Abstract
Successful execution of many behavioral goals relies on well-organized patterns of saccadic eye movements, and in complex tasks, these patterns can reveal the component processes underlying task performance. The present study examined the pattern of eye movements in a visual search task to provide evidence of attentional control impairments in people with schizophrenia (PSZ). We tested PSZ(N=38) and non-psychiatric control subjects (NCS,N=35) in a task that was designed to stress top-down control by pitting task goals against bottom-up salience. Participants searched for either a low-contrast (nonsalient) or a high-contrast (salient) target among low- and high-contrast distractors. By examining fixations of the low- and high-contrast items, we evaluated the ability of PSZ and NCS to focus on low-salience targets and filter out high-salience distractors (or vice versa). When participants searched for a salient target, both groups successfully focused on relevant, high-contrast stimuli and filtered out target-mismatched, low-contrast stimuli. However, when searching for a non-salient target, PSZ were impaired at efficiently suppressing high-contrast(salient) distractors. Specifically, PSZ were more likely than NCS to fixate and revisit salient distractors, and they dwelled on these items longer than did NCS. The results provide direct evidence that PSZ are impaired in their ability to utilize top-down goals to overcome the prepotent tendency to focus attention on irrelevant but highly salient information.
Keywords: Guided visual search, Saccadic eye movements, Top-down control, Schizophrenia
General Scientific Summary:
Attentional control impairments have been long recognized as fundamental aspects of cognitive dysfunction in schizophrenia. Using precise measures of eye position, this study shows that individuals with schizophrenia are highly prone to oculomotor capture by salient, but irrelevant stimuli, thus manifesting a diminished ability to exert cognitive control over what is attended to in the visual environment.
INTRODUCTION
Schizophrenia is associated with impairment to cognitive functions that are crucial for successful occupational and interpersonal functioning (Green et al.,2004). Among these impairments are attentional deficits in tasks that require selective processing of goal-relevant information in the presence of more salient, but task irrelevant, information (Luck & Gold,2008). Models of optimal visual search behavior involve integrating top-down information with the oculomotor system to direct eye movements to those locations that yield the most goal-relevant information (e.g., Henderson et al.,2007; Peters & Itti,2007; Pomplun,2006; Zelinsky et al.,2006). However, it is challenging to scan the visual environment in an optimal fashion when goal-irrelevant stimuli are more salient than goal-relevant stimuli.
In people with schizophrenia(PSZ), cognitive control impairments would be expected when bottom-up signals are unavailable, especially when bottom-up salience is incompatible with current goals. Previous studies of top-down control of attention in schizophrenia have yielded mixed results. Some evidence is consistent with impaired top-down attentional control in PSZ (e.g. Fuller et al.,2006; Gold et al.,2007; Dima et al.,2010). For example, substantial deficits arise when task-irrelevant distractors are more salient than task-relevant, to-be-remembered items in a spatial memory task.(Hahn et al., 2010). A recent study (126 PSZ,122 NCS) also found that PSZ were impaired when top-down control was needed to guide visual search but performed the task efficiently when guided by bottom-up inputs(Gold et al.,2017). However, PSZ can efficiently encode task-relevant visual stimuli into working memory(WM) and suppress the encoding of equally salient distractors(Gold, et al.,2006). Behavioral and electrophysiological evidence also shows that shifting attention to a single, salient target in a visual search array is unimpaired in PSZ (Luck et al.,2006). Furthermore, PSZ manifest intact top-down target selection and distractor filtering when distractors do not share target features. For instance, physically salient distractors presented simultaneously with or immediately following a set of to-be-remembered objects do not cause substantial WM impairments in PSZ (Erickson et al.,2014). Likewise, PSZ do not show exaggerated capture of attention by salient but irrelevant “pop-out” colors (Leonard et al.,2014).
Most studies report manual reaction times (RTs) as the primary behavioral measure. RTs however, reflect the combined influence of multiple stages of processing, which both increases trial-by-trial variability and decreases the ability to determine which specific stages of processing differ between groups. By contrast, eye tracking makes it possible to draw strong inferences about attention and distraction by directly assessing which items were selected for further processing, thus providing information beyond traditional manual response metrics. (Hortsmann et al.,2017).
