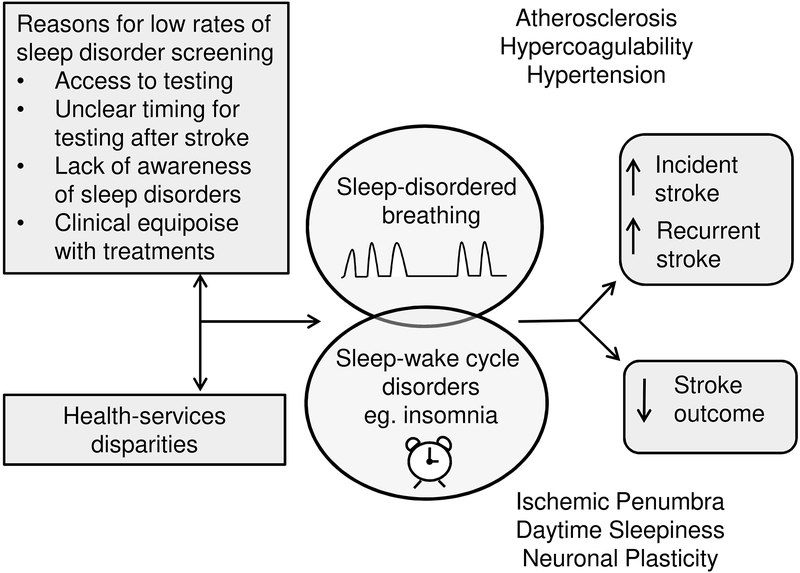
Stroke is the leading cause of serious long-term disability in the US, leaving less than half of survivors able to return directly home.1 Stroke recurrence, estimated as high as 17% over 5 years, also remains unacceptably high.1 The use of lytic drugs and endovascular devices have revolutionized the care of select stroke patients in the acute setting. However, enormous challenges remain in changing the trajectory of stroke recovery for the vast majority of patients who do not qualify or remain disabled after these treatments, and also in preventing the accumulating disability associated with stroke recurrence.
The role of sleep disorders in stroke outcome and recurrence has become a pressing question. Despite estimates of greater than 50% prevalence of sleep disorders after stroke, only about 6% of stroke survivors are offered formal sleep testing and an estimated 2% complete such testing in the 3-month post-stroke period.2 The reasons for the low rate of screening are at least partly related to the lack of awareness regarding sleep disorders among stroke providers (Figure 1). This review evaluates the role of sleep disorders, including sleep disordered breathing (SDB) and sleep-wake cycle disorders, in stroke etiology and examines the impact of their treatment on stroke outcome.
Figure 1:
Potential causes and consequences of untreated sleep disorders after stroke with proposed mechanisms leading to increased risk of stroke and poor stroke outcome.
Physiology and anatomy of breathing during sleep
During sleep, ventilation is reduced compared to wake, in parallel with the restorative and toning-down changes that occur to heart rate, temperature and blood pressure. Volitional or behavioral input on breathing are absent during sleep; only brainstem neurons, peripheral chemoreceptors and respiratory muscle afferents regulate breathing.3 Groups of chemoreceptive neurons in the brainstem, including those of the dorsolateral pons, nucleus solitarius and ventral medullary respiratory column, respond to changes in the partial pressure of carbon dioxide and oxygen and thereby serve as a pacemaker regulating the breathing rhythm.4 Along with effects on the breathing pattern, these brainstem neurons cause a reduction in the upper airway tone at sleep onset through reduced activity of airway dilator muscles, especially the genioglossus, which forms the bulk of the tongue.3 Alternatively, the chemoreceptive neurons of the brainstem can detect increased carbon dioxide by peripheral chemoreceptors and initiate increased activity of the dilator muscles in response to airway resistance or collapse.3
The stability of sleep can be affected by brief (3 to 15 second) arousals that occur in response to changes in airflow. Respiratory events include 10 seconds or longer breathing cessation (apneas) and 30% or greater airflow reduction with associated oxygen desaturation or arousals (hypopneas). These are summed in a sleep study to generate an apnea-hypopnea index (AHI)—the number of respiratory events per hour of sleep. The AHI in OSA is typically classified as mild (5 to 14 per hour), moderate (15 to 29 per hour) or severe (≥ 30 per hour). Respiratory provoked arousals during sleep when breathing is otherwise reliant on respiratory and chemical control feedback mechanisms serve to terminate the apnea or hypopnea by opening the airway in response to collapse and to increase the rate of ventilation in response to hypercapnia.3 Although the arousals during sleep may play an important compensatory role in people with SDB, they may also have deleterious effects on sleep stability and other physiologic parameters before and after stroke.
