Abstract

Developing cheap, stable, and efficient electrocatalysts is of extreme importance in the effort to replace noble metal electrocatalysts for use in the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER). We report a three-dimensional self-supported Cu3P nanobush (NB) catalyst directly grown on a copper mesh via a one-step method. This nanostructure exhibits a superior catalytic activity of achieving a current density of 10 mA cm–2 at 120 mV and exhibits a long-term stability in acid solutions. It shows a Tafel slope of 72 mV dec–1 and an onset potential of −44 mV. This catalyst displays a good catalytic activity in basic electrolytes, reaching a current density of 10 mA cm–2 at the overpotential values of 252 and 380 mV for HER and OER, respectively. The bifunctional Cu3P NB/Cu catalyst exhibits better catalytic performances than the Pt/C and IrO2 catalysts in a two-electrode electrolyzer for overall water splitting.
Introduction
Energy consumption and environmental pollution have aroused widespread concern worldwide. It is essential to develop clean, environmentally friendly, and sustainable energy sources to substitute fossil fuels, owing to the accelerated depletion and global environmental concerns connected with the burning of fossil fuels.1−3 Hydrogen is considered to be one of the best candidates to replace fossil fuels. Water electrolysis, consisting of hydrogen and oxygen evolution reactions (HER and OER, respectively), is a very promising and sustainable approach for energy conversion, storage, and utilization, such as in clean hydrogen generation, rechargeable metal–air cells, and fuel cells.4,5 Platinum-based catalysts are the high-efficiency catalysts for HER, and Ir- or Ru-based compounds show the highest activity for OER. The practical application of such catalysts is restricted by the scarcity and expensiveness of noble metals, and this limitation has encouraged great efforts to design and develop non-noble metal catalysts with low price and outstanding catalytic performance for HER and OER. In recent years, studies on highly active, earth-abundant catalysts have revealed some kinds of alternative materials, such as transition metal sulfides,6,7 carbides,8,9 nitrides,10,11 selenides,12,13 and even metal-free materials.14 In addition, many non-noble metal catalysts grounded on the oxides/hydroxides of cobalt,15,16 iron,17,18 nickel,19,20 copper,21,22 and carbon-based materials,23 have been studied for their availability as OER catalysts.
Transition metal phosphides (TMPs) are efficient and stable electrocatalysts because of their low electrical resistance and good corrosion resistance. In the past few years, innumerable efforts have been made in the preparation of TMPs for their use as HER electrocatalysts, including MoP,24 CoP,25 Co2P,26 Ni5P4,27 and FeP.28 Recent studies have shown that three-dimensional (3D) self-supported electrodes, such as Ni5P4/Ni foil,29 Ni5P4–Ni2P/Ni foam,30 FeP NAs/Ti plate,31 WP NAs/CC,32 CoP NS arrays/Ti plate,33 and CoP NW/CC,34 exhibit excellent HER catalytic performance when compared with the planar electrodes. The structure of 3D self-supported electrodes has the advantage of not requiring the use of a polymer binder between the electrocatalysts and the electrodes, thereby avoiding covering of the active sites and improving the catalytic performance.
Copper phosphide (Cu3P) is a type of special catalyst that is usually applied in lithium batteries because of its high specific capacitance and outstanding charge–discharge properties.35−37 The combination of Cu3P with another semiconductor or a metal to form a heterojunction has been used in photocatalysis or photoelectrocatalysis.38−40 Recent studies have shown that Cu3P is an excellent electrocatalyst for use in HER.41,42 Herein, we adopted a one-step method for the synthesis of a Cu3P nanobush (NB) on commercial copper meshes, for its use as bifunctional catalysts for efficient whole water decomposition. The obtained 3D self-supported nanostructure exhibits a superior HER activity, with an onset overpotential of −44 mV, a Tafel slope of 72 mV dec–1, and the corresponding overpotential of 120 mV at a current density of 10 mA cm–2 in acidic solutions. Most interestingly, Cu3P NB/Cu serves as an active, cheap, and bifunctional electrocatalyst for HER and OER in basic solutions, thus making it a promising catalyst to split water for energy conversion purposes.
Results and Discussion
The fabrication process of the Cu3P NB is illustrated in Figure 1. Red P and a copper mesh were heated to 450 °C for 30 min under an Ar gas flow. The original amaranthine color of the pristine copper mesh turned black after the phosphorization process, thus suggesting that the surface of the copper mesh was converted into metal phosphides. The micromorphological change was confirmed by scanning electron microscopy (SEM). Notably, the flexibility of the copper mesh was still maintained after the phosphorization process.
Figure 1.
Schematic of the preparation process of a Cu3P NB via the phosphorization of a Cu mesh.
The influence of the phosphating temperature on the nanostructures formed was first investigated, as shown in Figure 2a–d. Sparse nanowires were observed on the Cu mesh at 420 °C. With the increase in temperature, the NB was formed on the Cu mesh. The NB, which consisted of uniform nanowires with diameters ranging from 500 nm to 1.3 μm and tens of micrometers in length, was formed on a Cu mesh at 450 °C (Figure S1). The agglomeration of nanowires and bulk blocks appeared at the higher temperatures of 550 and 650 °C, respectively. A mechanism for the growth of Cu3P NB/Cu was proposed on the basis of these observations. The solid red P was turned into vapor when red phosphorus was heated to a certain temperature. The surface of the Cu mesh was first phosphorized into a thin film, as confirmed by SEM and elemental mapping analysis, as shown in Figure S2. Cu3P NB/Cu was sequentially formed on this Cu3P film on the basis of the interactional crowding-out mechanism.43 The electrochemical tests in Figure 2e,f showed that the product synthesized at 450 °C exhibited the most activity for HER.
Figure 2.

SEM images of the Cu3P NB electrodes at different phosphating temperatures: (a) 420, (b) 450, (c) 550, and (d) 650 °C. Electrocatalytic tests at different phosphating temperatures. (e) Polarization curves and (f) Tafel plots.
