 The synthesis and biological evaluation of two phomopsolide natural products (D and E) and two analogues is presented.
The synthesis and biological evaluation of two phomopsolide natural products (D and E) and two analogues is presented.
Abstract
The synthesis of two stable phomopsolide natural products (D and E) and two analogues is presented. The cytotoxicities of these four compounds are surveyed and compared across a panel of NCI-cancer cell lines. This analysis found moderate cytotoxicities (2–50 μM) for the majority of the cell lines with phomopsolide D being more active than phomopsolide E and the 7-oxa analogue being commensurately more active than the 7-aza analogue.
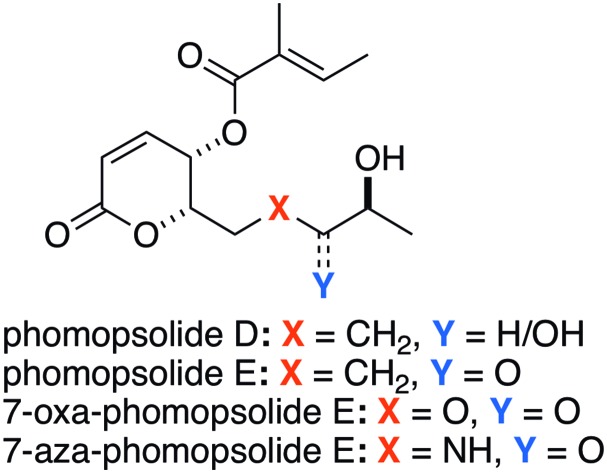
The phomopsolide class of polyketide natural products is made up of five 6-substituted 5,6-dihydro-5-hydroxypyran-2-ones that are all acylated with a tiglate ester group at the 5-position (Fig. 1).1,2 The structural variation in this class of natural products exists in the n-pentyl side chain at the 6-position (Fig. 1). The structural range of the known phomopsolides A–E consists of having or not having a C-6/7 alkene in the E- or Z-configuration and oxidation in either the alcohol or ketone stage at C-8. All known phomopsolides have an (S)-configured alcohol group at the C-9 position. The initial discovery of the phomopsolides by Grove1 and Stierle2 was a result of a program aimed at finding antiboring/antifeeding natural products that would prevent the elm bark beetle (Hylurgopinus rufipes) from spreading the fungus Ophiostoma ulmi associated with Dutch elm disease.3,4 The five phomopsolides were also found to exhibit antimicrobial activity (e.g., S. aureus).5
Fig. 1. The phomopsolide natural products (A–E).
As a result of their unique structure and biological activity, phomopsolides have garnered a fair amount of attention from the synthetic community. This interest has resulted in a total of seven successful syntheses of phomopsolide natural products. In addition, there have been several syntheses of related analogues. The first synthesis was done by Noshita in 1994 obtaining phomopsolide B.6 Synthetic interest in the phomopsolides returned in the following century, with our syntheses of phomopsolide C and phomopsolide D, in 2002 and 2004, respectively.7,8 This was followed up the following year with a synthesis of phomopsolide C by Blechert.9 Over the subsequent decade, other reports appeared detailing the syntheses of phomopsolide C, in particular, those by Prasad10 and Yadav,11 as well as the synthesis of the C-4,5-bis-epimer of phomopsolide by Atmakur.12 Most recently, we reported a synthesis of phomopsolide E, which built upon our de novo approach to phomopsolide D.13 Over this same time period, we have also investigated the synthesis of phomopsolide stereoisomers and congeners, such as 7-oxa-phomopsolide E,14 7-aza-phomopsolide E15 and related C-4/C-5 stereoisomers.7,12–14
Over the years, we have developed various de novo asymmetric approaches to polyketide natural products,16 with an emphasis on those that contain pyranone rings.17 Our interest in the phomopsolides came as part of a larger ongoing effort to study the structure activity relationship (SAR) of natural products, with a particular focus on stereochemistry (S-SAR). These SAR/S-SAR studies have covered a wide range of structural motifs (e.g., polyketides,18 as well as a range of carbohydrates19) and biological activities (e.g., anticancer, antibacterial, antiviral and related protein targets). In this regard, our efforts towards the phomopsolides are part of a long-term effort aimed at providing materials for systematic study of their SAR. These practical realities caused us to focus on phomopsolides D and E, due to an instability we found with the C-6/7 alkene. Herein we give the full account of our efficient syntheses of two phomopsolide natural products (D and E) and two analogue natural products (7-oxa and 7-aza) (Fig. 2). In addition, we detail, for the first time, our use of a comprehensive cancer cell cytotoxicity screen to comparatively evaluate the validity of the 7-oxa- and 7-aza-substitutions.
