Abstract
Dysregulated transforming growth factor beta (TGFβ) signaling is observed in a variety of human cancers. TGFβ is produced in large quantities by many tumor types and is known to be pro-oncogenic. Therapeutic strategies directed against TGFβ signaling using neutralizing antibodies and small molecular inhibitors have been developed. However, TGFβ is also found to function as a tumor suppressor. This switch from a tumor suppressor in premalignant stages of tumorigenesis to a tumor promoter in later stages of the disease poses great challenges in TGFβ-targeted cancer therapy. It remains unclear what mechanisms underlie the dual role of TGFβ and what factors mediate the switch. In the past, most work on dissecting underlying mechanisms was focused on differential regulation of signaling pathways by tumor cell autonomous TGFβ signaling. Recent progress in elucidating TGFβ effects on host immune/inflammatory reactions in the tumor microenvironment and distant organs brings exciting new perspectives to the field.
Keywords: TGFβ, Tumor suppressor, Metastasis, Immune, Inflammation, Tumor microenvironment
1. TGFβ and cancer metastasis: challenges in clinical application of TGFβ-targeted therapy
Alterations in transforming growth factor beta (TGFβ) signaling are common in human cancers. TGFβ is often produced in large quantities in many tumors. High TGFβ activity is present in aggressive, highly proliferative gliomas and is associated with a poor prognosis in patients [1]. Tgfbr2 mutation in colon cancers is associated with a favorable outcome after adjuvant chemotherapy [2]. In mouse models, enhancement of TGFβ signaling by expression of a constitutively active TGFβ1 or TβRI in mammary epithelial cells in conjunction with c-Neu or PyVmT expression increases pulmonary metastases [3], and consistently, systemic inhibition of TGFβ signaling suppresses pulmonary metastases [3]. TGFβ signaling is also a well-known mediator in the “vicious cycle” of osteolytic bone metastases [4]. TGFβ promotes tumor progression by many mechanisms. Among these is dysregulation of cyclindependent kinase inhibitors, alteration in cytoskeletal architecture which is often implicated in epithelial to mesenchymal transition increases in proteases and extracellular matrix formation, decreases in immune surveillance, and increases in angiogenesis.
However, TGFβ also functions as a tumor suppressor in early tumor development. In a number of human cancers, mutations in the genes encoding TβRI and TβRII (Tgfbrl and Tgfbr2, respectively) or decreases in expression and phosphorylation of other components of this pathway have been reported. In mouse models, the conditional knockout of Tgfbr2 in different tumor models has resulted in the development of much more aggressive tumors [5–8]. These data support TGFβ as a tumor suppressor, through which TGFβ inhibits cell cycle progression, increases apoptosis, and suppresses the expression of growth factors, cytokines, and chemokines.
This dual role of TGFβ poses a great challenge for TGFβ-targeted therapy. In fact, multiple clinical trials are underway with various approaches, including neutralizing antibodies and small molecular inhibitors (Fig. 1). Apparently, the therapeutic effects of TGFβ antagonism treatment rest on our understanding of the mechanisms by which TGFβ switches from a tumor suppressor to a tumor promoter. The goal of developing therapies is to abolish the tumor-promoting effect of TGFβ while maintaining its tumor-suppressive properties. It is clear that the function of TGFβ as a tumor suppressor or a tumor promoter depends on context and stage of tumor progression [9], but what factors mediate this switch of TGFβ signaling in function from tumor suppressor to tumor promoter? When does this switch occur?
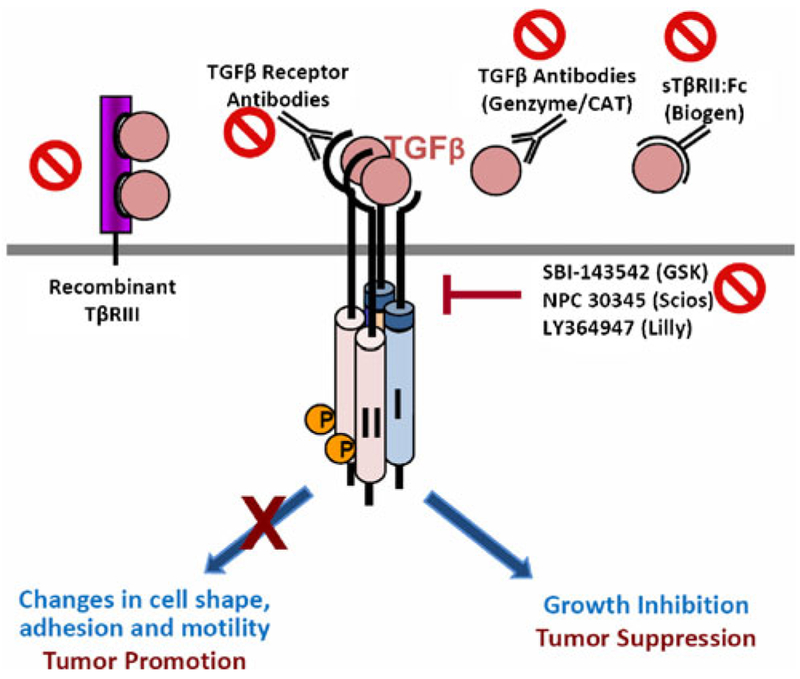
Fig. 1.
Strategies in TGFβ-targeted therapy. A variety of antagonists against TGFβ have been developed for cancer therapy, which include neutralizing antibodies to eliminate TGFβ ligands (recombinant TβRIII, TGFβ antibody, sTβRII:Fc fusion protein), or to block ligand-receptor binding (TGFβ receptor antibody), as well as small molecule kinase inhibitors against the TGFβ receptors. The challenge for a successful cancer therapy relies on how to precisely aim at the tumor-promoting activity of TGFβ but spare its tumor-suppressing function
A good body of literature support that the diverse autonomous tumor cell signaling pathways play significant roles, which is often characterized by changes in signal intensity and connectivity of SMAD-dependent and independent pathways. The TGFβ ligands (TGFβ1, 2, and 3) signal through the type I and type II TGFβ receptors (TβRI and TβRII, respectively). Canonical signaling proceeds with phosphorylation of Smad2 and Smad3, which then combined with Smad4 to enter the nucleus and modulate transcription in cooperation with other transcription factors, co-activators and co-repressors. Additionally, TGFβ binding to its receptors activates many non-canonical signaling pathways including PI3-kinase, MAP kinase, and small GTPase pathways (Fig. 2).
