Abstract
Glucocorticoids are standard of care for many chronic inflammatory conditions, including juvenile dermatomyositis (JDM) and anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV). We sought to define pharmacodynamic biomarkers of therapeutic efficacy and safety concerns of glucocorticoid treatment for these two disorders. Previous proteomic profiling of patients with Duchenne muscular dystrophy (DMD) and inflammatory bowel disease (IBD) treated with glucocorticoids identified candidate biomarkers for efficacy and safety concerns of glucocorticoids. Serial serum samples from patients with AAV (n = 30) and JDM (n = 12) were obtained during active disease, and after treatment with glucocorticoids. For AAV, 8 of 11 biomarkers of the anti-inflammatory response to glucocorticoids were validated (P-value ≤0.05; CD23, macrophage-derived cytokine, interleukin-22 binding protein, matrix metalloproteinase-12, T lymphocyte surface antigen Ly9, fibrinogen gamma chain, angiopoietin-2 [all decreased], and protein C [increased]), as were 5 of 7 safety biomarkers (P-value ≤0.05; afamin, matrix metalloproteinase-3, insulin growth factor binding protein-5, angiotensinogen, leptin [all increased]). For JDM, 10 of 11 efficacy biomarkers were validated (P-value ≤0.05; all proteins except fibrinogen gamma chain) and 6 of 7 safety biomarkers (P-value ≤0.05; AAV proteins plus growth hormone binding protein). The identified efficacy biomarkers may be useful as objective outcome measures for early phase proof-of-concept studies when assessing novel anti-inflammatory drugs in JDM and AAV, and likely in other inflammatory disorders. Similarly, safety biomarkers may also be helpful assessing toxicity of alternatives to glucocorticoids.
Keywords: Glucocorticoids, Biomarker, Juvenile dermatomyositis, Vasculitis, Anti-inflammatory
1. Introduction
A pharmacodynamic biomarker is one that reflects a drug’s pharmacological actions and the subsequent physiologic consequences. Some pharmacodynamic biomarkers are tissue- and disease-specific, while others may be more specific to the mechanism of action of the therapy. Glucocorticoids have similar pharmacological actions and physiologic consequences when used to treat inflammatory diseases. We hypothesized that we could identify peripheral blood-based biomarkers specific for glucocorticoid action and effect that were not reflective of downstream, disease-specific processes. The objective of this study was to validate previously-described glucocorticoid-responsive pharmacodynamic biomarkers in both patients with anti-neutrophil antibody-associated vasculitis (AAV) and patients with juvenile dermatomyositis (JDM). If meaningful and reproducible serum biomarkers specific for the anti-inflammatory efficacy of glucocorticoids were identified, then these could be used in proof-of-concept studies of alternative, glucocorticoid-sparing therapies. For example, to assess the potential for a new or repositioned anti-inflammatory drug in early phase trials in AAV or JDM, pharmacodynamic biomarkers for anti-inflammatory effect could be monitored as an objective outcome measure demonstrating proof-of-concept of efficacy. Pharmacodynamic biomarkers could also be correlated with exposure, thereby aiding in dose selection or extrapolation of data between age groups or indications. Chronic glucocorticoid use is associated with extensive safety concerns [1], some of which are already monitored clinically by blood biomarkers: insulin resistance measured by fasting insulin and glucose; adrenal suppression measured by first in morning cortisol; bone morbidities measured by markers of bone turnover. Expanding a panel of pharmacodynamic safety biomarkers could aid in the development of alternative dosing regimens of glucocorticoids in AAV and JDM, and in testing of novel steroidal drugs that may reduce side effects.
Anti-neutrophil cytoplasmic antibody (ANCA) – associated vasculitis (AAV) is a group of rare organ-and life-threatening multisystem diseases involving inflammation and destruction of small blood arteries [2]. Induction of remission in new or relapsing AAV is accomplished by several months of high-dose daily oral glucocorticoids combined with another immunosuppressive agent, usually cyclophosphamide or rituximab, followed by a maintenance phase of treatment with often chronic daily low-dose glucocorticoids along with another immunosuppressive agent [3]. Due to the intensity and duration of the treatment, the overall burden to patients with AAV in terms of glucocorticoid toxicity and permanent damage is substantial, including bone fragility, muscle weakness, weight gain, diabetes mellitus, and many other health problems [4,5]. Patients with AAV recognize and report significant and fast-acting clinical benefit from the anti-inflammatory properties of glucocorticoids, but report also suffering from other effects of the drugs [6].
Juvenile dermatomyositis (JDM) is a rare disease with an incidence rate in the United States of 3.2 children per million per year [7] and is the most common (85%) member of the juvenile inflammatory myopathies. Children with JDM display a classic heliotrope rash, Gottron’s papules, symmetrical proximal muscle weakness, and elevated muscle-derived enzymes in the blood. JDM is quite heterogeneous, with myositis-specific antibodies identifying specific disease patterns [8]. Muscle biopsy shows perifascicular muscle fiber atrophy, an associated progressive capillary occlusion, and a mononuclear inflammatory infiltrate [9]. As in the treatment of AAV, prolonged use of glucocorticoids, alone or in combination with a variety of biologics and immunomodulators, is considered standard of care [8,10,11]. However, glucocorticoids are well-recognized to deleteriously affect the quality of life in children, leading to increased risk of bone fragility fractures, cataracts, hypertension, volatile moods, and weight gain. These side effects become a significant aspect of morbidity associated with use of glucocorticoids in rheumatic diseases, for both the child and the child’s family [12]. Growth stunting can be particularly negatively impactful for children who are treated chronically with glucocorticoids.