Fig. 2. Phomopsolides D and E and their congeners.

The synthesis of phomopsolides D and E that we finally settled on is outlined in (Scheme 1). The route is a de novo asymmetric route, as it derives the absolute and relative stereochemistry in two catalytic asymmetric reactions. The first being a Sharpless dihydroxylation of diene 5 and the second being a reagent controlled Noyori reduction of acylfuran 6. Specifically, a two-step oxidation/protection process converted dienone 5 into acylfuran 6, which can be diastereoselectively reduced to form furfuryl alcohol 7 with excellent stereocontrol and yield. The Achmatowicz oxidation/hydration rearrangement was used to convert furfuryl alcohol 7 into 8-hydroxypyranone 8. A subsequent two-step Jones oxidation and Luche reduction reaction sequence was used to convert 8 into a 2 : 1 mixture of pyranones 9a and 9b (75%). A combination of retentive (tiglic acid/DCC, 82%) and invertive (tiglic acid/PPh3/DEAD) ester formations converted the mixture of diastereomers into an acetonide protected natural product, which was easily deprotected to form phomopsolide D (HCl(aq), 95%). Then in a three-step sequence, phomopsolide D was selectively oxidized to give phomopsolide E. This began with the selective protection of the diol at the C-8 position with a TBDPS group.20 Jones oxidation was then used to oxidize the resulting alcohol into a ketone, which could be deprotected with HF/Py to give the natural product phomopsolide E (2) (62% yield in 3 steps).
Scheme 1. De novo syntheses toward phomopsolide D (1) and phomopsolide E (2).
We next turned to the synthesis of the 7-oxa- and 7-aza-phomopsolide analogues 3 and 4 (Scheme 2). The synthesis began with suitably protected oxa- and aza-substituted acylfurans 11a and 11b, with TBS and Boc protecting groups, respectively. Both acylfurans 11a and 11b were asymmetrically reduced under the Noyori conditions, oxidatively rearranged under the Achmatowicz conditions and oxidized with the Jones reagent to give enediones 12a and 12b. Luche reduction of the enediones 12a and 12b gave a mixture of diastereomers, which were separated and acylated in a retentive (tiglic acid/DCC) to give esters 13a and 13b. Finally, a three step deprotection, acylation and deprotection sequence on 13a and 13b gave the two desired analogue targets, 7-oxa-phomopsolide E (3) and 7-aza-phomopsolide E (4).
Scheme 2. Syntheses of 7-oxa-phomopsolide E (3) and 7-aza-phomopsolide E (4).
