Abstract
Coat proteins play multiple roles in the life cycle of a membrane-bound transport intermediate, functioning in lipid bilayer remodeling, cargo selection and targeting to an acceptor compartment. The Coat Protein complex II (COPII) coat is known to act in each of these capacities, but recent work highlights the necessity for numerous accessory factors at all stages of transport carrier existence. Here, we review recent findings that highlight the roles of COPII and its regulators in the biogenesis of tubular COPII-coated carriers in mammalian cells that enable cargo transport between the endoplasmic reticulum and ER-Golgi intermediate compartments, the first step in a series of trafficking events that ultimately allows for the distribution of biosynthetic secretory cargoes throughout the entire endomembrane system.
Keywords: COPII coat, early secretory pathway, endoplasmic reticulum, ER exit site, posttranslational modification, Sec16A, Tango1/cTAGE5, TFG
1 ∣. INTRODUCTION
The first membrane fission and fusion steps in the mammalian secretory pathway are mediated by Coat Protein complex II (COPII), which promotes the formation of transport carriers at unique, ribosome-free subdomains of the endoplasmic reticulum (ER) to facilitate the delivery of cargoes, including an array of membrane proteins and lipids, to juxtaposed ER-Golgi intermediate compartment (ERGIC).1-5 A combination of in vitro biochemical assays, proteomic studies, single particle electron microscopy-based techniques and X-ray crystallography approaches have been used to provide mechanistic insights into COPII coat assembly and disassembly. Collectively, this work has led to a much improved understanding of how COPII, together with a variety of regulatory factors, support transport carrier fission and fusion at the ER/ERGIC interface.
The initial description of COPII was based on studies using yeast as a model system and highlighted the assembly of a 10-nm-thick electron dense coat on ~60 to 65 nm vesicles that were able to fuse with Golgi membranes following GTP hydrolysis-mediated disassembly.6 In contrast, contemporaneous studies demonstrated that ER to Golgi transport in mammalian cells requires the sequential actions of COPII and another coat complex (COPI), with COPII acting specifically at the ER to enable cargo transport to pre-Golgi intermediates composed of vesicular-tubular clusters, now commonly referred to as ERGIC membranes.7,8 Thus, unlike yeast, the activity of COPII in the mammalian secretory pathway is largely restricted to a ~300 to 500 nm space between the ER and ERGIC and is unlikely to play a direct role in ERGIC to Golgi trafficking.1
2 ∣. THE COPII COAT
The COPII coat complex consists of two proteinaceous layers that are capable of deforming highly curved ER membranes to generate cargoladen transport intermediates. The inner layer is composed of a flexible Sar1-Sec23-Sec24 lattice, while the outer cage is made up of Sec13-Sec31 heterotetramers.9-12 Assembly of COPII complexes is initiated by activation of Sar1, a small Ras-like GTPase, which is mediated by the integral membrane guanine nucleotide exchange factor (GEF) Sec12/PREB.13-15 In mammals, two Sar1 isoforms are expressed in most tissues, and although in vitro studies have defined only modest differences in their activities, Sar1A and Sar1B (~90% sequence identity) have been suggested to play distinct roles in vivo, particularly in the ER export of chylomicrons within enterocytes of the small intestine.16-20 Patients lacking Sar1B (or expressing mutant forms predicted to impair nucleotide or membrane binding) exhibit fat malabsorption disorders due to impaired chylomicron (often >200 μm in size) secretion into the bloodstream, even though Sar1A is present and expressed normally.16,21 These data suggest that Sar1B is uniquely suited to drive the formation of large COPII-coated transport carriers (some more than 1 μm in diameter) in vivo. In contrast, most cultured cells grown in vitro can tolerate individual inhibition of either Sar1A or Sar1B, while their co-depletion leads to potent cargo trafficking defects.22,23
The crystal structure of mammalian Sar1A is characteristic of other Ras superfamily GTPases with two mobile switch regions and a core of six central beta-strands positioned between three flanking alpha helices24,25 (Figure 1). The guanine-nucleotide-binding pocket contains a highly conserved GxxxxGKT Walker A motif (P-loop; residues 32-39) and serves as a docking site for Sec12 to catalyze GTP loading.15,27 Mutation of threonine 39 to asparagine generates a dominant negative isoform, which is constitutively bound to GDP and blocks COPII carrier formation.7,28,29 Conversely, mutation of histidine 79 to glycine within switch II dramatically reduces GTP hydrolysis and enables constitutive association of Sar1 with Sec23-Sec24 heterodimers, its major effector.7,28 Importantly, Sar1H79G strongly potentiates membrane tubulation, but potently blocks COPII-mediated transport in cells, suggesting a key role for GTP hydrolysis during carrier scission.18,30-36
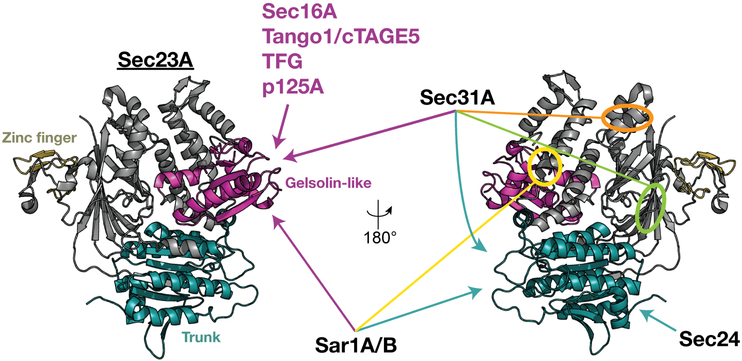
FIGURE 1.
Sec23 acts as an interaction hub for control over COPII carrier assembly and disassembly. An illustration highlighting the various interaction partners that have been identified for Sec23A/B. Numerous regulators are believed to compete for binding with the Sec23 gelsolin-like domain, enabling a stepwise assembly and disassembly pathway for COPII carrier biogenesis. The structure of Sec23A depicted is adapted from PDB 5VNO26
Unlike other members of the Ras superfamily that use myristoyl or prenyl modifications for membrane association, Sar1 isoforms employ a unique amino-terminal amphipathic helix to sample membranes.33 However, only when activated by Sec12 is Sar1GTP able to stably penetrate the bilayer to initiate membrane remodeling and COPII coat assembly.30,37 Importantly, active Sar1 exhibits curvature sensitivity, showing enhanced affinity for bent membranes.30 Thus, as the membrane becomes more highly deformed, Sar1 is able to bind more tightly, thereby promoting the formation of a tubular transport intermediate. Consistent with this idea, electron and fluorescence microscopy-based in vitro studies have demonstrated that Sar1 is capable of forming organized lattices on artificial liposomes, converting them into tubules and even detached vesicles at high concentrations.31 The intrinsic GTPase activity of Sar1 is also stimulated by the presence of highly curved membranes.30 Ultimately, Sar1 likely concentrates in regions of elevated curvature, such as the necks of budding transport carriers, where it can facilitate rapid fission upon GTP hydrolysis.38 Although speculative, Sar1B may uniquely drive the generation of large, unconventional COPII carriers by exhibiting slower GTPase activity in cells as compared to Sar1A and providing additional time to enclose sizable substrates including chylomicrons within a carrier ahead of neck scission.
Sculpting of COPII carriers is further governed by Sar1GTP- mediated recruitment of a Sec23-Sec24 lattice composed of repeating ~15 nm bowtie-shaped units that form a helical array.9,10,37,39 Crystallographic and electron microscopy-based analysis of the membrane proximal region of yeast Sec23-Sec24 shows it to be concave, with a positively charged surface that is conserved in their mammalian paralogs and may stabilize curvature of the underlying phospholipid bilayer.10 Even though the proteins exhibit limited sequence similarity, the overall folds of Sec23 and Sec24 are closely related, and they are linked via intimate hydrogen bonding and van der Waals interactions, which join their trunk domains to generate an intersubunit beta-sheet (Figure 1). The trunk domain of Sec23 further contacts the switch II region of Sar1, but the neighboring helical domain is most critical for recognizing switch I and the active GTP-bound state.10 Additionally, the gelsolin-like domain of Sec23 plays a key role in promoting GTP hydrolysis on Sar1 by providing a catalytic arginine finger in trans to the active site, thereby serving as a guanine nucleotide activating protein (GAP).10,40 Thus, somewhat paradoxically, completion of the inner layer of the COPII coat drives GTP hydrolysis on Sar1, promoting disassembly from the membrane surface. However, the integrity of Sar1GTP-Sec23-Sec24 complexes must be sufficiently long-lived to enable completion of COPII carrier biogenesis. Based on in vitro studies, the lifetime of such complexes is ~30 seconds, suggesting that COPII carriers at ER subdomains form at a similar rate as compared to other vesicle biogenesis events, including clathrin-mediated budding from the cell surface.41-43
Like Sar1, mammals express two isoforms of Sec23 that are ~85% identical to one another. Although mutations in Sec23B have been implicated in anemia and various forms of cancer, and the Sec23-AF382L mutation causes craniolenticulosutural dysplasia, the paralogs appear to be largely redundant for function and only vary by distinct tissue expression profiles.44-47 Both isoforms stably interact with any of the four Sec24 isoforms (A-D), which serve as adaptors for numerous integral membrane secretory proteins, including receptors for lumenal cargoes that must be guided out of the ER.48 Degenerate motifs enable a wide range of clients to be sorted into COPII carriers, with structural studies highlighting binding pockets on Sec24A and Sec24B that can associate with LxxLE and DxE sorting signals, while Sec24C and Sec24D harbor surface grooves for the IxM packaging motif.49-52 Additionally, other cargo binding domains may exist on each Sec24 paralog, further expanding their ability to capture a multitude of cargoes.50,53 Although Sec24A and Sec24B exhibit significant overlap in cargo selectivity, mutational analysis in mice has uncovered a potentially unique role for Sec24A in PCSK9 secretion, a key regulator of plasma cholesterol levels, which is mediated by the PCSK9 receptor Surf4.54,55 However, this selectively may only be the result of differences in the expression profiles of Sec24A and Sec24B. Consistent with this idea, Sec24A exhibits 5- to 10-fold higher expression as compared to Sec24B in the mouse liver.55 Similarly, conditional deletion of Sec24C in murine neural progenitors results in extensive neuronal cell death, but the phenotype can be rescued by ectopic expression of Sec24D.56,57
Inner COPII coat assembly enables Sec13-Sec31 recruitment and outer cage formation in cells. Specifically, based on the analysis of yeast protein fragments, the extended proline-rich domain of Sec31 binds directly to a surface on Sec23 that includes the gelsolin, trunk and β-barrel domains, and extends over switch II of GTP-bound Sar158 (Figure 1). These data explain the ordered process by which Sar1GTP-Sec23-Sec24 complexes must first associate with membranes, followed by outer cage assembly. However, the outer cage also drives organization of the inner coat to form a coherent lattice, with homotypic Sec23 interactions largely governing lateral associations.59 Based on indirect measurements of GTP hydrolysis on Sar1, which leverage changes in tryptophan fluorescence believed to accompany the transition of Sar1 from the GTP- to the GDP-bound state, binding of Sec31 has been suggested to accelerate Sar1 GTPase activity.41 The crystal structure of the yeast Sar1-Sec23-Sec31 ternary complex suggests a mechanism by which this occurs, involving optimization of the geometry of the histidine within switch II of Sar1 and a nucleophilic water molecule that bridges the imidazole side chain and the gamma phosphate of a GTP analog.58 However, in the absence of an analogous structure for a metazoan COPII complex, the extent to which Sec31 activates GTP hydrolysis in animal cells remains ill-defined.
