SUMMARY
The ergot diseases of grasses, caused by members of the genus Claviceps, have had a severe impact on human history and agriculture, causing devastating epidemics. However, ergot alkaloids, the toxic components of Claviceps sclerotia, have been used intensively (and misused) as pharmaceutical drugs, and efficient biotechnological processes have been developed for their in vitro production. Molecular genetics has provided detailed insight into the genetic basis of ergot alkaloid biosynthesis and opened up perspectives for the design of new alkaloids and the improvement of production strains; it has also revealed the refined infection strategy of this biotrophic pathogen, opening up the way for better control. Nevertheless, Claviceps remains an important pathogen worldwide, and a source for potential new drugs for central nervous system diseases.
1. INTRODUCTION
1.1 Claviceps spp.—the ergot fungi
Members of the fungal ascomycetous genus Claviceps parasitize more than 600 monocotyledonous plants of the Poaceae, Juncaceae and Cyperaceae, including economically important crop plants such as rye, wheat, barley, rice, corn, millet and oat (Bové, 1970). Most of the 30–36 species in this group (Taber, 1985) have a defined, narrow host range. Claviceps purpurea (Fries ex Fries) Tulasne, the most widely known species in Europe, is exceptional, as it infects more than 400 plant species with a disease known as ergot. The common name ‘Ergot Fungus’ is derived from the French word for spur (‘argot’) and refers to the dark sclerotia protruding from the ripe grass ear in the final disease stages (Fig. 5I).
Figure 5.
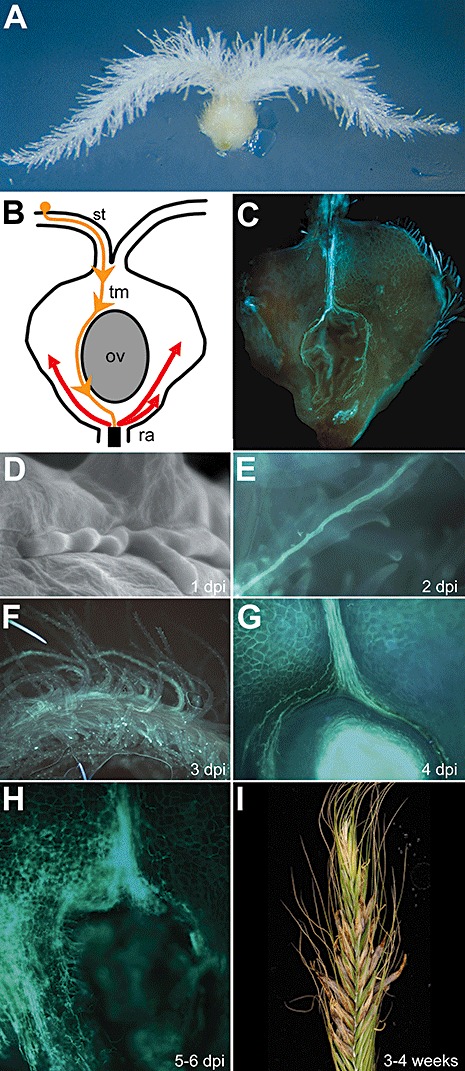
Major aspects of the life cycle of Claviceps purpurea. (A) An in vitro method to cultivate isolated rye ovaries on agar plates allows microscopic observation of the early pathogenic development of C. purpurea. (B/C) Schematic overview of fungal infection within the rye ovary and the corresponding fluorescence micrograph of a KOH/aniline blue‐stained infected rye ovary. After attachment of spores (asco‐ or conidiospores) to the exposed stigmatic hairs of the ovary style, hyphae penetrate the plant cell wall and the fungus grows within the stigmatic hair cells (yellow line). Growth is mainly observed in one direction, i.e. towards the style. Within the style, hyphae from different spores join and grow further towards the transmitting tissue. After the micropylar region is reached, hyphae grow further towards the base of the ovary. Fungal growth stops after a narrow interface between the plant's vascular bundles and the fungus has been established. From this point on, massive colonization of the whole ovary occurs by highly branched fungal hyphae, indicated as red arrows in (B). Fungal hyphae in (C) are stained in light green. (D) Scanning electron micrograph of a C. purpurea hypha penetrating a stigmatic hair. (E–H) Fluorescence micrographs of infected rye ovaries at different time points (dpi, days post‐inoculation) of colonization. Hyphae grow in stigmatic hairs (1–2 dpi), join in the style and grow towards the transmitting tissue (3 dpi), surround the ovule and grow further down to the ovary base (4 dpi), where massive branching and colonization of the whole ovary starts (5–6 dpi). (I) Three to four weeks post‐inoculation the sclerotia are visible as large brown structures on infected rye ears. ov, ovule; ra, rachilla; st, style; tm, transmitting tissue.
These structures contain the physiologically highly active ergot alkaloids, tri‐ or tetracyclic derivatives of prenylated tryptophan, that give the fungus its significance. In the Middle Ages, vast epidemics of so‐called St. Anthony's Fire disease were caused by the consumption of ergot‐contaminated rye bread (Fig. 1). However, midwives used ergot sclerotia as an aid in child birth (and abortion). This dual role of ergot persisted over the centuries. Ergot disease is feared by farmers as, in most countries, regulatory limits of between 0.05% and 0.3% of ergot in grain are standard and can render the grain too poisonous for use, whereas seed loss (5–10%) is almost negligible (Alderman et al., 1998). There has also been considerable commercial interest because of the effects of ergot alkaloids on the mammalian vasculature and central nervous system (CNS); these effects can be used to treat several diseases (Mukherjee and Menge, 2000). Scientific interest in this fungus continues, because the development of new defence strategies against ergot disease and strain improvement programmes for biotechnological purposes require a detailed understanding of the biology, physiology and genetics of this fungus.
Figure 1.

The temptation of St. Antonius. Detail of the ‘retable d'Issenheim’ (Grünewald, Agression de saint Antoine par les démons, 1512–1516 © musée d'Unterlinden, Colmar). The painting describes the symptoms of an ergot alkaloid poisoning: hallucinations and ‘burning’ limbs/gangrene.
1.2 The story of ergot: use, abuse and poisoning
There are very early references to ergot in history: for example, a description on an Assyrian tablet in 600 bc referred to it as a ‘noxious pustule in the ear of grain’. Around 350 bc, in one of the sacred books of the Parsees, descriptions were found of ‘noxious grasses that cause pregnant women to drop the womb and die in childbed’ (reviewed, for example, in de Costa, 2002; Schiff, 2006).
The intake of ergot alkaloids through the consumption of sclerotia by humans (usually caused by eating contaminated rye bread) or animals causes ergotism. The symptoms of this malady vary, probably depending on the particular profiles of the alkaloids present in the contaminated flour (Eadie, 2003). Two syndromes with different symptoms have been described: convulsive ergotism (Ergotismus convulsivus), characterized by paranoia and hallucinations, twitches and spasms, and gangrenous ergotism (Ergotismus gangraenosus), which causes loss of peripheral sensation, oedema and, finally, the loss of affected tissues (described, for example, in Eadie, 2003). Ergotism is thought to have occurred in ancient times (de Costa, 2002; van Dongen and de Groot, 1995), but had its peak level in Europe in the Middle Ages when the disease affected many thousands of people. A monastic order especially cared for the afflicted, the patron saint of which was St. Anthony (Fig. 1). The malady itself was known at that time as ignis sacer (holy fire), or St. Anthony's Fire, because of the burning sensations in the limbs (Matossian, 1989).
The first well‐documented epidemic of ergotism was in ad 944–945, when about 20 000 people living in Paris and the Aquitane region of France, at that time about one‐half of the population, died of the effects of ergot poisoning (Matossian, 1989; Schiff, 2006). Matossian (1989) also speculates that the slow recovery after the plague epidemics in Europe in the 14th century was at least partly caused by reduced fertility as a result of ergot toxifications. Ergot poisoning is nowadays widely believed to have influenced social history, such as the witch trials of Salem and in Norway during the 17th century (Alm, 2003; Caporael, 1976) and even mystic religious movements (Packer, 1998). Already in the late 16th and beginning of the 17th centuries, observations of the link between ergot‐contaminated rye and disease were made (Bauer, 1973), but it was not until the 19th century that the mycologist Louis Rene Tulasne revealed the correlation between infected rye and ergotism (Tulasne, 1853). This led to increased efforts to reduce ergot contamination in rye (for example, by flotation of seeds to remove sclerotia); therefore the occurrence of larger epidemics became rare. The most recent epidemic in Germany occurred in 1879–1881; however, there have been more recent epidemics in parts of Russia (1926–1927) (Barger, 1931; Eadie, 2003) and Ethiopia (1977–1978) (Urga et al., 2002), and, in India, outbreaks continued until the late 20th century (Tulpule and Bhat, 1978). Even today, ergot alkaloid contamination represents a major problem in agriculture and requires continuous control measures (Krska and Crews, 2008).
In contrast with this deleterious impact on human history, ergot alkaloids also have benefits for humankind, such as the ancient use of ergot alkaloids for medicinal purposes. Ergot was used as an aid to accelerate parturition or to initiate abortion by midwives in Europe; the first written reference of this is by Adam Lonicer (1582) in his ‘Kreuterbuch’ (reviewed, for example, in van Dongen and de Groot, 1995). This was a risky treatment because the dosage of active ingredients in sclerotia is difficult to control, ranging from 0.01% to 1% of dry weight (Didek‐Brumek et al., 1996). Rupture of the uterus as a result of hypercontraction was a significant risk. Since the 19th century, ergot alkaloids have mainly been used to prevent excessive bleeding during or after childbirth, which, even today, accounts for almost one‐half of all the postpartum maternal mortalities in developing countries (Li et al., 1996). In addition, ergot alkaloids have been used for the treatment of migraine since the second half of the 19th century (Waokes, 1868; Eulenburg, 1883; cited in Eadie, 2003) and for Parkinson's disease (see below).