To provide a more direct test of the hypothesis that PSZ have attentional control deficits, when challenged by highly salient distractors, we tracked eye position while PSZ and NCS performed a visual search task in which top-down goals and bottom-up salience were orthogonally manipulated. Capitalizing on the ability of eye tracking to determine which items were selected for foveal processing, we examined whether high-salience distractors were more likely to capture and hold attention in PSZ than in non-psychiatric control subjects(NCS).
Specifically, we used stimulus arrays comprising a mixture of low-contrast(low-salience) items and high-contrast(high-salience) items. The target was a circle with a gap on the top or bottom, and distractors were circles with a gap on the left or right. Participants were informed with a cue whether the target would be low- or high-contrast on each trial, and once they found it, they were to indicate whether the gap was at the top or bottom using gamepad keys. Because gap position on a given item cannot be readily perceived with peripheral vision, the most efficient strategy is to shift gaze toward items of the cued contrast level, avoiding items of the uncued contrast level. When the cued target was high-contrast (i.e. Salient), the goal of searching target-matched items was consistent with the bottom-up tendency to fixate high-contrast items, making it easy to avoid fixating the low-contrast distractor items. However, when the cued target was low-contrast, more intensive top-down control was required to guide attention to the goal-relevant low-contrast items and avoid fixating the goal-irrelevant high-contrast items. If PSZ are impaired in top-down control, they would be particularly impaired at directing gaze to the relevant contrast level on these trials.
We predicted that, during low-contrast search, PSZ would fixate high-contrast distractors more frequently and dwell upon them longer relative to NCS, whereas PSZ would not exhibit exaggerated processing of low-contrast distractors when searching for a high-contrast target. This pattern would provide direct evidence that visual search is impaired in PSZ due to deficits in using top-down control to suppress oculomotor capture by salient (high-contrast) stimuli.
The predicted RT effect could be explained on purely psychometric grounds as a greater deficit on a more difficult task, but by examining eye movement patterns we can directly determine the nature of attentional control failure. That is, there are many ways in which PSZ might be impaired relative to NCS, but our design allows us to determine whether PSZ are more likely than NCS to fixate and dwell on high-contrast distractors when low-contrast distractors are task-relevant, indicating impaired control per se.
METHODS
Participants.
We tested 38 outpatients from the Maryland Psychiatric Research Center and other community outpatient clinics meeting DSM-IV criteria for schizophrenia(N=32) or schizoaffective disorder(N=6), and 35 matched NCS with no history of psychiatric or substance abuse disorder and no first-degree relative with mental illness, recruited by advertisements posted on the Internet and in local libraries and businesses. Demographic information is summarized in Table 1, and additional neurocognitive measures are presented in supplemental materials (Table S1).
Table 1:
Participant Characteristics
| NCS (N=35) | PSZ (N=38) | Statistic | p-value | |
|---|---|---|---|---|
| Age | 36.83 (11.88) | 39.06 (10.34) | t=−0.827 | 0.411 |
| Gender (M | F) | 20| 13 | 23 | 13 | φ =0.08 | 0.78 |
| Race (African American | Caucasian | Other) | 13| 19 | 1 | 12 |21 | 2 | φ =0.37 | 0.83 |
| Participant Education | 15 (1.86) | 13.06 (2.37) | t= 3.762 | < .001 |
| WRAT 4 | 110.14(14.86) | 94.88(10.95) | t= 4.82 | < .001 |
| MCT Overall | 50.25 (14.92) | 30.49 (14.18) | t= 5.627 | < .001 |
| Total CPZ | 440.7 (365.2) | |||
| BPRS Total | 35.42 (7.7) | |||
| SANS Total | 27.42 (12.22) |
WRAT 4-Wide Range Achievement Test 4; MCT Overall-MATRICS Consensus Cognitive Battery Overall Score; CPZ-Total Chlorpromazine equivalents, calculated using the Andreasen method (Andreasen et al., 2010); BPRS-Brief Psychiatric Rating Scale; SANS-Scale for the Assessment of Negative Symptoms
Procedure.
