Abstract
Antibodies have become one of the most successful therapeutics for a number of oncology and inflammatory diseases. So far, central nervous system (CNS) indications have missed out on the antibody revolution, while they remain ‘hidden’ behind several hard to breach barriers. Among the various antibody modalities, single-domain antibodies (sdAbs) may hold the ‘key’ to unlocking the access of antibody therapies to CNS diseases. The unique structural features of sdAbs make them the smallest monomeric antibody fragments suitable for molecular targeting. These features are of particular importance when developing antibodies as modular building blocks for engineering CNS-targeting therapeutics and imaging agents. In this review, we first introduce the characteristic properties of sdAbs compared to traditional antibodies. We then present recent advances in the development of sdAbs as potential therapeutics across brain barriers, including their use for the delivery of biologics across the blood–brain and blood–cerebrospinal fluid (CSF) barriers, treatment of neurodegenerative diseases and molecular imaging of brain targets.
Keywords: single-domain antibodies, neurodegenerative diseases, brain imaging, blood–brain barrier, delivery
1. Introduction to sdAbs
1.1. Structure and Characteristics
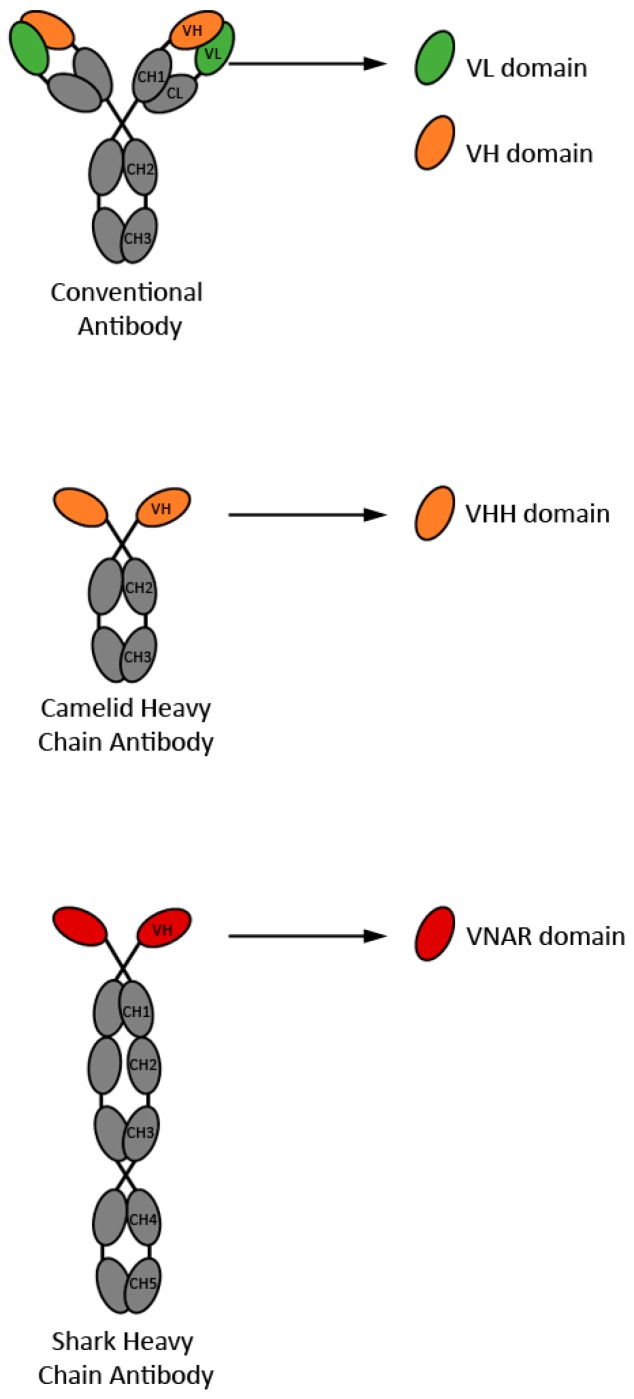
The concept of single-domain antibodies (sdAbs) originated in the 90’s, with the proof-of-concept experiments demonstrating sdAbs as bone fide antigen binding fragments [1], and the discovery of camelid [2] and shark [3] heavy chain-only antibodies (HCAbs). Single-domain antibodies can be derived from the antigen binding variable domains of homodimeric, light-chain lacking immunoglobulins, such as camelid HCAbs [4] and shark immunoglobulin new antigen receptors (IgNARs) [5], variable light chain (VL) or variable heavy chain (VH) domains of tetrameric—typically human—conventional immunoglobulins [6] (Figure 1). The variable domains of camelid HCAbs and shark IgNARs are referred to as VHHs (or nanobodies) and VNARs, respectively. While VHHs and VNARs are almost without exception non-aggregating and highly soluble, the opposite is true for VHs and VLs. However, various strategies have been developed to successfully obtain aggregation resistant and soluble human VHs and VLs ([7,8] and references therein) including transgenic mice technology [9,10]. Human VH and VL domains are of interest because of their human nature, a property that presumably makes them less immunogenic in humans compared to camelid VHHs or nurse shark VNARs.
Figure 1.
Schematic representation of the four types of sdAbs described in the current review. Antibody constant domains are in grey, whereas antibody variable domains from which sdAbs are derived are in color.
The desirable biophysical, biochemical, and structural properties of sdAbs, particularly those from natural repertoires are generally well known, and have been described in several reviews [4,5,6,11,12]. Despite their significantly smaller combining site, consisting of only three complementarity-determining regions (CDRs) or hypervariable loops—as opposed to six for conventional antibodies such as monoclonal antibodies (mAbs)—sdAbs demonstrate comparable antigen binding affinities. Interrelated properties such as small size (12–15 kDa vs. 150 kDa for mAbs); strict monomericity; high solubility, including at therapeutic doses; aggregation resistance; chemical, physical and protease stability; efficient folding /refolding; good recombinant expression, notably in economic microbial expression systems such as yeast and E. coli; excellent shelf life; excellent manufacturability; and low cost of production make sdAbs an attractive alternative to other antibody formats such as mAbs, Fabs (fragments antigen binding), and scFvs (single chain variable fragments) as therapeutic and diagnostic agents. Resistance to aggregation is particularly noteworthy as it significantly reduces the risk of immunogenicity. Furthermore, their small size and frequently extended CDR3 make sdAbs the antibody of choice when targeting recessed epitopes of proteins such as enzymes’ active sites or receptors’ cavities. Longer CDR3s also increase the combining site’s surface area, and to a significant degree compensate for the absence of VL CDRs. In addition, their fast blood clearance and effective tissue penetration, attributed to their small size, make sdAbs ideal imaging agents, e.g., against tumors. In this respect, the high stability and folding properties of sdAbs provide flexibility for labeling reactions with optimal outcomes. Modularity is another hallmark of sdAbs, and becomes a key property when engineering sdAb-based multimeric and multispecific constructs as CNS diagnostics and therapeutics (see Section 1.3).
1.2. Single-Domain Antibody Libraries and Selection
Single-domain antibodies have been typically isolated from display libraries mostly phage-displayed, although other display platforms also exist, such as yeast and ribosome display [13]. While VHHs and VNARs have been obtained from all types of libraries including immune, non-immune, semi-synthetic/synthetic libraries [4,14,15], human VHs and VLs have been most commonly obtained from synthetic libraries [6]. Unlike immune VNARs and VHHs that have high affinities as a result of in vivo somatic hypermutation, sdAbs obtained from non-immune or synthetic/semi-synthetic sdAb libraries are of low affinity, and often require further mutation for improved affinity and function. However, more recently, human VHs have also been isolated from immune VH display libraries derived from HCAb-producing transgenic mice that are immunized with a target antigen of interest [9,10]. While these VHs display high solubility, stability and affinity of immune VHHs, they are advantageously expected to be less immunogenic.
Constructing natural repertoire sdAb libraries is well established and relatively straightforward [4,15,16]. Human VH and VL synthetic libraries are typically built on a single scaffold with demonstrated good biophysical properties, such as high thermostability, solubility, and expression ([6,8] and references therein, [17,18]). Library diversity generation entails introducing random or specific amino acids at all or selected positions in the three CDRs. Owing to their small size and single-domain nature, in contrast to more complex, multidomain scFvs and Fabs, sdAbs lend themselves to a facile and straightforward library construction, and are not associated with VH/VL mispairing phenomenon that occurs during the construction of scFv and Fab libraries, which adversely affects the library quality.
In its simplest and most commonly practiced format, the selection, or panning, the process for the isolation of sdAbs from phage-displayed libraries, involves selecting for a single property—affinity for the target antigen. Most commonly, this involves exposure of a library to an antigen immobilized on a microtitre plate, washing away unbound phage, and eluting the bound phage molecules, which are amplified for another round of panning. Depending on the type of library, between two and four rounds of panning are typically sufficient to obtain around half a dozen sdAbs with affinity for the target antigen. With human sdAb libraries, affinity selection may be coupled with selection for stability for a more efficient isolation of aggregation-resistant binders [6].
One of the great advantages of antibody library display technologies over hybridoma technology for the isolation of mAbs is the capability to drive, in some measure, the selection process towards isolating antibodies with specific properties. For example, by panning in the presence of proteases it has been possible to isolate sdAbs with enhanced protease resistance [19]. In the context of this review, it is especially noteworthy that sdAbs that transmigrate across an in vitro human blood–brain barrier (BBB) model have been isolated [20].
1.3. Modular Building of Multispecific Molecules
Their small size and monomeric nature make sdAbs ideal building blocks for the construction of multivalent and multispecific therapeutic and imaging molecules of improved function and potency (compared to monomeric versions) with good development capacity and manufacturability [11,21,22]. For example, bivalent or bispecific sdAbs have been generated by linking two identical or two different sdAbs using a short spacer sequence [23,24,25,26,27,28,29,30,31,32]. Successful generation of trivalent bispecific and tetravalent bispecific sdAbs—where sdAb moieties are linked through short linker sequences—have also been reported [28,29,33,34,35,36]. Monospecific pentavalent sdAbs have been constructed by fusing sdAbs to the N- or C-terminus of the verotoxin 1B (VT1B) subunit [37]. Similarly, fusing different sdAbs to the N- and C-terminus of VT1B has yielded bispecific decavalent molecules [38]. Bivalent monospecific or tetravalent bispecific sdAbs can also be made by fusing sdAbs to an antibody Fc fragment [8,39,40,41,42,43]; this has the added advantage of greatly extending the serum half-life of sdAbs [44] and imparting effector functions such as antibody-dependent cellular cytotoxicity (ADCC) [42] or complement-dependent cytotoxicity (CDC) [45]. Single-domain antibodies should also be ideal molecules for constructing bivalent and bispecific antibodies incorporating a heterodimeric Fc region [46]. More complex constructs such IgG-sdAb fusions have also been reported [47]. For therapeutic applications, sdAbs have been linked to enzymes or toxins, either by cloning or by chemical conjugation [48,49,50,51,52,53].
1.4. Developing sdAbs as CNS Diagnostics or Therapeutics
Treating CNS disorders remains one of the greatest challenges in modern medicine. Although several promising therapeutics are developed every year, their failure to reach brain target prevents their advancement to the clinic. This is mainly due to the presence of the BBB acting as a gatekeeper to maintain brain homeostasis and protect neurological capabilities [54]. The BBB is composed of specialized endothelial cells sealed together by tight junctions to form a physical barrier lining the brain blood vessels. These cells differ from endothelial cells lining peripheral vessels by their lack of fenestrations and limited pinocytic activity thereby restricting transcellular transport. The brain endothelial layer is surrounded by pericytes and astrocyte end-feet, which are essential for maintaining the integrity of the BBB. In addition, several efflux transporters are present at the BBB and function to remove unwanted molecules from the brain. Although this restrictive physiology is necessary to prevent undesirable blood-borne material from penetrating the brain, it also limits the effective delivery of CNS therapeutics. Therefore, agents designed for use as CNS diagnostics or therapeutics must be delivered to sites of action via administration routes that circumvent the BBB, such as intrathecal/intraventricular, intracerebral administration, or combined with delivery technologies that increase their penetration across the BBB upon systemic administration.
The brain neuropil is packed with interacting cells, including neurons, neuronal processes, and various types of glial cells. The brain extracellular space (ECS), filled with brain extracellular fluid, is tight and very convoluted—modeling studies estimate its width between 35 and 60 nm [55]. Any compound administered directly into the neuropil will diffuse through the ECS to distances inversely proportional to the size of the molecule. Monoclonal antibodies exhibit limited diffusion in the brain ECS due to their large size and interactions with the extracellular matrix (ECM). Single-domain antibodies have a distinct advantage as intracerebrally administered reagents/therapeutics, achieving diffusion across longer distances from the site of injection [55]. In addition, the lack of a Fc fragment reduces their interactions with the ECM and brain efflux via an FcRn-mediated reverse transcytosis. A recent study on the brain biodistribution of antibodies via perivascular transport after intrathecal infusion in rodents [56] demonstrated both deeper brain penetration and broader brain exposure of a smaller VHH fragment compared to a full mAb. This study demonstrated that sdAbs are advantageous as a CNS therapeutic antibody modality developed for intracerebral (local) or intrathecal administration. This is particularly relevant for brain diseases originating from or confined to a specific brain area, such as Parkinson’s disease.