Central and obstructive sleep apnea
Two types of SDB, central sleep apnea (CSA) and obstructive sleep apnea (OSA), vary considerably in their etiology, prevalence, relative improvement after stroke and effects on stroke outcome. Central apneas occur most commonly in heart failure and opioid use but can also be observed after stroke due to distinct brain lesions involving autonomic and volitional respiratory centers.4–6 Absent airflow in OSA results from upper airway occlusion or narrowing, which may be related to decreased activity of airway dilator muscles during sleep with the tongue falling backward or anatomical features reducing upper airway diameter, including craniofacial structures, enlarged tonsils and obesity.4 Obstructive apneas or hypopneas occur despite activity of the thoracic muscles (diaphragm and intercostal muscles).4 Obstructive events can be distinguished from central events during polysomnography by detecting respiratory effort through the activity of chest and abdominal respiratory belts. Both CSA and OSA can disrupt sleep through the frequent arousals and desaturations, leading to fragmentation of sleep architecture and excessive daytime sleepiness.3
The development of CSA, which includes both Cheyne-Stokes periodic breathing and ataxic breathing pattern with primarily central apneas, is uncommon after stroke and thought to be related to disruption of respiratory networks involved in the regulation of breathing.5 In a meta-analysis of SDB after ischemic or hemorrhagic stroke or transient ischemic attack (TIA), 72% of patients had an AHI of at least 5 per hour but only 7% of patients had primarily central apneas.7 Further, CSA tends to improve after acute stroke. In a study of 161 patients with first-ever stroke or TIA who underwent portable sleep studies within 48 to 72 hours from admission and again after 3 months, the rate of SDB decreased from 71% to 62% with a significant reduction in central apneas but not obstructive apneas.8 Additionally, the effects of CSA on stroke outcome and the role of early therapy for CSA after stroke are unclear. In a prospective study of stroke rehabilitation patients with a 10-year follow-up period, the risk of death was significantly higher among patients with OSA compared to controls but not among patients with CSA.9 Continuous positive airway pressure (CPAP), the gold standard treatment for OSA, is only effective in about 50% of patients with CSA4 and has unclear benefit in stroke outcome.
The estimated prevalence of OSA after stroke or TIA is over 70%.7 The reasons for such high rates remain unclear though are at least partly related to positional sleep apnea, stroke-related upper airway tone changes and untreated OSA preceding the stroke. Multiple studies suggest that OSA is more often a preexisting condition prior to stroke rather than the consequence of brain injury. The findings from a prospective study of sleep apnea evaluations both before and after stroke and TIA show a similar frequency of OSA, suggesting it is a common predisposing condition.8 Further, most prior studies have found no link between the prevalence or severity of OSA and stroke subtype, topography or stroke severity.8,10 Also, while SDB may improve over time after stroke, like other stroke-related deficits, over half of stroke patients have an AHI > 10 per hour even after 3 months.8
Effects of obstructive sleep apnea on stroke occurrence, recurrence and recovery
If the development of OSA precedes stroke in the majority of patients, what roles does it play in the etiology of stroke? Population-based epidemiologic studies show that OSA independently predisposes to stroke. In a prospective analysis of 1,189 healthy participants in the Wisconsin Sleep Cohort study, an AHI ≥ 20 per hour was associated with an increased risk of stroke over the next 4 years (unadjusted OR 4.31; 95%CI 1.31–14.15; p=0.02), though this relationship was not significant after adjustment for potential confounders.11 In the community-based Sleep Heart Health Study, 5,422 healthy participants without stroke were evaluated with polysomnography and followed for a median of 8.7 years.12 An AHI > 15 per hour was 30% more common among participants who had an ischemic stroke compared to those who remained stroke-free. Men with moderate or severe OSA had an almost threefold increased risk of ischemic stroke compared to those without OSA, with an estimated 6% increased risk of stroke per unit increase in the AHI from 5 to 25 per hour, whereas women had an increased risk of stroke only with an AHI > 25 per hour. In an observational cohort study of 1,022 clinic patients referred for sleep evaluation, patients with OSA had a nearly two-fold increase in stroke or death from any cause after a median of 3.4 years independent of known vascular risk factors and with increased risk associated with OSA severity.13 A meta-analysis of 17 population or clinic-based, prospective cohort studies revealed that moderate to severe OSA significantly increased cardiovascular risk, in particular the risk of fatal or non-fatal stroke (RR 2.02, 95%CI 1.4–2.9).14