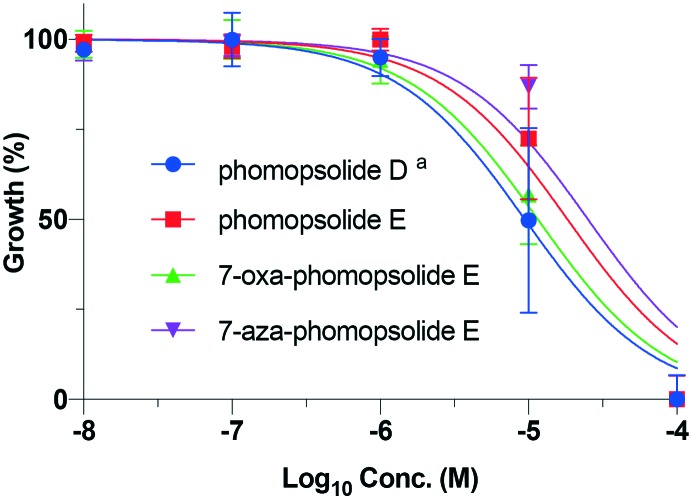
With the synthesis of the four phomopsolides (1–4) in hand, we next turned to their evaluation for cytotoxicity against the NCI 60 cell line panel from 10 μM to 100 μM.21,22 The cell lines used are maintained by the Developmental Therapeutics Program at the National Cancer Institute (for information regarding the cell lines, see: ; https://dtp.cancer.gov).23 In general, the compounds (1–4) demonstrated good cytotoxicity against the whole class of cell lines with IC50 ranging from a low value of 2 μM to a high value of 49 μM. The average data for the four phomopsolides across the full 60 cell line panel are plotted in Fig. 3 and outlined in Table 1. Not all the IC50 S-curves suitably flattened out at 10 μM, which negatively affected the accuracy of the IC50 calculations. As a result, the data from these experiments were excluded from this analysis. This phenomenon is more likely to occur for less cytotoxic compounds, such as 7-aza-phomopsolide E (4), for which only 38 of the 60 cell lines gave data that could be included (Fig. 3/Table 1). The other, more active, phomopsolides (1–3) had interpretable data giving IC50 values in 53, 57 and 58 of the 60 cell lines tested, respectively.
Fig. 3. Average phomopsolide (1–4) cytotoxicity curves against the NCI cell lines. aData for 53 out of the 60 cell lines are presented; bdata for 57 out of the 60 cell lines are presented; cdata for 58 out of the 60 cell lines are presented; ddata for 38 out of the 60 cell lines are presented.
Table 1. IC50 for the NCI cell lines.
| Compound | Average IC50 | IC50 range |
| Phomopsolide D a | 14 μM | ±14 μM |
| Phomopsolide E b | 23 μM | ±16 μM |
| 7-Oxa-phomopsolide E c | 15 μM | ±10 μM |
| 7-Aza-phomopsolide E d | 28 μM | ±18 μM |
aData for 53 out of the 60 cell lines are presented.
bData for 57 out of the 60 cell lines are presented.
cData for 58 out of the 60 cell lines are presented.
dData for 38 out of the 60 cell lines are presented.
Some general observations can be drawn from the panel-cell line average data, such as: 1) phomopsolide D (IC50 = 14 μM) was in general more active than phomopsolide E (IC50 = 23 μM); 2) phomopsolide D and 7-oxa-phomopsolide E (IC50 = 15 μM) were the most similar in terms of cytotoxicity and the most cytotoxic; whereas, 3) 7-aza phomopsolide E (IC50 = 28 μM) exhibited the least cytotoxicity and was the most similar to phomopsolide E. This analysis, of course, is limited by the fact that there is a significant variation across the panel of 60 cell lines, as evidenced by the large and overlapping ranges of IC50 values for compounds 1–4. To try to control for these variations, we looked at the average IC50 values of compounds 1–4 for the nine different tissue types in the panel (cf., Fig. 4–12/Tables 2–10). This analysis more precisely shows (less overlapping ranges of IC50) the same overall trend of compounds 1 and 3 being more similar and active and compounds 2 and 4 being more similar and less active.
Fig. 4. Average phomopsolide (1–4) cytotoxicity curves against melanoma cancer cell lines (LOX IMVI, MALME-3M, M14, SK-MEL-2, SK-MEL-28, SK-MEL-5, UACC-257, and UACC-62) against the log10 concentration of the compound introduced to the cells. aData for 7 out of the 8 cell lines are presented.
Fig. 5. Average phomopsolide (1–4) cytotoxicity curves against CNS cancer cell lines (SF-268, SF-295, SF-539, SNB-19, SNB-75, and U251), excluding outliers, against the log10 concentration of the compound introduced to the cells. aData for 5 out of the 6 cell lines are presented. bData for 4 out of the 6 cell lines are presented.