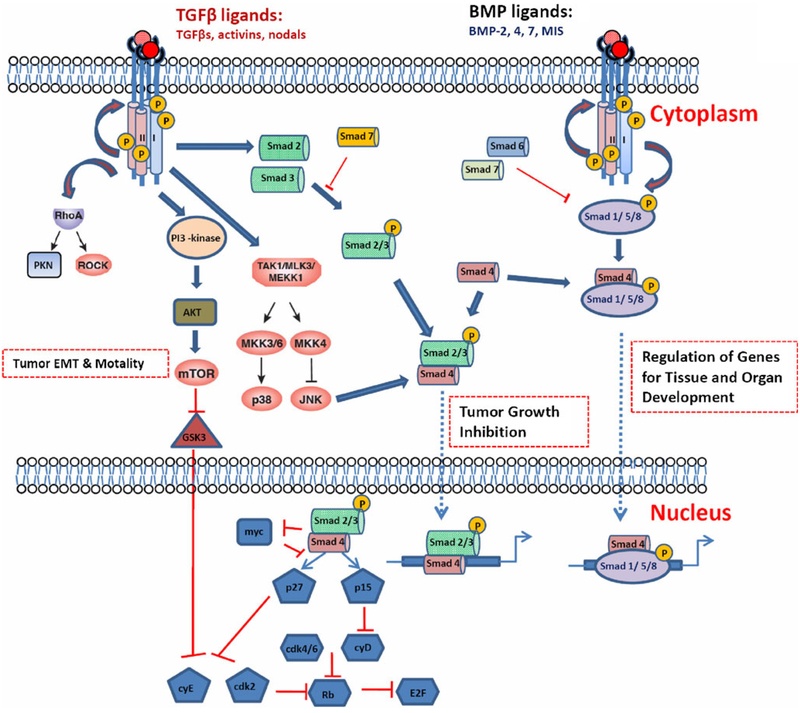
Fig. 2.
Simplified TGFβ signaling pathways. The TGFβ ligand transduces its signal through type I and type II TGFβ receptors. Canonical signaling leads to phosphorylation of Smad2 and Smad3, which then combine with Smad4, and translocate into the nucleus and mediate growth inhibition. Smad7 functions as a negative mediator in this process that blocks Smad2/3 phosphorylation. TGFβ also activates many non-canonical signaling pathways through interaction with the same receptors. It includes activation of small Rho GTPases, p38 kinase, and PI3 kinase pathways. These are important in regulating tumor cell migration and metastasis. In addition, bone morphogenetic proteins (BMPs) signal through Smad1, Smad5, or Smad8, which interact with Smad4 and activate or repress targeted gene transcription important for tissue and organ development. Smad6 and 7 are natural antagonists in TGFβ signaling pathway
It is postulated that SMAD-dependent pathways mediate the growth inhibition of TGFβ signaling, whereas the SMAD-independent pathways likely contribute to the tumor-promoting effect of the TGFβ signaling cascade, synergizing with the oncogenic amplification of MYC, activating mutations of RAS, as well as inactivating mutations of downstream effectors such as retinoblastoma and cyclin-dependent kinase inhibitors. However, recent data dispute this hypothesis. Recently, Smad signaling was found to be responsible for lung metastasis through induction of angiopoietin-like 4 (ANGPTL4) by TGFβ [10]. High TGFβ-Smad activity is present in aggressive, highly proliferative gliomas and confers poor prognosis in patients with gliomas [1]. Interestingly, mutant-p53 may promote Smad-mediated tumor metastasis [11]. In addition, it has also been found that ubiquitination tightly controls Smad4 activity [12]. Phosphorylation of Smad1 and Smad5 through ALK5, the L45 loop motif of the type I TGF receptor, mediates the pro-tumor activity of the TGFβ “switch” in breast cancer [13]. These data show a very diverse and complex role of TGFβ signaling. Yet, the host immune/inflammatory cells may hold the answer [14].
2. Tumor microenvironment: an indispensible participant in metastasis
Tumor metastasis accounts for the majority of cancerassociated death in patients. There are very few effective treatment options. During metastasis, tumor cells must disseminate, intravasate into circulation at the primary tumor site, travel through the vascular systems, arrest in capillary beds, and subsequently extravasate into the organ parenchyma. In a hostile distant organ, they must escape host immune surveillance in order to survive and grow. Evidence from recent years strongly suggests that the tumor microenvironment is an indispensible participant in this process. It provides a favorable microenvironment for tumor cells to not only escape from host immune surveillance, but also induce the formation of new blood vessels and invade the vasculature. Tumor is described as unhealed chronic inflamed wound, filled with various inflammatory cells (Fig. 3) including Tie2+, VEGFR1+, CD11b+, and F4/80+ subpopulations, as well as the innate and adaptive immune cells—natural killer T cells (NKT), T, and B cells. There is also an abundance of cancerassociated fibroblasts and endothelial progenitor cells [15]. These stromal cells collectively create an environment that favors tumor progression by providing growth factors, proangiogenic factors, proteases, and adhesion molecules that facilitate tumor cell proliferation, angiogenesis, invasion, and metastasis [15–19]. This very dynamic tumor microenvironment likely serves as a selective pressure for tumor cell variants through genomic instability, genomic heterogeneity, and epigenetic alterations [20].