Pharmacodynamic biomarkers of chronic glucocorticoids have been previously defined in a study of two disorders in children: Duchenne muscular dystrophy (DMD) and pediatric inflammatory bowel disease (IBD) [13,14]. In Hathout et al. [13], cross-sectional proteomics profiling tested over 1129 proteins using the SOMAscan™ aptamer method in glucocorticoid-naïve patients with DMD compared with patients with DMD treated with glucocorticoids, identifying biomarkers that were significantly different between cohorts. These candidate biomarkers were then validated in sera from another cohort of patients with DMD before and after glucocorticoid treatment. The majority of these same proteins were then confirmed in a cohort of pediatric patients with IBD using SOMAscan™ panels measured before and after chronic therapy with glucocorticoids. Eleven pharmacodynamic biomarkers of efficacy were identified; these were inflammation-associated proteins that were (with the exception of one anti-inflammatory protein) decreased by glucocorticoids. An additional 7 pharmacodynamic biomarkers of safety concerns were identified, which were increased by glucocorticoids. Biomarkers were pre-specified as reflective of efficacy or safety based upon the known functions of the protein, and the direction of change. Some biomarkers were shown to be elevated in patients with DMD compared with untreated healthy boys of a similar age, and decreased with prednisone treatment (i.e. MDC, IGFBP2, ITGA1 ITGB1, FGG). Others were decreased in patients with DMD compared with controls, and levels increased with prednisone treatment (i.e. GHBP, angiotensinogen, afamin) [13]. In the current study, we evaluated this panel of pharmacodynamic biomarkers for glucocorticoid efficacy (n = 11) and safety (n = 7) as candidate biomarkers in patients with both AAV and JDM.
2. Experimental
2.1. Patients and samples
Protocols and studies were approved by the Institutional Review Boards at all participating centers in the Vasculitis Clinical Research Consortium and at Ann & Robert H. Lurie Children’s Hospital of Chicago. Adult subjects and parents provided informed consent; for children, age-appropriate assent was obtained. Serum samples were accessioned from two established biobanks. Samples from patients with AAV were obtained from the Vasculitis Clinical Research Center (VCRC) Biospecimen Repository, with links to clinical data in the VCRC Clinical Data Repository. Thirty patients with AAV were selected to have matched serum samples with i) a study visit at a time of active disease off prednisone, and ii) sample at a follow-up visit at a time of remission on prednisone (n = 60 samples tested).
Serum samples from patients with JDM were obtained from the CureJM Center of Excellence, located in the Stanley Manne Children’s Research Institute of Chicago, affiliated with the Ann & Robert H. Lurie Children’s Hospital and Northwestern’s Feinberg School of Medicine. The Myositis Center is supported by the Cure JM Foundation, and hosts the Juvenile Myositis Registry and Biological Sample Repository. In addition to other samples, sera from children with JDM were generally collected at clinic visits every six months, aliquoted, and stored at −80 °C. Standardized Disease Activity Scores (DAS), developed by the Myositis Center and used internationally, were used for clinical characterization of the patients [15–17]. Twelve patients with JDM were selected who had matched serum samples with i) a study visit at a time of active disease off prednisone (baseline diagnosis or disease flare), and ii) sample at a follow-up visit on prednisone (n = 24 samples tested).
2.2. Proteomic profiling
Banked serum samples were thawed, aliquoted (~100 μL), stored at −80 °C, and delivered to SomaLogic, Inc. (Boulder CO) on dry ice for proteomic profiling of 1305 proteins using SOMAscan™, a proprietary aptamer panels testing system, as previously described [13,18]. All samples (n = 96) were run in parallel at three dilutions of subject sera (40%, 1%, 0.005%), enabling detection of proteins from fM to μM range. SOMAscan™ data are reported in Relative Fluorescence Units (RFU’s). Values from 95% of the proteins assayed showed a coefficient of variation < 12.6%. After data normalization, a data filter was applied to limit analyses to 11 pre-specified exploratory pro-inflammatory efficacy biomarkers and 7 pre-specified exploratory safety biomarkers. Ten efficacy biomarkers were previously noted to decrease with glucocorticoid treatment: (cell differentiation marker 23 [CD23], macrophage derived chemokine [MDC], interleukin 22 binding protein [IL22BP], lymphotoxin a1/b2 [LTa1/b2], insulin-like growth factor binding protein 2 [IGFBP-2], integrin A1B1 [ITGA1 ITGB1], angiopoietin 2 [ANGPT2], T lymphocyte antigen Ly9 [Ly9], fibrinogen gamma chain [FGG], and matrix metalloproteinase-12 [MMP-12]). These 10 proteins are inflammation-related, while Protein C is an anti-inflammatory protein that has been shown to increase with glucocorticoid therapy [13]. The 7 candidate safety biomarkers were previously demonstrated to be increased by glucocorticoids in DMD and IBD: insulin, leptin, matrix metalloproteinase-3 [MMP-3], afamin [AFM], angiotensinogen [AGT], insulin-like growth factor binding protein-5 [IGFBP-5], and growth hormone binding protein [GHBP] [13].
2.3. Statistical analysis
SOMAscan™ data are reported in Relative Fluorescence Units (RFU’s). Pre-treatment baseline RFU’s were compared to post-glucocorticoid RFU’s. All protein RFU’s were log (base 10) transformed and normality of each verified by the Shapiro-Wilk normality test. Student’s paired t-test and the Wilcoxon signed rank test were used to test the changes in protein expression from pre- to post-glucocorticoid treatment. Though 1310 proteins were evaluated in the aptamer panel, we used a data filter to limit analysis to only the pre-specified candidate proteins (11 efficacy, 7 safety biomarkers). Thus, we did not adjust for multiple testing due to restricted analyses. An alpha of 0.05 was used as the threshold for significance.