However, the majority of CNS diseases can be considered ‘whole-brain’ diseases, even when they initially affect more localized brain regions. The brain’s vascular network is particularly dense, and thus each brain capillary supplies only few neurons, the diffusion distance of compounds, including antibodies, delivered across the BBB to their neuronal targets is only ~25 µm. Transvascular (cross-BBB) brain delivery would therefore achieve a more global brain distribution of antibodies, regardless of their size, since these diffusion distances are readily achievable even by mAbs. Systemic delivery of therapeutic antibodies targeting the CNS could be improved using ‘carrier’ molecules selected or engineered for the ability to traverse the BBB.
In the following sections, sdAbs that have been developed as delivery agents across the BBB, as treatments against the most common neurodegenerative diseases and as neuroimaging tools are descried and summarized in Table 1.
Table 1.
Overview of single-domain antibodies developed for central nervous system applications.
| Product Name | Target | sdAb Type | Source | References |
|---|---|---|---|---|
| BBB Shuttles | ||||
| TBX4 | TfR1 | VNAR | Synthetic phage-displayed VNAR library | [66] |
| IGF1R-3 | IGF1R | VHH | Immune phage-displayed VHH library | [75,76] |
| FC5 | TMEM-30A | VHH | Non-immune phage-displayed VHH library | [20,70,71,72,73,74] |
| FC44 | Unknown | VHH | Non-immune phage-displayed VHH library | [20,70,71] |
| Neurodegenerative diseases | ||||
| Alzheimer’s disease | ||||
| B10 | Mature Aβ (1-40) fribrils and protofibrils | VHH | Synthetic phage-displayed VHH library | [84] |
| KW1 | Non-fibrillar Aβ (1-40) oligomers | VHH | Synthetic phage-displayed VHH library | [86,87] |
| ni3A | Aβ (1-42) deposits | VHH | Non-immune phage-displayed VHH library | [88,89,90] |
| V31-1 | Monomers and small Aβ (1-42) oligomers | VHH | Immune phage-displayed VHH library | [91] |
| PrioAD12 | Aβ (1-40) peptide | VHH | Immune phage-displayed VHH library | [92] |
| PrioAD13 | Aβ (1-42) peptide | VHH | Immune phage-displayed VHH library | [92] |
| PrioAD120 | Tau (1-16) peptide | VHH | Immune phage-displayed VHH library | [92] |
| VH1.27, VH1.28, VH2.8 | Aβ (1-42) peptide | VH | Immune phage-displayed mouse VH library | [93] |
| Aβ (1-10), Aβ (3-12), Aβ (6-15), Aβ (9-18), Aβ (12-21), Aβ (15-24), Aβ (18-27), Aβ (21-30), Aβ (24-33), Aβ (27-36), Aβ (30-39), Aβ (33-42) |
AB monomers, soluble oligomers or fibrils | VH | Grafted amyloid-motif antibodies (Gammabody) | [94,95,96,97] |
| DesAb-Aβ | Aβ (15-21) peptide | VH | Gammabody | [98] |
| Parkinson’s disease | ||||
| αSyn (69-78) | αSyn fibrils | VH | Gammabody | [95] |
| DesAb-D, DesAb-E, DesAb-F |
αSyn (61-67) or αSyn (70-76) peptide | VH | Gammabody | [98] |
| NbSyn2 | Monomeric αSyn and mature fibrils | VHH | Immune phage-displayed VHH library | [101,102,104] |
| NbSyn87 | Monomeric αSyn(A53T) and mature fibrils | VHH | Immune phage-displayed VHH library | [102,103,107,108] |
| VH14 | Monomeric αSyn | VH | Non-immune yeast-displayed human scFv library | [106,107,108] |
| Huntington’s disease | ||||
| VL12.3 | Htt protein | VL | Non-immune yeast-displayed human scFv library | [110,111,112,113] |
| Happ1, Happ3 | Htt protein | VL | Non-immune phage-displayed human scFv library | [112,113] |
| iVHH1, iVHH2, iVHH3, iVHH4 | Htt protein | VHH | Immune phage-displayed VHH library | [114] |
| Prion diseases | ||||
| PrioV3 | PrPc and PrPsc | VHH | Immune phage-displayed VHH library | [92,117] |
| Nb484 | MoPrP (23-230) | VHH | Immune phage-displayed VHH library | [118] |
| Glioblastoma multiforme | ||||
| Nb237 | TRIM28 | VHH | Immune phage-displayed VHH library | [122] |
| Nb141 | β-actin | VHH | Immune phage-displayed VHH library | [122] |
| Nb10 | ACTB/NUCL complex | VHH | Immune phage-displayed VHH library | [123] |
| Nb79 | VIM | VHH | Immune phage-displayed VHH library | [123] |
| Nb179 | NAP1L1 | VHH | Immune phage-displayed VHH library | [123] |
| Nb225 | TUFM | VHH | Immune phage-displayed VHH library | [123] |
| Nb314 | DPYSL2 and MTHFD1 | VHH | Immune phage-displayed VHH library | [123] |
| Nb394 | CRMP1 | VHH | Immune phage-displayed VHH library | [123] |
| Nb395 | ALYREF | VHH | Immune phage-displayed VHH library | [123] |
| Nb206 | TUFM | VHH | Immune phage-displayed VHH library | [124] |
| VH-9.7 | GSC | VH | Non-immune yeast-displayed human scFv library | [125] |
| C-C7 | Dynactin-1-p150Glued | VHH | Non-immune phage-displayed VHH library | [126] |
| ENb1, ENb2 | EGFR | VHH | Immune phage-displayed VHH library | [127,130] |
| Neuroimaging | ||||
| EG(2) | EGFR | VHH | Immune phage-displayed VHH library | [136] |
| sdAb 4.43 | IGFBP7 | VHH | Immune phage-displayed VHH library | [142,144,145] |
| FC5 | TMEM-30A | VHH | Non-immune phage-displayed VHH library | [148] |
| R3VQ | Aβ (1-42) peptide | VHH | Immune phage-displayed VHH library | [135] |
| A2 | Phospho-Tau protein | VHH | Immune phage-displayed VHH library | [135] |
| mVHH A10, mVHH E9, mVHH E3 |
GFAP | VHH | Immune ribosome-displayed VHH library | [151] |
2. Single-Domain Antibodies as Delivery Agents across the BBB
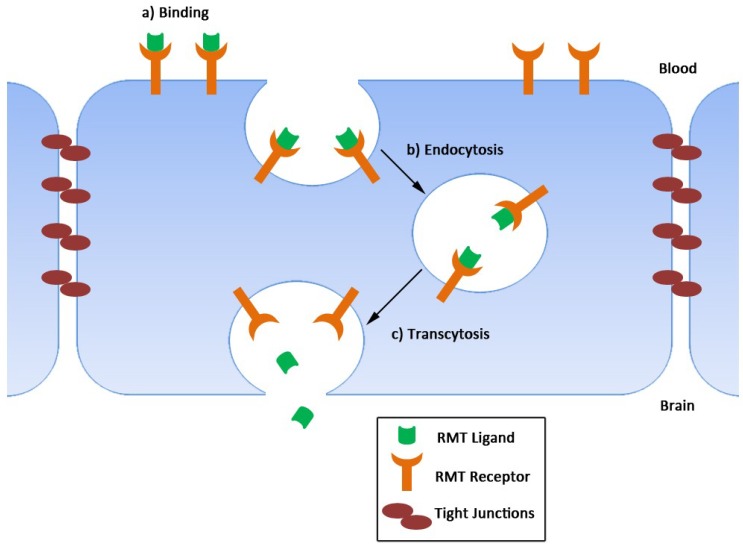
The most investigated method to deliver macromolecules into the brain is via receptor-mediated transcytosis (RMT) [57]. This process is crucial for the proper delivery of macromolecules essential for brain function such as vitamins, proteins and nutrients. They use naturally occurring transport systems to shuttle between the blood and the brain. Such systems can potentially be ‘hi-jacked’ to facilitate the delivery of therapeutics into brain.
The process of RMT is initiated by ligand binding to its receptor expressed at the luminal face of endothelial cells to trigger internalization of the receptor-ligand complex into endosomal vesicles (Figure 2). These vesicles then travel inside the cytoplasm of the cell via a complex vesicular sorting pathway to finally fuse with the abluminal surface of endothelial cells and deliver their cargo in the brain parenchyma. The receptor is then recycled at the luminal cell surface. Currently, the main RMT receptors that have been studied are the transferrin receptor (TfR) and insulin receptor (IR) [57]. Ligands against these receptors, including different antibody formats, have been used as carriers to deliver their therapeutic cargo inside the brain [7,58,59,60,61,62,63,64,65]. Single-domain antibodies present numerous advantages over conventional antibodies as potential transvascular brain delivery vectors including small size, low non-specific interactions with tissues expressing high levels of Fc receptors (e.g., liver, spleen), remarkable stability against harsh conditions and low immunogenicity (see Section 1). In the case of IR, although there are examples of mAbs and peptides specific for this receptor, no IR-specific sdAbs have been described to date. The only example of a sdAb targeting TfR is a VNAR, termed TBX4, which was obtained from a synthetic library following a combination of in vitro and in vivo phage display techniques [66]. When fused to an immunoglobulin Fc backbone, this antibody was enriched in the brain parenchyma of mice following vein tail injection. Furthermore, bispecific variants of this antibody fused to a CD20 targeting agent were able to reach aberrant B cells in the brain and induced cell toxicity. The use of this VNAR for the delivery of a variety of biologics to the brain is currently under investigation.
Figure 2.
Representation of the receptor-mediated transcytosis (RMT) process. (a) Initially, an RMT ligand binds to a specific RMT receptor on the luminal cell membrane, which (b) leads to the internalization of both receptor and ligand in intracellular vesicles via endocytosis. (c) These vesicles then travel within the cell cytoplasm to reach the abluminal membrane where fusion of endosomes with the cell membrane releases the vesicular cargo inside the brain.
Although there is now growing evidence that the use of the aforementioned receptors for therapeutic delivery into the brain is a promising avenue, recent studies suggest that these may not be ideal RMT targets [67,68,69]. Major drawbacks associated with these receptors include their ubiquitous expression in numerous peripheral organs and their involvement in essential physiological functions, which may raise significant safety concerns. Therefore, the chase for alternative RMT receptors with more optimal BBB crossing properties continues.
FC5 and FC44, two camelid VHHs of a non-immune phage-displayed library, were isolated by phenotypic panning for their ability to interact and internalize into the human brain cerebromicrovascular endothelial cells (HBECs) [20]. The two antibodies were found to transmigrate in an in vitro rat BBB model, and to accumulate in the brain at high levels following tail vein injection in rodents [20,70]. The investigation of their mechanism of action led to the finding that it was RMT-mediated [71], and in the case of FC5, the receptor was later identified as transmembrane domain protein 30A (TMEM-30A) [72]. Engineered fusions of FC5 to the human IgG1 Fc in a monovalent or bivalent format showed increased migration across the BBB in vitro, and achieved a significantly higher brain exposure in vivo compared to the control antibody-Fc [73]. FC5-Fc constructs were also detected in brain vessels and in the brain parenchyma in rat brain sections. Furthermore, the conjugation of FC5 antibodies with impermeable analgesic peptides, dalargin or neuropeptide Y, induced an important analgesic effect in a thermal hyperalgesia model whereas the systemic administration of the neuropeptides alone had no suppressive effect. Although all antibody formats were able to reduce hyperalgesia, the bivalent and monovalent Fc fusions showed a pronounced increase in the response at equal dose compared to the VHH suggesting that improving serum pharmacokinetics plays a determining role in the pharmacological potency of FC5. In addition to peptides, the CNS delivery of a monoclonal antibody antagonist of metabotropic glutamate receptor 1 (mGluR1) was successfully achieved using the BBB-crossing VHH FC5 [74]. Following intravenous injection in a rat model of persistent inflammatory pain, the BBB-mGluR1 bispecific antibody co-localized with thalamic neurons involved in mGluR1-mediated pain processing, and subsequently inhibited hyperalgesia.
Insulin growth factor 1 receptor (IGF1R) has been identified as a potential RMT candidate based on observation that its ligand, IGF-1 is transported across the BBB. A panel of VHHs, targeting the ectodomain of this receptor was isolated from a phage-displayed immune library [75]. Following humanization, the ability of several IGF1R-binding VHHs to transmigrate in rat and human BBB models in vitro was confirmed [75,76]. When expressed in fusion with a murine antibody fragment, the resultant IGF1R-specific VHHs were found to significantly increase brain and CSF exposure in mice and rats compared to the control [75]. IGF1R-targeting VHHs conjugated to galanin—A systemically restricted neuroactive peptide that produces analgesia by binding GalR1 and GalR2 receptors expressed in the brain [77]—Induced a strong analgesic effect in a rat model of inflammatory hyperalgesia following a single dose injection [75], suggesting the ability of these IGF1R-binding VHHs to act as delivery carriers across the BBB.