Fig. 6. Average phomopsolide (1–4) cytotoxicity curves against breast cancer cell lines (MCF7, NCI/ADR-RES, MDA-MB-231/ATCC, HS 578T, MDA-MB-435, and T-47D) against the log10 concentration of the compound introduced to the cells. aData for 3 out of the 4 cell lines are presented.
Fig. 7. Average phomopsolide (1–4) cytotoxicity curves against prostate cancer cell lines (PC-3 and DU-145) against the log10 concentration of the compound introduced to the cells.
Fig. 8. Average phomopsolide (1–4) cytotoxicity curves against renal cancer cell lines (786-0, A498, ACHN, CAKI-1, RXF 393, SN12C, TK-10 and UO-31) against the log10 concentration of the compound introduced to the cells. aData for 7 out of the 8 cell lines are presented.
Fig. 9. Average phomopsolide (1–4) cytotoxicity curves against ovarian cancer cell lines (IGROV1, OVCAR-3, OVCAR-4, OVCAR-5, OVCAR-8 and SK-OV-3) against the log10 concentration of the compound introduced to the cells. aData for 4 out of the 6 cell lines are presented.
Fig. 10. Average phomopsolide (1–4) cytotoxicity curves against leukemia cancer cell lines (CCRF-CEM, HL-60(TB), K-562, MOLT-4, and RPMI-8226) against the log10 concentration of the compound introduced to the cells. aData for 2 out of the 5 cell lines are presented. bData for 4 out of the 5 cell lines are presented.
Fig. 11. Average phomopsolide (1–4) cytotoxicity curves against non-small cell lung cancer cell lines (A549/ATCC, EKVX, HOP-62, NCI-H226, NCI-H23, NCI-H322M, NCI-H460, and NCI-H522) against the log10 concentration of the compound introduced to the cells.
Fig. 12. Average phomopsolide (1–4) cytotoxicity curves against colon cancer cell lines (COLO 205, HCC-2998, HCT-116, HCT-15, HT29, KM12, and SW-620) against the log10 concentration of the compound introduced to the cells. aData for 6 out of the 7 cell lines are presented.
Table 2. IC50 for the melanoma cancer cell lines.
| Compound | Average IC50 | IC50 range |
| Phomopsolide D a | 15 μM | ±4 μM |
| Phomopsolide E | 24 μM | ±7 μM |
| 7-Oxa-phomopsolide E | 13 μM | ±3 μM |
| 7-Aza-phomopsolide E | 28 μM | ±9 μM |
aData for 7 out of the 8 cell lines are presented.
Table 3. IC50 for the CNS cancer cell lines.
| Compound | Average IC50 | IC50 range |
| Phomopsolide D | 17 μM | ±5 μM |
| Phomopsolide E a | 26 μM | ±11 μM |
| 7-Oxa-phomopsolide E | 16 μM | ±5 μM |
| 7-Aza-phomopsolide E b | 29 μM | ±15 μM |
aData for 5 out of the 6 cell lines are presented.
bData for 4 out of the 6 cell lines are presented.
Table 4. IC50 for the breast cancer cell lines.
| Compound | Average IC50 | IC50 range |
| Phomopsolide D | 11 μM | ±3 μM |
| Phomopsolide E | 20 μM | ±6 μM |
| 7-Oxa-phomopsolide E | 12 μM | ±2 μM |
| 7-Aza-phomopsolide E a | 26 μM | ±10 μM |
aData for 3 out of the 4 cell lines are presented.
Table 5. IC50 for the prostate cancer cell lines.
| Compound | Average IC50 | IC50 range |
| Phomopsolide D | 18 μM | ±9 μM |
| Phomopsolide E | 23 μM | ±15 μM |
| 7-Oxa-phomopsolide E | 16 μM | ±7 μM |
| 7-Aza-phomopsolide E | 25 μM | ±18 μM |
Table 6. IC50 for the renal cancer cell lines.
| Compound | Average IC50 | IC50 range |
| Phomopsolide D a | 13 μM | ±4 μM |
| Phomopsolide E a | 25 μM | ±8 μM |
| 7-Oxa-phomopsolide E | 15 μM | ±4 μM |
| 7-Aza-phomopsolide E | 30 μM | ±11 μM |
aData for 7 out of the 8 cell lines are presented.