Sec31 isoforms are comprised of multiple domains, including a series of seven amino-terminal WD40 repeats that form a 7-bladed beta-propeller, another WD40 repeat (beta-blade) that co-assembles with Sec13, a series of alpha-helices that create an alpha-solenoid, an ancestral coatomer element (ACE1) domain, a proline-rich domain that contacts Sar1GTP-Sec23-Sec24, and another carboxyl-terminal alpha-helical domain.9,12 The much smaller Sec13 also contains a series of WD40 repeats (six) and forms a 7-bladed beta-propeller after incorporating the beta-blade of Sec31. Sec13-Sec31 dimers assemble into heterotetramers via a domain swap between two ACE1 domains to create an overall rod-like structure that is approximately 28 nm in length.12 Further co-assembly of four Sec31 beta-propellers at the termini of four distinct rods form one of many vertices within a cage.11,60 Additionally, the angle between the terminal beta-propeller domains and alpha-helical central rod is flexible, creating a hinge that allows the cage to vary in dimension, depending on the size of the transport carrier it must encase.12,60,61 A second potential hinge was identified more recently, which allows the beta-propeller domains of Sec13 and Sec31 to move relative to one another.62 Based on cryoEM studies, the underlying inner coat is positioned such that Sec23 protomers appear below each vertex and in the middle of triangular faces of the outer cage, and Sec24 subunits are located in the middle of square faces of cuboctahedrons or pentagons of icosidodecahedrons, providing sufficient space to accommodate the cytosolic domains of integral membrane cargoes.59 Direct association between the inner and outer coat was recently confirmed using yeast proteins, although the cryoEM density failed to overlap completely with the crystal structure of the Sar1GTP-Sec23-Sec31 interface, potentially due to the inherent flexibility of proline-rich domains.37 Unlike components of the inner coat, only a single Sec13 locus is found in mammals. However, two Sec31 paralogs have been identified, which are only ~47% identical. Based on subcellular localization studies, Sec31A and Sec31B distributions do not overlap precisely, raising the question of whether the two subunits are able to co-assemble into a single cage.63 Regardless, the diversity of COPII components expressed in mammals enables numerous types of carriers to be generated that can vary not only in size and subunit composition, but also cargo content.
3 ∣. REGULATION OF COPII COAT ASSEMBLY
Although COPII-mediated carrier formation has been reconstituted using minimal or chemically defined systems in vitro,32,39 several regulators of this process have been identified in mammalian cells that either facilitate localized COPII subunit assembly at specialized ER subdomains or promote coat disassembly, which is necessary after carrier scission to enable fusion with neighboring ERGIC membranes. One major driver of ER subdomain selection is the large scaffolding protein Sec16A, which interacts directly with several components of the COPII machinery.64-66 Its depletion reduces the number of COPII budding sites on the ER and attenuates the rate at which secretory cargoes leave the ER. At a biochemical level, only half of Sec16A has been well characterized, with little known about its first 1000 amino acids, beyond their dispensability for localization in overexpression studies.67 The remaining protein is comprised of three domains with varying roles in regulating COPII dynamics. The carboxyl-terminal domain (CTD) exhibits a conserved function for binding to Sec12, which helps to enrich the integral membrane GEF for Sar1 at ER subdomains to make them competent for COPII carrier formation.68 The CTD additionally harbors a proline-rich region, which likely facilitates Sec23 recruitment via its gelsolin-like domain (Figure 1). This interaction may also obstruct outer COPII coat assembly, as the Sec31 proline-rich domain must also associate with the same surface on Sec23 to direct overall COPII coat architecture.58,59 This potential role for Sec16A in delaying Sec31 action may also extend to nonmetazoan systems. Specifically, the carboxyl-terminal half of yeast Sec16 has been suggested to impede the stimulatory effect of Sec31 on Sar1 GTP hydrolysis.69 Surprisingly, the effect appears to be mediated by an association between Sec16 and the COPII cargo adaptor Sec24. This interaction could offer a unique sensing mechanism to delay outer coat assembly until transport carriers are first loaded with cargo. A mutation in Sec24 that disrupts its association with Sec16 leads to the formation of smaller transport carriers, suggesting that accelerated GTP hydrolysis results in premature scission.69 By extension, slowing GTP hydrolysis should promote the formation of larger COPII carriers. Further studies are necessary to demonstrate whether mammalian Sec16A directly or indirectly regulates the Sar1 GTPase cycle.
Upstream of the CTD is the central conserved domain (CCD) of Sec16A, which is found in all Sec16 paralogs across evolution. The CCD is comprised of a beta-blade followed by a helical ACE1 domain, highly similar to the organization of Sec31 isoforms.70 Analogously, Sec16 binds to Sec13 as a heterotetramer, in which Sec13 associates with the beta-blade of Sec16, and the ACE1 domains dimerize to form an extended ~16.5 nm rod.70 Unlike Sec31, however, Sec16 lacks an amino-terminal beta-propeller, preventing its assembly into cage-like structures. Thus, Sec16 recruits Sec13 to ER subdomains, but again may act to impede outer coat assembly by sequestering Sec13 until the appropriate time in the COPII coat assembly pathway.
Upstream of the Sec16A CCD is an ER localization determinant (ELD), which was recently shown to bind to the proline-rich domains of two members of the Tango1/cTAGE5 family (Tango1L and Tango1S) that govern the ER export of several collagen isoforms in large COPII transport carriers.71-73 Surprisingly, while both Tango1 isoforms are required for efficient collagen secretion, only Tango1L harbors a SH3-like domain that can engage HSP47, a collagen-specific molecular chaperone, suggesting that Tango1 family members may possess additional roles in regulating COPII-mediated transport beyond acting merely as a cargo receptor.74 Consistent with this idea, loss of both Tango1L and Tango1S was shown to cause Sec16A to be partially redistributed from ER subdomains and reduce overall COPII coat assembly.71 Conversely, depletion of Sec16A causes Tango1 isoforms to disperse throughout the ER network. Thus, Tango1 and Sec16A play complementary roles in directing the distribution of COPII budding sites on the ER, although it continues to remain unclear how these subdomains are initially formed or maintained. Tango1 additionally plays an important role in localizing a related family member cTAGE5, which also functions in ER collagen export without directly associating with cargo.75 The interaction between Tango1 and cTAGE5 is mediated by coiled coil domains found in the cytosolic sides of each protein. A second flanking coiled coil domain in cTAGE5 also contacts Sec12, facilitating its specific accumulation at COPII budding sites on the ER, but without affecting GEF activity.76,77 In contrast, neither of the Tango1 coiled coil domains exhibit an ability to associate with Sec12. Structural analysis of these coiled coil motifs should provide mechanistic insight into the specificity of their associations.