Along with functioning as an independent risk factor for stroke, OSA can also increase the risk of stroke through effects on traditional stroke risk factors, especially hypertension. In the Wisconsin Sleep Cohort Study, a dose-response association was noted between the AHI at baseline and the development of hypertension, where participants with moderate OSA compared to those with an AHI of 0 had approximately three times the odds of having hypertension at a 4-year follow-up.15 The presumed mechanism through which OSA may lead to hypertension is through high levels of sympathetic activity during apneas and the associated blood pressure surges during sleep, which are enhanced further by arousals and sleep fragmentation.12 In a study comparing blood pressure and sympathetic nerve activity among patients with OSA to age- and sex-matched subjects without OSA, sympathetic nerve activity was significantly higher in those with OSA during both wakefulness and sleep.16 In this study, apnea-induced nocturnal hypoxemia resulted in progressive increases in sympathetic nerve activity with subsequent surges in blood pressure as high as 240/130 mm Hg upon the cessation of apneas.16 Along with direct increases in oxidative stress, the effects of the OSA-related sympathetic activity likely contribute to sustained daytime hypertension and a blunted response to nocturnal dipping, the normal toning down pattern of sleep where blood pressure typically decreases by at least 10% of awake values.17 Such a non-dipping blood pressure pattern has been noted in 48–84% of patients with OSA, with increased frequency associated with OSA severity.18
Once stroke has occurred, observational studies suggest that OSA has a negative effect on outcome by predisposing to recurrent stroke, increasing the risk of mortality and worsening functional recovery. In a prospective cohort study of 166 patients with ischemic stroke who underwent a sleep study about 2 months after the acute hospitalization, CPAP was offered to 96 patients (58%) with an AHI of at least 20 per hour.19 After a period of 7 years, patients with moderately severe OSA who could not tolerate CPAP had a nearly three-fold increased risk of non-fatal cardiovascular events, particularly recurrent stroke, compared to those who were either adherent to CPAP, those without OSA, or those with mild disease combined (HR 2.87, 95% CI 1.1–7.7; p=0.03). The number needed to treat to prevent one nonfatal vascular event with CPAP was 5 patients (95% CI 2–19). In another prospective cohort study of 132 patients undergoing stroke rehabilitation and followed over 10 years, the presence of moderately severe OSA in patients after stroke was associated with a 75% increase in the risk of early death compared to patients without OSA independent of disability and traditional stroke risk factors.9 Several observational studies suggest that OSA may also be a predictor of poor functional outcome after stroke, increasing the risk of short-term neurologic worsening and of long-term dependency. In a study evaluating blood pressure and OSA severity among 41 patients with acute ischemic stroke, sleep apnea severity was associated with worsened stroke severity on day 1 and modified Rankin scale scores at hospital discharge.20 In this study, the non-dipping blood pressure pattern was more common among patients with OSA and was strongly associated with severe strokes and poor outcomes. In another study of 61 patients with ischemic and hemorrhagic stroke admitted to a rehabilitation unit, OSA was found in 60% of patients and was significantly and independently associated with worse functional impairment and a 40% longer rehabilitation stay.21 The mechanism by which OSA may lead to worse stroke recovery is unknown though thought to possibly be related to the deleterious effects on the injured brain, including fragmented sleep in the setting of impaired cognitive function and the effects of intermittent hypoxemia on the ischemic penumbra and on neuronal plasticity.22
Treatment of obstructive sleep apnea
Prevention of stroke
The gold-standard treatment for OSA is CPAP. Alternative treatments, including oral mandibular advancement appliances and upper airway surgery, have not been studied after stroke and are not practical in the acute stroke setting. Along with issues related to tolerance with high rates of patients discontinuing CPAP entirely and reduced adherence, barriers for CPAP use after stroke also include logistic difficulties and access to sleep apnea testing for patients with severe disability or immobility. Nonetheless, if proven effective in stroke prevention and recovery, CPAP could be a noninvasive and relatively inexpensive intervention that could improve stroke outcome and help prevent second stroke.