Table 7. IC50 for the ovarian cancer cell lines.
| Compound | Average IC50 | IC50 range |
| Phomopsolide D | 18 μM | ±6 μM |
| Phomopsolide E a | 24 μM | ±10 μM |
| 7-Oxa-phomopsolide E | 16 μM | ±6 μM |
| 7-Aza-phomopsolide E a | 28 μM | ±14 μM |
aData for 4 out of the 6 cell lines are presented.
Table 8. IC50 for the leukemia cancer cell lines.
| Compound | IC50 (μM) | IC50 range |
| Phomopsolide D a | 4 μM | ±3 μM |
| Phomopsolide E b | 16 μM | ±9 μM |
| 7-Oxa-phomopsolide E a | 8 μM | ±5 μM |
| 7-Aza-phomopsolide E b | 30 μM | ±14 μM |
aData for 2 out of the 5 cell lines are presented.
bData for 4 out of the 5 cell lines are presented.
Table 9. IC50 for the non-small cell lung cancer cell lines.
| Compound | Average IC50 | IC50 range |
| Phomopsolide D | 18 μM | ±6 μM |
| Phomopsolide E | 26 μM | ±8 μM |
| 7-Oxa-phomopsolide E | 19 μM | ±5 μM |
| 7-Aza-phomopsolide E | 27 μM | ±9 μM |
Table 10. IC50 for the colon cancer cell lines.
| Compound | Average IC50 | IC50 range |
| Phomopsolide D a | 9 μM | ±3 μM |
| Phomopsolide E | 18 μM | ±5 μM |
| 7-Oxa-phomopsolide E | 11 μM | ±3 μM |
| 7-Aza-phomopsolide E | 25 μM | ±8 μM |
aData for 6 out of the 7 cell lines are presented.
When the cytotoxicities for the phomopsolides (1–4) were plotted for only the eight melanoma cell lines (i.e., LOX IMVI, MALME-3M, M14, SK-MEL-2, SK-MEL-28, SK-MEL-5, UACC-257, and UACC-62), very similar activities and trends were seen (Fig. 4/Table 2), albeit with a smaller non-overlapping variation. The trends for the average data against the melanoma cell lines showed very similar trends, such as: 1) phomopsolide D (IC50 = 15 μM) and 7-oxa-phomopsolide E (IC50 = 13 μM) were the most active and exhibited very similar activities; whereas, 2) phomopsolide E (IC50 = 24 μM) and 7-aza-phomopsolide E (IC50 = 28 μM) were the least active and similar in terms of cytotoxicity.
When the cytotoxicities of the phomopsolides (1–4) are plotted against the six CNS cancer cell lines (i.e., SF-268, SF-295, SF-539, SNB-19, SNB-75, and U251), the average activities and relative trends were similar (Fig. 5/Table 3) with only slightly overlapping IC50 ranges, such as: 1) phomopsolide D (IC50 = 17 μM) and 7-oxa-phomopsolide E (IC50 = 16 μM) were the most active and exhibited very similar activities; whereas, 2) phomopsolide E (IC50 = 26 μM) and 7-aza-phomopsolide E (IC50 = 29 μM) were the least active and similar in terms of cytotoxicity.
When the cytotoxicities of the phomopsolides (1–4) are plotted against the six breast cancer cell lines (i.e., MCF7, NCI/ADR-RES, MDA-MB-231/ATCC, HS 578T, MDA-MB-435, and T-47D), the average activities and relative trends were similar with only slightly overlapping IC50 ranges (Fig. 6/Table 4), such as: 1) phomopsolide D (IC50 = 11 μM) and 7-oxa-phomopsolide E (IC50 = 12 μM) were the most active and exhibited very similar activities; whereas, 2) phomopsolide E (IC50 = 20 μM) and 7-aza-phomopsolide E (IC50 = 26 μM) were the least active and similar in terms of cytotoxicity.