All members of the Tango1/cTAGE5 family also bind to the inner COPII coat protein Sec23 via its gelsolin-like domain72 (Figure 1). Based on X-ray crystallography studies, the interaction is mediated by tripeptide PPP motifs within the proline-rich domain of Tango1, of which there are seven that associate with a series of surface aromatic residues on Sec23.78 Thus, individual Tango1/cTAGE5 proteins can recruit multiple Sec23 protomers, likely in complex with Sec24 subunits, as a transport carrier begins to assemble. It remains unclear whether these associations occur in synergy with Sec16A-mediated Sec23 binding or in competition, as they likely share a common interface. However, given the interdependent roles for Sec16A and Tango1/cTAGE5 in establishing subdomains on the ER capable of COPII budding, it appears more probably that they function together to drive inner coat assembly. In contrast, regions on the ER devoid of these factors are unlikely to support COPII carrier biogenesis, even though Sar1 may continually sample the membrane via its flexible amphipathic helix.30 Taken together, current evidence suggests a model in which Sec16A and members of the Tango1/cTAGE5 family concentrate Sec12 to enable local GTP loading onto Sar1, which deforms and tubulates ER microdomains, while simultaneously recruiting Sec23-Sec24 complexes to further transform membrane tubules into cargo-laden transport carriers. Based on several electron microscopy-based studies, the ER/ERGIC interface in mammalian cells is largely populated by clustered, dumbbell-shaped transport intermediates, as well as more spherical, detached vesicles.79-81 The presence of these dumbbell-shaped structures is most consistent with the initial formation of COPII-coated tubules, with GTPase activity on Sar1 generating intermittent constrictions at areas of elevated concentration. Ultimately, a fraction of these more highly bent regions undergo scission, perhaps stochastically due to a locally elevated GTP hydrolysis, enabling cargoes to be separated from the ER and begin their movement in an anterograde manner. In the case of highly elongated cargoes, such as fibrillar collagens that can reach ~300 nm in length and ~1.5 nm in diameter,82 steric hindrance may be sufficient to prevent scission reactions that would otherwise cause cargoes to become inappropriately severed.
Alternatively, members of the Tango1/cTAGE5 family may play more active roles in driving the formation of large COPII-coated transport intermediates. Tango1L possesses a Tether of ERGIC at ER (TEER) domain, a coiled coil motif that is capable of binding to ERGIC membranes in trans.83 Although the mechanism by which this occurs remains unclear, direct recruitment of ERGIC to sites of COPII carrier formation on the ER could provide an additional reservoir of membrane to generate transport intermediates. Given the enormous surface area of the ER, it is questionable whether membrane ever becomes limiting during collagen export, but local deposition from an alternative source could augment the kinetics of carrier biogenesis. Based on super resolution imaging studies, Tango1L has been suggested to assemble into ring structures that encircle COPII carriers at their base on ER membranes, potentially providing a scaffold onto which ERGIC membranes could be recruited.84,85 However, other non-diffraction-limited approaches have failed to resolve such rings.86,87 Instead, these structures may only form in response to non-physiological accumulation of collagen within the ER, which was necessary to initially observe Tango1 ring assembly.84 In contrast, more recent studies suggest that Tango1, together with Hsp47, cTAGE5 and Sec12, actually enter the large COPII carriers that enable collagen export from the ER.87 These factors are subsequently retrieved from ERGIC and Golgi membranes via the action of retrograde COPI transport, while collagen continues on its journey through the secretory pathway toward the cell surface. Importantly, by trafficking on large COPII carriers, Tango1/cTAGE5 family members would promote continual Sec12 GEF activity and maintain Sar1 in a GTP bound state. These actions may limit membrane constriction associated with GTP hydrolysis on Sar1 along the length of transport carriers, thereby ensuring fibrillar collagens can be properly packaged in large COPII carriers. Potentially in a similar manner, the fatty acid binding protein FABP5 was shown recently to bind Sec12 and enhance GTP loading onto Sar1, thereby promoting the formation of large COPII transport carriers capable of packaging collagens as well as large lipoproteins.88
Unlike conventional COPII-coated transport carriers that generally range in size from 50 to 200 nm and have been repeatedly resolved at the ER/ERGIC interface using electron microscopy-based approaches, larger COPII carriers have been more challenging to visualize in a native setting. This is at least in part due to the cell lines that have been chosen for analysis, most of which do not produce high levels of fibrillar collagens or other large cargoes. Although recent studies highlight the existence of large carriers that contain collagen and stain positively for COPII subunits, in most cases, their destinations are unclear.89 Instead, these carriers may participate in a noncanonical form of autophagy, which targets misfolded procollagens to the lysosome for degradation.90 In the absence of a large number of transport intermediates to analyze, some have even argued that collagens in the early secretory pathway may not even use a transport intermediate.91 Instead, direct connections between ER and ERGIC membranes could enable collagens to be exported from the ER in the absence of a vesicular carrier. Formation of these tubular connections would still require the COPII machinery to drive membrane remodeling and members of the Tango1/cTAGE5 family to recruit collagen and ERGIC membranes and maintain the activity of Sar1, but mechanisms to maintain the unique identities of the ER and ERGIC in such a model still require elucidation.92 Additionally, examination of native collagen trafficking by leveraging CRISPR/Cas9 technology to tag their endogenous isoforms would dramatically improve on current approaches that use overexpression, which likely activate ER stress responses and the unfolded protein response (UPR) and may lead to artifacts.93
Assembly of the outer COPII cage, mediated largely by contacts between Sec31 and Sec23, likely displaces several of the regulatory factors that initially stimulate formation of the inner adaptor layer of the COPII coat. Based on direct competition assays, the proline-rich domain of Sec31 exhibits higher affinity for the Sec23 gelsolin-like domain as compared to members of the Tango1/cTAGE5 family.78 By displacing these factors, Sec31 acts to downregulate further GTP loading onto Sar1 and may shift the equilibrium toward Sar1 GTP hydrolysis, which is predicted to promote detachment of COPII carriers from the ER. Recruitment of Sec13-Sec31 heterotetramers to the ER has been suggested to be regulated by several factors. Based on immunoprecipitation studies, soluble Sec13-Sec31 complexes interact with the large phospholipase A1-like factor p125A.94-97 The carboxyl-terminal region of p125A binds to acidic phospholipids, facilitating its targeting to COPII budding sites that are enriched for phos-phatidic acid and phosphatidylinositol 4-phosphate, while its central domain (residues ~260-600) is sufficient for interaction with Sec31.94,98 Although a mechanistic understanding of this association is lacking, biochemical studies further suggest that p125A binds to Sec23 via its adjacent, amino-terminal proline-rich domain, potentially serving as a bridge to bring Sec31 into close proximity of the inner coat98 (Figure 1). Overexpression studies also suggest that p125A displaces Sec16A from COPII complexes, further promoting outer COPII cage assembly as carriers emerge from the ER and leave Sec16A behind.94 The Sec31 binding partner ALG-2 (also known as PDCD6) has also been proposed to facilitate an interaction between the outer COPII cage and the inner coat. Specifically, ALG-2 associates with the proline-rich domain of Sec31 and alters its conformation to increase its affinity for Sec23.99 Again, the structural basis for this phenomenon has yet to be defined, but in vitro studies suggest that the presence of ALG-2 slows the rate at which COPII carriers bud from the ER. Thus, ALG-2 appears to facilitate interaction between the two layers of the COPII coat, while negatively regulating carrier scission, potentially via negative regulation of GTP hydrolysis on Sar1 until COPII assembly can be completed. Notably, ALG-2 harbors a calcium binding EF-hand, and the localization of ALG-2 to COPII budding sites requires the presence of calcium.100 Additionally, the calcium binding protein Annexin A11 further promotes stable association of Sec31 with COPII budding sites, using ALG-2 as an adaptor.101 Together, these data suggest that cytosolic calcium levels regulate COPII carrier formation, linking early secretory pathway function to various signaling cascades that are dependent on calcium influx, including the ER stress response and the UPR.102 Nonetheless, Sec23 remains the most prominent recruiting factor for the outer COPII components, best illustrated by the effect of a relatively subtle missense mutation (F382L) in Sec23A that underlies Cranio-lenticulo-sutural dysplasia, which results in impaired Sec13-Sec31 assembly at ER subdomains.17,47 Strikingly, patients harboring this mutation accumulate coated tubules that are readily observed in skin fibroblasts, further indicating that outer coat assembly plays a major role in regulating carrier scission, as opposed to initial carrier formation.47
4 ∣. REGULATION OF COPII COAT DISASSEMBLY AT THE ER/ERGIC INTERFACE
To enable efficient secretory cargo transport from the ER to the ERGIC, COPII coat assembly must be balanced by rapid disassembly following transport carrier scission. Based on the distribution and dynamics of COPII complexes in cells, the lifetime of the coat is brief and largely relegated to small ~500 nm areas scattered over the entire ER network, which extends throughout cells, but is most highly concentrated in the peri-nuclear region. Super resolution imaging has demonstrated that essentially all ER subdomains that produce COPII carriers are juxtaposed to ERGIC membranes, even in areas densely packed by Golgi cisternae, suggesting that COPII coat disassembly is coupled to fusion with ERGIC, as opposed to direct fusion with the Golgi.79 Many have questioned the origin of ERGIC membranes, with a consensus view suggesting that homotypic fusion of COPII carriers initially produces tubular-vesicular clusters that become enriched with ERGIC markers, and subsequent homotypic and/or heterotypic fusion events maintain the identity of the organelle.8,103 A major requirement for such an organization is a mechanism to locally tether COPII carriers for a sufficient period of time to allow coat complexes to disassemble and expose SNAREs necessary to drive fusion events. Recent work suggests that COPII carrier tethering is mediated by small ring-like homo-oligomers composed of Trk-fused gene (TFG) subunits.79,86,104
TFG was originally described as a fusion partner for the TrkA receptor tyrosine kinase, generated following a chromosomal translocation and functioning as a potent driver of cell transformation.105 Subsequently, several additional oncogenic TFG fusion proteins have been identified, each carrying the amino-terminal portion of TFG, including its PB1 domain and adjacent coiled coil motif.106 Structural analysis using single particle electron microscopy has demonstrated that this region of TFG is sufficient to form ~11 nm octameric rings, with a central ~4 nm pore.79 These data highlight the ability of the TFG amino-terminus to multimerize fusion partners, many of which are kinase domains that require co-assembly to elicit activity. However, oligomerization alone was shown to be insufficient to enable the TrkA kinase domain to transform cells. Instead, localization to the ER/ERGIC interface plays a critical role in oncogenesis, although the precise downstream targets that cause cells to become transformed remain undefined.104
Based on the analysis by circular dichroism, the carboxyl-terminal portion of TFG (residues 125-400) is largely unstructured, with a propensity to self-associate, thereby linking TFG octamers into a higher order meshwork.79 These characteristics are highly reminiscent of synapsin, another disordered protein, which was shown recently to undergo phase separation to generate liquid droplets.107 However, unlike other liquid droplet-like, membraneless organelles such as P granules or PML nuclear bodies, phase separated synapsin is able to capture small liposomes in vitro and play a key role in clustering synaptic vesicles in neuronal synapses. Analogously, phase separated TFG may tether COPII-coated transport carriers at the ER/ERGIC interface.86 Consistent with this idea, overexpression of TFG leads to the formation of large spherical structures in cells that sequester COPII carriers.79 Moreover, depletion of TFG leads to the accumulation of COPII carriers throughout the cytoplasm, no longer restricted to the ER/ERGIC interface.79,86 Biochemical studies have demonstrated that the last ~20 amino acids of TFG binds directly to Sec23 with high affinity, and its disordered domain is enriched with proline residues (>16%) that likely facilitate binding to the Sec23 gelsolin-like domain86 (Figure 1). These findings provide a potential molecular basis for hypothetical TFG liquid droplets to tether COPII-coated transport intermediates at the ER/ERGIC interface with specificity, although additional studies are required to validate this idea. Strikingly, in the absence of COPII carrier formation, TFG fails to accumulate near ER subdomains, suggesting that the presence of COPII-coated transport carriers is a prerequisite for concentrated assembly of TFG in cells.86 Beyond binding to Sec23 and tethering COPII carriers, TFG has also been shown to interact with ALG-2 in a calcium-dependent manner.108 in vitro studies suggest that this association may enhance co-assembly of TFG octamers into larger complexes. However, depletion of ALG-2 has minimal impact on the levels of TFG at the ER/ERGIC interface.108 Further work is necessary to define the mechanistic basis for TFG assembly in the early secretory pathway and how this process is regulated.