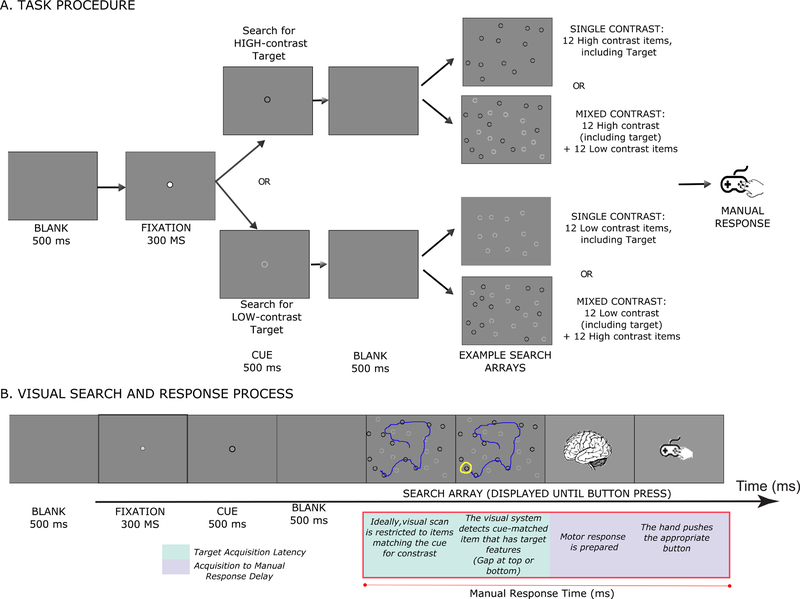
The task is illustrated in Figure 1 (Supplementary materials contain further details on methods and apparatus). Target contrast varied randomly across trials. For key mixed-contrast trials, the stimulus array consisted of equal numbers of low-contrast and high-contrast items. At the beginning of each trial, a cue indicated if the target was a high-contrast or low-contrast item. We included single-contrast trials in which all the items were low- or high-contrast, as a control \to rule out any effects of contrast that were independent of the need to selectively filter out a subset of items.
Figure 1. A. Task Procedure.
Each trial began with a fixation screen containing a solid circle appearing at the center. Participants were required to maintain steady fixation for 500 ms, after which the fixation circle was replaced with a cue circle. The cue indicating target contrast was either High-contrast or low contrast. The screen was then blanked, followed by the search display which remained visible until a manual response was made. Participants indicated the presence of the gap at the top or bottom of the target item by pushing one of two buttons on a game controller. A 500-ms blank interval was interposed between the response and the onset of the next trial. Each participant underwent 160 trials with all four trial types randomized. B. Guided Visual Search and Response Process. This panel depicts the visual search process and attentional guidance to the target location prior to execution of a response.
RESULTS
Manual response accuracy was uniformly high for both groups across all trial types (supplemental material). Trials with incorrect manual responses were excluded. Our main analyses focused on number of low- and high-contrast items fixated on each trial and time required to find the target and make the manual response (see supplemental materials for details of analysis procedures).
Time to Target
As illustrated in Figure 1B, manual response time(RT) can be broken into two components, amount of time required for gaze to reach the target (target acquisition time) and time between target acquisition and the manual response (acquisition-to-response time). Target acquisition latency provides a purer and more robust measure of processes required to find the target than does RT, because it factors out processes required to determine the target’s gap location, select the appropriate manual response, and execute that response (which are captured by the acquisition-to-response time). The process of selecting the relevant contrast level and avoiding the irrelevant contrast level was necessary only for mixed-contrast trials, so separate ANOVAs were conducted for single-contrast and mixed-contrast trials, with factors of Group and Target Contrast. Table 2 contains the statistical test results (unless otherwise noted, these statistics will not be provided in the main text).