3. Results
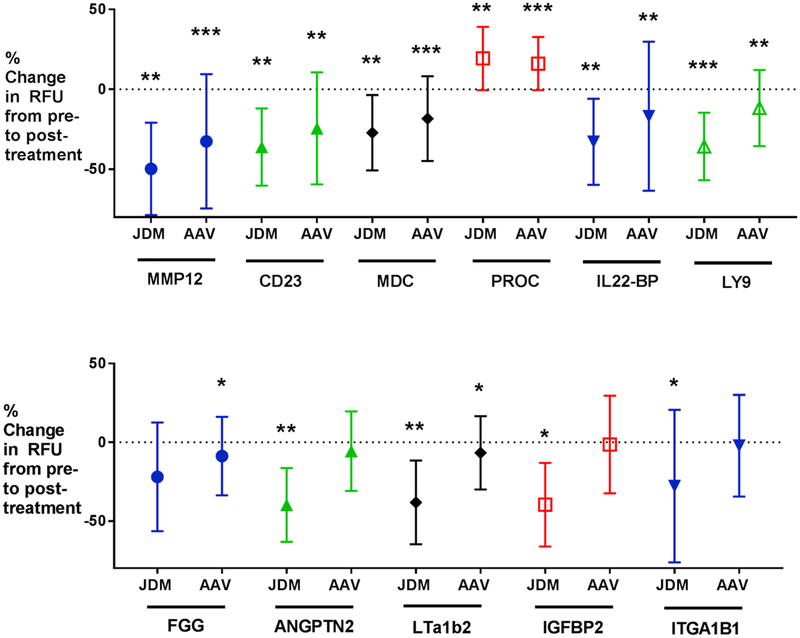
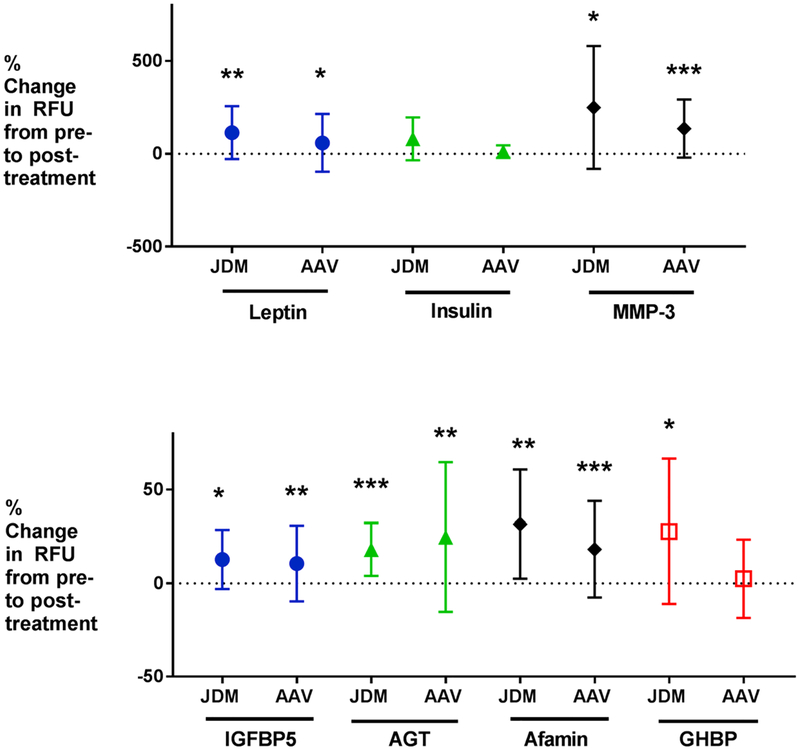
Details regarding the demographic and clinical information for the patients with AAV are reported in Table 1. Data for AAV candidate efficacy and safety biomarkers are shown in Table 2. Eight of 11 efficacy biomarkers reached significance by paired t-test (CD23, MDC, IL22BP, LTa1/b2, Ly9, FGG, MMP-12, and protein C) and 8 of 11 by Wilcoxon signed rank test (CD23, MDC, IL22BP, Ly9, FGG, MMP-12, ANGPT2, and protein C) (P-value ≤0.05). Only IGFBP-2 and ITGA1 ITGB1 did not reach significance by either method, and thus are not likely to be biomarkers for glucocorticoid efficacy in AAV. Percent change from baseline for efficacy biomarkers in AAV is shown in Fig. 1. Five of 7 safety biomarkers were elevated in AAV with glucocorticoid therapy (P-value ≤0.05): afamin, matrix metalloproteinase-3, insulin growth factor binding protein-5, angiotensinogen, and leptin. Non-fasting insulin and GHBP did not reach significance in the AAV cohort. Percent increases from baseline for safety biomarkers in AAV are shown in Fig. 2.
Table 1.
Demographics and clinical information for patients with anti-neutrophil cytoplasmic antibody-associated vasculitis.
| Sex | 12 males, 18 females |
| Type of AAV | 23 GPA, 5 EGPA, 2MPA |
| Average age (range) | 52.4 years (3.18–81.2) |
| Average duration of prednisone therapy (range) from baseline to follow up | 3months (1–5) |
| Concomitant immunomodulatory medications at baseline (n = 30 baseline) | 13 patients: 3 azathioprine; 8 methotrexate; 1 leflunomide; 1 leflunomide and rituximab |
| Concomitant immunomodulatory medications at follow up (n = 30 follow up) | 21 patients: 6 azathioprine; 10 methotrexate; 1 cyclophosphamide; 2 leflunomide; 1 leflunomide and rituximab; 1 rituximab |
| Average BVAS/WG Score Baseline (range) | 2.9 (1–7) |
| Average BVAS/WG Score Follow up (range) | 0 (0) |
Abbreviations: GPA = granulomatosis with polyangiitis; EGPA = eosinophilic granulomatosis with polyangiitis; MPA = microscopic polyangiitis; BVAS/WG = Birmingham vasculitis activity score for Wegener’s granulomatosis.
Table 2.
Data summary for candidate efficacy and safety biomarkers in anti-neutrophil antibody-associated vasculitis.