Only a few randomized controlled trials have evaluated the use of CPAP in secondary prevention. In one small, secondary prevention trial evaluating cardiovascular survival, 140 ischemic stroke patients were randomized within 3 to 6 days to early CPAP or to conventional treatment.23 Although the mean time to the next cardiovascular event was longer in the CPAP group (14.9 vs. 7.9 months, p=0.044), the overall cardiovascular event-free survival after 2 years was similar in both groups. Limitations of this study included the per-protocol analysis, small sample size and poor study retention, with a 20% dropout among patients assigned to CPAP. In the multicenter Sleep Apnea Cardiovascular Endpoints (SAVE) study, 2717 patients with established coronary artery or cerebrovascular disease and a diagnosis of moderate-to-severe OSA were randomized to CPAP or usual care.24 After a mean follow-up of 3.7 years, treatment with CPAP did not prevent recurrent cardiovascular events though the mean CPAP usage time of 3.3 hours per night did not meet the study’s criterion for CPAP adherence, ≥ 4 hours of use per night over the first 2 years, making it problematic to estimate actual treatment efficacy. In a prespecified, one-to-one propensity score-matched analysis, participants with ≥ 4 hours of use per night compared to matched participants in the control group had a lower risk of stroke (relative risk, 2.29; 95% CI, 1.05–4.99; p=0.04). This trend was further evaluated in a recent meta-analysis examining both primary and secondary stroke prevention in seven randomized controlled trials assessing the occurrence of major cardiovascular events and the treatment of moderate-to-severe obstructive sleep apnea with CPAP.25 Treatment with CPAP for a mean duration of 3.5 hours per night at a mean follow-up of 37 months did not demonstrate significant reduction in the incidence of major cardiovascular events though serial sensitivity analyses excluding studies with poor CPAP adherence showed an incremental risk reduction in major adverse cardiovascular events. With the exclusion of the SAVE trial, there was a significant 58% risk reduction of major cardiovascular events. A subgroup analysis showed similar results, demonstrating a decreased incidence of major cardiovascular events when CPAP was consistently used for ≥ 4 hours per night. Thus, the shortage of data from randomized trials demonstrating clear efficacy of CPAP in reducing stroke occurrence or recurrence may be related to not achieving an adequate dose of CPAP treatment.
Improving stroke outcome
While observational studies have demonstrated worse recovery in stroke survivors with OSA, the benefit gained from treatment with CPAP on functional recovery remains unknown. Some studies have shown an improvement in stroke severity, depressive symptoms, motor and cognitive recovery and sleepiness,22,23,26–29 while others have shown no difference in stroke severity, anxiety, depression, sleepiness and fatigue (Table 1).30,31 In many of these trials, CPAP adherence and study retention may have been insufficient to produce a clear neurologic or functional benefit. Also, studies with high CPAP adherence and participant retention which were able to show short-term benefits after stroke were typically confined to the supportive inpatient environment. Despite these mixed results, a pooled analysis of 5 studies including nearly 400 ischemic stroke patients with OSA demonstrated greater short-term neurological improvement with CPAP than without.32 The authors concluded that further investigation with an adequately powered randomized controlled trial was needed, though addressing these twin issues of poor tolerance and adherence will be fundamental to realizing the full benefits, if any, of CPAP on stroke recovery.
Table 1.
Randomized controlled trials of CPAP and stroke recovery where treatment was initiated on average within 1 month of stroke and for a treatment period of at least 4 weeks.
| Study | Patients treated with CPAP | Treatment duration and location | Attrition rate in CPAP group | Average adherence, hours/night | Outcomes |
|---|---|---|---|---|---|
| Sandberg et al. (2001)26 | 33 with AHI ≥ 15 | 28 days within rehabilitation unit | 2/33 (6%) | 4.1 ± 3.6 (16/31 with > 4 h/night) |
Improvement in depression but not ADL, cognitive function or delirium |
| Hsu et al. (2006)31 | 15 with AHI ≥ 30 | 8 weeks within hospital and home | 4/15 (27%) | 1.4 (2/15 with > 6 h/night) |
No improvement in ADL, stroke severity, cognitive function, anxiety, depression, sleepiness or QOL |
| Bravata et al. (2011)27 | 16 with airflow limitation on auto-titrating CPAP | 30 days within hospital and home | 9/31 (29%) | 5.1 ± 2.3 (10/16 with ≥ 4 h/night on ≥ 75% nights) |
Improvement in stroke severity (with greater improvement associated with more CPAP use) |
| Brown et al. (2011)30 | 15 with AHI ≥ 5 | 3 months within home | 7/15 (47%) | Estimated < 1 (median 53 hours over median 16 days) | No improvement in sleepiness, fatigue, ADL, depression or stroke severity |
| Parra et al. (2011)23 | 71 with AHI ≥ 25 | 24 months within hospital and home | 20/71 (28%) | 5.3 ± 1.9 |
Improvement in stroke severity and ADL at 1 month but not 3, 12, 24 months and no difference in QOL |
| Ryan et al. (2011)22 | 25 with AHI ≥ 15 | 4 weeks within rehabilitation unit | 3/25 (12%) | 4.96 ± 2.25 | Improvement in stroke severity, sleepiness, motor function, depression but not cognitive function, walking, sustained attention or executive function |
