Abstract
Neutral, flexible ditopic phosphine (P–P) or phosphine oxide (O=P–P=O) donors, rigid anionic bis-chelating oxygen donors, and Re2(CO)10 were used to assemble ten phosphine oxide (P=O)-donor-based neutral monocyclic M2LL′-, bicyclic M4L2L″-, and bicyclic M4LL′2-type supramolecular coordination complexes (SCCs). A soft ditopic phosphine donor was transformed into a hard ditopic phosphine oxide donor, during the formation of the cyclic complexes 1–3, 5–6, and 9–10. Complexes 4, 7, and 8 were obtained using a hard P=O donor ligand. These SCCs were characterized using elemental analysis, FTIR, NMR, and single-crystal X-ray diffraction analysis. The absorption properties of 1–8 were studied using absorption UV–vis spectroscopic methods, and the results were analyzed using theoretical calculations. The results revealed that the neutral P=O donor significantly influenced the photophysical properties by enhancing the absorption coefficient in the visible region.
Introduction
Designing supramolecular coordination complexes (SCCs), using various preprogrammed metal-based acceptors and organic-donor frameworks, has gained considerable research interest in the past two decades because of their potential applications in various fields including host–guest encapsulation, catalysis, and medicine.1−8 Among the available metal-directed approaches, the fac-Re(CO)3-core-based method is one of the well-known processes for synthesizing heteroleptic SCCs with various sizes, shapes, and functionalities.2−5 Owing to their high significance in several important areas, research is being channeled toward synthesizing new SCCs with improved properties and designing a one-step synthetic approach for multicomponent assembly. In this direction, ditopic phosphine oxide donors have been recently introduced in place of neutral ditopic nitrogen donors, which are one of the few organic-framework-building units in heteroleptic rhenium(I)-based SCCs.6a It is noteworthy that phosphine oxide donor ligands have been used as bridging ligands between the metal centers in metal–organic polymers and discrete lanthanide-based complexes.7−11 However, the use of the P=O-donor-based ligands as framework-building units for a discrete rhenium(I)-based SCCs is limited.6a In addition, the idea of using hydroxyl/carboxyl groups as the sources of neutral and anionic oxygen donors is quite popular now for synthesizing the SCCs.1−8 However, the use of oxygen donors from the P=O unit for synthesizing the SCCs is rare.6−8 Although the P=O donor can coordinate like the nitrogen donor, the electronic differences between these two donors result in SCCs with different photophysical properties. Initial results revealed that moving from the N-donor- to the P=O-donor-based building units significantly increases the absorbance of rhenium(I)-based cyclic complexes.6a As a continuation of our previous approach, that is, mixing Re2(CO)10, rigid bis-chelating HO∩O–O∩OH donor (H2-L = H2-CA = chloranilic acid), and flexible phosphine (P–P)/phosphine oxide (O=P–P=O) donor for the development of the P=O-donor-based strong visible-light-absorbing SCCs (Scheme 1),6a we plan to modulate a spacer and an organic group attached to phosphorous of flexible organic ligands and the substitution on bis-chelating O donor that would expectedly result in similar SCCs with different photophysical properties.
Scheme 1. Synthetic Approach to a P=O-Donor-Based SCC from the P Donor6a.
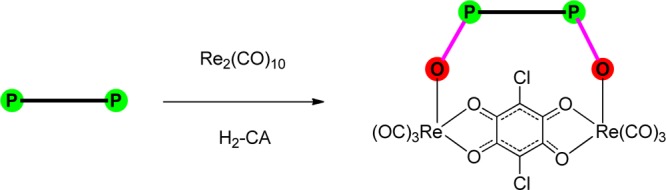
Herein, we report the preparation and characterization of seven neutral monocyclic SCCs 1–7 and three bicyclic SCCs 8–10. The complexes 1–3, 5–6, and 9–10 were obtained by treating the neutral ditopic phosphine donors [1,4-bis(diphenylphosphino)butane, L1; 1,3-bis(diphenylphosphino)propane, L2; 1,3-bis(diphenylphosphinomethyl)benzene, L3], bis-chelating HO∩O–O∩OH donor (chloranilic acid, H2-CA; 2,5-dihydroxy-1,4-benzoquinone, H2-DHBQ; tetrahydroxy-1,4-benzoquinone, H4-thq), and Re2(CO)10 in a one-pot approach.6a In this method, the soft phosphine donor was used as an indirect starting material for the oxygen donor ligand, which in turn got transformed to a hard phosphine oxide donor during the reaction (Scheme 1). Complexes 4, 7, and 8 were obtained using 1,4-bis(dimethylphosphoryl)-2,3,5,6-tetramethylbenzene (L4) or 1,2,4,5-tetrakis(dimethylphosphoryl)benzene (L5) as a neutral hard phosphine oxide donor, H2-CA/H2-DHBQ, and Re2(CO)10. The complexes were characterized using elemental analysis and 1H- and 31P-NMR spectroscopic methods. The molecular structures of the SCCs were further analyzed using single-crystal X-ray diffraction analysis. The absorption properties of 1–8 were examined using UV–vis spectroscopic methods and were analyzed using time-dependent density functional theory (TDDFT) calculations.
Results and Discussion
Complexes 1–10 were prepared by treating Re2(CO)10, a phosphine donor (L1/L2/L3)/phosphine oxide donor (L4/L5), H2-CA/H2-DHBQ/H4-thq·xH2O, and mesitylene/toluene using a solvothermal method (Schemes 2 and 3). The complexes were stable in air and moisture. Complexes 1–8 were soluble in polar organic solvents, whereas 9 and 10 were sparingly soluble. The 31P{1H} NMR spectra of 1–10 displayed a single sharp chemical resonance peak in the range of δ 27–30 in DMSO-d6 (Figures S1–S7), indicating the presence of single symmetrical complexes. The disappearance of the peak at δ −11, which corresponds to the phosphine P of L3, and the appearance of the new peak at –δ 27 for 3, 6, and 10 indicated the presence of O=P–CH2–C6H4–CH2–P=O building units in these complexes. This observation indicated that the highly oxophilic phosphine ligand could be transformed into phosphine oxide, in the presence of air under solvothermal conditions.12 The free ligand also exhibited a peak at δ 28, corresponding to the P=O donor unit. This clearly confirmed that the starting phosphine donor was transformed into phosphine oxide.
Scheme 2. Synthesis of Monocyclic SCCs 1–7 and Bicyclic SCC 8.
Scheme 3. Synthesis of Bicyclic SCCs 9 and 10.
Compared with those of the free ligand, the 1H NMR spectra of 1–10 showed very clear chemical resonance peaks both in the aromatic and in the aliphatic regions (Figures S8–S14). In particular, the protons of the methylene −CH2– group showed a doublet (∼δ 3.8), with a coupling constant of 13.8 (JPH), consistent with the presence of the phosphine oxide donor unit in the complexes 3−4, 6−8, and 10.13 A similar chemical resonance peak of CH2 was also observed in the case of free 1,4-bis(diphenylphosphinomethyl)benzene dioxide and its coordination complex.13
Crystal Structures of Metallacycles 1–5 and 7–10
The molecular structures of 1–5 and 7–10 were proved using single-crystal X-ray diffraction analysis (Figures 1–3 and S15–S17, and Tables S1–S3). Both solvothermal and recrystallization methods did not yield good-quality crystals of complex 6. Complexes 1–5 and 7 would be represented as a [1 + 1] assembly, that is, a single molecular clip [{(Re(CO)3)(μ-L)(Re(CO)3)}] capped by a ditopic O=P–P=O ligand. Complex 1 adopted a pseudorectangular structure (Figure S15), which was similar to complex I.6a The neutral O=P–P=O donor unit coordinated the molecular clip using its two oxygen atoms and took a syn conformation mode with anti-cofaciality. The rhenium atom adopted distorted octahedral geometry and was coordinated with three carbon atoms and three oxygen atoms.
Figure 1.

Molecular structures of 2 (A) and 5 (B). H atoms are omitted for clarity. Color code: gray = C, turquoise = H, red = O, pink = P, green = Cl and orange = Re.
Figure 3.

Molecular structures of 9 (A) and 10 (B). H atoms are omitted for clarity. Atoms in the cyclic frameworks are shown in the ball model; each cyclic bond is colored differently; and CO groups and phenylene attached to the P atoms are shown as thin sticks. Color code: gray = C, red = O, pink = P, and orange = Re.
Complexes 2 and 5 were isostructural (Figure 1A,B). Because of the boatlike conformation of the [{(Re(CO)3)(μ-L)(Re(CO)3)}] motif in 2 and 5, the structures of these complexes differed slightly from the dinuclear complexes I and 1. Although the CA2–/DHBQ2– moiety was planar in the complex, this unit was below the plane of Re···Re atoms. The two O=P groups (O11=P2–P1=O12 for 2 and O5=P1–P2=O6 for 5) pushed the CA2–/DHBQ2– moiety from the plane of Re···Re atoms in 2 and 5. The distance between the two rhenium atoms in the [Re(μ-L)Re] unit was 7.84/7.87 Å, which was significantly shorter than that for complexes I and 1 (dRe···Re = 8.08/8.08 Å for I/1). In both the complexes, the O=P–P=O donor unit acted as a molecular clip and adopted a syn-conformation. The conformation of O=P–(CH2)3–P=O in this complex was comparable with that of the uncoordinated ligand, except for the parallel arrangement of the two phenylene units.13 Because of the metal coordination, the bond distances of P=O in these complexes were marginally longer than that in the free phosphine ligand.