| Protein | Mean RFU’s Pre- treatment | Mean RFU’s Posttreatment | Mean difference Pre- to Post- treatment | Ratio (Post/ Pre) | Longitudinal paired t-test (P-value) | Wilcoxon signed rank test (P-value) |
|---|---|---|---|---|---|---|
| Candidate Efficacy biomarkers | ||||||
| MMP-12 | 2840 | 1469 | −1370 | 0.52 | < 0.0001*** | < 0.0001*** |
| CD23 | 6122 | 3639 | −2483 | 0.59 | 0.0015** | < 0.0001*** |
| MDC | 1857 | 1416 | −440 | 0.76 | 0.0002*** | < 0.0001*** |
| Protein C | 1945 | 2194 | 249 | 1.13 | 0.0002*** | 0.0003*** |
| IL-22BP | 4025 | 2934 | −1091 | 0.73 | 0.0017** | 0.002** |
| LY9 | 4868 | 4134 | −733 | 0.85 | 0.003** | 0.003** |
| FGG | 11,867 | 10,624 | −1243 | 0.90 | 0.018* | 0.036* |
| ANGPT2 | 197 | 181 | −16.6 | 0.92 | 0.055 | 0.041* |
| LT a1/b2 | 196 | 177 | −19.3 | 0.90 | 0.043* | 0.084 |
| IGFBP-2 | 614 | 565 | −49.3 | 0.92 | 0.297 | 0.393 |
| ITGA1 ITGB1 | 851 | 752 | −99.3 | 0.88 | 0.236 | 0.761 |
| Candidate Safety biomarkers | ||||||
| Afamin | 31,807 | 36,914 | 5106 | 1.16 | 0.0002*** | < 0.0001*** |
| Leptin | 10,154 | 11,280 | 1126 | 1.11 | 0.015* | 0.012** |
| Angiotensinogen | 7260 | 8737 | 1476 | 1.20 | 0.006** | 0.002** |
| MMP-3 | 3832 | 7345 | 3512 | 1.92 | < 0.0001*** | 0.0001*** |
| IGFBP-5 | 1595 | 1755 | 159 | 1.10 | 0.006** | 0.0003*** |
| GHBP | 1619 | 1608 | −11.4 | 0.99 | 0.926 | 0.655 |
| Insulin | 288 | 325 | 37.2 | 1.13 | 0.355 | 0.198 |
P-value ≤0.05;
P-value ≤0.01;
P-value ≤0.001
Abbreviations: MMP12 = matrix metalloproteinase-12; MDC = macrophage derived cytokine; IL-22 BP = interleukin-22 binding protein; LY9 = T lymphocyte antigen Ly9; FGG = fibrinogen gamma chain; ANGPT2 = angiopoietin-2; LT a1/b2 = lymphotoxin a1/b2; IGFBP-2 = insulin-like growth factor binding protein-2; ITGA1 ITGB1 = Integrin A1 B1; MMP-3 = matrix metalloproteinase-3; IGFBP-5 = insulin-like growth factor binding protein-5; GHBP = growth hormone binding protein.
Fig. 1.
Shown are plots of mean change in Relative Fluorescence Units (RFU’s) from baseline and standard deviation for 11 efficacy biomarkers in AAV and JDM. Significance is noted as follows: *P-value ≤0.05; **P-value ≤0.01; ***P-value ≤0.001. Eight of 11 biomarkers reached significance in AAV; 10 of 11 reached significance in JDM (by paired t-test). All biomarkers were decreased by glucocorticoids, except for Protein C. These changes were in the same direction as seen in previous studies in IBD and DMD [13,14]. Abbreviations: AAV = anti-neutrophil antibody-associated vasculitis; JDM = juvenile dermatomyositis; IBD = Inflammatory Bowel Disease; DMD = Duchenne muscular dystrophy; MMP-12 = matrix metalloproteinase-12; MDC = macrophage derived cytokine; PROC = Protein C; IL22-BP = IL22 binding protein; LY9 = T lymphocyte antigen Ly9; FGG = fibrinogen gamma chain; ANGPT2 = angiopoietin-2; IGFBP-2 = insulin-like growth factor binding protein-2; ITGA1 ITGB1 = integrin A1 B1.
Fig. 2.
Shown are plots of mean change in Relative Fluorescence Units (RFU’s) from baseline and standard deviation for 7 safety biomarkers in AAV and JDM. Significance is noted as follows:*P-value ≤ 0.05; **P-value ≤0.01; ***P-value ≤0.001. Five of 7 biomarkers reached significance in AAV; 6 of 7 reached significance in JDM (by paired t-test). All biomarkers were increased by glucocorticoids. These changes were in the same direction as seen in previous studies in IBD and DMD [13,14]. Abbreviations: AAV = anti-neutrophil antibody-associated vasculitis; JDM = juvenile dermatomyositis; IBD = Inflammatory Bowel Disease; DMD = Duchenne muscular dystrophy; MMP-3 = matrix metalloproteinase-3; AGT = angiotensinogen; IGFBP-5 = insulin-like growth factor binding protein-5; GHBP = growth hormone binding protein.
Details regarding the demographic and clinical information for the patients with JDM are shown in Table 3. Seven of the 12 patients were newly diagnosed and receiving their initial course of glucocorticoids. SOMAscan™ data for candidate safety and efficacy biomarkers in JDM are shown in Table 4. Ten of 11 efficacy biomarkers validated (P-value ≤0.05; all proteins except FGG) in the JDM cohort by paired t-test, and all 11 validated (P-value ≤0.05) by Wilcoxon signed rank test. Percent change from baseline for efficacy biomarkers in JDM is shown in Fig. 1. Furthermore, 6 of 7 safety biomarkers were increased in the JDM cohort (P-value ≤0.05; all AAV proteins plus growth hormone binding protein [GHBP]) by paired t-test, and 5 of 7 safety biomarkers were significant by Wilcoxon signed rank test (P-value ≤0.05; all AAV proteins). Percent increases from baseline for safety biomarkers in JDM are shown in Fig. 2.
Table 3.
Demographics and clinical information for patients with of juvenile dermatomyositis.
| Sex | 10 female, 2 male |
| Average age in years (range) | 9.9 (3.4–16.9) |
| Average oral prednisone dose at the time of follow up sample in mg/kg/day (range) | 0.3 (0.08–0.7) |
| *6 patients were also receiving weekly IV solumedrol pulses at the time of follow up sample (range 1–29.5 mg/kg) | |
| Average duration of prednisone therapy in months from baseline to follow up (range) | 6.9 (0.62–16.33) |
| Concomitant immunomodulatory medications at baseline (n = 12 baseline) | 1 mycophenylate mofetil (MMF) |
| Concomitant immunomodulatory medications at follow up (n = 12 follow up) | 10 patients: |
| 1 IV immunoglobulin (IVIG), cyclosporine, MMF, and hydroxychloroquine; 1 IVIG, cyclosporine, | |
| and MMF; 2 MMF; | |
| 1 cyclosporine and MMF; 2 methotrexate; | |
| 2 methotrexate and hydroxychloroquine; | |
| 1 methotrexate and MMF | |
| Average DAS Baseline (range) | Skin: 5.6 (2–7) |
| Muscle: 3.1 (1–9) | |
| Total: 8.7 (6–16) | |
| Average DAS Follow up (range) | Skin: 3.3 (0–7) |
| Muscle: 1.7 (0–6) | |
| Total: 5 (0–14) |
Abbreviations: DAS = Disease Activity Score.