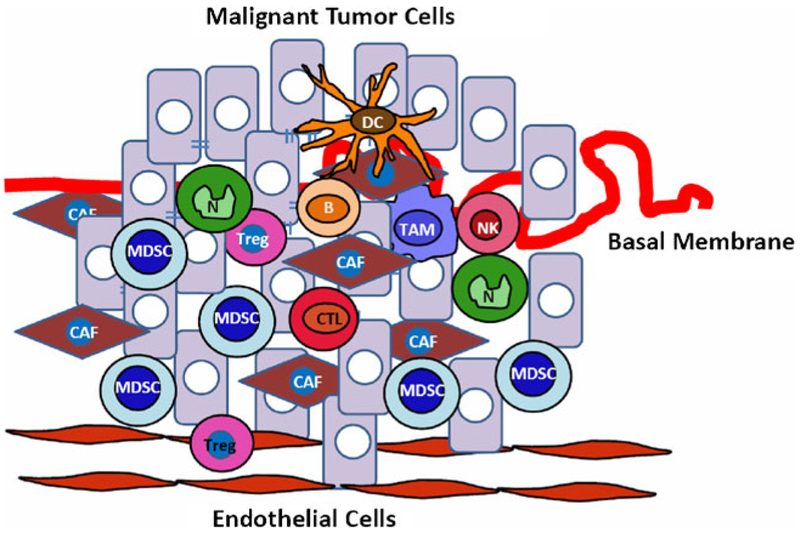
Fig. 3.
Host cell infiltration during tumorigenesis. A variety of host bone marrow-derived cells, which include CD8+ CTL (cytotoxic T lymphocyte), natural killer (NK), regulatory T cell (Treg) and B cell lymphocyte, as well as macrophages, neutrophils, and Gr-1+CD11b+ cells or MDSCs, infiltrate into tumors and create a microenvironment that favors tumor growth and progression
Tumor-infiltrating lymphoid cells include cytotoxic T lymphocytes (CTL), regulatory T cells (Treg), NKT, and B cells. CTL proliferation and function is usually suppressed under tumor conditions through various mechanisms: suppression of the transcription of genes encoding multiple key proteins such as perforin, granzymes, and cytotoxins that act through the granule exocytosis pathway [21, 22]; loss of the colocalization of the T-cell receptor and CD8 [23]; the development of other T-cell types such as tumor- infiltrating gamma/delta T cells that suppress T and dendritic cell function through Toll-like receptor 8 ligands, MyD88, TRAF6, IKKα, IKKβ, and p38α molecules [24]; and aberrant expression of stress-inducible NKG2D ligands (MIC) by later stage tumor cells, which causes sustained NKG2D costimulation and population expansion of immunosuppressive T cells [25]. CD4+CD25+ Tregs are induced in the tumor microenvironment. TGFβ is well known to induce Foxp3 expression and generates these Treg cells [26, 27]. Proinflammatory CD4+ T cells play a tumor-promoting role in the HPV16 mouse model (a transgenic model of multi-stage squamous carcinogenesis induced by human papillomavirus oncogenes), and deletion of CD4+ T cells results in a delayed neoplastic progression with a lower incidence of tumors [28]. IL-4-expressing CD4+ T lymphocytes regulate phenotype and function of CD11b+F4/80+ macrophages, which in turn enhance EGFR signaling in mammary epithelial cells promoting invasion and metastasis [29]. Chronic activation of B cells is indicated in potentiating carcinoma development. Genetic elimination of mature T and B lymphocytes in a transgenic mouse model of K14-HPV16 limits neoplastic progression to development of epithelial hyperplasias, which can be corrected by adoptive transfer of B lymphocytes or serum from HPV16 mice [30]. In fact, B cells, humoral immunity, and activating FcyRs are required for establishing chronic inflammatory programs that promote de novo carcinogenesis [31]. TGFβ (and/or IL-10) is responsible for Breg-mediated immune suppression [32, 33]. B-cell depletion could therapeutically enhance antitumor immune responses by decreasing IL-10 production [32]. Complement C5a in a tumor microenvironment recruits myeloid-derived suppressor cells that suppress CTL function, promoting tumor growth [34]. The NKT cell is a new natural killer cell type that produces IL-13, which signals through the IL-4R-STAT6 pathway to regulate the production of TGFβ in Gr-1+CD11b+ myeloid cells [35–37].
Tumor-infiltrating myeloid cells include three major cell types: Gr-1+CD11b+ immature myeloid cells, tumorassociated macrophages (TAM), and tumor-associated neutrophils (TAN). Gr-1+CD11b+ cells are a heterogeneous set of immature myeloid cells, while TAM and TAN are well differentiated. It is remains to be seen how these three populations of cells are interrelated in phenotype and function under tumor conditions and in the tumor microenvironment. Gr-1+CD11b+ cells are also called myeloid immune suppressor cells (MISCs) or immune-derived suppressor cells. They are well known for their immune suppressive effects [38–41]. In addition, Gr-1+CD11b+ myeloid cells produce MMP9 and TGFβ [18, 19], which has a profound impact on tumor progression and metastasis through modulation of tumor vascularization and tumor cell invasion [18, 19]. TAMs are identified as Mac-1 (CD11b+) and/or F4/80+. They have been shown to promote tumor progression and metastasis [42–48] through elevated CSF-1 production and enhanced EGF signaling in cancer cells [43]. TAN, identified as CD11b+Ly6G+ cells, have only recently been reported. TGFβ regulates N1–N2 polarization of neutrophils [49]. This N1–N2 polarization of neutrophils may mirror the M1–M2 polarization of macrophages that are defined by interferon-γ and IL-4 production as Th1 and Th2 cells, respectively [50].
In summary, a variety of immune cells infiltrate into tumors constituting the tumor microenvironment. The outcome of tumor development to some extent is determined by the type of immune response. CTL, Th1 cells, and NK cells likely mediate anti-tumor immunity through the production of IL-2 and IFNγ, which inhibit tumor development and progression. The probable outcome of the immune response involving B cells and activation of humoral immunity and/or a Th2 polarized response is likely promoting tumor development and progression via production ofIL-4, −5, −6, −10, and −13, as well as GMCSF [51].