The above studies provided evidence for the feasibility of using sdAbs as carriers targeting a new generation of RMT receptors for the development of CNS therapeutics.
3. Single-Domain Antibodies as Treatments against Neurodegenerative Diseases
3.1. Protein-Misfolding Diseases (PMDs)
A vast majority of neurodegenerative diseases are associated with misfolded proteins that interact with each other to form large aggregates referred to as amyloid fibrils [78]. These complexes are insoluble, highly organized and extremely stable, and their accumulation is toxic to the cell. Although all PMDs share a common mechanism of action, the nature of the misfolded proteins differs between each disorder and dictates the identity of the disease. Alzheimer’s disease is caused by the accumulation of amyloid β (Aβ) peptides and Tau proteins, whereas the aggregation of α-synucleins (αSyn) is at the origin of Parkinson’s disease. Similarly, aggregates formed by huntingtin (Htt) proteins lead to Huntington’s disease while Prion disease is associated with the conversion of the normal, cellular prion protein (PrPc) into its disordered scrapie isoform (PrPsc), which accumulates into large oligomers. The formation of fibrils is a complex phenomenon involving several intermediate distinct structures [78,79]. The identification and characterization of the various species formed during the process is essential for the development of early diagnostic tools and new therapeutic strategies. This is, however, an extremely difficult task due to the insolubility and heterogeneity of the different forms involved in the process of fibril formation. This explains the lack of effective treatments against the devastating PMDs to date.
Single-domain antibodies represent a promising asset for the treatment of PMDs since they possess unique characteristics allowing them to access unprecedented epitopes (see Section 1). In addition, their high specificity and stability ensured the targeting of specific species under harsh solubilizing conditions along the process of fibril maturation. In this next section, we will review recent advances in the use of sdAbs for the diagnostic and treatment of the main PMDs affecting the CNS.
3.1.1. Alzheimer’s Disease (AD)
The first description of AD goes back to 1906 [80]. However, it took several decades until it was finally established as a major neurodegenerative disorder. It is now considered the main cause of dementia accounting for up to 80% of all cases [81]. Patients affected with the disease suffer several symptoms including the loss of memory and cognitive functions. This is believed to be due to two main phenomena. First, the extracellular accumulation of Aβ peptides made of 39–42 amino acids to form amyloid plaques in the CNS [82] and second, the aggregation of hyperphosphorylated Tau proteins into Tau tangles inside neurons [83]. The presence of these aggregates or their precursor forms severely affects the normal function of neurons leading to cell death. In recent years, several efforts have been deployed to generate antibody fragments against Aβ and Tau aggregates in view of developing novel therapeutics for AD.
VHH B10 emerged from a synthetic phage-displayed library panned against biotinylated Aβ (1-40) fibrils [84]. This antibody was shown to bind specifically to mature amyloid fibrils as well as to protofibrils which were defined as the aggregated species forming prior to the assembly of more stable mature fibrils [85]. VHH did not interact with disaggregated peptides or other non-fibrillar Aβ oligomers. In addition, the authors demonstrated the antibody’s ability to stabilize protofibrils upon interacting with it, thus inhibiting mature fibril formation. However, B10 did not have the ability to disintegrate preformed fibrils. Similarly, another VHH isolated from a synthetic phage-displayed library, was shown to interact specifically with non-fibrillar Aβ (1-40) oligomers and prevent the formation of mature fibrils [86]. The antibody could not induce the disaggregation of already formed fibrils. In their report, the authors immobilized biotinylated Aβ (1-40) oligomers to select a conformation-specific binder that they named KW1. They demonstrated that the addition of KW1 to preformed Aβ oligomers prevented their synaptotoxic effect. Nevertheless, another report published two years later showed that Aβ oligomers formed in the presence of the same KW1 antibody were highly toxic [87], which seems to indicate a time-sensitive beneficial effect by the VHH. ni3A is a VHH that was isolated from a non-immune phage-displayed library using Aβ (1-42) as antigen [88]. This VHH bound to its target with high specificity and affinity and showed BBB-crossing abilities in vitro [89]. When tested in vivo [90], ni3A successfully detected Aβ deposits in a transgenic mouse model of AD, suggesting its potential as a diagnostic tool.
In contrast to the VHHs described above, three additional ones were isolated from a phage-displayed library made from the blood of a llama immunized with a mixture of Aβ (1-42) monomers, small oligomers and fibrils [91]. These antibodies bound specifically to monomers and small oligomers formed exclusively by Aβ (1-42) but not to higher molecular-mass aggregates or fibrils or to Aβ (1-40)-originating species. One VHH in particular, V31-1, was found to inhibit the formation of amyloid fibrils and to prevent the toxic cellular effect of Aβ oligomers [91]. Another immunization campaign—this time using brain homogenates from an AD patient as immunogen in alpacas—Led to the identification of three VHHs, PrioAD12, PrioAD13 and PrioAD120 targeting Aβ (1-40), Aβ (1-42) or Tau (1-16) peptides, respectively [92]. PrioAD12 had the ability to detect Aβ plaques in brain sections from an individual affected with AD while no detection (staining) was observed on sections from a normal brain. Finally, Aβ-specific VHs were isolated following immunization of a mouse with Aβ (1-42) peptides and construction of a phage-displayed VH library [93]. Selected antibodies were found to interact with different regions of the full-length peptide and inhibit its cell toxicity. Moreover, one VH (VH1.27), when tested for its ability to clear amyloid deposits in a mouse model of AD following intracranial injection was shown to significantly reduce the amyloid burden compared to the control.
Perchiacca and colleagues developed a new strategy to generate a series of sdAbs against disordered proteins [94,95,96,97]. They used defined algorithms to select motifs within disordered proteins that are predicted to participate in amyloid formation based on charge, hydrophobicity and propensity to form β-sheets [94]. They subsequently grafted peptides corresponding to the selected motifs into the CDR3 of a human VH with good solubility characteristics. By using their technique, the authors generated a pool of antibodies against amyloidogenic epitopes within Aβ (1-42) peptides. The VHs demonstrated specific and sensitive recognition of Aβ monomers, soluble oligomers or fibrillar intermediates depending on the region covered by the grafted peptide and prevented toxicity induced by the targeted conformers [94]. It was later demonstrated that binding of the VHs with their amyloidogenic target led to the assembly of Aβ-VHs non-toxic complexes thereby preventing the formation of mature amyloid fibrils [95]. This technique was extended to construct one VH specific for an aggregation-prone epitope within αSyn with the ability to inhibit fibrillization by the protein.
A similar grafting method was used to generate additional VHs targeting Aβ (1-42) or αSyn [98]. In this case, complementary peptides to the target sequence were designed based on interactions between amino acid sequences in the Protein Data Bank (PDB) and inserted into the CDR3 of a human VH. Resulting antibodies all showed specific binding to their respective target. In addition, one anti-αSyn VH was tested for its neutralization potency in in vitro assays and demonstrated the ability to significantly reduce the aggregation of the targeted protein [98].
3.1.2. Parkinson’s Disease (PD)
PD represents the second most common neurological disorder affecting approximately 10 million people worldwide, and this number is predicted to increase over the coming years due to population aging [99]. Hallmarks of the disease include the loss or degeneration of dopamine producing neurons leading to severe motor control impairment. At the molecular level, PD is associated with the appearance of intracellular fibrillar aggregates known as Lewy bodies (LB) or Lewy neurites composed mostly of αSyn [100]. These large inclusions are responsible for neuronal cell death. Therefore, antibodies targeting the small, αSyn protein represent a promising treatment against PD.
Three sdAbs recognizing αSyn have been described in addition to the ones mentioned in the previous section [94,101,102,103]. First, following immunization of a dromedary with monomeric αSyn and subsequent construction and screening of a phage-displayed library, a VHH (NbSyn2) interacting with the soluble form of the protein was identified [101]. Based on nuclear magnetic resonance (NMR) spectroscopy and X-ray crystallography, the epitope of NbSyn2 was mapped to the C-terminus of αSyn within the last four residues [104]. Interestingly, VHH also interacted with amyloid fibrils formed by αSyn suggesting that this region of the protein remains exposed following its aggregation. In this same report, the authors demonstrated that the binding of NbSyn2 to αSyn did not induce any structural changes nor did it have any effect on the kinetics of formation of fibrils [101]. However, the affinity of the binding decreased as the process of fibril formation progressed suggesting that there might be conformational rearrangements of the C-terminal region of αSyn upon fibril maturation.
The same group isolated a second VHH (NbSyn87) from a phage-displayed library generated from the blood of an immunized llama this time using a mutant of αSyn (A53T) [102], which has been associated with early onset of PD [105]. This antibody also interacted with a region encompassing the C-terminus of the monomeric αSyn distinct from the NbSyn2 epitope and had the ability to bind to amyloid fibrils without structural consequences. As was observed for NbSyn2, there was a time-dependent decrease in the affinity of the antibody for its amyloid target. Further characterization of NbSyn2 and NbSyn87 led to the observation that both VHHs could inhibit the formation of mature fibrils in vitro [103]. They also had the ability to induce the conversion of αSyn from more stable oligomers into less stable oligomers significantly reducing the cellular toxicity caused by the protein.
The last αSyn-specific sdAb is a human VH (VH14) against the non-amyloid component (NAC) region of monomeric αSyn, which was selected from a non-immune yeast-displayed scFv library [106]. Although it was shown to have the highest affinity for its target, this antibody failed to rescue the cytotoxicity induced by αSyn. Nevertheless, fusion of this domain antibody to a proteosomal targeting PEST motif increased its solubility and conferred the ability to induce αSyn clearance thereby reducing the toxic effect associated with protein aggregation both in situ [107] and in vivo [108]. When compared to NbSyn87-PEST, the VH14-PEST fusion demonstrated a more pronounced effect suggesting that the NAC region of αSyn is a preferable therapeutic target.
3.1.3. Huntington’s Disease (HD)
HD is caused by an autosomal dominantly inherited CAG trinucleotide repeat expansion in the Htt gene [109]. Due to its high tendency to aggregate, the resulting mutant protein is at the origin of neuronal anomalies leading to cell death. In patients, this translates into numerous psychiatric and motor dysfunctions. There appears to be an inverse correlation between the length of expansion and age of onset. Although there are currently no curative treatments for HD, it certainly represents one of the most treatable neurological disorder since the molecular triggers are clearly defined. In this regard, sdAbs binding to mutant Htt have the potential to reduce its associated toxicity.
The use of a non-immune yeast-displayed scFv library led to the isolation of a human VL sdAb targeting the first 20 amino acids of the Htt protein [110]. The VL showed the same affinity for its target compared to its precursor scFv while achieving higher levels of cytoplasmic expression. However, inhibition of Htt aggregation demonstrated in a cell-free in vitro assay as well as in mammalian cells was only modest, requiring high amounts of the sdAb. In view of increasing the potency of VL, the same group submitted it to mutagenesis to remove its disulfide bond for efficient expression of natively folded sdAbs in the cytoplasm and subsequently increase its binding affinity [111]. The mutant, VL12.3, was able to strongly inhibit the formation of Htt aggregates and rescue cell toxicity in rat and yeast HD models. Adenoviral-delivery of this sdAb was shown to significantly improve behavior and neuropathology in a lentiviral mouse model of HD [112]. In contrast, when injected in transgenic HD mice, the antibody was found to increase the severity of the disease leading to a higher mortality rate. This was later attributed to a higher nuclear retention of Htt in the presence of VL12.3 in the transgenic HD mouse model [113].
Similarly, two more VL domain antibodies (Happ1 and Happ3) targeting the proline-rich region of Htt were selected from a non-immune phage-displayed human scFv library [113]. Their capacity to reduce Htt-induced toxicity in cell culture increased compared to their scFv predecessors. Furthermore, they both had a greater ability to prevent neurodegeneration in a brain slice model of HD. Their mechanism of action involved an increased turnover rate of mutant Htt. Adenoviral-delivery of Happ1 demonstrated its efficacy in vivo in different mouse models of HD in which marked reduction of the disease-associated symptoms was observed following administration of the sdAb [112].
The first VHHs (iVHH1–iVHH4) against the N-terminal region of Htt have been isolated from an immunized llama using phage display technologies [114]. Although the functionality of these sdAbs remains to be examined, they were found to interact with purified human wild-type and mutant Htt and also co-immunoprecipitated with both species following incubation with human HD brain lysates.
3.1.4. Prion Diseases
Prion diseases, also known as transmissible spongiform encephalopathies (TSEs) comprise of a group of fatal transmissible neurodegenerative diseases caused by the misfolding of the cellular prion protein (PrPc) into the abnormally shaped scrapie prion protein (PrPsc). The emergence of the diseased state of the prion protein (PrP) can be spontaneous, genetic, or acquired [115]. In all cases, each newly formed PrPsc acts as a template and promotes the conversion of more PrPc leading to the assembly of large insoluble amyloid fibrils associated with neurotoxicity and spongiform change in the brain parenchyma. The most common TSE is the Creutzfeldt-Jakob disease with a new incidence rate of about 1–2 cases per million of population worldwide [116]. People suffering from this disorder show a wide variety of psychiatric symptoms that rapidly progress leading ultimately to death. Despite the severity of prion diseases, only two sdAbs targeting PrPs have been generated to date.
