 Bacteria employ a number of mechanisms to resist the effects of antibiotics, including the formation of biofilms.
Bacteria employ a number of mechanisms to resist the effects of antibiotics, including the formation of biofilms.
Abstract
Bacteria employ a number of mechanisms to resist the effects of antibiotics, including the formation of biofilms. We explored the anti-biofilm capabilities of a library of compounds based upon a 2-aminoquinazoline (2-AQ) scaffold against Mycobacterium smegmatis. This study resulted in the identification of 2-AQ derivatives with biofilm inhibition activity against M. smegmatis.
Biofilms are an assembly of surface associated bacteria that are encased in an extracellular polymeric substance (EPS).1 The EPS is typically composed predominantly of polysaccharides, and shields bacteria embedded within the biofilm from some microbicides and the host immune response.2 Within a biofilm, bacteria are upwards of 1000-fold more resistant to antibiotics when compared to their planktonic (free-floating) brethren, which often renders antibiotic treatments ineffective against biofilm-based infections.3 Given the central importance of biofilms within certain diseases, small molecules that control biofilm formation may serve as key adjuvants that, when paired with conventional antibiotics, lead to combinational therapies for the effective eradication of bacteria within a biofilm state.4
Mycobacterium tuberculosis is a slow growing pathogen that causes tuberculosis (TB). TB is predominantly an infection of the lungs and can be classified as a latent infection or active disease. Active TB disease patients present symptoms including chest pain, cough, fatigue, weight loss, and fever.5 TB now ranks above HIV/AIDS as the leading cause of death from a single infectious agent.6 Globally, it is estimated that one-third of the population is infected with TB.6
Current treatment for TB is a long process that lasts a minimum of six months. Generally, a patient is treated with isoniazid, rifampin and pyrazinamide for two months followed by isoniazid and rifampin for four months, with the overall goal of eradicating persistent TB reservoirs within the body to prevent disease relapse. Challenges with this regimen include ensuring patient completion and substantial drug toxicity due to extended antibiotic therapy.7
Recently it has been shown that M. tuberculosis readily forms biofilm-like communities on cellular debris in vitro, and in these communities the bacteria are more resistant to treatment with isoniazid.8M. tuberculosis biofilms have also been shown to survive treatment with isoniazid and rifampin while maintaining a minimum inhibitory concentration (MIC) 50 times greater than planktonic bacteria.9 It has been hypothesized that these biofilm-like communities may be the source of persistent TB reservoirs within an infected host.10 Therefore, the development of small molecules with anti-biofilm properties against mycobacteria could potentially be employed as adjuvants in combination with standard TB antibiotics, leading to a reduction of treatment time and increased patient compliance.
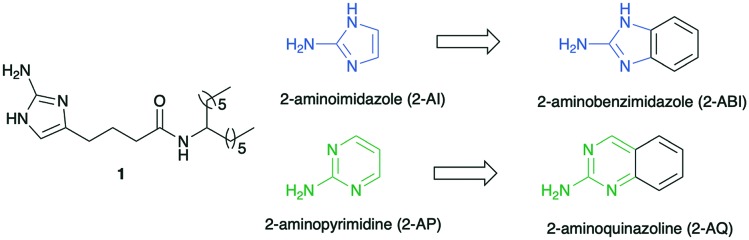
M. smegmatis is used as a model bacterium for M. tuberculosis since it is a non-pathogenic, faster growing bacterium. Furthermore, M. smegmatis shares over 2000 genetic homologs with M. tuberculosis including cell wall structure and resistance mechanisms.11 Previously we have shown that molecules identified as anti-biofilm molecules against M. smegmatis, showed anti-biofilm properties against M. tuberculosis, thus validating the use of M. smegmatis as a model organism for the discovery of anti-biofilm agents against M. tuberculosis. Additionally, these anti-biofilm compounds effectively reversed biofilm-based antibiotic tolerance when paired with isoniazid. The lead compound from these initial studies was the 2-aminoimidazole (2-AI) derivative 1 (Fig. 1), which was found to inhibit M. smegmatis biofilm formation with an IC50 value of 5.3 μM (where IC50 is defined as the concentration at which the compounds inhibits 50% biofilm formation in comparison to untreated control).8
Fig. 1. Active compound 1 and proposed structural modifications.