Surprisingly, as opposed to binding COPII carriers via the outer cage, TFG directly associates with the inner adaptor layer and utilizes the same interface on Sec23 as Sec31 uses for binding (Figure 1). Based on biochemical competition assays, TFG exhibits an ability to disrupt the association between the two COPII subunits, suggesting that it promotes outer coat disassembly at the ER/ERGIC interface, while simultaneously restricting the distribution of partially coated carriers.86 Exposure of the inner coat may also facilitate its association with the downstream TRAPP tethering complex, which functions on ERGIC membranes to promote COPII carrier fusion and cargo delivery. In particular, the TRAPPC3 subunit of the TRAPP complex, which exhibits a flattened alpha-beta fold, has been proposed to bind to Sec23.109,110 The interaction does not affect GAP activity, but little else is known regarding the mechanism of association or whether it would affect the ability of inner COPII-coated carriers to remain partitioned with putative TFG liquid droplets or be released for ERGIC fusion. Alternatively, dissociation of the inner COPII coat may be facilitated by GTP hydrolysis on Sar1 that remains associated with the transport carrier after scission from the ER.41 Although some biochemical data suggest that Sar1 is released from transport carriers rapidly after the budding process, localization studies have yet to define its distribution at the ER/ERGIC interface.6 Thus, it remains very possible that GTP-bound Sar1 persists on COPII carriers following scission, and local tethering mediated by TFG provides sufficient time for GTP hydrolysis to enable inner coat disassembly.
Although deletion of TFG results in early embryo lethality, point mutations have been identified in several patients suffering from various forms of neurological disease.86,111,112 Analysis of these mutant alleles has underscored the importance of the TFG ring-like architecture in regulating COPII-mediated cargo transport. Specifically, mutations in the PB1 domain or the coiled coil domain underlie early onset forms of complicated hereditary spastic paraplegia (HSP) and result in defective ring assembly without impairing the ability of TFG to homo-oligomerize.111 Although mutations in other factors that form liquid droplets in neurons have been suggested to promote their aggregation leading to neuronal toxicity, localization studies indicate that amino-terminal mutations in TFG cause it to become more diffusely distributed.111,113,114 These data suggest an alternative mechanism for neurodegeneration in HSP patients harboring TFG mutations. Consistent with this idea, stem-cell-derived neurons harboring the TFG (p.R106C) mutation exhibit a defect in axon pathfinding, likely caused by impaired trafficking of high molecular weight adhesion molecules that normally mediate axon bundling and their maintenance.111 Other mutations that affect the disordered domain of TFG have been implicated in late onset sensory neuropathies.112 However, the impact of these mutations to TFG structure and function remain to be defined.
5 ∣. REGULATION OF COPII CARRIER FORMATION AND TRANSPORT MEDIATED BY POSTTRANSLATIONAL MODIFICATIONS
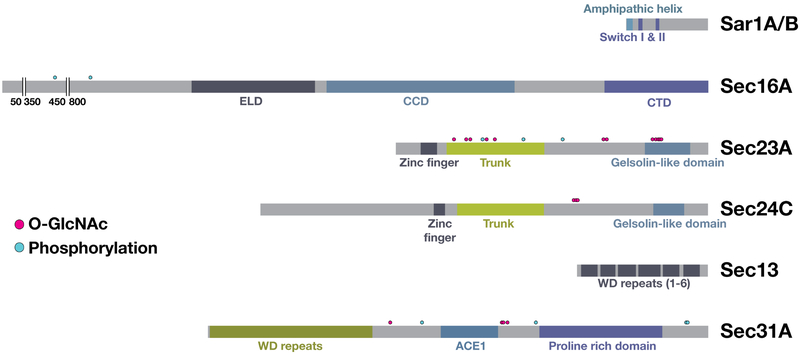
Posttranslational modifications (PTMs) play important regulatory roles in cell signaling, protein-protein interactions and membrane remodeling processes. Although numerous PTMs have been mapped on components of the early secretory pathway, we still have only an elementary understanding of their contributions to membrane trafficking. β-linked N-acetylglucosamine (O-GlcNAc) modifications are found frequently at serine and threonine residues on both nuclear and cytoplasmic proteins.115,116 These modifications are highly reversible and cycle on and off rapidly throughout the lifetime of the protein. In animals, O-GlcNAc transferase (OGT) is responsible for the addition of this modification, while O-GlcNAcase (OGA) mediates its removal.117 Based on mass spectrometry-based analysis, O-GlcNAc modified sites have been identified on Sec23A, Sec24C, Sec31A and TFG118 (Figure 2). In general, O-GlcNAcylation of COPII subunits is high during interphase and low during mitosis, when early secretory pathway trafficking is suspended.119 The roles of O-GlcNAc modification vary, with those found on Sec31A clustered within its hinge region, suggesting a potential function in regulating the flexibility of the outer COPII cage and thereby impacting cargo selection, while O-GlcNAc modified sites on Sec23A and Sec24C appear to regulate protein-protein interactions.118 Although the specific binding partners of the inner COPII coat proteins that require OGT activity have yet to be identified, mutation of one modified site in Sec23A (S184A) within its trunk domain results in defective collagen export from the ER, suggesting that the regulators may play a role in controlling Sar1 GTPase activity.118 TFG also undergoes O-GlcNAc modification, which may promote co-assembly of octamers to enhance the kinetics of liquid droplet formation. Alternatively, modification of TFG could enable the recruitment of a binding partner, similar to that brought in by Sec23A or Sec24C, to further support large carrier transport. Consistent with this idea, depletion studies suggest that TFG plays a particularly critical role in the anterograde movement of large COPII cargoes.111,120
FIGURE 2.
Components of the COPII transport system are subject to posttranslational modifications that regulate their functions. An illustration showing the distribution of posttranslationally modified residues on COPII components and several regulators of COPII carrier transport
PTM by the E3 ubiquitin ligase Cullin-3 (CUL3) has also been proposed to facilitate the formation of unconventionally large COPII carriers. Specifically, the addition of mono-ubiquitin to Sec31A has been suggested to augment the size of the COPII coat, although a mechanistic basis for this effect remains unresolved.121,122 A major hurdle has been identifying a specific ubiquitin-modified site on Sec31A that mediates enlargement of the outer COPII cage, and mutational analysis has failed to specify any individual lysine residues in Sec31A that are required for collagen secretion from the ER. Several adaptors are necessary for Sec31A ubiquitinylation by CUL3, including KLHL12, which harbors a series of Kelch-like repeats that form a β-propeller tertiary structure, and the calcium binding factors ALG-2 and Pef1 that can associate to form heterodimers.121-123 Based on co-immunoprecipitation studies, all five proteins form a complex, with KLHL12 present as a dimer.122 Additionally, Pef1 must itself be ubiquitin-modified by CUL3 to enable Sec31A ubiquitinylation, ensuring that only active CUL3 complexes are recruited to budding sites to drive large COPII carrier formation.122 The requirement for Pef1 and ALG-2 further suggests that the formation of large COPII carriers may rely on transient increases in cytosolic calcium. However, direct evidence to support this idea is lacking. Moreover, recent data now argue against a direct role for CUL3-KLHL12 in collagen secretion, implicating them instead in regulation of UPR signaling and collagen biosynthesis.124 An alternative explanation for the role of ubiquitin-modification in the early secretory pathway has also been suggested by studies showing that misfolded forms of procollagen are targeted to the lysosome via noncanonical autophagic transport carriers that originate at or near the ER. Instead of mediating large COPII carrier formation, it is possible that ubiquitin-modification of Sec31A facilitates recognition of sites harboring cargoes destined for lysosomal degradation, as opposed to secretion.90 With clear evidence accumulating that COPII functions in the generation of membranes necessary for autophagosome formation, the role of ubiquitin-modification in generating large secretory carriers may require substantial revision.
