Abstract
Growing evidence suggests that exposure to environmental contaminants contributes to the current diabetes epidemic. Inorganic arsenic (iAs), a drinking water and food contaminant, is one of the most widespread environmental diabetogens according to epidemiological studies. Several schemes have been proposed to explain the diabetogenic effects of iAs exposure; however, the exact mechanism remains unknown. We have shown that in vitro exposure to low concentrations of arsenite (iAsIII) or its trivalent methylated metabolites, methylarsonite (MAsIII) and dimethylarsinite (DMAsIII), inhibits glucose-stimulated insulin secretion (GSIS) from isolated pancreatic islets, with little effect on insulin transcription or total insulin content. The goal of this study was to determine if exposure to trivalent arsenicals impairs mitochondrial metabolism, which plays a key role in the regulation of GSIS in β cells. We used a Seahorse extracellular flux analyzer to measure oxygen consumption rate (OCR), a proxy for mitochondrial metabolism, in cultured INS-1 832/13 β cells exposed to iAsIII, MAsIII, or DMAsIII and stimulated with either glucose or pyruvate, a final product of glycolysis and a substrate for the Krebs cycle. We found that 24-h exposure to 2 μM iAsIII or 0.375–0.5 μM MAsIII inhibited OCR in both glucose- and pyruvate-stimulated β cells in a manner that closely paralleled GSIS inhibition. In contrast, 24-h exposure to DMAsIII (up to 2 μM) had no effects on either OCR or GSIS. These results suggest that iAsIII and MAsIII may impair GSIS in β cells by inhibiting mitochondrial metabolism, and that at least one target of these arsenicals is pyruvate decarboxylation or downstream reactions.
Keywords: Arsenic, Insulin secretion, β cells, Mitochondrial respiration, Diabetes
Introduction
Chronic exposure to inorganic arsenic (iAs) has been associated with the development of multiple disorders including cancer, cardiovascular disease, neurological dysfunction, and traits of metabolic syndrome, including impaired glucose homeostasis (Wang et al. 2007; Paul et al. 2011; Martinez et al. 2011; Moon et al. 2012; Tyler and Allan 2014). In 2011, a National Toxicology Program (NTP) expert panel reviewed published data on the association between chronic exposure to iAs in drinking water and diabetes. While the panel concluded that there was sufficient evidence to link chronic, high-level exposure to iAs (> 150 ppb) with risk of diabetes (Maull et al. 2012), additional mechanistic studies were recommended to determine the causal relationship between iAs exposure and diabetes as well as to identify mechanisms underlying the pancreatic β-cell dysfunction and insulin resistance that were reported in individuals exposed to iAs.
In 2013, we showed that in vitro exposure to arsenite (iAsIII) inhibits glucose-stimulated insulin secretion (GSIS) in isolated murine pancreatic islets with little or no effect on insulin mRNA level or insulin content, suggesting a defect in the insulin secretory machinery rather than in insulin synthesis (Douillet et al. 2013). We also examined GSIS in islets exposed to the trivalent methylated metabolites of iAs, methylarsonite (MAsIII) and dimethylarsinite (DMAsIII), and found that these metabolites are more potent inhibitors of GSIS than iAsIII. These data imply that the metabolism of iAs can impact its diabetogenic potential, thus adding a layer of complexity to the association between iAs exposure and the risk of developing diabetes. Although this and other studies provide evidence linking iAs exposure to inhibition of insulin secretion in pancreatic islets, the molecular mechanisms behind this effect remain largely unknown.
Insulin secretion by β cells is a complex process that involves multiple steps, starting with the uptake and oxidation of glucose and production of pyruvate by glycolysis. Pyruvate is shuttled into the mitochondria where it serves as a substrate for the energy (ATP) producing pathways, which are dependent on mitochondrial respiration. ATP generated in these pathways is responsible for the closure of ATP-sensitive potassium channels, leading to β-cell membrane depolarization, calcium influx, and insulin exocytosis (Fu et al. 2013). Thus, optimal mitochondrial function, which is coupled with the oxygen consuming electron transport chain in the inner mitochondrial membrane, is critically important for insulin secretion by the β cell (Maechler 2013). In 2010, Fu and co-workers observed a minor, but statistically significant reduction in oxygen consumption in glucose-stimulated INS-1 832/13 β cells exposed to iAsIII, suggesting a possible impairment in mitochondrial metabolism and ATP production (Fu et al. 2010). Our present study builds on these findings by examining the rate of oxygen consumption in INS-1 832/13 β cells exposed not only to iAsIII, but also to its methylated trivalent metabolites, MAsIII and DMAsIII, which were found to be more potent than iAsIII as inhibitors of GSIS in isolated pancreatic islets (Douillet et al. 2013). Our results suggest that both acute and 24-h exposures to iAsIII and MAsIII diminish GSIS in β cells by inhibiting oxygen-dependent mitochondrial metabolism, and that both arsenicals target the pathways of pyruvate metabolism in mitochondria. Notably, though previously shown to be an inhibitor of GSIS in intact islets, DMAsIII inhibited GSIS in INS-1 832/13 cells only in acute experiments using potentially cytotoxic concentrations and had no significant effects on oxygen consumption under either acute or a 24-h exposure scenario.
Materials and methods
Cell culture
Rat insulinoma cells expressing human pre-proinsulin, INS-1 832/13 (Hohmeier et al. 2000), were cultured at 5% CO2 and 37 °C in RPMI 1640 medium supplemented with 10% FBS, 10 mM HEPES, 2 mM L-glutamine, 1 mM sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin (all from Gibco, Waltham, MA), and 0.05 mM β-mercaptoethanol (Sigma, St. Louis, MO).