The nanowire structures were further characterized using X-ray diffraction (XRD). Figure 3a shows that all diffraction peaks are in accordance with the hexagonal structure of Cu3P (PDF 02-1623). The diffraction peaks at 28.7°, 36.2°, 39.3°, 41.8°, 45.0°, 46.5°, 47.3°, 52.5°, 53.8°, 56.7°, 59.1°, 66.7°, 69.5°, 72.0°, 73.3°, and 78.3° were assigned to the planes of (111), (112), (202), (211), (300), (113), (212), (220), (221), (311), (222), (223), (321), (410), (322), and (314), respectively. The lack of other observable peaks indicated that the as-synthesized Cu3P was a pure phase. The energy-dispersive X-ray spectroscopy (EDS) analysis displayed that the atomic ratio of Cu and P was 3:1, thus further confirming the formation of the Cu3P phase (Figure 3b). A high-resolution transmission electron microscopy (HRTEM) image showed the distinct lattice fringes with interplanar distances of 2.01 and 1.74 Å; these distances could be indexed to the (300) and (220) planes of Cu3P, respectively (Figure 3c). The corresponding SAED pattern also confirmed the polycrystalline structure of Cu3P, as shown in the inset of Figure 3c. Figure 3d exhibits the transmission electron microscopy (TEM) image and the corresponding elemental mapping patterns of Cu and P for the nanostructure, thus clearly demonstrating that the Cu and P elements were uniformly distributed on the nanowire.
Figure 3.

Characterization of the morphology and structure of Cu3P NB/Cu. (a) XRD pattern, (b) EDS spectrum, (c) HRTEM image (inset illustrates the selected-area electron diffraction (SAED) pattern), and (d) TEM image and the corresponding elemental mapping pattern.
The chemical states and compositions of individual elements in the as-synthesized Cu3P NB/Cu were characterized by X-ray photoelectron spectroscopy (XPS) in Figure 4. Figure 4a indicates three peaks at approximately 932.9, 934.6, and 944.6 eV for the Cu 2p3/2 energy level, which are attributed to Cuδ+ in Cu3P, oxidized Cu species, and the satellite of a Cu 2p3/2 peak, respectively. The three peaks with high bonding energy appeared at 952.7, 954.5, and 962.8 eV for the Cu 2p1/2 energy level, which were attributed to Cuδ+ in Cu3P, oxidized Cu species, and the satellite of the Cu 2p1/2 peak, respectively.42,44Figure 4b shows double peaks in the phosphorus region. The peak at 133.6 eV corresponds to the oxidized phosphorus in the form of phosphate. The binding energy of the peak at 129.7 eV is lower than that of red phosphorus at 130.2 eV.44 These results suggest that charge transfer occurs between Cu and P, thus demonstrating that Cu and P are positively charged and negatively charged, respectively, in which Cu may act as the hydride-acceptor center and P may act as the proton-acceptor center, which is similar to a hydrogenase.45−47
Figure 4.
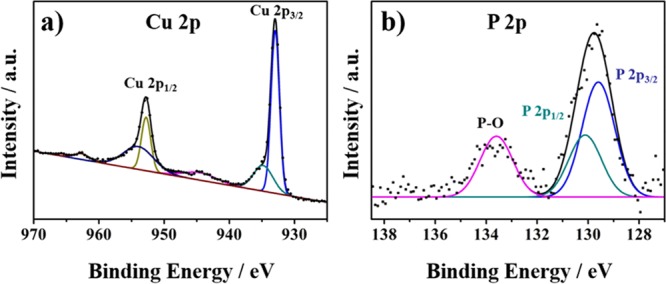
XPS patterns of Cu3P NB/Cu: (a) Cu 2p and (b) P 2p.
The electrocatalytic activity of Cu3P NB/Cu for HER was tested in 0.5 M H2SO4 with a three-electrode electrochemical configuration with a Ag/AgCl electrode as the reference electrode and a Pt foil as the counter electrode. Figure 5a shows the polarization curves of these electrodes without iR correction. The onset potential is the potential in which the current density is 1 mA cm–2.30 The onset potential of Cu3P/Cu is −44 mV, whereas the onset potential of Pt/C is close to 0 mV at a current density of 1 mA cm–2, thus suggesting a superior catalytic activity for HER. This electrode required overpotentials of 117, 147, and 283 mV to achieve current densities of 10, 20, and 100 mA cm–2, respectively. Tafel plots in Figure 5b were obtained from the linear portion conformed to the Tafel equation (η = a + b log j, where b is the Tafel slope and j is the current density). The Tafel slope of Pt/C is 30 mV dec–1, which was the same as previously reported values.48 Cu3P NB/Cu exhibited a Tafel slope of 72 mV dec–1, thus indicating that the HER process takes place via a Volmer–Heyrovsky mechanism, and the electrochemical desorption step (discharge step) is the rate-limiting step.26,49,50 By applying the extrapolation method to the Tafel plot, the exchange current density of Cu3P NB/Cu was calculated to be 0.25 mA cm–2. These parameters are comparable to or superior to the values from the recently reported non-noble HER catalysts in acidic solutions (Table S1).
Figure 5.