Table 2:
Statistics
| I.Manual RT | II.Target Acquisition Latency | III.Delay between Target Acquisition Latency and Manual RT | IV.Proportion of target mismatched items visited (mixed-contrast) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trial Type | F | p | η2p | F | p | η2p | F | p | η2p | F | p | η2p |
| A. Single Contrast | ||||||||||||
| Target Contrast | 29.94 | <.001 | 0.3 (L:0.13 U:0.44) | 21.08 | <.001 | 0.23 (L:0.08 U:0.38) | 0.73 | 0.39 | 0.01 (L:0.00 U:0.10) | - | - | - |
| Group | 46.5 | <.001 | 0.4 (L:0.22 U:0.53) | 42.29 | <.001 | 0.37 (L:0.20 U:0.51) | 24.27 | <.001 | 0.26 (L:0.10 U:0.40) | - | - | - |
| Target Contrast X Group | 1.57 | 0.22 | 0.02 (L:0.00 U:0.12) | 0.01 | 0.91 | 0 (L:0.00 U:0.03) | 1.07 | 0.31 | 0.01 (L:0.00 U:0.11) | - | - | - |
| B. Mixed-Contrast | ||||||||||||
| Target Contrast | 43.24 | <.001 | 0.38 (L:0.20 U:0.51) | 29.37 | <.001 | 0.3 (L:0.13 U:0.44) | 4.27 | 0.042 | 0.06 (L:0.00 U:0.18) | 11.16 | 0.001 | 0.14 (L:0.02 U:0.28) |
| Group | 37.53 | <.001 | 0.35 (L:0.17 U:0.49) | 22.11 | <.001 | 0.24 (L:0.08 U:0.39) | 75.67 | <.001 | 0.52 (L:0.35 U:0.63) | 29.76 | <.001 | 0.30 (L:0.13 U:0.44) |
| Target Contrast X Group | 1.14 | 0.129 | 0.02 (L:0.00 U:0.11) | 4.6 | 0.035 | 0.06 (L:0.00 U:0.19) | 2.07 | 0.15 | 0.03 (L:0.00 U:0.14) | 25.83 | <.001 | 0.27 (L:0.11 U:0.42) |
(L&U: Lower and Upper limits on partial eta-squared, 95%)
Overall RT.
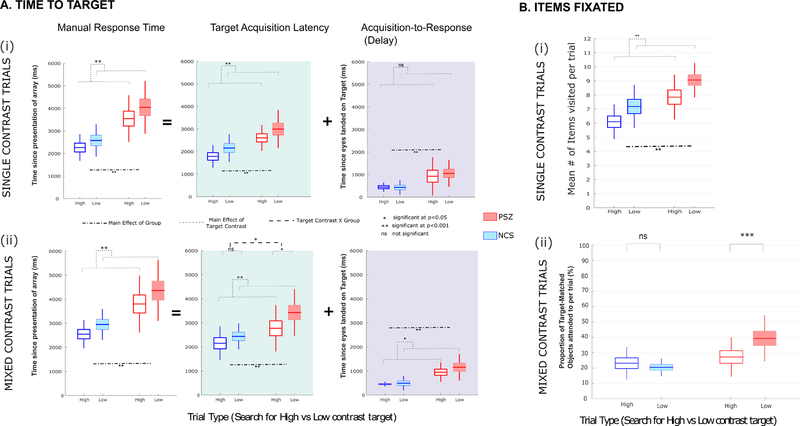
For single-contrast trials [Figure 2A(i), left;Table 2(A(I)], manual RT was significantly slower in both groups for low-contrast arrays than for high-contrast arrays. . Manual RT was also considerably slower in PSZ than in NCS (main effect of Group). The difference of manual RT between high- and low-salience stimuli was slightly but non-significantly greater in PSZ than in NCS (interaction of Group by Contrast).
For mixed-contrast trials [Figure 2A(ii) left;Table 2(B(I)], PSZ were significantly slower than NCS (main effect of group). Both groups were slowed by several hundred milliseconds for low-contrast targets relative to high-contrast targets (significant main effect of target contrast). As predicted, the difference of manual RTs between high- and low-contrast targets was greater in PSZ than in NCS, but the interaction between Target Contrast and Group was not significant for this measure.
Figure 2. A. Time to Target.
Three different measures of timing are depicted. The left side (unshaded) shows the manual RT. The other two measures break the manual RT into two components: the amount of time required for gaze to reach (acquire) the target (target acquisition latency, middle panels; and the delay between target acquisition and the manual response [acquisition-to-response time, right panels]. The top panels depict these measures for Single-contrast trials, and the bottom panel for mixed-contrast trials. Time from onset of search array is shown, separated by trial type. B. Items Fixated: The figure displays the proportion of unique target-mismatched distractors relative to all items fixated.
Target Acquisition Latency.
This was computed as time from the display onset to the time that gaze first reached the target stimulus, such that effective guidance to the target would manifest in shorter latencies.
For single-contrast trials [Figure 2A(i),middle;Table 2(A(II)], both groups took longer to find the target when searching low-contrast arrays versus high-contrast arrays (main effect of Target Contrast). Target acquisition latency was considerably slower in PSZ than in NCS (main effect of Group). The increase in target acquisition latency for low-salience stimuli did not differ between PSZ than in NCS (Nonsignificant interaction between Target Contrast and Group).