With an increased understanding of the chemistry and pharmacology of ergot alkaloids and the first isolation of ergotamine as a pure substance by Stoll in 1918, a significant role in modern medicine for this class of compounds began to emerge (Gröger and Floss, 1998; Sinz, 2008). The ergot alkaloids produced by C. purpurea (mainly ergotamine, ergocryptine and related ergopeptine alkaloids, plus simpler lysergyl amides; all derivatives of the basic ergoline ring structure; see 2, 3) can be readily converted to d‐lysergic acid by alkaline or acid hydrolysis, providing a convenient starting material for pharmaceuticals or illicit drugs.
Figure 2.
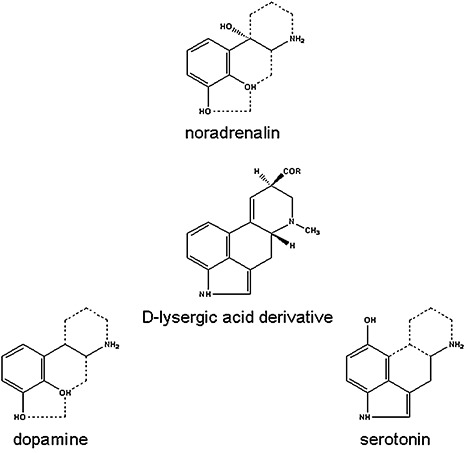
Ergot alkaloids: comparison of the chemical structures of the ergoline ring system with neurotransmitters.
Figure 3.
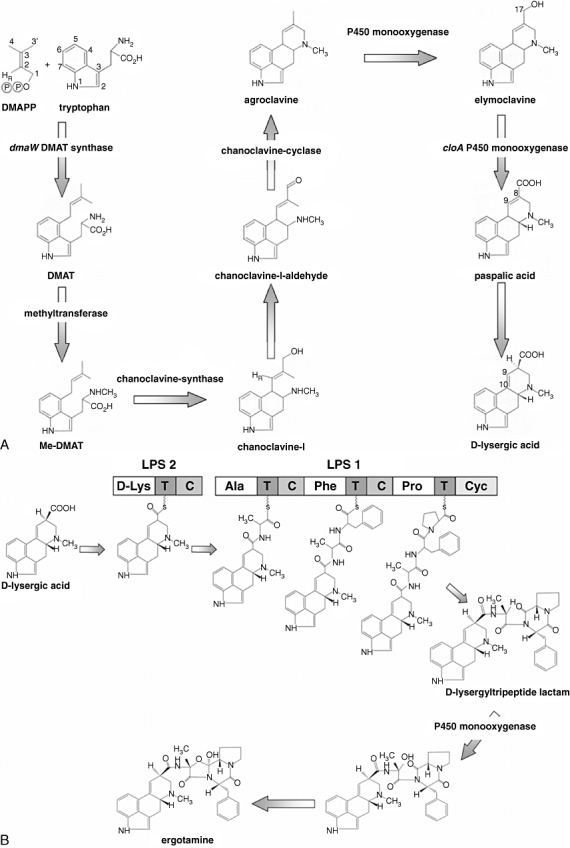
Ergot alkaloid biosynthesis in Claviceps purpurea. Synthesis of lysergic acid (A) and the ergopeptide ergotamine (B). For gene designations, see Table 1. DMAPP, dimethylallylpyrophosphate; DMAT, 4‐dimethylallyltryptophan; LPS, lysergyl peptidyl synthetase. Modified after Haarmann et al. (2005).
The most famous semi‐synthetic ergot alkaloid is the lysergic acid diethylamide (LSD). This compound, first synthesized accidentally in a series of lysergyl amides by Albert Hofmann in 1938 (Hofmann, 1978), is the most potent hallucinogen known (Nichols, 2001). Originally tested as an experimental antidepressant drug and for the treatment of alcoholism and schizophrenia, early tests revealed severe problems, including paranoia, potentially fatal loss of judgement and flashbacks. Nevertheless, the drug was widely used by psychotherapists and psychiatrists for the therapy of neuroses, sexual dysfunctions and anxiety, by scientists for the study of neuronal signal transmission, hallucinations and schizophrenia, and even by the secret service for special interrogation techniques (Hofmann, 1993; Stafford and Golightly, 1967). Based on the work of Leary (1969) and others, LSD became a widely consumed psychedelic drug. Thus, C. purpurea had an impact on a whole generation of drug‐using youngsters until, starting from 1966, the drug was declared illegal and most dangerous by the United Nations (UN) and by the US government (Flieger et al., 1997). Quite recently, clinical interest in the therapeutic potential of ergoline hallucinogens is beginning to emerge again, for example in the treatment of autism (Fantegrossi et al., 2008; Sigafoos et al., 2007).
2. ERGOT ALKALOIDS
2.1 Pharmacological activities
The pharmacological effects of the ergot alkaloids as a group are complex and variable, with the net result of their actions depending on the effects at different receptors of the CNS. The pharmacological activities arise most probably from the structural similarity between d‐lysergic acid‐derived compounds and three important neurotransmitters; the structures of noradrenalin, dopamine and serotonin (5‐hydroxytryptamine, 5‐HT) can be mapped almost entirely onto the d‐lysergic acid ring structure (Fig. 2). Many ergot alkaloids can probably interact with receptors for all three of these neurotransmitters (Berde and Stürmer, 1978), as either an agonist or antagonist, or even in a dual role as partial agonist and antagonist, depending on the substituents attached to the carboxyl group of d‐lysergic acid (Stadler and Giger, 1984). This broad specificity can provoke undesired side‐effects and the approaches to improve natural ergot alkaloids as therapeutic agents involve narrowing the specificity of the compounds by chemical modification (Vendrell et al., 2007). Interestingly, the derivatives of d‐isolysergic acid, the stereoisomer of d‐lysergic acid, show little or no pharmacological activity, making a total chemical synthesis of ergot alkaloids challenging.
In general, ergopeptines exert vasoconstrictive and sympatholytic–adrenolytic effects because of their affinity for adrenergic receptors (for a recent review, see Görnemann et al., 2008); however, the introduction of unnatural side‐chains has drastic effects. Thus, the simple derivative dihydroergotamine can affect α1‐ and α2‐adrenergic receptors and has a much more dominant adrenolytic effect with a concomitant reduction in the vasoconstrictive effect (Villalon et al., 1999; Willems et al., 1999). Dihydroergotamine is mainly used in the treatment of migraine (Tfelt‐Hansen and Koehler, 2008). Dihydroergotoxin, a mixture of several ergopeptines, finds application as an antihypertensive agent and in the treatment of cerebral dysfunction in the elderly (de Groot et al., 1998; Wadworth and Crisp, 1992). Ergotoxines, including ergocryptine, have been shown to have an inhibitory effect on the release of the peptide hormone prolactin. Thus, 2‐bromo‐ergocryptine (bromocriptine) is also effective in cases of hyperprolactinaemia, a condition that can result in reproductive disorders, such as galactorrhoea, prolactin‐dependent mammary carcinoma, amenorrhoea, acromegaly or anovulation, and long‐term complications, e.g. osteoporosis (Crosignani, 2006). Bromocriptine also has affinity to dopaminergic receptors, which became the basis for its use in the treatment of Parkinson's disease (Thobois, 2006).
In general, the broad activity spectrum of natural or semi‐synthetic ergot alkaloids renders them ‘dirty’ drugs, which are being increasingly substituted by synthetic derivatives with a more defined mode of action. Nevertheless, they still play a major role in the treatment of migraine and degenerative diseases of the CNS (Sinz, 2008).
2.2 Biosynthesis: the ergot alkaloid gene cluster
Much of the knowledge about the biosynthesis of ergot alkaloids stems from feeding experiments with radiolabelled putative precursors or intermediates added to rye ears infected with C. purpurea or to fermentation cultures of C. purpurea, Claviceps fusiformis or Claviceps paspali (Keller and Tudzynski, 2002). The subsequent analysis of the biotransformation products established the biosynthetic building blocks of the ergoline ring system as tryptophan, the methyl group of methionine and an isoprene unit derived from mevalonic acid (Fig. 3) (for a recent review, see Schardl et al., 2006).
The pathway leading to ergopeptines starts with the isoprenylation of tryptophan, yielding 4‐dimethylallyltryptophan (DMAT). The enzyme catalysing this determinant step, dimethylallyl tryptophan synthase (DMATS), was the first to be characterized in detail (Gebler and Poulter, 1992). As might be expected for the determinant step, DMATS is subject to strict regulation: tryptophan serves as inducer, whereas elymoclavine or agroclavine causes feedback regulation of the enzyme (Cheng et al., 1980).
Based on oligonucleotides derived from a partial amino acid sequence of the purified enzyme, the gene dmaW encoding DMATS was cloned, originally from a C. fusiformis strain (Tsai et al., 1995), and later from C. purpurea strain P1 (Tudzynski et al., 1999). A chromosome walking approach starting from dmaW led to the detection of a cluster of 14 genes, shown (or predicted) to encode ergot alkaloid biosynthetic enzymes (Fig. 4) (Haarmann et al., 2005; Lorenz et al., 2007; Tudzynski et al., 1999).
Figure 4.

Ergot alkaloid gene cluster in Claviceps purpurea. Orientation of arrows indicates direction of transcription. For gene designations, see Table 1. Modified after Haarmann et al. (2005).
Recently, in order to facilitate discussion of the genes and comparisons amongst ergot alkaloid‐producing fungi, a systematic set of names for the genes of the ergot alkaloid pathway has been agreed upon by the groups involved in the analysis (Schardl et al., 2006). Claviceps purpurea alkaloid cluster genes that have not yet been functionally characterized are designated easA through easH (ergot alkaloid synthesis). Genes whose products have been biochemically characterized are named according to the enzyme activities of the encoded proteins. A list of the genes and their shown or predicted function is given in Table 1.
Table 1.