In complex 3 (Figure S16), the bis-chelating CA2– unit was slightly twisted, that is, deviated from the planarity. However, the coordination mode and the bonding nature of the CA2– unit were similar to those of the same moiety in complexes I and 2. The O=P–CH2–C6H4–CH2–P=O unit acted as a molecular clip and adopted an anti-conformation with respect to the plane of the central benzene core. The free ligand O=P–CH2–C6H4–CH2–P=O also took a similar arrangement.13 The bond distances of P=O (1.508 and 1.512 Å) were slightly longer than the bond distances found in the free ligand (1.482 and 1.488 Å),13 which indicated that the coordination of P=O with rhenium was responsible for the bond elongation. The central benzene plane was vertical to the plane of the CA2– unit (dihedral angle = 90°), and its arene CH group (C13-H) was directed toward the center of the CA2– unit (dH···centroid = 2.8350 Å). The distances between P···P, P=O···O=P, and Re···Re were 7.690, 6.707, and 8.148 Å, respectively, which suggested that the ditopic O=P–CH2–C6H4–CH2–P=O donor was an ideal molecular-coordinating clip to the [{(CO)3Re)(μ-CA)(Re(CO)3}] unit. It is known that a ditopic phosphine oxide unit acted as an eight-membered chelating ligand in Er-L3-based complexes.13
In complexes 4 and 7, the planar CA2–/DHBQ2– unit coordinated two rhenium atoms symmetrically (Figure S17). The coordination mode and bonding nature of the CA2–/DHBQ2– unit were similar to those of the same moiety in complexes I and 1. The phosphine oxide unit adopted a syn conformation with respect to the plane of the central benzene core and coordinated as a molecular clip. The central tetramethylphenylene core was closely parallel (dihedral angle = 29°/36°) to the plane of the CA2–/DHBQ2– unit.
Complex 8 adopted a M4L2L′-type bicyclic structure and was composed of four fac-Re(CO)3 moieties, two DHBQ2– units, and a neutral tetratopic O=P donor unit (Figure 2). The complex was regarded as the [2 + 1] assembly product of two molecular clips, [{(Re(CO)3)(μ-L)(Re(CO)3)}], bridged by a bis-ditopic P=O donor ligand. The three six-membered rings (DHBQ2–···benzene···DHBQ2–) are arranged like a ladder (distance (centroid) = 4.26 Å; dihedral angle = 37°). The bonding nature of both DHBQ2– and L5 in the complex 8 was normal and was similar to the bicyclic complex II.6a
Figure 2.

Molecular structure of 8 (ball and stick view). H atoms are omitted for clarity. Atoms in the cyclic frameworks are shown in the ball model; each cyclic bonds are colored differently. Color code: gray = C, red = O, pink = P, and orange = Re.
Complexes 9 and 10 adopted a M4LL′2-type bicyclic structure and thus could be regarded as [1 + 2] assembly products with one [{(Re(CO)3)(μ-thq)(Re(CO)3)}] and two O=P–P=O units (Figure 3).6a The rhenium atom in the complexes adopted distorted octahedral geometry, with a C3O3 donor environment. The hexadentate 12-electron donor thq4– unit used all its oxygen atoms to coordinate with four rhenium atoms and adopted two μ2:η1:η2:η1 modes. The delocalization of π-electrons in the C6O6 ring was separated into two parts, [complex 9, part 1: (−O19–Re4–O18–C29–C30(O19)–C31–O20–Re1–O19) and part 2: (−O22–Re3–O21–C32–C33(O22)–C34–O17–Re5–O22); and complex 10, part 1: (−O5–Re2–O6–C35–C36(O5)–C37–O4–Re1-O5) and part 2: (−O2–Re3–O1–C32–C33(O2)–C34–O3–Re5–O2)], and confined to two chelating motifs, which were again separated by two C–C single bonds (complex 9: C29–C34/C31–C32 = 1.493/1.474 Å; and complex 10: C35–C34/C37–C32 = 1.503/1.500 Å). The average (av) of the C–C and C–O bond distances of the thq4– unit (av C–C = 1.417 Å, av C–O = 1.306 Å) in these complexes and that of the free H4-thq unit (av C–C = 1.438 Å, av C–O = 1.317 Å) and complex II (Scheme 2)6a were similar, which confirmed that the C6O6 ring was a tetra-anionic motif.14,6a
Each O=P–P=O donor unit adopted a syn-conformation and bound two rhenium cores in these complexes. However, the choice of the two metal cores by the O=P–P=O donor among the four metal centers in the [{(CO)3Re}4(μ-thq)] core was different from complex to complex. The spacer, −(CH2)3– and −CH2–(C6H4)–CH2–, present in the P=O donor unit played an important role in the selection of the two metal ions in the [{(CO)3Re}4(μ-thq)] motif.
In complex 9, the molecular clip of O=P–P=O donor units coordinated two rhenium atoms, which were separated by four atoms [part I (black color): Re4–O18–C–C–O17–Re5 and part II (blue color): Re1–O20–C31–C32–O21–Re3 in Figure 3A]. These two rhenium cores were not located in the fused bis-chelating motif, and this led to the formation of the 13-membered cyclic core in the complex. Two such cyclic systems, one above and the other below the plane of the [((CO)3Re)4(μ-thq)] motif, were present in complex 9. In complex 10, O=P–P=O donor units coordinated two rhenium atoms that were separated by only one oxygen atom [part I (black color): Re2–O5–Re1 and part II (blue color): Re3–O22–Re5] and were the part of the fused bis-chelating motif. Each cycle in complex 10 gave rise to a 12-membered monocyclic ring. It is worth mentioning that the bridging nature of the two O=P–(CH2)4–P=O donor units of the [((CO)3Re)4(thq)] motif in M4LL′2-complex III was different from the bridging nature of P=O donor units in complexes 9 and 10 (Scheme S1). The central benzene core was vertical to the plane of the thq4– unit in complex 10, and its arene CH group (C5-H) was projected toward one of the edges of the thq4– unit (dH···centroid = 2.8350 Å). In addition to that the intramolecular edge-to-face C–H···π interactions were found between the two oppositely arranged phenylene units of the O=P–P=O motif (dcentroid···centroid = 4.89 Å and dihedral angle = 84°; Figure 3).
Photophysical Studies of Complexes
The dinuclear complexes 1–7 and the tetranuclear complex 8 showed a broad and structureless visible-light absorption band covering the entire visible spectrum (350–710 nm) in THF (Figure 4, Table 1). The complexes also displayed an UV absorption band centered at ∼300 nm. The spectral patterns of these complexes (1–8) were similar to those of complexes I and II, with minor shifts in the absorption maxima in the visible region.6a On the basis of previous studies, the broad low-energy absorptions in these complexes were assigned to a mixture of metal-to-ligand charge-transfer transitions (MLCT, Re → CA2–/DHBQ2–) and ligand-centered (intraligand charge-transfer, ILCT and/or ligand-to-ligand charge-transfer, LLCT) electronic transitions. The high-energy bands can be assigned to the ILCT and LLCT transitions.
Figure 4.
Absorption spectra of I−II and 1–8 in THF.
Table 1. UV–Vis Data for I–II and 1–10 in (THF/DMSO).
| compound | anionic unit in complex | P=O unit of Ln | visible-light absorption region (nm) | λmax (nm) | ε (M–1 cm–1) |
|---|---|---|---|---|---|
| I | CA | R2R′PO of L1 | 360–700 | 500 | 9824 |
| 1 | DHBQ | R2R′PO of L1 | 350–700 | 488 | 10 387 |
| 2 | CA | R2R′PO of L2 | 350–700 | 496 | 11 620 |
| 5 | DHBQ | R2R′PO of L2 | 350–700 | 482 | 5610 |
| 3 | CA | R2R′PO of L3 | 365–710 | 497 | 4130 |
| 6 | DHBQ | R2R′PO of L3 | 350–700 | 480 | 4146 |
| 4 | CA | (R′′O)2R′PO of L4 | 350–710 | 495 | 11 170 |
| 7 | DHBQ | (R′′O)2R′PO of L4 | 350–680 | 472 | 18 324 |
| II | CA | (R′′O)2R′PO of L5 | 360–700 | 500 | 14 768 |
| 8 | DHBQ | (R′′O)2R′PO of L5 | 340–700 | 472 | 26 796 |
| 9 | thq | R2R′PO of L2 | 400–600 | 450 | 6788 |
| 10 | thq | R2R′PO of L3 | 370–600 | 391 | 7972 |
R = phenyl, R′ = CH2, and R′′ = CH3.
