a protruding tip and that this complex may act as a bridge mediating aphid transmission. In all, it is likely that there may be a more complicated involvement of motifs, including KITC, PTK and DAG, than is currently envisioned in the mediation of the aphid transmission of potyviruses.
In this study, we have demonstrated that residue H256 of CP and residue R455 of HC‐Pro, as well as residue G12 on the DAG motif, are critical for the CP–HC‐Pro interaction and aphid transmission of SMV, although further work is needed to clarify the involvement of these motifs in the aphid transmission of other potyviruses. We have also demonstrated that mutations on these amino acid residues and residue T310 of HC‐Pro abolish the aphid transmissibility of SMV. Taken together with previous studies (Atreya and Pirone, 1993; Blanc et al., 1997; Peng et al., 1998; Ruiz‐Ferrer et al., 2005; Torrance et al., 2006), these findings lead us to consider more complex molecular interactions bridging virus particles and aphid stylets than the simple involvement of HC‐Pro as a bridge, including interactions between HC‐Pros, interactions between CP and HC‐Pro and interactions with VPg. As it is assumed that the structural properties of HC‐Pro strongly influence the aphid transmissibility of potyviruses, further extensive studies will be required to obtain structural information on HC‐Pro in aphid transmission and to characterize the interactions between HC‐Pro and either aphid stylets or VPg to better understand the mode of action of HC‐Pro in aphid transmission.
EXPERIMENTAL PROCEDURES
Yeast two‐hybrid assay
YTHS was conducted on the basis of the MATCHMAKER Two‐Hybrid System 2 (Clontech, Mountain View, CA, USA), as described previously (2004, 2006). CP and HC‐Pro cistrons and their truncated versions (Fig. 1) were amplified from cDNA of SMV strain G7H by PCR using the exTaq polymerase (TaKaRa, Shiga, Japan) and appropriate primer pairs (Table S1; see Supporting Information). CP and its truncations were fused downstream of GAL4‐AD by in‐frame insertion utilizing the NcoI site of pACT2. HC‐Pro and its truncations were fused downstream of GAL4‐BD utilizing the EcoRI site of pAS2‐1. Amino acid substitution mutants of CP and HC‐Pro were constructed by PCR‐based, site‐directed mutagenesis using appropriate primers (Table S1; see Supporting Information) as described previously (Nassal and Rieger, 1990). The sequences of all constructed clones were validated by DNA sequencing.
Yeast competent cells were co‐transformed with the pACT2 and pAS2‐1 fusion derivatives by the lithium acetate method (Schiestl and Gietz, 1989), and then selected on SD/–LWHA or SD/–LW medium. The interactions between SV40 large T antigen(84–708) (pTD1‐1) and either murine p53(72–390) (pVA3‐1) or human lamin C(66–230) (pLAM5′‐1) served as positive and negative controls, respectively. All interactions were confirmed by α‐galactosidase activity assays using X‐α‐Gal reagent (Clontech). α‐Galactosidase activity was assayed for 2 days on SD/–LW and SD/–LWHA agar plates containing X‐α‐Gal (Kang et al., 2004).
BiFC assay
To construct the binary vector for BiFC, the PZP‐GFP construct (provided by Dr Steven Lommel, North Carolina State University, Raleigh) was modified by replacing the GFP region with either His6‐tagged nYFP or His6‐tagged cYFP (Fig. 3a). These YFP fragments were amplified by PCR from pSITE‐3CA using appropriate primer pairs (Table S1; see Supporting Information). The resulting constructs were named PZP‐nYFP and PZP‐cYFP, respectively. CP and HC‐Pro cistrons and their amino acid mutants were amplified by PCR from the clones used in YTHS, and inserted in‐frame into either PZP‐nYFP or PZP‐cYFP utilizing StuI and SpeI sites (Fig. 3a). The sequences of all constructed clones were validated by DNA sequencing.