For mixed-contrast trials [Figure 2A(ii), middle;Table 2(B(II)], both groups were slowed in target acquisition for low-contrast targets relative to high-contrast targets. PSZ were significantly slower than NCS in acquiring the target. Importantly, the additional time to acquire the target for low-contrast relative to high-contrast targets was greater in PSZ than in NCS (significant main effects of Target Contrast, group and Group by Contrast interaction). Thus, the cost of sifting through high-contrast distractors to find a low-contrast target was greater in PSZ than in NCS, indicative of impaired top-down control of attention.
Delay from Acquisition to Manual Response.
We also computed the delay between acquisition latency and the manual reaction time (Figure 2,right columns). In single-contrast trials [Figure 2A(i);Table 2(A(III))], we found that the delay between target selection and manual button press was minimal in NCS, whereas the delay was substantial in PSZ (significant main effect of Group). For mixed-contrast trials [Figure 2A(ii),right;Table 2(B(III)], the delay between initial fixation on the target and manual response was greater for low-contrast trials, with the PSZ group manifesting much longer delays (main effects of Group and Target Contrast). PSZ thus manifested deficits in response activation even after the target was acquired through visual search, and this impairment was slightly greater when searching for a low-contrast target in a mixed-contrast array. However, the Target Contrast by Group interaction did not reach significance.
Fixation measures
Number of items visited.
As seen in Figure 2B(i), for single-contrast trials, PSZ sampled a significantly higher number of distractor items before finding the target for both low- and high-contrast trials. For both groups, participants fixated fewer items on high-contrast trials than on low-contrast trials, reflecting greater efficiency in discrimination of high-contrast targets from the background. This benefit of high contrast was uniform across both groups for single-contrast trials as evidenced by lack of a significant Group by Contrast interaction effect (Supplementary Materials, Table S2.I.A displays all the statistics)
For mixed-contrast trials, we quantified mean number of distractors scanned, and determined if they were: Target-matched versus Target-mismatched. To quantify search selectivity, we computed the proportion of fixations that were directed to the target-mismatched distractors relative to all items fixated [target-mismatched ÷ (target-matched+target-mismatched), displayed in Figure 2B(bottom)]. For NCS, the proportion of target-mismatched items fixated was uniform across low and high-contrast target search. However, in PSZ, we observed a substantial increase in the proportion of target-mismatched items fixated when searching for a low-contrast target, thus providing further evidence for greater attentional capture by more salient (high-contrast) distractor stimuli. This result was corroborated by a significant Group by Target Contrast interaction when these proportions were submitted to an ANOVA (Table 2.IV). Thus, selectivity is compromised in PSZ when distractors are more salient than target stimuli as reflected in both the latency of target acquisition and in the number of fixations made to irrelevant, but salient distractors. For more detailed statistics on all count-based individual measures, see Supplemental Material, Table S2I).
According to the revisiting hypothesis (e.g.Peterson et al.,2001), in difficult visual search lower search efficiency may occur because participants scan some distractors repeatedly to ensure that they have not missed the target. We thus examined group and trial type differences in the frequency of revisiting an item previously fixated in the mixed-contrast condition. The proportion of target-mismatched items revisited was significantly higher for PSZ in low-contrast trials (Figure S2.A(ii), Table S2.A(iii) in supplemental material). This pattern also held true for dwell times, where PSZ tended to fixate on target-mismatched distractors for longer than NCS (main effect of Group), particularly when searching for a low-contrast target (significant Target Contrast by Group interaction; Figure S2.B and Table S2.B in supplemental materials).
Correlational Analyses.
We examined the relative contributions of dwell times, the fixation measures, and the target acquisition-button press delay to manual RTs using a stepwise (backward) regression approach, including group membership as a potential predictor variable. Figure S3 in Supplemental Material displays relationships between the key measures. Guided by results for proportions of items visited, we focused this analysis on low-contrast target trials in which non-selectivity (assayed by number of target-mismatched items visited) may have contributed to inefficient visual search performance. We tested whether number of target-mismatched, high-contrast items fixated still accounted for variance in RT after removing variance related to selectivity (i.e., Target-matched variables). Besides target acquisition-button press delay (standardized beta, β=0.48,p <0.001), mean number of target-mismatched items emerged as the strongest predictor (β=0.28, p =0.007) of RT, followed by dwell time on target mismatched distractors (β =0.196, p =0.05). (Supplemental Table S3). Using this approach, in an initial regression across both groups with manual RT as the outcome variable, only mean number of target-mismatched items fixated followed by dwell time on target mismatched distractors were significant predictors. When broken down using separate regressions for each group (Table S3.B), we observed that in PSZ, significant predictors also included mean number of target-mismatched items (in addition to target acquisition-button press delay). Only target acquisition-button press delay was significant for NCS. Taken together, these results indicate that impaired visual search performance in PSZ was largely driven by failures in top down control of attention, as revealed by attending to target-mismatched distractors more frequently.