As part of a greater program directed towards the identification of anti-biofilm agents, we have established that both 2-aminopyrimidines and 2-aminobenzimidazoles have anti-biofilm properties.12,13 Given the structural relationship between our 2-AI derivatives and the 2-aminobenzimidazole ring (essentially a 2-AI ring appended to a benzene ring), we hypothesized that performing a similar structural modification of the 2-aminopyrimidine ring, to yield 2-aminoquinazolines (2-AQ), would also deliver active anti-biofilm agents (Fig. 1). Furthermore, given the success of quinazoline-based drugs, 2-AQs with anti-biofilm properties may be suitable for future in vivo studies.14,15 Herein we report the synthesis of a pilot library of 2-AQs and establish, for the first time, that this heterocycle has potent anti-biofilm activity against M. smegmatis.
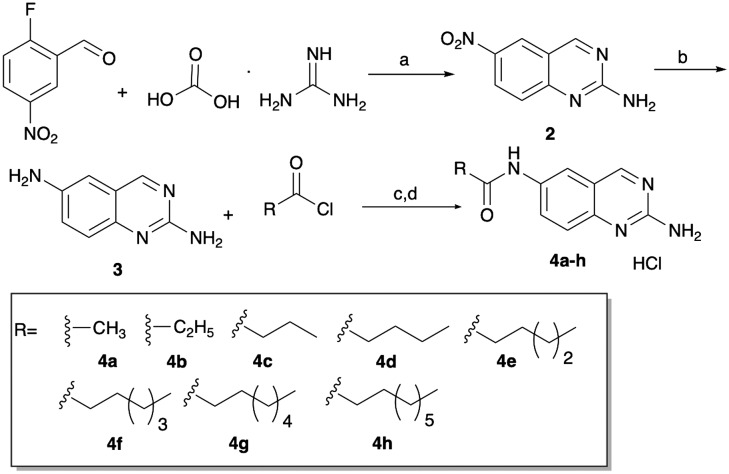
This study was initiated with the synthesis of 2,6-diaminoquinazoline 3 (Scheme 1), that we envisioned would subsequently be employed for selective acylation at N-6, to deliver the pilot library for screening. 2,6-Diaminoquinazoline 3 was synthesized by cyclizing commercially available 2-fluoro-5-nitrobenzaldehyde with guanidine carbonate salt to yield 6-nitro-2-aminoquinazoline 2, which, following hydrogenation, delivered 3.16 This intermediate was then acylated with alkyl acid chlorides (to initially mimic 1) and then was treated with HCl and methanol to generate the HCl salts 4a–h (Scheme 1). With these initial eight compounds in hand, we tested their ability to inhibit the formation of M. smegmatis biofilms (Table 1) using a crystal violet reporter assay.12 We noted a trend where activity correlated to alkyl chain length, as activity increased from methyl to pentyl, with activity then dropping off. Of these initial compounds, the pentyl derivative 4e was the most active, returning an IC50 of 23 μM.
Scheme 1. Synthetic route to afford 6 substituted 2-aminoquinazoline analogues. aK2CO3, ACN, molecular sieves, 72 °C, 16 h; bH2, 10% Pd/carbon, rt, 8 h; cK3PO4, dry THF, 0 °C to rt, 10 h; dHCl/MeOH.
Table 1. IC50 values of initial compounds 4a–h.
| IC50 (μM) | IC50 (μM) | ||
| 4a | 54 ± 4 | 4e | 23 ± 2 |
| 4b | 42 ± 3 | 4f | 49 ± 2 |
| 4c | 37 ± 3 | 4g | >100 |
| 4d | 26 ± 1 | 4h | >100 |
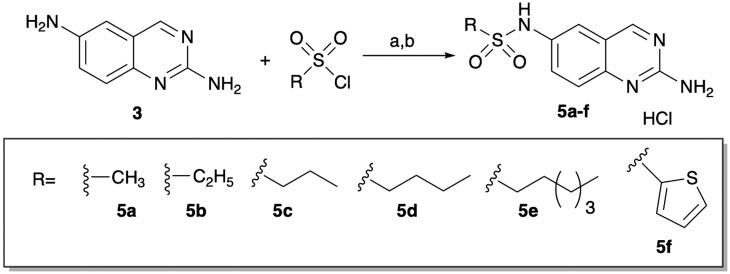
The necessity of the amide linker was probed by synthesizing a limited number of sulfonamide derivatives. These derivatives (5a–f) were synthesized from 2,6-diaminoquinazoline by reaction with the appropriate sulfonyl chloride, followed by generation of the HCl salt (Scheme 2). All these compounds were less active than the corresponding amide derivatives (Table 2).