Assignment of spectral bands was further confirmed by TDDFT calculations using a THF solvation model (Figures S18 and S19; Tables S4–S11). The visible-light absorptions of 1–7 were largely ascribed to the transition from the highest occupied molecular orbitals (HOMOs) to the lowest unoccupied molecular orbital (LUMO). In the HOMOs, the electron density was predominantly localized on the Re atoms, with a significant electron distribution on the three CO groups and the CA2–/DHBQ2– units; the contribution from the phosphate group is negligible. The electron density of the LUMO was localized over the CA2–/DHBQ2– units (91%). This observation clearly indicated that MLCT (Re → CA2–/DHBQ2–), LLCT (CO → CA2–/DHBQ2–), and ILCT (CA2–/DHBQ2– → CA2–/DHBQ2–) transitions were responsible for the absorption at longer wavelengths. The higher-energy transitions at ∼306–338 nm occurred mainly because of the ligand transitions with minor contribution from the MLCT transitions (Re → CA2–/DHBQ2–). A close inspection of the relevant molecular orbitals revealed that the electron density of the HOMOs was predominantly localized over the O=P–P=O and/or the DHBQ2–/CA2– units. The electron density of the LUMO was localized on the CA2–/DHBQ2– units. These studies clearly indicated that the higher-energy transitions for complexes 1–3, 5, and 6 occurred because of a combination of ILCT (DHBQ2–/CA2– → CA2–/DHBQ2–) and LLCT (phenylene of O=P–P=O → CA2–/DHBQ2–). Complexes 4, 7, and 8 displayed higher-energy LLCT (phenylene of O=P–P=O → CA2–/DHBQ2–) transitions.
Complexes 9 and 10 showed a broad absorption between 400 and 600 nm. A similar absorption was found in complex II possessing O=P–P=O and thq4– units. The visible absorption range of 9 and 10 was smaller than those of the other complexes.
Compared with free H2-CA (λmax = 442 nm, ε = 264 M–1 cm–1) and H2-DHBQ, these complexes exhibited red-shifted absorptions with several-times-higher molar absorption coefficients. All complexes displayed strong absorptions (ε > 10 000 M–1 cm–1) in the visible region, except for complexes 3, 5, and 6 (ε < 6000 M–1 cm–1). These results further indicated that the absorption maxima (λmax) in the visible region were blue-shifted by 12–18 nm on replacing the CA2– unit by a DHBQ2– unit in complexes 1–8; no influence was observed while changing the phosphine oxide donor unit. Furthermore, the molar absorption coefficients (ε) of the complexes at λmax ≈ 500 nm were significantly enhanced/reduced either by replacing the CA2– unit by a DHBQ2– unit or by tuning the methylene spacer of the O=P–(CH2)n–P=O (n = 4 or 3) donors in complexes I, 1, 2, and 5; no smooth trend in the molar absorption coefficient of the complexes was observed by either of the two changes. However, replacing O=P–(CH2)n–P=O (L1 or L2) by O=P–CH2–mC6H4–CH2–P=O (L3), possessing an arene spacer, dramatically reduces the ε values in the visible region. Replacing CA2– with DHBQ2– did not affect the molar absorption coefficient in complexes 3 and 6. This result indicates that the arene spacer in the complexes plays a significant role in decreasing the ε value, probably because of the noncovalent interactions between the arene spacer and the bis-chelating unit in complex 6. Close inspection of the molecular structure of 6 clearly indicates the CH···π interactions between the arene and the CA2–/DHBQ2– moiety.
For the phosphate ester-donor-based complexes 4, 7, II, and 8, replacing CA2– by DHBQ2– enhances the molar absorption coefficient remarkably. These results further imply that both the phosphate ester group and the anionic unit influence the photophysical properties of complexes 4, 7, II, and 8. Complexes II and 8 displayed higher ε values than complexes 4 and 7, probably because of the presence of nearly two units of 4 and 7 in complexes II and 8, respectively.
Conclusions
The P=O-donor-based SCCs, containing rhenium(I)carbonyl cores, bis-chelating O∩O–O∩O donors, and ditopic phosphine oxide donors, linked to butyl, propyl, xylene, or substituted xylene, were reported. Using a one-pot multicomponent solvothermal approach, these SCCs were synthesized by a spontaneous transformation of the soft phosphine into a hard phosphine oxide, in the presence of a bis-chelating oxygen donor ligand and Re2(CO)10. Direct combination of O=P–P=O, bis-chelating oxygen donor ligand, and Re2(CO)10 resulted in the formation of phosphine oxide-donor-based SCCs. The formation of the reported SCCs 1–8 experimentally supported the fact that the proposed synthetic approach was feasible. The results confirmed that a change to a neutral oxygen donor of phosphine oxide from a neutral nitrogen building unit significantly improves the absorbance of the SCCs in the visible region. In addition, the results predicted that the P=O donor of the ester ligand is a suitable building unit to make strong visible-light-absorbing rhenium-based SCCs. The construction of SCCs using ditopic/tritopic/multitopic P=O donors with conjugated spacer and acyclic complexes with different organic groups on P are under way.
Experimental Section
General Data
Re2(CO)10, H2-CA, H2-DHBQ, H4-thq·xH2O, (HCHO)n, HBr, P(OMe)3, 1,2,4,5-tetramethylbenzene, toluene, mesitylene, L1 (97%, Spectrochem, India), L2 (97%, Spectrochem, India), and L3 were used as received. Ligands L4 and L5 were prepared.15,16 Solvents hexane and toluene were purified using conventional procedures. NMR spectra were recorded on Bruker Avance III 400 and 500 MHz instruments. FTIR spectra were recorded on a JASCO 5300 FTIR spectrometer. Elemental analyses were performed on a Flash EA series 1112 CHNS analyzer. ESI-TOF mass spectra were recorded on a Bruker maXis mass spectrometer. Spectroscopic-grade solvents, tetrahydrofuran (THF, Finar) and dimethyl sulfoxide (DMSO, Spectrochem), were used as received. Absorption spectra were recorded on a UV-3600 Shimadzu UV–vis–NIR spectrophotometer.
General Synthetic Approach for 1–10
Re2(CO)10, H2-CA (or) H2-DHBQ (or) H4-thq·xH2O, mesitylene (or) toluene (∼10 mL), and hexane (∼6 mL) were kept in a Teflon vessel. The vessel was kept in the stainless steel solvothermal bomb. It was placed in an oven. The oven was programmed at 160 °C for 48 h and cooled to room temperature. Crystals or powder found in the bomb were filtered, washed with hexane, and air-dried. In a few cases, crystals were obtained by adding hexane to the clear solution obtained in the bomb and keeping as such at room temperature.
Synthesis of [{(Re(CO)3)(μ-DHBQ)(Re(CO)3)}(L1)] (1)
Dark crystals of 1 were obtained using Re2(CO)10 (100.1 mg, 0.1534 mmol), H2-DHBQ (21.7 mg, 0.1540 mmol), L1 (65.9 mg, 0.1545 mmol), and mesitylene (20 mL). Yield: 47% (81.2 mg, weight of crystals). 1H NMR (500 MHz, DMSO-d6): 7.76–7.47 (m, 20H, ArH), 5.79 (s, 1H, ArH), 2.39 (m, 4H, PCH2), 1.53 (m, 2H, PCH2CH2). 31P{1H} NMR (500 MHz, DMSO-d6): 30.1 (s). Anal. Calcd for C40H30O12P2Re2: C, 42.25; H, 2.66. Found: C, 42.36; H, 2.61. IR (KBr, cm–1): 2016 (s), 1896 (s), 1518 (s), 1145 (s).
Synthesis of [{(Re(CO)3)(μ-CA)(Re(CO)3)}(L2)] (2)
Dark crystals of 2 were obtained using Re2(CO)10 (100 mg, 0.1532 mmol), H2-CA (32 mg, 0.1532 mmol), L2 (63 mg, 0.1532 mmol), and mesitylene (10 mL). Yield: 45% (82.1 mg, weight of crystals). 1H NMR (400 MHz, DMSO-d6): δ 7.72–7.47 (m, 20H, ArH), 2.58–2.54 (m, 4H, PCH2), 1.68–1.55 (m, 2H, PCH2CH2). 31P{1H} NMR (400 MHz, DMSO-d6): δ 29.95 (s). Anal. Calcd for C39H26Cl2O12P2Re2: C, 39.30; H, 2.20. Found: C, 39.15; H, 2.41. IR (KBr, cm–1): 2010 (s), 1884 (s), 1528 (s), 1144 (s).
Synthesis of [{(Re(CO)3)(μ-CA)(Re(CO)3)}(L3)]·3C7H8 (3·3C7H8)
Dark crystals of 3 were obtained using Re2(CO)10 (101.4 mg, 0.1551 mmol), H2-CA (32.9 mg, 0.1583 mmol), L3 (73.9 mg, 0.1546 mmol), and toluene (10 mL). Yield: 44% (105.6 mg, weight of crystals). 1H NMR (500 MHz, DMSO-d6): δ 7.78–7.45 (m, 20H, ArH), 7.13 (s, 1H, ArH), 6.95–6.91 (m, 3H, ArH), 3.75 (d, 2JHP = 14 Hz, 4H, CH2). 31P{1H} NMR (500 MHz, DMSO-d6): δ 27.49 (s). Anal. Calcd for C44H28Cl2O12P2Re2: C, 42.14; H, 2.25. Found: C, 42.25; H, 2.21. IR (KBr, cm–1): 2022 (s), 1912 (s), 1512 (s), 1151 (s).
Synthesis of [{(Re(CO)3)(μ-CA)(Re(CO)3)}(L4)] (4)
Dark crystals of 4 were obtained using Re2(CO)10 (100 mg, 0.1532 mmol), H2-CA (32.02 mg, 0.1532 mmol), L4 (57.96 mg, 0.1532 mmol), and mesitylene (8 mL). Yield: 42% (73.4 mg, weight of crystals). 1H NMR (400 MHz, DMSO-d6): δ 3.55 (d, 2JHP = 10.64 Hz, 4H, CH2), 3.38–3.29 (m, 12H, OCH3), 2.22 (s, 12H, CH3). 31P{1H} NMR (400 MHz, DMSO-d6): δ 29.62 (s). ESI-TOF-MS. Calcd for C28H28Cl2O16P2Re2 ([M + H]+): m/z 1126.9411; found: m/z 1126.9146. Anal. Calcd for C28H28Cl2O16P2Re2: C, 29.87; H, 2.51. Found: C, 29.72; H, 2.62. IR (KBr, cm–1): 2022 (s), 1907 (s), 1501 (s), 1184 (s).