Agroinfiltration into N. benthamiana was performed as described previously (Koscianska et al., 2005; Seo et al., 2009a). Briefly, Agrobacterium strain GV2260 was transformed with each of the constructed clones by electroporation. Agrobacteria harbouring each clone were grown at 30 °C overnight in yeast extract phosphate (YEP) medium with kanamycin (50 µg/mL) and acetosyringone (20 µm). The cultures were harvested by centrifugation, resuspended in infiltration medium [Murashige and Skoog (MS) salts, 10 mm 2‐(N‐morpholino)ethanesulphonic acid (MES), pH 5.6, 200 µm acetosyringone] and incubated at 30 °C for a minimum of 2 h. Agrobacterium cultures [at an optical density at 600 nm (OD600) = 0.5] were mixed in pairwise combinations together with agrobacteria harbouring PZP‐TBSV p19 (expressing p19 silencing suppressor of Tomato bushy mosaic virus; Powers et al., 2008) and infiltrated onto the abaxial surface of leaves. p19 was used to enhance the ectopic expression of heterologous genes. At 3 dpi, epidermal cells of agroinfiltrated leaves were observed using epifluorescence microscopy (Carl Zeiss, Inc., Jena, Germany).
Construction of SMV mutant clones
Individual mutations G12E, H246A, R249A, D250A and H256A of CP and R455A of HC‐Pro were introduced into pSMV‐G7H by site‐directed mutagenesis using appropriate primers (Table S1; see Supporting Information) (Nassal and Rieger, 1990; Seo et al., 2009c). Briefly, CP mutations were first introduced into pTSMV‐3T (containing the fragment of pSMV‐G7H from NIa to NOS terminator; Seo et al., 2009b) utilizing XhoI sites, and then these 3′ fragments carrying each mutation were released and inserted into pSMV(dPml) utilizing PmlI sites (Seo et al., 2009b). The mutation R455A of HC‐Pro was first introduced into pSMV(dSpe) (Seo et al., 2009b) utilizing BamHI and SpeI sites, and the 3′ large fragment of pSMV‐G7H released by SpeI digestion was reinserted into pSMV(dSpe) utilizing the SpeI site. The resulting constructs were named pSMV‐CPG12E, pSMV‐CPH246A, pSMV‐CPR249A, pSMV‐CPD250A, pSMV‐CPH256A and pSMV‐HCR455A.
To utilize the NIa‐Pro proteolytic activity for the precessing of HC‐Pro, a cloning site (XbaI) and the additional NIa‐Pro cleavage site were inserted into the polyprotein open reading frame (ORF) between the P1 and P3 cistrons (Fig. 4). The P1 region from the EcoRI site to the 3′ end of P1 was amplified using a primer pair (5′‐TGTGCGCATCGAATTCTGT‐3′ and 5′‐ACGCGTTCTAGAGTAGTGCTGAATATCATCCA‐3′; EcoRI and XbaI sites are shown in italic), and cloned into the pGEM‐T Easy vector (Promega, Madison, WI, USA). The P3 region from the 5′ end to the SpeI site was also amplified using a primer pair (5′‐GCTCTAGA GAGTCTGTCTCGTTGCAGTCTGGTGAAGTGCAACAGAGAATG‐3′ and 5′‐CCYTGCAGYACACTAGTCATTTG‐3′; XbaI and SpeI sites are given in italic; the nucleotide sequences for the NIa‐Pro cleavage site are shown in bold). The amplified fragment was digested with XbaI and SpeI, and cloned downstream of P1 in the pGEM‐T Easy vector, which was opened with XbaI and SpeI. This resultant clone was digested with EcoRI and SpeI, and the P1/P3 region harbouring a XbaI site and the NIa‐Pro cleavage site was extracted and introduced into the appropriate sites within pSMV(dSpe) to produce pSMV(dSpe)dHC. Finally, the 3′ large fragment of pSMV‐G7H, released by SpeI digestion, was reinserted into pSMV(dSpe)dHC utilizing the SpeI site. The resulting clone was designated pSMVdHC and used for the insertion of HC‐Pro mutants between the P1 and P3 cistrons. HC‐Pro mutants were amplified by PCR from the clones used in YTHS using appropriate primer pairs (Table S1; see Supporting Information), and inserted in frame into pSMVdHC utilizing the XbaI site. The sequences of all constructed clones were validated by DNA sequencing. Mechanical inoculations of each construct were performed on soybean leaves (cv. Somyungkong) as described previously (Seo et al., 2009c).