3. TGFβ is a key regulator of inflammation and tumor microenvironment
Recent evidence strongly suggests that TGFβ is one of the critical regulators in the inflammatory reaction that orchestrates the tumor microenvironment. Specific deletion of Tgfbr2 in different types of epithelial cells including mammary [7], pancreatic [5], intestinal [6], colon [52], and head-and-neck squamous cell carcinomas [53] results in increased tumor progression and metastasis, which is mediated through inflammation and the tumor microenvironment [14, 19, 54]. Interestingly, this complete abrogation of TGFβ signaling correlates with reduced relapse-free survival in four human breast cancer data sets, especially in patients with estrogen receptor-positive tumors [55]. Deletion of SMAD4, the down-stream mediator of TGFβ signaling, in a colon cancer model results in an increased recruitment of CCR1+ myeloid cells (CD34+) that promote tumor invasion [56].
In addition to epithelial cells, TGFβ signaling in stromal cells also plays a significant role in tumor development and growth. Loss of the TGFβ type II receptor in a subset of fibroblasts (FSP promoter driving cre expression) leads to intraepithelial dysplasia in prostate cancer and invasive squamous cell carcinoma in the fore-stomach through upregulation of TGFα, MSP- and HGF-mediated signaling networks [57]. This is often accompanied by increased abundance of stromal cells and inflammatory cell infiltration. Deletion of TGFβ signaling in T lymphocytes results in the development of carcinomas throughout the GI tract, which is also accompanied by increased inflammatory gene expression of IL-5, IL-6, and IL-13 resulting in inflammatory cell infiltration [58].
The effects of TGFβ on inflammation and tumor microenvironment may be regulated through nuclear factor kappa B (NF-κB), a master regulator of inflammatory reactions and a critical mediator in tumor development and progression. For instance, TGFβ1 negatively regulates NF- κB activation through Smad7 [59], a critical mediator of TGFβ signaling thus blocking proinflammatory TNF signals [60]. Mice deficient in SMAD3 develop colon cancer due to increased inflammation caused by Helicobacter infection [61]. In TGFβ1-deficient mice, inflammation causes precancerous lesions to progress into colon cancer [62]. Recently, it was found that tumor-infiltrating RANKL (receptor activator of NF-κB ligand)-expressing cells activate nuclear IKKα and inhibit the transcription of the tumor metastasis suppressor Maspin, thereby promoting tumor metastasis [63]. Additionally, TGFβ cross talks with inflammatory pathways through the modulation of IL-1 [64]. These findings demonstrate that TGFβ, a tumor suppressor, also functions as an inflammation suppressor. When it is deleted in the epithelial or stromal compartment, it causes an increased inflammatory reaction that promotes tumor development and progression. But, contradictory to the above observations, over-expression of TGFβ 1 in head and neck epithelia results in inflammation, angiogenesis, and epithelial hyperproliferation [65]. It is unclear what underlies these different observations, and whether there are different chemokine/chemokine receptor mechanisms involved in deletion of TGFβ signaling versus increased TGFβ signaling.
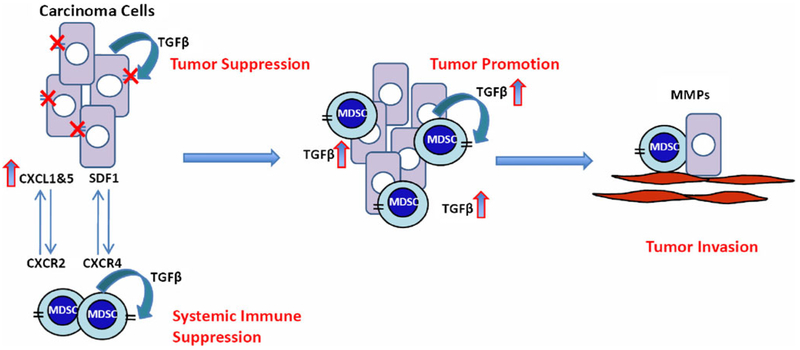
Our recent work suggests that TGFβ regulates the production of chemokine/chemokine receptors that are important for inflammatory cell recruitment. This includes stromal derived factor 1 (SDF-1 or CXCL12), key regulators of hematopoietic stem and progenitor cell trafficking between the peripheral circulation and targeted tumor tissues. SDF-1 mediates its effects on chemotaxis through its receptor, CXCR4, which is highly expressed on putative stem and progenitor cells [15, 16]. In studies carried out in our lab, we found that TGFβ also suppresses expression of CXCL1 and CXCL5. Deleted or diminished TGFβ signaling in tumor epithelial cells significantly elevates the expression of CXCL1 and CXCL5 [19, 54]. CXCL5 interacts with the CXCR2 chemokine receptor to recruit Gr-1+CD11b+ myeloid suppressor cells to the tumor microenvironment [19]. As myeloid progenitor cells, Gr-1 +CD11b+ cells express high levels of CXCR4, which interacts with SDF-1 in the tumor microenvironment to mediate the recruitment of these myeloid cells (Fig. 3). In the tumor microenvironment, these myeloid cells produce large quantities of matrix metalloproteases including MMP2, MMP13, and MMP14. Interestingly, tumors that lack TGFβ signaling produce significantly higher levels of TGF β 1 than the control tumors, probably due to increased infiltration of Gr-1+CD11b+ myeloid cells. The increased production of MMPs and TGFβ1 enhances tumor invasion and metastasis (Fig. 4).
Fig. 4.
Potential mechanisms by which TGFβ signaling switches from a tumor suppressor to a tumor promoter. TGFβ functions through the type II receptor on tumor cells to exhibit growth inhibition of carcinoma cells. TβRII deletion/mutation or downregulation leads to an increased CXCL1-CXCL5/CXCR2 and SDF-1/CXCR4 chemokine/chemokine receptor signaling, and subsequent recruitment of host-derived immature myeloid Gr-1+CD11b+ cells into tumors. These infiltrating myeloid cells produce large quantity of TGFβ1 and MMPs that suppress the host immune system and concurrently promote tumor invasion. The effect of Gr-1+CD11b+ cells on the tumor microenvironment and host immune surveillance constitutes a tumor-promoting mechanism of TGF β signaling
Aside from the tumor microenvironment, a recent report also demonstrates an important role of TGFβ in the distant premetastatic lung. In the premetastatic lungs of tumorbearing mice, TGFβ regulates the production of chemoattractants S100A8 and S100A9, which attract Mac1+ myeloid cells and activate mitogen-activated protein kinase p38 in tumor cells. This activation confers invasive activity via the development of pseudopodia (invadopodia) [66]. In addition, TGFβ also induces tumor cells to produce angiopoietin-like 4 that disrupts vascular endothelial cell–cell junctions and decreases the integrity of lung capillaries, facilitating trans-endothelial passage of tumor cells [10].