Genes of the ergot alkaloid cluster of Claviceps purpurea, former nomenclature and (putative) function.
| Gene | Former nomenclature | (Putative) function |
|---|---|---|
| easA | cpox3 | Reductase/dehydrogenase |
| lpsB | cpps2 | LPS subunit 2 |
| cloA | cpP450‐1 | Elymoclavine oxygenase |
| easC | cpcat2 | Reductase/dehydrogenase |
| easD | cpox2 | Reductase/dehydrogenase |
| easE | cpox1 | Reductase/dehydrogenase |
| easF | orfB | Methyltransferase |
| easG | orfA | Reductase/dehydrogenase (NmrA‐like protein) |
| dmaW | cpd1 | DMAT synthase |
| easH1 | orfC | Oxygenase/hydroxylase |
| lpsA1 | cpps1 | LPS subunit 1 |
| easH2 | orfE | Hydroxylase |
| lpsA2 | cpps4 | LPS subunit 1 |
DMAT, 4‐dimethylallyltryptophan; LPS, lysergyl peptidyl synthetase.
In addition to dmaW, seven enzyme‐encoding eas cluster genes have so far been functionally analysed by gene disruption and analysis of intermediates in C. purpurea P1. These include four non‐ribosomal peptide synthetase (NRPS) genes, lpsA1/A2, lpsB and C. Biochemical evidence has shown that the final steps of ergopeptine synthesis in C. purpurea include a complex of two interacting NRPSs (a new finding in fungi): d‐lysergyl peptidyl synthetase 2 (LPS2), catalysing the activation of lysergic acid, and LPS1, forming the tripeptide moiety (Fig. 3B; Riederer et al., 1996). Functional analysis has shown that lpsA1 and lpsA2 both encode LPS1 enzymes: LPS1‐1 necessary for the synthesis of the major alkaloid of strain P1, ergotamine, and LPS1‐2 for the synthesis of ergocryptine (Haarmann et al., 2008; Tudzynski et al., 1999). The gene lpsC probably encodes a monomodular NRPS enzyme that catalyses the formation of ergonovine (= ergometrine), an ergopeptine with a single amino acid side‐chain (I. Ortel and U. Keller, FU Berlin, personal communication). Thus, this set of NRPS genes encodes a highly flexible natural combinatory system unique in eukaryotes: activated lysergic acid formed by LPS2 can be used as a recipient for the addition of several peptide moieties, explaining the high variability of the ergot peptide alkaloid spectrum in C. purpurea strains.
Of the enzymes involved in the pathway from DMAT to lysergic acid, to date two genes have been identified by a knock‐out approach: the gene product of cloA has been shown to catalyse the conversion of elymoclavine to paspalic acid (see Fig. 3) (Haarmann et al., 2006), and easE encodes the chanoclavine synthase (N. Lorenz and P. Tudzynski, unpublished data). The role of the gene product of easC, a putative catalase, is yet to be determined. Heterologous expression of easC in Pichia demonstrated catalase activity; a ΔeasC mutant produces no detectable amounts of alkaloids or intermediates, and the transcription of the other cluster genes is down‐regulated, suggesting a regulatory function (T. Haarmann, N. Lorenz, S. Giesbert and P. Tudzynski, unpublished data).
2.3 Biotechnology
The industrial production of ergot alkaloids began with the patent of A. Stoll on the production of ergotamine tartrate, which was used by Sandoz as early as 1921; Sandoz dominated the world market in alkaloid production up to the 1950s, when competitors such as Boehringer Ingelheim, Galena, Gedeon Richter, Eli Lilley and Farmitalia appeared (see review by Cvak, 1999).
Claviceps purpurea, C. fusiformis and C. paspali were generally used as production strains. These species differ considerably with respect to their potential to synthesize specific alkaloids: only C. purpurea produces peptide alkaloids, whereas single lysergic acid derivatives are produced by C. paspali, and C. fusiformis is used to produce clavine alkaloids.
Originally, the field production of alkaloids on rye or triticale was the major production method, but the submersed production with specially designed strains soon prevailed (Keller and Tudzynski, 2002; Tudzynski et al., 2001). Today, the production of single clavine alkaloids or paspalic/lysergic acid is of major importance as a basis for semi‐synthetic drug development; the annual production of all ergopeptines is estimated to reach 5000–8000 kg, whereas about 10 000–15 000 kg of lysergic acid is (legally) produced annually (Schiff, 2006).
3. ERGOT: A HIGHLY SPECIALIZED PLANT DISEASE
3.1 Life cycle and disease development in C. purpurea
The major aspects of the life cycle of C. purpurea are presented in Fig. 5. Primary infection by C. purpurea is initiated by ascospores ejected from perithecia, which are formed in spring from germinating overwintering structures, the sclerotia. The fungus can infect a broad range of grasses or cereals, but is highly organ specific, as it infects only the ovaries. The mechanism underlying this specificity is unclear, but may be mediated by interaction with the surface of the stigmatic hairs where the spores germinate at anthesis. The germ tubes penetrate the plant cuticle (there is no evidence for the involvement of physical pressure and no specific infection structures are formed). After penetration, the fungus follows the path of the pollen tube, growing in thick hyphal bundles almost without any branching. Only at the base of the ovary do the hyphae leave the pollen tube path and tap the vascular tissue; only then is a ‘normal’ branched mycelium formed (the so‐called sphacelium), which colonizes the whole ovary and produces conidia, which are secreted (about 7 days post infection, dpi), together with plant phloem exudates, as honeydew and initiate a secondary infection by drop inoculation or transfer by insects. About 2 weeks post‐infection, the production of honeydew ceases and the development of sclerotia is initiated; they serve as overwintering structures and exclusively (at least in pathogenic field isolates) contain the ergot alkaloids.
This infection cycle has the following specific aspects that make C. purpurea an interesting model system for plant pathologists and cell biologists alike.
-
1
Strict organ specificity, the molecular basis of which is not yet understood.
-
2
Lack of detectable plant defence reactions; this is probably a result of mimicry of pollen tube growth, although molecular analyses suggest that the fungus is recognized but that defence reactions are repressed (see below).
-
3
Strict polar, oriented, almost unbranched growth in the first infection stage, recapitulating, for example, the oriented growth of axons; the cues/signals guiding this oriented growth are still unknown.
-
4
Biotrophic life style: the fungus shows no necrotrophic growth symptoms (necroses, rapid host cell death) in any phase of infection; it grows mostly intercellularly, but intracellular hyphae have also been described which could have haustoria‐like function. However, C. purpurea can be easily grown in culture; it is an ecologically obligate pathogen obtaining nutrients in planta exclusively from living tissue and maintaining host cell viability for extended periods.
These interesting features have stimulated a detailed molecular analysis of this system in recent years (see below).
3.2 Economic impact of ergot today
Today, several methods have been developed to reduce the risk of ergot infection in most cereal crops with the consequence that ergotism as a human disease has almost been eliminated. These methods include changes in crop rotation, deeper ploughing and sifting out of the sclerotia (Mielke, 1993). In addition, the application of fungicides, breeding for disease resistance and crossing of natural rye with hybrid rye reduce the infection of rye with C. purpurea (summarized in Mielke, 2000).
In the European Union, the amount of ergot in grain used for human food is restricted to 0.05% at a maximum. This corresponds to an average of 1 mg alkaloids per kilogram based on an average alkaloid content of about 0.2% (Appelt and Ellner, 2008). For animal feed, 0.1% grain samples containing ≤0.1% of sclerotia or pieces thereof can be tolerated. Similar standards are applied in the USA and Canada (Mirdita et al., 2008).
Selling grain exceeding these thresholds leads to financial suffering. According to Betz et al. (1998) incurred costs of seed cleaning amount to more than 2€/dt of grain. After cleaning of the grain, the costly disposal of ergot as hazardous waste increases the economical losses evoked by ergot infestation (Münzing, 1999).
In Germany, as a result of changes in cultivation and crop rotation, ergot did not cause any severe problems in rye cultivation until the 1980s. However, at the beginning of the 1990s, increased occurrence of infection in the cultivation of hybrid rye with C. purpurea was observed (Mielke, 2000). The increasing cultivation of hybrid rye itself seems to have contributed to the increased occurrence of ergot infestation since the 1980s (Mielke, 1993). Another reason might be the increasing cultivation of triticale (cited in Mielke, 2000); in addition, the grass borders around the field perimeters may contribute to the infection of rye as they can serve as alternative hosts for C. purpurea.
Underlining the average infection data for C. purpurea, annual investigations of the Federal Research Institute of Nutrition and Food (Max Rubner Institute) between 1995 and 2004 revealed a relatively constant level of ergot contamination in the analysed rye samples of about 0.11% w/w. Nevertheless, depending on different climatic and weather conditions, as well as differences in the employed cultivars, the contamination of rye with ergot differs considerably (Lindhauer et al., 2005). In 1998, massive infestation of rye in some parts of Germany led to almost a total loss of the harvest (Mielke, 2000). In addition, in the US Midwest in 2005, widespread occurrence of ergot in barley was reported (Schwarz et al., 2006).
In addition, ergot causes problems by poisoning grazing animals as a result of the consumption of ergot alkaloids. Ergot alkaloids ingested by livestock may be ultimately derived from two different sources. Ergot alkaloids produced by Claviceps spp. on ears of pasture grasses and feed grain crops that are ingested by livestock (Blaney et al., 2000) are particularly problematic if the animals are allowed to graze on grass that is flowering (Cross, 2003). However, ergot alkaloids are also produced by endophytic fungi of the genera Epichloë, Neotyphodium and Balansia belonging to the family Clavicipitaceae (Schardl et al., 2009; Zhang et al., 2006). These fungi are symbionts of many grasses of the subfamily Pooideae and colonize the intercellular spaces of all the above‐ground parts of the plant, including the reproductive tissues, but do not infect the root (Schardl, 1996). The sexual endophytes are classified as Epichloë and Balansia species, whereas their asexual counterparts are termed Neotyphodium and Ephelis species, respectively. The endophytes form systemic infections and derive nutrients from the extracellular substrates of the hosts. Many, but not all, of these fungi produce ergot alkaloids when associated with their host plant. They are important agents of biological plant protection against insects and grazing vertebrates (Bush et al., 1997; Schardl and Phillips, 1997). In addition, it has been shown for tall fescue infected with Neotyphodium coenophialum and for perennial ryegrass infected with Neotyphodium lolii symbioses that the grasses exhibit enhanced competitiveness, improved root growth and increased drought and mineral stress tolerance (Arechavaleta et al., 1989; Malinowski and Belesky, 2000; Popay and Bonos, 2005).