RNA extraction, virus detection and real‐time RT‐PCR
Total RNAs were extracted from soybean leaves using the TRI Reagent method (MRC, Cincinnati, OH, USA) according to the manufacturer's instructions. SMV detection was performed by RT‐PCR as described previously (Seo et al., 2009c). Real‐time RT‐PCR was performed using Power SYBR® Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) and a 7500 Real Time PCR System (Applied Biosystems), as described previously (Seo et al., 2009c). The ubiquitin gene was used as an internal control to standardize the different samples.
Nucleotide sequencing
DNA sequencing was performed using the dideoxynucleotide termination method and an ABI Prism 3730 XL DNA Analyzer (Applied Biosystems) located at the National Instrumentation Center for Environmental Management of Seoul National University, according to the manufacturer's instructions.
Aphid transmission assay
Aphids (A. glycines) were reared in controlled environment chambers on soybeans. Aphids, previously starved for 2 h, were placed on soybean leaves (cv. Somyungkong) showing SMV symptoms and allowed to probe for 10 min. Then, 15 aphids were transferred to new healthy soybean seedlings (cv. Somyongkong) and allowed to feed for 24 h before being killed with an insecticide. Inoculated plants were maintained for 3 weeks and SMV infection was verified by RT‐PCR.
Supporting information
Fig. S1 Identification of amino acid residues crucial for interaction between HC‐Pro and N‐terminal truncated CP in YTHS.
Fig. S2 Interactions between CP mutants and wt HC‐Pro.
Fig. S3 Interactions between wt CP and HC‐Pro mutants.
Table S1 Primers used for clone constructions in this study.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
ACKNOWLEDGEMENTS
We thank Dr T. L. Sit for reviewing the manuscript. This research was supported in part by grants from the National Research Foundation (Nos. R01‐2008‐000‐10087‐0 & R11‐2008‐062‐02003‐0) funded by the Ministry of Education, Science and Technology (MEST) and the Rural Development Administration (No. 20080401‐034‐010‐009‐02‐00). JKS was supported by a graduate research fellowship from MEST through the Brain Korea 21 Project.
REFERENCES
- Adams, M.J. , Antoniw, J.F. and Beaudoin, F. (2005) Overview and analysis of the polyprotein cleavage sites in the family Potyviridae. Mol. Plant Pathol. 6, 471–487. [DOI] [PubMed] [Google Scholar]
- Atreya, C.D. and Pirone, T.P. (1993) Mutational analysis of the helper component‐proteinase gene of a potyvirus: effects of amino acid substitutions, deletions, and gene replacement on virulence and aphid transmissibility. Proc. Natl. Acad. Sci. USA, 90, 11919–11923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atreya, C.D. , Raccah, B. and Pirone, T.P. (1990) A point mutation in the coat protein abolishes aphid transmissibility of a potyvirus. Virology, 178, 161–165. [DOI] [PubMed] [Google Scholar]
- Baratova, L.A. , Efimov, A.V. , Dobrov, E.N. , Fedorova, N.V. , Hunt, R. , Badun, G.A. , Ksenofontov, A.L. , Torrance, L. and Järvekülg, L. (2001) In situ spatial organization of Potato virus A coat protein subunits as assessed by tritium bombardment. J. Virol. 75, 9696–9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc, S. , Lopez‐Moya, J.J. , Wang, R. , Garcia‐Lampasona, S. , Thornbury, D.W. and Pirone, T.P. (1997) A specific interaction between coat protein and helper component correlates with aphid transmission of a potyvirus. Virology, 231, 141–147. [DOI] [PubMed] [Google Scholar]
