Atherosclerotic cardiovascular disease is the major cause of morbidity and mortality in diabetes mellitus (1). Atherosclerosis occurs earlier and with greater severity in the diabetic population leading to a much higher risk of myocardial infarction, stroke, and limb ischemia and amputation. While numerous factors contribute to the etiology of atherosclerosis, oxidative stress and inflammation play a fundamental role and both processes are exacerbated in diabetes. Given the rapidly growing worldwide incidence of diabetes, there is a critical need for new therapies that targets atherogenesis and its clinical manifestations in diabetic patients.
The gasotransmitters nitric oxide (NO), carbon monoxide (CO), and hydrogen sulfide (H2S) have emerged as crucial regulators of vascular disease in diabetes (2). While NO and CO have been extensively studied, less is known regarding the role of H2S in diabetes. H2S is a colorless, water soluble gas with the characteristic smell of rotten eggs. It is generated by the metabolism of cysteine by the enzymes cystathionine β-synthase and cystathionine γ-lyase or by the concerted action of cysteine amino transferase and 3-mercaptopyruvate sulfurtransferase (Figure 1). H2S is also produced non-enzymatically from glucose, glutathione, thiosulfate, and sulfur-containing proteins and by the bacterial reduction of sulfur in the intestinal tract (3,4). Although long considered a toxic gas, studies in the past decade have revealed important physiologic roles for H2S. H2S promotes blood flow by dilating blood vessels and inhibiting platelet aggregation (3,5). It also exerts potent antioxidant, anti-apoptotic, anti-inflammatory, and angiogenic responses. H2S elicits many of its biological effects by targeting proteins for S-sulfhydration where sulfur is added to the thiol groups of reactive cysteine residues resulting in the formation of hydropersulfide (6). More recently, H2S has been shown to mitigate endothelial dysfunction, retinopathy, cardiomyopathy, and nephropathy in experimental animal models of diabetes (7–10), highlighting the protective nature of this molecule.
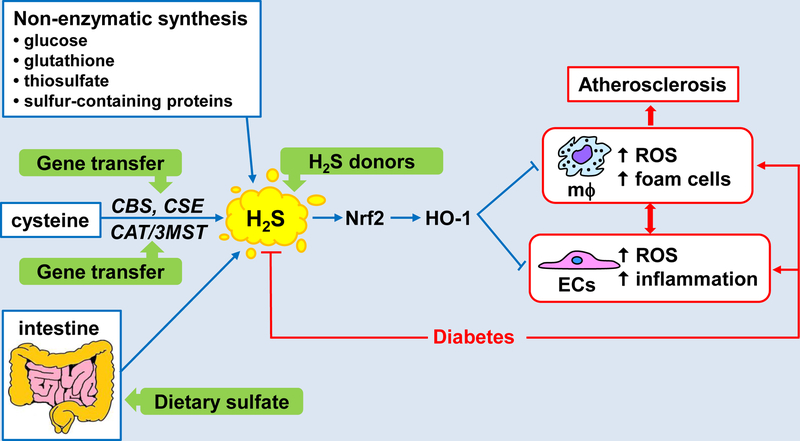
Figure 1.
Regulation and function of H2S in diabetes-accelerated atherosclerosis. H2S is generated by the metabolism of cysteine by the enzymes cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) or by the concerted action of cysteine amino transferase (CAT) and 3-mercaptopyruvate sulfurtransferase (3MST). H2S is also produced non-enzymatically from glucose, glutathione, thiosulfate, and sulfur-containing proteins and by the bacterial reduction of sulfur in the intestinal tract. Circulating levels of H2S are depressed in diabetes but restoration of H2S via the administration of H2S donor molecules, gene delivery of H2S generating enzymes, and/or dietary sulfate supplementation leads to the activation of the Nrf2-HO-1 signaling axis which inhibits the development of atherosclerosis by blocking diabetes-induced oxidative and inflammatory stress in endothelial cells (ECs) and reactive oxygen species (ROS) and foam cell formation by macrophages (mϕ).