Treatment
Twenty-four-hour exposure
INS-1 832/13 cells were exposed to iAsIII (sodium arsenite, > 99% pure; Sigma-Aldrich, St. Louis, MO), MAsIII (methylarsine oxide, > 98% pure), or DMAsIII (iododimethylarsine, > 98% pure) for 24 h prior to measuring oxygen consumption or GSIS. MAsIII and DMAsIII were provided by Dr. William Cullen (University of British Columbia, Vancouver, Canada) and by Dr. Chris Le (University of Alberta, Edmonton, Canada).
Acute exposures
iAsIII, MAsIII, or DMAsIII were introduced into the cell culture medium during the measurement of oxygen consumption or GSIS and effects were observed following addition.
GSIS
INS-1 832/13 cells were seeded at a density of 100,000 cells/well in a 96-well plate 24 h prior to treatment with arsenicals. Following arsenical treatment, cells were placed in a secretion assay buffer (SAB), pH 7.4, containing 114 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.16 mM MgSO4, 20 mM Hepes, 2.5 mM CaCl2, 0.2% bovine serum albumin, 25.5 mM NaHCO3, and 0 mM glucose for 40 min. Cells were then incubated in 2.5 mM glucose SAB for 1 h. Finally, cells were incubated 2 h in 16.7 mM glucose SAB. For acute arsenic exposures and exposure to mitochondrial inhibitors, INS-1 832/13 cells were seeded at a density of 1,000,000 cells/well in a 12-well plate or at 100,000 cells/well in a 96-well plate, respectively, 48 h prior to the GSIS assay. The cells were then incubated in 0 mM glucose SAB for 40 min followed by a 1-h incubation in 2.5 mM glucose SAB. Next, the cells were incubated in 16.7 mM glucose containing either arsenicals, as indicated, or uncouplers or inhibitors of the electron transport chain in mitochondria. In both experiments, medium was collected for the 2.5 and 16.7 mM glucose incubation and cells were lysed for protein normalization. The amount of insulin secreted from cells into the media was determined using a Rat/Mouse Insulin ELISA (Millipore, Billerica, MA).
Oxygen consumption rate (OCR) measurement
INS-1 832/13 cells were seeded at a density of 50,000 cells/well in a Seahorse XFe96 well plate 24 h prior to treatment with arsenicals. Following 24-h arsenic treatment, cells were incubated in 0 mM glucose SAB for 40 min in a 5% CO2, 37 °C incubator, followed by incubation in 2.5 mM glucose SAB (without NaHCO3) for 1 h without supplementation of CO2 at 37 °C. The plate was then placed in the Seahorse XFe96 analyzer (Agilent, Santa Clara, CA) and either 16.7 mM glucose or 10 mM pyruvate was added following baseline OCR measurements. For the mitochondrial stress test, INS-1 832/13 cells treated with arsenicals and untreated cells were incubated in 0 and 2.5 mM glucose SAB as above, then 16.7 mM glucose was added and OCR measured for 30 min. Next, 2 μM oligomycin, 4 μM FCCP (carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone), and a 1 μM mix of rotenone/antimycin A (all from Sigma, St. Louis, MO) were added sequentially with 18-min OCR reads in between each addition. For acute exposures, INS-1 832/13 cells were incubated in 0 and 2.5 mM glucose SAB as above, then 16.7 mM glucose was added and OCR monitored for 18 min. Next, each arsenical was added at indicated concentrations and OCR was monitored for 2 h.
Cell viability: MTT assay
INS-1 832/13 cells were seeded at a density of 100,000 cells/well in a 96-well plate 24 h prior to treatment with arsenicals. Following 24-h (24 h) or acute (2-h) treatment, cells were incubated with 0.5 mg/ml MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) for 1 h in phenol red free RPMI 1640 cell culture medium. Medium was removed, cells were lysed with DMSO, and absorbance at 570 nm was recorded by a Synergy HT plate reader (Biotek, Winooski, VT).
Cell viability: CellTox™ green assay
INS-1 832/13 cells were seeded at a density of 100,000 cells/well in a 96 well plate. 24 h after plating, arsenicals were added along with CellTox™ Green Dye using the express, no-step addition at dosing method for the CellTox™ Green Cytotoxicity Assay (Promega, Madison, WI). Fluorescence at 485Ex/520EM was measured at 0.5, 1, 2, and 24 h following addition of arsenicals in a Synergy HT plate reader. Between each time point assessment, cell plates were returned to normal cell culture incubation conditions as described above.
Analysis of arsenic
The speciation analysis of arsenic using hydride generation-cryotrapping-atomic absorption spectrometry was performed to determine arsenic content and chemical forms in INS-1 832/13 cells exposed to trivalent arsenicals. The detection limits of the method, calibration procedures and methods used for quality assurance have been previously described (Hernández-Zavala et al. 2008).
Statistics
Data were analyzed using a one- or two-way Anova with Dunnet’s post-test comparison or student’s t test. Data are represented as mean ± standard error of the mean (SEM) or as mean ± standard deviation (SD) as indicated; p values less than 0.05 are statistically significant. Statistical analyses were performed using GraphPad Prism version 7.0 (Graph-Pad Software Inc., California).