| Aaronson et al. (2016)29 | 20 with AHI ≥ 15 | 4 weeks within rehabilitation unit | 6/20 (30%) | 2.5 ± 2.8 (7/20 with > 4 h/night for ≥ 5 days/week) | Improvement in attention and executive function (with greater improvement associated with more CPAP use) but not other domains of cognitive function, stroke severity, ADL, sleep quality, sleepiness, fatigue, depression or anxiety |
AHI= apnea-hypopnea index; CPAP= continuous positive airway pressure; ADL= activities of daily living; QOL= quality of life
To date, no randomized trials of CPAP after stroke have been sufficiently powered and demonstrated adequate treatment adherence. The upcoming, phase 3 Sleep for Stroke Management And Recovery Trial (Sleep SMART; NCT03812653) will evaluate in over 3,000 participants the treatment of OSA with CPAP on both secondary stroke prevention and acute stroke recovery within the NIH StrokeNet, a network of more than 200 stroke trial sites across the U.S. In order to address issues related to adherence, Sleep SMART will randomize to either CPAP or control only patients with at least 4 hours of CPAP use on a single-night run-in. As such, Sleep SMART may be able to define, for the first time, the role of adequate CPAP therapy within a sufficiently powered study.
Adherence to treatment with continuous positive airway pressure
Long-term adherence to CPAP among stroke patients is lower than in individuals without stroke, with estimates ranging between 12 to 25% when CPAP is initiated soon after a stroke. A dose-response relationship between CPAP use and stroke outcome likely exists,24 and the optimum cutoff for CPAP use necessary to improve stroke outcome may need to be higher than for other symptomatic benefits. Yet treatment with CPAP during the early period after stroke is a challenge for stroke survivors, and the burden of CPAP therapy can be overwhelming.
Compared to the general OSA population, stroke patients with OSA have less daytime sleepiness, making altering patient perceptions of symptomatic benefit from CPAP particularly challenging. Recent trials, however, have shed some light on potential ways to improve CPAP tolerance and adherence. Early CPAP adherence has been shown to be strongly predictive of long-term CPAP use. Among 275 patients with established cardiovascular disease who were randomized into the active CPAP arm of the multicenter SAVE trial, adherence to CPAP declined from 4.4 ± 2 hours per night at 1 month to 3.3 ± 2.4 hours per night at 12 months and the only predictors of CPAP adherence at one year were early CPAP use and initial side effects from CPAP.33 In an effort to improve early tolerance, many studies have focused on interventions to optimize CPAP use and minimize side effects, including closer monitoring, increased encouragement and education, and continual CPAP adjustments to troubleshoot potential side effects with treatment.22,23,27 Improvement in CPAP adherence in stroke patients has also been linked to intensive support by nursing staff and other providers. In a randomized trial during stroke rehabilitation where the average daily use of CPAP was 4.96 hours over a one month treatment period, nurses were trained to administer CPAP to study patients with increased inpatient monitoring and support, which likely influenced environmental and social factors for adherence.22 Psychological variables, including coping strategies, may also influence CPAP adherence and behavioral interventions to increase patient motivation to use CPAP have shown some success in the general population. However, in one recent randomized controlled trial, Sleep Apnea in Transient Ischemic Attack and Stroke (SLEEP TIGHT), patients with recent stroke or TIA who were assigned to an enhanced intervention with behavioral therapy showed similar CPAP use after 12 months compared to patients with the standard intervention.34
Sleep-wake cycle disturbance and stroke
The association of sleep and stroke extends beyond SDB to disorders of the sleep-wake cycle, including long and short sleep duration, circadian rhythm disorders and insomnia. Approximately half of stroke survivors have insomnia.35 In a study utilizing polysomnography and Multiple Sleep Latency Tests 12 months after stroke, sleep latencies were longer and sleep efficiency was worse among motor-impaired, right hemisphere stroke patients compared with age and sex-matched controls.36 These findings were not related to SDB or periodic limb movements, which did not differ between the groups. Insomnia has also been associated with higher rates of incident stroke and worse post-stroke outcome. A Taiwan administrative data study examined 21,438 people with insomnia and 64,314 without who were age and sex matched.37 Those with insomnia had a 54% increased risk of stroke in the ensuing four years. In a study of 123 inpatient rehabilitation patients, insomnia but not SDB was associated with worse activities of daily living ability and less rehabilitation improvements after adjusting for confounders.38 As a treatment, Cognitive Behavior Therapy (CBT) has shown preliminary efficacy as a treatment for insomnia post-stroke.39
Other disturbances of the sleep may also predispose to cerebrovascular disease. People who have rotating shift work may have increased stroke risk, perhaps related to sleep-wake cycle disruption. A report from the Nurses’ Health Study suggested a 4% increased ischemic stroke risk in nurses with rotating night shift work schedules.40 Suboptimal sleep duration has also been linked to increased risk of stroke. In a meta-analysis of 16 prospective studies, a J-shaped association was observed between sleep duration and total stroke with the lowest risk noted in people sleeping for 7 hours.41 Longer sleepers had a higher risk of stroke than short sleepers with a 13% increased risk of total stroke for every 1 hour increment of sleep duration above 7 hours.