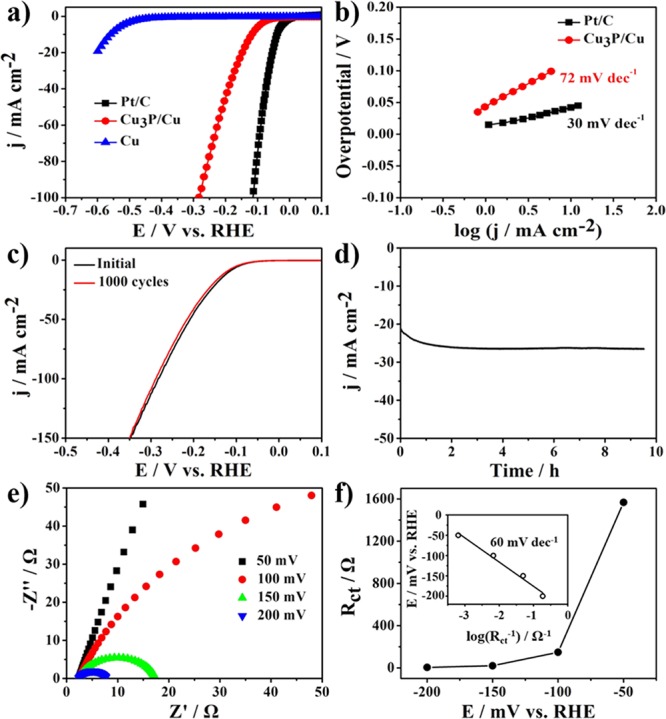
Electrocatalytic activity of the as-prepared Cu3P NB/Cu electrode for HER in 0.5 M H2SO4. (a) Polarization curves, (b) Tafel plots, (c) polarization curves of Cu3P NB/Cu before and after 1000 CV scanning, (d) current density curve related to time for Cu3P NB/Cu at the overpotential of 150 mV for at least 9 h, (e) Nyquist plots measured at different overpotentials, and (f) the Faraday resistance (Rct)-dependent overpotential plot; the inset shows the linearity between the overpotential and log(Rct–1).
The durability of Cu3P NB/Cu was investigated under continuous cyclic voltammetry (CV) cycles with a 0.5 M H2SO4 electrolyte over the potential range between −0.5 and 0.3 V versus reversible hydrogen electrode (RHE) with a scan rate of 100 mV s–1. Figure 5c shows a comparison of the polarization curve before and after 1000 cycles. Only slight decreases in the current density were observed after 1000 cycles, thus indicating that the Cu3P NB/Cu electrode has an excellent cycle life in acidic environments. The current density curve related to time under a static overpotential of 150 mV suggested that the catalytic activity of the as-synthesized Cu3P NB/Cu was maintained for at least 9 h, as shown in Figure 5d.
Electrochemical impedance spectroscopy (EIS) was performed to identify the interfacial properties and catalytic kinetics of the as-synthesized Cu3P NB/Cu catalyst in the HER process. A Bode plot of Cu3P revealed a typical one-time constant process in acidic media, as shown in Figure S3.51,52 The experimental data were fitted using an equivalent circuit consisting of a series resistance (Rs) with a parallel resistance (Rct, CPE), in which Rs is the ohmic resistance mainly resulting from the electrolyte and all contact resistances, and the time constant Rct-CPE illustrates Faraday resistance (Rct) at the interface between the nanostructures and the electrolyte.30,52 The value of Rs is the same, but the value of Rct varies with different voltages, as shown in Figure 5e. The Rct-dependent overpotential curve was plotted, as shown in Figure 5f. The Tafel slope is also obtained from the impedance data by plotting log(Rct–1) versus the overpotential (as shown in the inset of Figure 5f), which is smaller than that obtained by fitting the linear regions of Tafel plots in Figure 5b. The Tafel slope achieved in this method reflects only the charge transfer kinetics without regarding the catalyst resistance.53
The electrocatalytic performance of Cu3P NB/Cu for HER in 1.0 M KOH solution was further investigated. Commercial Pt/C catalysts loaded on Cu mesh and bare Cu mesh were tested in control experiments. A Cu3P NB/Cu electrode exhibited an onset potential of 121 mV and a Tafel slope of 150 mV dec–1. This electrode required an overpotential of 252 mV to achieve 10 mA cm–2. This catalytic performance was better than the recently reported non-noble catalysts in alkaline electrolytes (Table S2). The linear sweep voltammetry (LSV) curves in Figure 6c showed a negligible loss in the activity after 1000 cycles of CV scanning. The current density curve related to time at 300 mV is also shown in the inset of Figure 6c. These tests demonstrated that Cu3P NB/Cu displays an excellent stability and durability under alkaline conditions. EIS of the Cu3P NB/Cu electrode in Figure 6d revealed a low charge transfer resistance of 12 Ω, indicating a fast charge transfer process in alkaline media.
Figure 6.

(a) Polarization curves for HER, (b) Tafel plots, (c) polarization curves before and after 1000 CV cycles (the inset shows the current density curve related to time for Cu3P NB/Cu at 300 mV for 10 h), (d) Nyquist plot at 300 mV for HER, (e) polarization curves for OER, and (f) polarization curves before and after 1000 CV cycles (the inset shows the current density curve related to time for Cu3P NB/Cu at 420 mV for 10 h). All measurements were taken in 1.0 M KOH.
The OER performance of Cu3P NB/Cu in alkaline solutions was further investigated in a three-electrode configuration by using the commercial IrO2 and bare Cu mesh as controls. The LSV curves are depicted in Figure 6e. The polarization curve of Cu3P NB/Cu showed a wide and prominent anodic peak at 1.41 V before OER. This anodic peak was ascribed to the oxidation of Cu in Cu3P NB/Cu according to the SEM and XPS characterization results (Figures S5 and S6),54−56 thus indicating that the metal oxide/hydroxide may be the active site for the OER. The OER current density of Cu3P NB/Cu increased rapidly and exceeded that of the commercial IrO2 at 1.60 V. The onset overpotential of OER and the overpotential at 10 mA cm–2 were 320 and 380 mV obtained by scanning from 1.8 to 1.1 V to avoid the oxidation peak, as shown in Figure S4. The catalytic performance was better than that of the reported catalysts (Table S3). The stability of the catalyst was also evaluated by performing CV sweeps between 1.2 and 1.8 V versus RHE in 1.0 M KOH at a scan rate of 100 mV s–1. The polarization curves of Cu3P NB/Cu after 1000 CV scanning compared with the initial one showed negligible current change, as shown in Figure 6f. The current density of long-term electrochemical stability remained 90% after a continuous operation of 10 h at 420 mV in alkaline media, as shown in the inset of Figure 6f. The Cu3P NB/Cu electrode exhibited a robust catalytic activity for OER in a strong alkaline electrolyte.