Results
Effects of acute arsenical exposures on OCR and GSIS
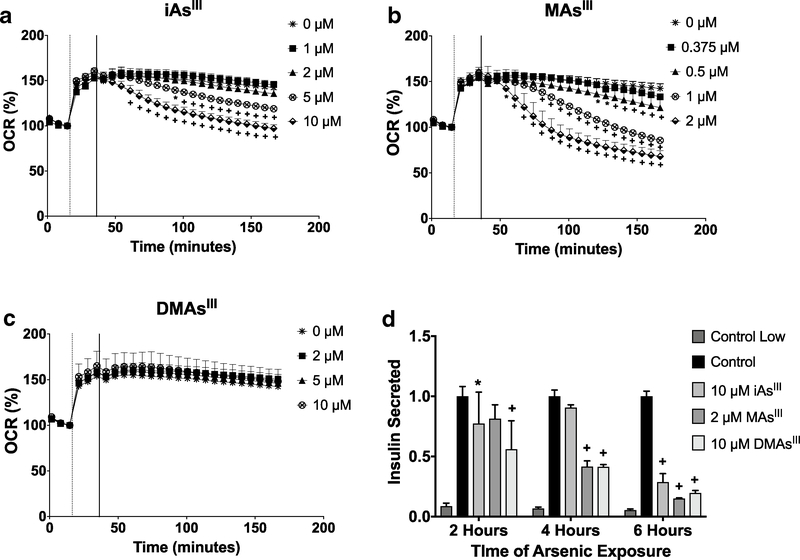
OCR was measured for 2 h following addition of iAsIII, MAsIII, or DMAsIII to the INS-1 832/13 cell monolayer in high glucose (16.7 mM) SAB medium. The addition of iAsIII (5 or 10 μM) or MAsIII (0.375–2 μM) triggered a dose-dependent decrease in OCR that continued during the 2-h exposure window (Fig. 1a, b). In contrast, addition of DMAsIII (up to 10 μM) had no significant effect on OCR (Fig. 1c). The effects of the acute exposure on GSIS were also examined. Insulin secretion was measured in INS-1 832/13 cells cultured in high glucose medium and exposed for 2, 4 and 6 h to 10 μM iAsIII, 2 μMAsIII, or 10 μM DMAsIII. Exposures to iAsIII and MAsIII significantly inhibited GSIS (Fig. 1d), starting at 2 and 4 h, respectively. Surprisingly, a marked inhibition was also observed in cells exposed to 10 μM DMAsIII, the concentration that had no effect on OCR (Fig. 1d).
Fig. 1.
Oxygen consumption and GSIS in INS-1 832/13 cells during acute exposures to iAsIII, MAsIII, and DMAsIII. OCR was measured in INS-1 832/13 cells stimulated with 16.7 mM glucose (marked with vertical dotted line) and following injection of arsenicals (marked with vertical solid line): iAsIII (a), MAsIII (b) or DMAsIII (c). OCR was monitored at 6-min intervals. GSIS was measured in INS-1 832/13 cells cultured in 16.7 mM glucose after acute (2, 4, 6 h) exposure to arsenicals (d). Each time point was normalized to the high glucose control. Mean ± SD is shown for four technical replicates (OCR), and mean + SD is shown for three technical replicates (GSIS). *p < 0.05 and +p < 0.01 for comparisons of treated versus untreated (0 μM) cells
Effects of 24-h exposures on OCR
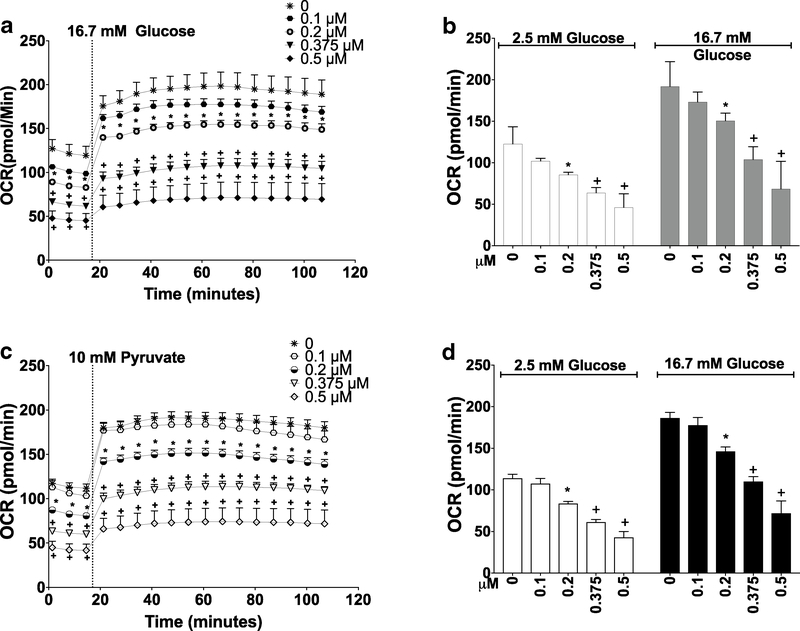
OCR was measured in INS-1 832/13 cells exposed for 24 h to iAsIII, MAsIII, or DMAsIII. Two energetic substrates, glucose and pyruvate, were used to stimulate mitochondrial respiration to determine whether the glycolytic pathway in the cytoplasm or mitochondrial pathways (e.g., Krebs Cycle or oxidative phosphorylation) are targeted by the arsenicals. Exposure to iAsIII resulted in a dose-dependent decrease in OCR in both low glucose (2.5 mM) and high glucose (16.7 mM) media; however, this decrease was only significant in cells exposed to 2 μM iAsIII (Fig. 2a, b). Essentially the same dose-dependent inhibition of OCR by iAsIII was also found in cells stimulated with pyruvate (Fig. 2c, d). MAsIII (0.2–0.5 μM) also inhibited glucose- and pyruvate-stimulated OCR in a dose-dependent manner, and it was a more potent inhibitor than iAsIII in all experimental conditions (Fig. 3). The 24-h exposure to DMAs™ (up to 2 μM) had no statistically significant effects on OCR in cells stimulated with either glucose or pyruvate (Suppl. Figure 1).
Fig. 2.
Oxygen consumption in INS-1 832/13 cells after 24-h exposures to iAsIII. OCR was measured in INS-1 832/13 cells stimulated with 16.7 mM glucose (a, b) or 10 mM pyruvate (c, d) following 24-h exposure to iAsIII. a, c Additions of 16.7 mM glucose or 10 mM pyruvate are marked by dotted lines. b, d OCR values recorded at low glucose (2.5 mM), high glucose (16.7 mM), and pyruvate (10 mM) were averaged to quantify the overall effects of the exposure. Mean + SEM is shown for four biological replicates. *p < 0.05, +p < 0.01 for comparisons of treated versus untreated (0 μM) cells
Fig. 3.