The first one, PrioV3 was isolated from a phage-displayed VHH library generated from the blood of dromedaries immunized with brain homogenates from scrapie-infected mice adsorbed on magnetic beads [117]. The VHH showed high affinity binding to a linear epitope at the C-terminus of both PrPc and PrPsc. PrioV3 was shown to cross the BBB in vitro in rat and human brain endothelial cell lines via RMT [92,117]. Moreover, when injected intravenously in rats, the VHH was detected in the brain parenchyma suggesting its ability to cross the BBB in vivo. It also had the capacity to reduce PrPc expression and PrPsc accumulation in prion-permissive cells following its addition to the culture medium. When the treatment was prolonged over four days, PrPsc was undetectable by Western blot suggesting complete and permanent inhibition of its replication by the antibody. Similar results were obtained in vivo in mice inoculated with scrapie-infected brain homogenates receiving a weekly dose of PrioV3 [92,117]. This treatment severely abrogated the accumulation of PrPsc in the spleen of the animals. Finally, PrioV3 showed no sign of neurotoxicity in vitro.
Nb484 was selected from a pool of 14 VHHs identified following llama immunization with murine PrPs and construction of phage-displayed VHH libraries [118]. This specific VHH showed the highest affinity for human PrPs. Assessment of its neutralizing properties revealed that the antibody could delay the formation of fibrils and abrogate the expression of PrPsc in scrapie-infected murine cells. In addition, Nb484 was used as a crystallization chaperone, allowing the solution of the first crystal structures of the full length human PrPc and a C-terminal truncated version of the protein, revealing novel structural insights on the early events of the conversion of PrPc into PrPsc.
3.2. Glioblastoma Multiforme (GBM)
GBM is the most common type of brain tumors with the emergence of approximately 1000 new cases every year worldwide [119]. It is a highly aggressive malignancy showing rapid growth, intensive vascularization and predominant necrosis. Current treatments generally consist of maximal surgical resection followed by radiotherapy and chemotherapy. However, even with the use of these interventions, the prognosis remains extremely low and patients usually succumb to the disease within the first two years following diagnosis. This is in part due to the highly invasive nature of GBM and the difficulty of surgically removing all tumor cells. In addition, there is now growing evidence that the presence of chemotherapy and radiotherapy resistant stem-like cells within the tumor contributes to the resilience and recurrence of GBM [120]. Since early stages of the disease are mostly asymptomatic, current therapeutic strategies also suffer from late diagnosis. Alternative tools for the treatment and diagnosis of GBM are therefore urgently needed. Here we will review the different applications for sdAbs to improve current therapeutic modalities against GBM.
In view of identifying novel biomarkers for GBM, Jocevzka and colleagues prepared a phage-displayed VHH library from the blood of a llama immunized with a human GBM cell line enriched in stem-like cells [121,122]. Following several rounds of selection using protein extracts from diverse biological samples, three GBM-specific VHHs were designated for further characterization. Identification of their antigen by mass spectrometry revealed two proteins, Trim28 and β-actin, which showed enrichment in GBM compared to control samples. The relevance of these proteins as GBM biomarkers remains to be determined. Using a similar approach, the same group isolated seven additional VHHs specifically interacting with GBM antigens [123]. Initial Western blot and qPCR analyses complemented with bioinformatics demonstrated differential expression of some of the identified proteins in GBM compared to low grade gliomas suggesting their potential application as glioma class differentiation markers. Moreover, one antigen, mitochondrial translation elongation factor (TUFM) was isolated in a second independent screening by the same authors in which the selection of a VHH specific for GBM stem cells (GSCs) was achieved [124]. The specificity of the VHH for its target was confirmed by immunocytochemistry, and cytotoxicity assays demonstrated its profound effect on GSC growth.
VH-9.7 is a GSC-binding human VH that emerged from a non-immune yeast-displayed human scFv library using a patient-derived GSC line for selection [125]. This sdAb showed selective binding to five GSC lines and successfully identified GSCs in mouse brain xenografts by flow cytometry. Its ability to detect and localize to GSCs was also demonstrated in vivo in mice harboring orthotopic GSC xenografts following intravenous injection of a fluorophore-conjugated VH-9.7.
The following study aimed to develop novel strategies to target GBM vasculature using an in vivo panning technique to isolate camelid phage-displayed sdAbs specifically accumulating in tumor vessels [126]. This led to the identification of the C-C7 VHH, which was later shown to target a distinct population of tumor vessels in mice xenografts as well as in GBM patient samples. The antibody also had the capacity to accumulate in the tumor vasculature following injection in mice harboring orthotopic xenografts while no antibodies were detected in normal brain vessels. Using a yeast-two-hybrid method, the antigen of C-C7 was identified as Dynactin-1-p150Glued, which was expressed exclusively on activated endothelial cells and may represent a valuable tumor vessel target. The antibody presented here could be used to assess the level of angiogenesis in GBM patients and determine the severity of the disease.
Finally, VHHs targeting the epidermal growth factor receptor (EGFR) have been investigated as GBM therapeutic agents [127]. EGFR is well known to be overexpressed and mutated in a wide variety of tumors including GBM and has been extensively studied as an anti-cancer target [128,129]. VHHs targeting this receptor were isolated from an immune phage-displayed library following llama immunization with overexpressing cell preparations [130]. These antibodies (ENb1 and ENb2) were selected specifically for their ability to prevent binding of the EGF ligand to the receptor via competitive elution strategies. When engineered for sustained on-site delivery by neural-stem cells, the VHHs were shown to localize specifically in the tumor environment and inhibit EGFR signaling in vitro and to significantly reduce tumor growth in mouse models of malignant and invasive GBMs [127].
4. Single-Domain Antibodies as Neuroimaging Tools
4.1. Single-Domain Antibodies as Targeted Molecular Imaging Agents
Molecular imaging using advance and hybrid imaging modalities such as computed tomography (CT), positron emission tomography (PET), single photon emission computed tomography (SPECT), magnetic resonance imaging (MRI), and optical imaging, provide noninvasive means to characterize physiological processes and correlate molecular alterations with clinical outcomes. These technologies are improving early disease diagnosis, surgical guidance, patient stratification, and treatment monitoring [131]. Molecular imaging has advanced significantly during the last few decades through the identification of novel molecular targets and the development of multifunctional contrast agents along with new imaging instrumentation and analysis tools to extract quantitative data.
Targeted molecular imaging consists of an imaging probe linked to an agent that targets a specific biomarker of clinical relevance. Targeted molecular imaging agents have unique requirements that often differ from those of targeted therapeutic agents. In both cases, a high expression of the target antigen in the diseased versus normal tissue is required. However, for a targeted molecular imaging agent, a short half-life in circulation is preferable. Standard mAbs have a long half-life with slow liver clearance, which is a major hindrance for imaging applications, where a high contrast at early time points is critical for clinical applications [132]. The small size of sdAbs enables good tissue penetration and a fast clearance of the unbound fraction primarily via renal filtration (~60 kDa cutoff) [133,134,135]. This also allows the use of short-lived radionuclides, such as 68Ga (t1/2 = 68 min) or 18F (t1/2 = 109.8 min) for PET imaging which significantly reduces the patient’s exposure to radiation. A first-in human PET study using an anti-HER2 sdAb labeled with 68Ga-NOTA in female patients with metastatic breast cancer showed that imaging at 60–90 min provides suitable contrast to detect small and large tumor lesions with a fast blood clearance of the sdAb, such that only 7.2% of initial activity was remaining at 90 min [131].
The unique modularity of sdAbs to be engineered in different multivalent formats, including monomers, dimers, and pentamers provide additional flexibility to fulfill their antigen binding and pharmacokinetic characteristics to specific applications [136]. For instance, anti-EGFR sdAbs in monomer and pentamer formats showed to be particularly suitable for molecular optical imaging of glioblastoma tumors due to their respective short half-lives of 40 min and 80 min, while the same sdAbs engineered into a bivalent format fused with human IgG Fc have better potential to be exploited for therapeutic applications due to their extended half-lives (12.5 h) and enhanced avidity [136].
4.2. Single-Domain Antibodies for Imaging Brain Tumor Vasculature
The brain tumor vasculature represents a readily reachable target for molecular imaging due to its direct access via blood perfusion after intravenous administration. For brain tumors, such as GBM, assessment of tumor angiogenesis can provide information on the severity of the disease and guide appropriate treatment regimens [137,138]. Various tumor vascular targets that are overexpressed in the disease brain tissue and not in normal brain have been previously exploited by molecular targeted moieties for the non-invasive assessment of tumor angiogenesis using PET, optical imaging, and MRI. These include vascular endothelial growth factor receptor 2 (VEGFR2) [139], endothelial cell adhesion molecules (αvβ3 and αvβ5 integrins) [140], and insulin-like growth factor binding protein 7 (IGFBP7) [141,142]. IGFBP7, in particular, is a secreted protein that accumulates in the basement membrane of tumor endothelial cells, and its expression is believed to be associated with higher-grade gliomas [141,142,143]. Since the tumor’s malignancy is highly correlated with the degree of angiogenesis [137,138], the use of anti-IGFBP7 sdAb linked to a contrast agent for tumor vascular imaging could aid in the diagnosis and clinical management of brain tumors. In preclinical mouse models of GBM, an anti-IGFBP7 sdAb linked to a fluorophore was capable of non-invasively imaging the degree of angiogenesis [142]. Furthermore, bimodal optical-MRI contrast agents were developed by the bio-conjugation of anti-IGFBP7 sdAbs and the near-infrared fluorophore Cy5.5 to the surface of two types of nanoparticles, gadolinium-coated lipid particles for T1-weighted MRI imaging [144] and PEG functionalized-iron oxide nanoparticles for T2-weighted MRI imaging [145]. In both cases, after intravenous administration, the agents elicited an increased MRI contrast enhancement and fluorescent signal in a xenograft GBM tumor compared to a non-targeted nanoparticle. The molecular localization of the anti-IGFBP7 sdAb in the tumor brain vessels was further demonstrated by fluorescence microscopy [142].
4.3. Single-Domain Antibodies for Imaging Brain Targets
Due to the presence of the BBB, which limits the access of most biologics (i.e., proteins, peptides, antibodies) to the brain, radioligands used for PET imaging of CNS targets have been based on small molecular weight (<500 Da) molecules [146]. Targeted radioligands utilizing antibodies, antibody fragments, or sdAbs have been mainly developed for peripheral targets and used in different applications, including the detection of tumor markers, monitoring inflammatory processes, and visualization of antitumor immune responses [147]. However, a variety of strategies have been employed to allow the delivery of protein molecules across the BBB using both disruptive and non-disruptive methods.
As previously described, transmigration of antibodies across the BBB via RMT is a non-disruptive method for gaining access to brain targets (see Section 2). Using this strategy molecular imaging probes coupled to BBB carriers can be shuttled to the brain. For instance, taking advantage of the modularity of the BBB-transmigrating FC5 sdAb, a lipid-based nanoparticle was designed to encapsulate the anti-cancer drug doxorubicin and to display on its surface both a near-infrared (NIR) imaging agent and FC5 sdAb [148]. Upon intravenous injection in mice, in vivo optical imaging indicated increased brain delivery of the FC5-targeted versus non-targeted doxorubicin-containing liposomes. The optical fluorescent signal detected in vivo in the brain parenchyma correlated with the amount of doxorubicin delivered in the brain and measured ex vivo. Thus, this method allows for a non-invasive estimation of drug delivery into the brain.
BBB transmigration of macromolecules may also be achieved via adsorptive-mediated endocytosis through non-specific, charge-based interactions with the endothelial cell surface [149]. Endothelial cells are characterized by the presentation of negatively-charged clathrin-coated pits at the luminal surface, which can bind cationic proteins and facilitate their penetration through the BBB [150]. In an AD in vivo two-photon imaging study, sdAbs, selected for a basic isoelectric point (i.e., due to cationic amino acids) and for their binding to brain Aβ deposits or Tau inclusions were able to penetrate the BBB and bind to their respective brain target in vivo [135]. Interestingly, it was suggested that, in addition to the basic isoelectric pI, the molecular size of the sdAb was an important factor in the BBB penetration capability, as larger constructs (i.e., sdAb dimers) demonstrated reduced BBB penetration [151].
Some sdAbs have also been shown to interact with intracellular targets (i.e., penetrate cells) [152]. For instance, VHHs against the astrocyte marker glial fibrillary acidic protein (GFAP) has been shown to cross the BBB, reach the brain tissue, and penetrate into astrocytes, as demonstrated by immunofluorescence studies on injected animal tissue sections [151]. This feature of sdAbs has the potential to open up “difficult to access” intracellular targets in the brain or within brain cell subtypes.