In addition to these benefits for endophyte‐infected grasses, deleterious effects on livestock may occur. Toxicosis problems suffered by animals grazing on tall fescue were first noted in the 1930s, and in 1990 the estimated economic loss for the beef cattle industry in the USA was more than $600 million (Hoveland, 1993). Affected livestock show loss of appetite and reduced weight gain, fat necrosis, loss of body temperature control (hyperthermia), convulsions, rough hair coats and reduced fertility, and lactating cows show reduced milk production (Porter and Thompson, 1992; Schmid and Osborne, 1993; Thomson and Stuedemann, 1993). Affected animals, particularly horses, can also suffer increased frequencies of still‐births, where foals are characteristically delivered several weeks after normal gestation and show distortions in bone structure (Cross et al., 1995). Another symptom is fescue foot, usually seen in the winter months, where affected animals suffer from dry gangrene of the extremities.
The effects of ergot alkaloid poisoning are mainly attributed to the ergopeptine ergovaline; however, transport across ruminant gastric membranes is much higher for intermediate lysergyl compounds than for ergopeptines, suggesting that intermediate ergot alkaloids may also play a significant role (Hill et al., 2001).
3.3 New epidemics: ergot of sorghum
During the past decades, ergot of sorghum has emerged to become a worldwide threat to the sorghum industry, and has gained great economical importance because of its rapid spread throughout the world. Sorghum (Sorghum bicolor) is the world's fifth most important cereal crop, which is grown on 45 million hectares and is used for food, livestock feeding, beverage production and industrial purposes. Ergot of sorghum affects non‐fertilized ovaries in sorghum male ‐sterile plants and infects hybrids if there is pollen sterility at flowering time. The disease is caused by three different Claviceps species, namely C. africana, C. sorghi and C. sorghicola. In 1917, sorghum ergot caused by C. sorghi was first described in India (McRae, 1917; cited in Pažoutováet al., 2000) and, thereafter, in 1923 in Kenya (Bandyopadhyay et al., 1998), where it was identified as C. africana (Frederickson et al., 1991). The disease did not account for considerable economical losses until the introduction of male‐sterile sorghum in the 1960s (Bandyopadhyay et al., 1998; Pažoutová and Frederickson, 2005), which is highly susceptible to infection with Claviceps spp. Consequently, together with the development and expansion of F1 hybrid seed production, ergot spread across Africa and Asia (reviewed in Bandyopadhyay et al., 1998; Pažoutováet al., 2000). Economic losses are caused by a reduction in seed quality and a reduced yield, creation of difficulties in harvesting and threshing, and problems in the international trade of contaminated seed. In addition, as outlined above, alkaloid‐containing sclerotia carry the risk of intoxication of livestock when being fed with ergot‐contaminated sorghum (Blaney et al., 2000). As a consequence, the limit for sclerotia/sphacelia in grains for stockfeed has been reduced to 0.1% w/w in Australia (Chakraborty and Ryley, 2008).
Over the past decades, ergot of sorghum has become a global threat. Losses of up to 80% in India and 25% in Zimbabwe have been reported (Bandyopadhyay et al., 1998). Probably because of its ability to produce airborne secondary conidia, it is C. africana that has emerged as the dominant causal agent of ergot sorghum worldwide, almost completely replacing C. sorghi in India (Pažoutováet al., 2000).
In 1995, the first occurrence of sorghum ergot caused by C. africana outside Africa and Asia was reported in Brazil, where it caused a rapid and widespread epidemic. Subsequently, the disease has rapidly distributed to South, Central and North America and the Caribbean (summarized, for example, in Bandyopadhyay et al., 1998). The ability of C. africana to produce (asexual) airborne secondary conidia (Frederickson et al., 1993) that travel moderate distances is thought to be one of the reasons for its rapid international dispersal. In 1997, ergot of sorghum caused widespread economic loss in the Texas Panhandle (Prom et al., 2005), where 95% of the hybrid sorghum seed supply of the USA is produced. In 2002, sorghum ergot affected 87% and, in 2004, 90% of the seed production fields in the Panhandle (Workneh et al., 2006).
Hybrid seed is especially affected as the disease raises difficulties not only in the production of hybrid seed, but also with seed distribution, as the seed obtained is restricted.
After its introduction into the Americas in 1995, C. africana was reported in Queensland, Australia in 1996 (Ryley et al., 1996) and, within a month, the disease had spread over an area of approximately 70 000 km2 (Bandyopadhyay et al., 1998). Today, the disease causes between 30% and 100% losses in nurseries and parent seed production (Chakraborty and Ryley, 2008). The origin of ergot introduction into Australia was probably India or another South Asian country. However, the means by which C. africana spread to Brazil in 1995 and to Australia in 1996 still remains to be elucidated (Bandyopadhyay et al., 1998; Chakraborty and Ryley, 2008; Pažoutová and Frederickson, 2005). Nevertheless, it seems to be beyond any doubt that climatic conditions, such as low temperatures and humid weather before and during flowering, contribute to ergot outbreaks and the rapid distribution of the disease (see, for example, Bandyopadhyay et al., 1998; Ryley and Chakraborty, 2008; Workneh and Rush, 2006).
4. MOLECULAR ASPECTS OF HOST–PATHOGEN INTERACTION
Claviceps purpurea is regarded as a plant pathogen, as reproduction of the infected ear is altered. However, protection from grazing animals may balance this reproductive cost, implying that a beneficial ‘partnership’ of host and pathogen has evolved. A complex network of growth and developmental regulation to overcome plant defence mechanisms must underlie the unique infection pattern. Some of the strategies invented by the fungus to accomplish biotrophic growth in its model host Secale cereale (rye) are discussed here. An invaluable tool in analysing in detail the function of genes and in identifying virulence/pathogenicity factors is a knock‐out approach. In Table 2, the knock‐out mutants currently available are described.
Table 2.
Functional analysis (knock‐out) of potential Claviceps purpurea virulence genes.
| Gene product | Gene | Impact on pathogenicity | Other defects | Reference |
|---|---|---|---|---|
| Cellobiohydrolase | cpcel1 | None | Müller et al. (1997) | |
| Endo‐1,4‐β‐xylanase | cpxyl1 | None | J. Scheffer et al., WWU Muenster, unpublished data | |
| Endo‐1,4‐β‐xylanase | cpxyl2 | Slight delay of colonization | J. Scheffer et al., WWU Muenster, unpublished data | |
| cpxyl1/cpxyl2 | Slight delay of colonization | J. Scheffer et al., WWU Muenster, unpublished data | ||
| Polygalacturonases | cppg1/cppg2 | Non‐pathogenic | Oeser et al. (2002) | |
| Cu/Zn superoxide dismutase | cpsod1 | Reduced growth rate | Moore et al. (2002) | |
| Catalase | cpcat1 | None | Garre et al. (1998) | |
| NADPH oxidase | cpnox1 | Significant reduction of virulence | Giesbert et al. (2008) | |
| NADPH oxidase | cpnox2 | Increase of virulence/honeydew formation | No ripe sclerotia | D. Buttermann et al., unpublished data |
| Multicopper oxidase | cpmco1 | None | S. Moore et al., WWU Muenster, unpublished data | |
| Transcription factor | cptf1 | Significant reduction of virulence | Reduction of catalase activity | Nathues et al. (2004) |
| MAPK | cpmk1 | Non‐pathogenic | Mey et al. (2002b) | |
| MAPK | cpmk2 | Significant reduction of virulence | Defective in conidiation and cell wall structure | Mey et al. (2002a) |
| Serine/threonine kinase | cpcot1 | Non‐pathogenic | Cell shape and branching strongly affected | Scheffer et al. (2005a) |
| Small GTPase | cpcdc42 | Non‐pathogenic | Hyperbranching, hypersporulation | Scheffer et al. (2005b) |
| Small GTPase | rac | Non‐pathogenic | Cell shape and branching strongly affected | Rolke and Tudzynski (2008) |
| PAK kinase | cla4 | Non‐pathogenic | Cell shape and branching strongly affected | Rolke and Tudzynski (2008) |
| Pentahydrophobin | cpph1 | None | Mey et al. (2003) | |
| Histidine kinase | cphk1 | Reduction of virulence (conidia only) | Significantly reduced germination rate, increased resistance to menadione | Nathues et al. (2007) |
| Stretch‐activated ion channel | cpmid1 | Non‐pathogenic | Increased sensitivity to cell wall stress | J. Bormann and P. Tudzynski, unpublished data |
| Subunit involved in non‐homologous end‐joining | ku70 | Haarmann et al. (2008) |
GTPase, guanosine triphosphatase; MAPK, mitogen‐activated protein kinase; NADPH, reduced nicotinamide adenine dinucleotide phosphate; PAK, p21‐activated kinase.
Phytopathogenic fungi have generated different mechanisms to overcome the first defence barrier, the plant cell wall. One is the formation of specialized penetration structures applying great force, called appressoria. Penetration structures have never been observed in C. purpurea, suggesting that penetration must be achieved by loosening of the cell wall by enzyme secretion. Grasses have developed a special cell wall type containing mainly glucuronoarabinoxylans, cellulose and β‐1,3‐glucan, and only small amounts of pectin. The detection of xylanase and β‐1,3‐glucanase (Giesbert et al., 1998; Tenberge, 1999) activity in planta indicates a role for cell wall‐degrading enzymes (CWDEs) in fungal host colonization. Cellulolytic activity, however, has been studied extensively, but could not be correlated with penetration (Müller et al., 1997). After penetration of the outer wall, the fungus grows mainly intercellularly, deriving nutrients from the degradation products of the pectic material present in the middle lamella (Tenberge et al., 1996). Double deletion of two putative endo‐polygalacturonase genes showed drastic effects on pathogenicity, although colonization of the ovaries was not completely abolished, indicating that other enzymes compensate for the loss of galacturonase activity (Oeser et al., 2002).