4. Other tumor suppressors in inflammation and the tumor microenvironment
Do tumor suppressors other than TGFβ function as inflammatory suppressors? p53 is a very important tumor suppressor, regulating cell cycle, check point control, DNA damage/repair, and induction of apoptosis [67]. It is the most commonly mutated gene in human cancers [68]. p53 mutations in mice are associated with inflammation, bile duct injury, and fibrosis, which is similar to intrahepatic cholangiocarcinoma, a lethal human malignancy of the biliary epithelium [69]. p53 is also frequently mutated in ulcerative colitis-associated dysplastic lesions and colorectal cancer. The increased frequency of specific p53 mutations in non-cancerous ulcerative colitis may confer susceptibility to the development of colorectal cancer through an increased level of nitric oxide in the microenvironment [70, 71]. Combined loss of p53 and Atr, an essential genome maintenance regulator, leads to severe defects in hair follicle regeneration and deterioration of the intestinal epithelium. This is associated with increased inflammatory Mac1+Gr1+ cell infiltration [72]. Head and neck squamous cell carcinomas with mutated p53 show differentially expressed gene signatures compared to those with wild-type p53. NF-kB phospho-p65 is inversely associated with p53 [73].
Pten, a tumor suppressor with lipid and protein phosphatase activity, is a major regulator of PI3K signaling [74]. Genetic inactivation of Pten in stromal fibroblasts of mouse mammary glands accelerates the initiation, progression, and malignant transformation of mammary epithelial tumors, which is associated with the massive remodeling of extracellular matrix (ECM), innate immune cell infiltration, and increased angiogenesis [75]. This loss of Pten also leads to increased expression, phosphorylation, and recruitment of Ets2, a protein that modulates gene expression associated with inflammation, angiogenesis, and ECM [75].
APC tumor suppressor gene is mutated in the Min mouse in which multiple benign polyps develop in the intestine. A compound heterozygote of APC and Smad4 also leads to intestinal polyps which develop into more malignant tumors characterized by extensive stromal cell proliferation, submucosal invasion, and cell-type heterogeneity. These tumors show increased CD34+ immature myeloid cell infiltration, elevated matrix metalloproteinases MMP9 and MMP2, as well as increased CC-chemokine receptor CCR1 [56, 76].
Semaphorin 3B (SEMA3B), a putative tumor suppressor, is expressed at high levels in many invasive and metastatic human cancers. SEMA3B suppresses primary tumor growth but also induces the production of IL-8 by tumor cells, which in turn, leads to the recruitment of tumor-associated macrophages and metastatic dissemination to the lung [77]. SEMA3B seems to possess dual functions in tumor development. It inhibits primary tumor growth, but enhances metastatic dissemination.
In addition to tumor suppressor, metastasis suppressor is also a very interesting class of molecules that are important in tumor host interaction. A metastasis suppressor is defined as a molecule whose expression results in the inhibition of a cancer cell’s ability to metastasize while having little or no effect on primary tumor growth [78]. More than 20 of them have been found, including Ecadherin, Nm23, BRMS1, and others. The majority of disseminated cells, which should be fully malignant, persist in a potentially dormant state for a prolonged period. They do not proliferate immediately at secondary sites. While genetic mutations or epigenetic changes may be required for a cell or group of cells to separate and survive at distance from the primary tumor, the microenvironment within secondary tissues plays a substantial role in influencing whether disseminated cells survive and proliferate. Studying these suppressor proteins helps to dissect interactions among cancer cells and their microenvironment hopefully identifying targets for inhibiting metastatic growth and prolonged disease-free survival [79].
5. Anti-metastasis effect of TGFβ antagonistis dependent on host inflammation/inflammation responses
Increasing evidence supports that tumor metastasis is a coordinated event between tumor cells and host cells through inflammation. In fact, inflammatory cells produce much more TGFβ than tumor cells [19, 36]. Gr−1+CD11b+ cells infiltrate into tumors in response to a loss of TGFβ signaling, yet tumor cells with deletion of Tgfbr2 produced more TGFβ than their control counterpart [19]. Indeed, recent developments in the field point out that the efficacy of TGFβ antagonist therapy may not derive from a direct effect on tumor cells as was originally thought, but rather through mechanisms acting on the host compartment. Blocking the presence of TGFβ may reduce the suppression of host immune surveillance. This effect is particularly important as TGFβ antagonists may be combined with antitumor immunotherapy. Secondly, TGFβ antagonism may abrogate the tumor-promoting effect of Gr-1+CD11b+ cells in the tumor microenvironment by neutralizing the high levels of TGFβ1 that they produce [19]. Third, TGFβ antagonism could be very effective in blocking the “vicious cycle” of bone metastasis as TGFβ signaling is a key player in the differentiation of osteoclasts that destroy the bone [4]. Lastly, TGFβ antagonism may be effective for patients directly after radiation or chemotherapy because there is a surge in TGFβ production after these treatments [80].
TGFβ is a critical regulator of the tumor microenvironment and distant premetastatic organ conditions. TGFβ exerts systemic immune suppression and significantly inhibits host tumor immune surveillance [26, 27, 81]. Neutralizing TGFβ in preclinical mouse models enhances CD8+ T-cell and natural killer cell-mediated anti-tumor immune response [82–84]. It also increases neutrophilattracting chemokines and results in the recruitment and activation of neutrophils with an anti-tumor phenotype [49]. In addition to the systemic effects, TGFβ also regulates the infiltration of inflammatory/immune cells and cancerassociated fibroblasts into the tumor microenvironment, which affects the signaling cascade in tumor cells. This emerging understanding of TGFβ regulation on the interface between tumor cells and host immune cells may provide new insight for the development of successful TGFβ antagonist treatments. Perhaps profiling of the inflammatory Gr-1+CD11b+ cells in the tumor microenvironment could be used as a biomarker for patient selection in the ongoing phase I/II clinical trials of TGFβ-based therapy. This is supported by recent findings indicating that these immune/inflammatory responses are reliable markers for predicting clinical outcome in human hepatocellular carcinoma [85] and colorectal tumors [86], as well as breast cancer [87]. These studies also point out a novel therapeutic strategy for advanced cancers.