In this issue, Xie et al. (11) further address the role of H2S in diabetes and demonstrate for the first time that this gas suppresses diabetes-accelerated atherosclerosis. They show that daily systemic administration of the slow-releasing H2S donor GYY4137 decreases atherosclerotic lesion size in atheroprone, streptozotocin-induced diabetic mice fed a high fat diet, independent of any change in circulating blood glucose or cholesterol. The anti-atherosclerotic effect of GYY4137 is associated with reductions in macrophage content within the plaque and decreases in the production of superoxide and expression of the adhesion receptors, intercellular adhesion molecule-1 and vascular cell adhesion molecule-1, in the aortic endothelium. Comparable effects are also observed when peritoneal macrophages or endothelial cells are exposed to high concentrations of glucose and oxidized low density lipoprotein that mimics the in vivo environment encountered in diabetes. However, the protective actions of GYY4137 are lost if GYY4137 is depleted of H2S or if a structural analogue of GYY4137 that lacks sulfur is used, indicating that H2S mediates the actions of GYY4137. They also demonstrate that GYY4137 activates the transcription factor Nrf2 via the specific S-sulfhydration of cysteine-151 in Keap1, a repressor protein that binds Nrf2 and promotes its degradation by the ubiquitin proteasome pathway (12). Significantly, deletion or silencing of Nrf2 abolishes the anti-atherogenic action of GYY4137 in diabetic mice and cells, illustrating the essential role of Nrf2. In addition, GYY4137 stimulates the expression of heme oxygenase-1 (HO-1) in a Nrf2-dependent manner, and depletion or inhibition of HO-1 abolishes the cellular actions of GYY4137, implicating HO-1 in the anti-atherogenic effects of H2S.
Notably, Xie and coworkers (11) detect significantly lower levels of plasma H2S in diabetic mice that is corrected by the administration of GYY4137. A reduction in circulating H2S has also been noted in other diabetic animal models and diabetic patients (7,13–15), supporting the presence of a H2S deficiency state in diabetes. The cause for this decline is not known but may reflect alterations in the global activity of H2S-generating enzymes, the liberation of H2S from other sources, the microbial reduction of sulfate in the intestine, and/or the metabolism of H2S in diabetes. Clearly, further studies are needed to address this issue and establish optimal circulating concentrations of H2S needed to maintain vascular homeostasis in diabetes.
The study by Xie et al. (11) represents an important advance in the field and identifies H2S as a novel therapeutic target in diabetes-accelerated atherosclerosis. The finding that Nrf2 functions as the initial transducer of the anti-atherogenic action of H2S is somewhat surprising given the controversial role of Nrf2 in atherosclerosis (16), but it is in-line with a recent report showing that Nrf2 activation represses atherosclerosis in a mouse model of diabetes (17). The discovery that HO-1 is the downstream target of Nrf2 that mediates the anti-atherogenic effects of H2S in macrophages and endothelial cells is less surprising given the known anti-atherogenic properties of its products, biliverdin and CO (18). However, it provides additional evidence that cross-talk between signaling gases occurs in diabetes (2), and this may contribute to the vasoprotective actions of H2S. Moving forward, it will be important to extend this work in male mice to females, as sex differences in the cardiovascular consequences of diabetes exist (19).
A schematic diagram depicting the beneficial actions of H2S in diabetes-accelerated atherosclerosis is shown in Figure 1. In this model, restoration of circulating concentrations of H2S in diabetes leads to the activation of the Nrf2-HO-1 signaling pathway which limits the development of atherosclerosis by blocking diabetes-induced oxidative and inflammatory stress in endothelial cells and formation of reactive oxygen species and foam cells in macrophages. Several strategies can be employed to augment H2S levels in diabetes. The use of H2S-releasing compounds is a highly feasible near term approach. Aside from inorganic salts and natural H2S donors, many synthetic compounds have been developed that possess superior H2S release kinetics and pharmacokinetic profiles (20). Alternatively, endogenous circulating levels of H2S may be increased by gene delivery of H2S generating enzymes or by the supplementation of dietary sulfur that is readily converted to H2S by the gut microbiome. Future translational studies utilizing these approaches will determine the clinical success of this odorous gas in treating diabetes-associated macrovascular disease.
Acknowledgement
W.D. is supported by the American Heart Association Midwest Affiliate (15GRNT25250015).