When the cytotoxicities of the phomopsolides (1–4) are plotted against the two prostate cancer cell lines (i.e., PC-3 and DU-145), the average activities and relative trends were similar, however with much more overlapping IC50 values (Fig. 7/Table 5). Once again, the greatest cytotoxicities were seen for phomopsolide D (IC50 = 18 μM) and 7-oxa-phomopsolide E (IC50 = 16 μM); whereas, 2) phomopsolide E (IC50 = 23 μM) and 7-aza-phomopsolide E (IC50 = 25 μM) were the least active and similar in terms of cytotoxicity.
When the cytotoxicities of the phomopsolides (1–4) are plotted against the eight renal cancer cell lines (i.e., 786-0, A498, ACHN, CAKI-1, RXF 393, SN12C, TK-10 and UO-31), the average activities and relative trends were similar with only slightly overlapping IC50 ranges (Fig. 8/Table 6), such as: 1) phomopsolide D (IC50 = 13 μM) and 7-oxa-phomopsolide E (IC50 = 15 μM) were the most active and exhibited very similar activities; whereas, 2) phomopsolide E (IC50 = 25 μM) and 7-aza-phomopsolide E (IC50 = 30 μM) were the least active and similar in terms of cytotoxicity.
When the cytotoxicities of the phomopsolides (1–4) are plotted against the six ovarian cancer cell lines (i.e., IGROV1, OVCAR-3, OVCAR-4, OVCAR-5, OVCAR-8 and SK-OV-3), the average activities and relative trends were similar, albeit with overlapping IC50 ranges (Fig. 9/Table 7). Phomopsolide D (IC50 = 18 μM) and 7-oxa-phomopsolide E (IC50 = 16 μM) were again the most active and phomopsolide E (IC50 = 24 μM) and 7-aza-phomopsolide E (IC50 = 28 μM) were similar in terms of cytotoxicity and again the least active.
Of the NCI cancer cell lines, the leukemia cell lines showed the greatest sensitivity to the most active phomopsolide, compound 1, with an IC50 of 4 μM. When the cytotoxicities of the phomopsolides (1–4) are plotted against the five leukemia cancer cell lines (i.e., CCRF-CEM, HL-60(TB), K-562, MOLT-4, and RPMI-8226), the average activities and relative trends were similar, with only slightly overlapping ranges of IC50 (Fig. 10/Table 8). For instance, phomopsolide D (IC50 = 4 μM) and 7-oxa-phomopsolide E (IC50 = 8 μM) were the most active and very similar in activity; whereas, 2) phomopsolide E (IC50 = 16 μM) and 7-aza-phomopsolide E (IC50 = 30 μM) were similar in activity and the least cytotoxic.
When the cytotoxicities of the phomopsolides (1–4) are plotted against the eight non-small cell lung cancer cell lines (i.e., A549/ATCC, EKVX, HOP-62, NCI-H226, NCI-H23, NCI-H322M, NCI-H460, and NCI-H522), the average activities and relative trends were similar with only slightly overlapping IC50 ranges, (Fig. 11/Table 9), such as: 1) phomopsolide D (IC50 = 18 μM) and 7-oxa-phomopsolide E (IC50 = 19 μM) were the most active and exhibited very similar activities; whereas, 2) phomopsolide E (IC50 = 26 μM) and 7-aza-phomopsolide E (IC50 = 27 μM) were the least active and similar in terms of cytotoxicity.
Finally, when the cytotoxicities of the phomopsolides (1–4) are plotted against the seven colon cancer cell lines (i.e. COLO 205, HCC-2998, HCT-116, HCT-15, HT29, KM12, and SW-620), the average activities and relative trends were similar and the range of cytotoxicities was small (Fig. 12/Table 10). Phomopsolide D (IC50 = 9 μM) and 7-oxa-phomopsolide E (IC50 = 11 μM) were again the most active and very similar in activity. Once again, phomopsolide E (IC50 = 18 μM) and 7-aza-phomopsolide E (IC50 = 25 μM) were the least active and similar in terms of cytotoxicity.