Acknowledgments
We thank Dr B.R. Achyut for graphic assistance and Nan Roche for editorial assistance.
References
- 1.Bruna A, Darken RS, Rojo F, Ocana A, Penuelas S, Arias A, et al. (2007). High TGFbeta-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell, 11, 147–160 [DOI] [PubMed] [Google Scholar]
- 2.Watanabe T, Wu TT, Catalano PJ, Ueki T, Satriano R, Haller DG, et al. (2001). Molecular predictors of survival after adjuvant chemotherapy for colon cancer. New England Journal of Medicine, 344, 1196–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arteaga CL (2006). Inhibition of TGFbeta signaling in cancer therapy. Current Opinion in Genetics & Development, 16, 30–37. [DOI] [PubMed] [Google Scholar]
- 4.Mundy GR (2002). Metastasis to bone: Causes, consequences and therapeutic opportunities. Nature Reviews. Cancer, 2, 584–593. [DOI] [PubMed] [Google Scholar]
- 5.Ijichi H, Chytil A, Gorska AE, Aakre ME, Fujitani Y, Fujitani S, et al. (2006). Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-beta signaling in cooperation with active Kras expression. Genes & Development, 20, 3147–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munoz NM, Upton M, Rojas A, Washington MK, Lin L, Chytil A, et al. (2006). Transforming growth factor beta receptor type II inactivation induces the malignant transformation of intestinal neoplasms initiated by Apc mutation. Cancer Research, 66, 9837–9844. [DOI] [PubMed] [Google Scholar]
- 7.Forrester E, Chytil A, Bierie B, Aakre M, Gorska AE, Sharif-Afshar AR, et al. (2005). Effect of conditional knockout of the type II TGF-beta receptor gene in mammary epithelia on mammary gland development and polyomavirus middle T antigen induced tumor formation and metastasis. Cancer Research, 65, 2296–2302. [DOI] [PubMed] [Google Scholar]
- 8.Guasch G, Schober M, Pasolli HA, Conn EB, Polak L, & Fuchs E (2007). Loss of TGFbeta signaling destabilizes homeostasis and promotes squamous cell carcinomas in stratified epithelia. Cancer Cell, 12, 313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massague J (2008). TGFbeta in cancer. Cell, 134, 215–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, et al. (2008). TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell, 133, 66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adorno M, Cordenonsi M, Montagner M, Dupont S, Wong C, Hann B, et al. (2009). A mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell, 137, 87–98. [DOI] [PubMed] [Google Scholar]
- 12.Dupont S, Mamidi A, Cordenonsi M, Montagner M, Zacchigna L, Adorno M, et al. (2009). FAM/USP9x, a deubiquitinating enzyme essential for TGFbeta signaling, controls Smad4 monoubiquitination. Cell, 136, 123–135. [DOI] [PubMed] [Google Scholar]
- 13.Liu IM, Schilling SH, Knouse KA, Choy L, Derynck R, & Wang XF (2009). TGFbeta-stimulated Smad1/5 phosphorylation requires the ALK5 L45 loop and mediates the pro-migratory TGFbeta switch. EMBO Journal, 28, 88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang L, & Moses HL (2008). Transforming growth factor beta: Tumor suppressor or promoter? Are host immune cells the answer? Cancer Research, 68, 9107–9111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, et al. (2008). HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell, 13, 206–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balkwill F, & Coussens LM (2004). Cancer: An inflammatory link. Nature, 431, 405–406. [DOI] [PubMed] [Google Scholar]
- 17.Coussens LM, & Werb Z (2002). Inflammation and cancer. Nature, 420, 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang L, Debusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, et al. (2004). Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell, 6, 409–421. [DOI] [PubMed] [Google Scholar]
- 19.Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, et al. (2008). Abrogation of TGFbeta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell, 13, 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bristow RG, & Hill RP (2008). Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nature Reviews. Cancer, 8, 180–192 [DOI] [PubMed] [Google Scholar]
- 21.Thomas DA, & Massague J (2005). TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell, 8, 369–380. [DOI] [PubMed] [Google Scholar]
- 22.Trapani JA (2005). The dual adverse effects of TGF-beta secretion on tumor progression. Cancer Cell, 8, 349–350. [DOI] [PubMed] [Google Scholar]
- 23.Demotte N, Stroobant V, Courtoy PJ, Van Der Smissen P, Colau D, Luescher IF, et al. (2008). Restoring the association of the T cell receptor with CD8 reverses anergy in human tumor-infiltrating lymphocytes. Immunity, 28, 414–424. [DOI] [PubMed] [Google Scholar]
- 24.Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, & Wang RF (2007). Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity, 27, 334–348. [DOI] [PubMed] [Google Scholar]
- 25.Groh V, Smythe K, Dai Z, & Spies T (2006). Fas-ligand-mediated paracrine T cell regulation by the receptor NKG2D in tumor immunity. Nature Immunology, 7, 755–762. [DOI] [PubMed] [Google Scholar]
- 26.Wahl SM, Wen J, & Moutsopoulos N (2006). TGF-beta: A mobile purveyor of immune privilege. Immunological Reviews, 213, 213–227. [DOI] [PubMed] [Google Scholar]
- 27.Li MO, & Flavell RA (2008). TGF-beta: A master of all T cell trades. Cell, 134, 392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daniel D, Meyer-Morse N, Bergsland EK, Dehne K, Coussens LM, & Hanahan D (2003). Immune enhancement of skin carcinogenesis by CD4+ T cells. Journal of Experimental Medicine, 197, 1017–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, et al. (2009). CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell, 16, 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Visser KE, Korets LV, & Coussens LM (2005). De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell, 7, 411–423. [DOI] [PubMed] [Google Scholar]