A two-electrode configuration was constructed with Cu3P NB/Cu as both the anode and the cathode to determine its catalytic activity for overall water splitting. The electrodes were first tested separately in a three-electrode system to estimate their individual performance in the relevant reactions, as shown in Figure 7b. The combination of Pt/C and IrO2 was used as the benchmark catalyst for comparison. The alkaline water electrolysis was then conducted with a two-electrode setup. The Cu3P/Cu(+)//Cu3P/Cu(−) system reached 10 and 20 mA cm–2 at cell voltages of only 1.85 and 1.94 V, respectively, which is close to Co3O4/TM//Co3O4/TM (1.82 V) and better than CoxPO4/CoP (1.91 V), NiCo2O4/CC//NiCo2O4/CC (1.98 V), and commercial electrolyzers (1.8–2.0 V).57−60 These results are superior to the values of the IrO2(+)//Pt/C(−) catalyst. The long-term electrochemical stability of the electrolyzer was operated at a given overpotential of 1.88 V. The current density remained 93.8% after a continuous operation of 12 h, as shown in Figure 7c, thus suggesting the superior durability of Cu3P/Cu electrodes. The optical photograph in Figure 7c shows vigorous bubble accumulation and release on the two electrodes. Two AA batteries were successfully used to drive the water splitting with Cu3P NB/Cu as both the anode and the cathode, as shown in Figure S7. Thus, Cu3P NB/Cu is a good candidate bifunctional catalyst for overall water splitting in practical applications.
Figure 7.
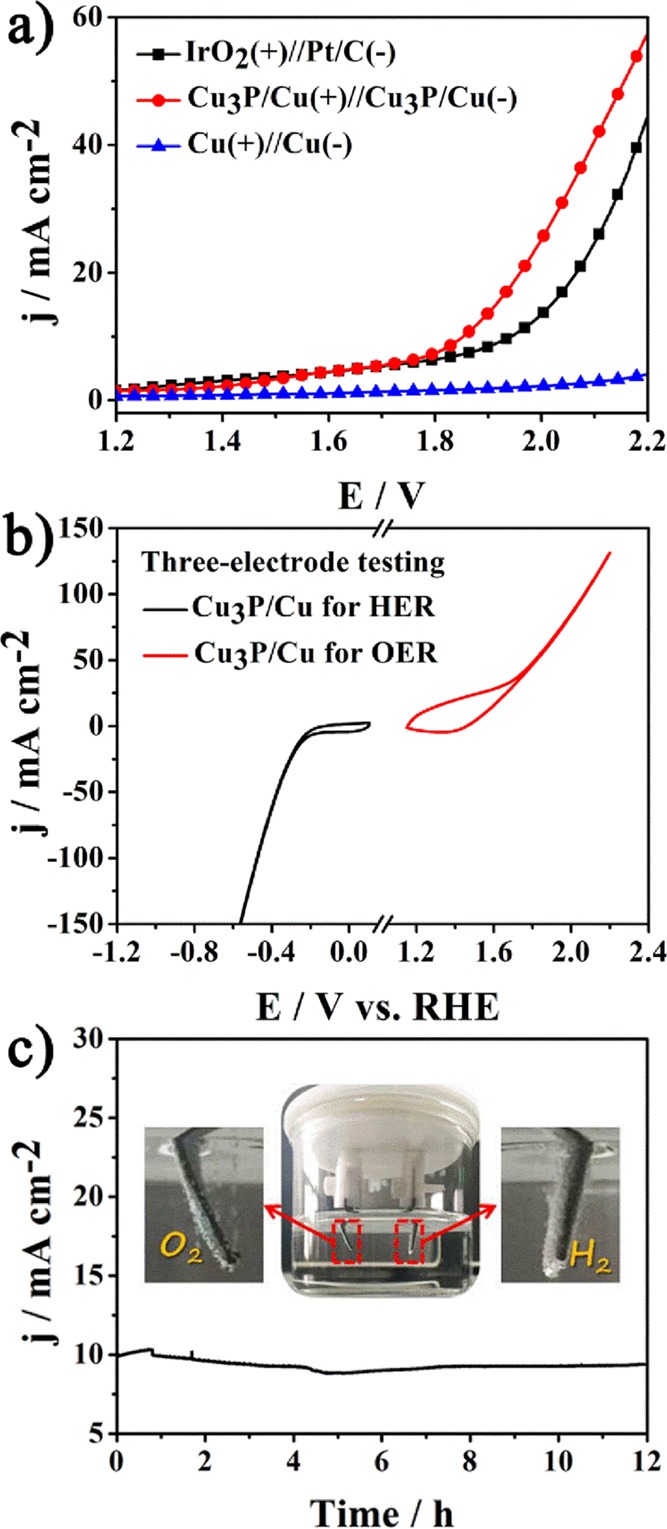
(a) Polarization curves of different catalysts for overall water splitting. (b) Performance of the individual electrodes tested in a three-electrode electrochemical cell setup. (c) Time-dependent current density curve of Cu3P NB/Cu at 10 mA cm–2 and optical photograph of Cu3P NB/Cu for overall water splitting. All measurements were taken in 1.0 M KOH.
Conclusions
A Cu3P NB on the copper mesh was prepared through a simple one-step method. The catalyst exhibits an excellent catalytic activity for overall water splitting as a bifunctional catalyst. The excellent catalytic performance is attributed to the structure and composition of the catalyst. The 3D nanostructure provides more active sites, owing to the high specific surface area, and promotes gas production and bubble release. Cu3P shows a good electrical conductivity that decreases the resistance of the catalytic system. This low-cost, stable Cu3P NB/Cu is directly used as a bifunctional catalyst in a two-electrode alkaline electrolyzer for hydrogen and oxygen production.
Experimental Section
Materials
The Cu mesh was purchased from Anping Shuangpeng Co., Ltd. (China). Red phosphorous (P) was obtained from Alfa Aesar Chemicals Co., Ltd. (China). The hydrochloric acid (HCl) aqueous solution, isopropanol, acetone, ethanol, potassium hydroxide (KOH), and sulfuric acid (H2SO4) were obtained from Beijing Reagent Co. (China), and these reagents were of analytical grade. The water with a resistivity of 18.2 MΩ cm–1 was used in all experiments.