One of the most intriguing and disturbing hypotheses about the relationship of sleep disturbances and stroke is that it is, in part, responsible for the increased stroke risk among poor and minority populations.42 Different data sources find that populations who reside in inner cities are frequently kept awake at night by loud environmental sounds such as industrial plants, sirens and public and private transport vehicles. These result in reduced total sleep, insomnia, reduced sleep efficiency and excessive daytime sleepiness.43 Several studies have suggested increased stroke risk in low socio-economic status neighborhoods. It is possible that reduced sleep duration and reduced sleep quality are links between neighborhood disadvantage and stroke.44
Conclusion
It is apparent that there is an association of both SDB and other sleep impairments with stroke. Sleep disturbances appear to be both a stroke risk factor for, and worsened by stroke. As such, remedies to reduce sleep impairments may have important roles in both primary and secondary stroke prevention. On-going clinical trials will determine whether prompt treatment of SDB will improve outcome and reduce second stroke. Further, increased attention on reducing insomnia through cognitive behavior therapy, and the general recognition of the importance of sleep may improve non-SDB sleep disturbances. Sleep disturbances, while equally prevalent among different race/ethnic populations,45 are more profoundly felt by the poor and minority groups due to these populations’ more prevalent location in cities where noise, light and other impediments to sleep are more common.
The anatomy, physiology and clinical data paint a coherent picture of sleep disturbance as both risk for stroke and linked to poor stroke outcome. Researchers and clinicians are poised to bring this often overlooked stroke risk factor into the limelight with new vigor.
ACKNOWLEDGMENTS
SOURCES OF FUNDING
NIH R01NS38916 (LBM)
Footnotes
CONFLICTS OF INTEREST/DISCLOSURES
Both authors are investigators in the NIH funded Sleep SMART trial.
REFERENCES
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: A report from the american heart association. Circulation. 2017;135:e146–e603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown DL, Jiang X, Li C, Case E, Sozener CB, Chervin RD, et al. Sleep apnea screening is uncommon after stroke [published online September 27, 2018]. Sleep Med. 2018. 10.1016/j.sleep.2018.09.009. Accessed March 15, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckert DJ, Malhotra A, Jordan AS. Mechanisms of apnea. Prog Cardiovasc Dis. 2009;51:313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Javaheri S, Barbe F, Campos-Rodriguez F, Dempsey JA, Khayat R, Javaheri S, et al. Sleep apnea: Types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69:841–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hermann DM, Siccoli M, Kirov P, Gugger M, Bassetti CL. Central periodic breathing during sleep in acute ischemic stroke. Stroke. 2007;38:1082–1084 [DOI] [PubMed] [Google Scholar]
- 6.Siccoli MM, Valko PO, Hermann DM, Bassetti CL. Central periodic breathing during sleep in 74 patients with acute ischemic stroke - neurogenic and cardiogenic factors. J Neurol. 2008;255:1687–1692 [DOI] [PubMed] [Google Scholar]
- 7.Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and tia patients: A meta-analysis. J Clin Sleep Med. 2010;6:131–137 [PMC free article] [PubMed] [Google Scholar]
- 8.Parra O, Arboix A, Bechich S, Garcia-Eroles L, Montserrat JM, Lopez JA, et al. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. Am J Respir Crit Care Med. 2000;161:375–380 [DOI] [PubMed] [Google Scholar]
- 9.Sahlin C, Sandberg O, Gustafson Y, Bucht G, Carlberg B, Stenlund H, et al. Obstructive sleep apnea is a risk factor for death in patients with stroke: A 10-year follow-up. Arch Intern Med. 2008;168:297–301 [DOI] [PubMed] [Google Scholar]