Synthesis of [{(Re(CO)3)(μ-DHBQ)(Re(CO)3)}(L2)] (5)
Pale brown crystals of 5 were obtained using Re2(CO)10 (101.1 mg, 0.1532 mmol), H2-DHBQ (21.8 mg, 0.1532 mmol), L2 (63.4 mg, 0.1532 mmol), and mesitylene (10 mL). Yield: 25% (43.3 mg, weight of crystals). 1H NMR (400 MHz, DMSO-d6): δ 7.73–7.47 (m, 20H, ArH), 5.78 (s, 1H, ArH), 2.58–2.54 (m, 4H, PCH2), 1.65–1.57 (m, 2H, PCH2CH2). 31P{1H} NMR (400 MHz, DMSO-d6): δ 29.93 (s). Anal. Calcd for C39H28O12P2Re2: C, 41.71; H, 2.51. Found: C, 41.62; H, 2.58. IR (KBr, cm–1): 2016 (s), 1896 (s), 1523 (s), 1151 (s).
Synthesis of [{(Re(CO)3)(μ-DHBQ)(Re(CO)3)}(L3)] (6)
Brown powder of 6 was obtained using Re2(CO)10 (100.6 mg, 0.1551 mmol), H2-DHBQ (22.2 mg, 0.1532 mmol), L3 (73.2 mg, 0.1532 mmol), and toluene (10 mL). Yield: 14% (25.5 mg). 1H NMR (400 MHz, DMSO-d6): δ 7.79–7.45 (m, 20H, ArH), 7.12 (s, 1H, ArH), 6.92 (m, 3H, ArH), 5.79 (s, 1H, ArH), 3.75 (d, 2JHP = 13.92 Hz, 4H, CH2). 31P{1H} NMR (400 MHz, DMSO-d6): δ 27.51 (s). Anal. Calcd for C44H30O12P2Re2: C, 44.59; H, 2.55. Found: C, 44.65; H, 2.61. IR (KBr, cm–1): 2011 (s), 1890 (s), 1528 (s), 1167 (s) cm–1.
Synthesis of [{(Re(CO)3)(μ-DHBQ)(Re(CO)3)}(L4)] (7)
Dark crystals of 7 were obtained using Re2(CO)10 (100 mg, 0.1532 mmol), H2-DHBQ (21.9 mg, 0.1532 mmol), L4 (58.4 mg, 0.1532 mmol), and mesitylene (8 mL). Yield: 49% (79.1 mg, weight of crystals). 1H NMR (400 MHz, DMSO-d6): δ 5.79 (s, 1H, ArH), 3.55 (d, 2JHP = 10.8 Hz, 4H, CH2), 3.34–3.29 (m, 12H, OCH3), 2.22 (s, 12H, CH3). 31P{1H} NMR (400 MHz, DMSO-d6): δ 29.62 (s). ESI-TOF-MS. Calcd for C28H30O16P2Re2 ([M + H]+): m/z 1057.0170; found: m/z 1056.9374. Anal. Calcd for C28H30O16P2Re2: C, 31.82; H, 2.86. Found: C, 31.74; H, 2. 79. IR (KBr, cm–1): 2016 (s), 1901 (s), 1167 (s).
Synthesis of [{(Re(CO)3)(μ-DHBQ)(Re(CO)3)}2(L5)] (8)
Dark crystals of 8 were obtained using a mixture of Re2(CO)10 (100.9 mg, 0.1546 mmol), H2-DHBQ (22.2 mg, 0.1584 mmol), L5(43.9 mg, 0.077 mmol), and mesitylene (20 mL). Yield: 48% (71 mg). 1H NMR (500 MHz, DMSO-d6): 7.16 (s, 1H, ArH), 5.79 (s, 1H, ArH), 3.59 (d, 2JHP = 10.45 Hz, 4H, CH2), 3.42–3.38, (m, 24H, OCH3). 31P{1H} NMR (500 MHz, DMSO-d6): 28.69 (s). Anal. Calcd for C42H38O32P4Re4: C, 26.23; H, 1.99. Found: C, 26.31; H, 1.92. IR (KBr, cm–1): 2011 (s) and 1885 (s).
Synthesis of [{(Re(CO)3)2(μ-thq)(Re(CO)3)2}(L2)2] (9)
Dark crystals of 9 were obtained using Re2(CO)10 (100 mg, 0.1532 mmol), H4–thq·xH2O (26.3 mg, 0.1532 mmol), L2 (63 mg, 0.1532 mmol), and mesitylene (8 mL). Yield: 54% (88.9 mg, weight of crystals). 1H NMR (400 MHz, DMSO-d6): δ 7.73–7.48 (m, 20H, ArH), 2.58–2.52 (m, 4H, PCH2), 1.66–1.59 (m, 2H, PCH2CH2). 31P{1H} NMR (400 MHz, DMSO-d6): δ 29.88 (s). Anal. Calcd for C72H52O22P4Re4: C, 40.45; H, 2.45. Found: C, 40.36; H, 2.51. IR (KBr, cm–1): 2022 (s), 1896 (s), 1145 (s).
Synthesis of [{(Re(CO)3)2(μ-thq)(Re(CO)3)2}(L3)2] (10)
Dark crystals of 10 were obtained using Re2(CO)10 (100.7 mg, 0.1532 mmol), H4-thq·xH2O (13.5 mg, 0.0766 mmol), L3 (73.2 mg, 0.1532 mmol), and toluene (8 mL). Yield: 36% (62.6 mg, weight of crystals). 1H NMR (500 MHz, DMSO-d6): δ 7.78–7.46 (m, 20H, ArH), 7.13 (s, 2H, ArH), 6.95–6.92 (m, 6H, ArH). 3.75 (d, 2JPH = 14 Hz, 8H, CH2). 31P{1H} NMR (500 MHz, DMSO-d6): δ 27.48 (s). Anal. Calcd for C82H56O22P4Re4: C, 43.54; H, 2.50. Found: C, 43.41; H, 2.56. IR (KBr, cm–1): ν = 2027 (s), 1890 (s), 1145 (s).
X-ray Crystallography
Intensity data of crystals of 1–3, 5, 7–8, and 10 were collected on a Bruker D8 Quest diffractometer [λ(Mo Kα) = 0.71073 Å]. Intensity data of 4 were collected on an Oxford Xcalibur S diffractometer. Intensity data of 9 were collected on a Rigaku Saturn 724+ CCD diffractometer. The structures were solved by direct methods using SHELXS-9717 and refined using the SHELXL-2014/7 program (within the WinGX program package).17b,17c Non-H atoms were refined anisotropically. Some of the lattice solvent molecules could not be modeled, and hence their contribution to the intensities was excluded using the SQUEEZE option in PLATON.17d The crystallographic data of 1–5 and 7–10 are provided in Tables S1–S2.
Computational Section
The atomic coordinates were obtained from the X-ray crystal structures of 1–5 and 7–8. The ground-state geometry optimizations of 1–8 were performed in the gas phase using the B3LYP method. A Stuttgart–Dresden (SDD) basis set for the rhenium atom and the 6-311G* basis set for all other atoms were used using the Gaussian 09 program package.18−21 Geometry optimizations were achieved without any constraints. To exactly analyze the absorption spectra, UV–visible spectral analysis for vertical excitations from the ground state was computed using TDDFT using the IEFPCM (integral equation formalism polarizable continuum model) solvent model with THF. The TDDFT analysis was carried out, and the B3LYP functional and SDD/6-311G* basis set for 1–8 were used in the optimization step. A total of the lowest 100 singlet excited states and their corresponding oscillator strengths were determined using a TDDFT calculation for 1–8. The GaussSum programs were used to calculate the percentage contribution of various groups and the electronic spectral simulation.
Acknowledgments
We thank the University of Hyderabad and the DST-SERB (EMR/2015/000627), for the financial support. We thank Prof. R. Murugavel for the use of his Single Crystal X-ray Diffraction Facility established through a DAE-SRC Outstanding Investigator Award.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.6b00187.
NMR spectra, molecular structures of the complexes, tables, and Cartesian coordinates of DFT optimized geometries (PDF)
Crystallographic information of 1 (CIF)
Crystallographic information of 2 (CIF)
Crystallographic information of 3 (CIF)
Crystallographic information of 4 (CIF)
Crystallographic information of 5 (CIF)
Crystallographic information of 7 (CIF)
Crystallographic information of 8 (CIF)
Crystallographic information of 9 (CIF)
Crystallographic information of 10 (CIF)
The authors declare no competing financial interest.
Dedication
This paper is dedicated to Prof. Kuang-Lieh Lu, Institute of Chemistry, Academia Sinica, Taipei 115, Taiwan, for his outstanding contribution in the field of rhenium based supramolecular coordination complexes.