Range of DAS for skin is 0–9, muscle 0–11 and total 0–20.
Table 4.
Data summary for candidate efficacy and safety biomarkers in juvenile dermatomyositis.
| Protein | Mean RFU’s Pre-treatment | Mean RFU’s Posttreatment | Mean difference Pre- to Post-treatment | Ratio (Post/ Pre) | Longitudinal paired t-test (P-value) | Wilcoxon signed rank test (P-value) |
|---|---|---|---|---|---|---|
| Candidate Efficacy biomarkers | ||||||
| MMP-12 | 3709 | 1441 | −2268 | 0.39 | 0.0057** | 0.001*** |
| CD23 | 11,293 | 6587 | −4706 | 0.58 | 0.0013*** | 0.001*** |
| MDC | 2964 | 2169 | −795 | 0.73 | 0.0028** | 0.0034** |
| Protein C | 1565 | 1911 | 346 | 1.22 | 0.0015** | 0.0034** |
| IL-22BP | 6716 | 4230 | −2486 | 0.63 | 0.0068** | 0.0024** |
| LY9 | 8012 | 4802 | −3210 | 0.6 | 0.0005*** | 0.001*** |
| FGG | 13,299 | 7898 | −5401 | 0.59 | 0.0726 | 0.0425* |
| ANGPT2 | 410 | 217 | −193 | 0.53 | 0.0018** | 0.001*** |
| LT a1/b2 | 576 | 307 | −269 | 0.53 | 0.0017** | 0.0024** |
| IGFBP-2 | 599 | 295 | −304 | 0.49 | 0.0172* | 0.001*** |
| Integrin A1B1 | 1963 | 974 | −989 | 0.5 | 0.0162* | 0.0122** |
| Candidate Safety biomarkers | ||||||
| Afamin | 27,200 | 34,491 | 7291 | 1.27 | 0.0022** | 0.0034** |
| Leptin | 4645 | 8085 | 3440 | 1.74 | 0.0091** | 0.0068** |
| Angiotensinogen | 5876 | 6949 | 1073 | 1.18 | 0.0009*** | 0.0015** |
| MMP-3 | 936 | 3256 | 2320 | 3.48 | 0.0171* | 0.001*** |
| IGFBP-5 | 1537 | 1703 | 166 | 1.11 | 0.018* | 0.0269* |
| GHBP | 1211 | 1457 | 246 | 1.2 | 0.0463* | 0.064 |
| Insulin | 336 | 470 | 134 | 1.4 | 0.1532 | 0.2661 |
P-value ≤0.05;
P-value ≤0.01;
P-value ≤0.001
Abbreviations: MMP12 = matrix metalloproteinase-12; MDC = macrophage derived cytokine; IL-22 BP = interleukin-22 binding protein; LY9 = T lymphocyte antigen Ly9; FGG = fibrinogen gamma chain; ANGPT2 = angiopoietin-2; LT a1/b2 = lymphotoxin a1/b2; IGFBP-2 = insulin-like growth factor binding protein-2; ITGA1 ITGB1 = integrin A1 B1; MMP-3 = matrix metalloproteinase-3; IGFBP-5 = insulin-like growth factor binding protein-5; GHBP = growth hormone binding protein.
Candidate biomarkers were similarly evaluated in subjects with Duchenne muscular dystrophy and pediatric IBD before and after several weeks of therapy with glucocorticoids. In these analyses, mixed effects linear regression models were used with terms for time of glucocorticoid use, and age [13,14]. To compare effect of glucocorticoids on changes in these proteins across 4 inflammatory diseases, P-values from these previous published analyses are shown compared with P-values from the current AAV and JDM analyses (P-values from Wilcoxon signed rank tests are shown) (Table 5).
Table 5.
Comparison of P-values in patients with different inflammatory diseases using a longitudinal assessment change in protein RFU’s before and after glucocorticoid treatment (P-values shown for DMD and IBD are from Hathout, et al., 2016 [13]).
| Biomarker | Function | DMD | IBD | AAV | JDM | |
|---|---|---|---|---|---|---|
| Number of subjects Type of Test | N= 4 | N= 11 | N= 30 | N= 12 | ||
| mixed effects linear regression | mixed effects linear regression | Wilcoxon signed rank test | Wilcoxon signed rank test | |||
| Efficacy | ||||||
| MMP-12 | Macrophage migration; resolution of acute inflammation | 0.002** | 0.068 | < 0.0001*** | 0.001*** | |
| CD23 | B cell activation, receptor on activated T cells, IgE binding | 0.027* | 0.026* | < 0.0001*** | 0.001*** | |
| MDC | Chemokine secreted by macrophages and dendritic cells | 0.015* | 0.014** | < 0.0001*** | 0.0034** | |
| Protein C | Serine protease with anti-inflammatory functions | 0.023* | 0.063 | 0.0003*** | 0.0034** | |
| IL-22BP | T-cell mediated inflammation | 0.015* | 0.038* | 0.002** | 0.0024** | |
| Ly9 | Activation of Natural Killer T cells | 0.01** | 0.003** | 0.003** | 0.001*** | |
| FGG | Acute phase reactant upregulated by IL-6 | 0.06 | 0.03* | 0.036* | 0.0425* | |
| ANGPT2 | Inflammation/angiogenesis | 0.023* | 0.38 | 0.041* | 0.001*** | |
| LT a1/b2 | B cell activation | 0.001*** | 0.422 | 0.084 | 0.0024** | |
| IGFBP-2 | Activated T cell proliferation | 0.015* | < 0.001*** | 0.393 | 0.001*** | |
| ITGA1 ITGB1 | T cell migration, T cell activation | 0.05* | 0.084 | 0.761 | 0.0122** | |
| Safety | ||||||
| Afamin | Biomarker for type 2 DM/metabolic syndrome | 0.015* | 0.001*** | < 0.0001*** | 0.0034** | |
| Leptin | Regulates appetite | 0.022* | 0.005** | 0.012** | 0.0068** | |
| AGT | Blood pressure regulation | 0.003** | 0.033* | 0.002** | 0.0015** | |
| MMP-3 | Extracellular matrix binding | 0.007** | 0.03* | 0.0001*** | 0.001*** | |
| IGFBP-5 | 0.002** | 0.122 | 0.0003*** | 0.0269* | ||
| GHBP | Extracellular domain of growth hormone receptor | 0.026* | 0.001*** | 0.655 | 0.064 | |
| Insulin | Glucose metabolism | 0.032* | < 0.001*** | 0.198 | 0.266 | |
P-value ≤0.05;
P-value ≤0.01;
P-value ≤0.001.