Neurocognitive assessment correlates.
We investigated relationships between guided visual search in the mixed-contrast trials and clinical measures of attention and WM using Pearson correlations using a Bonferroni corrected α of 0.002. Table S4 in supplemental material provides the correlation values. We were particularly interested in correlations with symbol digit substitution because a prior study using eye movements reported prolonged dwell times and more revisits to the key area in PSZ (Elahipanah et al.,2011; Bachman et al.,2010).
Interestingly, in PSZ, the symbol coding measure was strongly associated with proportion of target-mismatched(high-contrast) distractors during non-salient search(r=−0.52,p=0.001). When considering associations at an uncorrected α(0.05), mean dwell time (ms) on target-mismatched distractors(r=−0.37,p=0.03) was also associated with symbol coding. Furthermore, we observed negative correlations between the proportion of target-mismatched distractors visited during non-salient search and the Continuous Performance Task average d-prime (d’) measure(r=−0.59,p<0.001). We also observed a relationship between proportion of target-mismatched distractors visited during non-salient search and overall score on the MCCB (r= −0.42,p=0.013). Thus, these highly specific eye movement measures share variance with overall level of neuropsychological performance as well as with measures of processing speed and sustained attention. It is likely that an underlying impairment in cognitive control is implicated in the deficits in both the eye movement metrics and more general neuropsychological measures, thereby accounting for the correlations between these measurement domains.
DISCUSSION
Clinical observations and experimental studies have long indicated that attention is dysfunctional in PSZ (e.g.McGhie & Chapman,1961;Nuechterlein & Dawson,1984), but the specific nature of this impairment remains unclear. . We have proposed that this impairment reflects a failure of the attentional control mechanisms that determine what sources of information should be attended rather than an impairment in the mechanisms that actually implement the selective processing of these sources (Luck & Gold,2008). Several experiments have now shown that implementation of selection is intact in PSZ under conditions designed to produce minimal challenge on attentional control systems (e.g. Gold et al.,2006,2017). Our goal was to test the hypothesis that impairments would arise when control was challenged by the need to avoid the prepotent tendency to direct attention toward highly salient inputs. Consistent with this hypothesis, we found that PSZ were unimpaired at directing gaze away from nonsalient items when searching for a salient target but were impaired at directing gaze away from salient items when searching for a nonsalient target.
Our results expand previous findings showing diminished visual search efficiency in PSZ (e.g. Gold et al.,2007) by unraveling the source of the RT deficit, specifically, inability to avoid fixating (and revisiting) salient distractors when searching for a nonsalient target. Unlike many cognitive control measures where it is necessary to make inferences about processes underlying performance and the deficits that lead to task failure, measures of gaze direction provide direct evidence of failures in top down control in the face of salient bottom-up competition.
Our results are broadly consistent with studies using paradigms such as the Stroop task (e.g.Hepp et al.,1996), expectancy versions of the AX CPT (e.g.Servan-Schreiber et al.,1996), and anti-saccade tasks (e.g.Fukushima et al.,1988). In all these cases, PSZ demonstrate deficits in the ability to inhibit goal-irrelevant, but highly prepotent responses. However, these paradigms focus on competition in late, motor response processes, and the role of cognitive control in the selection of perceptual inputs has rarely been examined in schizophrenia. Hahn et al.(2010) demonstrated deficits in attentional control over WM encoding when PSZ were to selectively store static stimuli in memory and avoid storing more salient flickering stimuli. Maruff and colleagues(1996) demonstrated, using a covert orienting of attention task, that PSZ were unable to inhibit reflective shifts of attention to salient peripheral cues even though they were aware that the target would never appear at the cued location. Our findings thus contribute to this sparse literature showing deficits in control during the selection of appropriate inputs. It is also noteworthy that eye movement measures were more discriminating of diagnostic group status than were measures of overall manual reaction time, suggesting that eye movement measures may offer a more specific measure of cognitive control processes.