In biology, fungal hyphae, axons, pollen tubes and root hairs are the most prominent examples of apical growth (Harris, 2006; Palanivelu and Preuss, 2000). Claviceps purpurea grows in the transmitting tissue, mimicking pollen tube growth, until the ovary base is reached and the pollen tube path is abandoned. The growth direction must be highly regulated at this stage of colonization as the fungus grows mainly without branching. After reaching the nutrient‐rich phloem exudate, the fungus becomes highly branched and colonizes the entire ovary. The signals guiding and restricting fungal growth in the earliest infection stage are unknown. One hypothesis is based on the observation that the fungus follows and feeds on a pectin‐rich trace in the transmitting tissue (Tenberge et al., 1996). During pectin degradation, calcium ions are released, putatively functioning as a guidance cue and possibly as a branching inhibitor. It has long been known that a tip high calcium gradient is necessary for polar growth (Jackson and Heath, 1993; Silverman‐Gavrila and Lew, 2000). To elucidate whether high calcium levels in the surrounding medium are sensed by C. purpurea, functional analysis of a stretch‐activated calcium channel (Mid1) was undertaken. Loss of mid1 leads to non‐pathogenicity and increased sensitivity to cell wall stress, indicating that either ion sensing or cell wall composition are important for host colonization. An overall reduction in growth rate was suppressed on medium containing higher percentages of agar, indicating that cell wall defects of the mutant may impair force generation (J. Bormann, WWU Muenster, unpublished data). In this regard, it is interesting that deletion of the gene cpcot1, encoding an NDR‐like kinase (Cpcot1), not only evoked hyperbranching, decreased growth rate and a penetration defect, but the wild‐type phenotype was partial restored when grown under an overlay of 8% agar (Scheffer et al., 2005a). It is still unclear whether imposed pressure on hyphae during intercellular in planta growth plays a general role in growth regulation. Claviceps purpurea mutants that show altered morphology and/or altered cell wall structure are often found to be impaired in host colonization. For instance, a mutant lacking the mitogen‐activated protein kinase (MAPK) Cpmk2 (homologue to yeast Slt2) is more sensitive to lysing enzyme, shows hyperbranched and coiled hyphae and reduced virulence; reduced virulence was also shown for Δcpmk1 (lacking a homologue of yeast Fus3/Kss1) (2002a, 2002b). Activation of MAPK depends on a two‐component sensor histidine kinase system. A class X histidine kinase has an influence on pathogenicity, stress tolerance and spore germination in C. purpurea (Nathues et al., 2007). These examples indicate that either cell wall stability is important to generate pressure during in planta growth or that altered gene regulation in these mutants may elicit a host response.
Known elicitors of host defence include cell wall components, extracellular enzymes produced by the pathogen, and secreted small molecules, such as reactive oxygen species (ROS). However, ROS are known to act in the earliest defence reactions of plants. A first‐hand role of these molecules is reinforcement of the host cell wall by cross‐linking of cell wall proteins. Also triggered by ROS is the hypersensitive response (HR), a plant defence programme causing rapid necrosis at the proximate infection site, and induction of late defence genes in the surrounding tissue (Baker and Orlandi, 1995). As no HR was observed during C. purpurea infection of rye, the fungus might have evolved a strong antioxidant machinery to detoxify plant ROS. Two mechanisms, the expression of ROS‐scavenging enzymes, such as superoxide dismutases and catalases, and the accumulation of non‐enzymatic antioxidants, such as ascorbic acid, mannitol, glutathione etc., are well studied systems causing ROS detoxification. Targeted inactivation of a Cu–Zn‐superoxide dismutase (SOD)‐encoding gene (cpsod1) (Moore et al., 2002) and of a gene (cpcat1) coding for two catalase isoforms (Garre et al., 1998) had no influence on plant colonization. However, deletion of a bZIP‐transcription factor‐encoding gene (cptf1) led to activity loss of all four catalase isoforms and to a significantly reduced pathogenicity. In these mutants, significant amounts of H2O2 were detected around the hyphae and in distal, not yet colonized, plant tissue. This was never observed during infection by the wild‐type (Nathues et al., 2004).
In contrast with the above‐mentioned hypothesis that C. purpurea copes with an oxidative burst of the plant by secreting detoxifying enzymes, the latter observations point to a role of these enzymes in repressing ROS secretion by the fungus itself to prevent elicitor function, which, in turn, can trigger ROS production by the plant.
In recent years, ROS production by fungi has become a topic of extensive research, and an increasing number of publications have shown that the production of superoxide (O2 ‐) by NADPH oxidases plays a crucial role in pathogenicity (for example, Segmüller et al., 2008), symbiosis (Tanaka et al., 2006), sexual and asexual development (Lara‐Ortiz et al., 2003; Malagnac et al., 2004) and polarity establishment (Semighini and Harris, 2008). In C. purpurea, Cpnox1 (class A, according to the nomenclature of Aguirre et al., 2005) has an impact on the germination of conidia and pathogenicity: Δcpnox1 mutants can penetrate the host epidermis, but are impaired in the colonization of plant ovarian tissue; only in rare cases were visible infection symptoms, such as the production of honeydew or sclerotia, observed (Giesbert et al., 2008). In contrast, deletion of Cpnox2, coding for a type B NADPH oxidase, leads to an increased amount and duration of honeydew production. Interestingly, despite the inverse effect of Cpnox1 and 2 in early plant colonization, both mutants show a defect in sclerotia development (D. Buttermann, WWU Muenster, unpublished data).
Several studies have shown that deletion of the O2 ‐‐producing Nox system leads to elevated levels of ROS (for example, Malagnac et al., 2004). Therefore, it is speculative whether the deletion of C. purpurea Cpnox1/2 leads to an overall decreased or increased level of ROS, probably counter‐balanced by the action of catalases or alternative ROS‐producing mechanisms. Nox enzyme regulation can contribute to the understanding of this complex mechanism. Studies from the close relative of C. purpurea, Epichloe festucae, an endophyte of grasses, recently described the interaction of the small guanosine triphosphatase (GTPase) Rac with the regulatory enzyme NoxR as crucial for the activation of NoxA (Tanaka et al., 2008).
The role of a NoxR homologue in C. purpurea is presently under investigation, and initial results have indicated a similar regulatory system as described for E. festucae, as NoxR interacts with Rac in a yeast two‐hybrid assay (S. Giesbert and D. Buttermann, WWU Muenster, unpublished data).
Functional analysis of Rac in C. purpurea revealed that the small GTPase is indispensable for pathogenicity. Deletion of Rac has drastic effects on hyphal organization and development. The latter is not surprising as, in addition to its proposed role in NOX activation, Rac is known to activate various signalling pathways, among others via interaction with p21‐activated kinases (PAKs). PAKs are known to be involved in a variety of signalling cascades influencing cytoskeleton organization and cell motility. A C. purpurea PAK mutant Δcla4 showed, in all other analysed phenotypic characteristics, the same features as observed for Δrac. Expression studies of genes encoding ROS scavenging and generating enzymes indicate a function of Rac and Cla4 in ROS homeostasis (Rolke and Tudzynski, 2008). Functional analysis of another small GTPase, Cdc42, revealed that the protein is dispensable for normal penetration. Yet, fungal colonization stopped before the transmitting tissue was reached. Cytological analysis by transmission electron microscopy (TEM) showed massive production of H2O2 (detected by CeCl3 staining) around the hyphae, indicating an imbalance of ROS homeostasis, as described for Δrac and Δcla4 mutants (Scheffer et al., 2005b). The role of the small GTPases Rac and Cdc42 and the PAK Cla4 in NOX complex regulation is currently under investigation.
Nowadays, the genomes of an increasing number of organisms from all kingdoms have been sequenced. Genetic analysis can be speeded up by bypassing screening and sequencing DNA regions of interest by hand. However, full genome sequencing is cost‐intensive and not feasible currently in C. purpurea. Considering this, a C. purpurea expressed sequence tag (EST) library containing nearly 10 000 ESTs derived from axenic and in planta material was established. The C. purpurea EST library was split into one‐quarter of clones derived from axenic culture and three‐quarters from in planta material (days 6–20 post‐inoculation). cDNA clones were sequenced and processed for macroarray analysis. Eighty per cent of the ESTs could be analysed; they were assembled into 1273 true contigs and 3079 singlet contigs, and blasted to a database [BlastX: non‐redundant; National Center for Biotechnology Information (NCBI); Altschul et al., 1990]; bioinformatic evaluation showed interesting parallels with comparable EST analyses in other pathogenic fungi, and will help in the identification of potential candidate virulence genes (B. Oeser et al., WWU Muenster, unpublished data). Macroarrays are currently used for target gene analyses of signalling components: for example, to compare the expression profiles of the two GTPase mutants (Cdc42, Rac) and the PAK mutant (Cla4) to learn more about the target genes affected by the respective gene deletions. Although the phenotypes of the rac and cla4 mutants are indistinguishable, the array results depict interesting differences in gene expression. Detailed analysis of these data will be helpful to decipher differences in the respective signalling pathways (Y. Rolke and J. von Sengbusch, WWU Muenster, unpublished data).
Genomic tools are also now available for the study of the host response; because of the high homology between rye and wheat genomes, genomic arrays of wheat have been successfully used to study gene expression in rye florets infected with C. purpurea. Interestingly, infection leads to a significant modification of the plant's transcriptome, indicating that—in contrast with the classical view of perfect mimicry—the plant ‘sees’ the pathogen (B. Oeser et al., WWU Muenster, unpublished data). As these analyses included material collected at 5 dpi, it is possible that the mimicry phase is restricted to the very first days of infection.
5. PERSPECTIVES
Claviceps spp., the ergot fungus, as outlined above, has had a significant impact on human history, social development, agriculture, pharmacology and biotechnology. It has kept its Janus‐faced importance throughout the centuries, as a supplier of dangerous toxins and valuable pharmaceuticals; it has influenced a whole generation of recreational drug users, and will help to establish new strategies for the treatment of important degenerative diseases, which will have enormous economic importance. Last, but not least, it serves as a model system to understand host–pathogen interaction, with its special aspects of organ specificity, oriented in planta growth and (probably) suppression of the host defence. Nevertheless, after so many years of research, no real effective control strategy has been developed; therefore, Claviceps is not yet history, but a persistent threat to modern agriculture. It is also at the dawn of the development of new pharmaceutical applications.