Abbreviations: AAV = anti-neutrophil antibody-associated vasculitis; JDM = juvenile dermatomyositis; MMP12 = matrix metalloproteinase-12; MDC = macrophage derived cytokine; IL-22 BP = interleukin-22 binding protein; LY9 = T lymphocyte antigen Ly9; FGG = fibrinogen gamma chain; ANGPT2 = angiopoietin-2; LTa1/b2 = lymphotoxin a1/b2; IGFBP-2 = insulin-like growth factor binding protein-2; ITGA1 ITGB1 = integrin A1 B1; AGT = angiotensinogen; MMP-3 = matrix metalloproteinase-3; IGFBP-5 = insulin-like growth factor binding protein-5; GHBP = growth hormone binding protein.
4. Discussion
Glucocorticoids are used by about 1% of the US population, and are among the most commonly prescribed medications [19]. The dramatic anti-inflammatory effects of glucocorticoids were first noted in patients with arthritis in the 1940s and this work led to the award of the 1950 Nobel Prize for medicine. However, the extensive side effects lead to significant reductions in quality of life of patients who take glucocorticoids chronically, especially in children and the elderly. The development of alternatives to glucocorticoids have focused on dissociative steroidal drugs and targeted biologics. While effective replacements for glucocorticoids have emerged in some indications, patients with many chronic inflammatory disorders, such as AAV and JDM, still rely on glucocorticoids as necessary therapy to mitigate their disease and associated symptoms. There remains a significant need for alternative and glucocorticoid-sparing therapies, either new or repositioned, for patients with AAV and JDM.
The assessment of clinical effectiveness and safety of treatments for many inflammatory diseases requires long-term study, as drugs are used to induce and maintain remission. But it is unethical and challenging to withhold standard of care therapy in early-phase trials of novel anti-inflammatory drugs in many patients. The possibility of receiving placebo is a major deterrent for enrollment in trials and raises ethical concerns, especially in rare pediatric disorders, or when equipoise is reduced by proven efficacy in adults [20,21]. Development of pharmacodynamic biomarkers reflective of aspects of efficacy and safety of glucocorticoids may facilitate the development of safer replacement therapies, while also decreasing burdens on patients and families. Because serum biomarkers are considered objective outcome measures of drug effect, an early-phase biomarker-focused clinical trial design might require study of only a few patients, and for only a short treatment period, in effect resulting in a ‘clinical de-risking’ of a new or repositioned therapy in a specific indication, such as AAV or JDM. These data may be particularly beneficial for conditions with small numbers of patients (who may have choices of clinical trials), or in pediatrics, where there may be more inherent tension involved in enrolling patients when other therapies exist.
In this current study we evaluated candidate glucocorticoid-responsive efficacy and safety biomarkers in patients with AAV and JDM who were responsive to therapy with glucocorticoids. JDM is a pediatric disorder, and the previous groups studied, DMD and IBD, were also pediatric subjects. AAV is a chronic inflammatory state mostly occurring in adults, often over the age of 60 years. Thus, this study extends our pharmacodynamic biomarker studies to a broad age range of subjects, and across four distinct chronic inflammatory disorders. We found that the large majority of biomarkers were validated as glucocorticoid-responsive in both AAV and JDM. This finding supports our hypothesis that these biomarkers are reflective of drug effect, and not indicative of disease-specific factors.
A limitation of our study is that samples were obtained within observational, natural history studies, and not within the context of clinical trials. Dosing of prednisone/prednisolone, as well as the timing of blood collection, was inconsistent. No exposure-response relationship could be identified in this study because pharmacokinetic data were not available. Moreover, values could be affected by Circadian rhythms and fasting status. For example, many of the baseline samples in the IBD cohort were drawn at the time of diagnostic colonoscopy, when the patient was fasting [14]. However, the same was not necessarily true of patients with AAV and JDM, likely contributing to the lack of consistent change in non-fasting insulin. A potential confounding variable is that patients in both cohorts were exposed to concomitant immunomodulatory medications that changed between the pre- and post-prednisone visits. DMD, IBD, JDM, and AAV are quite heterogeneous inflammatory diseases, with different longitudinal courses and clinical responses to glucocorticoids. However, despite significant heterogeneity of these diseases and exposures, most biomarkers were glucocorticoid-responsive across all four diseases, thereby clearly demonstrating a treatment-specific effect.
Clinical de-risking based on pharmacodynamic biomarkers may generate early-phase data that could reduce cost, risk, and time for patients, while providing confidence that enables a clinical development program to proceed. Using these biomarkers as objective outcome measures, it may be possible to conduct exploratory clinical trials with small numbers of patients, and for short durations of treatment time, while minimizing withholding standard of care for extended periods of time. If treatment with vamorolone results in normalization of pharmacodynamic efficacy biomarkers, or a change in biomarkers well-correlated with glucocorticoid exposure, then a larger trial could be designed and conducted using clinical outcome measures. In other rare or pediatric disease populations, this approach could be utilized by other anti-inflammatory or next-generation steroidal drug development programs for proof-of-concept studies, drug repositioning, and in support of extrapolation of efficacy between patient populations.