Oxygen consumption in INS-1 832/13 cells after 24-h exposures to MAsIII. OCR was measured in INS-1 832/13 cells stimulated with 16.7 mM glucose (a, b) or 10 mM pyruvate (c, d) following 24-h exposure to MAsIII. a, c Additions of 16.7 mM glucose or 10 mM pyruvate are marked by dotted lines. b, d: OCR values recorded at low glucose (2.5 mM), high glucose (16.7 mM), and pyruvate (10 mM) were averaged to quantify the overall effects of the exposure. Mean + SEM is shown for four biological replicates. *p < 0.05, +p < 0.01 for comparison of treated versus untreated (0 μM) cells
Effects of 24-h exposure on mitochondrial respiration parameters
The mitochondrial stress test was performed to further assess the effects of arsenicals on β-cell function. Here, OCR was measured in glucose-stimulated INS-1 832/13 cells exposed for 24 h to arsenicals and in the control cells before and after a sequential addition of oligomycin (ATP synthase inhibitor), FCCP (uncoupler), and rotenone with antimycin A (inhibitors of complex I and III, respectively). Maximal respiration (i.e., respiration in presence of the uncoupler FCCP), spare respiratory capacity, non-mitochondrial respiration, and proton leak were assessed following the established procedures and formulas (for details see Suppl. Figure 2). Interestingly, maximal respiration was decreased following exposure to all concentrations of iAsIII, and was independent of a change in basal OCR at the 0.5 and 1 μM exposure levels (Fig. 4a). 24-h exposure to 0.375 or 0.5 μM MAsIII also elicited a significant reduction in maximal respiration (Fig. 4a). A significant reduction in spare respiratory capacity was observed following 24-h exposure to all concentrations of iAsIII and to 0.5 μM MAsIII (Fig. 4b) and was consistent with the decrease in maximal respiration. Nonmitochondrial respiration was not significantly altered by either iAsIII or MAsIII exposure (Fig. 4c). However, proton leak was decreased for most exposures (Fig. 4d).
Fig. 4.
Mitochondrial respiration parameters of INS-1 832/13 cells after 24-h exposure to iAsIII or MAsIII. OCR was measured in INS-1 832/13 cells following 24-h exposure to iAsIII or MAsIII. Maximal respiration (a), spare respiratory capacity (b), non-mitochondrial respiration (c), and proton leak (d) were measured following sequential addition of: 16.7 mM glucose, oligomycin, FCCP, and a Rotenone/Antimycin A mix. Mean + SD is shown for six to seven technical replicates with two biological replicates. *p < 0.05, +p < 0.01 for comparison of treated versus control cells
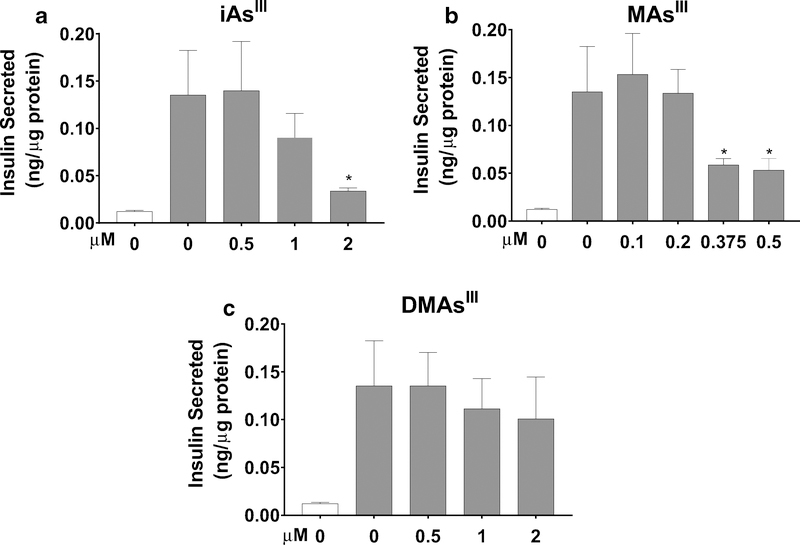
Effects of 24-h exposures and Mitochondrial Inhibitors on GSIS in INS-1 832/13 cells
Since the insulin secretory pathway is tightly coupled with mitochondrial respiration and because the acute exposures to iAsIII and MAsIII inhibited GSIS (Fig. 1d), we performed a GSIS assay in INS-1 832/13 cells after 24-h exposures. Consistent with results of the acute experiments, 24-h exposure to iAsIII inhibited GSIS in a dose-dependent manner, but as observed with 24-h OCR inhibition, the inhibition of GSIS was statistically significant only in cells exposed to 2 μM iAsIII (Fig. 5a). Similarly, MAsIII suppressed GSIS at the concentrations that also inhibited OCR (0.375 and 0.5 μM) (Fig. 5b). The 24-h exposure of up to 2 μM DMAsIII, unlike the acute exposure to 10 μM DMAsIII, had no statistically significant impact on GSIS (Fig. 5c). As a proof of concept, we have also examined insulin secretion by INS-1 832/13 cells that were exposed to oligomycin and rotenone, two inhibitors used in the mitochondrial stress experiments. Here, 2-h exposures to either oligomycin or rotenone resulted in a marked inhibition of GSIS (Suppl. Figure 3), confirming that GSIS in these cells is indeed linked to mitochondrial respiration and ATP synthesis.
Fig. 5.
Glucose stimulated insulin secretion in INS-1 832/13 cells after 24-h exposures to iAsIII, MAsIII, and DMAsIII. INS-1 832/13 cells were exposed to iAsIII (a), MAsIII (b), or DMAsIII (c) for 24 h prior to GSIS. Insulin secretion was measured after stimulation with 2.5 mM glucose (clear bars) followed by 16.7 mM glucose (gray bars). Mean + SD is shown for three biological replicates. *p < 0.05 for comparison of treated versus untreated (0 μM) cells
Arsenic speciation in INS-1 832/13 Cells after 24-h exposures
Speciation analysis of arsenic was performed in the INS-1 832/13 cells exposed for 24 h to iAsIII, MAsIII, or DMAsIII. The majority of arsenic found in the cells (> 98%) was represented by species to which the cells were exposed, suggesting that INS-1 832/13 cells cannot methylate or demethylate trivalent arsenicals (Suppl. Table 1).