A major ancillary finding was a substantial delay between the time the eyes landed on the target and execution of the motor response in PSZ. This delay likely involves a motor component and a decisional component. RT slowing is widely documented in PSZ(Nuechterlein,1977), and event-related potential studies have shown a deficit in d the lateralized readiness potential (LRP) suggesting slowing in the in selection and preparation of appropriate manual responses(e.g. Kappenman et al.,2012). Additionally, the fact that greater slowing of manual response generation was observed after the eyes landed on a low contrast target (relative to a high contrast target) suggests that perceptual decision-making processes are involved as well. We are unaware of prior evidence implicating this type of post target-acquisition slowing of RT, and additional experiments are needed to evaluate the role of decisional vs. response activation slowing. To rule out a medication confound, we examined correlations between all acquisition-response delay measures and CPZ-equivalent antipsychotic dose but found no suggestive relationships (p>0.56).
Finally, we acknowledge that some caution is needed in extrapolating these results to real-world scenarios. . Future work may include studying visual search in more naturalistic settings and investigating the influence of multiple feature attributes on guidance of attention (Wolfe & Horowitz,2017). This is especially important to unravel when studying visual processing and cognitive control in clinical populations.
Supplementary Material
Acknowledgments
The current study was funded by NIH grant R01MH065034 awarded to JMG and SJL. All participants gave written informed consent before taking part in the study. The protocol was approved by the Institutional Review Board at the University of Maryland, Baltimore. (Protocol No. HP00054557: Attention, Working Memory, and Brain Electrophysiology).
References
- Andreasen NC, Pressler M, Nopoulos P, Miller D, & Ho BC (2010). Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biological psychiatry, 67(3), 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachman P, Reichenberg A, Rice P, Woolsey M, Chaves O, Martinez D, … & Glahn DC (2010). Deconstructing processing speed deficits in schizophrenia: application of a parametric digit symbol coding test. Schizophrenia research, 118(1–3), 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dima D, Dietrich DE, Dillo W, & Emrich HM (2010). Impaired top-down processes in schizophrenia: A DCM study of ERPs. NeuroImage, 52(3), 824–832. [DOI] [PubMed] [Google Scholar]
- Elahipanah A, Christensen BK, & Reingold EM (2011). What can eye movements tell us about Symbol Digit substitution by patients with schizophrenia? Schizophrenia research, 127(1–3), 137–143. [DOI] [PubMed] [Google Scholar]
- Erickson MA, Hahn B, Leonard CJ, Robinson B, Gray B, Luck SJ, & Gold J (2014). Impaired working memory capacity is not caused by failures of selective attention in schizophrenia. Schizophrenia bulletin, 41(2), 366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima J, Fukushima K, Chiba T, Tanaka S, Yamashita I, & Kato M (1988). Disturbances of voluntary control of saccadic eye movements in schizophrenic patients. Biological psychiatry, 23(7), 670–677. [DOI] [PubMed] [Google Scholar]
- Fuller RL, Luck SJ, Braun EL, Robinson BM, McMahon RP, & Gold JM (2006). Impaired control of visual attention in schizophrenia. Journal of abnormal psychology, 115(2), 266. [DOI] [PubMed] [Google Scholar]
- Gaspelin N, & Luck SJ (2017). The role of inhibition in avoiding distraction by salient stimuli. Trends in cognitive sciences [DOI] [PMC free article] [PubMed]
- Gold JM, Fuller RL, Robinson BM, McMahon RP, Braun EL, & Luck SJ (2006). Intact attentional control of working memory encoding in schizophrenia. Journal of Abnormal Psychology, 115(4), 658. [DOI] [PubMed] [Google Scholar]
- Gold JM, Fuller RL, Robinson BM, Braun EL, & Luck SJ (2007). Impaired top–down control of visual search in schizophrenia. Schizophrenia research, 94(1–3), 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Robinson B, Leonard CJ, Hahn B, Chen S, McMahon RP, & Luck SJ (2017). Selective attention, working memory, and executive function as potential independent sources of cognitive dysfunction in schizophrenia. Schizophrenia bulletin [DOI] [PMC free article] [PubMed]
- Green MF, Kern RS, & Heaton RK (2004). Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophrenia research, 72(1), 41–51. [DOI] [PubMed] [Google Scholar]
- Hahn B, Robinson BM, Kaiser ST, Harvey AN, Beck VM, Leonard CJ, … & Gold JM (2010). Failure of schizophrenia patients to overcome salient distractors during working memory encoding. Biological psychiatry, 68(7), 603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson JM, Brockmole JR, Castelhano MS, & Mack M (2007). Visual saliency does not account for eye movements during visual search in real-world scenes. In Eye movements (pp. 537-III).