Conclusions
In conclusion, the synthesis of two phomopsolide natural products and two related analogues was achieved, which were subsequently analyzed for cytotoxicity across a panel of NCI-cancer cell lines. The data suggest that there is comparable biological activity in all four phomopsolides. Phomopsolide D and 7-oxo-phomopsolide E present the highest cytotoxicities with nearly identical activities in most of the cancer cell types, with phomopsolide E and 7-aza-phomopsolide E being consistently less active.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the National Science Foundation (CHE-1565788) and the National Institutes of Health (AI144196-01 and AI142040) for their support of this work. AZA would like to thank the King Abdullah Scholarship Program for a fellowship. SAF would like to thank the Northeastern University Office of Undergraduate Research and Fellowships for the Undergraduate Research and Creative Endeavor Award.
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c9md00121b
References
- Grove J. F. J. Chem. Soc., Perkin Trans. 1. 1985;1:865–869. [Google Scholar]
- Stierle D. B., Stierle A. A., Ganser B. J. Nat. Prod. 1997;60:1207–1209. doi: 10.1021/np970338f. [DOI] [PubMed] [Google Scholar]
- Becker H. Agric. Res. 1996;44:4–8. [Google Scholar]
- Santamour F. S., Bentz S. E. J. Arboric. 1995;21:122–131. [Google Scholar]
- Noshita T., Sugiyama T., Yamashita K., Oritani T. Biosci., Biotechnol., Biochem. 1994;58:740. doi: 10.1271/bbb.59.1921. [DOI] [PubMed] [Google Scholar]
- Noshita T., Sugiyama T., Yamashita K., Oritani T. Biosci., Biotechnol., Biochem. 1994;58:740–744. [Google Scholar]
- Harris J. M., O'Doherty G. A. Tetrahedron Lett. 2002;43:8195–8199. [Google Scholar]
- Li M., O'Doherty G. A. Tetrahedron Lett. 2004;45:6407–6411. [Google Scholar]
- Michaelis S., Blechert S. Org. Lett. 2005;7:5513–5516. doi: 10.1021/ol052332k. [DOI] [PubMed] [Google Scholar]
- Prasad K. R., Gutala P. Tetrahedron. 2012;68:7489–7493. [Google Scholar]
- Reddy D. V., Sabitha G., Yadav J. S. Tetrahedron Lett. 2015;56:4112–4114. [Google Scholar]
- Emmadi N. R., Bingi C., Kumar C. G., Yedla P., Atmakur K. Synthesis. 2014;46:2945–2950. [Google Scholar]
- Harris J. M., Li M., O'Doherty G. A., Heterocycles, 2019, 9910.3987/COM-18-S(F)96 , , ASAP . [Google Scholar]
- Li M., Scott J. G., O'Doherty G. A. Tetrahedron Lett. 2004;45:1005–1009. [Google Scholar]
- Aljahdali A. Z., Freedman S. A., Li M., O'Doherty G. A. Tetrahedron. 2018;74:7121–7126. [Google Scholar]
- (a) Goins C. M., Sudasinghe T. D., Liu X., Wang Y., O'Doherty G. A., Ronning D. R. Biochemistry. 2018;57:2383. doi: 10.1021/acs.biochem.8b00152. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Liu X., Wang Y., Duclos R. I., O'Doherty G. A. ACS Med. Chem. Lett. 2018;9:274. doi: 10.1021/acsmedchemlett.8b00050. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Liu X., Wang Y., O'Doherty G. A. Asian J. Org. Chem. 2015;4:994. [Google Scholar]; (d) Mulzer M., Tiegs B., Wang Y., Coates G. W., O'Doherty G. A. J. Am. Chem. Soc. 2014;136:10814. doi: 10.1021/ja505639u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Hunter T. J., Wang Y., Zheng J., O'Doherty G. A. Synthesis. 