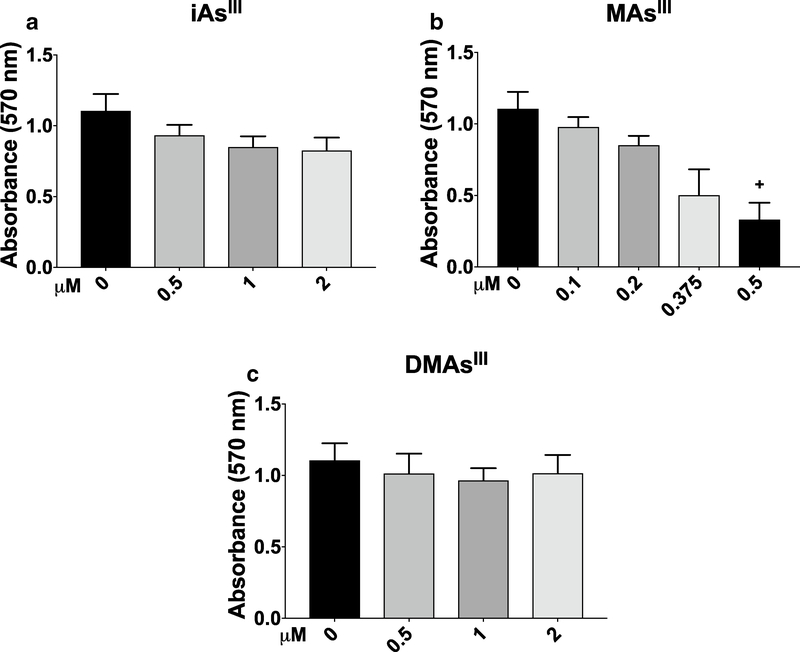
Cell viability after exposure to arsenicals
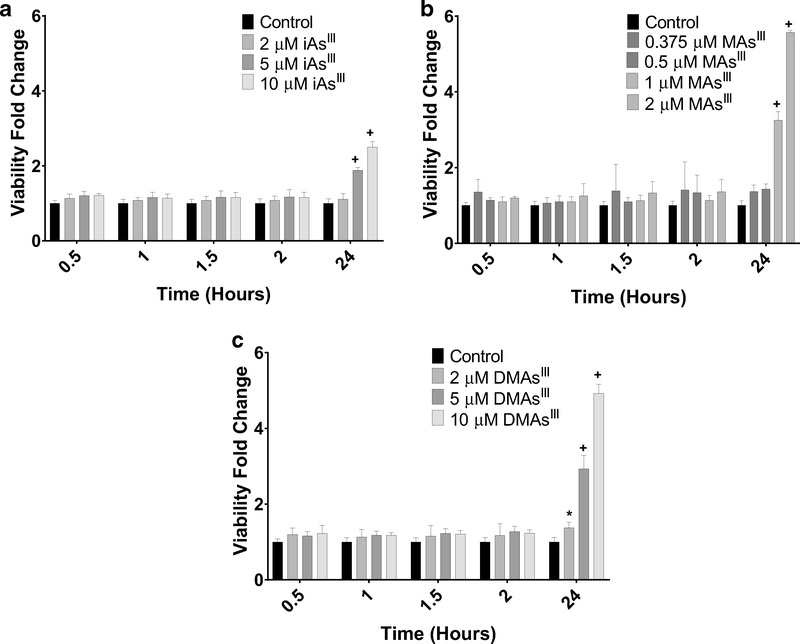
Two separate assays were used to evaluate viability of INS-1 832/13 cells exposed to trivalent arsenicals: the MTT assay, which measures the metabolic fitness of cells (Janjic and Wollheim 1992), and the CellTox™ Green assay, which measures cell membrane integrity and is closely linked to cell death (Koen et al. 2016). Results of the MTT assay indicate a decrease in the metabolic activity of INS-1 832/13 cells exposed for 24 h to 0.2, 0.375, or 0.5 μM MAsIII, but no statistically significant effects were found for iAsIII or DMAsIII exposure (Fig. 6). A significant decrease in metabolic activity was also found in cells exposed acutely (for 2 h) to 5 or 10 μM iAsIII or 0.5 or 2 μM MAsIII, but not in cells exposed to up to 10 μM DMAsIII (Suppl. Figure 4). Using the CellTox™ Green assay, no significant effects on cell viability were found during the acute (0.5, 1, or 1.5 h) exposures to up to 10 μM iAsIII, 2 μM MAsIII, or 10 μM DMAsIII (Fig. 7). However, 24-h exposures to ≥ 5 μM iAsIII, ≥ 1 μM MAsIII, or ≥ 2 μM DMAsIII decreased INS-1 832/13 cell viability (Fig. 7); notably, OCR was inhibited by lower concentrations.
Fig. 6.
Viability of INS-1 832/13 cells after 24-h exposure to iAsIII, MAsIII and DMAsIII: The MTT assay INS-1 832/13 cells were exposed for 24 h to iAsIII (a), MAsIII (b), or DMAsIII (c) and cell viability was measured by the MTT assay. Mean + SEM is shown for three biological replicates. +p < 0.01 for comparisons of treated versus untreated (0 μM) cells
Fig. 7.