Beyond ubiquitin and O-GlcNAc modifications, COPII components are also subject to phosphorylation, although most sites have yet to be unambiguously mapped and only a selected few have been assigned a functional or regulatory role. Casein Kinase II (CKII) has been implicated in Sec31A phosphorylation, which may impede its association with the inner COPII coat125 (Figure 2). Specifically, two regions of Sec31A are phosphorylated by CKII, the alpha-solenoid (S527 and S799) and its carboxyl-terminal alpha-helical domain (S1163 and T1165). Although none of the phosphorylated residues are positioned within known Sec31A binding interfaces, mutations within the alpha-solenoid that block phosphorylation stabilize association with Sec23.125 Thus phosphorylation of Sec31A may either delay outer coat assembly to facilitate cargo loading and prevent premature carrier scission, or potentially help drive disassembly of the outer cage after carrier scission is complete. Structural analysis is necessary to provide further insights into the role of Sec31A phosphorylation during COPII-mediated membrane transport, and localization studies are required to determine CKII distribution at the ER/ERGIC interface, which will shed light on the timing of its action in the early secretory pathway.
Further regulation of COPII coat assembly is mediated by phosphorylation of Sec23 isoforms by the Unc-51 like kinase (ULK1), an enzyme most often associated with regulating the autophagy pathway in response to nutrient availability (Figure 2). When modified on sites within the trunk and β-barrel domains of Sec23A (S207, S312 and T405), its interaction with Sec31A is dramatically reduced, suggesting that ULK1 activation impedes full COPII coat assembly required for secretory protein export from the ER, and shifts its function toward the biogenesis of donor membranes needed for autophagosome formation.126 ULK1 has also been shown to phosphorylate Sec23B within the trunk domain (S186) (Figure 2), but in this case, phosphorylation disrupts an interaction with the F-box protein FBXW5, which normally targets Sec23B for proteasome-mediated degradation.127 In addition to stabilizing Sec23B, phosphorylation of Sec23B by ULK1 inhibits its binding to Sec24C and Sec24D, but not Sec24A or Sec24B, and allows for redistribution of Sec23B to ERGIC membranes to fuel autophagosome biogenesis.127 At a mechanistic level, it remains unclear how phosphorylation of Sec23B would influence Sec24 binding in an isoform-specific manner, but impaired association with Sec24C and Sec24D would likely reduce overall secretory protein efflux from the ER. Together, these findings highlight multiple pathways by which ULK1 activation retunes the role of the COPII machinery to meet cellular demands in response to changing environmental conditions. However, the major question of how COPII components relocalize from ER subdomains to ERGIC membranes in response to starvation remains unanswered.
One possibility is that ULK1 targets additional components in the early secretory pathway to enable transport of COPII machinery across the ER/ERGIC interface. In particular, Sec16A has been shown to be phosphorylated by ULK1 and its paralog ULK2 (Figure 2). Both kinases associate with the CCD domain of Sec16A and promote phosphorylation upstream of their binding site (S846).128 A mutant isoform of Sec16A, which cannot be phosphorylated, exhibits reduced accumulation at ER subdomains and also impairs recruitment of the Sec24C cargo adaptor.128 Although one interpretation of these data argues that ULK1 activity plays a constitutive role in supporting anterograde trafficking of biosynthetic cargoes, an alternative view predicts that active ULK kinases selectively stimulate the secretion of only certain cargo to ERGIC membranes in response to starvation, one of which may be Sec12, which would enable Sar1GTP accumulation to drive the formation of LC3 lipidation-active vesicles for autophagosome biogenesis.129-131 However, Sec16A has also been identified as an ERK7 kinase substrate during serum or amino acid starvation, with phosphorylation within its carboxyl-terminus leading to dispersal from ER subdomains.132 Further work is needed to better understand how various signaling pathways converge on the early secretory pathway during nutrient deprivation.
Lastly, Sec16A has also been shown to be a target of ERK2/MAPK1 in actively proliferating cells, downstream of growth factor stimulation. Phosphorylation by ERK2 within the amino-terminal half of Sec16A (T415) increases the number of Sec16A-marked ER subdomains, potentially augmenting the capacity of COPII-mediated membrane trafficking133 (Figure 2). Based on high throughput, mass spectrometry-based analysis, several other components of the early secretory pathway are also subject to PTM, both under steady state conditions and following various forms of exogenous stimulation. We are now only beginning to understand the complex regulatory systems in place to modulate secretory efflux, which likely occurs in a cell type specific manner. In the future, it will be critical to decipher these pathways to fully appreciate how membrane transport at the ER/ERGIC interface influences overall tissue homeostasis.
6 ∣. CONCLUSIONS AND PERSPECTIVES
Over the last 25 years, the field has witnessed a dramatic expansion in the defined roles for COPII in membrane transport, with numerous implications for normal human development and disease. The impacts of pathological point mutations that affect early secretory pathway components are now becoming understood not only at the cellular level but also at a structural level, which should enable the development of therapeutic approaches to combat disease. Nevertheless, some very fundamental questions remain unresolved. How are COPII budding sites on the ER defined? What is the structure of a native, intact COPII coat? How are transport intermediates organized at the ER/ERGIC interface? What imparts vectoral transport between ER and ERGIC membranes in the absence of cytoskeletal elements? What drives full COPII coat disassembly? For several of these problems, improvements in focused ion beam technology and cryoEM-based techniques should provide conclusive answers. With high resolution views of COPI and clathrin coats now defined, solution of the COPII coat structure is likely forthcoming.134-137 However, it will be critical to examine coat architecture and underlying membrane morphology in several mammalian cell types, which express a variety of cargoes, including fibrillar collagens or chylomicrons. In this regard, use of genome-edited human stem cells that express native levels of functionally tagged COPII subunits (to enable correlative light and electron microscopy) and differentiated to distinct cell fates is necessary to obtain physiologically relevant structures that are likely to be present in developing tissues. For now, we propose a working model for COPII-mediated transport that includes a series of steps that act in a coordinated fashion at the ER/ERGIC interface (Figure 3). The next 25 years will undoubtedly yield an array of new and exciting findings that detail additional regulatory aspects governing function of the early secretory pathway, with the prospect of a full mechanistic understanding of COPII-mediated trafficking just over the horizon.
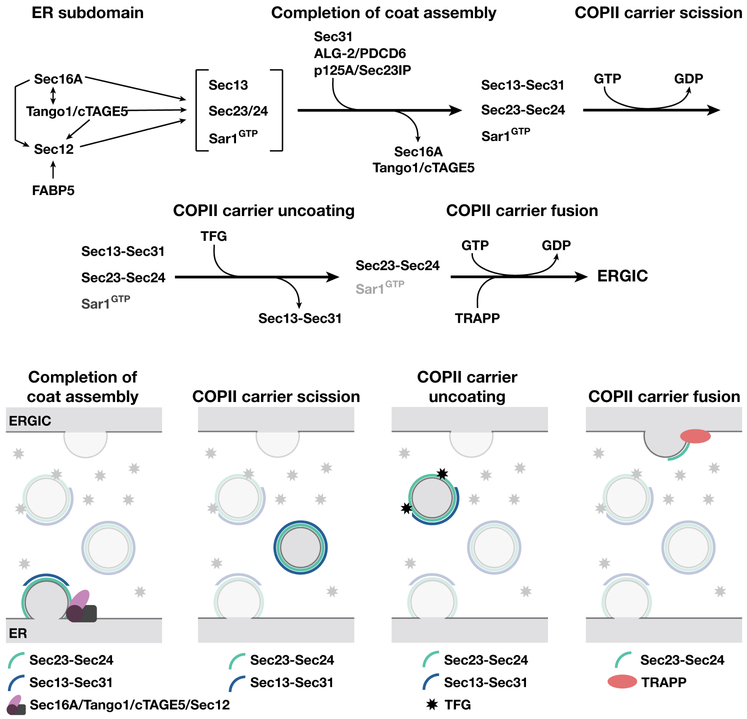
FIGURE 3.
Working model for COPII-mediated transport at the ER/ERGIC interface. Based on current findings, we proposed a speculative model highlighting a set of sequential regulatory steps that ensure COPII coat assembly and disassembly during cargo transport from the ER to ERGIC membranes
ACKNOWLEDGMENTS
This work was supported in part by the National Institutes of Health (NIH)/NIGMS R01GM110567 (to A.A.) and T32GM008688 (to J.P.). We thank members of the Audhya lab for suggestions and critically reading this manuscript.