ACKNOWLEDGEMENTS
We thank M. Nicholson for critical reading of the manuscript, N. Lorenz, D. Buttermann, B. Oeser and J. Bormann for discussions and sharing of data prior to publication, and the Deutsche Forschungsgemeinschaft (DFG) for financial support.
REFERENCES
- Aguirre, J. , Ríos‐Momberg, M. , Hewitt, D. and Hansberg, W. (2005) Reactive oxygen species and development in microbial eukaryotes. Trends Microbiol. 13, 111–118. [DOI] [PubMed] [Google Scholar]
- Alderman, S.C. , Coats, D.D. , Crowe, F.J. and Butler, M.D. (1998) Occurrence and distribution of ergot and estimates of seed loss in Kentucky bluegrass grown for seed in central Oregon. Plant Dis. 82, 89–93. [DOI] [PubMed] [Google Scholar]
- Alm, T. (2003) The witch trials of Finnmark, northern Norway, during the 17th century: evidence for ergotism as a contributing factor. Econ. Bot. 57, 403–416. [Google Scholar]
- Altschul, S.F. , Gish, W. , Miller, W. , Myers, E.W. and Lipman, D.J. (1990) Basic local alignment search tool. J. Mol. Biol. 215, 403–410. [DOI] [PubMed] [Google Scholar]
- Appelt, M. and Ellner, F.M. (2008) Ergot alkaloids—relevance and first own results of incidences in 2007. ALVA-Mitteilungen, 6, 27–30. [Google Scholar]
- Arechavaleta, M. , Bacon, C.W. , Hoveland, C.S. and Radcliff, D.E. (1989) Effects of the tall fescue endophyte on plant response to environmental stress. Agron. J. 81, 83–90. [Google Scholar]
- Baker, C.J. and Orlandi, E.W. (1995) Active oxygen in plant pathogenesis. Annu. Rev. Phytopathol. 33, 299–321. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay, R. , Frederickson, D.E. , McLaren, N.W. , Odvody, G.N. and Ryley, M.J. (1998) Ergot: a new disease threat to sorghum in the Americas and Australia. Plant Dis. 82, 356–367. [DOI] [PubMed] [Google Scholar]
- Barger, G. (1931) Ergot and Ergotism. London: Gurney & Jackson. [Google Scholar]
- Bauer, V.H. (1973) Das Antoniusfeuer in Kunst und Medizin. Berlin, Heidelberg, New York: Springer Verlag. [Google Scholar]
- Berde, B. and Stürmer, E. (1978) Introduction to the pharmacology of ergot alkaloids and related compounds In: Ergot Alkaloids and Related Compounds (Berde B. and Schild H.O., eds), pp. 1–28. Berlin, Heidelberg, New York: Springer. [Google Scholar]
- Betz, H.G. , Müller, R. , Wilde, P. and Wortmann, H. (1998) Mutterkorn vermeiden. Auswertungs‐ und Informationsdienst für Ernährung , Landwirtschaft und Forsten 1361, 3–16. [Google Scholar]
- Blaney, B.J. , McKenzie, R.A. , Walters, J.R. , Taylor, L.F. , Bewg, W.S. , Ryley, M.J. and Maryam, R. (2000) Sorghum ergot (Claviceps africana) associated with agalactia and feed refusal in pigs and dairy cattle. Aust. Vet. J. 78, 102–107. [DOI] [PubMed] [Google Scholar]
- Bové, F.J. (1970) The Story of Ergot. Basle: S. Karger. [Google Scholar]
- Bush, L.P. , Wilkinson, H.H. and Schardl, C.L. (1997) Bioprotective alkaloids of grass‐fungal endophyte symbioses. Plant Physiol. 114, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporael, L.R. (1976) Ergotism: the Satan loosed in Salem? Science, 192, 21–26. [DOI] [PubMed] [Google Scholar]
- Chakraborty, S. and Ryley, M.J. (2008) Sorghum ergot can develop without local Claviceps africana inoculum from nearby infected plants. Plant Pathol. 57, 484–492. [Google Scholar]
- Cheng, L.J. , Robbers, J.E. and Floss, H.G. (1980) End‐product regulation of ergot alkaloid formation in intact cells and protoplasts of Claviceps species, strain SD58. J. Nat. Prod. 43, 329–339. [Google Scholar]
- De Costa, C. (2002) St. Anthony's fire and living ligatures: a short history of ergometrine. Lancet, 359, 1768–1770. [DOI] [PubMed] [Google Scholar]
- Crosignani, P.G. (2006) Current treatment issues in female hyperprolactinaemia. Eur. J. Obstet. Gynaecol. Reprod. Biol. 125, 152–164. [DOI] [PubMed] [Google Scholar]
- Cross, D.L. (2003) Ergot alkaloid toxicity In: Mycology, Clavicipitacean Fungi: Evolutionary Biology, Chemistry, Biocontrol and Cultural Impacts, Vol. 19 (White J.F., Jr, Bacon C.W., Hywel‐Jones N.L. and Spatafora J.W., eds), pp. 475–494. New York, Basle: Marcel Dekker Inc. [Google Scholar]
- Cross, D.L. , Redmond, L.M. and Strickland, J.R. (1995) Equine fescue toxicosis: signs and solutions. J. Anim. Sci. 73, 899–908. [DOI] [PubMed] [Google Scholar]
- Cvak, L. (1999) Industrial production of ergot alkaloids In: Ergot, the Genus Claviceps, Vol. 6 (Kren V. and Cvak L., eds), pp. 373–409. Amsterdam: Harwood Academic Publishers. [Google Scholar]
- Didek‐Brumec, M. , Gaberc‐Porekar, V. , Alcevic, M. (1996) Relationship between the Claviceps life cycle and productivity of ergot alkaloids. Crit Rev Biotechnol. 16, 257–299. [Google Scholar]
- Van Dongen, P.W.J. and De Groot, A.N.J.A. (1995) History of ergot alkaloids from ergotism to ergometrine. Eur. J. Obstet. Gynaecol. Reprod Biol. 60, 109–116. [DOI] [PubMed] [Google Scholar]
- Eadie, M.J. (2003) Convulsive ergotism: epidemics of the serotonin syndrome? Lancet Neurol. 2, 429–434. [DOI] [PubMed] [Google Scholar]
- Fantegrossi, W.E. , Murnane, A.C. and Reissig, C.J. (2008) The behavioral pharmacology of hallucinogens. Biochem. Pharmacol. 75, 17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flieger, M. , Wurst, M. and Shelby, R. (1997) Ergot alkaloids—sources, structures and analytical methods. Folia Microbiologica, 42, 3–30. [DOI] [PubMed] [Google Scholar]
- Frederickson, D.E. , Mantle, P.G. and De Milliano, W.A.J. (1991) Claviceps africana sp. nov., the distinctive ergot pathogen of sorghum in Africa. Mycol. Res. 95, 1101–1107. [Google Scholar]
- Frederickson, D.E. , Mantle, P.G. and De Milliano, W.A.J. (1993) Windborne spread of ergot disease (Claviceps africana) in sorghum A‐lines in Zimbabwe. Plant Pathol. 42, 368–377. [Google Scholar]
- Garre, V. , Müller, U. and Tudzynski, P. (1998) Cloning, characterization, and targeted disruption of cpcat1, coding for an in planta secreted catalase of Claviceps purpurea . Mol. Plant–Microbe Interact. 11, 772–783. [DOI] [PubMed] [Google Scholar]
- Gebler, J.C. and Poulter, D. (1992) Purification and characterization of dimethylallyl tryptophan synthase from Claviceps purpurea . Arch. Biochem. Biophys. 296, 308–313. [DOI] [PubMed] [Google Scholar]
- Giesbert, S. , Lepping, H‐B. , Tenberge, K.B. and Tudzynski, P. (1998) The xylanolytic system of Claviceps purpurea: cytological evidence for secretion of xylanases in infected rye tissue and molecular characterization of two xylanase genes. Phytopathology, 88, 1020–1030. [DOI] [PubMed] [Google Scholar]
- Giesbert, S. , Schürg, T. , Scheele, S. and Tudzynski, P. (2008) The NADPH oxidase Cpnox1 is required for full pathogenicity of the ergot fungus Claviceps purpurea . Mol. Plant Pathol. 9, 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görnemann, T. , Jähnichen, S. , Schurad, B. , Latté, K.P. , Horowski, R. , Tack, J. , Flieger, M. and Pertz, H.H. (2008) Pharmacological properties of a wide array of ergolines at functional alpha1‐adrenoceptor subtypes. Naunyn-Schmiedeberg's Arch. Pharmacol. 376, 321–330. [DOI] [PubMed] [Google Scholar]
- Gröger, D. and Floss, H.G. (1998) Biochemistry of ergot alkaloids—achievements and challenges In: The Alkaloids: Chemistry and Biology, Vol. 50. (Cordell G.A., ed.), pp. 171–218. London: Academic Press. [Google Scholar]
- De Groot, A.N. , Van Dongen, P.W. , Vree, T.B. , Hekster, Y.A. and Van Roosmalen, J. (1998) Ergot alkaloids. Current status and review of clinical pharmacology and therapeutic use compared with other oxytocics in obstetrics and gynaecology. Drugs, 56, 523–535. [DOI] [PubMed] [Google Scholar]
- Haarmann, T. , Lorenz, N. and Tudzynski, P. (2008) Use of a nonhomologous end joining deficient strain (Δku70) of the ergot fungus Claviceps purpurea for identification of the nonribosomal peptide synthetase gene involved in ergotamine biosynthesis. Fungal Genet. Biol. 45, 35–44. [DOI] [PubMed] [Google Scholar]
- Haarmann, T. , Machado, C. , Lübbe, Y. , Correia, T. , Schardl, C.L. , Panaccione, D.G. and Tudzynski, P. (2005) The ergot alkaloid gene cluster in Claviceps purpurea: extension of the cluster sequence and intraspecies evolution. Phytochemistry, 66, 1312–1320. [DOI] [PubMed] [Google Scholar]