In summary, sdAbs hold promise for dynamic imaging compared to other antibody-based agents due to their small size that allows better tissue penetration, rapid and homogeneous tumor/brain accumulation and fast blood clearance, which results in high tissue-to-background noise ratios. Single-domain antibodies are versatile, stable in very harsh conditions (pH, temperature), easy to conjugate to different imaging probes, and relatively safe due to their high specificity.
5. Conclusions
Singe-domain antibody technologies are ‘coming of age’ with many being tested in clinical trials. Several notable advantages of this compact antibody format, including ease of engineering, stability, recognition of unusual epitopes, and versatility for creating bi- and multifunctional molecules, have resulted in sdAbs being poised to address some of the most difficult target and disease spaces, most notably those of the CNS. CNS diseases are among the most difficult to treat not only because therapeutic targets (e.g., misfolded proteins, ion channels and G-protein coupled receptors) are very complex, but also because they are ‘hidden’ behind brain barriers and are thus difficult to access systemically. Selectivity of targeting of receptor/channel subtypes, often in specific activation states, specific targeting of point mutations, or epitopes ‘embedded’ in misfolded proteins present unique challenges, often difficult to address by either synthetic molecules or mAbs. While ‘precision’ targeting of desired epitopes is achievable by both sdAbs and mAbs, compact sdAb format could improve access to hidden epitopes. One distinct advantage of this format is improved diffusion in brain tissue after direct intracerebral administration, and enhanced brain tissue penetration after intrathecal infusion via perivascular flow. Furthermore, sdAbs are proving to be a versatile format for designing BBB carriers that could be easily combined in various display linkages (mono-, bi-, multivalent) with therapeutic monoclonal antibodies and other therapeutic cargos (peptides, proteins, nanocarriers, and imaging agents). The pipeline of sdAbs, both camelid and human, raised against CNS targets from naïve or immune libraries and tested in preclinical models is growing with prospects for entry into clinical testing in the near future. With parallel and significant progress in the development of BBB-delivery technologies based on sdAbs, the field of CNS, so far dominated by small molecule therapeutics, is slowly but steadily progressing into a new era of biological treatments, most notably antibody therapies for chronic neurodegenerative diseases.
Author Contributions
K.B., U.I., M.M., J.T., R.M. and D.S. wrote and proof-read the manuscript. K.B. assembled the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ward E.S., Gussow D., Griffiths A.D., Jones P.T., Winter G. Binding activities of a repertoire of single immunoglobulin variable domains secreted from Escherichia coli. Nature. 1989;341:544–546. doi: 10.1038/341544a0. [DOI] [PubMed] [Google Scholar]
- 2.Hamers-Casterman C., Atarhouch T., Muyldermans S., Robinson G., Hamers C., Songa E.B., Bendahman N., Hamers R. Naturally occurring antibodies devoid of light chains. Nature. 1993;363:446–448. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg A.S., Avila D., Hughes M., Hughes A., McKinney E.C., Flajnik M.F. A new antigen receptor gene family that undergoes rearrangement and extensive somatic diversification in sharks. Nature. 1995;374:168–173. doi: 10.1038/374168a0. [DOI] [PubMed] [Google Scholar]
- 4.Muyldermans S. Nanobodies: Natural single-domain antibodies. Annu. Rev. Biochem. 2013;82:775–797. doi: 10.1146/annurev-biochem-063011-092449. [DOI] [PubMed] [Google Scholar]
- 5.Konning D., Zielonka S., Grzeschik J., Empting M., Valldorf B., Krah S., Schroter C., Sellmann C., Hock B., Kolmar H. Camelid and shark single domain antibodies: Structural features and therapeutic potential. Curr. Opin. Struct. Boil. 2017;45:10–16. doi: 10.1016/j.sbi.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Kim D.Y., Hussack G., Kandalaft H., Tanha J. Mutational approaches to improve the biophysical properties of human single-domain antibodies. Biochim. Biophys. Acta. 2014;1844:1983–2001. doi: 10.1016/j.bbapap.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Yu Y.J., Atwal J.K., Zhang Y., Tong R.K., Wildsmith K.R., Tan C., Bien-Ly N., Hersom M., Maloney J.A., Meilandt W.J., et al. Therapeutic bispecific antibodies cross the blood-brain barrier in nonhuman primates. Sci. Transl. Med. 2014;6:261ra154. doi: 10.1126/scitranslmed.3009835. [DOI] [PubMed] [Google Scholar]
- 8.Henry K.A., Kim D.Y., Kandalaft H., Lowden M.J., Yang Q., Schrag J.D., Hussack G., MacKenzie C.R., Tanha J. Stability-Diversity Tradeoffs Impose Fundamental Constraints on Selection of Synthetic Human VH/VL Single-Domain Antibodies from In Vitro Display Libraries. Front. Immunol. 2017;8:1759. doi: 10.3389/fimmu.2017.01759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janssens R., Dekker S., Hendriks R.W., Panayotou G., van Remoortere A., San J.K., Grosveld F., Drabek D. Generation of heavy-chain-only antibodies in mice. Proc. Natl. Acad. Sci. USA. 2006;103:15130–15135. doi: 10.1073/pnas.0601108103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drabek D., Janssens R., de Boer E., Rademaker R., Kloess J., Skehel J., Grosveld F. Expression Cloning and Production of Human Heavy-Chain-Only Antibodies from Murine Transgenic Plasma Cells. Front. Immunol. 2016;7:619. doi: 10.3389/fimmu.2016.00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steeland S., Vandenbroucke R.E., Libert C. Nanobodies as therapeutics: Big opportunities for small antibodies. Drug Discov. Today. 2016;21:1076–1113. doi: 10.1016/j.drudis.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Porter C.B.a.A. VNARS: An ancient and unique repertoire of small molecules that deliver small, soluble, stable and high affinity binders of proteins. Antibodies. 2015;4:240–258. doi: 10.3390/antib4030240. [DOI] [Google Scholar]
- 13.Hoogenboom H.R. Selecting and screening recombinant antibody libraries. Nat. Biotechnol. 2005;23:1105–1116. doi: 10.1038/nbt1126. [DOI] [PubMed] [Google Scholar]
- 14.Zielonka S., Empting M., Grzeschik J., Konning D., Barelle C.J., Kolmar H. Structural insights and biomedical potential of IgNAR scaffolds from sharks. mAbs. 2015;7:15–25. doi: 10.4161/19420862.2015.989032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovaleva M., Ferguson L., Steven J., Porter A., Barelle C. Shark variable new antigen receptor biologics—A novel technology platform for therapeutic drug development. Expert Opin. Boil. Ther. 2014;14:1527–1539. doi: 10.1517/14712598.2014.937701. [DOI] [PubMed] [Google Scholar]
- 16.Baral T.N., MacKenzie R., Arbabi Ghahroudi M. Single-domain antibodies and their utility. Curr. Protoc. Immunol. 2013;103:2–17. doi: 10.1002/0471142735.im0217s103. [DOI] [PubMed] [Google Scholar]
- 17.Schneider D., Xiong Y., Hu P., Wu D., Chen W., Ying T., Zhu Z., Dimitrov D.S., Dropulic B., Orentas R.J. A Unique Human Immunoglobulin Heavy Chain Variable Domain-Only CD33 CAR for the Treatment of Acute Myeloid Leukemia. Front. Oncol. 2018;8:539. doi: 10.3389/fonc.2018.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henry K.A., Tanha J. Performance evaluation of phage-displayed synthetic human single-domain antibody libraries: A retrospective analysis. J. Immunol. Methods. 2018;456:81–86. doi: 10.1016/j.jim.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Hussack G., Riazi A., Ryan S., van Faassen H., MacKenzie R., Tanha J., Arbabi-Ghahroudi M. Protease-resistant single-domain antibodies inhibit Campylobacter jejuni motility. Protein Eng. Des. Sel. PEDS. 2014;27:191–198. doi: 10.1093/protein/gzu011. [DOI] [PubMed] [Google Scholar]
- 20.Muruganandam A., Tanha J., Narang S., Stanimirovic D. Selection of phage-displayed llama single-domain antibodies that transmigrate across human blood-brain barrier endothelium. FASEB J. 2002;16:240–242. doi: 10.1096/fj.01-0343fje. [DOI] [PubMed] [Google Scholar]
- 21.Iezzi M.E., Policastro L., Werbajh S., Podhajcer O., Canziani G.A. Single-Domain Antibodies and the Promise of Modular Targeting in Cancer Imaging and Treatment. Front. Immunol. 2018;9:273. doi: 10.3389/fimmu.2018.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ubah O.C., Buschhaus M.J., Ferguson L., Kovaleva M., Steven J., Porter A.J., Barelle C.J. Next-generation flexible formats of VNAR domains expand the drug platform’s utility and developability. Biochem. Soc. Trans. 2018;46:1559–1565. doi: 10.1042/BST20180177. [DOI] [PubMed] [Google Scholar]
- 23.Els Conrath K., Lauwereys M., Wyns L., Muyldermans S. Camel single-domain antibodies as modular building units in bispecific and bivalent antibody constructs. J. Boil. Chem. 2001;276:7346–7350. doi: 10.1074/jbc.M007734200. [DOI] [PubMed] [Google Scholar]
- 24.Holt L.J., Basran A., Jones K., Chorlton J., Jespers L.S., Brewis N.D., Tomlinson I.M. Anti-serum albumin domain antibodies for extending the half-lives of short lived drugs. Protein Eng. Des. Sel. PEDS. 2008;21:283–288. doi: 10.1093/protein/gzm067. [DOI] [PubMed] [Google Scholar]
- 25.Coppieters K., Dreier T., Silence K., de Haard H., Lauwereys M., Casteels P., Beirnaert E., Jonckheere H., Van de Wiele C., Staelens L., et al. Formatted anti-tumor necrosis factor alpha VHH proteins derived from camelids show superior potency and targeting to inflamed joints in a murine model of collagen-induced arthritis. Arthritis Rheum. 2006;54:1856–1866. doi: 10.1002/art.21827. [DOI] [PubMed] [Google Scholar]
- 26.Simmons D.P., Abregu F.A., Krishnan U.V., Proll D.F., Streltsov V.A., Doughty L., Hattarki M.K., Nuttall S.D. Dimerisation strategies for shark IgNAR single domain antibody fragments. J. Immunol. Methods. 2006;315:171–184. doi: 10.1016/j.jim.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 27.De Bernardis F., Liu H., O’Mahony R., La Valle R., Bartollino S., Sandini S., Grant S., Brewis N., Tomlinson I., Basset R.C., et al. Human domain antibodies against virulence traits of Candida albicans inhibit fungus adherence to vaginal epithelium and protect against experimental vaginal candidiasis. J. Infect. Dis. 2007;195:149–157. doi: 10.1086/509891. [DOI] [PubMed] [Google Scholar]