Funding information
National Institute of General Medical Sciences, Grant/Award Numbers: GM008688, GM110567; National Institutes of Health, Grant/Award Numbers: T32GM008688, R01GM110567
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Hanna MG, Peotter JL, Frankel EB, Audhya A. Membrane transport at an organelle interface in the early secretory pathway: take your coat off and stay a while: evolution of the metazoan early secretory pathway. Bioessays. 2018;40:e1800004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zanetti G, Pahuja KB, Studer S, Shim S, Schekman R. COPII and the regulation of protein sorting in mammals. Nat Cell Biol. 2011;14:20–28. [DOI] [PubMed] [Google Scholar]
- 3.Budnik A, Stephens DJ. ER exit sites—localization and control of COPII vesicle formation. FEBS Lett. 2009;583:3796–3803. [DOI] [PubMed] [Google Scholar]
- 4.Barlowe C, Helenius A. Cargo capture and bulk flow in the early secretory pathway. Annu Rev Cell Dev Biol. 2016;32:197–222. [DOI] [PubMed] [Google Scholar]
- 5.Aridor M COPII gets in shape: lessons derived from morphological aspects of early secretion. Traffic. 2018;19:823–839. [DOI] [PubMed] [Google Scholar]
- 6.Barlowe C, Orci L, Yeung T, et al. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. [DOI] [PubMed] [Google Scholar]
- 7.Aridor M, Bannykh SI, Rowe T, Balch WE. Sequential coupling between COPII and COPI vesicle coats in endoplasmic reticulum to Golgi transport. J Cell Biol. 1995;131:875–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Appenzeller-Herzog C, Hauri HP. The ER-Golgi intermediate compartment (ERGIC): in search of its identity and function. J Cell Sci. 2006;119:2173–2183. [DOI] [PubMed] [Google Scholar]
- 9.Stagg SM, LaPointe P, Razvi A, et al. Structural basis for cargo regulation of COPII coat assembly. Cell. 2008;134:474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bi X, Corpina RA, Goldberg J. Structure of the Sec23/24-Sar1 pre-budding complex of the COPII vesicle coat. Nature. 2002;419:271–277. [DOI] [PubMed] [Google Scholar]
- 11.Stagg SM, Gürkan C, Fowler DM, et al. Structure of the Sec13/31 COPII coat cage. Nature. 2006;439:234–238. [DOI] [PubMed] [Google Scholar]
- 12.Fath S, Mancias JD, Bi X, Goldberg J. Structure and organization of coat proteins in the COPII cage. Cell. 2007;129:1325–1336. [DOI] [PubMed] [Google Scholar]
- 13.Nakańo A, Muramatsu M. A novel GTP-binding protein, Sar1p, is involved in transport from the endoplasmic reticulum to the Golgi apparatus. J Cell Biol. 1989;109:2677–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barlowe C, Schekman R. SEC12 encodes a guanine-nucleotide-exchange factor essential for transport vesicle budding from the ER. Nature. 1993;365:347–349. [DOI] [PubMed] [Google Scholar]
- 15.Weissman JT, Plutner H, Balch WE. The mammalian guanine nucleotide exchange factor mSec12 is essential for activation of the Sar1 GTPase directing endoplasmic reticulum export. Traffic. 2001;2:465–475. [DOI] [PubMed] [Google Scholar]
- 16.Jones B, Jones EL, Bonney SA, et al. Mutations in a Sar1 GTPase of COPII vesicles are associated with lipid absorption disorders. Nat Genet. 2003;34:29–31. [DOI] [PubMed] [Google Scholar]
- 17.Fromme JC, Ravazzola M, Hamamoto S, et al. The genetic basis of a craniofacial disease provides insight into COPII coat assembly. Dev Cell. 2007;13:623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long KR, Yamamoto Y, Baker AL, et al. Sar1 assembly regulates membrane constriction and ER export. J Cell Biol. 2010;190:115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fryer LG, Jones B, Duncan EJ, et al. The endoplasmic reticulum coat protein II transport machinery coordinates cellular lipid secretion and cholesterol biosynthesis. J Biol Chem. 2014;289:4244–4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loftus AF, Hsieh VL, Parthasarathy R. Modulation of membrane rigidity by the human vesicle trafficking proteins Sar1A and Sar1B. Biochem Biophys Res Commun. 2012;426:585–589. [DOI] [PubMed] [Google Scholar]
- 21.Levy E, Poinsot P, Spahis S. Chylomicron retention disease: genetics, biochemistry, and clinical spectrum. Curr Opin Lipidol. 2019;30(2):134–139. 10.1097/MOL.0000000000000578. [DOI] [PubMed] [Google Scholar]
- 22.Cutrona MB, Beznoussenko GV, Fusella A, Martella O, Moral P, Mironov AA. Silencing of mammalian Sar1 isoforms reveals COPII-independent protein sorting and transport. Traffic. 2013;14:691–708. [DOI] [PubMed] [Google Scholar]
- 23.Sané AT, Seidman E, Peretti N, et al. Understanding chylomicron retention disease through Sar1b Gtpase gene disruption: insight from cell culture. Arterioscler Thromb Vasc Biol. 2017;37:2243–2251. [DOI] [PubMed] [Google Scholar]
- 24.Huang M, Weissman JT, Beraud-Dufour S, et al. Crystal structure of Sar1-GDP at 1.7 A resolution and the role of the NH2 terminus in ER export. J Cell Biol. 2001;155:937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao Y, Bian C, Yuan C, et al. An open conformation of switch I revealed by Sar1-GDP crystal structure at low Mg2+. Biochem Biophys Res Commun. 2006;348:908–915. [DOI] [PubMed] [Google Scholar]
- 26.Ma W, Goldberg E, Goldberg J. ER retention is imposed by COPII protein sorting and attenuated by 4-phenylbutyrate. Elife. 2017;8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMahon C, Studer SM, Clendinen C, Dann GP, Jeffrey PD, Hughson FM. The structure of Sec12 implicates potassium ion coordination in Sar1 activation. J Biol Chem. 2012;287:43599–43606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rowe T, Aridor M, McCaffery JM, Plutner H, Balch WE. COPII vesicles derived from mammalian endoplasmic reticulum (ER) microsomes recruit COPI. J Cell Biol. 1996;135:895–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aridor M, Fish KN, Bannykh S, et al. The Sar1 GTPase coordinates biosynthetic cargo selection with endoplasmic reticulum export site assembly. J Cell Biol. 2001;152:213–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanna MG 4th, Mela I, Wang L, et al. Sar1 GTPase activity is regulated by membrane curvature. J Biol Chem. 2016;291:1014–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hariri H, Bhattacharya N, Johnson K, Noble AJ, Stagg SM. Insights into the mechanisms of membrane curvature and vesicle scission by the small GTPase Sar1 in the early secretory pathway. J Mol Biol. 2014;426:3811–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schindler AJ, Schekman R. In vitro reconstitution of ER-stress induced ATF6 transport in COPII vesicles. Proc Natl Acad Sci USA. 2009;106:17775–17780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee MC, Orci L, Hamamoto S, Futai E, Ravazzola M, Schekman R. Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle. Cell. 2005;122:605–617. [DOI] [PubMed] [Google Scholar]
- 34.Bacia K, Futai E, Prinz S, et al. Multibudded tubules formed by COPII on artificial liposomes. Sci Rep. 2011;1:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schenk NA, Dahl PJ, Hanna MG 4th, et al. A simple supported tubulated bilayer system for evaluating protein-mediated membrane remodeling. Chem Phys Lipids. 2018;215:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bielli A, Haney CJ, Gabreski G, Watkins SC, Bannykh SI, Aridor M. Regulation of Sar1 NH2 terminus by GTP binding and hydrolysis promotes membrane deformation to control COPII vesicle fission. J Cell Biol. 2005;171:919–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hutchings J, Stancheva V, Miller EA, Zanetti G. Subtomogram averaging of COPII assemblies reveals how coat organization dictates membrane shape. Nat Commun. 2018;9:4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurokawa K, Suda T, Nakano A. Sar1 localizes at the rims of COPII-coated membranes in vivo. J Cell Sci. 2016;129:3231–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuoka K, Orci L, Amherdt M, et al. COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell. 1998;93:263–275. [DOI] [PubMed] [Google Scholar]
- 40.Yoshihisa T, Barlowe C, Schekman R. Requirement for a GTPase-activating protein in vesicle budding from the endoplasmic reticulum. Cell. 2004;118:591–605. [DOI] [PubMed] [Google Scholar]
- 41.Antonny B, Madden D, Hamamoto S, Orci L, Schekman R. Dynamics of the COPII coat with GTP and stable analogues. Nat Cell Biol. 2001;3:531–537. [DOI] [PubMed] [Google Scholar]
- 42.Ehrlich M, Boll W, Van Oijen A, et al. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118:591–605. [DOI] [PubMed] [Google Scholar]
- 43.Li D, Shao L, Chen BC, et al. ADVANCED IMAGING. Extended-resolution structured illumination imaging of endocytic and cytoskeletal dynamics. Science. 2015;349:aab3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khoriaty R, Hesketh GG, Bernard A, et al. Functions of the COPII gene paralogs SEC23A and SEC23B are interchangeable in vivo. Proc Natl Acad Sci USA. 2018;115:E7748–E7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwarz K, Iolascon A, Verissimo F, et al. Mutations affecting the secretory COPII coat component SEC23B cause congenital dyserythropoietic anemia type II. Nat Genet. 2009;41:936–940. [DOI] [PubMed] [Google Scholar]