2016;48:1700. [Google Scholar]; (b) Cuccarese M. F., Wang Y., Beuning P. J., O'Doherty G. A. ACS Med. Chem. Lett. 2014;5:522. doi: 10.1021/ml4005039. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wang Y., O'Doherty G. A. J. Am. Chem. Soc. 2013;135:9334. doi: 10.1021/ja404401f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Hunter T. J., O'Doherty G. A. Org. Lett. 2001;3:2777. doi: 10.1021/ol016399t. [DOI] [PubMed] [Google Scholar]; (e) Smith C. M., O'Doherty G. A. Org. Lett. 2003;5:1959. doi: 10.1021/ol0345529. [DOI] [PubMed] [Google Scholar]
- (a) Harris J. M., O'Doherty G. A. Org. Lett. 2000;2:2983. doi: 10.1021/ol000179i. [DOI] [PubMed] [Google Scholar]; (b) Harris J. M., O'Doherty G. A. Tetrahedron. 2000;57:5161. [Google Scholar]
- (a) Li M., Li Y., Ludwik K. A., Sandusky Z. M., Lannigan D. A., O'Doherty G. A. Org. Lett. 2017;19:2410–2413. doi: 10.1021/acs.orglett.7b00945. [DOI] [PubMed] [Google Scholar]; (b) Ludwik K. A., Campbell J. P., Li M., Li L., Sandusky Z. M., Pasic L., Sowder M. E., Brenin D. R., Pietenpol J. A., O'Doherty G. A., Lannigan D. A. Mol. Cancer Ther. 2016;15:2598–2608. doi: 10.1158/1535-7163.MCT-16-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Bajaj S. O., Shi P., Beuning P. J., O'Doherty G. A. Med. Chem. Commun. 2014;5:1138–1142. doi: 10.1039/C4MD00095A. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Cai H., Wang H.-Y. L., Venkatadri R., Fu D.-X., Forman M., Bajaj S. O., Li H., O'Doherty G. A., Arav-Boger R. ACS Med. Chem. Lett. 2014;5:395–399. doi: 10.1021/ml400529q. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Hinds J. W., McKenna S. B., Sharif E. U., Wang H.-Y. L., Akhmedov N. G., O'Doherty G. A. ChemMedChem. 2013;8:63–69. doi: 10.1002/cmdc.201200465. [DOI] [PubMed] [Google Scholar]; (f) Shi P., Silva M., Wu B., Wang H.-Y. L., Akhmedov N. G., Li M., Beuning P., O'Doherty G. A. ACS Med. Chem. Lett. 2012;3:1086–1090. doi: 10.1021/ml300303g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrocchi-Fantoni G., Servi S. J. Chem. Res., Synop. 1986;6:199. [Google Scholar]
- (a) Alley M. C., Scudiero D. A., Monks P. A., Hursey M. L., Czerwinski M. J., Fine D. L., Abbott B. J., Mayo J. G., Shoemaker R. H., Boyd M. R. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]; (b) Grever M. R., Schepartz S. A., Chabner B. A. Semin. Oncol. 1992;19:622–638. [PubMed] [Google Scholar]; (c) Boyd M. R., Paull K. D. Drug Dev. Res. 1995;34:91–109. [Google Scholar]; (d) Shoemaker R. H. Nat. Rev. Cancer. 2006;6:813–823. doi: 10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]
- (a) Yu X., O'Doherty G. A. Org. Lett. 2008;10:4529–4532. doi: 10.1021/ol801817f. [DOI] [PubMed] [Google Scholar]; (b) Iyer A., Zhou M., Azad N., Elbaz H., Wang L., Rogalsky D. K., Rojanasakul Y., O'Doherty G. A., Langenhan J. M. ACS Med. Chem. Lett. 2010;1:326–330. doi: 10.1021/ml1000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker R. H. Nat. Rev. Cancer. 2006;6:813–823. doi: 10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.