Preparation of Cu3P NB/Cu
The Cu mesh was first cleaned by sonication in 4 M HCl for 10 min to get rid of the surface oxide layer. The mesh was then sequentially washed with acetone, ethanol, and deionized water and finally dried with nitrogen gas flow. Typically, a piece of the Cu mesh and approximately 0.4 g of red P were placed at two different locations of the tube furnace. The furnace was purged with argon (Ar, 99.99%) for 30 min, heated to 450 °C under the heating rate of 5 °C min–1, and then maintained for 30 min at this temperature. Finally, the furnace was naturally cooled down to ambient temperature. Argon was maintained during the whole process. To investigate the impact of the phosphating temperature on the electrocatalytic activity, the phosphorization process was also conducted at 420, 550, and 650 °C, whereas the other parameters remained unchanged.
Characterization
The structure was characterized by XRD using a Bragg–Brentano diffractometer (D8-tools, Germany) equipped with a Cu Kα (λ = 0.15418 nm) emitting source. Scanning electron microscope images were taken on a JSM-6700F field-emission scanning electron microscope from JEOL Co., Japan. TEM, HRTEM, and SAED were carried out using a JEM-2000EX instrument from JEOL Co., Japan. XPS was measured on an Escalab-250 instrument from Thermo Fisher Scientific, USA with a hemisphere detector and a monochromatic Al Kα radiation source (1486.6 eV).
Electrochemical Measurements
All electrochemical tests were performed with a CHI660E electrochemical workstation. The performances of HER and OER were carried out with a standard three-electrode system. The Ag/AgCl electrode and Pt foil were the reference electrode and the counter electrode, whereas the Cu3P NB/Cu sample was the working electrode. In all measurements, all potentials mentioned were recorded versus the RHE. To measure the HER activity of Cu3P NB/Cu in acid, the electrolyte was first purged by N2 (ultrahigh-grade purity) for 30 min to remove oxygen. LSV was performed in 0.5 M H2SO4 at a scan rate of 2 mV s–1. EIS, used to determine the polarization of HER, was conducted in potentiostatic mode from 100 000 to 0.01 Hz under the amplitude of 10 mV at different overpotentials.
To determine the OER activity of the Cu3P NB/Cu electrode in 1.0 M KOH, the electrolyte was degassed by highly purified O2 for 30 min to ensure the saturation of the electrolyte. The electrode was activated by 25 CV sweeps from 1.20 to 1.80 V versus RHE in 1.0 M KOH at a scan rate of 50 mV s–1. LSV was performed at a scan rate of 5 mV s–1. Reverse scans were carried out with CV over the potential range from 1.8 to 1.0 V at a constant scan rate of 5 mV s–1. The stability was tested by CV between 1.2 and 1.8 V versus RHE.
Electrochemical measurements of overall water splitting were taken with a two-electrode setup in a 1.0 M KOH solution with Cu3P NB/Cu electrodes as both the anode and the cathode.
A 20 wt % Pt/C (or IrO2) working electrode was prepared on Cu mesh. First, 20 mg of 20 wt % Pt/C was dispersed in a mixed solution consisting of 0.95 mL of distilled water and 50 μL of Nafion solution (5 wt %, Sigma-Aldrich) under 30 min of sonication. Next, 20 μL of this dispersion was then loaded onto Cu mesh and then dried at room temperature.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2016YFA0200400), the National Natural Science Foundation of China (nos. 21275064 and 51372095), the Specialized Research Fund for the Doctoral Program of Higher Education (20130061110035), and the Graduate Innovation Fund of Jilin University (Project 2016075).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.6b00366.
Diameter distribution of the as-prepared Cu3P nanowires, growth schematic of Cu3P nanostructures, Bode plot of Cu3P, reverse scan of LSV curves of Cu3P NB/Cu for OER, morphology characterization after OER testing, XPS spectra of Cu3P after OER and 10 nm depth profile after OER test, optical photograph of overall water splitting powered by two AA batteries, and comparison of the HER and OER electrocatalytic activity (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Faber M. S.; Jin S. Earth-abundant inorganic electrocatalysts and their nanostructures for energy conversion applications. Energy Environ. Sci. 2014, 7, 3519–3542. 10.1039/c4ee01760a. [DOI] [Google Scholar]
- Zou X.; Zhang Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. 10.1039/c4cs00448e. [DOI] [PubMed] [Google Scholar]
- Dresselhaus M. S.; Thomas I. L. Alternative energy technologies. Nature 2001, 414, 332–337. 10.1038/35104599. [DOI] [PubMed] [Google Scholar]
- Jin H.; Wang J.; Su D.; Wei Z.; Pang Z.; Wang Y. In situ cobalt–cobalt oxide/N-doped carbon hybrids as superior bifunctional electrocatalysts for hydrogen and oxygen evolution. J. Am. Chem. Soc. 2015, 137, 2688–2694. 10.1021/ja5127165. [DOI] [PubMed] [Google Scholar]