- 46.Yehia L, Niazi F, Ni Y, et al. Germline heterozygous variants in SEC23B are associated with Cowden syndrome and enriched in apparently sporadic thyroid cancer. Am J Hum Genet. 2015;97:661–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyadjiev SA, Fromme JC, Ben J, et al. Cranio-lenticulo-sutural dysplasia is caused by a SEC23A mutation leading to abnormal endoplasmic-reticulum-to-Golgi trafficking. Nat Genet. 2006;38:1192–1197. [DOI] [PubMed] [Google Scholar]
- 48.Wendeler MW, Paccaud JP, Hauri HP. Role of Sec24 isoforms in selective export of membrane proteins from the endoplasmic reticulum. EMBO Rep. 2007;8:258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mancias JD, Goldberg J. Structural basis of cargo membrane protein discrimination by the human COPII coat machinery. EMBO J. 2008;27:2918–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mossessova E, Bickford LC, Goldberg J. SNARE selectivity of the COPII coat. Cell. 2003;114:483–495. [DOI] [PubMed] [Google Scholar]
- 51.Miller EA, Beilharz TH, Malkus PN, et al. Multiple cargo binding sites on the COPII subunit Sec24p ensure capture of diverse membrane proteins into transport vesicles. Cell. 2003;114:497–509. [DOI] [PubMed] [Google Scholar]
- 52.Nishimura N, Balch WE. A di-acidic signal required for selective export from the endoplasmic reticulum. Science. 1997;277:556–558. [DOI] [PubMed] [Google Scholar]
- 53.Barlowe C Molecular recognition of cargo by the COPII complex: a most accommodating coat. Cell. 2003;114:395–397. [DOI] [PubMed] [Google Scholar]
- 54.Chen XW, Wang H, Bajaj K, et al. SEC24A deficiency lowers plasma cholesterol through reduced PCSK9 secretion. Elife. 2013;2:e00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Emmer BT, Hesketh GG, Kotnik E, et al. The cargo receptor SURF4 promotes the efficient cellular secretion of PCSK9. Elife. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang B, Joo JH, Mount R, et al. The COPII cargo adapter SEC24C is essential for neuronal homeostasis. J Clin Invest. 2018;128:3319–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adams EJ, Chen XW, O'Shea KS, Ginsburg D. Mammalian COPII coat component SEC24C is required for embryonic development in mice. J Biol Chem. 2014;289:20858–20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bi X, Mancias JD, Goldberg J. Insights into COPII coat nucleation from the structure of Sec23.Sar1 complexed with the active fragment of Sec31. Dev Cell. 2007;13:635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhattacharya N, O'Donnell J, Stagg SM. The structure of the Sec13/31 COPII cage bound to Sec23. J Mol Biol. 2012;420:324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Noble AJ, Zhang Q, O'Donnell J, et al. A pseudoatomic model of the COPII cage obtained from cryo-electron microscopy and mass spectrometry. Nat Struct Mol Biol. 2013;20:167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Copic A, Latham CF, Horlbeck MA, D'Arcangelo JG, Miller EA. ER cargo properties specify a requirement for COPII coat rigidity mediated by Sec13p. Science. 2012;335:1359–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paraan M, Bhattacharya N, Uversky VN, Stagg SM. Flexibility of the Sec13/31 cage is influenced by the Sec31 C-terminal disordered domain. J Struct Biol. 2018;204:250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stankewich MC, Stabach PR, Morrow JS. Human Sec31B: a family of new mammalian orthologues of yeast Sec31p that associate with the COPII coat. J Cell Sci. 2006;119:958–969. [DOI] [PubMed] [Google Scholar]
- 64.Watson P, Townley AK, Koka P, Palmer KJ, Stephens DJ. Sec16 defines endoplasmic reticulum exit sites and is required for secretory cargo export in mammalian cells. Traffic. 2006;7:1678–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bhattacharyya D, Glick BS. Two mammalian Sec16 homologues have nonredundant functions in endoplasmic reticulum (ER) export and transitional ER organization. Mol Biol Cell. 2007;18:839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iinuma T, Shiga A, Nakamoto K, et al. Mammalian Sec16/p250 plays a role in membrane traffic from the endoplasmic reticulum. J Biol Chem. 2007;282:17632–17639. [DOI] [PubMed] [Google Scholar]
- 67.Hughes H, Budnik A, Schmidt K, et al. Organisation of human ER-exit sites: requirements for the localisation of Sec16 to transitional ER. J Cell Sci. 2009;122:2924–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Montegna EA, Bhave M, Liu Y, Bhattacharyya D, Glick BS. Sec12 binds to Sec16 at transitional ER sites. PLoS One. 2012;7:e31156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kung LF, Pagant S, Futai E, et al. Sec24p and Sec16p cooperate to regulate the GTP cycle of the COPII coat. EMBO J. 2012;31:1014–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Whittle JR, Schwartz TU. Structure of the Sec13-Sec16 edge element, a template for assembly of the COPII vesicle coat. J Cell Biol. 2010;190:347–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maeda M, Katada T, Saito K. TANGO1 recruits Sec16 to coordinately organize ER exit sites for efficient secretion. J Cell Biol. 2017;216:1731–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saito K, Chen M, Bard F, et al. TANGO1 facilitates cargo loading at endoplasmic reticulum exit sites. Cell. 2009;136:891–902. [DOI] [PubMed] [Google Scholar]
- 73.Maeda M, Saito K, Katada T. Distinct isoform-specific complexes of TANGO1 cooperatively facilitate collagen secretion from the endoplasmic reticulum. Mol Biol Cell. 2016;27:2688–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ishikawa Y, Ito S, Nagata K, Sakai LY, Bächinger HP. Intracellular mechanisms of molecular recognition and sorting for transport of large extracellular matrix molecules. Proc Natl Acad Sci USA. 2016;113:E6036–E6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saito K, Yamashiro K, Ichikawa Y, et al. cTAGE5 mediates collagen secretion through interaction with TANGO1 at endoplasmic reticulum exit sites. Mol Biol Cell. 2011;22:2301–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saito K, Yamashiro K, Shimazu N, Tanabe T, Kontani K, Katada T. Concentration of Sec12 at ER exit sites via interaction with cTAGE5 is required for collagen export. J Cell Biol. 2014;206:751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tanabe T, Maeda M, Saito K, Katada T. Dual function of cTAGE5 in collagen export from the endoplasmic reticulum. Mol Biol Cell. 2016;27:2008–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma W, Goldberg J. TANGO1/cTAGE5 receptor as a polyvalent template for assembly of large COPII coats. Proc Natl Acad Sci USA. 2016;113:10061–10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Johnson A, Bhattacharya N, Hanna M, et al. TFG clusters COPII-coated transport carriers and promotes early secretory pathway organization. EMBO J. 2015;34:811–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mironov AA, Mironov AA Jr, Beznoussenko GV, et al. ER-to-Golgi carriers arise through direct en bloc protrusion and multistage maturation of specialized ER exit domains. Dev Cell. 2003;5:583–594. [DOI] [PubMed] [Google Scholar]
- 81.Zeuschner D, Geerts WJ, van Donselaar E, et al. Immuno-electron tomography of ER exit sites reveals the existence of free COPII-coated transport carriers. Nat Cell Biol. 2006;8:377–383. [DOI] [PubMed] [Google Scholar]
- 82.Buehler MJ. Nanomechanics of collagen fibrils under varying cross-link densities: atomistic and continuum studies. J Mech Behav Biomed Mater. 2008;1:59–67. [DOI] [PubMed] [Google Scholar]
- 83.Santos AJ, Raote I, Scarpa M, Brouwers N, Malhotra V. TANGO1 recruits ERGIC membranes to the endoplasmic reticulum for procollagen export. Elife. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Raote I, Ortega Bellido M, Pirozzi M, et al. TANGO1 assembles into rings around COPII coats at ER exit sites. J Cell Biol. 2017;216:901–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Raote I, Ortega-Bellido M, Santos AJ, et al. TANGO1 builds a machine for collagen export by recruiting and spatially organizing COPII, tethers and membranes. Elife. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hanna MG 4th, Block S, Frankel EB, et al. TFG facilitates outer coat disassembly on COPII transport carriers to promote tethering and fusion with ER-Golgi intermediate compartments. Proc Natl Acad Sei USA. 2017;114:E7707–E7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yuan L, Kenny SJ, Hemmati J, Xu K, Schekman R. TANGO1 and SEC12 are copackaged with procollagen I to facilitate the generation of large COPII carriers. Proc Natl Acad Sci USA. 2018;115:E12255–E12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Melville D, Gorur A, Schekman R. Fatty-acid binding protein 5 modulates the SAR1 GTPase cycle and enhances budding of large COPII cargoes. Mol Biol Cell. 2019;30:387–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gorur A, Yuan L, Kenny SJ, Baba S, Xu K, Schekman R. COPII-coated membranes function as transport carriers of intracellular procollagen I. J Cell Biol. 2017;216:1745–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Omari S, Makareeva E, Roberts-Pilgrim A, et al. Noncanonical autophagy at ER exit sites regulates procollagen turnover. Proc Natl Acad Sci USA. 2018;115:E10099–E10108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McCaughey J, Stevenson NL, Cross S, Stephens DJ. ER-to-Golgi trafficking of procollagen in the absence of large carriers. J Cell Biol. 2019;218(3):929–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Raote I, Malhotra V. Protein transport by vesicles and tunnels. J Cell Biol. 2019;218(3):737–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Karagöz GE, Acosta-Alvear D, Walter P. The unfolded protein response: detecting and responding to fluctuations in the protein-folding capacity of the endoplasmic reticulum. Cold Spring Harb Perspect Biol. 2019. 10.1101/cshperspect.a033886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Klinkenberg D, Long KR, Shome K, Watkins SC, Aridor M. A cascade of ER exit site assembly that is regulated by p125A and lipid signals. J Cell Sci. 2014;127:1765–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tani K, Mizoguchi T, Iwamatsu A, Hatsuzawa K, Tagaya M. p125 is a novel mammalian Sec23p-interacting protein with structural similarity to phospholipid-modifying proteins. J Biol Chem. 1999;274:20505–20512. [DOI] [PubMed] [Google Scholar]