- 28.Muller M.R., Saunders K., Grace C., Jin M., Piche-Nicholas N., Steven J., O’Dwyer R., Wu L., Khetemenee L., Vugmeyster Y., et al. Improving the pharmacokinetic properties of biologics by fusion to an anti-HSA shark VNAR domain. mAbs. 2012;4:673–685. doi: 10.4161/mabs.22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steven J., Muller M.R., Carvalho M.F., Ubah O.C., Kovaleva M., Donohoe G., Baddeley T., Cornock D., Saunders K., Porter A.J., et al. In Vitro Maturation of a Humanized Shark VNAR Domain to Improve Its Biophysical Properties to Facilitate Clinical Development. Front. Immunol. 2017;8:1361. doi: 10.3389/fimmu.2017.01361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nosenko M.A., Atretkhany K.N., Mokhonov V.V., Efimov G.A., Kruglov A.A., Tillib S.V., Drutskaya M.S., Nedospasov S.A. VHH-Based Bispecific Antibodies Targeting Cytokine Production. Front. Immunol. 2017;8:1073. doi: 10.3389/fimmu.2017.01073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beirnaert E., Desmyter A., Spinelli S., Lauwereys M., Aarden L., Dreier T., Loris R., Silence K., Pollet C., Cambillau C., et al. Bivalent Llama Single-Domain Antibody Fragments against Tumor Necrosis Factor Have Picomolar Potencies due to Intramolecular Interactions. Front. Immunol. 2017;8:867. doi: 10.3389/fimmu.2017.00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darling T.L., Sherwood L.J., Hayhurst A. Intracellular Crosslinking of Filoviral Nucleoproteins with Xintrabodies Restricts Viral Packaging. Front. Immunol. 2017;8:1197. doi: 10.3389/fimmu.2017.01197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Z., Schmidt D., Liu W., Li S., Shi L., Sheng J., Chen K., Yu H., Tremblay J.M., Chen X., et al. A novel multivalent, single-domain antibody targeting TcdA and TcdB prevents fulminant Clostridium difficile infection in mice. J. Infect. Dis. 2014;210:964–972. doi: 10.1093/infdis/jiu196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Roy M., Ververken C., Beirnaert E., Hoefman S., Kolkman J., Vierboom M., Breedveld E., t Hart B., Poelmans S., Bontinck L., et al. The preclinical pharmacology of the high affinity anti-IL-6R Nanobody(R) ALX-0061 supports its clinical development in rheumatoid arthritis. Arthritis Res. Ther. 2015;17:135. doi: 10.1186/s13075-015-0651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desmyter A., Spinelli S., Boutton C., Saunders M., Blachetot C., de Haard H., Denecker G., Van Roy M., Cambillau C., Rommelaere H. Neutralization of Human Interleukin 23 by Multivalent Nanobodies Explained by the Structure of Cytokine-Nanobody Complex. Front. Immunol. 2017;8:884. doi: 10.3389/fimmu.2017.00884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harmsen M.M., Fijten H.P., Dekker A., Eble P.L. Passive immunization of pigs with bispecific llama single-domain antibody fragments against foot-and-mouth disease and porcine immunoglobulin. Vet. Microbiol. 2008;132:56–64. doi: 10.1016/j.vetmic.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J., Tanha J., Hirama T., Khieu N.H., To R., Tong-Sevinc H., Stone E., Brisson J.R., MacKenzie C.R. Pentamerization of single-domain antibodies from phage libraries: A novel strategy for the rapid generation of high-avidity antibody reagents. J. Mol. Boil. 2004;335:49–56. doi: 10.1016/j.jmb.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 38.Stone E., Hirama T., Tanha J., Tong-Sevinc H., Li S., MacKenzie C.R., Zhang J. The assembly of single domain antibodies into bispecific decavalent molecules. J. Immunol. Methods. 2007;318:88–94. doi: 10.1016/j.jim.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J., Liu X., Bell A., To R., Baral T.N., Azizi A., Li J., Cass B., Durocher Y. Transient expression and purification of chimeric heavy chain antibodies. Protein Expr. Purif. 2009;65:77–82. doi: 10.1016/j.pep.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 40.Ubah O.C., Steven J., Kovaleva M., Ferguson L., Barelle C., Porter A.J.R., Barelle C.J. Novel, Anti-hTNF-alpha Variable New Antigen Receptor Formats with Enhanced Neutralizing Potency and Multifunctionality, Generated for Therapeutic Development. Front. Immunol. 2017;8:1780. doi: 10.3389/fimmu.2017.01780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kovaleva M., Johnson K., Steven J., Barelle C.J., Porter A. Therapeutic Potential of Shark Anti-ICOSL VNAR Domains is Exemplified in a Murine Model of Autoimmune Non-Infectious Uveitis. Front. Immunol. 2017;8:1121. doi: 10.3389/fimmu.2017.01121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D’Eall C., Pon R.A., Rossotti M.A., Krahn N., Spearman M., Callaghan D., van Faassen H., Hussack G., Stetefeld J., Butler M., et al. Modulating antibody-dependent cellular cytotoxicity of epidermal growth factor receptor-specific heavy-chain antibodies through hinge engineering. Immunol. Cell Boil. 2019:1–12. doi: 10.1111/imcb.12238. [DOI] [PubMed] [Google Scholar]
- 43.Rossotti M.A., Henry K.A., van Faassen H., Tanha J., Callaghan D., Hussack G., Arbabi-Ghahroudi M., MacKenzie C.R. Camelid single-domain antibodies raised by DNA immunization are potent inhibitors of EGFR signaling. Biochem. J. 2019;476:39–50. doi: 10.1042/BCJ20180795. [DOI] [PubMed] [Google Scholar]
- 44.Henry K.A., Kandalaft H., Lowden M.J., Rossotti M.A., van Faassen H., Hussack G., Durocher Y., Kim D.Y., Tanha J. A disulfide-stabilized human VL single-domain antibody library is a source of soluble and highly thermostable binders. Mol. Immunol. 2017;90:190–196. doi: 10.1016/j.molimm.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 45.Schutze K., Petry K., Hambach J., Schuster N., Fumey W., Schriewer L., Rockendorf J., Menzel S., Albrecht B., Haag F., et al. CD38-Specific Biparatopic Heavy Chain Antibodies Display Potent Complement-Dependent Cytotoxicity Against Multiple Myeloma Cells. Front. Immunol. 2018;9:2553. doi: 10.3389/fimmu.2018.02553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Von Kreudenstein T.S., Escobar-Carbrera E., Lario P.I., D’Angelo I., Brault K., Kelly J., Durocher Y., Baardsnes J., Woods R.J., Xie M.H., et al. Improving biophysical properties of a bispecific antibody scaffold to aid developability: Quality by molecular design. mAbs. 2013;5:646–654. doi: 10.4161/mabs.25632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brinkmann U., Kontermann R.E. The making of bispecific antibodies. mAbs. 2017;9:182–212. doi: 10.1080/19420862.2016.1268307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cortez-Retamozo V., Backmann N., Senter P.D., Wernery U., De Baetselier P., Muyldermans S., Revets H. Efficient cancer therapy with a nanobody-based conjugate. Cancer Res. 2004;64:2853–2857. doi: 10.1158/0008-5472.CAN-03-3935. [DOI] [PubMed] [Google Scholar]
- 49.Baral T.N., Magez S., Stijlemans B., Conrath K., Vanhollebeke B., Pays E., Muyldermans S., De Baetselier P. Experimental therapy of African trypanosomiasis with a nanobody-conjugated human trypanolytic factor. Nat. Med. 2006;12:580–584. doi: 10.1038/nm1395. [DOI] [PubMed] [Google Scholar]
- 50.Yu Y., Li J., Zhu X., Tang X., Bao Y., Sun X., Huang Y., Tian F., Liu X., Yang L. Humanized CD7 nanobody-based immunotoxins exhibit promising anti-T-cell acute lymphoblastic leukemia potential. Int. J. Nanomed. 2017;12:1969–1983. doi: 10.2147/IJN.S127575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Behdani M., Zeinali S., Karimipour M., Khanahmad H., Schoonooghe S., Aslemarz A., Seyed N., Moazami-Godarzi R., Baniahmad F., Habibi-Anbouhi M., et al. Development of VEGFR2-specific Nanobody Pseudomonas exotoxin A conjugated to provide efficient inhibition of tumor cell growth. New Biotechnol. 2013;30:205–209. doi: 10.1016/j.nbt.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 52.Tian B., Wong W.Y., Hegmann E., Gaspar K., Kumar P., Chao H. Production and characterization of a camelid single domain antibody-urease enzyme conjugate for the treatment of cancer. Bioconj. Chem. 2015;26:1144–1155. doi: 10.1021/acs.bioconjchem.5b00237. [DOI] [PubMed] [Google Scholar]
- 53.Tian B., Wong W.Y., Uger M.D., Wisniewski P., Chao H. Development and Characterization of a Camelid Single Domain Antibody-Urease Conjugate That Targets Vascular Endothelial Growth Factor Receptor 2. Front. Immunol. 2017;8:956. doi: 10.3389/fimmu.2017.00956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abbott N.J., Patabendige A.A., Dolman D.E., Yusof S.R., Begley D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 55.Wolak D.J., Thorne R.G. Diffusion of macromolecules in the brain: Implications for drug delivery. Mol. Pharm. 2013;10:1492–1504. doi: 10.1021/mp300495e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pizzo M.E., Wolak D.J., Kumar N.N., Brunette E., Brunnquell C.L., Hannocks M.J., Abbott N.J., Meyerand M.E., Sorokin L., Stanimirovic D.B., et al. Intrathecal antibody distribution in the rat brain: Surface diffusion, perivascular transport and osmotic enhancement of delivery. J. Physiol. 2018;596:445–475. doi: 10.1113/JP275105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones A.R., Shusta E.V. Blood-brain barrier transport of therapeutics via receptor-mediation. Pharm. Res. 2007;24:1759–1771. doi: 10.1007/s11095-007-9379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pardridge W.M., Buciak J.L., Friden P.M. Selective transport of an anti-transferrin receptor antibody through the blood-brain barrier in vivo. J. Pharmacol. Exp. Ther. 1991;259:66–70. [PubMed] [Google Scholar]
- 59.Yu Y.J., Zhang Y., Kenrick M., Hoyte K., Luk W., Lu Y., Atwal J., Elliott J.M., Prabhu S., Watts R.J., et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci. Transl. Med. 2011;3:84ra44. doi: 10.1126/scitranslmed.3002230. [DOI] [PubMed] [Google Scholar]
- 60.Niewoehner J., Bohrmann B., Collin L., Urich E., Sade H., Maier P., Rueger P., Stracke J.O., Lau W., Tissot A.C., et al. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron. 2014;81:49–60. doi: 10.1016/j.neuron.2013.10.061. [DOI] [PubMed] [Google Scholar]
- 61.Haqqani A.S., Delaney C.E., Brunette E., Baumann E., Farrington G.K., Sisk W., Eldredge J., Ding W., Tremblay T.L., Stanimirovic D.B. Endosomal trafficking regulates receptor-mediated transcytosis of antibodies across the blood brain barrier. J. Cereb. Blood Flow Metab. 2018;38:727–740. doi: 10.1177/0271678X17740031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coloma M.J., Lee H.J., Kurihara A., Landaw E.M., Boado R.J., Morrison S.L., Pardridge W.M. Transport across the primate blood-brain barrier of a genetically engineered chimeric monoclonal antibody to the human insulin receptor. Pharm. Res. 2000;17:266–274. doi: 10.1023/A:1007592720793. [DOI] [PubMed] [Google Scholar]
- 63.Boado R.J., Zhou Q.H., Lu J.Z., Hui E.K., Pardridge W.M. Pharmacokinetics and brain uptake of a genetically engineered bifunctional fusion antibody targeting the mouse transferrin receptor. Mol. Pharm. 2010;7:237–244. doi: 10.1021/mp900235k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Giugliani R., Giugliani L., de Oliveira Poswar F., Donis K.C., Corte A.D., Schmidt M., Boado R.J., Nestrasil I., Nguyen C., Chen S., et al. Neurocognitive and somatic stabilization in pediatric patients with severe Mucopolysaccharidosis Type I after 52 weeks of intravenous brain-penetrating insulin receptor antibody-iduronidase fusion protein (valanafusp alpha): An open label phase 1-2 trial. Orphanet J. Rare Dis. 2018;13:110. doi: 10.1186/s13023-018-0849-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pardridge W.M., Boado R.J., Patrick D.J., Ka-Wai Hui E., Lu J.Z. Blood-Brain Barrier Transport, Plasma Pharmacokinetics, and Neuropathology Following Chronic Treatment of the Rhesus Monkey with a Brain Penetrating Humanized Monoclonal Antibody Against the Human Transferrin Receptor. Mol. Pharm. 2018 doi: 10.1021/acs.molpharmaceut.8b00730. [DOI] [PubMed] [Google Scholar]
- 66.Walsh F.S., Wicher K., Szary J., Stocki P., Demydchuk M., Rutkowski L. Delivery of a CD20 transferrin receptor VNAR bispecific antibody to the brain for CNS lymphoma; Proceedings of the AACR Annual Meeting; Washington, DC, USA. 1–5 April 2017. [Google Scholar]
- 67.Bahney J., von Bartheld C.S. The Cellular Composition and Glia-Neuron Ratio in the Spinal Cord of a Human and a Nonhuman Primate: Comparison with Other Species and Brain Regions. Anat. Rec. 2018;301:697–710. doi: 10.1002/ar.23728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Couch J.A., Yu Y.J., Zhang Y., Tarrant J.M., Fuji R.N., Meilandt W.J., Solanoy H., Tong R.K., Hoyte K., Luk W., et al. Addressing safety liabilities of TfR bispecific antibodies that cross the blood-brain barrier. Sci. Transl. Med. 2013;5:183ra57. doi: 10.1126/scitranslmed.3005338. [DOI] [PubMed] [Google Scholar]
- 69.Webster C.I., Stanimirovic D.B. A gateway to the brain: Shuttles for brain delivery of macromolecules. Ther. Deliv. 2015;6:1321–1324. doi: 10.4155/tde.15.78. [DOI] [PubMed] [Google Scholar]
- 70.Haqqani A.S., Caram-Salas N., Ding W., Brunette E., Delaney C.E., Baumann E., Boileau E., Stanimirovic D. Multiplexed evaluation of serum and CSF pharmacokinetics of brain-targeting single-domain antibodies using a NanoLC-SRM-ILIS method. Mol. Pharm. 2013;10:1542–1556. doi: 10.1021/mp3004995. [DOI] [PubMed] [Google Scholar]
- 71.Abulrob A., Sprong H., Van Bergen en Henegouwen P., Stanimirovic D. The blood-brain barrier transmigrating single domain antibody: Mechanisms of transport and antigenic epitopes in human brain endothelial cells. J. Neurochem. 2005;95:1201–1214. doi: 10.1111/j.1471-4159.2005.03463.x. [DOI] [PubMed] [Google Scholar]