- 10.Brown DL, Mowla A, McDermott M, Morgenstern LB, Hegeman G 3rd, Smith MA, et al. Ischemic stroke subtype and presence of sleep-disordered breathing: The basic sleep apnea study. J Stroke Cerebrovasc Dis. 2015;24:388–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O’Connor GT, Resnick HE, et al. Obstructive sleep apnea-hypopnea and incident stroke: The sleep heart health study. Am J Respir Crit Care Med. 2010;182:269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041 [DOI] [PubMed] [Google Scholar]
- 14.Dong JY, Zhang YH, Qin LQ. Obstructive sleep apnea and cardiovascular risk: Meta-analysis of prospective cohort studies. Atherosclerosis. 2013;229:489–495 [DOI] [PubMed] [Google Scholar]
- 15.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384 [DOI] [PubMed] [Google Scholar]
- 16.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loredo JS, Nelesen R, Ancoli-Israel S, Dimsdale JE. Sleep quality and blood pressure dipping in normal adults. Sleep. 2004;27:1097–1103 [DOI] [PubMed] [Google Scholar]
- 18.Wolf J, Hering D, Narkiewicz K. Non-dipping pattern of hypertension and obstructive sleep apnea syndrome. Hypertens Res. 2010;33:867–871 [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Garcia MA, Campos-Rodriguez F, Soler-Cataluna JJ, Catalan-Serra P, Roman-Sanchez P, Montserrat JM. Increased incidence of nonfatal cardiovascular events in stroke patients with sleep apnoea: Effect of cpap treatment. Eur Respir J. 2012;39:906–912 [DOI] [PubMed] [Google Scholar]
- 20.Selic C, Siccoli MM, Hermann DM, Bassetti CL. Blood pressure evolution after acute ischemic stroke in patients with and without sleep apnea. Stroke. 2005;36:2614–2618 [DOI] [PubMed] [Google Scholar]
- 21.Kaneko Y, Hajek VE, Zivanovic V, Raboud J, Bradley TD. Relationship of sleep apnea to functional capacity and length of hospitalization following stroke. Sleep. 2003;26:293–297 [DOI] [PubMed] [Google Scholar]
- 22.Ryan CM, Bayley M, Green R, Murray BJ, Bradley TD. Influence of continuous positive airway pressure on outcomes of rehabilitation in stroke patients with obstructive sleep apnea. Stroke. 2011;42:1062–1067 [DOI] [PubMed] [Google Scholar]
- 23.Parra O, Sanchez-Armengol A, Bonnin M, Arboix A, Campos-Rodriguez F, Perez-Ronchel J, et al. Early treatment of obstructive apnoea and stroke outcome: A randomised controlled trial. Eur Respir J. 2011;37:1128–1136 [DOI] [PubMed] [Google Scholar]
- 24.McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. Cpap for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–931 [DOI] [PubMed] [Google Scholar]
- 25.Khan SU, Duran CA, Rahman H, Lekkala M, Saleem MA, Kaluski E. A meta-analysis of continuous positive airway pressure therapy in prevention of cardiovascular events in patients with obstructive sleep apnoea. Eur Heart J. 2018;39:2291–2297 [DOI] [PubMed] [Google Scholar]
- 26.Sandberg O, Franklin KA, Bucht G, Eriksson S, Gustafson Y. Nasal continuous positive airway pressure in stroke patients with sleep apnoea: A randomized treatment study. Eur Respir J. 2001;18:630–634 [DOI] [PubMed] [Google Scholar]
- 27.Bravata DM, Concato J, Fried T, Ranjbar N, Sadarangani T, McClain V, et al. Continuous positive airway pressure: Evaluation of a novel therapy for patients with acute ischemic stroke. Sleep. 2011;34:1271–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minnerup J, Ritter MA, Wersching H, Kemmling A, Okegwo A, Schmidt A, et al. Continuous positive airway pressure ventilation for acute ischemic stroke: A randomized feasibility study. Stroke. 2012;43:1137–1139 [DOI] [PubMed] [Google Scholar]