- 31.Andreu P, Johansson M, Affara NI, Pucci F, Tan T, Junankar S, et al. (2010). FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell, 17, 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inoue S, Leitner WW, Golding B, & Scott D (2006). Inhibitory effects of B cells on antitumor immunity. Cancer Research, 66, 7741–7747. [DOI] [PubMed] [Google Scholar]
- 33.Mauri C, & Ehrenstein MR (2008). The ‘short’ history of regulatory B cells. Trends in Immunology, 29, 34–40. [DOI] [PubMed] [Google Scholar]
- 34.Markiewski MM, DeAngelis RA, Benencia F, Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C, et al. (2008). Modulation of the antitumor immune response by complement. Nature Immunology, 9, 1225–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, et al. (2000). NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nature Immunology, 1, 515–520. [DOI] [PubMed] [Google Scholar]
- 36.Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, et al. (2003). Transforming growth factorbeta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: Abrogation prevents tumor recurrence. Journal of Experimental Medicine, 198, 1741–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terabe M, & Berzofsky JA (2007). NKT cells in immunoregulation of tumor immunity: A new immunoregulatory axis. Trends in Immunology, 28, 491–496. [DOI] [PubMed] [Google Scholar]
- 38.Serafini P, Borrello I, & Bronte V (2006). Myeloid suppressor cells in cancer: Recruitment, phenotype, properties, and mechanisms of immune suppression. Seminars in Cancer Biology, 16, 53–65. [DOI] [PubMed] [Google Scholar]
- 39.Marx J (2008). Cancer immunology. Cancer’s bulwark against immune attack: MDS cells. Science, 319, 154–156. [DOI] [PubMed] [Google Scholar]
- 40.Marigo I, Dolcetti L, Serafini P, Zanovello P, & Bronte V (2008). Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunological Reviews, 222, 162–179. [DOI] [PubMed] [Google Scholar]
- 41.Gabrilovich DI, & Nagaraj S (2009). Myeloid-derived suppressor cells as regulators of the immune system. Nature Reviews. Immunology, 9, 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pollard JW (2004). Tumour-educated macrophages promote tumour progression and metastasis. Nature Reviews. Cancer, 4, 71–78. [DOI] [PubMed] [Google Scholar]
- 43.Condeelis J, & Pollard JW (2006). Macrophages: Obligate partners for tumor cell migration, invasion, and metastasis. Cell, 124, 263–266. [DOI] [PubMed] [Google Scholar]
- 44.Mantovani A, Schioppa T, Porta C, Allavena P, & Sica A (2006). Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Reviews, 25, 315–322. [DOI] [PubMed] [Google Scholar]
- 45.Mantovani A, Allavena P, Sica A, & Balkwill F (2008). Cancer-related inflammation. Nature, 454, 436–444. [DOI] [PubMed] [Google Scholar]
- 46.Pollard JW (2009). Trophic macrophages in development and disease. Nature Reviews. Immunology, 9, 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joyce JA, & Pollard JW (2009). Microenvironmental regulation of metastasis. Nature Reviews. Cancer, 9, 239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mantovani A (2009). Cancer: Inflaming metastasis. Nature, 457, 36–37. [DOI] [PubMed] [Google Scholar]
- 49.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. (2009). Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell, 16, 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mantovani A (2009). The yin-yang of tumor-associated neutrophils. Cancer Cell, 16, 173–174. [DOI] [PubMed] [Google Scholar]
- 51.de Visser KE, Eichten A, & Coussens LM (2006). Paradoxical roles of the immune system during cancer development. Nature Reviews. Cancer, 6, 24–37. [DOI] [PubMed] [Google Scholar]
- 52.Biswas S, Chytil A, Washington K, Romero-Gallo J, Gorska AE, Wirth PS, et al. (2004). Transforming growth factor beta receptor type II inactivation promotes the establishment and progression of colon cancer. Cancer Research, 64, 4687–4692. [DOI] [PubMed] [Google Scholar]
- 53.Lu SL, Herrington H, Reh D, Weber S, Bornstein S, Wang D, et al. (2006). Loss of transforming growth factor-beta type II receptor promotes metastatic head-and-neck squamous cell carcinoma. Genes & Development, 20, 1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bierie B, Stover DG, Abel TW, Chytil A, Gorska AE, Aakre M, et al. (2008). Transforming growth factor-beta regulates mammary carcinoma cell survival and interaction with the adjacent microenvironment. Cancer Research, 68, 1809–1819. [DOI] [PubMed] [Google Scholar]
- 55.Bierie B, & Moses HL (2009). Gain or loss of TGFbeta signaling in mammary carcinoma cells can promote metastasis. Cell Cycle, 8, 3319–3327. [DOI] [PubMed] [Google Scholar]
- 56.Kitamura T, Kometani K, Hashida H, Matsunaga A, Miyoshi H, Hosogi H, et al. (2007). SMAD4-deficient intestinal tumors recruit CCR1(+) myeloid cells that promote invasion. Nature Genetics, 39, 467–475. [DOI] [PubMed] [Google Scholar]
- 57.Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, et al. (2004). TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science, 303, 848–851. [DOI] [PubMed] [Google Scholar]
- 58.Kim BG, Li C, Qiao W, Mamura M, Kasprzak B, Anver M, et al. (2006). Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature, 441, 1015–1019. [DOI] [PubMed] [Google Scholar]
- 59.Monteleone G, Mann J, Monteleone I, Vavassori P, Bremner R, Fantini M, et al. (2004). A failure of transforming growth factor-beta1 negative regulation maintains sustained NF-kappaB activation in gut inflammation. J Biol Chem, 279, 3925–3932. [DOI] [PubMed] [Google Scholar]
- 60.Hong S, Lee C, & Kim SJ (2007). Smad7 sensitizes tumor necrosis factor induced apoptosis through the inhibition of antiapoptotic gene expression by suppressing activation of the nuclear factor-kappaB pathway. Cancer Research, 67, 9577–9583. [DOI] [PubMed] [Google Scholar]