- Hepp HH, Maier S, Hermle L, & Spitzer M (1996). The Stroop effect in schizophrenic patients. Schizophrenia research, 22(3), 187–195. [DOI] [PubMed] [Google Scholar]
- Horstmann G, Becker S, & Ernst D (2017). Dwelling, rescanning, and skipping of distractors explain search efficiency in difficult search better than guidance by the target. Visual Cognition, 25(1–3), 291–305. [Google Scholar]
- Kappenman ES, Kaiser ST, Robinson BM, Morris SE, Hahn B, Beck VM, … & Luck SJ (2012). Response activation impairments in schizophrenia: evidence from the lateralized readiness potential. Psychophysiology, 49(1), 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, & Maunsell JH (2010). The effect of attention on neuronal responses to high and low contrast stimuli. Journal of neurophysiology, 104(2), 960–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CJ, Robinson BM, Hahn B, Gold JM, & Luck SJ (2014). Enhanced distraction by magnocellular salience signals in schizophrenia. Neuropsychologia, 56, 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, & Gold JM (2008). The construct of attention in schizophrenia. Biological psychiatry, 64(1), 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Fuller RL, Braun EL, Robinson B, Summerfelt A, & Gold JM (2006). The speed of visual attention in schizophrenia: Electrophysiological and behavioral evidence. Schizophrenia research, 85(1–3), 174–195. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Kappenman ES, Fuller RL, Robinson B, Summerfelt A, & Gold JM (2009). Impaired response selection in schizophrenia: evidence from the P3 wave and the lateralized readiness potential. Psychophysiology, 46(4), 776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruff P, Pantelis C, Danckert J, Smith D, & Currie J (1996). Deficits in the endogenous redirection of covert visual attention in chronic schizophrenia. Neuropsychologia, 34(11), 1079–1084. [DOI] [PubMed] [Google Scholar]
- McGhie A, Chapman J. Disorders of attention and perception in early schizophrenia. Br J Med Psychol 1961;34:103–116. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH (1977). Reaction time and attention in schizophrenia: A critical evaluation of the data and theories. Schizophrenia Bulletin, 3(3), 373. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, & Dawson ME (1984). Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophrenia bulletin, 10(2), 160–203. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF. MATRICS Consensus Cognitive Battery, Manual Los Angeles, CA: MATRICS Assessment Inc; 2006. [Google Scholar]
- Peters RJ, & Itti L (2007, June). Beyond bottom-up: Incorporating task-dependent influences into a computational model of spatial attention. In Computer Vision and Pattern Recognition, 2007. CVPR’07. IEEE Conference on (pp. 1–8). IEEE. [Google Scholar]
- Peterson MS, Kramer AF, Wang RF, Irwin DE, & McCarley JS (2001). Visual search has memory. Psychological Science, 12(4), 287–292. [DOI] [PubMed] [Google Scholar]
- Pomplun M (2006). Saccadic selectivity in complex visual search displays. Vision research, 46(12), 1886–1900. [DOI] [PubMed] [Google Scholar]
- Servan-Schreiber D, Cohen JD, & Steingard S (1996). Schizophrenic deficits in the processing of context: A test of a theoretical model. Archives of general psychiatry, 53(12), 1105–1112. [DOI] [PubMed] [Google Scholar]
- Wechsler D Wechsler Abbreviated Scale of Intelligence. (WASI): Psychological Corporation; 1999. [Google Scholar]
- Wolfe JM, & Horowitz TS (2017). Five factors that guide attention in visual search. Nature Human Behaviour, 1(3), 0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinsky G, Zhang W, Yu B, Chen X, & Samaras D (2006). The role of top-down and bottom-up processes in guiding eye movements during visual search. In Advances in neural information processing systems (pp. 1569–1576).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.