- Peng Z.; Jia D.; Al-Enizi A. M.; Elzatahry A. A.; Zheng G. Electrocatalysts: From water oxidation to reduction: Homologous Ni–Co based nanowires as complementary water splitting electrocatalysts. Adv. Energy Mater. 2015, 5, 1570050. 10.1002/aenm.201570030050. [DOI] [Google Scholar]
- Jaramillo T. F.; Jørgensen K. P.; Bonde J.; Nielsen J. H.; Horch S.; Chorkendorff I. Identification of Active Edge Sites for Electrochemical H2 Evolution from MoS2 Nanocatalysts. Science 2007, 317, 100–102. 10.1126/science.1141483. [DOI] [PubMed] [Google Scholar]
- Xie J.; Zhang H.; Li S.; Wang R.; Sun X.; Zhou M.; Zhou J.; Lou X. W. D.; Xie Y. Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv. Mater. 2013, 25, 5807–5813. 10.1002/adma.201302685. [DOI] [PubMed] [Google Scholar]
- Liao L.; Wang S.; Xiao J.; Bian X.; Zhang Y.; Scanlon M. D.; Hu X.; Tang Y.; Liu B.; Girault H. H. A nanoporous molybdenum carbide nanowire as an electrocatalyst for hydrogen evolution reaction. Energy Environ. Sci. 2014, 7, 387–392. 10.1039/c3ee42441c. [DOI] [Google Scholar]
- Vrubel H.; Hu X. Molybdenum boride and carbide catalyze hydrogen evolution in both acidic and basic solutions. Angew. Chem., Int. Ed. 2012, 51, 12703–12706. 10.1002/anie.201207111. [DOI] [PubMed] [Google Scholar]
- Cao B.; Veith G. M.; Neuefeind J. C.; Adzic R. R.; Khalifah P. G. Mixed close-packed cobalt molybdenum nitrides as non-noble metal electrocatalysts for the hydrogen evolution reaction. J. Am. Chem. Soc. 2013, 135, 19186–19192. 10.1021/ja4081056. [DOI] [PubMed] [Google Scholar]
- Chen W.-F.; Sasaki K.; Ma C.; Frenkel A. I.; Marinkovic N.; Muckerman J. T.; Zhu Y.; Adzic R. R. Hydrogen-evolution catalysts based on non-noble metal nickel–molybdenum nitride nanosheets. Angew. Chem., Int. Ed. 2012, 51, 6131–6135. 10.1002/anie.201200699. [DOI] [PubMed] [Google Scholar]
- Wang F.; Li Y.; Shifa T. A.; Liu K.; Wang F.; Wang Z.; Xu P.; Wang Q.; He J. Selenium-enriched nickel selenide nanosheets as a robust electrocatalyst for hydrogen generation. Angew. Chem. 2016, 128, 7033–7038. 10.1002/ange.201602802. [DOI] [PubMed] [Google Scholar]
- Kong D.; Wang H.; Lu Z.; Cui Y. CoSe2 nanoparticles grown on carbon fiber paper: An efficient and stable electrocatalyst for hydrogen evolution reaction. J. Am. Chem. Soc. 2014, 136, 4897–4900. 10.1021/ja501497n. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Qu L.; Shi G.; Liu J.; Chen J.; Dai L. N,P-codoped carbon networks as efficient metal-free bifunctional catalysts for oxygen reduction and hydrogen evolution reactions. Angew. Chem., Int. Ed. 2016, 55, 2230–2234. 10.1002/anie.201510495. [DOI] [PubMed] [Google Scholar]
- Danilovic N.; Subbaraman R.; Strmcnik D.; Chang K.-C.; Paulikas A. P.; Stamenkovic V. R.; Markovic N. M. Enhancing the alkaline hydrogen evolution reaction activity through the bifunctionality of Ni(OH)2/metal catalysts. Angew. Chem., Int. Ed. 2012, 51, 12495–12498. 10.1002/anie.201204842. [DOI] [PubMed] [Google Scholar]
- Yin Q.; Tan J. M.; Besson C.; Geletii Y. V.; Musaev D. G.; Kuznetsov A. E.; Luo Z.; Hardcastle K. I.; Hill C. L. A fast soluble carbon-free molecular water oxidation catalyst based on abundant metals. Science 2010, 328, 342–345. 10.1126/science.1185372. [DOI] [PubMed] [Google Scholar]
- Smith R. D. L.; Prévot M. S.; Fagan R. D.; Zhang Z.; Sedach P. A.; Siu M. K. J.; Trudel S.; Berlinguette C. P. Photochemical route for accessing amorphous metal oxide materials for water oxidation catalysis. Science 2013, 340, 60–63. 10.1126/science.1233638. [DOI] [PubMed] [Google Scholar]
- Friebel D.; Louie M. W.; Bajdich M.; Sanwald K. E.; Cai Y.; Wise A. M.; Cheng M.-J.; Sokaras D.; Weng T.-C.; Alonso-Mori R.; Davis R. C.; Bargar J. R.; Nørskov J. K.; Nilsson A.; Bell A. T. Identification of highly active Fe sites in (Ni, Fe)OOH for electrocatalytic water splitting. J. Am. Chem. Soc. 2015, 137, 1305–1313. 10.1021/ja511559d. [DOI] [PubMed] [Google Scholar]
- Gao M.; Sheng W.; Zhuang Z.; Fang Q.; Gu S.; Jiang J.; Yan Y. Efficient water oxidation using nanostructured α-nickel-hydroxide as an electrocatalyst. J. Am. Chem. Soc. 2014, 136, 7077–7084. 10.1021/ja502128j. [DOI] [PubMed] [Google Scholar]
- Trotochaud L.; Young S. L.; Ranney J. K.; Boettcher S. W. Nickel–iron oxyhydroxide oxygen-evolution electrocatalysts: The role of intentional and incidental iron incorporation. J. Am. Chem. Soc. 2014, 136, 6744–6753. 10.1021/ja502379c. [DOI] [PubMed] [Google Scholar]
- Du J.; Chen Z.; Ye S.; Wiley B. J.; Meyer T. J. Copper as a robust and transparent electrocatalyst for water oxidation. Angew. Chem., Int. Ed. 2015, 54, 2073–2078. 10.1002/anie.201408854. [DOI] [PubMed] [Google Scholar]
- Yu F.; Li F.; Zhang B.; Li H.; Sun L. Efficient electrocatalytic water oxidation by a copper oxide thin film in borate buffer. ACS Catal. 2015, 5, 627–630. 10.1021/cs501510e. [DOI] [Google Scholar]