- 61.Maggio-Price L, Treuting P, Zeng W, Tsang M, Bielefeldt- Ohmann H, & Iritani BM (2006). Helicobacter infection is required for inflammation and colon cancer in SMAD3-deficient mice. Cancer Research, 66, 828–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Engle SJ, Ormsby I, Pawlowski S, Boivin GP, Croft J, Balish E, et al. (2002). Elimination of colon cancer in germ-free transforming growth factor beta 1-deficient mice. Cancer Research, 62, 6362–6366. [PubMed] [Google Scholar]
- 63.Luo JL, Tan W, Ricono JM, Korchynskyi O, Zhang M, Gonias SL, et al. (2007). Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature, 446, 690–694. [DOI] [PubMed] [Google Scholar]
- 64.Lu T, Tian L, Han Y, Vogelbaum M, & Stark GR (2007). Dose-dependent cross-talk between the transforming growth factor-beta and interleukin-1 signaling pathways. Proceedings of the National Academy of Sciences of the United States ofAmerica, 104, 4365–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu SL, Reh D, Li AG, Woods J, Corless CL, Kulesz- Martin M, et al. (2004). Overexpression of transforming growth factor beta1 in head and neck epithelia results in inflammation, angiogenesis, and epithelial hyperproliferation. Cancer Research, 64, 4405–4410. [DOI] [PubMed] [Google Scholar]
- 66.Hiratsuka S, Watanabe A, Aburatani H, & Maru Y (2006). Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nature Cell Biology, 8, 1369–1375. [DOI] [PubMed] [Google Scholar]
- 67.Kruse JP, & Gu W (2009). Modes of p53 regulation. Cell, 137, 609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hainaut P, & Hollstein M (2000). p53 and human cancer: The first ten thousand mutations. Advances in Cancer Research, 77, 81–137. [DOI] [PubMed] [Google Scholar]
- 69.Farazi PA, Zeisberg M, Glickman J, Zhang Y, Kalluri R, & DePinho RA (2006). Chronic bile duct injury associated with fibrotic matrix microenvironment provokes cholangiocarcinoma in p53-deficient mice. Cancer Research, 66, 6622–6627. [DOI] [PubMed] [Google Scholar]
- 70.Hussain SP, Amstad P, Raja K, Ambs S, Nagashima M, Bennett WP, et al. (2000). Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: A cancer-prone chronic inflammatory disease. Cancer Research, 60, 3333–3337. [PubMed] [Google Scholar]
- 71.Hussain SP, He P, Subleski J, Hofseth LJ, Trivers GE, Mechanic L, et al. (2008). Nitric oxide is a key component in inflammation-accelerated tumorigenesis. Cancer Research, 68, 7130–7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ruzankina Y, Schoppy DW, Asare A, Clark CE, Vonderheide RH, & Brown EJ (2009). Tissue regenerative delays and synthetic lethality in adult mice after combined deletion of Atr and Trp53. Nature Genetics, 41, 1144–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee TL, Yang XP, Yan B, Friedman J, Duggal P, Bagain L, et al. (2007). A novel nuclear factor-kappaB gene signature is differentially expressed in head and neck squamous cell carcinomas in association with TP53 status. Clinical Cancer Research, 13, 5680–5691. [DOI] [PubMed] [Google Scholar]
- 74.Salmena L, Carracedo A, & Pandolfi PP (2008). Tenets of PTEN tumor suppression. Cell, 133, 403–414. [DOI] [PubMed] [Google Scholar]
- 75.Trimboli AJ, Cantemir-Stone CZ, Li F, Wallace JA, Merchant A, Creasap N, et al. (2009). Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature, 461, 1084–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takaku K, Oshima M, Miyoshi H, Matsui M, Seldin MF, & Taketo MM (1998). Intestinal tumorigenesis in compound mutant mice of both Dpc4 (Smad4) and Apc genes. Cell, 92, 645–656. [DOI] [PubMed] [Google Scholar]
- 77.Rolny C, Capparuccia L, Casazza A, Mazzone M, Vallario A, Cignetti A, et al. (2008). The tumor suppressor semaphorin 3B triggers a prometastatic program mediated by interleukin 8 and the tumor microenvironment. Journal of Experimental Medicine, 205, 1155–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bodenstine TM, & Welch DR (2008). Metastasis suppressors and the tumor microenvironment. Cancer Microenvironment, 1, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Taylor J, Hickson J, Lotan T, Yamada DS, & Rinker- Schaeffer C (2008). Using metastasis suppressor proteins to dissect interactions among cancer cells and their microenvironment. Cancer Metastasis Reviews, 27, 67–73. [DOI] [PubMed] [Google Scholar]
- 80.Biswas S, Guix M, Rinehart C, Dugger TC, Chytil A, Moses HL, et al. (2007). Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. Journal of Clinical Investigation, 117, 1305–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li MO, Wan YY, Sanjabi S, Robertson AK, & Flavell RA (2006). Transforming growth factor-beta regulation of immune responses. Annual Review of Immunology, 24, 99–146. [DOI] [PubMed] [Google Scholar]
- 82.Nam JS, Terabe M, Mamura M, Kang MJ, Chae H, Stuelten C, et al. (2008). An anti-transforming growth factor beta antibody suppresses metastasis via cooperative effects on multiple cell compartments. Cancer Research, 68, 3835–3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nam JS, Terabe M, Kang MJ, Chae H, Voong N, Yang YA, et al. (2008). Transforming growth factor beta subverts the immune system into directly promoting tumor growth through interleukin-17. Cancer Research, 68, 3915–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fujita T, Teramoto K, Ozaki Y, Hanaoka J, Tezuka N, Itoh Y, et al. (2009). Inhibition of transforming growth factor-beta-mediated immunosuppression in tumor-draining lymph nodes augments antitumor responses by various immunologic cell types. Cancer Research, 69, 5142–5150. [DOI] [PubMed] [Google Scholar]
- 85.Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA, et al. (2006). Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell, 10, 99–111. [DOI] [PubMed] [Google Scholar]
- 86.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. (2006). Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science, 313, 1960–1964. [DOI] [PubMed] [Google Scholar]
- 87.Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, et al. (2008). Stromal gene expression predicts clinical outcome in breast cancer. Natural Medicines, 14, 518–527. [DOI] [PubMed] [Google Scholar]