- Haarmann, T. , Ortel, I. , Tudzynski, P. and Keller, U. (2006) Identification of the cytochrome P450 monooxygenase that bridges the clavine and ergoline alkaloid pathways. ChemBioChem. 7, 645–652. [DOI] [PubMed] [Google Scholar]
- Harris, S.D. (2006) Cell polarity in filamentous fungi: shaping the mold. Int. Rev. Cytol. 251, 41–77. [DOI] [PubMed] [Google Scholar]
- Hill, N.S. , Thompson, F.N. , Stuedemann, J.A. , Rottinghaus, G.W. , Ju, H.J. , Dawe, D.L. and Hiatt, E.E. (2001) Ergot alkaloid transport across ruminant gastric tissues. J. Anim. Sci. 79, 542–549. [DOI] [PubMed] [Google Scholar]
- Hofmann, A. (1978) Historical view on ergot alkaloids. Pharmacology, 16, 1–11. [DOI] [PubMed] [Google Scholar]
- Hofmann, A. (1993) LSD—mein Sorgenkind: Die Entdeckung einer Wunderdroge. Munich: Deutscher Taschbuch Verlag GmbH & Co KG. [Google Scholar]
- Hoveland, C. (1993) Importance and economic significance of the Acremonium endophytes to performance of animals and grass plants. Agric. Ecosyst. Environ. 44, 3–12. [Google Scholar]
- Jackson, S.L. and Heath, I.B. (1993) Roles of calcium ions in hyphal tip growth. Microbiol. Rev. 57, 367–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, U. and Tudzynski, P. (2002) Ergot alkaloids In: The Mycota X, Industrial Applications (Osiewacz H.D., ed.), pp. 157–172. Berlin, Heidelberg: Springer‐Verlag. [Google Scholar]
- Krska, R. and Crews, C. (2008) Significance, chemistry and determination of ergot alkaloids: a review. Food Addit. Contam. 25, 722–731. [DOI] [PubMed] [Google Scholar]
- Lara‐Ortiz, T. , Riveros‐Rosas, H. and Aguirre, J. (2003) Reactive oxygen species generated by microbial NADPH oxidase NoxA regulate sexual development in Aspergillus nidulans . Mol. Microbiol. 50, 1241–1255. [DOI] [PubMed] [Google Scholar]
- Leary, T. (1969) The effects of consciousness‐expanding drugs on prisoner rehabilitation. Psychedel. Rev. 10, 29–44. [Google Scholar]
- Li, X.F. , Fortney, J.A. , Kotelchuck, M. and Glover, L.H. (1996) The postpartum period: the key to maternal mortality. Int. J. Gynaecol. Obstet. 54, 1–10. [DOI] [PubMed] [Google Scholar]
- Lindhauer, M. , Münzing, K. , Seling, S. , Betsche, T. , Kersting, H.‐J. , Masloff, S. and Seifert, M. (2005) Hochwertiges Getreide durch kontinuierliche Qualitätserhebungen. Detmold: Forschungsreport 2 Federal Research Centre for Nutrition and Food. [Google Scholar]
- Lorenz, N. , Wilson, E.V. , Machado, C. , Schardl, C. and Tudzynski, P. (2007) Comparison of ergot alkaloid biosynthesis gene clusters in Claviceps species indicates loss of late pathway steps in evolution of C. fusiformis . Appl. Env. Microbiol. 73, 7185–7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malagnac, F. , Laluque, G. , Lepere, G. and Silar, P. (2004) Two NADPH oxidase isoforms are required for sexual reproduction and ascospore germination in the filamentous fungus Podospora anserina . Fungal Genet. Biol. 41, 982–997. [DOI] [PubMed] [Google Scholar]
- Malinowski, D.P. and Belesky, D.P. (2000) Adaptation of endophyte‐infected cool season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Sci. 40, 923–940. [Google Scholar]
- Matossian, M.K. (1989) Poisons of the Past: Molds, Epidemics and History. New Haven, CT: Yale University Press. [Google Scholar]
- Mey, G. , Correia, T. , Oeser, B. , Kershaw, M.J. , Garre, V. , Arntz, C. , Talbot, N.J. and Tudzynski, P. (2003) Structural and functional analysis of an oligomeric hydrophobin gene from Claviceps purpurea . Mol. Plant Pathol. 4, 31–41. [DOI] [PubMed] [Google Scholar]
- Mey, G. , Held, K. , Scheffer, J. , Tenberge, K.B. and Tudzynski, P. (2002a) CPMK2, a Slt2‐homologous MAP‐kinase, is essential for pathogenesis of Claviceps purpurea on rye: evidence for a second conserved pathogenesis‐related MAP‐kinase cascade in phytopathogenic fungi. Mol. Microbiol. 46, 305–318. [DOI] [PubMed] [Google Scholar]
- Mey, G. , Oeser, B. , Lebrun, M.H. and Tudzynski, P. (2002b) The biotrophic, non‐appressoria forming grass pathogen Claviceps purpurea needs a Fus3/Pmk1 homologous MAP kinase for colonization of rye ovarian tissue. Mol. Plant–Microbe Interact. 15, 303–312. [DOI] [PubMed] [Google Scholar]
- Mielke, H. (1993) Untersuchungen zur Bekämpfung des Mutterkorns. Nachrichtenbl. Deut. Pflanzenschutzd. 5/ 6, 97–102. [Google Scholar]
- Mielke, H. (2000) Studien über den Pilz Claviceps purpurea (Fries) Tulasne unter Berücksichtigung der Anfälligkeit verschiedener Roggensorten und der Bekämpfungsmöglichkeiten des Erregers. Mitt. Biol. Bundesanst. Land‐Forstwirtsch., Vol. 375, Parey Buchverlag Berlin.
- Mirdita, V. , Dhillon, B.S. , Geiger, H.H. and Miedaner, T. (2008) Genetic variation for resistance to ergot (Claviceps purpurea [Fr.] Tul.) among full‐sib families of five populations of winter rye (Secale cereale L. ). Theor. Appl. Genet. 118, 85–90. [DOI] [PubMed] [Google Scholar]
- Moore, S. , De Vries, O.M.H. and Tudzynski, P. (2002) The major Cu,Zn SOD of the phytopathogen Claviceps purpurea is not essential for pathogenicity. Mol. Plant Pathol. 3, 9–22. [DOI] [PubMed] [Google Scholar]
- Mukherjee, J. and Menge, M. (2000) Progress and prospects of ergot alkaloid research. Adv. Biochem. Eng. Biotechnol. 68, 1–20. [DOI] [PubMed] [Google Scholar]
- Müller, U. , Tenberge, K.B. , Oeser, B. and Tudzynski, P. (1997) Cel1, probably encoding a cellobiohydrolase lacking the substrate binding domain, is expressed in the initial infection phase of Claviceps purpurea on Secale cereale . Mol. Plant–Microbe Interact. 10, 268–279. [DOI] [PubMed] [Google Scholar]
- Münzing, K. (1999) Sicherung der Marktanforderungen für Qualitätsgetreide. Getreide, 5, 138–145. [Google Scholar]
- Nathues, E. , Jörgens, C. , Lorenz, N. and Tudzynski, P. (2007) The histidine kinase CpHK2 has impact on spore germination, oxidative stress and fungicide resistance, and virulence of the ergot fungus Claviceps purpurea . Mol. Plant Pathol. 8, 653–665. [DOI] [PubMed] [Google Scholar]
- Nathues, E. , Joshi, S. , Tenberge, K.B. , Von Den Driesch, M. , Oeser, B. , Bäumer, N. , Mihlan, M. and Tudzynski, P. (2004) CPTF1 a CREB‐like transcription factor, is involved in the oxidative stress response in the phytopathogen Claviceps purpurea and modulates ROS level in its host Secale cereale . Mol. Plant–Microbe Interact. 17, 383–393. [DOI] [PubMed] [Google Scholar]
- Nichols, D.E. (2001) LSD and its lysergamid cousins. Heffter Rev. Psychedel. Res. 2, 80–87. [Google Scholar]
- Oeser, B. , Heidrich, P. , Müller, U. , Tudzynski, P. and Tenberge, K.B. (2002) Polygalacturonase is a pathogenicity factor in the Claviceps purpurea/rye interaction. Fungal Genet. Biol. 36, 176–186. [DOI] [PubMed] [Google Scholar]
- Packer, S. (1998) Jewish mystical movements and the European ergot epidemics. Israel J. Psychatr. Relat. Sci. 35, 227–239. [PubMed] [Google Scholar]
- Palanivelu, R. and Preuss, D. (2000) Pollen tube targeting and axon guidance: parallels in tip growth mechanisms. Trends Cell Biol. 10, 517–524. [DOI] [PubMed] [Google Scholar]
- Pažoutová, S. , Bandyopadhyay, R. , Frederickson, D.E. , Mantle, P.G. and Frederiksen, R.A. (2000) Relations among sorghum ergot isolates from the Americas, Africa, India and Australia. Plant Dis. 84, 437–442. [DOI] [PubMed] [Google Scholar]
- Pažoutová, S. and Frederickson, D.E. (2005) Genetic diversity of Claviceps africana on sorghum and Hyparrhenia . Plant Pathol. 54, 749–763. [Google Scholar]
- Popay, A.J. and Bonos, S.A. (2005) Biotic responses in endophytic grasses In: Neotyphodium in Cool‐Season Grasses (Roberts C.A., West C.P. and Spiers D.E., eds), pp. 164–185. Ames, IA: Blackwell Publishing. [Google Scholar]
- Porter, J.K. and Thompson, F.N.J. (1992) Effects of fescue toxicosis on reproduction in livestock. J. Anim. Sci. 70, 1594–1603. [DOI] [PubMed] [Google Scholar]
- Prom, L.K. , Isakeit, T. , Odvody, G.N. , Rush, C.M. , Kaufman, H.W. and Montes, N. (2005) Survival of Claviceps africana within sorghum panicles at several Texas locations. Plant Dis. 89, 39–43. [DOI] [PubMed] [Google Scholar]
- Riederer, B. , Han, M. and Keller, U. (1996) D‐Lysergyl peptide synthetase from the ergot fungus Claviceps purpurea . J. Biol. Chem. 271, 27 524–27 530. [DOI] [PubMed] [Google Scholar]