- 72.Albulrob A., Stanimirovic D., Muruganandam A. Blood-Brain Barrier Epitopes and Uses Thereof. WO2007036021A1. Patent. 2007 Apr 5;
- 73.Farrington G.K., Caram-Salas N., Haqqani A.S., Brunette E., Eldredge J., Pepinsky B., Antognetti G., Baumann E., Ding W., Garber E., et al. A novel platform for engineering blood-brain barrier-crossing bispecific biologics. FASEB J. 2014;28:4764–4778. doi: 10.1096/fj.14-253369. [DOI] [PubMed] [Google Scholar]
- 74.Webster C.I., Caram-Salas N., Haqqani A.S., Thom G., Brown L., Rennie K., Yogi A., Costain W., Brunette E., Stanimirovic D.B. Brain penetration, target engagement, and disposition of the blood-brain barrier-crossing bispecific antibody antagonist of metabotropic glutamate receptor type 1. FASEB J. 2016;30:1927–1940. doi: 10.1096/fj.201500078. [DOI] [PubMed] [Google Scholar]
- 75.Stanimirovic D., Kemmerich K., Haqqani A.S., Sulea T., Arbabi-Ghahroudi M., Massie B., Gilbert R. Insulin-Like Growth Factor 1 Receptor-Specific Antibodies and Uses Thereof. US20170015748A1. U.S. Patent. 2017 Jan 19;
- 76.Ribecco-Lutkiewicz M., Sodja C., Haukenfrers J., Haqqani A.S., Ly D., Zachar P., Baumann E., Ball M., Huang J., Rukhlova M., et al. A novel human induced pluripotent stem cell blood-brain barrier model: Applicability to study antibody-triggered receptor-mediated transcytosis. Sci. Rep. 2018;8:1873. doi: 10.1038/s41598-018-19522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Robertson C.S., Cherian L., Shah M., Garcia R., Navarro J.C., Grill R.J., Hand C.C., Tian T.S., Hannay H.J. Neuroprotection with an erythropoietin mimetic peptide (pHBSP) in a model of mild traumatic brain injury complicated by hemorrhagic shock. J. Neurotrauma. 2012;29:1156–1166. doi: 10.1089/neu.2011.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chiti F., Dobson C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 79.Dobson C.M. Principles of protein folding, misfolding and aggregation. Semin. Cell Dev. Boil. 2004;15:3–16. doi: 10.1016/j.semcdb.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 80.Katzman R. Editorial: The prevalence and malignancy of Alzheimer disease. A major killer. Arch. Neurol. 1976;33:217–218. doi: 10.1001/archneur.1976.00500040001001. [DOI] [PubMed] [Google Scholar]
- 81.Barker W.W., Luis C.A., Kashuba A., Luis M., Harwood D.G., Loewenstein D., Waters C., Jimison P., Shepherd E., Sevush S., et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis. Assoc. Disord. 2002;16:203–212. doi: 10.1097/00002093-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 82.Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 83.Wang D.S., Dickson D.W., Malter J.S. Tissue transglutaminase, protein cross-linking and Alzheimer’s disease: Review and views. Int. J. Clin. Exp. Pathol. 2008;1:5–18. [PMC free article] [PubMed] [Google Scholar]
- 84.Habicht G., Haupt C., Friedrich R.P., Hortschansky P., Sachse C., Meinhardt J., Wieligmann K., Gellermann G.P., Brodhun M., Gotz J., et al. Directed selection of a conformational antibody domain that prevents mature amyloid fibril formation by stabilizing Abeta protofibrils. Proc. Natl. Acad. Sci. USA. 2007;104:19232–19237. doi: 10.1073/pnas.0703793104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Walsh D.M., Lomakin A., Benedek G.B., Condron M.M., Teplow D.B. Amyloid beta-protein fibrillogenesis. Detection of a protofibrillar intermediate. J. Boil. Chem. 1997;272:22364–22372. doi: 10.1074/jbc.272.35.22364. [DOI] [PubMed] [Google Scholar]
- 86.Morgado I., Wieligmann K., Bereza M., Ronicke R., Meinhardt K., Annamalai K., Baumann M., Wacker J., Hortschansky P., Malesevic M., et al. Molecular basis of beta-amyloid oligomer recognition with a conformational antibody fragment. Proc. Natl. Acad. Sci. USA. 2012;109:12503–12508. doi: 10.1073/pnas.1206433109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wacker J., Ronicke R., Westermann M., Wulff M., Reymann K.G., Dobson C.M., Horn U., Crowther D.C., Luheshi L.M., Fandrich M. Oligomer-targeting with a conformational antibody fragment promotes toxicity in Abeta-expressing flies. Acta Neuropathol. Commun. 2014;2:43. doi: 10.1186/2051-5960-2-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rutgers K.S., van Remoortere A., van Buchem M.A., Verrips C.T., Greenberg S.M., Bacskai B.J., Frosch M.P., van Duinen S.G., Maat-Schieman M.L., van der Maarel S.M. Differential recognition of vascular and parenchymal beta amyloid deposition. Neurobiol. Aging. 2011;32:1774–1783. doi: 10.1016/j.neurobiolaging.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rutgers K.S., Nabuurs R.J., van den Berg S.A., Schenk G.J., Rotman M., Verrips C.T., van Duinen S.G., Maat-Schieman M.L., van Buchem M.A., de Boer A.G., et al. Transmigration of beta amyloid specific heavy chain antibody fragments across the in vitro blood-brain barrier. Neuroscience. 2011;190:37–42. doi: 10.1016/j.neuroscience.2011.05.076. [DOI] [PubMed] [Google Scholar]
- 90.Nabuurs R.J., Rutgers K.S., Welling M.M., Metaxas A., de Backer M.E., Rotman M., Bacskai B.J., van Buchem M.A., van der Maarel S.M., van der Weerd L. In vivo detection of amyloid-beta deposits using heavy chain antibody fragments in a transgenic mouse model for Alzheimer’s disease. PLoS ONE. 2012;7:e38284. doi: 10.1371/journal.pone.0038284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lafaye P., Achour I., England P., Duyckaerts C., Rougeon F. Single-domain antibodies recognize selectively small oligomeric forms of amyloid beta, prevent Abeta-induced neurotoxicity and inhibit fibril formation. Mol. Immunol. 2009;46:695–704. doi: 10.1016/j.molimm.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 92.David M.A., Jones D.R., Tayebi M. Potential candidate camelid antibodies for the treatment of protein-misfolding diseases. J. Neuroimmunol. 2014;272:76–85. doi: 10.1016/j.jneuroim.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 93.Medecigo M., Manoutcharian K., Vasilevko V., Govezensky T., Munguia M.E., Becerril B., Luz-Madrigal A., Vaca L., Cribbs D.H., Gevorkian G. Novel amyloid-beta specific scFv and VH antibody fragments from human and mouse phage display antibody libraries. J. Neuroimmunol. 2010;223:104–114. doi: 10.1016/j.jneuroim.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Perchiacca J.M., Ladiwala A.R., Bhattacharya M., Tessier P.M. Structure-based design of conformation- and sequence-specific antibodies against amyloid beta. Proc. Natl. Acad. Sci. USA. 2012;109:84–89. doi: 10.1073/pnas.1111232108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ladiwala A.R., Bhattacharya M., Perchiacca J.M., Cao P., Raleigh D.P., Abedini A., Schmidt A.M., Varkey J., Langen R., Tessier P.M. Rational design of potent domain antibody inhibitors of amyloid fibril assembly. Proc. Natl. Acad. Sci. USA. 2012;109:19965–19970. doi: 10.1073/pnas.1208797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Julian M.C., Lee C.C., Tiller K.E., Rabia L.A., Day E.K., Schick A.J., 3rd, Tessier P.M. Co-evolution of affinity and stability of grafted amyloid-motif domain antibodies. Protein Eng. Des. Sel. PEDS. 2015;28:339–350. doi: 10.1093/protein/gzv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee C.C., Julian M.C., Tiller K.E., Meng F., DuConge S.E., Akter R., Raleigh D.P., Tessier P.M. Design and Optimization of Anti-amyloid Domain Antibodies Specific for beta-Amyloid and Islet Amyloid Polypeptide. J. Boil. Chem. 2016;291:2858–2873. doi: 10.1074/jbc.M115.682336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sormanni P., Aprile F.A., Vendruscolo M. Rational design of antibodies targeting specific epitopes within intrinsically disordered proteins. Proc. Natl. Acad. Sci. USA. 2015;112:9902–9907. doi: 10.1073/pnas.1422401112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hoehn M.M., Yahr M.D. Parkinsonism: Onset, progression, and mortality. Neurology. 1998;50:318. doi: 10.1212/WNL.50.2.318. [DOI] [PubMed] [Google Scholar]
- 100.Malek N., Swallow D., Grosset K.A., Anichtchik O., Spillantini M., Grosset D.G. Alpha-synuclein in peripheral tissues and body fluids as a biomarker for Parkinson’s disease—A systematic review. Acta Neurol. Scand. 2014;130:59–72. doi: 10.1111/ane.12247. [DOI] [PubMed] [Google Scholar]
- 101.De Genst E.J., Guilliams T., Wellens J., O’Day E.M., Waudby C.A., Meehan S., Dumoulin M., Hsu S.T., Cremades N., Verschueren K.H., et al. Structure and properties of a complex of alpha-synuclein and a single-domain camelid antibody. J. Mol. Boil. 2010;402:326–343. doi: 10.1016/j.jmb.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 102.Guilliams T., El-Turk F., Buell A.K., O’Day E.M., Aprile F.A., Esbjorner E.K., Vendruscolo M., Cremades N., Pardon E., Wyns L., et al. Nanobodies raised against monomeric alpha-synuclein distinguish between fibrils at different maturation stages. J. Mol. Boil. 2013;425:2397–2411. doi: 10.1016/j.jmb.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 103.Iljina M., Hong L., Horrocks M.H., Ludtmann M.H., Choi M.L., Hughes C.D., Ruggeri F.S., Guilliams T., Buell A.K., Lee J.E., et al. Nanobodies raised against monomeric a-synuclein inhibit fibril formation and destabilize toxic oligomeric species. BMC Boil. 2017;15:57. doi: 10.1186/s12915-017-0390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vuchelen A., O’Day E., De Genst E., Pardon E., Wyns L., Dumoulin M., Dobson C.M., Christodoulou J., Hsu S.T. 1H, 13C and 15N assignments of a camelid nanobody directed against human alpha-synuclein. Biomol. NMR Assign. 2009;3:231–233. doi: 10.1007/s12104-009-9182-4. [DOI] [PubMed] [Google Scholar]
- 105.Polymeropoulos M.H., Lavedan C., Leroy E., Ide S.E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 106.Lynch S.M., Zhou C., Messer A. An scFv intrabody against the nonamyloid component of alpha-synuclein reduces intracellular aggregation and toxicity. J. Mol. Boil. 2008;377:136–147. doi: 10.1016/j.jmb.2007.11.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Butler D.C., Joshi S.N., Genst E., Baghel A.S., Dobson C.M., Messer A. Bifunctional Anti-Non-Amyloid Component alpha-Synuclein Nanobodies Are Protective In Situ. PLoS ONE. 2016;11:e0165964. doi: 10.1371/journal.pone.0165964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chatterjee D., Bhatt M., Butler D., De Genst E., Dobson C.M., Messer A., Kordower J.H. Proteasome-targeted nanobodies alleviate pathology and functional decline in an alpha-synuclein-based Parkinson’s disease model. NPJ Park. Dis. 2018;4:25. doi: 10.1038/s41531-018-0062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Butler D.C., McLear J.A., Messer A. Engineered antibody therapies to counteract mutant huntingtin and related toxic intracellular proteins. Prog. Neurobiol. 2012;97:190–204. doi: 10.1016/j.pneurobio.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Colby D.W., Garg P., Holden T., Chao G., Webster J.M., Messer A., Ingram V.M., Wittrup K.D. Development of a human light chain variable domain (V(L)) intracellular antibody specific for the amino terminus of huntingtin via yeast surface display. J. Mol. Boil. 2004;342:901–912. doi: 10.1016/j.jmb.2004.07.054. [DOI] [PubMed] [Google Scholar]