Supplementary Material
References
- a Amouri H.; Desmarets C.; Moussa J. Confined nanospaces in metallocages: Guest molecules, weakly encapsulated anions, and catalyst sequestration. Chem. Rev. 2012, 112, 2015–2041. and references therein 10.1021/cr200345v. [DOI] [PubMed] [Google Scholar]; b Lehn J.-M. Perspectives in chemistry–steps towards complex matter. Angew. Chem., Int. Ed. 2013, 52, 2836–2850. 10.1002/anie.201208397. [DOI] [PubMed] [Google Scholar]; c Cook T. R.; Zheng Y.-R.; Stang P. J. Metal–organic frameworks and self-assembled supramolecular coordination complexes: Comparing and contrasting the design, synthesis, and functionality of metal–organic materials. Chem. Rev. 2013, 113, 734–777. 10.1021/cr3002824. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Inokuma Y.; Kawano M.; Fujita M. Crystalline molecular flasks. Nat. Chem. 2011, 3, 349–358. 10.1038/nchem.1031. [DOI] [PubMed] [Google Scholar]; e Wiester M. J.; Ulmann P. A.; Mirkin C. A. Enzyme mimics based upon supramolecular coordination chemistry. Angew. Chem., Int. Ed. 2011, 50, 114–137. 10.1002/anie.201000380. [DOI] [PubMed] [Google Scholar]; f Wang Z. J.; Clary K. N.; Bergman R. G.; Raymond K. N.; Toste F. D. A supramolecular approach to combining enzymatic and transition metal catalysis. Nat. Chem. 2013, 5, 100–103. 10.1038/nchem.1531. [DOI] [PubMed] [Google Scholar]; g Smulders M. M. J.; Riddell I. A.; Browne C.; Nitschke J. R. Building on architectural principles for three-dimensional metallosupramolecular construction. Chem. Soc. Rev. 2013, 42, 1728–1754. 10.1039/c2cs35254k. [DOI] [PubMed] [Google Scholar]; h Saalfrank R. W.; Maid H.; Scheurer A. Supramolecular coordination chemistry: The synergistic effect of serendipity and rational design. Angew. Chem., Int. Ed. 2008, 47, 8794–8824. 10.1002/anie.200702075. [DOI] [PubMed] [Google Scholar]; i Hiratani K.; Albrecht M. The tandem claisen rearrangement in the construction of building blocks for supramolecular chemistry. Chem. Soc. Rev. 2008, 37, 2413–2421. 10.1039/b719548f. [DOI] [PubMed] [Google Scholar]; j Ward M. D. Polynuclear coordination cages. Chem. Commun. 2009, 4487–4499. 10.1039/b906726b. [DOI] [PubMed] [Google Scholar]; k Han Y.-F.; Li H.; Jin G.-X. Host–guest chemistry with bi- and tetra-nuclear macrocyclic metallasupramolecules. Chem. Commun. 2010, 46, 6879–6890. 10.1039/c0cc00770f. [DOI] [PubMed] [Google Scholar]; l Saha M. L.; De S.; Pramanik S.; Schmittel M. Orthogonality in discrete self-assembly–survey of current concepts. Chem. Soc. Rev. 2013, 42, 6860–6909. 10.1039/c3cs60098j. [DOI] [PubMed] [Google Scholar]; m Frischmann P. D.; MacLachlan M. J. Metallocavitands: An emerging class of functional multimetallic host molecules. Chem. Soc. Rev. 2013, 42, 871–890. 10.1039/c2cs35337g. [DOI] [PubMed] [Google Scholar]; n Lippert B.; Miguel P. J. S. Metallatriangles and metallasquares: The diversity behind structurally characterized examples and the crucial role of ligand symmetry. Chem. Soc. Rev. 2011, 40, 4475–4487. 10.1039/c1cs15090a. [DOI] [PubMed] [Google Scholar]; o Sauvage J.-P.; Amabilino D. B. The beauty of knots at the molecular level. Top. Curr. Chem. 2011, 323, 107–125. 10.1007/128_2011_292. [DOI] [PubMed] [Google Scholar]; p Therrien B. Drug delivery by water-soluble organometallic cages. Top. Curr. Chem. 2011, 319, 35–55. 10.1007/128_2011_272. [DOI] [PubMed] [Google Scholar]; q Zangrando E.; Casanova M.; Alessio E. Trinuclear metallacycles: Metallatriangles and much more. Chem. Rev. 2008, 108, 4979–5013. 10.1021/cr8002449. [DOI] [PubMed] [Google Scholar]; r Chifotides H. T.; Dunbar K. R. Anion−π interactions in supramolecular architectures. Acc. Chem. Res. 2013, 46, 894–906. 10.1021/ar300251k. [DOI] [PubMed] [Google Scholar]; s Yam V. W.-W.; Au V. K.-M.; Leung S. Y.-L. Light-emitting self-assembled materials based on d8 and d10 transition metal complexes. Chem. Rev. 2015, 115, 7589–7728. 10.1021/acs.chemrev.5b00074. [DOI] [PubMed] [Google Scholar]; t Safont-Sempere M. M.; Fernández G.; Würthner F. Self-sorting phenomena in complex supramolecular systems. Chem. Rev. 2011, 111, 5784–5814. 10.1021/cr100357h. [DOI] [PubMed] [Google Scholar]; u Lim S. H.; Su Y.; Cohen S. M. Supramolecular tetrahedra of phosphines and coinage metals. Angew. Chem., Int. Ed. 2012, 51, 5106–5109. 10.1002/anie.201200730. [DOI] [PubMed] [Google Scholar]; v Cook T. R.; Vajpayee V.; Lee M. H.; Stang P. J.; Chi K.-W. Biomedical and biochemical applications of self-assembled metallacycles and metallacages. Acc. Chem. Res. 2013, 46, 2464–2474. 10.1021/ar400010v. [DOI] [PMC free article] [PubMed] [Google Scholar]; w Su C.-Y.; Cai Y.-P.; Chen C.-L.; Smith M. D.; Kaim W.; zur Loye H.-C. Ligand-directed molecular architectures: Self-assembly of two-dimensional rectangular metallacycles and three-dimensional trigonal or tetragonal prisms. J. Am. Chem. Soc. 2003, 125, 8595–8613. 10.1021/ja034267k. [DOI] [PubMed] [Google Scholar]; x Rohacova J.; Sekine A.; Kawano T.; Tamari S.; Ishitani O. Trinuclear and tetranuclear Re(I) rings connected with phenylene, vinylene, and ethynylene chains: Synthesis, photophysics, and redox properties. Inorg. Chem. 2015, 54, 8769–8777. 10.1021/acs.inorgchem.5b01397. [DOI] [PubMed] [Google Scholar]; y Sahara G.; Ishitani O. Efficient photocatalysts for CO2 reduction. Inorg. Chem. 2015, 54, 5096–5104. 10.1021/ic502675a. [DOI] [PubMed] [Google Scholar]; z Morimoto T.; Nishiura C.; Tanaka M.; Rohacova J.; Nakagawa Y.; Funada Y.; Koike K.; Yamamoto Y.; Shishido S.; Kojima T.; Saeki T.; Ozeki T.; Ishitani O. Ring-shaped Re(I) multinuclear complexes with unique photofunctional properties. J. Am. Chem. Soc. 2013, 135, 13266–13269. 10.1021/ja406144h. [DOI] [PubMed] [Google Scholar]
- a Dinolfo P. H.; Hupp J. T. Supramolecular coordination chemistry and functional microporous molecular materials. Chem. Mater. 2001, 13, 3113–3125. 10.1021/cm010176f. [DOI] [Google Scholar]; b Dinolfo P. H.; Coropceanu V.; Brédas J.-L.; Hupp J. T. A new class of mixed-valence systems with orbitally degenerate organic redox centers. Examples based on hexa-rhenium molecular prisms. J. Am. Chem. Soc. 2006, 128, 12592–12593. 10.1021/ja0623764. [DOI] [PubMed] [Google Scholar]; c Dinolfo P. H.; Williams M. E.; Stern C. L.; Hupp J. T. Rhenium-based molecular rectangles as frameworks for ligand-centered mixed valency and optical electron transfer. J. Am. Chem. Soc. 2004, 126, 12989–13001. 10.1021/ja0473182. [DOI] [PubMed] [Google Scholar]; d Dinolfo P. H.; Hupp J. T. Tetra-rhenium molecular rectangles as organizational motifs for the investigation of ligand-centered mixed valency: Three examples of full delocalization. J. Am. Chem. Soc. 2004, 126, 16814–16819. 10.1021/ja045457d. [DOI] [PubMed] [Google Scholar]; e Kumar A.; Sun S.-S.; Lees A. J. Directed assembly metallocyclic supramolecular systems for molecular recognition and chemical sensing. Coord. Chem. Rev. 2008, 252, 922–939. 10.1016/j.ccr.2007.07.023. [DOI] [Google Scholar]; f Kumar A.; Sun S.-S.. Photophysics and photochemistry of molecular recognition and sensing with metal-directed macrocyclic systems. In Molecular Self-Assembly: Advances and Applications; Dequan A. L., Ed.; Pan Stanford Publishing, 2012; pp 301–349. [Google Scholar]; g Thanasekaran P.; Lee C.-C.; Lu K.-L. One-step orthogonal-bonding approach to the self-assembly of neutral rhenium-based metallacycles: Synthesis, structures, photophysics, and sensing applications. Acc. Chem. Res. 2012, 45, 1403–1418. 10.1021/ar200243w. [DOI] [PubMed] [Google Scholar]