References
- 1.Wang CCL, Hess CN, Hiatt WR, Goldfine AB. Clinical update: cardiovascular disease in diabetes mellitus. Atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus – mechanisms, management, and clinical considerations. Circulation 2016;133:2459–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van den Born JC, Hammes HP, Greffrath W, van Goor H, Hillebrands JL on behalf of the DFG GRK International Training Group 1874 Diabetic Microvascular Complications (DIAMiCOM). Gasotransmitters in vascular complications of diabetes. Diabetes 2016; 65:331–335. [DOI] [PubMed] [Google Scholar]
- 3.Kolluru GK, Shen X, Bir SC, Kevil CG. Hydrogen sulfide chemical biology: pathophysiological roles and detection. Nitric Oxide 2013;35:5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen X, Carlstrom M, Borniquel S, Jadert C, Kevil CG, Lundberg JO. Microbiol regulation of host hydrogen sulfide bioavailability and metabolism. Free Radic. Biol. Med 2013;60:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polhemus DJ, Lefer DJ. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ. Res 2014;114:730–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, Snyder SH. H2S signals through protein S-sulfhydration . Sci. Signal 2009;2:ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki K, Olah G, Modis K, Coletta C, Kulp G, Gero D, Szoleczky P, Chang T, Zhou Z, Wu L, Wang R, Papapetropoulos A, Szabo C. Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc. Natl. Acad. Sci. USA 2011;108:13829–13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou X, Feng Y, Zhan Z, Chen J. Hydrogen sulfide alleviates diabetic nephropathy in a streptozotocin-induced diabetic rat model. J. Biol. Chem 2014;289:28827–28834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Si YF, Wang J, Guan J, Zhou L, Sheng Y, Zhao J. Treatment with hydrogen sulfide alleviates streptozotocin-induced diabetic retinopathy in rats. Br. J. Pharmacol 2013;169:619–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou X, An G, Lu X. Hydrogen sulfide attenuates the development of diabetic cardiomyopathy. Clin. Sci 2015;128:325–335. [DOI] [PubMed] [Google Scholar]
- 11.Xie L, Gu Y, Wen M, Zhao S, Wang W, Ma Y, Meng G, Han Y, Wang Y, Liu G, Moore PK, Wang X, Wang H, Zhang Z, Yu Y, Ferro A, Huang Z, Ji Y. Hydrogen sulfide induces keap1 S-sulfhydration and suppresses diabetes-accelerated atherosclerosis via Nrf2 activation. Diabetes. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kansanen E, Kuosmanen SM, Leionen H, Levonen AL. The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol. 2013;1:45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brancaleone V, Roviezzo F, Vellecco V, De Gruttola L, Buci M, Cirino G. Biosynthesis of H2S is impaired in non-obese diabetic (NOD) mice. Br. J. Pharmacol 2008;155:673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain SK, Bull R, Rains JL, Bass PF, Levine SN, reddy S, McVie R, Bocchini JA. Low levels of hydrogen sulfide in the blood of diabetes patients and streptozotocin-treated rats causes vascular inflammation? Antioxid. Redox Signal. 2010;12:1333–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whiteman M, Gooding KM, Whatmore JL, Ball CI, Mawson D, Skinner K, Tooke JE, Shore AC. Adiposity is a major determinant of plasma levels of the novel vasodilator hydrogen sulfide. Diabetologia 2010;53:1722–1726. [DOI] [PubMed] [Google Scholar]
- 16.Mimura J, Itoh K. Role of Nrf2 in the pathogenesis of atherosclerosis. Free Radic. Biol. Med 2015;88:221–232. [DOI] [PubMed] [Google Scholar]
- 17.Tan SM, Sharma A, Stefanovic N, Yuen DY, Karagiannis TC, Meyer C, Ward KW, Cooper ME, de Haan JB. Derivative of bardoxolone methyl, Dh404, in an inverse dose-dependent manner lessens diabetes-associated atherosclerosis and improve diabetic kidney disease. Diabetes 2104; 63:3091–3103. [DOI] [PubMed] [Google Scholar]
- 18.Durante W Protective role of heme oxygenase-1 against inflammation in atherosclerosis. Front. Biosci 2011;16:2372–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Regensteiner JG, Golden S, Huebschmann AG, Barrett-Connor E, Chang AY, Chyun D, Fox CS, Kim C, Mehta N, Reckelhoff JF, Reusch JEB, Rexrode KM, Sumner AE, Welty FK, Wenger NK, Anton B; on behalf of the American Heart Association Diabetes Committee of the Council of Lifestyle and Cardiometabolic Health, Council of Epidemiology and Prevention, Council of Functional Genomics and Translational Biology, and Council of Hypertension. Sex differences in the cardiovascular consequences of diabetes mellitus: a scientific statement by the American Heart Association. Circulation 2015;132:2424–2447. [DOI] [PubMed] [Google Scholar]
- 20.Song ZJ, Ng MY, Lee ZW, Dai W, Hagen T, Moore PK, Huang D, Deng LW, Tan CH. Hydrogen sulfide donors in research and drug development. Med. Chem. Commun 2014;5:557–570. [Google Scholar]