This approach of utilizing these same glucocorticoid-responsive serum biomarkers as pharmacodynamic measures of drug mechanism of action has recently been utilized in the development of vamorolone, a first-in-class dissociative steroidal drug being developed for Duchenne muscular dystrophy (DMD) [22]. A first-in-patient clinical trial of vamorolone in 48 boys with DMD pre-specified 7 of the glucocorticoid-responsive biomarkers shown in Table 5 as exploratory outcomes for anti-inflammatory mechanism of action [23]. The authors found that 4 of the 7 efficacy biomarkers robustly validated in JDM and AAV also showed robust dose-responsive decreases with 2 weeks of vamorolone treatment in boys with DMD [CD23, MDC, IL22BP, MMP12]. ITGA1/ITGA2 showed poor response to vamorolone, and likewise was not validated in AAV. Lymphotoxin a1/b1 and IGFBP2 were dose-responsive to vamorolone, but were also were variable in response to glucocorticoids in AAV. The data presented here may enable similar clinical de-risking trials of vamorolone in early-phase trials of AAV and JDM.
Acknowledgements
Supported by research grants from the National Institutes of Health (NICHD U54HD090254 Research in Pediatric Developmental Pharmacology Center [JvdA, YH, EPH, LSC]; NIAMS 1R43AR073547-01 [EPH, LMP], NIAMS R43AR073541-01 [JMD, PAM]). Research also supported by a grant from the Foundation to Eradicate Duchenne [EPH]. The Vasculitis Clinical Research Consortium (VCRC) (U54 AR057319) is part of the Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), National Center for Advancing Translational Science (NCATS). The VCRC is funded through collaboration between NCATS, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and has received funding from the National Center for Research Resources (U54 RR019497) [PAM]. The Myositis Center is supported by the Cure JM Foundation, and hosts the Juvenile Myositis Registry and Biological Sample Repository [LMP]. KN is supported by the National Institutes of Health (R21AI128248-02, R56NS097229-01 and K26OD011171), the Myositis Association and the US Department of Defense (W81XWH-11-1-0809).
Footnotes
Declaration of interest
LSC, JMD, JvdA, KN, and EPH are employees of ReveraGen BioPharma. KN, and EPH are co-founders of ReveraGen and own founder shares. LSC, JMD, JvdA own stock options of ReveraGen.
References
- [1].Bradford Rice J., White AG, Johnson M, Wagh A, Qin Y, Bartels-Peculis L, Ciepielewska G, Nelson WW, Quantitative characterization of the relationship between levels of extended corticosteroid use and related adverse events in a US population, Curr. Med. Res. Opin 34 (8) (2018) 1519–1527. [DOI] [PubMed] [Google Scholar]
- [2].Xiao H, Hu P, Falk RJ, Jennette JC, Overview of the pathogenesis of ANCA-associated vasculitis, Kidney Dis. 1 (4) (2016) 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yates M, Watts RA, Bajema IM, Cid MC, Crestani B, Hauser T, Hellmich B, Holle JU, Laudien M, Little MA, Luqmani RA, Mahr A, Merkel PA, Mills J, Mooney J, Segelmark M, Tesar V, Westman K, Vaglio A, Yalcindag N, Jayne DR, Mukhtyar C, EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis, Ann. Rheum. Dis 75 (9) (2016) 1583–1594. [DOI] [PubMed] [Google Scholar]
- [4].Seo P, Min YI, Holbrook JT, Hoffman GS, Merkel PA, Spiera R, Davis JC, Ytterberg SR, St. Clair EW, McCune WJ, Specks U, Allen NB, Luqmani RA, Stone JH, Damage caused by Wegener’s granulomatosis and its treatment: prospective data from the Wegener’s Granulomatosis Etanercept Trial (WGET), Arthritis 52 (7) (2005) 2168–2178. [DOI] [PubMed] [Google Scholar]
- [5].Robson J, Doll H, Suppiah R, Flossman O, Harper L, Höglund P, Jayne D, Mahr A, Westman K, Lugmani R, Glucocorticoid treatment and damage in the anti-neutrophil cytoplasm antibody-associated vasculitides: long-term data from the European vasculitis study group trials, Rheumatology 54 (3) (2015) 471–481. [DOI] [PubMed] [Google Scholar]
- [6].Robson JC, Dawson J, Cronholm PF, Ashdown S, Easley E, Kellom KS, Gebhart D, Lanier G, Milman N, Peck J, Luqmani RA, Shea JA, Tomasson G, Merkel PA, Patient perceptions of glucocorticoids in anti-neutrophil cytoplasmic antibody-associated vasculitis, Rheumatol. Int 38 (2018) 675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mendez EP, Lipton R, Ramsey-Goldman R, Roettcher P, Bowyer S, Dyer A, Pachman LM, US incidence of juvenile dermatomyositis, 1995–1998: results from the National Institute of Arthritis and Musculoskeletal Diseases Registry, Arthritis Rheum. 49 (3) (2003) 300–305. [DOI] [PubMed] [Google Scholar]
- [8].Pachman LM, Khojah AM, Advances in juvenile dermatomyositis: myositis specific antibodies aid in understanding disease heterogeneity, J. Pediatr 195 (2018) 16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Deakin CT, Yasin SA, Simou S, Arnold KA, Tansley SL, Betteridge ZE, McHugh NJ, Varsani H, Holton JL, Jacques TS, Pilkington CA, Nistala K, Wedderburn LR, Muscle biopsy findings in combination with myositis-specific autoantibodies aid prediction of outcomes in juvenile dermatomyositis, Arthritis Rheumatol. 