- a Thorp-Greenwood F. L.; Balasingham R. G.; Coogan M. P. Organometallic complexes of transition metals in luminescent cell imaging applications. J. Organomet. Chem. 2012, 714, 12–21. 10.1016/j.jorganchem.2012.01.020. [DOI] [Google Scholar]; b Wise M. D.; Ruggi A.; Pascu M.; Scopelliti R.; Severin K. Clathrochelate-based bipyridyl ligands of nanoscale dimensions: Easy-to-access building blocks for supramolecular chemistry. Chem. Sci. 2013, 4, 1658–1662. 10.1039/c3sc50155h. [DOI] [Google Scholar]; c Brasey T.; Buryak A.; Scopelliti R.; Severin K. Synthesis of a metallamacrocyclic Re(CO)3 complex using a tridentate bridging ligand. Eur. J. Inorg. Chem. 2004, 964–967. 10.1002/ejic.200300880. [DOI] [Google Scholar]; d Barbazán P.; Carballo R.; Casas J. S.; García-Martínez E.; Pereiras-Gabián G.; Sánchez A.; Vázquez-López E. M. Synthesis and characterization of new trimeric rhenium(I) complexes. The influence of steric factors on the size of pyrazolonaterhenium(I) metallomacrocycles. Inorg. Chem. 2006, 45, 7323–7330. 10.1021/ic061080w. [DOI] [PubMed] [Google Scholar]; e Coogan M. P.; Fernández-Moreira V.; Kariuki B. M.; Pope S. J. A.; Thorp-Greenwood F. L. A rhenium tricarbonyl 4′-oxo-terpy trimer as a luminescent molecular vessel with a removable silver stopper. Angew. Chem., Int. Ed. 2009, 48, 4965–4968. 10.1002/anie.200900981. [DOI] [PubMed] [Google Scholar]; f Carballo R.; Casas J. S.; García-Martínez E.; Pereiras-Gabián G.; Sánchez A.; Sordo J.; Vázquez-López E. M. Rhenium(I)-induced cyclization of thiosemicarbazones derived from β-keto esters. Inorg. Chem. 2003, 42, 6395–6403. 10.1021/ic030154q. [DOI] [PubMed] [Google Scholar]; g Boccalon M.; Iengo E.; Tecilla P. Metal–organic transmembrane nanopores. J. Am. Chem. Soc. 2012, 134, 20310–20313. 10.1021/ja310425j. [DOI] [PubMed] [Google Scholar]; h Boccalon M.; Iengo E.; Tecilla P. New meso-substituted trans-A2B2 di(4-pyridyl)-porphyrins as building blocks for metal-mediated self-assembling of 4 + 4 Re(I)–porphyrin metallacycles. Org. Biomol. Chem. 2013, 11, 4056–4067. 10.1039/c3ob40452h. [DOI] [PubMed] [Google Scholar]; i Laramée-Milette B.; Lachance-Brais C.; Hanan G. S. Synthesis of discrete Re(I) di- and tricarbonyl assemblies using a [4 × 1] directional bonding strategy. Dalton Trans. 2015, 44, 41–45. 10.1039/c4dt03077j. [DOI] [PubMed] [Google Scholar]; j Botana E.; Da Silva E.; Benet-Buchholz J.; Ballester P.; de Mendoza J. Inclusion of cavitands and calix[4]arenes into a metallobridged para-(1H-imidazo[4,5-f][3,8]phenanthrolin-2-yl)-expanded calix[4]arene. Angew. Chem., Int. Ed. 2007, 46, 198–201. 10.1002/anie.200603180. [DOI] [PubMed] [Google Scholar]; k Xiong J.; Liu W.; Wang Y.; Cui L.; Li Y.-Z.; Zuo J.-L. Tricarbonyl mono- and dinuclear rhenium(I) complexes with redox-active bis(pyrazole)–tetrathiafulvalene ligands: Syntheses, crystal structures, and properties. Organometallics 2012, 31, 3938–3946. 10.1021/om300159r. [DOI] [Google Scholar]; l Chang S.-Y.; Jang H.-Y.; Jeong K.-S. Quantitative comparison of kinetic stabilities of metallomacrocycle-based rotaxanes. Chem.—Eur. J. 2003, 9, 1535–1541. 10.1002/chem.200390176. [DOI] [PubMed] [Google Scholar]; m Yedukondalu M.; Ravikanth M. Re(I) bridged porphyrin dyads, triads and tetrads. J. Chem. Sci. 2011, 123, 201–214. 10.1007/s12039-011-0113-4. [DOI] [Google Scholar]
- a Manimaran B.; Vanitha A.; Karthikeyan M.; Ramakrishna B.; Mobin S. M. Self-assembly of selenium-bridged rhenium(I)-based metalla rectangles: Synthesis, characterization, and molecular recognition studies. Organometallics 2014, 33, 465–472. 10.1021/om400673f. [DOI] [Google Scholar]; b Nagarajaprakash R.; Divya D.; Ramakrishna B.; Manimaran B. Synthesis and spectroscopic and structural characterization of oxamidato-bridged rhenium(I) supramolecular rectangles with ester functionalization. Organometallics 2014, 33, 1367–1373. 10.1021/om400776m. [DOI] [Google Scholar]; c Nagarajaprakash R.; Kumar C. A.; Mobin S. M.; Manimaran B. Multicomponent self-assembly of thiolato- and selenato-bridged ester-functionalized rhenium(I)-based trigonal metallaprisms: Synthesis and structural characterization. Organometallics 2015, 34, 724–730. 10.1021/om501005n. [DOI] [Google Scholar]; d Govindarajan R.; Nagarajaprakash R.; Manimaran B. Synthesis, structural characterization, and host–guest studies of aminoquinonato-bridged Re(I) supramolecular rectangles. Inorg. Chem. 2015, 54, 10686–10694. 10.1021/acs.inorgchem.5b01543. [DOI] [PubMed] [Google Scholar]; e Nagarajaprakash R.; Govindarajan R.; Manimaran B. One-pot synthesis of oxamidato-bridged hexarhenium trigonal prisms adorned with ester functionality. Dalton Trans. 2015, 44, 11732–11740. 10.1039/C5DT01102G. [DOI] [PubMed] [Google Scholar]; f Orsa D. K.; Haynes G. K.; Pramanik S. K.; Iwunze M. O.; Greco G. E.; Krause J. A.; Ho D. M.; Williams A. L.; Hill D. A.; Mandal S. K. Synthesis, characterization, and fluorescence and cytotoxicity studies of a tetrarhenium molecular rectangle. Inorg. Chem. Commun. 2007, 10, 821–824. 10.1016/j.inoche.2007.04.006. [DOI] [Google Scholar]
- a Roy B.; Ghosh A. K.; Srivastava S.; D’Silva P.; Mukherjee P. S. A Pd8 tetrafacial molecular barrel as carrier for water insoluble fluorophore. J. Am. Chem. Soc. 2015, 137, 11916–11919. 10.1021/jacs.5b08008. [DOI] [PubMed] [Google Scholar]; b Bhat I. A.; Samanta D.; Mukherjee P. S. A Pd24 pregnant molecular nanoball: Self-templated stellation by precise mapping of coordination sites. J. Am. Chem. Soc. 2015, 137, 9497–9502. 10.1021/jacs.5b06628. [DOI] [PubMed] [Google Scholar]; c Samanta D.; Mukherjee P. S. Sunlight-induced covalent marriage of two triply interlocked Pd6 cages and their facile thermal separation. J. Am. Chem. Soc. 2014, 136, 17006–17009. 10.1021/ja511360e. [DOI] [PubMed] [Google Scholar]; d Samanta D.; Mukherjee P. S. Component selection in the self-assembly of palladium(II) nanocages and cage-to-cage transformations. Chem.—Eur. J. 2014, 20, 12483–12492. 10.1002/chem.201402553. [DOI] [PubMed] [Google Scholar]; e Mukherjee S.; Mukherjee P. S. Template-free multicomponent coordination-driven self-assembly of Pd(II)/Pt(II) molecular cages. Chem. Commun. 2014, 50, 2239–2248. 10.1039/C3CC49192G. [DOI] [PubMed] [Google Scholar]