- 29.Aaronson JA, Hofman WF, van Bennekom CA, van Bezeij T, van den Aardweg JG, Groet E, et al. Effects of continuous positive airway pressure on cognitive and functional outcome of stroke patients with obstructive sleep apnea: A randomized controlled trial. J Clin Sleep Med. 2016;12:533–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown DL, Chervin RD, Kalbfleisch JD, Zupancic MJ, Migda EM, Svatikova A, et al. Sleep apnea treatment after stroke (sats) trial: Is it feasible? J Stroke Cerebrovasc Dis. 2013;22:1216–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu CY, Vennelle M, Li HY, Engleman HM, Dennis MS, Douglas NJ. Sleep-disordered breathing after stroke: A randomised controlled trial of continuous positive airway pressure. J Neurol Neurosurg Psychiatry. 2006;77:1143–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsivgoulis G, Alexandrov AV, Katsanos AH, Barlinn K, Mikulik R, Lambadiari V, et al. Noninvasive ventilatory correction in patients with acute ischemic stroke: A systematic review and meta-analysis. Stroke. 2017;48:2285–2288 [DOI] [PubMed] [Google Scholar]
- 33.Chai-Coetzer CL, Luo YM, Antic NA, Zhang XL, Chen BY, He QY, et al. Predictors of long-term adherence to continuous positive airway pressure therapy in patients with obstructive sleep apnea and cardiovascular disease in the save study. Sleep. 2013;36:1929–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bravata DM, Sico J, Vaz Fragoso CA, Miech EJ, Matthias MS, Lampert R, et al. Diagnosing and treating sleep apnea in patients with acute cerebrovascular disease. J Am Heart Assoc. 2018;7:e008841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palomaki H, Berg A, Meririnne E, Kaste M, Lonnqvist R, Lehtihalmes M, et al. Complaints of poststroke insomnia and its treatment with mianserin. Cerebrovasc Dis. 2003;15:56–62 [DOI] [PubMed] [Google Scholar]
- 36.Sterr A, Kuhn M, Nissen C, Ettine D, Funk S, Feige B, et al. Post-stroke insomnia in community-dwelling patients with chronic motor stroke: Physiological evidence and implications for stroke care. Sci Rep. 2018;8:8409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu MP, Lin HJ, Weng SF, Ho CH, Wang JJ, Hsu YW. Insomnia subtypes and the subsequent risks of stroke: Report from a nationally representative cohort. Stroke. 2014;45:1349–1354 [DOI] [PubMed] [Google Scholar]
- 38.Huang RJ, Lai CH, Lee SD, Pai FY, Chang SW, Chung AH, et al. Objective sleep measures in inpatients with subacute stroke associated with levels and improvements in activities of daily living. Arch Phys Med Rehabil. 2018;99:699–706 [DOI] [PubMed] [Google Scholar]
- 39.Herron K, Farquharson L, Wroe A, Sterr A. Development and evaluation of a cognitive behavioural intervention for chronic post-stroke insomnia. Behav Cogn Psychother. 2018;46:641–660 [DOI] [PubMed] [Google Scholar]
- 40.Brown DL, Feskanich D, Sanchez BN, Rexrode KM, Schernhammer ES, Lisabeth LD. Rotating night shift work and the risk of ischemic stroke. Am J Epidemiol. 2009;169:1370–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He Q, Sun H, Wu X, Zhang P, Dai H, Ai C, et al. Sleep duration and risk of stroke: A dose-response meta-analysis of prospective cohort studies. Sleep Med. 2017;32:66–74 [DOI] [PubMed] [Google Scholar]
- 42.Jackson CL, Redline S, Emmons KM. Sleep as a potential fundamental contributor to disparities in cardiovascular health. Annu Rev Public Health. 2015;36:417–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Canivet C, Nilsson PM, Lindeberg SI, Karasek R, Ostergren PO. Insomnia increases risk for cardiovascular events in women and in men with low socioeconomic status: A longitudinal, register-based study. J Psychosom Res. 2014;76:292–299 [DOI] [PubMed] [Google Scholar]
- 44.Troxel WM, DeSantis A, Richardson AS, Beckman R, Ghosh-Dastidar B, Nugroho A, et al. Neighborhood disadvantage is associated with actigraphy-assessed sleep continuity and short sleep duration. Sleep. 2018;41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Lott LB, Lisabeth LD, Sanchez BN, Morgenstern LB, Smith MA, Garcia NM, et al. Prevalence of pre-stroke sleep apnea risk and short or long sleep duration in a bi-ethnic stroke population. Sleep Med. 2014;15:1582–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]