Viability of INS-1 832/13 cells after 24-h exposures to iAsIII, MAsIII and DMAsIII: CellTox™ Green. INS-1 832/13 cells were exposed for up to 24 h to iAsIII (a), MAsIII (b), or DMAsIII (c). Cell viability was measured using CellTox™ Green at 0.5, 1, 1.5, and 24 h. Values were normalized to the control at each time point. Mean + SD is shown for four technical replicates. *p < 0.05, +p < 0.01 for comparisons of treated versus untreated (control) cells
Discussion
iAs is a multi-target toxin that acts through a variety of mechanisms. In various cell types, exposure to iAsIII has been linked to defects in mitochondrial function, including damage to mitochondrial DNA, increased production of reactive oxygen species, decreased expression of Krebs cycle enzymes, and decreased mitochondrial membrane potential (Partridge et al. 2007; Sumedha and Miltonprabu 2015; Prakash et al. 2015). Fu and coworkers were the first to show that a prolonged (96-h ) iAsIII exposure suppresses mitochondrial respiration in the INS-1 832/13 β-cell line while inhibiting GSIS (Fu et al. 2010). These effects were associated with increased transcription of antioxidant genes and suppression of reactive oxygen species (ROS), suggesting that the GSIS inhibition was a result of down-regulation of intracellular ROS which may be required for GSIS regulation. These researchers did not identify targets of iAsIII in either the cytosolic or mitochondrial pathways of glucose metabolism. Additionally, no effects were examined of the methylated metabolites of iAs, MAsIII and DMAsIII, though they are generally more toxic than iAsIII and have been shown in our studies to inhibit GSIS in isolated islets (Douillet et al. 2013).
To address these gaps in knowledge, our study focused on OCR as an indirect measure of mitochondrial metabolism, which plays an essential role in regulating GSIS in β cells. We found that trivalent arsenicals, specifically iAsIII and MAsIII, inhibit OCR in clonal INS-1 832/13 β cells at concentrations that also inhibit GSIS. Notably, the degree of inhibition in the INS-1 832/13 cells stimulated with glucose was similar to that found in the cells stimulated with a metabolite of glucose, pyruvate. Supplementing INS-1 832/13 cells with pyruvate, did not rescue the OCR inhibition by iAsIII and MAsIII, suggesting that these arsenicals target pyruvate decarboxylation or metabolic steps downstream from this reaction, possibly Krebs cycle or electron transport which are coupled with oxygen consumption. Surprisingly, DMAsIII, which was a potent inhibitor of GSIS in intact isolated islets in our previous study (Douillet et al. 2013), did not inhibit either GSIS or OCR in the cultured INS-1 832/13 cells in the 24-h exposure experiments and inhibited only GSIS in the acute exposure experiments at high doses (10 μM). Differences in the results of the isolated islets and β-cell line may be due to the composition of islets in that they are of multiple cell types and the cell-to-cell crosstalk is essential for synchronization of insulin secretion from the β cells (Yang et al. 2011; Caicedo 2013). Thus, while not directly affecting β cells, DMAsIII may target the mechanisms involved in the cell-to-cell communication leading to an impairment of GSIS in intact islets. Furthermore, the requirement for the thiol-reactive reagent β-mercaptoethanol in the culture medium of INS-1 832/13 cells may impact the action of arsenicals in the cell, especially DMAsIII, which can bind with high affinity to the single thiol group of β-mercaptoethanol (Shen et al. 2013). An additional factor that plays a role in experiments involving DMAsIII exposure is the low stability of this arsenical; DMAsIII readily oxidizes to a non-toxic pentavalent analog, DMAsV (Gong et al. 2001). Thus, it is possible that DMAsIII oxidized during the 24-h exposures allowing the β cells in culture to recover and to restore both mitochondrial respiration and GSIS. This would also explain why the short-acute exposure to DMAsIII did inhibit GSIS. However, the fact that the acute exposure did not affect OCR suggests that mechanisms other than mitochondrial dysfunction may underlie GSIS inhibition by this arsenical.
Interestingly, both iAsIII and MAsIII inhibited maximal mitochondrial respiration (i.e., oxygen consumption in presence of an uncoupler) and spare respiratory capacity, suggesting a direct effect on electron carriers of the electron transport chain in the inner mitochondrial membrane. This finding is consistent with the previously reported inhibition of the mitochondrial electron transport in rat RLC-16 liver cells exposed to the trivalent arsenicals (Naranmandura et al. 2011). Conversely, there is no significant difference in nonmitochondrial respiration between any treatment groups, suggesting that the mitochondria are the primary targets and that OCR inhibition by the arsenicals is exclusively due to suppression of mitochondrial respiration. In other studies, arsenicals have been shown to inhibit essential reactions that feed into the electron transport chain including pyruvate dehydrogrenase, succinate dehydrogenase, α-ketoglutarate dehydrogenase, among other components of the Krebs cycle, which could generate a similar outcome of reduced OCR as observed in the present study (Petrick et al. 2001; Bergquist et al. 2009; Hosseini et al. 2013). In either scenario, iAsIII and MAsIII appear to inhibit either the reactions that feed into the electron transport chain or the electron transport chain itself.
In the present study, MAsIII was more potent than iAsIII as an inhibitor of OCR in INS-1 832/13 β cells. These findings are consistent with the results of another study conducted in vascular smooth muscle cells, which showed a decrease in OCR after exposure to 0.5 μM MAsIII, whereas exposure to 10 μM iAsIII had no significant effect on OCR (Pace et al. 2016). These data suggest that iAsIII and MAsIII may interact with different mitochondrial targets. A study examining electron transport in RLC-16 rat liver cells exposed to trivalent arsenicals further corroborate our findings. Here, in vitro exposure to MAsIII inhibited both complex II and complex IV of the electron transport chain, whereas DMAsIII only inhibited complex II, and iAsIII did not inhibit activity of any electron transport complexes (Naranmandura et al. 2011). Taken together, these data indicate that MAsIII, an intermediate product of iAs methylation, may significantly contribute to the diabetogenic effects of iAs exposure by disrupting key mechanisms regulating GSIS in β cells, including mitochondrial function.