- a Shankar B.; Elumalai P.; Shanmugam R.; Singh V.; Masram D. T.; Sathiyendiran M. New class of phosphine oxide donor-based supramolecular coordination complexes from an in situ phosphine oxidation reaction or phosphine oxide ligands. Inorg. Chem. 2013, 52, 10217–10219. 10.1021/ic401257w. [DOI] [PubMed] [Google Scholar]; b Shankar B.; Elumalai P.; Deval Sathiyashivan S.; Sathiyendiran M. Spheroid metallocavitands with eight calixarene-shaped receptors on the surface. Inorg. Chem. 2014, 53, 10018–10020. 10.1021/ic5014895. [DOI] [PubMed] [Google Scholar]; c Shankar B.; Sahu S.; Deibel N.; Schweinfurth D.; Sarkar B.; Elumalai P.; Gupta D.; Hussain F.; Krishnamoorthy G.; Sathiyendiran M. Luminescent dirhenium(I)-double-heterostranded helicate and mesocate. Inorg. Chem. 2014, 53, 922–930. 10.1021/ic4023135. [DOI] [PubMed] [Google Scholar]; d Shankar B.; Marimuthu R.; Sathiyashivan S. D.; Sathiyendiran M. Spheroid metallacycles and metallocavitands with calixarene- and/or cleft-shaped receptors on the surface. Inorg. Chem. 2016, 55, 4537–4544. 10.1021/acs.inorgchem.6b00371. [DOI] [PubMed] [Google Scholar]; e Gupta D.; Rajakannu P.; Shankar B.; Shanmugam R.; Hussain F.; Sarkar B.; Sathiyendiran M. Furan-decorated neutral Re(I)-based 2D rectangle and 3D trigonal prism. Dalton Trans. 2011, 40, 5433–5435. 10.1039/c0dt01227k. [DOI] [PubMed] [Google Scholar]; f Rajakannu P.; Elumalai P.; Shankar B.; Hussain F.; Sathiyendiran M. Rhenium(I) based metallocalix[4]arenes decorated with free functionalized benzimidazolyl units. Dalton Trans. 2013, 42, 11359–11362. 10.1039/c3dt51096d. [DOI] [PubMed] [Google Scholar]; g Elumalai P.; Kanagaraj R.; Marimuthu R.; Shankar B.; Kalita A. C.; Sathiyendiran M. Rhenium(I)-based bridgeless double metallocalix[4]arenes. Dalton Trans. 2015, 44, 11274–11277. 10.1039/C5DT00841G. [DOI] [PubMed] [Google Scholar]; h Rajakannu P.; Shankar B.; Yadav A.; Shanmugam R.; Gupta D.; Hussain F.; Chang C.-H.; Sathiyendiran M.; Lu K.-L. Adaptation toward restricted conformational dynamics: From the series of neutral molecular rotors. Organometallics 2011, 30, 3168–3176. 10.1021/om200269z. [DOI] [Google Scholar]; i Rajakannu P.; Mobin S. M.; Sathiyendiran M. Thiophene/furan units decorated unsymmetrical dinuclear metallocalix[4]arenes. J. Organomet. Chem. 2014, 771, 68–77. 10.1016/j.jorganchem.2014.03.028. [DOI] [Google Scholar]; j Gupta D.; Rajakannu P.; Shankar B.; Hussain F.; Sathiyendiran M. Synthesis and crystal structure of a wheel-shaped supramolecular coordination complex. J. Chem. Sci. 2014, 126, 1501–1506. 10.1007/s12039-014-0655-3. [DOI] [Google Scholar]
- a Zhao X.; Zhou F.; Liu Q.; Chen Q.-F.; Yang J.-Y.; Zhang W.-H.; Song Y.-L.; Lang J.-P. Diverse Tp*-capped W–Cu–S clusters from one-pot assembly involving in situ thiolation of phosphines. Inorg. Chem. 2016, 55, 1861–1871. 10.1021/acs.inorgchem.5b02765. [DOI] [PubMed] [Google Scholar]; b Wang J.-F.; Liu S.-Y.; Liu C.-Y.; Ren Z.-G.; Lang J.-P. Silver(I) complexes with a P–N hybrid ligand and oxyanions: Synthesis, structures, photocatalysis and photocurrent responses. Dalton Trans. 2016, 45, 9294–9306. 10.1039/C6DT01211F. [DOI] [PubMed] [Google Scholar]; c Qi H.-X.; Wang J.-F.; Ren Z.-G.; Ning J.-J.; Lang J.-P. Syntheses and structures of two gold(I) coordination compounds derived from P–S hybrid ligands and their efficient catalytic performance in the photodegradation of nitroaromatics in water. Dalton Trans. 2015, 44, 5662–5671. 10.1039/c5dt00167f. [DOI] [PubMed] [Google Scholar]; d Wu X.-Y.; Qi H.-X.; Ning J.-J.; Wang J.-F.; Ren Z.-G.; Lang J. P. One silver(I)/tetraphosphine coordination polymer showing good catalytic performance in the photodegradation of nitroaromatics inaqueous solution. Appl. Catal., B 2015, 168, 98–104. 10.1016/j.apcatb.2014.12.024. [DOI] [Google Scholar]; e Yang J.-H.; Wu X.-Y.; He R.-T.; Ren Z.-G.; Li H.-X.; Wang H.-F.; Lang J.-P. Degradation versus Expansion of the AgX Frameworks: Formation of Oligomeric and Polymeric Silver Complexes from Reactions of Bulk AgX with N,N-Bis(diphenylphosphanylmethyl)-2-aminopyridine. Cryst. Growth Des. 2013, 13, 2124–2134. 10.1021/cg4002048. [DOI] [Google Scholar]; f Huang Z.-J.; Cheng H.-J.; Dai M.; Ni C.-Y.; Li H.-X.; Hou K.-P.; Ren Z.-G.; Lang J.-P. Solvothermal syntheses and crystal structures of one 1D and two 3D [PbxIy]-based coordination polymers. Inorg. Chem. Commun. 2013, 31, 33–36. 10.1016/j.inoche.2013.02.016. [DOI] [Google Scholar]; g Sun S.; Ren Z.-G.; Yang J.-H.; He R.-T.; Wang F.; Wu X.-Y.; Gong W.-J.; Li H.-X.; Lang J.-P. Formation of N-heterocyclic diphosphine ligands from Ag(I)-assisted condensation reactions between bdppeda and formaldehyde and their binuclear silver(I) complexes. Dalton Trans. 2012, 41, 8447–8454. 10.1039/c2dt30860f. [DOI] [PubMed] [Google Scholar]; h Ren Z.-G.; Sun S.; Dai M.; Wang H.-F.; Lü C.-N.; Lang J.-P.; Sun Z.-R. Assembly of bicyclic or monocyclic clusters from [(η5-C5Me5)2Mo2(μ3-S)4(CuMeCN)2]2+ with tetraphosphine or N, P mixed ligands: Syntheses, structures and enhanced third-order NLO performances. Dalton Trans. 2011, 40, 8391–8398. 10.1039/c1dt10685f. [DOI] [PubMed] [Google Scholar]
- a Rosario-Amorin D.; Duesler E. N.; Paine R. T.; Hay B. P.; Delmau L. H.; Reilly S. D.; Gaunt A. J.; Scott B. L. Synthesis and coordination chemistry of phosphine oxide decorated dibenzofuran platforms. Inorg. Chem. 2012, 51, 6667–6681. 10.1021/ic300301d. [DOI] [PubMed] [Google Scholar]; b Redmond M. P.; Cornet S. M.; Woodall S. D.; Whittaker D.; Collison D.; Helliwell M.; Natrajan L. S. Probing the local coordination environment and nuclearity of uranyl(VI) complexes in non-aqueous media by emission spectroscopy. Dalton Trans. 2011, 40, 3914–3926. 10.1039/c0dt01464h. [DOI] [PubMed] [Google Scholar]; c Guseva E. V.; Naumova A. A.; Karimova D. T.; Sokolova A. V.; Gavrilova E. L.; Busygina T. E. Reaction of rhodium trichloride and dirhodium(II) acetate with trans-4,4′-bis(diethoxyphosphoryl)biphenyl-18-crown-6. Russ. J. Gen. Chem. 2012, 82, 1–8. 10.1134/S107036321201001X. [DOI] [Google Scholar]; d Cheng F.; Codgbrook H. L.; Hector A. L.; Levason W.; Reid G.; Webster M.; Zhang W. Gallium(III) halide complexes with phosphines, arsines and phosphine oxides–a comparative study. Polyhedron 2007, 26, 4147–4155. 10.1016/j.poly.2007.05.008. [DOI] [Google Scholar]; e Lees A. M. J.; Platt A. W. G. Complexes of lanthanide nitrates with bis(diphenylphosphino)methane dioxide. Inorg. Chem. 2003, 42, 4673–4679. 10.1021/ic0342954. [DOI] [PubMed] [Google Scholar]; f Peveling K.; Dannappel K.; Schürmann M.; Costisella B.; Jurkschat K. Structure-directing properties of lithium chloride in supramolecular {4-t-Bu-2,6-[P(O)(OEt)2]2C6H2}SiH2Ph·LiCl·2H2O. Intermolecular P=O→Li versus intramolecular P=O→(H)Si coordination. Organometallics 2006, 25, 368–374. 10.1021/om050824v. [DOI] [Google Scholar]
- a Atefi F.; McMurtrie J. C.; Turner P.; Duriska M.; Arnold D. P. meso-Porphyrinylphosphine Oxides: Mono- and bidentate ligands for supramolecular chemistry and the crystal structures of monomeric {[10,20-diphenylporphyrinatonickel(II)-5,15-diyl]-bis-[P(O)Ph2] and polymeric self-coordinated {[10,20-diphenylporphyrinatozinc(II)-5,15-diyl]-bis-[P(O)Ph2]}. Inorg. Chem. 2006, 45, 6479–6489. 10.1021/ic060372u. [DOI] [PubMed] [Google Scholar]; b Atefi F.; McMurtrie J. C.; Arnold D. P. Multiporphyrin coordination arrays based on complexation of magnesium(II) porphyrins with porphyrinylphosphine oxides. Dalton Trans. 2007, 2163–2170. 10.1039/b703589f. [DOI] [PubMed] [Google Scholar]; c Sinelshchikova A. A.; Nefedov S. E.; Enakieva Y. Y.; Gorbunova Y. G.; Tsivadze A. Y.; Kadish K. M.; Chen P.; Bessmertnykh-Lemeune A.; Stern C.; Guilard R. Unusual formation of a stable 2D copper porphyrin network. Inorg. Chem. 2013, 52, 999–1008. 10.1021/ic302257g. [DOI] [PubMed] [Google Scholar]; d Beletskaya I.; Tyurin V. S.; Tsivadze A. Y.; Guilard R.; Stern C. Supramolecular chemistry of metalloporphyrins. Chem. Rev. 2009, 109, 1659–1713. 10.1021/cr800247a. [DOI] [PubMed] [Google Scholar]; e Zhang X.; Ge C.; Yin J.; Zhao Y.; He C. Interpenetrating 2D manganese(II) coordination polymer supported by 4,4′-bis(dimethoxyphosphorylmethyl)-biphenyl ligands. Chin. J. Chem. 2009, 27, 1195–1198. 10.1002/cjoc.200990201. [DOI] [Google Scholar]; f Marchetti F.; Pettinari C.; Pizzabiocca A.; Drozdov A. A.; Troyanov S. I.; Zhuravlev C. O.; Semenov S. N.; Belousov Y. A.; Timokhin I. G. Syntheses, structures, and spectroscopy of mono- and polynuclear lanthanide complexes containing 4-acyl-pyrazolones and diphosphineoxide. Inorg. Chim. Acta 2010, 363, 4038–4047. 10.1016/j.ica.2010.08.006. [DOI] [Google Scholar]; g Liu X.; Guo-Cong; Liu B.; Chen W.-T.; Huang J.-S. A novel 2-D honeycomb-like lithium coordination polymer containing 42-membered rings. Cryst. Growth Des. 2005, 5, 841–843. 10.1021/cg049570r. [DOI] [Google Scholar]; h Spichal Z.; Jancarik A.; Mazal C.; Pinkas J.; Pekarkova P.; Necas M. Lanthanide coordination polymers with bis(diphenylphosphoryl)bicyclo[1.1.1]pentane. Polyhedron 2013, 62, 83–88. and references therein 10.1016/j.poly.2013.06.026. [DOI] [Google Scholar]; i Pekarkova P.; Spichal Z.; Taborsky P.; Necas M. Luminescent properties of europium complexes with bis(diphenylphosphino)alkane dioxides. Luminescence 2011, 26, 650–655. 10.1002/bio.1291. [DOI] [PubMed] [Google Scholar]