Scheme 2. Synthesis of sulfonamides. aPyridine, DCM, rt, 10 h; bHCl/MeOH.
Table 2. IC50 values of sulfonamides 5a–f.
| IC50 (μM) | IC50 (μM) | ||
| 5a | >100 | 5d | 60 ± 3 |
| 5b | 34 ± 2 | 5e | >100 |
| 5c | 36 ± 1 | 5f | >100 |
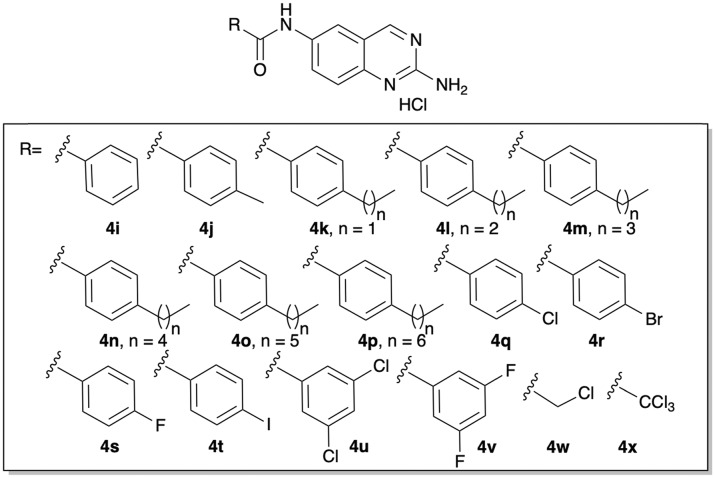
After establishing that the 2-AQ core motif was in fact a viable scaffold for the synthesis of anti-biofilm agents, we next turned to varying the identity of the alkyl chain. The first set of derivatives accessed, was a conservative change from alkyl to phenalkyl derivatives. Using the synthetic approach outlined in Scheme 1, we accessed derivatives 4i–p (Fig. 2). These compounds also demonstrated a dependence on chain length but were generally more active than our initial set of alkyl derivatives. The most active compound was the 4-propyl derivative 4l, which returned an IC50 of 16 μM (Table 3).
Fig. 2. Structure of phenalkyl and halide containing derivatives, 4i–x.
Table 3. IC50 values of phenalkyl derivatives 4i–p.
| IC50 (μM) | IC50 (μM) | ||
| 4i | 34 ± 3 | 4m | 39 ± 10 |
| 4j | 19 ± 3 | 4n | 39 ± 10 |
| 4k | 18 ± 4 | 4o | 65 ± 8 |
| 4l | 16 ± 4 | 4p | >100 |
Previous studies in our group on 2-AI derivatives have shown that replacing phenalkyl motifs with halogenated benzene motifs can deliver compounds with equipotent, if not more potent activity.17 Based on this precedent, we posited that the same general trend would be observed on the 2-AQ scaffold. The 4-chloro derivative 4q was initially synthesized to test the hypothesis, again using the approach outlined in Scheme 1. 4q was essentially equipotent to 4l and delivered an IC50 value of 15 μM. We then varied the identity of the halide and found that replacement of the chlorine with either fluorine (4s) or iodine (4t) abolished activity, while activity was diminished by substitution with bromine (4r), which returned an IC50 value of 44 μM. Di-substituted derivatives, 4u and 4v, were active, albeit less than 4q, and returned IC50 values of 42 and 46 μM respectively. Finally, we tested whether chlorination on the alkyl derivatives would generate compounds with equivalent or improved activity by synthesizing and testing compounds 4w and 4x. Both compounds were slightly more active than the corresponding methyl derivative 4a, with IC50 values of 43 and 50 μM respectively (Table 4).