- Qu K.; Zheng Y.; Dai S.; Qiao S. Z. Graphene oxide–polydopamine derived N,S-codoped carbon nanosheets as superior bifunctional electrocatalysts for oxygen reduction and evolution. Nano Energy 2016, 19, 373–381. 10.1016/j.nanoen.2015.11.027. [DOI] [Google Scholar]
- McEnaney J. M.; Crompton J. C.; Callejas J. F.; Popczun E. J.; Biacchi A. J.; Lewis N. S.; Schaak R. E. Amorphous molybdenum phosphide nanoparticles for electrocatalytic hydrogen evolution. Chem. Mater. 2014, 26, 4826–4831. 10.1021/cm502035s. [DOI] [PubMed] [Google Scholar]
- Popczun E. J.; Read C. G.; Roske C. W.; Lewis N. S.; Schaak R. E. Highly active electrocatalysis of the hydrogen evolution reaction by cobalt phosphide nanoparticles. Angew. Chem., Int. Ed. 2014, 53, 5427–5430. 10.1002/anie.201402646. [DOI] [PubMed] [Google Scholar]
- Huang Z.; Chen Z.; Chen Z.; Lv C.; Humphrey M. G.; Zhang C. Cobalt phosphide nanorods as an efficient electrocatalyst for the hydrogen evolution reaction. Nano Energy 2014, 9, 373–382. 10.1016/j.nanoen.2014.08.013. [DOI] [Google Scholar]
- Laursen A. B.; Patraju K. R.; Whitaker M. J.; Retuerto M.; Sarkar T.; Yao N.; Ramanujachary K. V.; Greenblatt M.; Dismukes G. C. Nanocrystalline Ni5P4: A hydrogen evolution electrocatalyst of exceptional efficiency in both alkaline and acidic media. Energy Environ. Sci. 2015, 8, 1027–1034. 10.1039/C4EE02940B. [DOI] [Google Scholar]
- Callejas J. F.; McEnaney J. M.; Read C. G.; Crompton J. C.; Biacchi A. J.; Popczun E. J.; Gordon T. R.; Lewis N. S.; Schaak R. E. Electrocatalytic and photocatalytic hydrogen production from acidic and neutral-pH aqueous solutions using iron phosphide nanoparticles. ACS Nano 2014, 8, 11101–11107. 10.1021/nn5048553. [DOI] [PubMed] [Google Scholar]
- Ledendecker M.; Calderón S. K.; Papp C.; Steinrück H.-P.; Antonietti M.; Shalom M. The synthesis of nanostructured Ni5P4 films and their use as a non-noble bifunctional electrocatalyst for full water splitting. Angew. Chem., Int. Ed. 2015, 54, 12361–12365. 10.1002/anie.201502438. [DOI] [PubMed] [Google Scholar]
- Wang X.; Kolen’ko Y. V.; Bao X.-Q.; Kovnir K.; Liu L. One-step synthesis of self-supported nickel phosphide nanosheet array cathodes for efficient electrocatalytic hydrogen generation. Angew. Chem., Int. Ed. 2015, 54, 8188–8192. 10.1002/anie.201502577. [DOI] [PubMed] [Google Scholar]
- Liu R.; Gu S.; Du H.; Li C. M. Controlled synthesis of FeP nanorod arrays as highly efficient hydrogen evolution cathode. J. Mater. Chem. A. 2014, 2, 17263–17267. 10.1039/c4ta03638g. [DOI] [Google Scholar]
- Pu Z.; Liu Q.; Asiri A. M.; Sun X. Tungsten phosphide nanorod arrays directly grown on carbon cloth: A highly efficient and stable hydrogen evolution cathode at all pH values. ACS Appl. Mater. Interfaces 2014, 6, 21874–21879. 10.1021/am5060178. [DOI] [PubMed] [Google Scholar]
- Pu Z.; Liu Q.; Jiang P.; Asiri A. M.; Obaid A. Y.; Sun X. CoP nanosheet arrays supported on a Ti plate: An efficient cathode for electrochemical hydrogen evolution. Chem. Mater. 2014, 26, 4326–4329. 10.1021/cm501273s. [DOI] [Google Scholar]
- Tian J.; Liu Q.; Asiri A. M.; Sun X. Self-supported nanoporous cobalt phosphide nanowire arrays: An efficient 3D hydrogen-evolving cathode over the wide range of pH 0–14. J. Am. Chem. Soc. 2014, 136, 7587–7590. 10.1021/ja503372r. [DOI] [PubMed] [Google Scholar]
- Stan M. C.; Klöpsch R.; Bhaskar A.; Li J.; Passerini S.; Winter M. Cu3P binary phosphide: Synthesis via a wet mechanochemical method and electrochemical behavior as negative electrode material for lithium-ion batteries. Adv. Energy Mater. 2013, 3, 231–238. 10.1002/aenm.201200655. [DOI] [Google Scholar]
- Ni S.; Ma J.; Lv X.; Yang X.; Zhang L. The fine electrochemical performance of porous Cu3P/Cu and the high energy density of Cu3P as anode for Li-ion batteries. J. Mater. Chem. A. 2014, 2, 20506–20509. 10.1039/C4TA03871A. [DOI] [Google Scholar]
- Bichat M.-P.; Politova T.; Pfeiffer H.; Tancret F.; Monconduit L.; Pascal J.-L.; Brousse T.; Favier F. Cu3P as anode material for lithium ion battery: Powder morphology and electrochemical performances. J. Power Sources 2004, 136, 80–87. 10.1016/j.jpowsour.2004.05.024. [DOI] [Google Scholar]
- Sun Z.; Yue Q.; Li J.; Xu J.; Zheng H.; Du P. Copper phosphide modified cadmium sulfide nanorods as a novel p–n heterojunction for highly efficient visible-light-driven hydrogen production in water. J. Mater. Chem. A 2015, 3, 10243–10247. 10.1039/C5TA02105G. [DOI] [Google Scholar]
- Dutta A.; Dutta S. K.; Mehetor S. K.; Mondal I.; Pal U.; Pradhan N. Oriented attachments and formation of ring-on-disk heterostructure Au–Cu3P photocatalysts. Chem. Mater. 2016, 28, 1872–1878. 10.1021/acs.chemmater.6b00050. [DOI] [Google Scholar]
- Dutta A.; Samantara A. K.; Adhikari S. D.; Jena B. K.; Pradhan N. Au nanowire-striped Cu3P platelet photoelectrocatalysts. J. Phys. Chem. Lett. 2016, 7, 1077–1082. 10.1021/acs.jpclett.6b00341. [DOI] [PubMed] [Google Scholar]