- Rolke, Y. and Tudzynski, P. (2008) The small GTPase Rac and the PAK kinase Cla4 in Claviceps purpurea: interaction and impact on polarity, development and pathogenicity. Mol. Microbiol. 68, 405–423. [DOI] [PubMed] [Google Scholar]
- Ryley, M.J. , Alcorn, J.L. , Kochman, J.K. , Kong, G.A. and Thompson, S.M. (1996) Ergot of Sorghum spp. in Australia. Austral. Plant Pathol. 25, 214. [Google Scholar]
- Ryley, M.J. and Chakraborty, S. (2008) Patterns of release of the secondary conidia of Claviceps africana, the sorghum ergot pathogen in Australia. Plant Pathol. 57, 473–483. [Google Scholar]
- Schardl, C.L. (1996) Epichloë species: fungal symbionts of grasses. Annu. Rev. Phytopathol. 34, 109–130. [DOI] [PubMed] [Google Scholar]
- Schardl, C.L. , Panaccione, D.G. and Tudzynski, P. (2006) Ergot alkaloids—biology and molecular biology. Alkaloids Chem. Biol. 63, 45–86. [DOI] [PubMed] [Google Scholar]
- Schardl, C.L. and Phillips, T.D. (1997) Protective grass endophytes where are they from and where are they going? Plant Dis. 81, 430–438. [DOI] [PubMed] [Google Scholar]
- Schardl, C.L. , Scott, B. , Florea, S. and Zhang, D. (2009) Epichloë endophytes: Clavicipitaceous symbionts of grasses In: The Mykota V: Plant Relationships, 2nd edn. (Deising H., ed.), pp. 275–306. Berlin, Heidelberg: Springer Verlag. [Google Scholar]
- Scheffer, J. , Chen, C. , Heidrich, P. , Dickman, M.B. and Tudzynski, P. (2005b) A CDC42 homologue in C. purpurea is involved in vegetative differentiation and is essential for pathogenicity. Eukaryot. Cell, 4, 1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer, J. , Ziv, C. , Yarden, O. and Tudzynski, P. (2005a) The COT1 homologue CPCOT1 regulates polar growth and branching and is essential for pathogenicity in Claviceps purpurea . Fungal Genet. Biol. 42, 107–118. [DOI] [PubMed] [Google Scholar]
- Schiff, P.L. (2006) Ergot and its alklaloids. Am. J. Pharmaceut. Educ. 70, 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid, S.P. and Osborne, T.G. (1993) Effects of endophyte‐infected tall fescue on animal performance. Agric. Ecosyst. Environ. 44, 233–262. [Google Scholar]
- Schwarz, P.B. , Neate, S.M. and Rottinghaus, G.E. (2006) Widespread occurrence of ergot in upper midwestern U.S. barley, 2005. Plant Dis. 90, 527. [DOI] [PubMed] [Google Scholar]
- Segmüller, N. , Kokkelink, L. , Giesbert, S. , Odinius, D. , Van Kan, J. and Tudzynski, P. (2008) NADPH oxidases are involved in differentiation and pathogenesis in Botrytis cinerea . Mol. Plant–Microbe Interact. 21, 808–819. [DOI] [PubMed] [Google Scholar]
- Semighini, C.P. and Harris, S.D. (2008) Regulation of apical dominance on Aspergillus nidulans hyphae by reactive oxygen species. Genetics, 179, 1919–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigafoos, J. , Green, V.A. , Edrisinha, C. and Lancioni, G.E. (2007) Flashback to the 1960s: LSD in the treatment of autism. Dev. Neurorehabil. 10, 75–81. [DOI] [PubMed] [Google Scholar]
- Silverman‐Gavrila, L.B. and Lew, R.R. (2000) Calcium and tip growth in Neurospora crassa . Protoplasma, 213, 203–217. [Google Scholar]
- Sinz, A. (2008) Die Bedeutung der Mutterkorn‐Alkaloide als Arzneistoffe. Pharm. Unserer Zeit. 4, 306–309. [DOI] [PubMed] [Google Scholar]
- Stadler, P.A. and Giger, R. (1984) Ergot alkaloids and their derivatives in medical chemistry and therapy In: Natural Products and Drug Development (Krosgard‐Larson P., Christensen C.H. and Kofod H., eds), pp. 463–485. Copenhagen: Munksgaard. [Google Scholar]
- Stafford, P.C. and Golightly, B.H. (1967) LSD—The Problem‐solving Psychedelic. New York: Award Books. [Google Scholar]
- Taber, W.A. (1985) Biology of Claviceps In: Biotechnology Series, Vol. 6, Biology of Industrial Microorganisms (Demain A.L. and Nadine A.S., eds), pp. 449–486. New York: The Benjamin Cummings Publishing Co., Inc. [Google Scholar]
- Tanaka, A. , Christensen, M.J. , Takemoto, D. , Park, P. and Scott, B. (2006) Reactive oxygen species play a role in regulating a fungus perennial ryegrass mutualistic association. Plant Cell, 18, 1052–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, A. , Takemoto, D. , Hyon, G‐S , Park, P. and Scott, B. (2008) NoxA activation by the small GTPase RacA is required to maintain a mutualistic symbiotic association between Epichloe festucae and perennial ryegrass. Mol. Microbiol. 68, 1165–1178. [DOI] [PubMed] [Google Scholar]
- Tenberge, K.B. (1999) Biology and life strategy of the ergot fungi In: Medicinal and Aromatic Plants—Industrial Profiles, Vol. 6. Ergot—The Genus Claviceps. (Kren V. and Cvak L., eds), pp. 25–56. Chur: Harwood Academic Publishers. [Google Scholar]
- Tenberge, K.B. , Homann, V. , Oeser, B. and Tudzynski, P. (1996) Structure and expression of two polygalacturonase genes of Claviceps purpurea oriented in tandem and cytological evidence for pectinolytic enzyme activity during infection of rye. Phytopathology, 86, 1084–1097. [Google Scholar]
- Tfelt‐Hansen, P.C. and Koehler, P.J. (2008) History of the use of ergotamine and dihydroergotamine in migraine from 1906 and onward. Cephalalgia, 28, 877–886. [DOI] [PubMed] [Google Scholar]
- Thobois, S. (2006) Proposed dose equivalence for rapid switch between dopamine receptor agonists in Parkinson's disease: a review of the literature. Clin. Ther. 28, 1–12. [DOI] [PubMed] [Google Scholar]
- Thomson, F.N. and Stuedemann, J.A. (1993) Pathophysiology of fescue toxicosis. Agric. Ecosyst. Environ. 44, 263–281. [Google Scholar]
- Tsai, H.F. , Wang, H. , Gebler, J.C. , Poulter, C.D. and Schardl, C.L. (1995) The Claviceps purpurea gene encoding dimethylallyltryptophan synthase, the committed step for ergot alkaloid biosynthesis. Biochem. Biophys. Res. Commun. 216, 119–125. [DOI] [PubMed] [Google Scholar]
- Tudzynski, P. , Correia, T. and Keller, U. (2001) Biotechnology and genetics of ergot alkaloids. Appl. Microbiol. Biotechnol. 57, 593–605. [DOI] [PubMed] [Google Scholar]
- Tudzynski, P. , Hölter, K. , Correia, T. , Arntz, C. , Grammel, N. and Keller, U. (1999) Evidence for an ergot alkaloid gene cluster in Claviceps purpurea . Mol. Gen. Genet. 261, 133–141. [DOI] [PubMed] [Google Scholar]
- Tulasne, L.‐R. (1853) Memoire sur l'ergot des glumacees. Ann. Sci. Nat. (Parie Botanique) 20, 5–56. [Google Scholar]
- Tulpule, P.G. and Bhat, R.V. (1978) Food toxins and their implication in human health. Indian J. Med. Res. 68, 99–108. [PubMed] [Google Scholar]
- Urga, K. , Debella, A. , W'Medihn, Y. , Agata, N. , Bayu, A. and Zewdie, W. (2002) Laboratory studies on the outbreak of gangrenous ergotism associated with consumption of contaminated barley in Arsi, Ethiopia. J. Health Dev. 16, 317–323. [Google Scholar]
- Vendrell, M. , Angulo, E. , Casadó, V. , Lluis, C. , Franco, R. , Albericio, F. and Royo, M. (2007) Novel ergopeptides as dual ligands for adenosine and dopamine receptors. J. Med. Chem. 50, 3062–3069. [DOI] [PubMed] [Google Scholar]
- Villalon, C.M. , De Vries, P. , Rabelo, G. , Centurion, D. , Sanchez‐Lopez, A. and Saxena, P.R. (1999) Canine external carotid vasoconstriction to methysergide, ergotamine and dihydroergotamine: role of 5‐HT1B/1D receptors and α2‐adrenoceptors. Br. J. Pharmacol. 126, 585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadworth, A.N. and Crisp, P. (1992) Co‐dergocrine mesylate. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in age‐related cognitive decline. Drugs Aging, 2, 153–173. [DOI] [PubMed] [Google Scholar]
- Willems, E.W. , Trion, M. , De Vries, P. , Heiligers, J.O.C. , Villalon, C.M. and Saxena, P.R. (1999) Pharmacological evidence that α1‐ and α2‐adrenoceptors mediate vasoconstriction of carotid arteriovenous anastomoses in anaesthetized pigs. Br. J. Pharmacol. 127, 1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workneh, F. , Narasimhan, B. , Srinivasan, R. and Rush, C.M. (2006) Assessment of regional site‐specific sorghum ergot severity potential using radar‐rainfall measurement. Plant Dis. 90, 704–707. [DOI] [PubMed] [Google Scholar]
- Workneh, F. and Rush, C.M. (2006) Weather factors associated with development of sorghum ergot in the Texas panhandle. Plant Dis. 90, 717–722. [DOI] [PubMed] [Google Scholar]
- Zhang, H.W. , Song, Y.C. and Tan, R.X. (2006) Biology and chemistry of endophytes. Nat. Prod. Rep. 23, 753–771. [DOI] [PubMed] [Google Scholar]