Although results of the present study strongly suggest that iAsIII and MAsIII impair mitochondrial function leading to impaired GSIS, the implications for human exposures and the development of diabetes remain unclear. There is no data on the concentrations of iAs or its methylated metabolites in pancreata of humans chronically exposed to iAs. Although β-cell dysfunction has been reported in individuals exposed to iAs (Del Razo et al. 2011; Gribble et al. 2012; Díaz-Villaseñor et al. 2013; Rhee et al. 2013; Peng et al. 2015), it is unclear if it was associated with β-cell loss or with impaired β-cell function. Our results suggest that to inhibit mitochondrial function, the concentrations of trivalent arsenicals would need to reach micromolar or sub-micromolar levels that may be toxic to β cells. The results of the CellTox™ Green membrane integrity assay show no or minor losses of cell viability at exposure levels that inhibited OCR in INS-1 832/13 cells. The MTT assay, which measures metabolic activity of the cells, suggests a reduction in the metabolic activity following 24-h exposure to MasIII. However, it has been previously reported that inhibition of metabolic activity as recorded by the MTT assay preceded any appreciable change in cell viability (cell death) (Green and Leeuwenburgh 2002). Notably, we have shown that inhibition of metabolic activity measured by MTT in primary hepatocytes exposed to iAsIII or MAsIII can be completely reversed as the arsenicals are converted to DMAs during a 24-h exposure (Styblo et al. 2000). Additionally, we see no appreciable loss of cell membrane integrity during the acute exposure, where OCR was significantly inhibited by very short exposures to iAsIII or MAsIII. The OCR inhibition under these conditions cannot be explained by changes in gene transcription, as suggested in Fu et al. (2010), but points again to direct interactions of the arsenicals with metabolic pathways that are linked to glucose metabolism and/or OCR. Overall, the results of the MTT assay performed in INS-1 832/13 cells combined with the results of the Seahorse XF assay are consistent with inhibition of mitochondrial metabolism and do not necessarily indicate cell death. More data on the concentrations of iAs and its metabolites in human tissues are needed to help to better explain the results of this study and to inform designs of future mechanistic studies examining effects of in vitro exposures of iAs in cultured cells or tissues.
In summary, this study is the first to compare the effects of iAsIII and its methylated trivalent metabolites, DMAsIII and MAsIII, on GSIS and OCR in a pancreatic β-cell model. The results suggest that inhibition of OCR by iAsIII and/or MAsIII may be one of the mechanisms by which iAs exposure impairs β-cell function, leading to diabetes in humans. Future studies should identify the specific metabolic steps and mechanisms that are targeted by iAs and its metabolites in β cells. Results of these studies may ultimately help to improve the understanding of how iAs affects mitochondria not only in β cells, but also in other cell types that regulate glucose homeostasis. Knowledge of the precise mechanisms underlying the diabetogenic effects of iAs and its metabolites could improve treatment strategies for diabetes associated with iAs exposure.
Supplementary Material
Acknowledgments
Funding This work was supported by a grant from the National Institutes of Health [R01ES022697], a National Research Service Award from the National Institute of Environmental Health Sciences, NIH [T32 ES007126], the UNC Nutrition Obesity Research Center funded by the National Institute of Diabetes and Digestive and Kidney Diseases [DK056350], and by a Grant from the National Institutes of Health [R01DK107481 to ELK].
Footnotes
Compliance with ethical standards
Electronic supplementary material The online version of this article (doi:10.1007/s00204-017-2074-y) contains supplementary material, which is available to authorized users.
References
- Bergquist ER, Fischer RJ, Sugden KD, Martin BD (2009) Inhibition by methylated organo-arsenicals of the respiratory 2-oxo-acid dehydrogenases. J Organomet Chem 694:973–980. doi: 10.1016/j.jorganchem.2008.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A (2013) Paracrine and autocrine interactions in the human islet: more than meets the eye. Semin Cell Dev Biol 24:11–21. doi: 10.1016/j.semcdb.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Razo LM, García-Vargas GG, Valenzuela OL et al. (2011) Exposure to arsenic in drinking water is associated with increased prevalence of diabetes: a cross-sectional study in the Zimapán and Lagunera regions in Mexico. Environ Health Glob Access Sci Source 10:73. doi: 10.1186/1476-069X-10-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Villaseñor A, Cruz L, Cebrián A et al. (2013) Arsenic exposure and calpain-10 polymorphisms impair the function of pancreatic beta-cells in humans: a pilot study of risk factors for T2DM. PLoS One 8:e51642. doi: 10.1371/journal.pone.0051642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douillet C, Currier J, Saunders J et al. (2013) Methylated trivalent arsenicals are potent inhibitors of glucose stimulated insulin secretion by murine pancreatic islets. Toxicol Appl Pharmacol 267:11–15. doi: 10.1016/j.taap.2012.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Woods CG, Yehuda-Shnaidman E et al. (2010) Low-level arsenic impairs glucose-stimulated insulin secretion in pancreatic beta cells: involvement of cellular adaptive response to oxidative stress. Environ Health Perspect 118:864–870. doi: 10.1289/ehp.0901608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Gilbert ER, Liu D (2013) Regulation of insulin synthesis and secretion and pancreatic beta-cell dysfunction in diabetes. Curr Diabetes Rev 9:25–53 [PMC free article] [PubMed] [Google Scholar]