- Blanc, S. , Ammar, E.D. , Garcia‐Lampasona, S. , Dolja, V.V. , Llave, C. , Baker, J. and Pirone, T.P. (1998) Mutations in the potyvirus helper component protein: effects on interactions with virions and aphid stylets. J. Gen. Virol. 79, 3119–3122. [DOI] [PubMed] [Google Scholar]
- Carrington, J.C. and Herndon, K.L. (1992) Characterization of the potyviral HC‐pro autoproteolytic cleavage site. Virology, 187, 308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , Zheng, H.Y. , Lin, L. , Adams, M.J. , Antoniw, J.F. , Zhao, M.F. , Shang, Y.F. and Chen, J.P. (2004) A virus related to Soybean mosaic virus from Pinellia ternata in China and its comparison with local soybean SMV isolates. Arch. Virol. 149, 349–363. [DOI] [PubMed] [Google Scholar]
- Chung, B.Y. , Miller, W.A. , Atkins, J.F. and Firth, A.E. (2008) An overlapping essential gene in the Potyviridae. Proc. Natl. Acad. Sci. USA, 105, 5897–5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citovsky, V. , Lee, L.Y. , Vyas, S. , Glick, E. , Chen, M.H. , Vainstein, A. , Gafni, Y. , Gelvin, S.B. and Tzfira, T. (2006) Subcellular localization of interacting proteins by bimolecular fluorescence complementation in planta. J. Mol. Biol. 362, 1120–1131. [DOI] [PubMed] [Google Scholar]
- Dombrovsky, A. , Huet, H. , Chejanovsky, N. and Raccah, B. (2005) Aphid transmission of a potyvirus depends on suitability of the helper component and the N terminus of the coat protein. Arch. Virol. 150, 287–298. [DOI] [PubMed] [Google Scholar]
- Domier, L.L. , Latorre, I.J. , Steinlage, T.A. , McCoppin, N. and Hartman, G.L. (2003) Variability and transmission by Aphis glycines of North American and Asian Soybean mosaic virus isolates. Arch. Virol. 148, 1925–1941. [DOI] [PubMed] [Google Scholar]
- Eggenberger, A.L. , Hajimorad, M.R. and Hill, J.H. (2008) Gain of virulence on Rsv1‐genotype soybean by an avirulent Soybean mosaic virus requires concurrent mutations in both P3 and HC‐Pro. Mol. Plant–Microbe Interact. 21, 931–936. [DOI] [PubMed] [Google Scholar]
- Evangelista, C. , Lockshon, D. and Fields, S. (1996) The yeast two‐hybrid system: prospects for protein linkage maps. Trends Cell Biol. 6, 196–199. [DOI] [PubMed] [Google Scholar]
- Fields, S. and Sternglanz, R. (1994) The two‐hybrid system: an assay for protein–protein interactions. Trends Genet. 10, 286–292. [DOI] [PubMed] [Google Scholar]
- Flasinski, S. and Cassidy, B.G. (1998) Potyvirus aphid transmission requires helper component and homologous coat protein for maximal efficiency. Arch. Virol. 143, 2159–2172. [DOI] [PubMed] [Google Scholar]
- Govier, D.A. and Kassanis, B. (1974a) Evidence that a component other than the virus particle is needed for aphid transmission of potato virus Y. Virology, 57, 285–286. [DOI] [PubMed] [Google Scholar]
- Govier, D.A. and Kassanis, B. (1974b) A virus‐induced component of plant sap needed when aphids acquire potato virus Y from purified preparations. Virology, 61, 420–426. [DOI] [PubMed] [Google Scholar]
- Guo, D. , Rajamaki, M.L. , Saarma, M. and Valkonen, J.P. (2001) Towards a protein interaction map of potyviruses: protein interaction matrixes of two potyviruses based on the yeast two‐hybrid system. J. Gen. Virol. 82, 935–939. [DOI] [PubMed] [Google Scholar]
- Hajimorad, M.R. , Eggenberger, A.L. and Hill, J.H. (2005) Loss and gain of elicitor function of Soybean mosaic virus G7 provoking Rsv1‐mediated lethal systemic hypersensitive response maps to P3. J. Virol. 79, 1215–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajimorad, M.R. , Eggenberger, A.L. and Hill, J.H. (2006) Strain‐specific P3 of Soybean mosaic virus elicits Rsv1‐mediated extreme resistance, but absence of P3 elicitor function alone is insufficient for virulence on Rsv1‐genotype soybean. Virology, 345, 156–166. [DOI] [PubMed] [Google Scholar]