- 96.Mizoguchi T, Nakajima K, Hatsuzawa K, et al. Determination of functional regions of p125, a novel mammalian Sec23p-interacting protein. Biochem Biophys Res Commun. 2000;279:144–149. [DOI] [PubMed] [Google Scholar]
- 97.Shimoi W, Ezawa I, Nakamoto K, et al. p125 is localized in endoplasmic reticulum exit sites and involved in their organization. J Biol Chem. 2005;280:10141–10148. [DOI] [PubMed] [Google Scholar]
- 98.Ong YS, Tang BL, Loo LS, Hong W. p125A exists as part of the mammalian Sec13/Sec31 COPII subcomplex to facilitate ER-Golgi transport. J Cell Biol. 2010;190:331–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.la Cour JM, Schindler AJ, Berchtold MW, Schekman R. ALG-2 attenuates COPII budding in vitro and stabilizes the Sec23/Sec31A complex. PLoS One. 2013;8:e75309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yamasaki A, Tani K, Yamamoto A, Kitamura N, Komada M. The Ca2+-binding protein ALG-2 is recruited to endoplasmic reticulum exit sites by Sec31A and stabilizes the localization of Sec31A. Mol Biol Cell. 2006;17:4876–4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shibata H, Kanadome T, Sugiura H, et al. A new role for annexin A11 in the early secretory pathway via stabilizing Sec31A protein at the endoplasmic reticulum exit sites (ERES). J Biol Chem. 2015;290:4981–4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bahar E, Kim H, Yoon H. ER stress-mediated signaling: action potential and Ca(2+) as key players. Int J Mol Sci. 2016;17:1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xu D, Hay JC. Reconstitution of COPII vesicle fusion to generate a pre-Golgi intermediate compartment. J Cell Biol. 2004;167:997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Witte K, Schuh AL, Hegermann J, et al. TFG-1 function in protein secretion and oncogenesis. Nat Cell Biol. 2011;13:550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Greco A, Mariani C, Miranda C, et al. The DNA rearrangement that generates the TRK-T3 oncogene involves a novel gene on chromosome 3 whose product has a potential coiled-coil domain. Mol Cell Biol. 1995;15:6118–6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen Y, Tseng SH. Targeting tropomyosin-receptor kinase fused gene in cancer. Anticancer Res. 2014;34:1595–1600. [PubMed] [Google Scholar]
- 107.Milovanovic D, Wu Y, Bian X, De Camilli P. A liquid phase of synapsin and lipid vesicles. Science. 2018;361:604–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kanadome T, Shibata H, Kuwata K, Takahara T, Maki M. The calcium-binding protein ALG-2 promotes endoplasmic reticulum exit site localization and polymerization of Trk-fused gene (TFG) protein. FEBS J. 2017;284:56–76. [DOI] [PubMed] [Google Scholar]
- 109.Kim YG, Sohn EJ, Seo J, et al. Crystal structure of bet3 reveals a novel mechanism for Golgi localization of tethering factor TRAPP. Nat Struct Mol Biol. 2005;12:38–45. [DOI] [PubMed] [Google Scholar]
- 110.Cai H, Yu S, Menon S, et al. TRAPPI tethers COPII vesicles by binding the coat subunit Sec23. Nature. 2007;445:941–944. [DOI] [PubMed] [Google Scholar]
- 111.Slosarek EL, Schuh AL, Pustova I, et al. Pathogenic TFG mutations underlying hereditary spastic paraplegia impair secretory protein trafficking and axon fasciculation. Cell Rep. 2018;24:2248–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yagi T, Ito D, Suzuki N. TFG-related neurologic disorders: new insights into relationships between endoplasmic reticulum and neurodegeneration. J Neuropathol Exp Neurol. 2016;75:299–305. [DOI] [PubMed] [Google Scholar]
- 113.Patel A, Lee HO, Jawerth L, et al. A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell. 2015;162:1066–1077. [DOI] [PubMed] [Google Scholar]
- 114.Wegmann S, Eftekharzadeh B, Tepper K, et al. Tau protein liquid-liquid phase separation can initiate tau aggregation. EMBO J. 2018;37:e98049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tarbet HJ, Toleman CA, Boyce M. A sweet embrace: control of protein-protein interactions by O-linked β-N-acetylglucosamine. Biochemistry. 2018;57:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu Rev Biochem. 2011;80:825–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hanover JA, Krause MW, Love DC. The hexosamine signaling pathway: O-GlcNAc cycling in feast or famine. Biochim Biophys Acta. 1800;2010:80–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cox NJ, Unlu G, Bisnett BJ, et al. Dynamic glycosylation governs the vertebrate COPII protein trafficking pathway. Biochemistry. 2018;57:91–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dudognon P, Maeder-Garavaglia C, Carpentier JL, Paccaud JP. Regulation of a COPII component by cytosolic O-glycosylation during mitosis. FEBS Lett. 2004;561:44–50. [DOI] [PubMed] [Google Scholar]
- 120.McCaughey J, Miller VJ, Stevenson NL, et al. TFG promotes organization of transitional ER and efficient collagen secretion. Cell Rep. 2016;15:1648–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jin L, Pahuja KB, Wickliffe KE, et al. Ubiquitin-dependent regulation of COPII coat size and function. Nature. 2012;482:495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McGourty CA, Akopian D, Walsh C, et al. Regulation of the CUL3 ubiquitin ligase by a calcium-dependent co-adaptor. Cell. 2016;167:525–538.e14. [DOI] [PubMed] [Google Scholar]
- 123.Canning P, Cooper CD, Krojer T, et al. Structural basis for Cul3 protein assembly with the BTB-Kelch family of E3 ubiquitin ligases. J Biol Chem. 2013;288:7803–7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim K, Park S, Kim J. Cullin3-RING ubiquitin ligases are intimately linked to the unfolded protein response of the endoplasmic reticulum. bioRxiv 428136. doi: 10.1101/428136 [DOI] [Google Scholar]
- 125.Koreishi M, Yu S, Oda M, Honjo Y, Satoh A. CK2 phosphorylates Sec31 and regulates ER-to-Golgi trafficking. PLoS One. 2013;8:e54382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gan W, Zhang C, Siu KY, Satoh A, Tanner JA, Yu S. ULK1 phosphorylates Sec23A and mediates autophagy-induced inhibition of ER-to-Golgi traffic. BMC Cell Biol. 2017;18:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jeong YT, Simoneschi D, Keegan S, et al. The ULK1-FBXW5-SEC23B nexus controls autophagy. Elife. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Joo JH, Wang B, Frankel E, et al. The noncanonical role of ULK/-ATG1 in ER-to-Golgi trafficking is essential for cellular homeostasis. Mol Cell. 2016;62:491–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ge L, Melville D, Zhang M, Schekman R. The ER-Golgi intermediate compartment is a key membrane source for the LC3 lipidation step of autophagosome biogenesis. Elife. 2013;2:e00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ge L, Zhang M, Schekman R. Phosphatidylinositol 3-kinase and COPII generate LC3 lipidation vesicles from the ER-Golgi intermediate compartment. Elife. 2014;3:e04135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ge L, Wilz L, Schekman R. Biogenesis of autophagosomal precursors for LC3 lipidation from the ER-Golgi intermediate compartment. Autophagy. 2015;11:2372–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zacharogianni M, Kondylis V, Tang Y, et al. ERK7 is a negative regulator of protein secretion in response to amino-acid starvation by modulating Sec16 membrane association. EMBO J. 2011;30:3684–3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Farhan H, Wendeler MW, Mitrovic S, et al. MAPK signaling to the early secretory pathway revealed by kinase/phosphatase functional screening. J Cell Biol. 2010;189:997–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dodonova SO, Diestelkoetter-Bachert P, von Appen A, et al. VESICULAR TRANSPORT. A structure of the COPI coat and the role of coat proteins in membrane vesicle assembly. Science. 2015;349:195–198. [DOI] [PubMed] [Google Scholar]
- 135.Avinoam O, Schorb M, Beese CJ, Briggs JA, Kaksonen M. ENDOCYTOSIS. Endocytic sites mature by continuous bending and remodeling of the clathrin coat. Science. 2015;348:1369–1372. [DOI] [PubMed] [Google Scholar]
- 136.Dodonova SO, Aderhold P, Kopp J, et al. 9 Å structure of the COPI coat reveals that the Arf1 GTPase occupies two contrasting molecular environments. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fotin A, Cheng Y, Sliz P, et al. Molecular model for a complete clathrin lattice from electron cryomicroscopy. Nature. 2004;432:573–579. [DOI] [PubMed] [Google Scholar]