- Gong Z, Lu X, Cullen WR, Le XC (2001) Unstable trivalent arsenic metabolites, monomethylarsonous acid and dimethylarsinous acid. J Anal At Spectrom 16:1409–1413. doi: 10.1039/B105834G [DOI] [Google Scholar]
- Green PS, Leeuwenburgh C (2002) Mitochondrial dysfunction is an early indicator of doxorubicin-induced apoptosis. Biochim Biophys Acta BBA—Mol Basis Dis 1588:94–101. doi: 10.1016/S0925-4439(02)00144-8 [DOI] [PubMed] [Google Scholar]
- Gribble MO, Howard BV, Umans JG et al. (2012) Arsenic exposure, diabetes prevalence, and diabetes control in the strong heart study. Am J Epidemiol 176:865–874. doi: 10.1093/aje/kws153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Zavala A, Matoušek T, Drobná Z et al. (2008) Speciation analysis of arsenic in biological matrices by automated hydride generation-cryotrapping-atomic absorption spectrometry with multiple microflame quartz tube atomizer (multiatomizer). J Anal At Spectrom 23:342–351. doi: 10.1039/b706144g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmeier HE, Mulder H, Chen G et al. (2000) Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49:424–430. doi: 10.2337/diabetes.49.3.424 [DOI] [PubMed] [Google Scholar]
- Hosseini M-J, Shaki F, Ghazi-Khansari M, Pourahmad J (2013) Toxicity of arsenic (III) on isolated liver mitochondria: a new mechanistic approach. Iran J Pharm Res IJPR 12:121–138 [PMC free article] [PubMed] [Google Scholar]
- Janjic D, Wollheim CB (1992) Islet cell metabolism is reflected by the MTT (tetrazolium) colorimetric assay. Diabetologia 35:482–485. doi: 10.1007/BF02342448 [DOI] [PubMed] [Google Scholar]
- Koen YM, Liu K, Shinogle H et al. (2016) Comparative toxicity and metabolism of N-acyl homologues of acetaminophen and its isomer 3′-hydroxyacetanilide. Chem Res Toxicol 29:1857–1864. doi: 10.1021/acs.chemrestox.6b00270 [DOI] [PubMed] [Google Scholar]
- Maechler P (2013) Mitochondrial function and insulin secretion. Mol Cell Endocrinol 379:12–18. doi: 10.1016/j.mce.2013.06.019 [DOI] [PubMed] [Google Scholar]
- Martinez VD, Vucic EA, Becker-Santos DD et al. (2011) Arsenic exposure and the induction of human cancers. J Toxicol 2011:e431287. doi: 10.1155/2011/431287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maull EA, Ahsan H, Edwards J et al. (2012) Evaluation of the association between arsenic and diabetes: a National Toxicology Program workshop review. Environ Health Perspect 120:1658–1670. doi: 10.1289/ehp.1104579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon K, Guallar E, Navas-Acien A (2012) Arsenic exposure and cardiovascular disease: an updated systematic review. Curr Atheroscler Rep 14:542–555. doi: 10.1007/s11883-012-0280-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranmandura H, Xu S, Sawata T et al. (2011) Mitochondria are the main target organelle for trivalent monomethylarsonous acid (MMA(III))-induced cytotoxicity. Chem Res Toxicol 24:1094–1103. doi: 10.1021/tx200156k [DOI] [PubMed] [Google Scholar]
- Pace C, Banerjee TD, Welch B et al. (2016) Monomethylarsonous acid, but not inorganic arsenic, is a mitochondria-specific toxicant in vascular smooth muscle cells. Toxicol In Vitro 35:188–201. doi: 10.1016/j.tiv.2016.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge MA, Huang SXL, Hernández-Rosa E et al. (2007) Arsenic induced mitochondrial DNA damage and altered mitochondrial oxidative function: implications for genotoxic mechanisms in mammalian cells. Cancer Res 67:5239–5247. doi: 10.1158/0008-5472.CAN-07-0074 [DOI] [PubMed] [Google Scholar]
- Paul DS, Walton FS, Saunders RJ, Styblo M (2011) Characterization of the impaired glucose homeostasis produced in C57BL/6 mice by chronic exposure to arsenic and high-fat diet. Environ Health Perspect 119:1104–1109. doi: 10.1289/ehp.1003324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Q, Harlow SD, Park SK (2015) Urinary arsenic and insulin resistance in US adolescents. Int J Hyg Environ Health 218:407–413. doi: 10.1016/j.ijheh.2015.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrick JS, Jagadish B, Mash EA, Aposhian HV (2001) Monomethylarsonous acid (MMAIII) and arsenite: LD50 in hamsters and in vitro inhibition of pyruvate dehydrogenase. Chem Res Toxicol 14:651–656. doi: 10.1021/tx000264z [DOI] [PubMed] [Google Scholar]
- Prakash C, Soni M, Kumar V (2015) Biochemical and molecular alterations following arsenic-induced oxidative stress and mitochondrial dysfunction in rat brain. Biol Trace Elem Res 167:121–129. doi: 10.1007/s12011-015-0284-9 [DOI] [PubMed] [Google Scholar]
- Rhee SY, Hwang Y-C, Woo J et al. (2013) Arsenic exposure and prevalence of diabetes mellitus in Korean adults. J Korean Med Sci 28:861–868. doi: 10.3346/jkms.2013.28.6.861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Li X-F, Cullen WR et al. (2013) Arsenic binding to proteins. Chem Rev 113:7769–7792. doi: 10.1021/cr300015c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styblo M, Razo LMD, Vega L et al. (2000) Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch Toxicol 74:289–299. doi: 10.1007/s002040000134 [DOI] [PubMed] [Google Scholar]
- Sumedha NC, Miltonprabu S (2015) Cardiac mitochondrial oxidative stress and dysfunction induced by arsenic and its amelioration by diallyl trisulphide. Toxicol Res 4:291–301. doi: 10.1039/C4TX00097H [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler CR, Allan AM (2014) The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: a review. Curr Environ Health Rep 1:132–147. doi: 10.1007/s40572-014-0012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S-L, Chang F-H, Liou S-H et al. (2007) Inorganic arsenic exposure and its relation to metabolic syndrome in an industrial area of Taiwan. Environ Int 33:805–811. doi: 10.1016/j.envint.2007.03.004 [DOI] [PubMed] [Google Scholar]
- Yang YHC, Szabat M, Bragagnini C et al. (2011) Paracrine signalling loops in adult human and mouse pancreatic islets: netrins modulate beta cell apoptosis signalling via dependence receptors. Diabetologia 54:828–842. doi: 10.1007/s00125-010-2012-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.