- a Maxim C.; Matni A.; Geoffroy M.; Andruh M.; Hearns N. G. R.; Clérac R.; Avarvari N. C3 symmetric tris(phosphonate)-1,3,5-triazine ligand: Homopolymetallic complexes and its radical anion. New J. Chem. 2010, 34, 2319–2327. 10.1039/c0nj00204f. [DOI] [Google Scholar]; b Sues P. E.; Lough A. J.; Morris R. H. Synthesis, characterization, and activity of yttrium(III) nitrate complexes bearing tripodal phosphine oxide and mixed phosphine–phosphine oxide ligands. Inorg. Chem. 2012, 51, 9322–9332. 10.1021/ic3010147. [DOI] [PubMed] [Google Scholar]; c Kaempfe P.; Stock N. Synthesis and characterization of the new copper tetraphosphonate Cu2[(HO3PCH2)4C6H2] 2H2O. Z. Anorg. Allg. Chem. 2008, 634, 714–717. 10.1002/zaac.200700460. [DOI] [Google Scholar]
- a Rodriguez-Palacios R.; Reyes-Lezama M.; Márquez-Pallares L.; Lemus-Santana A. A.; Sánchez-Guadarrama O.; Hopfl H.; Zúñiga-Villarreal N. Formation of carbonyl rhenium cryptates with alkali metal cations: Coordination chemistry studies of [Ph2P(E)NP(E)Ph2]−, E = O, S, Se towards ReBr(CO)5. Polyhedron 2010, 29, 3103–3110. 10.1016/j.poly.2010.08.011. [DOI] [Google Scholar]; b Lemus-Santana A. A.; Reyes-Lezama M.; Zúñiga-Villarreal N.; Toscano R. A.; Espinosa-Pérez G. E. Spontaneous formation of an organometallic–inorganic dirhenium carbonyl cryptate encapsulating a sodium cation. Organometallics 2006, 25, 1857–1860. 10.1021/om050912f. [DOI] [Google Scholar]
- a Barder T. E.; Buchwald S. L. Rationale behind the resistance of dialkylbiaryl phosphines toward oxidation by molecular oxygen. J. Am. Chem. Soc. 2007, 129, 5096–5101. 10.1021/ja0683180. [DOI] [PubMed] [Google Scholar]; b Hilliard C. R.; Bhuvanesh N.; Gladysz J. A.; Blümel J. Synthesis, purification, and characterization of phosphine oxides and their hydrogen peroxide adducts. Dalton Trans. 2012, 41, 1742–1754. 10.1039/c1dt11863c. [DOI] [PubMed] [Google Scholar]
- a Calcagno P.; Kariuki B. M.; Kitchin S. J.; Robinson J. M. A.; Philp D.; Harris K. D. M. Understanding the structural properties of a homologous series of bis-diphenylphosphine oxides. Chem.—Eur. J. 2000, 6, 2338–2349. . [DOI] [PubMed] [Google Scholar]; b Tan Y.-C.; Gan X.-M.; Stanchfield J. L.; Duesler E. N.; Paine R. T. Synthesis and coordination chemistry of 1,3-bis(diphenylphosphinomethyl)benzene P,P′-dioxide. Inorg. Chem. 2001, 40, 2910–2913. 10.1021/ic0012367. [DOI] [PubMed] [Google Scholar]
- Hettegger H.; Hosoya T.; Rosenau T. Chemistry of the redox series from hexahydroxybenzene to cyclohexanehexaone. Curr. Org. Synth. 2015, 13, 86–100. 10.2174/1570179412666150710182456. [DOI] [Google Scholar]
- a Hu X.; Dou W.; Xu C.; Tang X.; Zheng J.; Liu W. Lanthanide radii controlled one-dimensional polymer and dinuclear complexes and their fluorescent properties. Dalton Trans. 2011, 40, 3412–3418. 10.1039/c0dt01350a. [DOI] [PubMed] [Google Scholar]; b Zeller M.; Hunter A. D. Bis(dimethylphosphorylmethyl)durene. Acta Crystallogr., Sect. E: Struct. Rep. Online 2007, 63, o802–o803. 10.1107/S160053680700236X. [DOI] [Google Scholar]
- Wang L.; Tao X.-T.; Yang J.-X.; Yu W.-T.; Ren Y.; Xin Q.; Liu Z.; Jiang M.-H. Synthesis, structure and two-photon absorption properties of a new multi-branched compound, 1,2,4,5-tetrakis(4-pyridylvinyl)benzene. J. Solid State Chem. 2004, 177, 4293–4299. 10.1016/j.jssc.2004.08.036. [DOI] [Google Scholar]
- a Sheldrick G. M.Program for Crystal Structure Solution. SHELXS-97; University of Göttingen: Göttingen, Germany, 1997.; b Sheldrick G. M. A short history of SHELX. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, 64, 112–122. 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]; c Sheldrick G. M. Crystal structure refinement with SHELXL. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71, 3–8. 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Spek A. L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. 10.1107/S0021889802022112. [DOI] [Google Scholar]
- a Becke A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. 10.1063/1.464913. [DOI] [Google Scholar]; b Lee C.; Yang W.; Parr R. G. Development of the colle-salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B: Condens. Matter Mater. Phys 1988, 37, 785–789. 10.1103/PhysRevB.37.785. [DOI] [PubMed] [Google Scholar]; c Vosko S. H.; Wilk L.; Nusair M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. 10.1139/p80-159. [DOI] [Google Scholar]
- a McLean A. D.; Chandler G. S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11–18. J. Chem. Phys. 1980, 72, 5639–5648. 10.1063/1.438980. [DOI] [Google Scholar]; b Krishnan R.; Binkley J. S.; Seeger R.; Pople J. A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. 10.1063/1.438955. [DOI] [Google Scholar]
- a Stratmann R. E.; Scuseria G. E.; Frisch M. J. An efficient implementation of time-dependent density-functional theory for the calculation of excitation energies of large molecules. J. Chem. Phys. 1998, 109, 8218–8224. 10.1063/1.477483. [DOI] [Google Scholar]; b Bauernschmitt R.; Ahlrichs R. Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. Chem. Phys. Lett. 1996, 256, 454–464. 10.1016/0009-2614(96)00440-X. [DOI] [Google Scholar]; c Casida M. E.; Jamorski C.; Casida K. C.; Salahub D. R. Molecular excitation energies to high-lying bound states from time-dependent density-functional response theory: Characterization and correction of the time-dependent local density approximation ionization threshold. J. Chem. Phys. 1998, 108, 4439–4449. 10.1063/1.475855. [DOI] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.; Nakatsuji H.; Caricato M.; Li X.; Hratchian H. P.; Izmaylov A. F.; Bloino J.; Zheng G.; Sonnenberg J. L.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Montgomery J. A. Jr.; Peralta J. E.; Ogliaro F.; Bearpark M.; Heyd J. J.; Brothers E.; Kudin K. N.; Staroverov V. N.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Rega N.; Millam J. M.; Klene M.; Knox J. E.; Cross J. B.; Bakken V.; Adamo C.; Jaramillo J.; Gomperts R.; Stratmann R. E.; Yazyev O.; Austin A. J.; Cammi R.; Pomelli C.; Ochterski J. W.; Martin R. L.; Morokuma K.; Zakrzewski V. G.; Voth G. A.; Salvador P.; Dannenberg J. J.; Dapprich S.; Daniels A. D.; Farkas Ö.; Foresman J. B.; Ortiz J. V.; Cioslowski J.; Fox D. J.. Gaussian 09; Gaussian, Inc.: Wallingford, CT, 2009.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