- Hill, J.H. (1999) Soybean mosaic virus In: Compendium of Soybean Diseases (Hartman G.L., Sinclair J.B. and Rupe J.C., eds), pp. 70–71. St. Paul, MN: The American Phytopathological Society. [Google Scholar]
- Hu, C.D. and Kerppola, T.K. (2003) Simultaneous visualization of multiple protein interactions in living cells using multicolor fluorescence complementation analysis. Nat. Biotechnol. 21, 539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet, H. , Gal‐On, A. , Meir, E. , Lecoq, H. and Raccah, B. (1994) Mutations in the helper component protease gene of zucchini yellow mosaic virus affect its ability to mediate aphid transmissibility. J. Gen. Virol. 75, 1407–1414. [DOI] [PubMed] [Google Scholar]
- Jayaram, C. , Hill, J.H. and Miller, W.A. (1992) Complete nucleotide sequences of two soybean mosaic virus strains differentiated by response of soybean containing the Rsv resistance gene. J. Gen. Virol. 73, 2067–2077. [DOI] [PubMed] [Google Scholar]
- Kang, S.‐H. , Lim, W.‐S. and Kim, K.‐H. (2004) A protein interaction map of Soybean mosaic virus strain G7H based on the yeast two‐hybrid system. Mol. Cells, 18, 122–126. [PubMed] [Google Scholar]
- Kang, S.‐H. , Lim, W.‐S. , Hwang, S.‐H. , Park, J.‐W. , Choi, H.‐S. and Kim, K.‐H. (2006) Importance of the C‐terminal domain of Soybean mosaic virus coat protein for subunit interactions. J. Gen. Virol. 87, 225–229. [DOI] [PubMed] [Google Scholar]
- Kasschau, K.D. and Carrington, J.C. (2001) Long‐distance movement and replication maintenance functions correlate with silencing suppression activity of potyviral HC‐Pro. Virology, 285, 71–81. [DOI] [PubMed] [Google Scholar]
- Kendall, A. , McDonald, M. , Bian, W. , Bowles, T. , Baumgarten, S.C. , Shi, J. , Stewart, P.L. , Bullitt, E. , Gore, D. , Irving, T.C. , Havens, W.M. , Ghabrial, S.A. , Wall, J.S. and Stubbs, G. (2008) Structure of flexible filamentous plant viruses. J. Virol. 82, 9546–9554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y.H. , Kim, O.S. , Lee, B.C. , Moon, J.K. , Lee, S.C. and Lee, J.Y. (2003) G7H, a new Soybean mosaic virus strain: its virulence and nucleotide sequence of CI gene. Plant Dis. 87, 1372–1375. [DOI] [PubMed] [Google Scholar]
- Koscianska, E. , Kalantidis, K. , Wypijewski, K. , Sadowski, J. and Tabler, M. (2005) Analysis of RNA silencing in agroinfiltrated leaves of Nicotiana benthamiana and Nicotiana tabacum . Plant Mol. Biol. 59, 647–661. [DOI] [PubMed] [Google Scholar]
- Lin, L. , Shi, Y. , Luo, Z. , Lu, Y. , Zheng, H. , Yan, F. , Chen, J. , Chen, J. , Adams, M.J. and Wu, Y. (2009) Protein–protein interactions in two potyviruses using the yeast two‐hybrid system. Virus Res. 142, 36–40. [DOI] [PubMed] [Google Scholar]
- Llave, C. , Martinez, B. , Diaz‐Ruiz, J.R. and Lopez‐Abella, D. (2002) Amino acid substitutions within the Cys‐rich domain of the tobacco etch potyvirus HC‐Pro result in loss of transmissibility by aphids. Arch. Virol. 147, 2365–2375. [DOI] [PubMed] [Google Scholar]
- Magliery, T.J. , Wilson, C.G. , Pan, W. , Mishler, D. , Ghosh, I. , Hamilton, A.D. and Regan, L. (2005) Detecting protein–protein interactions with a green fluorescent protein fragment reassembly trap: scope and mechanism. J. Am. Chem. Soc. 127, 146–157. [DOI] [PubMed] [Google Scholar]
- Mayo, M.A. and Pringle, C.R. (1998) Virus taxonomy—1997. J. Gen. Virol. 79, 649–657. [DOI] [PubMed] [Google Scholar]