- Ma L.; Shen X.; Zhou H.; Zhu J.; Xi C.; Ji Z.; Kong L. Synthesis of Cu3P nanocubes and their excellent electrocatalytic efficiency for the hydrogen evolution reaction in acidic solution. RSC Adv. 2016, 6, 9672–9677. 10.1039/C5RA24427G. [DOI] [Google Scholar]
- Tian J.; Liu Q.; Cheng N.; Asiri A. M.; Sun X. Self-supported Cu3P nanowire arrays as an integrated high-performance three-dimensional cathode for generating hydrogen from water. Angew. Chem., Int. Ed. 2014, 53, 9577–9581. 10.1002/anie.201403842. [DOI] [PubMed] [Google Scholar]
- Hou H.; Xie Y.; Li Q. Large-scale synthesis of single-crystalline quasi-aligned submicrometer CuO ribbons. Cryst. Growth Des. 2005, 5, 201–205. 10.1021/cg049972z. [DOI] [Google Scholar]
- Pfeiffer H.; Tancret F.; Brousse T. Synthesis, characterization and electrochemical properties of copper phosphide (Cu3P) thick films prepared by solid-state reaction at low temperature: A probable anode for lithium ion batteries. Electrochim. Acta 2005, 50, 4763–4770. 10.1016/j.electacta.2005.02.024. [DOI] [Google Scholar]
- Liu P.; Rodriguez J. A. Catalysts for hydrogen evolution from the [NiFe] hydrogenase to the Ni2P (001) surface: The importance of ensemble effect. J. Am. Chem. Soc. 2005, 127, 14871–14878. 10.1021/ja0540019. [DOI] [PubMed] [Google Scholar]
- Liu P.; Rodriguez J. A.; Asakura T.; Gomes J.; Nakamura K. Desulfurization reactions on Ni2P (001) and α-Mo2C (001) surfaces: Complex role of P and C sites. J. Phys. Chem. B 2005, 109, 4575–4583. 10.1021/jp044301x. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Hong J.; Zheng J.; Huang Z.; Zhou J.; Xu R. Nickel–thiolate complex catalyst assembled in one step in water for solar H2 production. J. Am. Chem. Soc. 2011, 133, 20680–20683. 10.1021/ja208555h. [DOI] [PubMed] [Google Scholar]
- Popczun E. J.; McKone J. R.; Read C. G.; Biacchi A. J.; Wiltrout A. M.; Lewis N. S.; Schaak R. E. Nanostructured nickel phosphide as an electrocatalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2013, 135, 9267–9270. 10.1021/ja403440e. [DOI] [PubMed] [Google Scholar]
- Conway B. E.; Tilak B. V. Interfacial processes involving electrocatalytic evolution and oxidation of H2, and the role of chemisorbed H. Electrochim. Acta 2002, 47, 3571–3594. 10.1016/S0013-4686(02)00329-8. [DOI] [Google Scholar]
- Qi K.; Yu S.; Wang Q.; Zhang W.; Fan J.; Zheng W.; Cui X. Decoration of the inert basal plane of defect-rich MoS2 with Pd atoms for achieving Pt-similar HER activity. J. Mater. Chem. A 2016, 4, 4025–4031. 10.1039/C5TA10337A. [DOI] [Google Scholar]
- Losiewicz B. The structure, morphology and electrochemical impedance study of the hydrogen evolution reaction on the modified nickel electrodes. Int. J. Hydrogen Energy 2004, 29, 145–157. 10.1016/s0360-3199(03)00096-x. [DOI] [Google Scholar]
- Xiao P.; Sk M. A.; Thia L.; Ge X.; Lim R. J.; Wang J.-Y.; Lim K. H.; Wang X. Molybdenum phosphide as an efficient electrocatalyst for the hydrogen evolution reaction. Energy Environ. Sci. 2014, 7, 2624–2629. 10.1039/c4ee00957f. [DOI] [Google Scholar]
- Vrubel H.; Moehl T.; Grätzel M.; Hu X. Revealing and accelerating slow electron transport in amorphous molybdenum sulphide particles for hydrogen evolution reaction. Chem. Commun. 2013, 49, 8985–8987. 10.1039/c3cc45416a. [DOI] [PubMed] [Google Scholar]
- Wang G.; Huang J.; Chen S.; Gao Y.; Cao D. Preparation and supercapacitance of CuO nanosheet arrays grown on nickel foam. J. Power Sources 2011, 196, 5756–5760. 10.1016/j.jpowsour.2011.02.049. [DOI] [Google Scholar]
- Hsu Y.-K.; Chen Y.-C.; Lin Y.-G. Characteristics and electrochemical performances of lotus-like CuO/Cu(OH)2 hybrid material electrodes. J. Electroanal. Chem. 2012, 673, 43–47. 10.1016/j.jelechem.2012.03.019. [DOI] [Google Scholar]
- Hou C.-C.; Chen Q.-Q.; Wang C.-J.; Liang F.; Lin Z.; Fu W.-F.; Chen Y. Self-supported cedarlike semimetallic Cu3P nanoarrays as a 3D high-performance Janus electrode for both oxygen and hydrogen evolution under basic conditions. ACS Appl. Mater. Interfaces 2016, 8, 23037–23048. 10.1021/acsami.6b06251. [DOI] [PubMed] [Google Scholar]
- Yang L.; Qi H.; Zhang C.; Sun X. An efficient bifunctional electrocatalyst for water splitting based on cobalt phosphide. Nanotechnology 2016, 27, 23LT01. 10.1088/0957-4484/27/23/23lt01. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Fei H.; Ruan G.; Tour J. M. Porous cobalt-based thin film as a bifunctional catalyst for hydrogen generation and oxygen generation. Adv. Mater. 2015, 27, 3175–3180. 10.1002/adma.201500894. [DOI] [PubMed] [Google Scholar]
- Liu D.; Lu Q.; Luo Y.; Sun X.; Asiri A. M. NiCo2S4 nanowires array as an efficient bifunctional electrocatalyst for full water splitting with superior activity. Nanoscale 2015, 7, 15122–15126. 10.1039/C5NR04064G. [DOI] [PubMed] [Google Scholar]
- Zeng K.; Zhang D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. 10.1016/j.pecs.2009.11.002. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