68 (11) (2016) 2806–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Spencer CH, Rouster-Stevens K, Gewanter H, Syverson G, Modica R, Schmidt K, Emerty H, Wallace C, Grevich S, Nanda K, Zhao YD, Shenoi S, Tarvin S, Hong S, Lindsley C, Weiss JE, Passo M, Ede K, Brown A, Ardalan K, Bernal W, Stoll ML, Lang B, Carrasco R, Agaiar C, Feller L, Bukulmez H, Vehe R, Kim H, Schmeling H, Gerstbacher D, Hoeltzel M, Eberhard B, Sundel R, Kim S, Huber AM, Patwardhan A, Biologic therapies for refractory juvenile dermatomyositis: five yearsof experience of the childhood arthritis and rheumatology research alliance in North America, Pediatr. Rheumatol. Online J 15 (1) (2017) 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kishi T, Bayat N, Ward MM, Huber AM, Wu L, Mamyrova G, Targoff IN, Warren-Hicks WJ, Miller FW, Rider LG, Medications received by patients with juvenile dermatomyositis, Semin. Arthritis Rheum. (2018), 10.1016/j.semarthrit.2018.03.016 (pii: S0049–0172(17)30753–9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rouster-Stevens K, Nageswaran S, Arcury TA, Kemper KJ , How do parents of children with juvenile idiopathic arthritis (JIA) perceive their therapies, BMC Complement. Altern. Med 8 (2008) 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hathout Y, Conklin LS, Seol H, Gordish-Dressman H, Brown KJ, Morgenroth LP, Nagaraju K, Heier CR, Damsker JM, van den Anker JN, Henricson E, Clemens PR, Mah JK, McDonald C, Hoffman EP, Serum pharmacodynamic biomarkers for chronic corticosteroids treatment of children, Sci. Rep 6 (2016) 31727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Heier CR, Fiorillo AA, Chaisson EE, Gordish-Dressman H, Hathout Y, Damsker JM, Hoffman EP, Conklin LS, Identification of pathway – specific serum biomarkers of response to glucocorticoid and infliximab treatment in children with inflammatory bowel disease, Clin. Transl. Gastroenterol 7 (9) (2016) e192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bode RK, Klein-Gitelman MS, Miller ML, Lechman TS, Pachman LM, Disease activity score for children with juvenile dermatomyositis: reliability and validity evidence, Arthritis Rheum. 49 (1) (2003) 7–15. [DOI] [PubMed] [Google Scholar]
- [16].Ruperto N, Pistorio A, Ravelli A, Rider L, Pilkington C, Oliveira S, Wulffraat N, Espada G, Garay S, Cuttica R, Hofer M, Quartier P, Melo-Gomes J, Reed A, Wierzbowska M, Feldman B, Harjacek M, Huppertz H, Nielsen S, Flato B, Lahdenne P, Michels H, Murray KJ, Punaro L, Rennebohm R, Russo R, Balogh Z, Rooney M, Pachman LM, Wallace C, Hashkes P, Lovell DJ,Giannini EH, Gare B, Martini AA, Paediatric Rheumatology International Trials Organisation (PRINTO); Pediatric Rheumatology Collaborative Study Group (PRCSG), The Paediatric Rheumatology International Trials Organization provisional criteria for the evaluation of response to therapy in juvenile dermatomyositis, Arthritis Care Res. 62 (11) (2016) 1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rider LG, Aggarwal R, Pistorio A, Bayat N, Erman B, Feldman BM, Huber AM, Cimaz R, Cuttica RJ, de Oliveira SK, Lindsley CB, Pilkington CA, Punaro M, Ravelli A, Reed AM, Rouster-Stevens K, van Royen-Kerkhof A, Dressler F, Magalhaes CS, Constantin T, Davidson JE, Magnusson B, Russo R, Villa L, Rinaldi M, Rockette H, Lachenbruch PA, Miller FW, Vencovsky J, Ruperto N, International Myositis Assessment and Clinical Studies Group and the Paediatric Rheumatology International Trials Organisation, 2016 American College of Rheumatology/European League against rheumatism criteria for minimal, moderate, and major clinical response in juvenile dermatomyositis: an International Myositis Assessment and Clinical Studies Group/Paediatric Rheumatology International Trials Organisation collaborative initiative, Arthritis Rheumatol. 69(5) (2017) 911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kim CH, Tworoger SS, Stampfer MJ, Dillon ST, Gu X, Sawyer SJ, Chan AT, Libermann TA, Eliassen AH, Stability and reproducibility of proteomic profiles measured with an aptamer-based platform, Sci. Rep 8 (1) (2018) 8382, 10.1038/s41598-018-26640-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Overman RA, Yeh JY, Deal CL, Prevalence of oral glucocorticoid usage in the United States: a general population perspective, Arthritis Care Res. 65 (2) (2013) 294–298. [DOI] [PubMed] [Google Scholar]
- [20].Peay HL, Biesecker BB, Wilfond BS, Jarecki J, Umstead KL, Escolar DM, Tibben A, Barriers and facilitators to clinical trial participation among parents of children with pediatric neuromuscular disorders, Clin. Trials 15 (2) (2018) 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Turner D, Koletzko S, Griffiths AM, Hyams J, Dubinsky M, de Ridder L, Escher J, Lionetti P, Cucchiara S, Lentze MJ, Koletzko B, van Rheenen P, Russell RK, Mack D, Veereman G, Vermeire S, Ruemmele F, Use of placebo in pediatric inflammatory bowel diseases: a position paper from ESPGHAN, ECCO, PIBDnet, and the Canadian Children IBD Network, J. Pediatr. Gastroenterol. Nutr 62 (1) (2016) 183–187. [DOI] [PubMed] [Google Scholar]
- [22].Hoffman EP, Riddle V, Siegler MA, Dickerson D, Backonja M, Kramer WG, Nagaraju K, Gordish-Dressman H, Damsker JM, McCall JM, Phase 1 trial of vamorolone, a first-in-class steroid, shows improvement in side effects via biomarkers bridged to clinical outcomes, Steroids 134 (2018) 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Conklin LS, Damsker JM, Hoffman EP, Jusko WJ, Mavroudis PD, Schwartz BD, Mengle-Gaw LJ, Smith EC, Mah JK, Guglieri M, Nevo Y, Kuntz N, McDonald CM, Tulinius M, Ryan MM, Webster R, Castro D, Finkel RS, Smith AL, Morgenroth LP, Arrieta A, Shimony M, Jaros M, Shale P, McCall JM, Hathout Y, Nagaraju K, van den Anker J, Ward LM, Ahmet A, Cornish MR, Clemens PR, Phase IIa trial in Duchenne muscular dystrophy shows vamorolone is a first-in-class dissociative steroidal anti-inflammatory drug, Pharmacol. Res 136 (2018) 140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]