- Nassal, M. and Rieger, A. (1990) PCR‐based site‐directed mutagenesis using primers with mismatched 3′‐ends. Nucleic Acids Res. 18, 3077–3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, Y.H. , Kadoury, D. , Gal‐On, A. , Huet, H. , Wang, Y. and Raccah, B. (1998) Mutations in the HC‐Pro gene of zucchini yellow mosaic potyvirus: effects on aphid transmission and binding to purified virions. J. Gen. Virol. 79, 897–904. [DOI] [PubMed] [Google Scholar]
- Plisson, C. , Drucker, M. , Blanc, S. , German‐Retana, S. , Le Gall, O. , Thomas, D. and Plisson, C. (2003) Structural characterization of HC‐Pro, a plant virus multifunctional protein. J. Biol. Chem. 278, 23753–23761. [DOI] [PubMed] [Google Scholar]
- Powers, J.G. , Sit, T.L. , Qu, F. , Morris, T.J. , Kim, K.‐H. and Lommel, S.A. (2008) A versatile assay for the identification of RNA silencing suppressors based on complementation of viral movement. Mol. Plant–Microbe Interact. 21, 879–890. [DOI] [PubMed] [Google Scholar]
- Roudet‐Tavert, G. , German‐Retana, S. , Delaunay, T. , Delecolle, B. , Candresse, T. and Le Gall, O. (2002) Interaction between potyvirus helper component‐proteinase and capsid protein in infected plants. J. Gen. Virol. 83, 1765–1770. [DOI] [PubMed] [Google Scholar]
- Ruiz‐Ferrer, V. , Boskovic, J. , Alfonso, C. , Rivas, G. , Llorca, O. , López‐Abella, D. and López‐Moya, J.J. (2005) Structural analysis of tobacco etch potyvirus HC‐pro oligomers involved in aphid transmission. J. Virol. 79, 3758–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl, R.H. and Gietz, R.D. (1989) High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr. Genet. 16, 339–346. [DOI] [PubMed] [Google Scholar]
- Seo, J.‐K. , Kwon, S.‐J. , Choi, H.‐S. and Kim, K.‐H. (2009a) Evidence for alternate states of Cucumber mosaic virus replicase assembly in positive‐ and negative‐strand RNA synthesis. Virology, 383, 248–260. [DOI] [PubMed] [Google Scholar]
- Seo, J.‐K. , Lee, H.‐G. , Choi, H.‐S. , Lee, S.‐H. and Kim, K.‐H. (2009b) Infectious in vivo transcripts from a full‐length clone of Soybean mosaic virus strain G5H. Plant Pathol. J. 25, 54–61. [Google Scholar]
- Seo, J.‐K. , Lee, H.‐G. and Kim, K.‐H. (2009c) Systemic gene delivery into soybean by simple rub‐inoculation with plasmid DNA of a Soybean mosaic virus‐based vector. Arch. Virol. 154, 87–99. [DOI] [PubMed] [Google Scholar]
- Seo, J.‐K. , Lee, S.‐H. and Kim, K.‐H. (2009d) Strain‐specific cylindrical inclusion protein of Soybean mosaic virus elicits extreme resistance and a lethal systemic hypersensitive response in two resistant soybean cultivars. Mol. Plant–Microbe Interact. 22, 1151–1159. [DOI] [PubMed] [Google Scholar]
- Shukla, D.D. , Strike, P.M. , Tracy, S.L. , Gough, K.H. and Ward, C.W. (1988) The N and C termini of the coat proteins of potyviruses are surface‐located and the N terminus contains the major virus‐specific epitopes. J. Gen. Virol. 69, 1497–1508. [Google Scholar]
- Torrance, L. , Andreev, I.A. , Gabrenaite‐Verhovskaya, R. , Cowan, G. , Makinen, K. and Taliansky, M.E. (2006) An unusual structure at one end of potato potyvirus particles. J. Mol. Biol. 357, 1–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Identification of amino acid residues crucial for interaction between HC‐Pro and N‐terminal truncated CP in YTHS.
Fig. S2 Interactions between CP mutants and wt HC‐Pro.
Fig. S3 Interactions between wt CP and HC‐Pro mutants.
Table S1 Primers used for clone constructions in this study.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item
Supporting info item