Table 4. IC50 values of halide containing derivatives 4q–x.
| IC50 (μM) | IC50 (μM) | ||
| 4q | 15 ± 1 | 4u | 42 ± 4 |
| 4r | 44 ± 6 | 4v | 46 ± 3 |
| 4s | >200 | 4w | 43 ± 2 |
| 4t | >200 | 4x | 50 ± 2 |
Finally, we tested the toxicity of lead 2-AQ derivatives against both planktonic M. smegmatis cells as well as eukaryotic red blood cells (a standard test for antibiotic toxicity). M. smegmatis planktonic toxicity was quantified to determine if biofilm inhibition was occurring through an antibiotic/microbicidal mechanism or through a non-toxic mechanism and was established by comparing the growth curves of bacteria grown in the absence and presence of compound. The growth curves of bacteria treated with either 4l or 4q at 60 μM indicate that biofilm inhibition occurs through a non-toxic mechanism (Fig. S1†). Red blood cell toxicity was measured by quantifying hemolysis of lead compounds 4l and 4q as well as inactive compound 4p. At 500 μM (highest concentration tested), 4l, 4p and 4q caused 25%, 57%, and 24% lysis respectively compared to 1% Triton-X. At 200 μM, 4l, 4p and 4q effected 14%, 31% and 4% lysis respectively. Both active compounds, 4l and 4q, return a hemolytic index (defined here as concentration that causes hemolysis/IC50) of >33, indicating their relative non-toxic nature.
In conclusion, we have established for the first time that the 2-AQ heterocycle can form the basis for the construction of anti-biofilm molecules. In a proof of concept study, we have established that lead compounds 4l and 4q are low micromolar inhibitors of M. smegmatis biofilm formation, making them some of the most potent compounds reported for this application. These compounds modulate biofilms in a non-toxic manner and possess a relatively low hemolytic index. Given these properties, the 2-AQ heterocycle may form the basis for future in vivo experiments to study the impact inhibition of biofilm formation has upon TB treatment.
This work was funded by the National Institutes of Health (AI106733).
We purchased the red blood cells from Hemostat laboratories https://hemostat.com/
Conflicts of interest
Dr. Melander is co-founder of Agile Sciences, a biotechnology company seeking to commercialize small molecules with anti-biofilm activity.
Supplementary Material
Footnotes
†Electronic supplementary information (ESI) available: Experimental procedures and characterization data. See DOI: 10.1039/c9md00156e
References
- Mah T. F., O'Toole G. A. Trends Microbiol. 2001;9(1):34–39. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- Worthington R. J., Melander C. Anti-Infect. Agents. 2014;12(1):120–138. [Google Scholar]
- Lewis K. Antimicrob. Agents Chemother. 2001;45(4):999–1007. doi: 10.1128/AAC.45.4.999-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlan R. M., Costerton J. W. Clin. Microbiol. Rev. 2002;15(2):167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook L., Tuberculosis in Priority Medicines for Europe and the World “A Public Health Approach to Innovation”, World Health Organization, Geneva, Switzerland2013.
- World Health Organization and Global Tuberculosis Programme, Global tuberculosis control, WHO report. Global Tuberculosis Programme, Geneva, Switzerland, 2017, p. 15.
- Horsburgh Jr. C. R., Barry 3rd C. E., Lange C. N. Engl. J. Med. 2015;373(22):2149–2160. doi: 10.1056/NEJMra1413919. [DOI] [PubMed] [Google Scholar]
- Ackart D. F., Lindsey E. A., Podell B. K., Melander R. J., Basaraba R. J., Melander C. Pathog. Dis. 2014;70(3):370–378. doi: 10.1111/2049-632X.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulka K., Hatfull G., Ojha A. K. J. Visualized Exp. 2012;(60):3820. doi: 10.3791/3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M. Tuberculosis. 2014;94:11. doi: 10.1016/j.tube.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altaf M., Miller C. H., Bellows D. S., O'Toole R. Tuberculosis. 2010;90(6):333–337. doi: 10.1016/j.tube.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Lindsey E. A., Worthington R. J., Alcaraz C., Melander C. Org. Biomol. Chem. 2012;10(13):2552–2561. doi: 10.1039/c2ob06871k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey E. A., Brackett C. M., Mullikin T., Alcaraz C., Melander C. MedChemComm. 2012;3(11):1462–1465. doi: 10.1039/C2MD20244A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvam T. P., Kumar P. V. Res. Pharm. 2011;1:1. [Google Scholar]
- Shagufta, Ahmad I. MedChemComm. 2017;8:871. doi: 10.1039/c7md00097a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. M., Zhang M., Xiong B., Tan Z. D., Lv W., Jiang H. F. Org. Lett. 2014;16(22):6028–6031. doi: 10.1021/ol503052s. [DOI] [PubMed] [Google Scholar]
- Brackett C. M., Melander R. J., An I. H., Krishnamurthy A., Thompson R. J., Cavanagh J., Melander C. J. Med. Chem. 2014;57:7450. doi: 10.1021/jm501050e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.