- 111.Colby D.W., Chu Y., Cassady J.P., Duennwald M., Zazulak H., Webster J.M., Messer A., Lindquist S., Ingram V.M., Wittrup K.D. Potent inhibition of huntingtin aggregation and cytotoxicity by a disulfide bond-free single-domain intracellular antibody. Proc. Natl. Acad. Sci. USA. 2004;101:17616–17621. doi: 10.1073/pnas.0408134101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Southwell A.L., Ko J., Patterson P.H. Intrabody gene therapy ameliorates motor, cognitive, and neuropathological symptoms in multiple mouse models of Huntington’s disease. J. Neurosci. 2009;29:13589–13602. doi: 10.1523/JNEUROSCI.4286-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Southwell A.L., Khoshnan A., Dunn D.E., Bugg C.W., Lo D.C., Patterson P.H. Intrabodies binding the proline-rich domains of mutant huntingtin increase its turnover and reduce neurotoxicity. J. Neurosci. 2008;28:9013–9020. doi: 10.1523/JNEUROSCI.2747-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schut M.H., Pepers B.A., Klooster R., van der Maarel S.M., El Khatabi M., Verrips T., den Dunnen J.T., van Ommen G.J., van Roon-Mom W.M. Selection and characterization of llama single domain antibodies against N-terminal huntingtin. Neurol. Sci. 2015;36:429–434. doi: 10.1007/s10072-014-1971-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Harris D.A. Cellular biology of prion diseases. Clin. Microbiol. Rev. 1999;12:429–444. doi: 10.1128/CMR.12.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ladogana A., Puopolo M., Croes E.A., Budka H., Jarius C., Collins S., Klug G.M., Sutcliffe T., Giulivi A., Alperovitch A., et al. Mortality from Creutzfeldt-Jakob disease and related disorders in Europe, Australia, and Canada. Neurology. 2005;64:1586–1591. doi: 10.1212/01.WNL.0000160117.56690.B2. [DOI] [PubMed] [Google Scholar]
- 117.Jones D.R., Taylor W.A., Bate C., David M., Tayebi M. A camelid anti-PrP antibody abrogates PrP replication in prion-permissive neuroblastoma cell lines. PLoS ONE. 2010;5:e9804. doi: 10.1371/journal.pone.0009804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Abskharon R.N., Giachin G., Wohlkonig A., Soror S.H., Pardon E., Legname G., Steyaert J. Probing the N-terminal beta-sheet conversion in the crystal structure of the human prion protein bound to a nanobody. J. Am. Chem. Soc. 2014;136:937–944. doi: 10.1021/ja407527p. [DOI] [PubMed] [Google Scholar]
- 119.Veliz I., Loo Y., Castillo O., Karachaliou N., Nigro O., Rosell R. Advances and challenges in the molecular biology and treatment of glioblastoma-is there any hope for the future? Ann. Transl. Med. 2015;3:7. doi: 10.3978/j.issn.2305-5839.2014.10.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shibata M., Shen M.M. The roots of cancer: Stem cells and the basis for tumor heterogeneity. BioEssays News Rev. Mol. Cell. Dev. Boil. 2013;35:253–260. doi: 10.1002/bies.201200101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bourkoula E., Mangoni D., Ius T., Pucer A., Isola M., Musiello D., Marzinotto S., Toffoletto B., Sorrentino M., Palma A., et al. Glioma-associated stem cells: A novel class of tumor-supporting cells able to predict prognosis of human low-grade gliomas. Stem Cells. 2014;32:1239–1253. doi: 10.1002/stem.1605. [DOI] [PubMed] [Google Scholar]
- 122.Jovcevska I., Zupanec N., Kocevar N., Cesselli D., Podergajs N., Stokin C.L., Myers M.P., Muyldermans S., Ghassabeh G.H., Motaln H., et al. TRIM28 and beta-actin identified via nanobody-based reverse proteomics approach as possible human glioblastoma biomarkers. PLoS ONE. 2014;9:e113688. doi: 10.1371/journal.pone.0113688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jovcevska I., Zupanec N., Urlep Z., Vranic A., Matos B., Stokin C.L., Muyldermans S., Myers M.P., Buzdin A.A., Petrov I., et al. Differentially expressed proteins in glioblastoma multiforme identified with a nanobody-based anti-proteome approach and confirmed by OncoFinder as possible tumor-class predictive biomarker candidates. Oncotarget. 2017;8:44141–44158. doi: 10.18632/oncotarget.17390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Samec N., Jovcevska I., Stojan J., Zottel A., Liovic M., Myers M.P., Muyldermans S., Sribar J., Krizaj I., Komel R. Glioblastoma-specific anti-TUFM nanobody for in-vitro immunoimaging and cancer stem cell targeting. Oncotarget. 2018;9:17282–17299. doi: 10.18632/oncotarget.24629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zorniak M., Clark P.A., Umlauf B.J., Cho Y., Shusta E.V., Kuo J.S. Yeast display biopanning identifies human antibodies targeting glioblastoma stem-like cells. Sci. Rep. 2017;7:15840. doi: 10.1038/s41598-017-16066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.van Lith S.A., Roodink I., Verhoeff J.J., Makinen P.I., Lappalainen J.P., Yla-Herttuala S., Raats J., van Wijk E., Roepman R., Letteboer S.J., et al. In vivo phage display screening for tumor vascular targets in glioblastoma identifies a llama nanobody against dynactin-1-p150Glued. Oncotarget. 2016;7:71594–71607. doi: 10.18632/oncotarget.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.van de Water J.A., Bagci-Onder T., Agarwal A.S., Wakimoto H., Roovers R.C., Zhu Y., Kasmieh R., Bhere D., Van Bergen en Henegouwen P.M., Shah K. Therapeutic stem cells expressing variants of EGFR-specific nanobodies have antitumor effects. Proc. Natl. Acad. Sci. USA. 2012;109:16642–16647. doi: 10.1073/pnas.1202832109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ciardiello F., Tortora G. EGFR antagonists in cancer treatment. N. Engl. J. Med. 2008;358:1160–1174. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 129.Huang P.H., Xu A.M., White F.M. Oncogenic EGFR signaling networks in glioma. Sci. Signal. 2009;2:re6. doi: 10.1126/scisignal.287re6. [DOI] [PubMed] [Google Scholar]
- 130.Roovers R.C., Laeremans T., Huang L., De Taeye S., Verkleij A.J., Revets H., de Haard H.J., van Bergen en Henegouwen P.M. Efficient inhibition of EGFR signaling and of tumour growth by antagonistic anti-EFGR Nanobodies. Cancer Immunol. Immunother. CII. 2007;56:303–317. doi: 10.1007/s00262-006-0180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Keyaerts M., Xavier C., Heemskerk J., Devoogdt N., Everaert H., Ackaert C., Vanhoeij M., Duhoux F.P., Gevaert T., Simon P., et al. Phase I Study of 68Ga-HER2-Nanobody for PET/CT Assessment of HER2 Expression in Breast Carcinoma. J. Nucl. Med. 2016;57:27–33. doi: 10.2967/jnumed.115.162024. [DOI] [PubMed] [Google Scholar]
- 132.Freise A.C., Wu A.M. In vivo imaging with antibodies and engineered fragments. Mol. Immunol. 2015;67:142–152. doi: 10.1016/j.molimm.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xavier C., Vaneycken I., D’Huyvetter M., Heemskerk J., Keyaerts M., Vincke C., Devoogdt N., Muyldermans S., Lahoutte T., Caveliers V. Synthesis, preclinical validation, dosimetry, and toxicity of 68Ga-NOTA-anti-HER2 Nanobodies for iPET imaging of HER2 receptor expression in cancer. J. Nucl. Med. 2013;54:776–784. doi: 10.2967/jnumed.112.111021. [DOI] [PubMed] [Google Scholar]
- 134.Li Z., Krippendorff B.F., Sharma S., Walz A.C., Lave T., Shah D.K. Influence of molecular size on tissue distribution of antibody fragments. mAbs. 2016;8:113–119. doi: 10.1080/19420862.2015.1111497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li T., Vandesquille M., Koukouli F., Dudeffant C., Youssef I., Lenormand P., Ganneau C., Maskos U., Czech C., Grueninger F., et al. Camelid single-domain antibodies: A versatile tool for in vivo imaging of extracellular and intracellular brain targets. J. Control. Release. 2016;243:1–10. doi: 10.1016/j.jconrel.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 136.Iqbal U., Trojahn U., Albaghdadi H., Zhang J., O’Connor-McCourt M., Stanimirovic D., Tomanek B., Sutherland G., Abulrob A. Kinetic analysis of novel mono- and multivalent VHH-fragments and their application for molecular imaging of brain tumours. Br. J. Pharmacol. 2010;160:1016–1028. doi: 10.1111/j.1476-5381.2010.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Daumas-Duport C., Varlet P., Tucker M.L., Beuvon F., Cervera P., Chodkiewicz J.P. Oligodendrogliomas. Part I: Patterns of growth, histological diagnosis, clinical and imaging correlations: A study of 153 cases. J. Neuro-Oncol. 1997;34:37–59. doi: 10.1023/A:1005707203596. [DOI] [PubMed] [Google Scholar]
- 138.Daumas-Duport C., Tucker M.L., Kolles H., Cervera P., Beuvon F., Varlet P., Udo N., Koziak M., Chodkiewicz J.P. Oligodendrogliomas. Part II: A new grading system based on morphological and imaging criteria. J. Neuro-Oncol. 1997;34:61–78. doi: 10.1023/A:1005759220434. [DOI] [PubMed] [Google Scholar]
- 139.Olsson A.K., Dimberg A., Kreuger J., Claesson-Welsh L. VEGF receptor signalling—In control of vascular function. Nat. Rev. Mol. Cell Boil. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 140.Varner J.A., Cheresh D.A. Integrins and cancer. Curr. Opin. Cell Boil. 1996;8:724–730. doi: 10.1016/S0955-0674(96)80115-3. [DOI] [PubMed] [Google Scholar]
- 141.Pen A., Moreno M.J., Martin J., Stanimirovic D.B. Molecular markers of extracellular matrix remodeling in glioblastoma vessels: Microarray study of laser-captured glioblastoma vessels. Glia. 2007;55:559–572. doi: 10.1002/glia.20481. [DOI] [PubMed] [Google Scholar]
- 142.Iqbal U., Albaghdadi H., Luo Y., Arbabi M., Desvaux C., Veres T., Stanimirovic D., Abulrob A. Molecular imaging of glioblastoma multiforme using anti-insulin-like growth factor-binding protein-7 single-domain antibodies. Br. J. Cancer. 2010;103:1606–1616. doi: 10.1038/sj.bjc.6605937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nagakubo D., Murai T., Tanaka T., Usui T., Matsumoto M., Sekiguchi K., Miyasaka M. A high endothelial venule secretory protein, mac25/angiomodulin, interacts with multiple high endothelial venule-associated molecules including chemokines. J. Immunol. 2003;171:553–561. doi: 10.4049/jimmunol.171.2.553. [DOI] [PubMed] [Google Scholar]
- 144.Iqbal U., Albaghdadi H., Nieh M.P., Tuor U.I., Mester Z., Stanimirovic D., Katsaras J., Abulrob A. Small unilamellar vesicles: A platform technology for molecular imaging of brain tumors. Nanotechnology. 2011;22:195102. doi: 10.1088/0957-4484/22/19/195102. [DOI] [PubMed] [Google Scholar]
- 145.Tomanek B., Iqbal U., Blasiak B., Abulrob A., Albaghdadi H., Matyas J.R., Ponjevic D., Sutherland G.R. Evaluation of brain tumor vessels specific contrast agents for glioblastoma imaging. Neuro-Oncology. 2012;14:53–63. doi: 10.1093/neuonc/nor183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pardridge W.M. Blood-brain barrier drug targeting: The future of brain drug development. Mol. Interv. 2003;3:90–105, 151. doi: 10.1124/mi.3.2.90. [DOI] [PubMed] [Google Scholar]
- 147.Rashidian M., Keliher E.J., Bilate A.M., Duarte J.N., Wojtkiewicz G.R., Jacobsen J.T., Cragnolini J., Swee L.K., Victora G.D., Weissleder R., et al. Noninvasive imaging of immune responses. Proc. Natl. Acad. Sci. USA. 2015;112:6146–6151. doi: 10.1073/pnas.1502609112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Iqbal U., Abulrob A., Stanimirovic D.B. Integrated platform for brain imaging and drug delivery across the blood-brain barrier. Methods Mol. Boil. 2011;686:465–481. doi: 10.1007/978-1-60761-938-3_24. [DOI] [PubMed] [Google Scholar]
- 149.Herve F., Ghinea N., Scherrmann J.M. CNS delivery via adsorptive transcytosis. AAPS J. 2008;10:455–472. doi: 10.1208/s12248-008-9055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bickel U., Yoshikawa T., Pardridge W.M. Delivery of peptides and proteins through the blood-brain barrier. Adv. Drug Deliv. Rev. 2001;46:247–279. doi: 10.1016/S0169-409X(00)00139-3. [DOI] [PubMed] [Google Scholar]
- 151.Li T., Bourgeois J.P., Celli S., Glacial F., Le Sourd A.M., Mecheri S., Weksler B., Romero I., Couraud P.O., Rougeon F., et al. Cell-penetrating anti-GFAP VHH and corresponding fluorescent fusion protein VHH-GFP spontaneously cross the blood-brain barrier and specifically recognize astrocytes: Application to brain imaging. FASEB J. 2012;26:3969–3979. doi: 10.1096/fj.11-201384. [DOI] [PubMed] [Google Scholar]
- 152.Traenkle B., Emele F., Anton R., Poetz O., Haeussler R.S., Maier J., Kaiser P.D., Scholz A.M., Nueske S., Buchfellner A., et al. Monitoring interactions and dynamics of endogenous beta-catenin with intracellular nanobodies in living cells. Mol. Cell. Proteom. MCP. 2015;14:707–723. doi: 10.1074/mcp.M114.044016. [DOI] [PMC free article] [PubMed] [Google Scholar]